Abstract
Although a growing literature points to substantial variation in speech/language abilities related to individual differences in musical abilities, mainstream models of communication sciences and disorders have not yet incorporated these individual differences into childhood speech/language development. This article reviews three sources of evidence in a comprehensive body of research aligning with three main themes: (a) associations between musical rhythm and speech/language processing, (b) musical rhythm in children with developmental speech/language disorders and common comorbid attentional and motor disorders, and (c) individual differences in mechanisms underlying rhythm processing in infants and their relationship with later speech/language development. In light of converging evidence on associations between musical rhythm and speech/language processing, we propose the Atypical Rhythm Risk Hypothesis, which posits that individuals with atypical rhythm are at higher risk for developmental speech/language disorders. The hypothesis is framed within the larger epidemiological literature in which recent methodological advances allow for large-scale testing of shared underlying biology across clinically distinct disorders. A series of predictions for future work testing the Atypical Rhythm Risk Hypothesis are outlined. We suggest that if a significant body of evidence is found to support this hypothesis, we can envision new risk factor models that incorporate atypical rhythm to predict the risk of developing speech/language disorders. Given the high prevalence of speech/language disorders in the population and the negative long-term social and economic consequences of gaps in identifying children at-risk, these new lines of research could potentially positively impact access to early identification and treatment.
Keywords: developmental language disorder, dyslexia, rhythm, risk factor, specific language impairment
1 |. INTRODUCTION
Developmental speech/language disorders have a high prevalence (3–16%) in the population (National Academies of Sciences, Engineering, and Medicine, 2016) but many of these cases are identified late or are not identified at all. For example, epidemiological approaches involving screening large numbers of children consistently show prevalence rates of 7–8% for developmental language disorder (DLD; Tomblin et al., 1997), but only parents of a quarter of these children were aware their child had a speech or language problem. These low identification rates are relevant because speech/language disorders cause life-long difficulties in academic, social, and economic domains (Baker & Ireland, 2007; Beitchman, Nair, Clegg, & Patel, 1986; Cantwell & Baker, 1987; Catts, 1993; Conti-Ramsden, Durkin, Toseeb, Botting, & Pickles, 2018; Hall & Tomblin, 1978; Hubert-Dibon, Bru, Le Guen, Launay, & Roy, 2016; Law, Rush, Schoon, & Parsons, 2009; Paul & Cohen, 1984; Rice, Sell, & Hadley, 1991). Children with dyslexia and DLD are also far more likely to enter into the juvenile justice system (Snow, 2019). Importantly, long-term consequences could be attenuated with more efficient and earlier identification of the disorders and earlier intervention (Bowyer-Crane et al., 2008; Roberts & Kaiser, 2015; Snowling, 2013).
Recent work has highlighted the need to facilitate the identification of speech- and language-related developmental disorders and improve the design of early intervention by exploring risk factors in population-based samples (Raghavan et al., 2018). Converging evidence supports comorbidities among different speech/language disorders (e.g., Dyslexia and DLD; Bishop & Snowling, 2004; Catts, Adlof, Hogan, & Weismer, 2005) and also between speech/language disorders and motor disorders (e.g., developmental coordination disorder [DCD]; Kaplan, Dewey, Crawford, & Wilson, 2001; Scabar, Devescovi, Blason, Bravar, & Carrozzi, 2006; Selassie, Jennische, Kyllerman, Viggedal, & Hartelius, 2005; Zwicker, Missiuna, & Boyd, 2009) or attentional disorders (e.g., attention deficit hyperactivity disorder [ADHD], Box 1; Donaher & Richels, 2012; Kaplan et al., 2001; Kovac, Garabedian, Du Souich, & Palmour, 2001; Mueller & Tomblin, 2012; Redmond, 2016; Selassie et al., 2005; Westerlund, Bergkvist, Lagerberg, & Sundelin, 2002; Zwicker et al., 2009). This research suggests that it is unusual to have discrete, categorical developmental disorders, and that it may be more efficient to search for underlying deficits that can be identified across disorders in large samples of children. In accordance with these results, the possibility of the transdiagnostic approach has arisen in research, diagnosis and treatment of disorders (Mareva & Holmes, 2019).
BOX 1. Glossary of terms.
Attention deficit hyperactivity disorder.
Attention deficit hyperactivity disorder involves difficulties with attention, hyperactivity, and impulsivity (Biederman & Faraone, 2005), and is frequently associated with language impairment (e.g., Randell, Somerville-Brown, & Chen, 2018).
Developmental coordination disorder.
Developmental coordination disorder is characterized by impaired motor abilities, especially related to postural control, motor learning, and sensorimotor coordination that affect quality of life (Zwicker et al., 2009). Difficulties with language processing were also reported for DCD (e.g., Mirabella et al., 2017).
Developmental dyslexia.
Dyslexia is a neurodevelopmental disorder affecting word decoding, which in turn impacts spelling performance and the development of reading fluency (Snowling, 2013).
Developmental language disorder (DLD).
DLD is a disorder affecting language comprehension and/or production, with no known biomedical causes such as brain injury, acquired epileptic aphasia, neurodegenerative disease, cerebral palsy, hearing loss, intellectual disability, or autism spectrum disorder. DLD is likely to persist in middle childhood and beyond (Bishop et al., 2017). In accordance with recent recommendations (Bishop et al., 2017) we use the term developmental language disorder for children who were often referred to as children with specific language impairment in previous studies. Bishop et al. (2017) suggest eliminating the latter term because it is misleading to state that the impairment is specific to language. In addition, they suggest using the term disorder instead of impairment to convey greater seriousness and importance, and the naming consistent with the names of other developmental disorders (Autism spectrum disorder, developmental coordination disorder, attention deficit hyperactivity disorder) and compatible with the two main diagnostic systems (DSM-5 [American Psychiatric Association, 2013] and ICD-11 [World Health Organization, 2018]).
Heritability.
Heritability is the proportion of phenotypic variation that is due to genetic variation. Heritability analyses also allow for a comparison of the relative importance of genes and environment to the variation of traits within and across populations (Visscher, Hill, & Wray, 2008).
Mismatch negativity (MMN).
MMN appears when a regular feature of a sound sequence is infrequently omitted (Kujala, Tervaniemi, & Schröger, 2007; Näätänen, Tervaniemi, Sussman, Paavilainen, & Winkler, 2001).
Pleiotropy.
Pleiotropy occurs when one genetic locus affects more than one trait (Solovieff et al., 2013).
Rapid auditory processing.
The ability to process and categorize fast acoustic changes over a millisecond time range (e.g., detecting changes in pitch of tones when they are presented with very short [70 ms] inter-stimulus intervals; Benasich et al., 2006).
Risk factors for language disorders.
Risk factors are biological or environmental factors that are statistically associated with language disorders, but whose causal relationship to the language problem is unclear or partial (Bishop et al., 2017).
Stuttering.
Stuttering is a speech disorder characterized by frequent occurrences of repetitions or prolongations of sounds, syllables, or words that disrupt the rhythmic flow of speech (World Health Organization, 2010). Language impairments often co-occur with stuttering (Ntourou, Conture, & Lipsey, 2011).
Transdiagnostic approach.
The transdiagnostic approach focuses on underlying mechanisms that are relevant across a class of disorders instead of single diagnostic categories in research, diagnosis and treatment of disorders (see Sauer-Zavala et al., 2017).
In the current paper, we propose that atypical rhythm might be one of the underlying risk factors that has common biological underpinnings with, and may lead to, co-morbid impairments in speech/language processing. This hypothesis will be referred to as the Atypical Rhythm Risk Hypothesis. For the purpose of the Atypical Rhythm Risk Hypothesis, we define atypical rhythm as a general construct capturing any/all of the following terms: Impairments in rhythm/beat/ meter sensitivity, significantly weaker than normal rhythm ability/skill, poor dynamic attending, beat deafness (Sowiński & Dalla Bella, 2013), or time-based amusia (Peretz & Vuvan, 2017). Atypical rhythm can be classified by poor performance on any implicit or explicit perception or production task of rhythm or timing, such as rhythm discrimination, interval discrimination, rhythm, beat, or meter processing, and synchronization or entrainment. Atypical rhythm may also be described as a rhythm impairment or a rhythm disorder. While the underlying neural mechanisms giving rise to different manifestations of typical and atypical rhythm are of great interest (Fiveash, Bedoin, & Tillmann, submitted), in this article, we will primarily focus on the clinical significance of common biological risk factors across different manifestations of atypical rhythm.
In light of recent genetic epidemiological approaches showing shared genetic architecture between related, but clinically distinct traits (e.g., common heritability across many different brain disorders; Anttila et al., 2018), new avenues for the exploration of common risk factors, such as atypical rhythm, can now be pursued and eventually expanded into the genetic domain. There are several genetic and environmental risk factors proposed for speech/language disorders, and presumably, there are other risk factors still to be discovered (e.g., for a multifactorial view of language disorders focusing on dyslexia: Bishop, 2015; focusing on DLD: Bishop et al., 2017). In line with this multifactorial view of language disorders, we propose that, within a pool of risk factors, a generalized rhythm/timing deficit may interact with other genetic or environmental risk factors. We here synthesize evidence linking rhythm to speech/language development and propose an overarching theoretical framework as groundwork for testing the Atypical Rhythm Risk Hypothesis.
If future research supports the Atypical Rhythm Risk Hypothesis, new possibilities for incorporating rhythm tests into clinical practice may open up. For example, using atypical rhythm as a risk factor for the development of speech and language disorders could be beneficial for improving early identification of these disorders. Atypical timing skills can be measured with tasks targeting musical rhythm perception, which can be assessed earlier in development (around 7–10 months, see Kalashnikova, Goswami, & Burnham, 2019, or already in 2- to 3-day-old newborns, see Winkler, Haden, Ladinig, Sziller, & Honing, 2009) than primary symptoms of speech/language disorders (e.g., atypical reading in dyslexia and atypical expressive grammar in DLD that can be assessed at preschool age at the earliest, or atypical speech production in stuttering that can be assessed from age two at the earliest). In addition, rhythm tasks could be included in the language screening of preschool-age children. To develop the hypothesis of musical rhythm processing at infancy and early childhood as a risk factor for speech/language disorders, we will synthesize different lines of research investigating whether (a) musical rhythm and speech/language skills are associated, (b) rhythm is impaired in speech/language disorders and common comorbid attentional and motor disorders, and (c) individual differences in mechanisms underlying rhythm processing skills at infancy are related to language development and the presence/absence of speech/language disorders in childhood. We will also frame this hypothesis within the larger epidemiological literature that has recently experienced a series of methodological advances allowing for large-scale testing of shared underlying biology across clinically distinct disorders. We then outline a series of predictions for future work testing the Atypical Rhythm Risk Hypothesis.
2 |. PERCEPTUAL AND NEURAL MECHANISMS OF HUMAN RHYTHM PROCESSING ABILITY AND THEIR RELATION TO HIGHER-LEVEL LANGUAGE PROCESSES
Temporal regularities are present at multiple hierarchical levels in both music and speech. In the domain of music, this regularity is more salient as temporal intervals of the underlying beat are isochronous, whereas in speech there is a higher variability in intervals (e.g., also referred to as quasi-periodic: Peelle & Davis, 2012). When listening to music, a basic frequency serves as a temporal organizer; it is referred to as the pulse or beat and typically falls between 1 and 2 Hz (see London, 2004). Strong and weak beats are grouped into a hierarchical metrical structure, which is a cognitive construct of the listener (Lerdahl & Jackendoff, 1983). In spoken language, the rhythm of speech is carried by the so-called amplitude envelope, which captures information about duration, rhythm, tempo, and stress of speech (Goswami, 2019; Kotz, Ravignani, & Fitch, 2018; Myers, Lense, & Gordon, 2019). When the amplitude envelope is degraded, speech can become unintelligible (Ghitza, 2012). Similarly to musical rhythm, groupings of strong and weak accented speech events (such as stressed and unstressed syllables) form metrical structures. These stress patterns play a role both in language acquisition (Bernard & Gervain, 2012; de Carvalho, Dautriche, & Christophe, 2016; Dupoux, Pallier, Sebastian, & Mehler, 1997; Gervain & Werker, 2013; Jusczyk, Cutler, & Redanz, 1993) and speech processing (Bion, Benavides-Varela, & Nespor, 2011; Dilley & McAuley, 2008). At this point, we would like to emphasize that when we use the term rhythm processing in the paper both in relation to music and speech, we are also referring to the processing of beat and metrical structures of the stimuli as an aspect of rhythm processing.
Based on accumulating evidence in recent research (summarized below), several theories have outlined shared underlying processes for musical rhythm and speech processing: The dynamic attending theory (DAT; Jones, 1976, 2016; Jones & Boltz, 1989; Large & Jones, 1999), which inspired many of the later theories; the temporal sampling framework (TSF; Goswami, 2011, 2018); the sound envelope processing and synchronization and entrainment to pulse hypothesis (SEP; Fujii & Wan, 2014); the precise auditory timing hypothesis (PATH; Tierney & Kraus, 2014); and the OPERA hypothesis (Patel, 2011, 2012). Fiveash et al. (submitted) highlight three common elements in these theories and propose their combination as crucial for music rhythm and speech processing. (a) All theories emphasize the role of fine-grained auditory processing (precise, low-level processing of the acoustic signal) in music and speech as a necessary element underlying perception and transfer effects between domains. (b) Neural oscillations and their entrainment to the auditory stimuli play a role in structural processing (including hierarchical processing), temporal integration, and prediction of music and speech signals. According to the DAT (Jones, 2019; Large & Jones, 1999), endogenous brain oscillations synchronize with external regularities and predictable cues (e.g., beat or stress), help to structure the auditory input, and focus attention to important elements of the auditory stimulus and its presentation over time (see also Ghitza, 2011; Giraud & Poeppel, 2012; Peelle & Davis, 2012). (c) The role of sensorimotor coupling both in music rhythm and speech/language processing is included in several of these theories. The involvement of motor functions is not surprising in the case of speaking or when moving to music, but interestingly, motor areas are consistently found to be activated during the perception of music (Chen, Penhune, & Zatorre, 2008; Fujioka, Trainor, Large, & Ross, 2012; Gordon, Cobb, & Balasubramaniam, 2018; Grahn & Brett, 2007; Stephan, Lega, & Penhune, 2018) and speech (Glanz et al., 2018; Möttönen, Dutton, & Watkins, 2013; Wilson, Saygin, Sereno, & Iacoboni, 2004), even in the absence of overt movement.
In line with the central role of these three processes, several studies have shown overlapping brain activations in auditory and motor cortices for both musical rhythm and speech/language processing (Chen, Zatorre, & Penhune, 2006; Grahn & Brett, 2007; Keitel, Benwell, Thut, & Gross, 2018; Kotz et al., 2018). Kotz, Schwartze, and Schmidt-Kassow (2009) outlined a network involving frontal (dorsolateral prefrontal cortex), supplementary motor area (SMA) and basal ganglia regions (the pre-SMA-basal ganglia circuit). They suggest that this circuitry is involved in the processing of predictable sensory cues, such as beat in music or word stress/linguistic meter in speech. The authors also emphasize the role of neural oscillations and propose that the pre-SMA-basal ganglia circuit regulates the synchronization of neural oscillations with auditory stimuli and therefore plays a crucial role in predicting when the next event will occur in a sequence. Although cortical oscillations can be reliably measured in humans, oscillatory activity originating from subcortical structures, such as the basal ganglia, cannot be easily isolated. However, primate research has provided evidence that oscillations in the beta frequency band (which have been shown to play a crucial role in rhythm processing: Zanto, Large, Fuchs, & Kelso, 2005) originate from the basal ganglia, suggesting a similar function in humans (Merchant & Bartolo, 2018).
Another line of research emphasizes the shared nature of structural and hierarchical processing in language and music—including rhythm processing. According to Lashley (1951), language and music are both made up of sub-elements that need to be correctly ordered in the temporal domain. The organization of the sub-elements can be described by hierarchical, tree-like structures, in which lower levels are incorporated by higher levels, and are ordered according to specific rules (Fitch, 2017). Fitch and Martins (2014) proposed that the emergence of tree-like syntax in the grammatical structure of language in early humans was an evolutionary turning point that might also have coincided with the emergence of the metrical structure of rhythm in human musicality. In line with this proposal, hierarchical structures in language and music seem to be processed using similar cognitive and neural mechanisms (Patel, 2003, 2008). Although most of the studies investigating shared structural processing between music and language have focused on harmonic syntax or music processing in general (Fiveash, McArthur, & Thompson, 2018; Fiveash & Pammer, 2014; Herdener et al., 2014; Hoch, Poulin-Charronnat, & Tillmann, 2011; Jentschke & Koelsch, 2009; Jentschke, Koelsch, Sallat, & Friederici, 2008; Koelsch, Gunter, Wittfoth, & Sammler, 2005; Kunert, Willems, & Hagoort, 2016; Slevc, Rosenberg, & Patel, 2009; Steinbeis & Koelsch, 2008), recent evidence suggests some associations between linguistic and rhythmic syntax as well (Sun, Liu, Zhou, & Jiang, 2018).
Hierarchically organized neural oscillations, emphasized by several theories of musical rhythm and speech processing, might play a crucial role in the processing of hierarchically organized syntactic structures as well. According to the Metric Binding Theory by Jones (2019), it is the internal entrainment of multiple nested neural oscillators and their binding that support meter processing and enhances temporal predictions. It is possible that the same process might extend to language and allow for higher-level structure learning and processing. This hypothesis is supported by studies showing that neural oscillations entrain not only to physically marked beats and stressed syllables, but also to higher-level structures both in music (e.g., the metrical structure; Nozaradan, Peretz, Missal, & Mouraux, 2011) and language (e.g., syntactic structure; Ding, Melloni, Zhang, Tian, & Poeppel, 2015), which are not necessarily physically present in the signal (Fiveash et al., 2020; Tal et al., 2017). Efficient entrainment, defined here as the precise phase-locking of neural oscillations at the appropriate frequency, to higher-level structures in musical rhythm and language may also lead to improved prediction skills, possibly through attention allocation (e.g., Large & Jones, 1999; Schmidt-Kassow & Kotz, 2009). Entrainment and increased attention to important parts of the signal may not only facilitate temporal predictions (e.g., predicting when something will happen; predictive timing, Friston, 2005), but also lead to better predictions of what will happen next (predictive coding, Friston, 2005; Jones & Boltz, 1989; Koelsch, Vuust, & Friston, 2019). The predictions developed for what and when of incoming input allow for faster and more efficient processing of these events and their underlying structures, whether musical or linguistic. The possibility of such a link between predictive skills in rhythm and language is supported by preliminary evidence showing that children who are impaired in tapping tasks are also worse at making structure-based morpho-syntactic predictions in language (Persici, Stucchi, & Arosio, 2019).
The research findings reviewed above suggest that a shared network underlies musical rhythm and speech/language processing that supports the processing of surface-level features of musical rhythm and speech as well as the processing of syntactic structures in musical rhythm and language (Figure 1). In the following sections, we summarize evidence for associations between musical rhythm and speech/language processing in typical and atypical populations.
FIGURE 1.
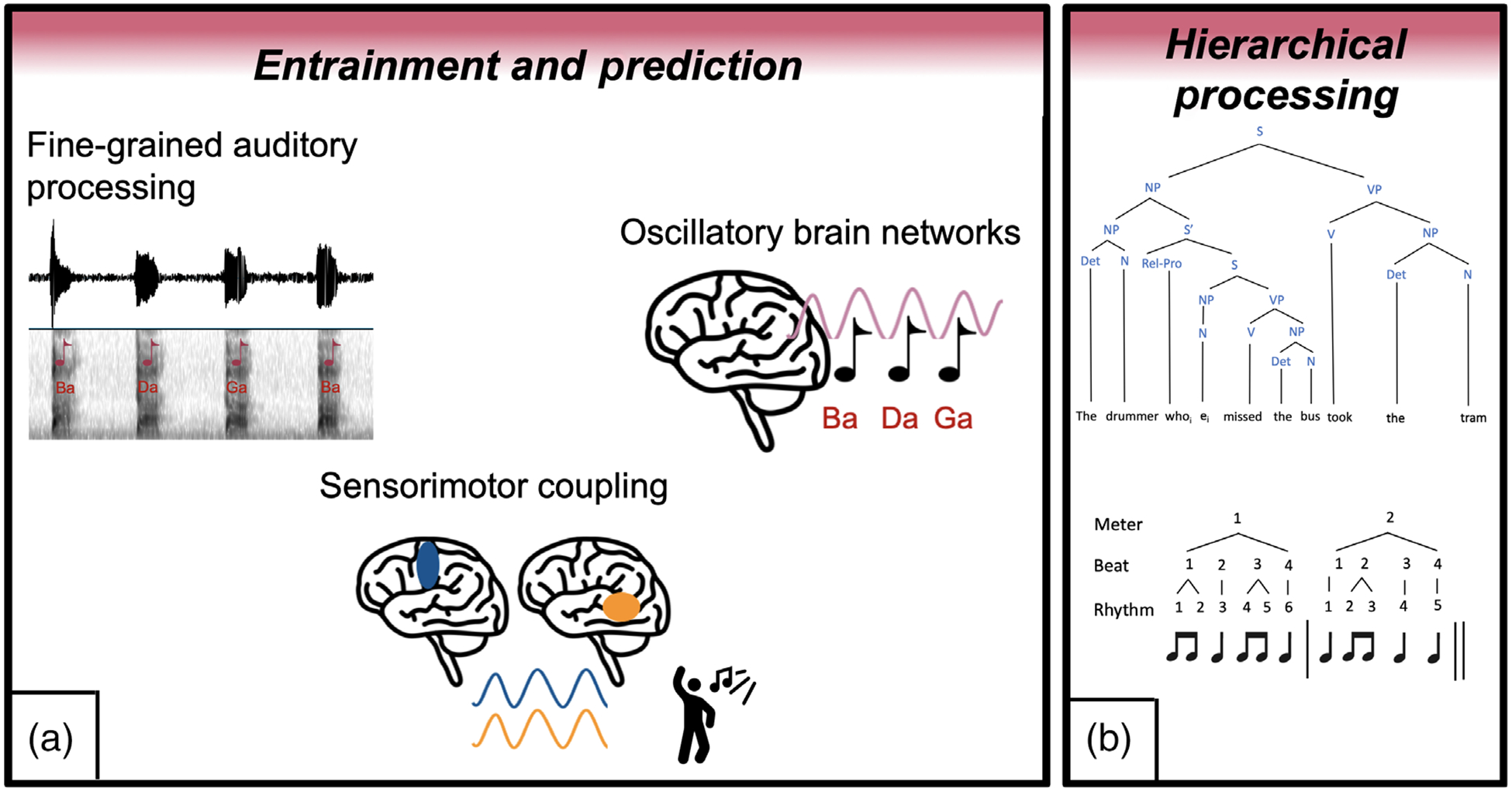
Shared underlying mechanisms for musical rhythm and speech/language processing. (a) Models of underlying mechanisms for musical rhythm and speech/language processing emphasize the role of fine-grained auditory processing, oscillatory brain networks and sensorimotor coupling (Fiveash et al., submitted). (b) Another line of literature emphasizes the shared role of processing of hierarchical structures in both musical rhythm and syntactic processing (e.g., Fitch & Martins, 2014; bottom figure is adapted from Heard & Lee, 2020)
3 |. INDIVIDUAL DIFFERENCES: A SYNTHESIS OF RESEARCH INVESTIGATING ASSOCIATIONS BETWEEN RHYTHM AND SPEECH/LANGUAGE IN TYPICALLY DEVELOPING INDIVIDUALS
Overlapping neural processes underlying musical rhythm and speech/language abilities are supported by a large body of literature showing associations between individual differences in language and rhythm skills (Anvari, Trainor, Woodside, & Levy, 2002; Degé, Kubicek, & Schwarzer, 2015; Douglas & Willatts, 1994; Gordon et al., 2015; Grube, Kumar, Cooper, Turton, & Griffiths, 2012; Holliman, Wood, & Sheehy, 2010; Magne, Jordan, & Gordon, 2016; Moritz, Yampolsky, Papadelis, Thomson, & Wolf, 2013; Ozernov-Palchik, Wolf, & Patel, 2018; Strait, Hornickel, & Kraus, 2011). For instance, beat synchronization and early literacy as well as spoken language skills are strongly linked (see Figure 2 showing data from Woodruff Carr et al., 2014). In addition, there is ample evidence of better performance on various language tasks after rhythm/music training in the typically developing population (Degé & Schwarzer, 2011; Linnavalli, Putkinen, Lipsanen, Huotilainen, & Tervaniemi, 2018; Patscheke, Degé, & Schwarzer, 2016; Rautenberg, 2015; Taub & Lazarus, 2012; Zhao & Kuhl, 2016). Moreover, several studies have found a short-term facilitating effect of regular rhythm on subsequent grammar task performance in typically developing children (Ladányi, Lukács, & Gervain, submitted; Bedoin, Brisseau, Molinier, Roch, & Tillmann, 2016; Canette et al., 2020; Chern, Tillmann, Vaughan, & Gordon, 2018; Przybylski et al., 2013). In addition, better speech/language skills, such as more efficient speech processing and word segmentation, have been reported for musicians compared to non-musicians (Brod & Opitz, 2012; François, Jaillet, Takerkart, & Schön, 2014; Marie, Magne, & Besson, 2011; Musacchia, Sams, Skoe, & Kraus, 2007; Sares, Foster, Allen, & Hyde, 2018; Zuk et al., 2013), although this advantage could originate from other differences between musicians and non-musicians beyond differences in rhythm skills. It is also important to note that individual differences in musical ability or aptitude in adults predict speech perception task performance beyond musical training (Mankel & Bidelman, 2018). Interestingly, evidence extends beyond surface-level auditory characteristics of speech and to deeper, hierarchically structured syntactic processing of language (Gordon, Jacobs, Schuele, & McAuley, 2015; Politimou, Dalla Bella, Farrugia, & Franco, 2019; Woodruff Carr et al., 2014). A complete review of correlations between rhythm and speech/language skills in typical development is beyond the scope of the present paper (see Fiveash et al., submitted).
FIGURE 2.
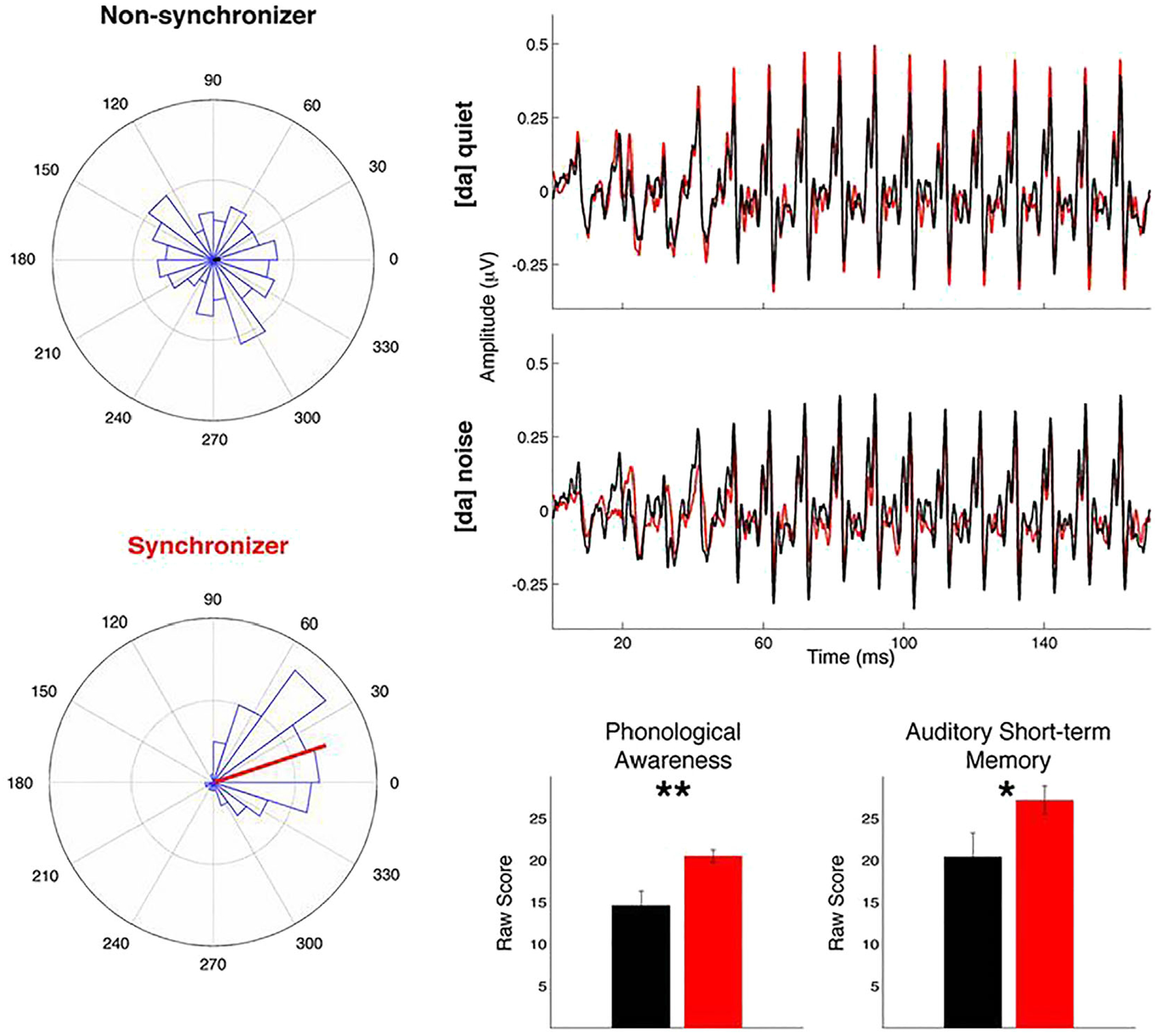
Rhythm production ability and early literacy skills. Convergent evidence across data acquired with various methods, in support of associations between rhythm skills and language in preschool-aged children (Reprinted with permission from Woodruff Carr, White-Schwoch, Tierney, Strait, and Kraus (2014)). Children that performed well on a musical beat synchronization task (here called synchronizers, in red, shown on the rose plots on left to have better drumming accuracy) encoded speech more efficiently (top right) and show significantly better phonological awareness than their non-synchronizer peers (bottom right). Synchronizers also performed better on a sentence repetition task (it is important to note that sentence repetition/imitation tasks not only require auditory perception and short-term memory but also reflect deeper access to the grammatical structure of language: see Klem et al., 2015)
4 |. ATYPICAL RHYTHM IN CHILDREN WITH ATYPICAL SPEECH/LANGUAGE DEVELOPMENT
Associations between rhythm and speech/language processing are strongly supported by recent research demonstrating that children with speech/language developmental disorders (e.g., dyslexia, DLD, stuttering), as well as children with speech/language impairments as co-morbid deficits in other developmental disorders (e.g., DCD, ADHD), often exhibit underlying timing deficits that could be contributing to the symptomatology within each pathology. In each of these disorders, research has revealed some evidence for associated timing impairments, even though specifics of these impairments differ (see a summary of this research in Table 1). Considering that there are high levels of comorbidity between disorders (Bishop & Snowling, 2004; Catts et al., 2005; Donaher & Richels, 2012; Kaplan et al., 2001; Kovac et al., 2001; Mueller & Tomblin, 2012; Redmond, 2016; Scabar et al., 2006; Selassie et al., 2005; Westerlund et al., 2002; Zwicker et al., 2009), it is likely that there are shared impairments in underlying neural mechanisms across different pathologies. We will return to possible etiologies of co-morbidities across these disorders in Section 6; here we focus on atypical rhythm in several highly prevalent developmental speech/language disorders. Together with Fiveash et al. (submitted), we suggest that common deficits in timing may be largely related to impaired fine-grained auditory processing, impaired tracking of rhythms via neural oscillations, and impaired sensorimotor coupling in the brain. We further propose that impaired hierarchical processing could result in both impaired processing of rhythmic structures and syntactic processing of language. Impairment in one or more of these underlying mechanisms appears to be associated with atypical speech/language processing, rhythm processing and/or motor impairments. In addition, we need to consider environmental factors and genetic family history, as further discussed below.
TABLE 1.
Summary of current literature investigating rhythm in children with atypical speech–language development
| Age | Task | Evidence for atypical rhythm? | ||
|---|---|---|---|---|
| Dyslexia | Colling, Noble, and Goswami (2017) | 9–10 years | Beat perception Tapping task | Yes |
| Cutini, Szucs, Mead, Huss, and Goswami (2016) | 12 years | Neural entrainment to amplitude-modulated noise | Yes (2 Hz) | |
| Frey, Francois, Chobert, Besson, and Ziegler (2019) | 10 years | Neural processing of speech sounds in silence, noise, and envelope conditions | Yes | |
| Goswami et al. (2002) | 11 years | Beat detection in amplitude-modulated sounds | Yes | |
| Goswami, Gerson, and Astruc (2010) | 7–13 years | Amplitude envelope onset (rise time) discrimination | Yes | |
| Goswami, Huss, Mead, Fosker, and Verney (2013) | 8–14 years | Beat perception | Yes | |
| Goswami et al. (2013) | 9 years | Syllable stress discrimination | Yes | |
| Goswami et al. (2016) | Discrimination of amplitude rise time | Yes | ||
| Temporal modulations of nursery rhymes | No but impaired acoustic learning during the experiment from low-pass filtered targets | |||
| Hämäläinen, Rupp, Soltész, Szücs, and Goswami (2012) | 19–29 years | Amplitude-modulated white noise | Yes at 2 Hz | |
| Huss, Verney, Fosker, Mead, and Goswami (2011) | 8–13 years | Amplitude envelope rise time perception | Yes | |
| Lee, Sie, Chen, and Cheng (2015) | 9–12 years | Rhythmic imitation | Yes | |
| Leong and Goswami (2014) | <40 years, mean: 22 years |
Rhythmic detection to identify amplitude-modulated nursery rhyme sentences | Yes | |
| Leong, Hämäläinen, Soltész, and Goswami (2011) | 17–41 years | Amplitude envelope onset (rise time) perception and syllable stress detection | Yes | |
| Lizarazu et al. (2015) | Children: 8–14 years; adults: 17–44 years |
Auditory neural synchronization | Yes | |
| Molinaro, Lizarazu, Lallier, Bourguignon, and Carreiras (2016) | Children: 8–14 years; adults: 22–37 years |
Neural synchronization to spoken sentences (MEG) | Yes | |
| Muneaux, Ziegler, True, Thomson, and Goswami (2004) | 11 years | Beat perception (slope) | Yes | |
| Overy (2000) | 6–7 years | Rhythm discrimination Tempo discrimination Meter reproduction | Yes, especially in meter reproduction | |
| Overy, Nicolson, Fawcett, and Clarke (2003) | 7–11 years | Tests of timing skills (rhythm copying, rhythm discrimination, song rhythm, tempo copying, tempo discrimination, song beat) | Yes | |
| Pasquini, Corriveau, and Goswami (2007) | 19–27 years | Rise time perception and temporal order judgment | Yes | |
| Persici et al. (2019) | 9–11 years | Tapping | Yes | |
| Power, Colling, Mead, Barnes, and Goswami (2016) | 12–14 years | Neural entrainment to speech syllables | Yes | |
| Soltész, Szűcs, Leong, White, and Goswami (2013) | Mean: 25.8 years |
Neural entrainment to tones presented at 2 or 1.5 Hz | Yes | |
| Surányi et al. (2009) | 8–9 years | Amplitude envelope rise time discrimination | Yes | |
| Thomson, Fryer, Maltby, and Goswami (2006) | 18–31 years | Basic auditory processing tasks (rise time, duration, and intensity discrimination) | Yes | |
| Tempo discrimination | No | |||
| Tapping (unimanual and bimanual) | Yes but only in the inter-tap-interval variability | |||
| Thomson and Goswami (2008) | 10 years | Rhythmic discrimination | No | |
| Paced and unpaced finger tapping | Yes | |||
| Wang, Huss, Hämäläinen, and Goswami (2012) | 9–10 years | Basic auditory processing tasks (rise time, duration, and intensity discrimination) | Yes | |
| Zuk et al. (2017) | 18–36 years | Speech syllable discrimination | Yes | |
| DLD | Bedoin et al. (2016) | 9–11 years | Rhythm discrimination | Yes |
| Corriveau and Goswami (2009) | 7–11 years | Paced and unpaced tapping | Yes in the paced condition | |
| Corriveau, Pasquini, and Goswami (2007) | 7–11 years | Amplitude envelope rise time and sound duration perception | Yes | |
| Cumming, Wilson, Leong, Colling, and Goswami (2015) | 6–12 years | Beat detection Tapping Speech/music task | Yes, especially in tapping | |
| Goswami et al. (2016) | 9 years | Discrimination of amplitude rise time | Yes | |
| Temporal modulations of nursery rhymes | ||||
| Richards and Goswami (2015) | 8–12 years | Stress perception task | Yes | |
| Richards and Goswami (2019) | 6–11 years | Stress pattern disruptions | Yes | |
| Sabisch, Hahne, Glass, von Suchodoletz, and Friederici (2009) | 8–10 years | Syntactic processing with prosody disruptions | Yes | |
| Sallat and Jentschke (2015) | 4–5 years | Rhythmic–melodic perception task | Yes | |
| Vuolo, Goffman, and Zelaznik (2017) | 4–5 years | Tapping and bimanual clapping | Yes, but only in the bimanual clapping task | |
| Weinert (1992) | 5–8 years | Rhythmic discrimination | Yes | |
| Wells and Peppé (2003) | 8 years | Prosody perception | Yes | |
| Zelaznik and Goffman (2010) | 6–8 years | Tapping and drawing to a metronome | Yes (but no in the timing skill in the manual domain) | |
| Stuttering | Chang, Chow, Wieland, and McAuley (2016) | 6–11 years | Auditory rhythm discrimination task | Yes |
| Falk, Müller, and Dalla Bella (2015) | 8–16 years | Finger tapping | Yes | |
| Olander, Smith, and Zelaznik (2010) | 4–6 years | Metronome clapping | Yes | |
| Toyomura, Fujii, and Kuriki (2011) | 18–55 years | Metronome-timed speech | No (yes in the normal speech condition) | |
| Wieland, McAuley, Dilley, and Chang (2015) | 6–11 years | Simple and complex rhythms discrimination | Yes | |
| DCD | Puyjarinet, Begél, Lopez, Dellacherie, and Dalla Bella (2017) | Children: 6–12 years; adults: 19–50 years |
Duration and beat perception Tapping | Yes |
| Rosenblum and Regev (2013) | 7–12 years | Metronome synchronization | Yes | |
| Trainor, Chang, Cairney, and Li (2018) | 6–7 years | Auditory duration and rhythm discrimination Oddball ERP paradigm | Yes | |
| ADHD | Carrer (2015) | 6–14 years | Rhythmic discrimination | Yes |
| Hove, Gravel, Spencer, and Valera (2017) | 20 years | Paced and unpaced finger tapping | Yes (in the standard task, not in the one with time shifts) | |
| Puyjarinet et al. (2017) | Children: 6–12 years; adults: 19–50 years |
Duration and beat perception Tapping | Yes | |
| Valera et al. (2010) | 10 years | Paced and unpaced tapping | Yes—greater within-subject variability | |
| Zelaznik et al. (2012) | 9 years | Spacebar press following a metronome | Yes |
Abbreviations: ADHD, attention deficit hyperactivity disorder; DCD, developmental coordination disorder; DLD, developmental language disorder.
4.1 |. Atypical rhythm in dyslexia
Recent research has shown that children with dyslexia are impaired in comparison to typically developing children in rhythm perception and production tasks. According to the Temporal Sampling Framework (TSF; Goswami, 2011), many of the processing deficits observed in dyslexia may be accounted for by inefficient entrainment of brain oscillations to sensory input, which in turn is theorized to affect not only rhythm processing but also phonological processing as well as other aspects of language processing. Studies investigating neural entrainment in individuals with dyslexia support this hypothesis by showing deficits in synchronization to the speech envelope (Leong & Goswami, 2014; Molinaro et al., 2016; Power et al., 2016) regardless of the language spoken (e.g., English: Goswami et al., 2010; English and Hungarian: Surányi et al., 2009; Chinese: Wang et al., 2012) and atypical neural entrainment to nonspeech stimuli compared to controls (Cutini et al., 2016; Frey et al., 2019). Further studies have shown impaired beat synchronization in individuals with dyslexia (Colling et al., 2017; Overy et al., 2003; Thomson & Goswami, 2008). Relatedly, rhythm, language, and reading skills are correlated: Individuals with dyslexia who show weaker performance in rhythm perception and production tasks also show weaker phonological awareness (Flaugnacco et al., 2014; Forgeard, Winner, Norton, & Schlaug, 2008; Goswami et al., 2010; Huss et al., 2011; Lee et al., 2015; Thomson & Goswami, 2008) and reading skills (Dellatolas, Watier, Le Normand, Lubart, & Chevrie-Muller, 2009; Flaugnacco et al., 2014; Goswami et al., 2002, 2010; Goswami, Huss, et al., 2013; Goswami, Mead, et al., 2013; Huss et al., 2011; Muneaux et al., 2004; Thomson & Goswami, 2008). Individuals with dyslexia also show impaired processing of rise-time information, and this deficit has been linked to inefficient entrainment of neural oscillations to the speech stream (Goswami et al., 2016; Huss et al., 2011; Leong, Hämäläinen, Soltész, & Goswami, 2011,_S1_Reference170; Thomson et al., 2006). Deficits in neural entrainment to higher-level structures throughout development may also result in impaired hierarchical processing skills. Interestingly, recent research suggests that children with dyslexia perform below age-matched peers in tasks that require the use of morphological information to predict incoming material (Persici et al., 2019).
These phonological and rhythmic deficits do not appear to fully recover later in development, though some studies suggest that deficits in the adult population may be constrained to the type of measure used (Leong & Goswami, 2014). Adults with dyslexia show significantly weaker synchronization and beat perception skills as compared to adults with typical development (Pasquini et al., 2007; Thomson et al., 2006), and exhibit impaired low-frequency neural entrainment, regardless of whether speech (Molinaro et al., 2016) or nonspeech (Hämäläinen et al., 2012; Lizarazu et al., 2015) stimuli were used. As in children, this temporal processing deficit in keeping time with an external stimulus is particularly disrupted at 2 Hz (Soltész et al., 2013), a frequency that is also important for speech perception, as it corresponds to the accented syllabic rate. Musical training may reduce these processing deficits in individuals with dyslexia, as it has been shown that musicians with dyslexia have better auditory temporal processing than nondyslexics (Bishop-Liebler, Welch, Huss, Thomson, & Goswami, 2014) and better amplitude information processing skills than nonmusicians with dyslexia (Zuk et al., 2017).
Building on these observed connections, a few studies have aimed to apply rhythm training approaches in children with dyslexia and found improved language- and reading-related skills after training (Bonacina, Cancer, Lanzi, Lorusso, & Antonietti, 2015; Flaugnacco et al., 2015; Habib et al., 2016; Overy, 2000; Thomson, Leong, & Goswami, 2013). Interestingly, even a short presentation of rhythmic musical primes improves grammatical processing of subsequently presented sentences in children (Przybylski et al., 2013) and adults (Canette et al., 2019) with dyslexia. These results further support the hypothesis that rhythm and language processing are related, and show that music rhythm training in the long-term and rhythm stimulation in the short-term may be useful approaches to improve language skills in addition to more traditional language-centered therapeutic methods (Schön & Tillmann, 2015).
4.2 |. Atypical rhythm in developmental language disorder
Children with DLD show difficulties in both speech and music rhythm processing (Bedoin et al., 2016; Cumming et al., 2015; Sallat & Jentschke, 2015; Weinert, 1992). They have weaker synchronization skills than controls when asked to tap with the beat (Corriveau & Goswami, 2009; Cumming et al., 2015), though synchronization deficits are not observed in all studies (Zelaznik & Goffman, 2010), or for all types of tapping tasks (Vuolo et al., 2017, in which differences in synchronization skills between typically developing and children with DLD were only found when participants were asked to use both hands in a clapping task compared to just one hand). Recent studies have demonstrated the presence of deficits in amplitude envelope and rise-time information processing for children with DLD (Corriveau et al., 2007; Goswami et al., 2016; Richards & Goswami, 2015). Impaired sensitivity to amplitude rise-time has been associated with poor performance on language and literacy measures (such as vocabulary attainment, phonological awareness, and reading; Corriveau et al., 2007) and speech stress processing (Cumming et al., 2015; Richards & Goswami, 2015). Similar patterns in children with DLD and dyslexia led Goswami to extend the TSF to DLD, suggesting shared underlying impairments across disorders (Goswami et al., 2016).
Several studies also reported difficulties in prosody processing in children with DLD compared to TD children (Fisher, Plante, Vance, Gerken, & Glattke, 2007; Richards & Goswami, 2019; Sabisch et al., 2009; Wells & Peppé, 2003), whereas others report intact prosody perception in DLD (Goffman, 2004). Weinert (1992) found that the ability to take advantage of prosodic information in children with DLD was associated with their performance on a rhythm discrimination task, suggesting that impaired processing of prosody and rhythm may be caused by an underlying impairment in the processing of temporal cues.
Similarly to dyslexia, the presentation of a regular rhythmic prime enhances subsequent grammatical sentence judgments in children with DLD compared to both irregular primes (Ladányi et al., submitted; Przybylski et al., 2013) and neutral non-musical auditory primes (Bedoin et al., 2016), supporting the hypothesis that rhythm and language processing are related and suggested that using rhythm in the therapy of children with DLD might facilitate speech/language therapy.
4.3 |. Atypical rhythm in stuttering
Recent research has suggested that speech dysfluency in stuttering is associated with impaired sensorimotor coupling (Chang et al., 2016; Hickok, Houde, & Rong, 2011) and a disruption to the production of timing cues from the basal ganglia (Alm, 2004; Toyomura et al., 2011). Individuals who stutter tend to be impaired in several types of rhythmic tasks, including unpaced tapping, which relies on internal time keeping (Olander et al., 2010). In addition, they show weaker synchronization to an external stimulus (Falk et al., 2015), and poorer rhythm discrimination (Wieland et al., 2015; see Figure 3) than typically developing peers. Impaired predictive timing via sensorimotor coupling has been suggested as the underlying cause of the rhythm deficits reported for individuals who stutter (Hickok et al., 2011). Interestingly, it has been shown that the addition of external auditory stimulation can attenuate stuttering, potentially because it provides an external rhythmic cue to compensate for the impaired internal time keeping (Toyomura et al., 2011). Singing also enhances fluency in speech, likely by regulating the temporal structure of the words (Falk, Maslow, Thum, & Hoole, 2016; Glover, Kalinowski, Rastatter, & Stuart, 1996; Wan, Rüber, Hohmann, & Schlaug, 2010).
FIGURE 3.
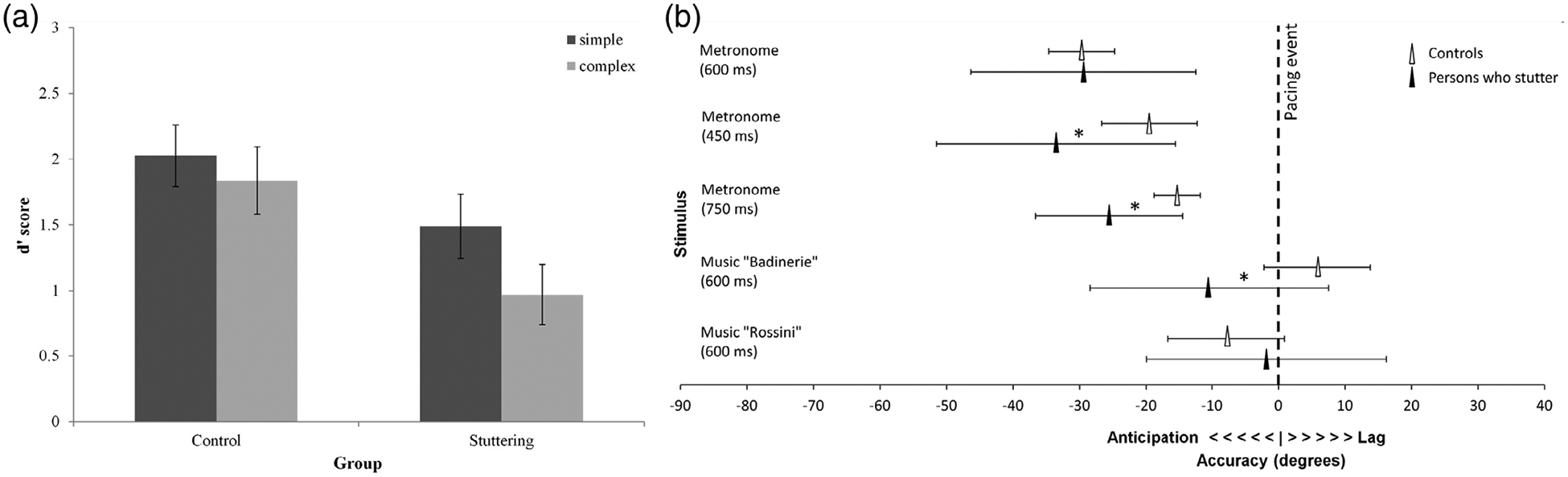
Rhythm perception and production difficulties in children with stuttering. (a) Children who stutter show impaired rhythm perception performance compared to TD children on a task requiring discrimination of simple and complex musical rhythmic sequences (Wieland et al., 2015). (b) Children and adolescents who stutter are less accurate than TD peers on synchronization tests, both tapping to a metronome at certain rates and tapping to the beat in music (Falk et al., 2015). Both figures are reprinted with permission from the original papers
4.4 |. Atypical rhythm in other speech disorders
Although several speech disorders are differentiated in the literature beyond stuttering (speech-sound disorders including articulation/phonological disorder, dysarthria, and childhood apraxia of speech, and voice disorders), most have a known physiological cause (e.g., cleft palate, impaired laryngeal structures, or brain trauma). At the same time, the underlying cause of some forms of articulation/phonological disorder and childhood apraxia is unknown, and they may have partly shared etiology and comorbidities with the other speech/language disorders discussed here. Articulation and phonological sequencing, which requires timing and motor skills, are often impaired in these children. Given the timing demands of sequencing, we believe it would be of great interest to investigate atypical rhythm in these populations. We are aware of one study that explored rhythm processing in individuals with speech difficulties (Alcock, Passingham, Watkins, & Vargha-Khadem, 2000). The authors investigated nine individuals (children and adults) belonging to the same family (KE family) showing both expressive and receptive speech and language impairments together with difficulties with nonverbal oral movements, linked to rare variants in the gene FOXP2. Affected family members performed worse both on rhythm perception and production tasks compared to control participants. Future work is needed to explore rhythm across different motor speech disorders.
4.5 |. Atypical rhythm in developmental coordination disorder
In contrast to numerous studies investigating the motor circuitry involved in musical rhythm processing in typically developing individuals (Merchant, Grahn, Trainor, Rohrmeier, & Fitch, 2015), and atypical rhythm processing in individuals with Parkinson’s Disease (Grahn & Brett, 2009; Harrington, Haaland, & Hermanowitz, 1998; O’Boyle, Freeman, & Cody, 1996),1 only a few studies have examined rhythm processing in children with DCD, a disorder characterized by impaired motor abilities, especially related to postural control, motor learning, and sensorimotor coordination that affect quality of life (Zwicker et al., 2009). Children with DCD show poorer synchronization to an external beat compared to typically developing children in synchronization tasks (Rosenblum & Regev, 2013), and children with both ADHD and DCD show even poorer synchronization compared to children with just ADHD or matched controls (Puyjarinet et al., 2017). However, all of these studies investigated performance in rhythm production tasks, which may be easily affected by inherent motor coordination deficits. Only Trainor et al. (2018) have investigated auditory timing with perceptual tasks in DCD; their first behavioral and neuroimaging evidence suggest that auditory perceptual timing (measured with duration and rhythmic discrimination tasks) may also be impaired in this population. Interestingly, motor impairments in children with DCD have also been associated with difficulties in language processing (Mirabella et al., 2017). Future research should now investigate more specifically the potential timing and/or perception deficits in DCD as well as whether and how the impairments in timing might be related to language processing skills in cases of DCD with atypical language development.
4.6 |. Atypical rhythm in attention deficit hyperactivity disorder
Recent work suggests that both children and adults with ADHD show poorer performance in paced and unpaced tapping and body movement synchronization tasks compared to controls (Amrani & Golumbic, 2019; Carrer, 2015; Hove et al., 2017; Noreika, Falter, & Rubia, 2013; Slater & Tate, 2018; Valera et al., 2010; Zelaznik et al., 2012), especially when synchronization requires beat extraction (Puyjarinet et al., 2017). Though it is difficult to disentangle the role of more generalized attentional deficits from deficits in temporal processing (and in particular, temporal attention and dynamic attending), this emerging literature points to difficulties with both synchronization and internal time-keeping in ADHD (see Falter & Noreika, 2014, for a review). Future work in larger ADHD samples with a variety of rhythm tasks is needed to tease apart various dimensions of rhythm processing, their potential deficits in ADHD, and how they relate to domain-general attentional deficits.
5 |. CAN ATYPICAL RHYTHM AT INFANCY PREDICT ATYPICAL SPEECH/LANGUAGE DEVELOPMENT?
The reported associations between rhythm and speech/language processing as well as atypical rhythm processing in speech/language disorders lead to the hypothesis that atypical musical rhythm processing skills at infancy could be used as a risk factor for speech/language disorders. This type of approach has been employed by Kalashnikova et al. (2019), who showed longitudinal evidence for a predictive relationship between temporal processing (measured with amplitude rise time) at infancy and oral language development. Infants’ performance on an amplitude envelope rise time discrimination task at 7–10 months of age-predicted children’s performance on vocabulary tests at three years of age. To examine this potential predictive relationship between temporal processing and language development further, we first summarize research about rhythm processing in infants to explore whether infants reliably process rhythm and whether it can be measured experimentally. Then, we discuss work exploring individual differences in underlying rhythm processing mechanisms and their relationship with later language development.
Experimental evidence suggests that rhythm processing starts to develop very early in life. A few infant studies showing behavioral (Hannon & Trehub, 2005; Phillips-Silver & Trainor, 2007; Zentner & Eerola, 2010) and electrophysiological (Cirelli, Spinelli, Nozaradan, & Trainor, 2016) evidence indicate that infants and newborns (Winkler et al., 2009) process rhythmic regularities (i.e., beat, meter) in musical stimuli. Infants are also sensitive to the rhythmic cues of speech. Newborns can discriminate between languages from different rhythmic categories (Mehler et al., 1988; Nazzi, Bertoncini, & Mehler, 1998; Ramus, 2000) and discriminate words with different patterns of lexical stress (Sansavini, Bertoncini, & Giovanelli, 1997). Further, infants exploit lexical stress for word segmentation (Dupoux et al., 1997; Jusczyk, 1999) and phrasal level prosody for grammar acquisition (Bernard & Gervain, 2012; de Carvalho, Dautriche, Lin, & Christophe, 2017; de Carvalho et al., 2016; Gervain, 2018; Gervain & Werker, 2013; see electrophysiological data for infant’s sensitivity to speech rhythm in Kalashnikova, Peter, Di Liberto, Lalor, & Burnham, 2018), suggesting an important role of speech rhythm in language development. Based on the research reviewed thus far, we suggest that infants may use the same mechanisms for processing rhythm in the two domains. Future research is needed to compare the benefits of measuring the processing of music rhythm versus speech rhythm in infants in order to predict speech/language development, as each domain has a unique set of constraints and advantages. However, the temporal regularity of music rhythm makes it a useful tool to measure neural entrainment in infants, especially under noisy testing conditions.
Taken together, these studies suggest that rhythm processing is functional from birth, and rhythm skills can be measured both behaviorally and physiologically. Aiming to use atypical rhythm as a risk factor for speech/language disorders also requires knowledge of whether infants show individual differences in rhythm processing and importantly, whether these differences might be related to later speech/language development as well as the presence/absence of speech/language disorders. Although we did not find any studies exploring these questions by measuring musical rhythm processing, numerous studies have investigated infants’ abilities related to fine-grained auditory processing—one of the shared fundamental aspects of rhythm sensitivity that we outlined in the Introduction based on Fiveash et al. (submitted). Neural entrainment of oscillations and sensorimotor coupling has been investigated by some studies, but to the best of our knowledge, no studies have investigated the relationship between rhythm and hierarchical processing of syntactic structures in infants. We are only aware of studies exploring rhythm-related mechanisms at infancy in relation to dyslexia or DLD; therefore, we discuss these results below. While we do not cover stuttering, DCD or ADHD in the remainder of this section, similar logic could be applied to testing the developmental precursors of rhythm processing and their predictive strength for language development in these populations.
5.1 |. Fine-grained auditory processing
The majority of studies exploring fine-grained auditory processing and its relationship to later speech/language development have investigated infants with a family history of language disorders (i.e., dyslexia and/or DLD). Several studies have shown altered neural responses to auditory stimuli in infants with a family history of dyslexia both for verbal stimuli (Leppanen, Pihko, Eklund, & Lyytinen, 1999; Lohvansuu, Hämäläinen, Ervast, Lyytinen, & Leppänen, 2018; Richardson, Leppänen, Leiwo, & Lyytinen, 2003; Thiede et al., 2019; van Herten et al., 2008; van Leeuwen et al., 2006) and nonverbal stimuli (Leppänen et al., 2010; Plakas, van Zuijen, van Leeuwen, Thomson, & van der Leij, 2013; van Zuijen et al., 2012), in comparison to infants without family history of dyslexia. Infants with a family history of language or reading difficulties showed less efficient rapid auditory processing according to both behavioral and electrophysiological measures compared to children without a family history of such difficulties (Benasich & Tallal, 2002; Benasich, Thomas, Choudhury, & Leppänen, 2002; Cantiani et al., 2016; Cantiani et al., 2019; Choudhury & Benasich, 2011; Choudhury, Leppanen, Leevers, & Benasich, 2007; Raschle, Stering, Meissner, & Gaab, 2014). Multiple measures of fine-grained auditory processing at infancy were also associated with individual differences in later language and literacy development (Benasich et al., 2002; Cantiani et al., 2016, 2019; Choudhury & Benasich, 2011; Guttorm et al., 2005; Guttorm, Leppänen, Hämäläinen, Eklund, & Lyytinen, 2010; Kalashnikova et al., 2019; Leppänen et al., 2010; Lohvansuu et al., 2018; van Zuijen, Plakas, Maassen, Maurits, & van der Leij, 2013).
In light of the studies reported above, consistent differences in fine-grained auditory processing between infants with and without a family history of language disorders suggest a shared underlying biology for fine-grained auditory processing and a family history of language disorders. Phenotypic associations occur as a result of a combination of shared genetics and shared environment. Auditory processing shows a moderate to high heritability (32–74%), depending on the exact mechanism measured (Brewer et al., 2016), suggesting a strong genetic component in the phenotypic association between family history of speech/language disorders and fine-grained auditory processing. These results suggest that fine-grained auditory processing is one of the risk factors that may increase risk of language disorder depending on the interplay between this and other risk factors, such as maternal education level or perinatal circumstances (Leppänen et al., 2010; Leppänen et al., 2011).
5.2 |. Oscillatory brain networks
We are aware of only one study investigating oscillatory brain activity in infants and its relationship to later speech/language development (Cantiani et al., 2019). In this study, oscillatory activity was measured in 6-month-old infants with or without a family history of language or reading impairment in a rapid auditory processing paradigm. The authors found a reduction in gamma power in infants with versus without a family history of language or reading difficulties, and concluded that atypical oscillatory activity might explain inefficient rapid auditory processing in infants (Heim, Friedman, Keil, & Benasich, 2011, for gamma oscillations with reduced power and attenuated phase-locking in children with impaired language or reading impairment). In addition, oscillatory measures were associated with expressive vocabulary at 20 months. These results suggest that (a) there is a phenotypic association between inefficient speech/language-related oscillatory activity and familial risk of language and reading disorders, and (b) the efficiency of oscillatory activity during auditory processing is associated with language development, although further research is needed to explore these associations. The relationship of oscillatory activity at infancy with language disorders later in school-aged children has not been investigated up to now.
5.3 |. Sensorimotor coupling
The third shared element underlying rhythm and speech/language processing proposed by Fiveash et al. (submitted) is sensorimotor coupling. We are not aware of any studies measuring the relationship between sensorimotor coupling and language development in infants, but a few studies have explored associations between the role of motor functions in general in infants and their relationship with speech/language disorders. Atypical motor development could also serve as a risk factor for speech/language disorders, as studies show impaired fine motor skills (e.g., in a peg moving task where small pegs are placed as fast as possible from a matrix to a vertical line of target holes) in children with dyslexia (Capellini, Coppede, & Valle, 2010; Gooch, Hulme, Nash, & Snowling, 2014) and DLD (DiDonato Brumbach & Goffman, 2014; Finlay & McPhillips, 2013; Flapper & Schoemaker, 2013; Hill, 2001; Jäncke, Siegenthaler, Preis, & Steinmetz, 2007). We are aware of two studies investigating the associations between motor skills at infancy and later speech/language disorders in the same group of children. Viholainen, Ahonen, Cantell, Lyytinen, and Lyytinen (2002) did not find a difference between motor development (measured by parent questionnaires about reaching developmental milestones) of infants with and without a family history of dyslexia. However, children with both a family history of dyslexia and slow motor development at infancy showed weaker language skills at 18 months (Viholainen et al., 2002) as well as slower reading at 7 years of age (Viholainen et al., 2006) than infants without a family history of dyslexia or with a family history of dyslexia but with fast motor development. Taken together, there is mixed evidence for motor impairments in individuals who develop speech/language disorders; further studies in larger samples are needed to disentangle these factors.
The research on infants reviewed here also suggests that the three mechanisms outlined above (fine-grained auditory processing, neural entrainment, and sensorimotor development) are related to speech/language development. Research still needs to determine whether hierarchical processing in infants is related to later speech/language disorders. Even though an impairment in a single domain does not seem to have a discrete oneto-one mapping to specific disorders, we believe that these findings are promising for the use of musical rhythm processing as a potential risk factor, in part because it involves each of the three mechanisms (and potentially other processes shared by musical rhythm and language processing, e.g., precision, emotion, repetition, and attention, see Patel, 2011). Therefore, it is possible that musical rhythm has a stronger association with speech/language disorders than the three mechanisms independently.
6 |. THE ATYPICAL RHYTHM RISK HYPOTHESIS
In light of the evidence reviewed thus far, we propose the Atypical Rhythm Risk Hypothesis, which posits that individuals with atypical rhythm processing are at higher risk for developmental speech/language disorders. We would like to emphasize that we do not assume that infants with impaired musical rhythm processing will definitively develop a speech/language disorder. Rather, we believe that impaired timing skills measured through music rhythm processing can serve as one risk factor in the prediction of speech/language disorders, in combination with other risk factors both known and still to be determined (Smoller et al., 2019). In practical clinical situations, early screening of rhythm processing with nonverbal, musical material might allow for referral to appropriate speech therapy services for additional testing if atypical rhythm is detected. Broad-based screening of rhythm as a risk factor could offer multiple advantages. First, rhythm skills are likely less affected by the language environment, thus eliminating false positives often occurring in the case of bilingual children when language screeners are used. Second, atypical rhythm may be an indicator of risk for several different speech and language disorders, whereas currently available speech/language screenings tend to be geared toward separate disorders (and thus a child screened for speech difficulties may have an undetected language problem). Third, simple computer-based rhythm assessments could be administered to preschoolers and school-aged children by various professionals (teachers, nurses, school counselors, pediatricians) who do not have specialized Speech–Language Pathology expertise, and then a much smaller number of the children showing atypical rhythm could be referred for SLP assessment, thus optimizing the use of resources. Therefore, our Hypothesis could affect clinical practice first in the screening of preschool-aged and school-aged children, and then could be extended to infant screening when more research and reliable rhythm tests will be available for infants. There are multiple existing behavioral paradigms for measuring rhythm abilities in older children and in adults that could potentially be used for screening. For instance, in rhythm discrimination paradigms (e.g., Gordon, 1979; Law & Zentner, 2012), participants are presented with rhythmic excerpts and asked to decide whether they are the same or different. Tasks from the Battery for the Assessment of Auditory Sensorimotor and Timing Abilities (BAASTA; Dalla Bella et al., 2017) such as tapping in synchrony with the beat of music or deciding whether a beat superimposed onto music is aligned with the beat of the music (see also the Beat Alignment Test, Iversen & Patel, 2008, and the child extension in Einarson & Trainor, 2016) could also inspire screening tasks. Future research should aim to develop rhythm tests with high test–retest reliability for even younger children and for infants.
Recent advances in population genetics analysis methods have highlighted the challenges and opportunities in identifying underlying causal biology that can account for clinically co-morbid conditions of complex traits, such as ADHD, depression, and many other psychiatric disorders (Demontis et al., 2019; Smoller et al., 2019; Tylee et al., 2018). Language and music phenotypes are complex traits, meaning they do not follow a Mendelian pattern of inheritance (Ruiz-Narváez, 2011). The heritability of complex traits is polygenic, that is, involving common genetic variants widely distributed across the genome (Wray et al., 2014), and very large sample sizes are needed to investigate the genetic basis of these traits (Deriziotis & Fisher, 2017; Niarchou et al., 2019). Cross-trait genetic correlation approaches in particular (see Bulik-Sullivan et al., 2015; Turley et al., 2018; Yang, Lee, Goddard, & Visscher, 2011) have revealed a surprising amount of shared underlying genetic architecture (pleiotropy; Solovieff, Cotsapas, Lee, Purcell, & Smoller, 2013) across a spectrum of neurodevelopmental and other health disorders (Anttila et al., 2018; Okbay et al., 2016; Watanabe et al., 2005). Recent findings of pleiotropy between ADHD and literacy development (Gialluisi, Andlauer, Mirza-Schreiber, et al., 2019; Verhoef et al., 2019) align with epidemiological evidence of comorbidity (Mueller & Tomblin, 2012), and pave the way for testing the presence and function of underlying shared deficits, such as atypical rhythm processing. The likelihood and feasibility of examining pleiotropy between rhythm and speech/language traits is demonstrated by recent reports of genetic correlations between rhythm and other cognitive and motor traits (i.e., processing speed and grip strength; Niarchou et al., 2019).
Family-based studies have shown that musical rhythm skills are moderately heritable (50%; Ullén, Mosing, Holm, Eriksson, & Madison, 2014), and our ongoing work with genome-wide approaches in a large sample now point to highly polygenic genetic architecture of musical rhythm (Niarchou et al., 2019). While the heritability of musicality traits is a relatively recent area of inquiry (see Gingras, Honing, Peretz, Trainor, & Fisher, 2015, for a review), more is known about the heritability of speech and language abilities. Family history of speech/language disorders has been identified as one of the strongest risk factors for speech/language disorders in offspring. In particular, the literature converges to show moderate to high heritability of speech and language abilities and in particular, of speech and language disorders (dyslexia: Friend, DeFries, & Olson, 2008; Harlaar, Spinath, Dale, & Plomin, 2005; Kirkpatrick, Legrand, Iacono, & McGue, 2011; DLD: Bishop & Hayiou-Thomas, 2008; Conti-Ramsden, Falcaro, Simkin, & Pickles, 2007; Hayiou-Thomas, 2008; stuttering: Dworzynski, Remington, Rijsdijk, Howell, & Plomin, 2007; van Beijsterveldt, Felsenfeld, & Boomsma, 2010; see Deriziotis & Fisher, 2017, for a review). Importantly, different developmental speech/language disorders as well as speech/language disorders and ADHD tend to co-occur in families (e.g., Carroll & Myers, 2010; Flax et al., 2003; Kovac et al., 2001; Lahey & Edwards, 1995; Mueller & Tomblin, 2012).
As reviewed in earlier sections, there is also converging evidence across approaches and populations for phenotypic correlations between rhythm and speech/language development. Given that phenotypic correlations are generally shown to be driven by some underlying genetic correlation or pleiotropy (Sodini, Kemper, Wray, & Trzaskowski, 2018), it is entirely possible that rhythm and speech/language development share some of their genetic architecture and are mediated through some degree of shared neural architecture. Genetically driven relationships between musical rhythm and speech/language phenotypes might be driven by pleiotropy, such that a common set of causal genes affects both phenotypes directly (Figure 4a), or mediated genetic pleiotropy (Figure 4b, c). In the case of biological pleiotropy, the same set of genes would affect the development of cortical and subcortical structures underlying musical rhythm processing and speech/language processing. Mediated genetic pleiotropy could occur in both directions: Genes might directly affect rhythm phenotypes, and then those phenotypes affect individual differences in acquisition of speech/language during development (i.e., via enhanced rhythm skills; Figure 4b), or genes could affect speech and language development, which affects the development of musical rhythm skills (Figure 4c); however, we believe this latter case is unlikely given the evidence from training studies reviewed in this article, and the more precise timing necessary for music rhythm processing (see Patel, 2011; Tierney & Kraus, 2014). Future research should investigate the genetic architecture of phenotypic associations between musical rhythm and speech/language processing.
FIGURE 4.
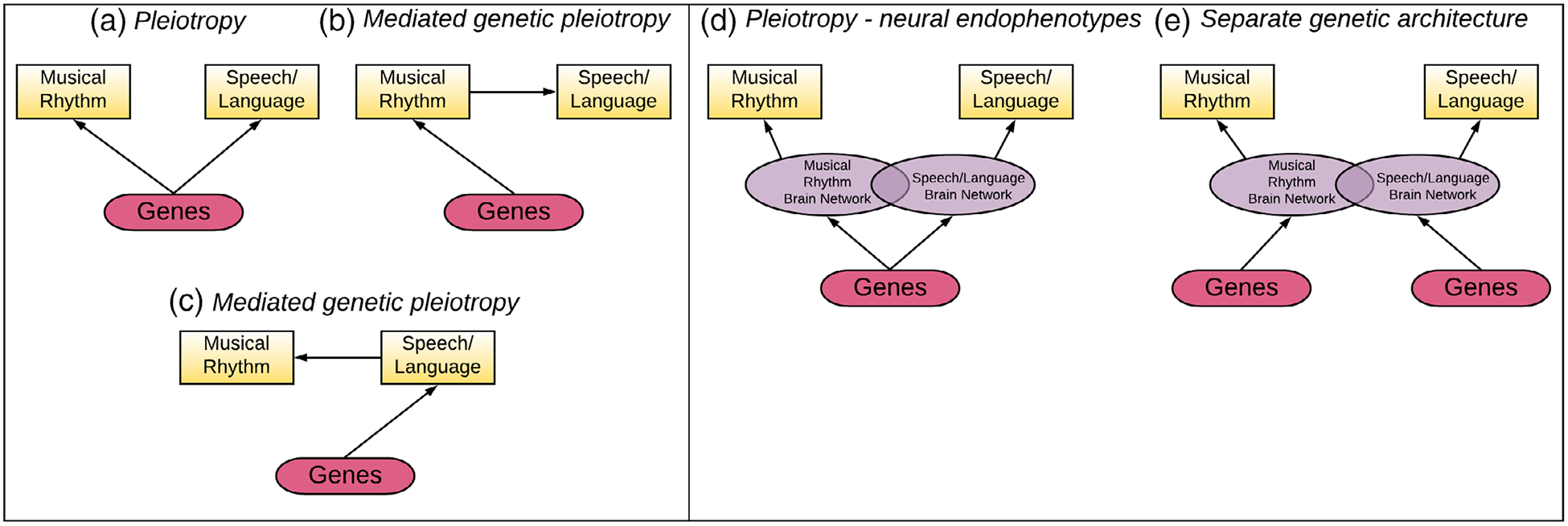
Pleiotropy scenarios for shared versus separate genetic architecture of rhythm and speech/language. The Atypical Rhythm Risk hypothesis predicts that associations between rhythm and speech/language are (a) in part driven by genetic pleiotropy, such that a common set of causal genes affects both phenotypes directly, or (b) mediated genetic pleiotropy, such that genes directly affect rhythm phenotypes, and those phenotypes in turn affect individual differences in acquisition of speech/language during development, or (c) genes directly affect speech/language phenotypes, and those phenotypes affect individual differences in rhythm development. These models should be tested against the null hypothesis of separate genetic architecture. Moreover, a key to understanding the dynamics between genes, brain and behavior will be to test mediating neural endophenotypes linked to (d) shared or (e) separate genetic architecture
It will also be important to investigate neural endophenotypes (corresponding to overlapping brain networks/mechanisms recruited by both music and speech), that could mediate the relationship between genes, brain, and behavior: A common set of genes may give rise to endophenotypic variation in the brain, that then in turn affects individual variation in both rhythm and language phenotypes (Figure 4d). It is also possible that individual variation in rhythm and language is driven by separate sets of genes, and that phenotypic correlations arise solely due to overlapping brain networks (separate genetic architecture, shown in Figure 4e). Statistical testing of these models will be necessary to disentangle the direction of causation for reported links between atypical rhythm and disordered speech/language acquisition.
The exact mechanisms by which phenotypic relationships are driven are also yet to be understood. If mediated pleiotropy underlies the phenotypic associations between musical rhythm and speech/language processing, it has to be determined what are the exact mechanisms driving the relationship and in what direction. For example, it has been suggested that sound envelope processing as well as synchronization and entrainment to the pulse are shared between music and speech rhythm (SEP hypothesis; Fujii & Wan, 2014), and that precise auditory timing via entrainment to music rhythm can have a positive influence on language processing (PATH hypothesis, Tierney & Kraus, 2014; see also Section 2). One possible scenario (Figure 4b) explaining this relationship would be that allelic variation in genes associated with typical (vs. atypical) rhythm (Niarchou et al., 2019) is involved in the development and maintenance of certain auditory-motor pathways in the brain, that are recruited during rhythmic synchronization and auditory timing, thus enhancing sensitivity to linguistic features of the speech signal and bolstering acquisition of grammar and phonology. In parallel, allelic variation associated with atypical rhythm could result in less-than-optimal development of auditory-motor pathways (measurable via poorer rhythm task performance), resulting in reduced sensitivity to phonological and grammatical information in the speech signal, and increasing the probability of dyslexia or developmental language disorder.
When new studies are deployed to test the Atypical Rhythm Risk Hypothesis, it will also be important to incorporate other risk factors for speech/language disorders. Among the several other risk factors that have been investigated for speech/language disorders (see Mascheretti, Andreola, Scaini, & Sulpizio, 2018), maternal education (Ozernov-Palchik & Gaab, 2016; Sun et al., 2013; Zhao, Zhang, Chen, Zhou, & Zuo, 2016) and, even more so, home literacy environment, seem to be the most important for development dyslexia (Dilnot, Hamilton, Maughan, & Snowling, 2017; Sénéchal & LeFevre, 2002; Storch & Whitehurst, 2001; Sun et al., 2013; Torppa, Eklund, van Bergen, & Lyytinen, 2015; Torppa, Poikkeus, Laakso, Eklund, & Lyytinen, 2006; van Bergen, van der Leij, & de Jong, 2014; Zhao et al., 2016). Preterm birth and birth weight are also found to be risk factors for later language development (Dilnot et al., 2017; Liu et al., 2016; Samuelsson et al., 2006). In DLD, low maternal education level, low 5-min Apgar score, being a male and not being a first child were consistently found to be risk factors according to a meta-analysis (Rudolph, 2017). In stuttering, preterm birth or harmful events before or at birth were proposed as risk factors (Ajdacic-Gross et al., 2010; Stromswold, 2006); less clear is the role of socioeconomic status for this disorder (Yairi & Ambrose, 2013). However, there is strong evidence that all of the risk factors listed in this paragraph arise from gene–environment interactions, and thus it is difficult to dissociate them from other genetic risk factors for speech/language disorders without carefully designed genetic models. Unfortunately, very large-scale population cohort studies such as UK Biobank have generally not included speech/language or musical variables in their massive data collection efforts to date, although genome-wide summary statistics on educational attainment, SES, and preterm birth are now widely available and could be incorporated into novel studies outlined here.
Although we believe that the Atypical Rhythm Risk Hypothesis is a promising view which could facilitate the identification of speech/language disorders, some questions might arise for the reader. One could ask whether associations between musical rhythm and speech/language processing may be explained by other shared processes, such as intelligence, working memory or other general cognitive functions. Although these processes are definitely involved in both domains, it does not undermine the Atypical Rhythm Risk Hypothesis. First, associations between musical rhythm and speech/language processing were found to be associated after controlling for the variance in general cognitive measures (e.g., Gordon, Shivers, et al., 2015). Second, if musical rhythm at infancy or early childhood proves to be a sufficient risk factor for speech/language disorders, for practical purposes it is irrelevant what the underlying shared processes are. One could also ask why we propose that a non-linguistic impairment might contribute to speech/language disorders. Even though this contradicts some prevalent domain-specific views about speech/language disorders, it is in line with the contemporary view of speech/language disorders stating that not only linguistic processes are impaired in these populations (e.g., Hill, 2001; Ullman & Pierpont, 2005). The reader could also wonder how rhythm tests would be integrated in clinical practice; we intentionally aim to be cautious and only focus in the current paper on research that must first be conducted before the potential integration of rhythm screeners into clinical practice. If research findings result in support of rhythm tests as a screener, the details of such implementation into clinical practice should be determined by experts from developmental research, speech–language pathology, and policy-makers depending on local and national systems in place.
7 |. CONCLUSION
Inefficient identification of speech/language problems has academic, social, and economic consequences both for the affected individuals, their families, and society (e.g., Conti-Ramsden et al., 2018; Snow, 2019). In the current paper, we reviewed evidence motivating the Atypical Rhythm Risk Hypothesis, which posits that individuals with atypical rhythm are at higher risk for developmental speech/language disorders. We reviewed different lines of research suggesting (a) shared underlying processes for musical rhythm and speech/language processing, (b) associations between musical rhythm and speech/language processing in typically developing populations and impaired musical rhythm processing in children with developmental disorders affecting speech/language skills, and (c) individual differences in mechanisms underlying rhythm processing in infants, which were associated with later speech/language development.
The Atypical Rhythm Risk Hypothesis and its theoretical framework presented here allow us to generate a series of predictions (presented in Box 2) about co-morbidities between rhythm and speech/language disorders and the shared underlying biology from genes to brain to behavior. For instance, one prediction of our theory is that if a screener for atypical rhythm is administered to a large population, individuals with rhythm deficits would show a higher prevalence of speech/language disorders. Associations between rhythm-related processes at infancy and language development reported by Kalashnikova et al. (2019) provide initial supporting evidence for this hypothesis. Furthermore, there is already some evidence for statistically increased prevalence rates of atypical rhythm in association with developmental disorders (Peretz & Vuvan, 2017) from a study of over 16,000 individuals, which identified 2.7% of their sample as “time-based amusics” and an additional 3.4% of the sample with general amusia/uncategorized deficits that included poor rhythm performance. In particular, the time-based amusia group had higher prevalence of dyslexia, speech disorders, and attentional disorders than controls, consistent with the framing of atypical rhythm as a risk factor across these disorders. Although the contribution of pitch-based amusia and deficits in other aspects of music perception to atypical speech/language development (i.e., Couvignou, Peretz, & Ramus, 2019) is beyond the scope of the current review, other elements of musicality should certainly be considered in the broader context of an influence of rhythm, melody, timbre, or harmony on speech and language development (see Brandt, Gebrian, & Slevc, 2012, for a model of how “musical hearing” may scaffold language acquisition).
BOX 2. Cumulative evidence and predictions of the Atypical Rhythm Risk Hypothesis for future research.
Current evidence for the Atypical Rhythm Risk Hypothesis
Individual differences in rhythm ability are associated with speech/language skills as well as task performance in TD children and adults; there is some overlap in neural circuitry.
Associations are found among underlying mechanisms of rhythm processing: fine-grained auditory processing, entrainment of neural oscillatory networks, motor function, and hierarchical processing; all are used in speech perception/production.
Rhythm impairments are prevalent in children with atypical speech-language development (e.g., dyslexia, DLD, stuttering) and in commonly co-morbid motor and attentional disorders.
Predictions of the Atypical Rhythm Risk Hypothesis
- Individual differences approaches
- Individual differences in rhythm sensitivity (measured across the lifespan with various behavioral and physiological methods) will predict significant variance in speech/language skills cross-sectionally and longitudinally. When assessing rhythm in infants, it will be important to discover the youngest age range at which atypical rhythm could predict risk for later speech/language disorders.
- Rhythm screening approaches
- We predict higher prevalence of speech/language disorders and more generally, lower linguistic performance/skills among individuals (children, adults) who show poor performance on rhythm tasks
- Young children with atypical rhythm sensitivity are more likely to develop speech/language disorders.
- Rhythm screening tests could eventually be used clinically to refer to Speech/Language Pathology services for more extensive testing.
- If the rhythm screening approach turns out to be promising, intervention programs, potentially incorporating a rhythm component, could be tested.
- Family-based predictions
- Parents with atypical rhythm sensitivity are more likely to have children with atypical rhythm sensitivity and who develop speech/language disorders.
- Predictive power could be improved by combining atypical rhythm and other risk factors for speech/language disorders into a “risk score”.
- Genetic correlations between rhythm and speech/language phenotypes
- We predict some degree of shared genetic architecture between rhythm and speech/language development that would account for phenotypic correlations.
- Shared underlying genetic architecture could affect overlapping neural circuitry as an endophenotype between rhythm and speech/language development.
- A causal influence of rhythm on language acquisition can be assessed with genetic modelling (See Figure 4), and alternative models can be compared.
- Gene-environment interactions could also be assessed with twin models or other family-based methods if there is data on speech/language and rhythm phenotypes available within a given (large) cohort.
The Atypical Rhythm Risk Hypothesis is in line with the transdiagnostic approach (Mareva & Holmes, 2019) emphasizing the need for large-scale epidemiological studies (e.g., Raghavan et al., 2018). This work needs to incorporate various known and to-be-determined risk factors into prediction models and disentangle gene–environment interactions, intermediary neural endophenotypes, and underlying biological mechanisms. Once genome-wide data for rhythm and language phenotypes from large enough samples are available, recently developed methods such as two-sample Mendelian Randomization (Zhu et al., 2018) may be used to begin to identify the hypothesized causal influence of rhythm on speech/language development (even when measured in separate samples) and to model other contributing variables. With new population-based efforts to assess individual differences in rhythm and speech/language abilities in tens and hundreds of thousands of participants (i.e., Niarchou et al., 2019, and ongoing work by the GenLang consortium), exploration of the hypothesized shared genetic architecture among these traits and other risk factors (e.g., Watanabe et al., 2005) is on the near horizon.
If a significant body of experimental evidence is found in favor of the hypothesized association between atypical rhythm and speech/language disorders, we can envision new risk factor models that incorporate atypical rhythm processing. Measuring rhythm processing could serve as a simple, easy-to-administer prescreening test that can be conducted with young infants and children and even in parents to identify familial risk of atypical rhythm. These screening efforts could be used as a tool to increase referrals to appropriate speech/language pathology services with the end goal of closing the gap in the identification and increasing access to early intervention to maximize long-term impact.
ACKNOWLEDGMENTS
The authors would like to thank Nancy Cox, Daniel Gustavson, Miriam Lense, Olivia Boorom, Catherine Bush, and J. Devin McAuley for fruitful discussions about the theoretical framework; Anna Kasdan and Rachana Nitin for assistance with figures; and Alyssa Scartozzi for assistance with editing. This project was supported by funding from the National Institute on Deafness and Other Communication Disorders, the Office of Behavioral and Social Sciences Research, and the Office of the Director, of the National Institutes of Health under Award Numbers DP2HD098859, R01DC016977, K18DC017383, and R03DC014802, and was supported by funding from the National Science Foundation (NSF 1926794) and an ANR grant (Grant Agreement number ANR-16-CE28-0012). The team “Auditory cognition and psychoacoustics” is part of the LabEx CeLyA (“Centre Lyonnais d’Acoustique”, ANR-10-LABX-60). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
Note that musical rhythm processing, speech/language functions, and motor impairments were reported in nondevelopmental disorders such as Parkinson’s disease and in acquired brain injuries (e.g., Ariatti, Benuzzi, & Nichelli, 2008; Grahn & Brett, 2009; Kotz & Gunter, 2015; Smith & Caplan, 2018). The investigation of the relationship between these impairments has a theoretical importance as well as clinical relevance. This article and the Atypical Rhythm Risk Hypothesis, however, focus on developmental disorders, and the extension to nondevelopmental disorders is beyond the scope of the paper.
REFERENCES
- Ajdacic-Gross V, Vetter S, Müller M, Kawohl W, Frey F, Lupi G, … Rössler W (2010). Risk factors for stuttering: A secondary analysis of a large data base. European Archives of Psychiatry and Clinical Neuroscience, 260(4), 279–286. 10.1007/s00406-009-0075-4 [DOI] [PubMed] [Google Scholar]
- Alcock KJ, Passingham RE, Watkins K, & Vargha-Khadem F (2000). Pitch and timing abilities in inherited speech and language impairment. Brain and Language, 75(1), 34–46. 10.1006/brln.2000.2323 [DOI] [PubMed] [Google Scholar]
- Alm PA (2004). Stuttering and the basal ganglia circuits: A critical review of possible relations. Journal of Communication Disorders, 37(4), 325–369. 10.1016/j.jcomdis.2004.03.001 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: APA. [Google Scholar]
- Amrani AK, & Golumbic EZ (2019). The Preferred Period Hypothesis Revisited: Rhythmic Preferences and Motor Tapping Precision in ADHD Adults and Controls. BioRxiv, 2019, 887802 10.1101/2019.12.24.887802 [DOI] [Google Scholar]
- Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, Duncan L, … Neale BM (2018). Analysis of shared heritability in common disorders of the brain. Science, 360(6395), eaap8757 10.1126/science.aap8757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anvari SH, Trainor LJ, Woodside J, & Levy BA (2002). Relations among musical skills, phonological processing, and early reading ability in preschool children. Journal of Experimental Child Psychology, 83(2), 111–130. 10.1016/s0022-0965(02)00124-8 [DOI] [PubMed] [Google Scholar]
- Ariatti A, Benuzzi F, & Nichelli P (2008). Recognition of emotions from visual and prosodic cues in Parkinson’s disease. Neurological Sciences, 29(4), 219–227. 10.1007/s10072-008-0971-9 [DOI] [PubMed] [Google Scholar]
- Baker SF, & Ireland JL (2007). The link between dyslexic traits, executive functioning, impulsivity and social self-esteem among an offender and non-offender sample. International Journal of Law and Psychiatry, 30(6), 492–503. 10.1016/j.ijlp.2007.09.010 [DOI] [PubMed] [Google Scholar]
- Bedoin N, Brisseau L, Molinier P, Roch D, & Tillmann B (2016). Temporally regular musical primes facilitate subsequent syntax processing in children with specific language impairment. Frontiers in Neuroscience, 10, 245 10.3389/fnins.2016.00245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitchman JH, Nair R, Clegg M, & Patel PG (1986). Prevalence of speech and language disorders in 5-year-old kindergarten children in the Ottawa-Carleton region. Journal of Speech and Hearing Disorders, 51(2), 98–110. 10.1044/jshd.5102.98 [DOI] [PubMed] [Google Scholar]
- Benasich AA, Choudhury N, Friedman JT, Realpe-Bonilla T, Chojnowska C, & Gou Z (2006). The infant as a prelinguistic model for language learning impairments: Predicting from event-related potentials to behavior. Neuropsychologia, 44(3), 396–411. 10.1016/j.neuropsychologia.2005.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasich AA, & Tallal P (2002). Infant discrimination of rapid auditory cues predicts later language impairment. Behavioural Brain Research, 136(1), 31–49. 10.1016/S0166-4328(02)00098-0 [DOI] [PubMed] [Google Scholar]
- Benasich AA, Thomas JJ, Choudhury N, & Leppänen PHT (2002). The importance of rapid auditory processing abilities to early language development: Evidence from converging methodologies. Developmental Psychobiology, 40(3), 278–292. 10.1002/dev.10032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C, & Gervain J (2012). Prosodic cues to word order: What level of representation? Frontiers in Psychology, 3(October), 1–6. 10.3389/fpsyg.2012.00451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, & Faraone SV (2005). Attention-deficit hyperactivity disorder. Lancet, 366(9481), 237–248. 10.1016/S0140-6736(05)66915-2 [DOI] [PubMed] [Google Scholar]
- Bion RAH, Benavides-Varela S, & Nespor M (2011). Acoustic markers of prominence influence infants’ and adults’ segmentation of speech sequences. Language and Speech, 54(1), 123–140. 10.1177/0023830910388018 [DOI] [PubMed] [Google Scholar]
- Bishop DV, & Hayiou-Thomas ME (2008). Heritability of specific language impairment depends on diagnostic criteria. Genes, Brain, and Behavior, 7(3), 365–372. 10.1111/j.1601-183X.2007.00360.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DVM (2015). The interface between genetics and psychology: Lessons from developmental dyslexia. Proceedings of the Royal Society B: Biological Sciences, 282(1806), 20143139 10.1098/rspb.2014.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DVM, & Snowling MJ (2004). Developmental Dyslexia and Specific Language Impairment: Same or different? Psychological Bulletin, 130(6), 858–886. 10.1037/0033-2909.130.6.858 [DOI] [PubMed] [Google Scholar]
- Bishop DVM, Snowling MJ, Thompson PA, Greenhalgh T, & the CATALISE-2 Consortium. (2017). Phase 2 of CATALISE: A multinational and multidisciplinary Delphi consensus study of problems with language development: Terminology. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 58(10), 1068–1080. 10.1111/jcpp.12721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop-Liebler P, Welch G, Huss M, Thomson JM, & Goswami U (2014). Auditory temporal processing skills in musicians with dyslexia. Dyslexia, 20(3), 261–279. 10.1002/dys.1479 [DOI] [PubMed] [Google Scholar]
- Bonacina S, Cancer A, Lanzi PL, Lorusso ML, & Antonietti A (2015). Improving reading skills in students with dyslexia: The efficacy of a sublexical training with rhythmic background. Frontiers in Psychology, 6, 1510 10.3389/fpsyg.2015.01510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer-Crane C, Snowling MJ, Duff FJ, Fieldsend E, Carroll JM, Miles J, … Hulme C (2008). Improving early language and literacy skills: Differential effects of an oral language versus a phonology with reading intervention. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 49(4), 422–432. 10.1111/j.1469-7610.2007.01849.x [DOI] [PubMed] [Google Scholar]
- Brandt A, Gebrian M, & Slevc LR (2012). Music and early language acquisition. Frontiers in Psychology, 3, 327 10.3389/fpsyg.2012.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer CC, Zalewski CK, King KA, Zobay O, Riley A, Ferguson MA, … Moore DR (2016). Heritability of non-speech auditory processing skills. European Journal of Human Genetics, 24(8), 1137–1144. 10.1038/ejhg.2015.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brod G, & Opitz B (2012). Does it really matter? Separating the effects of musical training on syntax acquisition. Frontiers in Psychology, 3, 543 10.3389/fpsyg.2012.00543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh P-R, … Neale BM (2015). An atlas of genetic correlations across human diseases and traits. Nature Genetics, 47, 1236 10.1038/ng.3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canette L-H, Fiveash A, Krzonowski J, Corneyllie A, Lalitte P, Thompson D, … Tillmann B (2019). Regular rhythmic primes boost P600 in grammatical error processing in dyslexic adults and matched controls. Neuropsychologia, 138, 107324 10.1016/j.neuropsychologia.2019.107324 [DOI] [PubMed] [Google Scholar]
- Canette L-H, Lalitte P, Bedoin N, Pineau M, Bigand E, & Tillmann B (2020). Rhythmic and textural musical sequences differently influence syntax and semantic processing in children. Journal of Experimental Child Psychology, 191, 104711 10.1016/j.jecp.2019.104711 [DOI] [PubMed] [Google Scholar]
- Cantiani C, Ortiz-Mantilla S, Riva V, Piazza C, Bettoni R, Musacchia G, … Benasich AA (2019). Reduced left-lateralized pattern of event-related EEG oscillations in infants at familial risk for language and learning impairment. NeuroImage: Clinical, 22, 101778 10.1016/j.nicl.2019.101778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantiani C, Riva V, Piazza C, Bettoni R, Molteni M, Choudhury N, … Benasich AA (2016). Auditory discrimination predicts linguistic outcome in Italian infants with and without familial risk for language learning impairment. Developmental Cognitive Neuroscience, 20, 23–34. 10.1016/j.dcn.2016.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantwell DP, & Baker L (1987). Clinical significance of childhood communication disorders: Perspectives from a longitudinal study. Journal of Child Neurology, 2(4), 257–264. 10.1177/088307388700200404 [DOI] [PubMed] [Google Scholar]
- Capellini SA, Coppede AC, & Valle TR (2010). Fine motor function of school-aged children with dyslexia, learning disability and learning difficulties. Pro-Fono Revista de Atualizacao Cientifica, 22(3), 201–208. 10.1590/s0104-56872010000300008 [DOI] [PubMed] [Google Scholar]
- Carrer LRJ (2015). Music and sound in time processing of children with ADHD. Frontiers in Psychiatry, 6, 127 10.3389/fpsyt.2015.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JM, & Myers JM (2010). Speech and language difficulties in children with and without a family history of dyslexia. Scientific Studies of Reading, 14(3), 247–265. 10.1080/10888430903150634 [DOI] [Google Scholar]
- Catts HW (1993). The relationship between speech-language impairments and reading disabilities. Journal of Speech, Language, and Hearing Research, 36(5), 948–958. 10.1044/jshr.3605.948 [DOI] [PubMed] [Google Scholar]
- Catts HW, Adlof SM, Hogan TP, & Weismer SE (2005). Are specific language impairment and dyslexia distinct disorders? Journal of Speech, Language, and Hearing Research, 48(6), 1378–1396. 10.1044/1092-4388(2005/096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-E, Chow HM, Wieland EA, & McAuley JD (2016). Relation between functional connectivity and rhythm discrimination in children who do and do not stutter. NeuroImage: Clinical, 12, 442–450. 10.1016/j.nicl.2016.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Penhune VB, & Zatorre RJ (2008). Moving on time: Brain network for auditory–motor synchronization is modulated by rhythm complexity and musical training. Journal of Cognitive Neuroscience, 20, 226–239. 10.1162/jocn.2008.20018 [DOI] [PubMed] [Google Scholar]
- Chen JL, Zatorre RJ, & Penhune VB (2006). Interactions between auditory and dorsal premotor cortex during synchronization to musical rhythms. NeuroImage, 32(4), 1771–1781. 10.1016/J.NEUROIMAGE.2006.04.207 [DOI] [PubMed] [Google Scholar]
- Chern A, Tillmann B, Vaughan C, & Gordon RL (2018). New evidence of a rhythmic priming effect that enhances grammaticality judgments in children. Journal of Experimental Child Psychology, 173, 371–379. 10.1016/j.jecp.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury N, & Benasich AA (2011). Maturation of auditory evoked potentials from 6 to 48 months: Prediction to 3 and 4 year language and cognitive abilities. Clinical Neurophysiology, 122(2), 320–338. 10.1016/j.clinph.2010.05.035 [DOI] [PubMed] [Google Scholar]
- Choudhury N, Leppanen PHT, Leevers HJ, & Benasich AA (2007). Infant information processing and family history of specific language impairment: Converging evidence for RAP deficits from two paradigms. Developmental Science, 10(2), 213–236. 10.1111/j.1467-7687.2007.00546.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli LK, Spinelli C, Nozaradan S, & Trainor LJ (2016). Measuring neural entrainment to beat and meter in infants: Effects of music background. Frontiers in Neuroscience, 10, 229 10.3389/fnins.2016.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colling LJ, Noble HL, & Goswami U (2017). Neural entrainment and sensorimotor synchronization to the beat in children with developmental dyslexia: An EEG study. Frontiers in Neuroscience, 11(July), 360 10.3389/fnins.2017.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti-Ramsden G, Durkin K, Toseeb U, Botting N, & Pickles A (2018). Education and employment outcomes of young adults with a history of developmental language disorder. International Journal of Language and Communication Disorders, 53(2), 237–255. 10.1111/1460-6984.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti-Ramsden G, Falcaro M, Simkin Z, & Pickles A (2007). Familal loading in specific language impairment: Patterns of differences across proband characteristics, gender and relative type. Genes, Brain, and Behavior, 6(3), 216–228. 10.1111/j.1601-183X.2006.00250.x [DOI] [PubMed] [Google Scholar]
- Corriveau K, Pasquini E, & Goswami U (2007). Basic auditory processing skills and Specific Language Impairment: A new look at an old hypothesis. Journal of Speech, Language, and Hearing Research, 50(3), 647–666. 10.1044/1092-4388(2007/046) [DOI] [PubMed] [Google Scholar]
- Corriveau KH, & Goswami U (2009). Rhythmic motor entrainment in children with speech and language impairments: Tapping to the beat. Cortex, 45(1), 119–130. 10.1016/j.cortex.2007.09.008 [DOI] [PubMed] [Google Scholar]
- Couvignou M, Peretz I, & Ramus F (2019). Comorbidity and cognitive overlap between developmental dyslexia and congenital amusia. Cognitive Neuropsychology, 36(1–2), 1–17. 10.1080/02643294.2019.1578205 [DOI] [PubMed] [Google Scholar]
- Cumming R, Wilson A, Leong V, Colling LJ, & Goswami U (2015). Awareness of rhythm patterns in speech and music in children with specific language impairments. Frontiers in Human Neuroscience, 9, 672 10.3389/fnhum.2015.00672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutini S, Szucs D, Mead N, Huss M, & Goswami U (2016). Atypical right hemisphere response to slow temporal modulations in children with developmental dyslexia. NeuroImage, 143, 40–49. 10.1016/j.neuroimage.2016.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla Bella S, Farrugia N, Benoit C-E, Begel V, Verga L, Harding E, & Kotz SA (2017). BAASTA: Battery for the Assessment of Auditory Sensorimotor and Timing Abilities. Behavior Research Methods, 49(3), 1128–1145. 10.3758/s13428-016-0773-6 [DOI] [PubMed] [Google Scholar]
- de Carvalho A, Dautriche I, & Christophe A (2016). Preschoolers use phrasal prosody online to constrain syntactic analysis. Developmental Science, 19(2), 235–250. 10.1111/desc.12300 [DOI] [PubMed] [Google Scholar]
- de Carvalho A, Dautriche I, Lin I, & Christophe A (2017). Phrasal prosody constrains syntactic analysis in toddlers. Cognition, 163, 67–79. 10.1016/j.cognition.2017.02.018 [DOI] [PubMed] [Google Scholar]
- Degé F, Kubicek C, & Schwarzer G (2015). Associations between musical abilities and precursors of reading in preschool aged children. Frontiers in Psychology, 6(August), 1–10. 10.3389/fpsyg.2015.01220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degé F, & Schwarzer G (2011). The Effect of a music program on phonological awareness in preschoolers. Frontiers in Psychology, 2, 124 10.3389/fpsyg.2011.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellatolas G, Watier L, Le Normand M-T, Lubart T, & Chevrie-Muller C (2009). Rhythm reproduction in kindergarten, reading performance at second grade, and developmental dyslexia theories. Archives of Clinical Neuropsychology, 24(6), 555–563. 10.1093/arclin/acp044 [DOI] [PubMed] [Google Scholar]
- Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, … Neale BM (2019). Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nature Genetics, 51(1), 63–75. 10.1038/s41588-018-0269-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deriziotis P, & Fisher SE (2017). Speech and language: Translating the genome. Trends in Genetics, 33(9), 642–656. 10.1016/j.tig.2017.07.002 [DOI] [PubMed] [Google Scholar]
- DiDonato Brumbach AC, & Goffman L (2014). Interaction of language processing and motor skill in children with specific language impairment. Journal of Speech, Language, and Hearing Research, 57(1), 158–171. 10.1044/1092-4388(2013/12-0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilley LC, & McAuley JD (2008). Distal prosodic context affects word segmentation and lexical processing. Journal of Memory and Language, 59(3), 294–311. 10.1016/j.jml.2008.06.006 [DOI] [Google Scholar]
- Dilnot J, Hamilton L, Maughan B, & Snowling MJ (2017). Child and environmental risk factors predicting readiness for learning in children at high risk of dyslexia. Development and Psychopathology, 29(1), 235–244. 10.1017/S0954579416000134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Melloni L, Zhang H, Tian X, & Poeppel D (2015). Cortical tracking of hierarchical linguistic structures in connected speech. Nature Neuroscience, 19, 158 10.1038/nn.4186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaher J, & Richels C (2012). Traits of attention deficit/hyperactivity disorder in school-age children who stutter. Journal of Fluency Disorders, 37(4), 242–252. 10.1016/j.jfludis.2012.08.002 [DOI] [PubMed] [Google Scholar]
- Douglas S, & Willatts P (1994). The relationship between musical ability and literacy skills. Journal of Research in Reading, 17(2), 99–107. 10.1111/j.1467-9817.1994.tb00057.x [DOI] [Google Scholar]
- Dupoux E, Pallier C, Sebastian N, & Mehler J (1997). A destressing “deafness” in French? Journal of Memory and Language, 36(3), 406–421. 10.1006/jmla.1996.2500 [DOI] [Google Scholar]
- Dworzynski K, Remington A, Rijsdijk F, Howell P, & Plomin R (2007). Genetic etiology in cases of recovered and persistent stuttering in an unselected, longitudinal sample of young twins. American Journal of Speech-Language Pathology, 16(2), 161–178. 10.1044/1058-0360(2007/021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einarson KM, & Trainor LJ (2016). Hearing the beat: Young children’s perceptual sensitivity to beat alignment varies according to metric structure. Music Perception: An Interdisciplinary Journal, 34(1), 56–70. 10.1525/mp.2016.34.1.56 [DOI] [Google Scholar]
- Falk S, Maslow E, Thum G, & Hoole P (2016). Temporal variability in sung productions of adolescents who stutter. Journal of Communication Disorders, 62, 101–114. 10.1016/j.jcomdis.2016.05.012 [DOI] [PubMed] [Google Scholar]
- Falk S, Müller T, & Dalla Bella S (2015). Non-verbal sensorimotor timing deficits in children and adolescents who stutter. Frontiers in Psychology, 6, 847 10.3389/fpsyg.2015.00847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falter CM, & Noreika V (2014). Time processing in developmental disorders: A comparative view In Subjective time: The philosophy, psychology, and neuroscience of temporality (pp. 557–597). Cambridge, MA: MIT Press. [Google Scholar]
- Finlay JCS, & McPhillips M (2013). Comorbid motor deficits in a clinical sample of children with specific language impairment. Research in Developmental Disabilities, 34(9), 2533–2542. 10.1016/j.ridd.2013.05.015 [DOI] [PubMed] [Google Scholar]
- Fisher J, Plante E, Vance R, Gerken L, & Glattke TJ (2007). Do children and adults with language impairment recognize prosodic cues? Journal of Speech, Language, and Hearing Research, 50(3), 746–758. 10.1044/1092-4388(2007/052) [DOI] [PubMed] [Google Scholar]
- Fitch WT (2017). Empirical approaches to the study of language evolution. Psychonomic Bulletin & Review, 24(1), 3–33. 10.3758/s13423-017-1236-5 [DOI] [PubMed] [Google Scholar]
- Fitch WT, & Martins MD (2014). Hierarchical processing in music, language, and action: Lashley revisited. Annals of the New York Academy of Sciences, 1316(1), 87–104. 10.1111/nyas.12406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiveash A, Bedoin N, & Tillmann B (submitted). Rhythmic processing of music and language: A review and implications for developmental disorders.
- Fiveash A, McArthur G, & Thompson WF (2018). Syntactic and non-syntactic sources of interference by music on language processing. Scientific Reports, 8(1), 17918 10.1038/s41598-018-36076-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiveash A, & Pammer K (2014). Music and language: Do they draw on similar syntactic working memory resources? Psychology of Music, 42(2), 190–209. 10.1177/0305735612463949 [DOI] [Google Scholar]
- Fiveash A, Schön D, Canette L-H, Morillon B, Bedoin N, & Tillmann B (2020). A stimulus-brain coupling analysis of regular and irregular rhythms in adults with dyslexia and controls. Brain and Cognition, 140, 105531 10.1016/j.bandc.2020.105531 [DOI] [PubMed] [Google Scholar]
- Flapper BCT, & Schoemaker MM (2013). Developmental Coordination Disorder in children with specific language impairment: Comorbidity and impact on quality of life. Research in Developmental Disabilities, 34(2), 756–763. 10.1016/j.ridd.2012.10.014 [DOI] [PubMed] [Google Scholar]
- Flaugnacco E, Lopez L, Terribili C, Montico M, Zoia S, & Schön D (2015). Music training increases phonological awareness and reading skills in developmental dyslexia: A randomized control trial. PLoS One, 10(9), e0138715 10.1371/journal.pone.0138715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaugnacco E, Lopez L, Terribili C, Zoia S, Buda S, Tilli S, … Schön D (2014). Rhythm perception and production predict reading abilities in developmental dyslexia. Frontiers in Human Neuroscience, 8, 392 10.3389/fnhum.2014.00392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flax JF, Realpe-Bonilla T, Hirsch LS, Brzustowicz LM, Barlett CW, & Tallal P (2003). Specific Language Impairment in families. Journal of Speech, Language, and Hearing Research, 46(3), 530–543. 10.1044/1092-4388(2003/043) [DOI] [PubMed] [Google Scholar]
- Forgeard M, Winner E, Norton A, & Schlaug G (2008). Practicing a musical instrument in childhood is associated with enhanced verbal ability and nonverbal reasoning. PLoS One, 3(10), e3566 10.1371/journal.pone.0003566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- François C, Jaillet F, Takerkart S, & Schön D (2014). Faster sound stream segmentation in musicians than in nonmusicians. PLoS One, 9(7), e101340 10.1371/journal.pone.0101340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey A, François C, Chobert J, Besson M, & Ziegler JC (2019). Behavioral and electrophysiological investigation of speech perception deficits in silence, noise and envelope conditions in developmental dyslexia. Neuropsychologia, 130, 3–12. 10.1016/j.neuropsychologia.2018.07.033 [DOI] [PubMed] [Google Scholar]
- Friend A, DeFries JC, & Olson RK (2008). Parental education moderates genetic influences on reading disability. Psychological Science, 19(11), 1124–1130. 10.1111/j.1467-9280.2008.02213.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K (2005). A theory of cortical responses. Philosophical Transactions of the Royal Society, B: Biological Sciences, 360(1456), 815–836. 10.1098/rstb.2005.1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, & Wan CY (2014). The role of rhythm in speech and language rehabilitation: The SEP hypothesis. Frontiers in Human Neuroscience, 8, 777 10.3389/fnhum.2014.00777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka T, Trainor LJ, Large EW, & Ross B (2012). Internalized timing of isochronous sounds is represented in neuromagnetic beta oscillations. Journal of Neuroscience, 32(5), 1791–1802. 10.1523/JNEUROSCI.4107-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervain J (2018). Gateway to language: The perception of prosody at birth In Bartos H, den Dikken M, Bánréti Z, & Váradi T (Eds.), Boundaries Crossed, at the Interfaces of Morphosyntax, Phonology, Pragmatics and Semantics. Studies in Natural Language and Linguistic Theory (Vol. 94). Cham: Springer; 10.1007/978-3-319-90710-9 [DOI] [Google Scholar]
- Gervain J, & Werker JF (2013). Prosody cues word order in 7-month-old bilingual infants. Nature Communications, 4(1), 1490 10.1038/ncomms2430 [DOI] [PubMed] [Google Scholar]
- Ghitza O (2011). Linking speech perception and neurophysiology: Speech decoding guided by cascaded oscillators locked to the input rhythm. Frontiers in Psychology, 2, 130 10.3389/fpsyg.2011.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza O (2012). On the role of theta-driven syllabic parsing in decoding speech: Intelligibility of speech with a manipulated modulation spectrum. Frontiers in Psychology, 3(July), 1–12. 10.3389/fpsyg.2012.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gialluisi A, Andlauer TFM, Mirza-Schreiber N, Moll K, Becker J, Hoffmann P, … Schulte-Körne G (2019). Genome-wide association scan identifies new variants associated with a cognitive predictor of dyslexia. Translational Psychiatry, 9, 77 10.1038/s41398-019-0402-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras B, Honing H, Peretz I, Trainor LJ, & Fisher SE (2015). Defining the biological bases of individual differences in musicality. Philosophical Transactions of the Royal Society, B: Biological Sciences, 370(1664), 20140092 10.1098/rstb.2014.0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud A-L, & Poeppel D (2012). Cortical oscillations and speech processing: Emerging computational principles and operations. Nature Neuroscience, 15(4), 511–517. 10.1038/nn.3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanz O, Derix J, Kaur R, Schulze-Bonhage A, Auer P, Aertsen A, & Ball T (2018). Real-life speech production and perception have a shared premotor-cortical substrate. Science Reports, 8(1), 8898 10.1038/s41598-018-26801-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover H, Kalinowski J, Rastatter M, & Stuart A (1996). Effect of instruction to sing on stuttering frequency at normal and fast rates. Perceptual and Motor Skills, 83(2), 511–522. 10.2466/pms.1996.83.2.511 [DOI] [PubMed] [Google Scholar]
- Goffman L (2004). Kinematic differentiation of prosodic categories in normal. Journal of Speech, Language, and Hearing Research, 47(5),1088–1102. 10.1044/1092-4388(2004/081) [DOI] [PubMed] [Google Scholar]
- Gooch D, Hulme C, Nash HM, & Snowling MJ (2014). Comorbidities in preschool children at family risk of dyslexia. Journal of Child Psychology and Psychiatry, 55(3), 237–246. 10.1111/jcpp.12139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CL, Cobb PR, & Balasubramaniam R (2018). Recruitment of the motor system during music listening: An ALE meta-analysis of fMRI data. PLoS One, 13(11), e0207213 10.1371/journal.pone.0207213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E (1979). Primary Measures of Music Audiation. Chicago, IL: GIA Publications. [Google Scholar]
- Gordon RL, Jacobs MS, Schuele CM, & McAuley JD (2015). Perspectives on the rhythm-grammar link and its implications for typical and atypical language development. Annals of the New York Academy of Sciences, 1337(1), 16–25. 10.1111/nyas.12683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon RL, Shivers CM, Wieland EA, Kotz SA, Yoder PJ, & Devin McAuley J (2015). Musical rhythm discrimination explains individual differences in grammar skills in children. Developmental Science, 18(4), 635–644. 10.1111/desc.12230 [DOI] [PubMed] [Google Scholar]
- Goswami U (2011). A temporal sampling framework for developmental dyslexia. Trends in Cognitive Sciences, 15(1), 3–10. 10.1016/j.tics.2010.10.001 [DOI] [PubMed] [Google Scholar]
- Goswami U (2018). A neural basis for phonological awareness? An oscillatory temporal-sampling perspective. Current Directions in Psychological Science, 27(1), 56–63. 10.1177/0963721417727520 [DOI] [Google Scholar]
- Goswami U (2019). Speech rhythm and language acquisition: An amplitude modulation phase hierarchy perspective. Annals of the New York Academy of Sciences, 1453, 67–78. 10.1111/nyas.14137 [DOI] [PubMed] [Google Scholar]
- Goswami U, Cumming R, Chait M, Huss M, Mead N, Wilson AM, … Fosker T (2016). Perception of filtered speech by children with Developmental Dyslexia and children with Specific Language Impairments. Frontiers in Psychology, 7, 791 10.3389/fpsyg.2016.00791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami U, Gerson D, & Astruc L (2010). Amplitude envelope perception, phonology and prosodic sensitivity in children with developmental dyslexia. Reading and Writing, 23(8), 995–1019. 10.1007/s11145-009-9186-6 [DOI] [Google Scholar]
- Goswami U, Huss M, Mead N, Fosker T, & Verney JP (2013). Perception of patterns of musical beat distribution in phonological developmental dyslexia: Significant longitudinal relations with word reading and reading comprehension. Cortex, 49(5), 1363–1376. 10.1016/j.cortex.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Goswami U, Mead N, Fosker T, Huss M, Barnes L, & Leong V (2013). Impaired perception of syllable stress in children with dyslexia: A longitudinal study. Journal of Memory and Language, 69(1), 1–17. 10.1016/j.jml.2013.03.001 [DOI] [Google Scholar]
- Goswami U, Thomson J, Richardson U, Stainthorp R, Hughes D, Rosen S, & Scott SK (2002). Amplitude envelope onsets and developmental dyslexia: A new hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 99(16), 10911–10916. 10.1073/pnas.122368599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn JA, & Brett M (2007). Rhythm and beat perception in motor areas of the brain. Journal of Cognitive Neuroscience, 19(5), 893–906. 10.1162/jocn.2007.19.5.893 [DOI] [PubMed] [Google Scholar]
- Grahn JA, & Brett M (2009). Impairment of beat-based rhythm discrimination in Parkinson’s disease. Cortex, 45, 54–61. 10.1016/j.cortex.2008.01.005 [DOI] [PubMed] [Google Scholar]
- Grube M, Kumar S, Cooper FE, Turton S, & Griffiths TD (2012). Auditory sequence analysis and phonological skill. Proceedings of the Royal Society B: Biological Sciences, 279(1746), 4496–4504. 10.1098/rspb.2012.1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttorm TK, Leppänen PHT, Hämäläinen JA, Eklund KM, & Lyytinen HJ (2010). Newborn event-related potentials predict poorer pre-reading skills in children at risk for dyslexia. Journal of Learning Disabilities, 43(5), 391–401. 10.1177/0022219409345005 [DOI] [PubMed] [Google Scholar]
- Guttorm TK, Leppänen PHT, Poikkeus AM, Eklund KM, Lyytinen P, & Lyytinen H (2005). Brain event-related potentials (ERPs) measured at birth predict later language development in children with and without familial risk for dyslexia. Cortex, 41(3), 291–303. 10.1016/S0010-9452(08)70267-3 [DOI] [PubMed] [Google Scholar]
- Habib M, Lardy C, Desiles T, Commeiras C, Chobert J, & Besson M (2016). Music and dyslexia: A new musical training method to improve reading and related disorders. Frontiers in Psychology, 7, 26 10.3389/fpsyg.2016.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall PK, & Tomblin JB (1978). A follow-up study of children with articulation and language disorders. Journal of Speech and Hearing Disorders, 43(2), 227–241. 10.1044/jshd.4302.227 [DOI] [PubMed] [Google Scholar]
- Hämäläinen JA, Rupp A, Soltész F, Szücs D, & Goswami U (2012). Reduced phase locking to slow amplitude modulation in adults with dyslexia: An MEG study. NeuroImage, 59(3), 2952–2961. 10.1016/j.neuroimage.2011.09.075 [DOI] [PubMed] [Google Scholar]
- Hannon EE, & Trehub SE (2005). Tuning in to musical rhythms: Infants learn more readily than adults. Proceedings of the National Academy of Sciences of the United States of America, 102(35), 12639–12643. 10.1073/pnas.0504254102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlaar N, Spinath FM, Dale PS, & Plomin R (2005). Genetic influences on early word recognition abilities and disabilities: A study of 7-year-old twins. Journal of Child Psychology and Psychiatry, 46(4), 373–384. 10.1111/j.1469-7610.2004.00358.x [DOI] [PubMed] [Google Scholar]
- Harrington DL, Haaland KY, & Hermanowitz N (1998). Temporal processing in the basal ganglia. Neuropsychology, 12(1), 3–12. 10.1037/0894-4105.12.1.3 [DOI] [PubMed] [Google Scholar]
- Hayiou-Thomas ME (2008). Genetic and environmental influences on early speech, language and literacy development. Journal of Communication Disorders, 41(5), 397–408. 10.1016/j.jcomdis.2008.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard M, & Lee YS (2020). Shared neural resources of rhythm and syntax: An ALE meta-analysis. Neuropsychologia, 137, 107284 10.1016/j.neuropsychologia.2019.107284 [DOI] [PubMed] [Google Scholar]
- Heim S, Friedman JT, Keil A, & Benasich AA (2011). Reduced sensory oscillatory activity during rapid auditory processing as a correlate of language-learning impairment. Journal of Neurolinguistics, 24(5), 538–555. 10.1016/j.jneuroling.2010.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdener M, Humbel T, Esposito F, Habermeyer B, Cattapan-Ludewig K, & Seifritz E (2014). Jazz drummers recruit language-specific areas for the processing of rhythmic structure. Cerebral Cortex, 24(3), 836–843. 10.1093/cercor/bhs367 [DOI] [PubMed] [Google Scholar]
- Hickok G, Houde J, & Rong F (2011). Sensorimotor in speech processing: Computational basis and neural organization. Neuron, 69(3), 407–422. 10.1016/j.neuron.2011.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EL (2001). Non-specific nature of specific language impairment: A review of the literature with regard to concomitant motor impairments. International Journal of Language and Communication Disorders, 36(2), 149–171. 10.1080/13682820010019874 [DOI] [PubMed] [Google Scholar]
- Hoch L, Poulin-Charronnat B, & Tillmann B (2011). The influence of task-irrelevant music on language processing: Syntactic and semantic structures. Frontiers in Psychology, 2, 112 10.3389/fpsyg.2011.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliman AJ, Wood C, & Sheehy K (2010). The contribution of sensitivity to speech rhythm and non-speech rhythm to early reading development. Educational Psychology, 30(3), 247–267. 10.1080/01443410903560922 [DOI] [Google Scholar]
- Hove MJ, Gravel N, Spencer RMC, & Valera EM (2017). Finger tapping and pre-attentive sensorimotor timing in adults with ADHD. Experimental Brain Research, 235(12), 3663–3672. 10.1007/s00221-017-5089-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert-Dibon G, Bru M, Le Guen CG, Launay E, & Roy A (2016). Health-related quality of life for children and adolescents with specific language impairment: A cohort study by a Learning Disabilities Reference Center. PLoS One, 11(11), 1–14. 10.1371/journal.pone.0166541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss M, Verney JP, Fosker T, Mead N, & Goswami U (2011). Music, rhythm, rise time perception and developmental dyslexia: Perception of musical meter predicts reading and phonology. Cortex, 47(6), 674–689. 10.1016/J.CORTEX.2010.07.010 [DOI] [PubMed] [Google Scholar]
- Iversen JR, & Patel AD (2008). The Beat Alignment Test (BAT): Surveying beat processing abilities in the general population In Miyazaki K, Adachi M, Nakajima Y, & Tsuzaki M (Eds.), Proceedings of the 10th International Conference on Music Perception and Cognition (pp. 465–468). Sapporo, Japan Adelaide: Causal Productions. [Google Scholar]
- Jäncke L, Siegenthaler T, Preis S, & Steinmetz H (2007). Decreased white-matter density in a left-sided fronto-temporal network in children with developmental language disorder: Evidence for anatomical anomalies in a motor-language network. Brain and Language, 102(1), 91–98. 10.1016/j.bandl.2006.08.003 [DOI] [PubMed] [Google Scholar]
- Jentschke S, & Koelsch S (2009). Musical training modulates the development of syntax processing in children. NeuroImage, 47(2), 735–744. 10.1016/j.neuroimage.2009.04.090 [DOI] [PubMed] [Google Scholar]
- Jentschke S, Koelsch S, Sallat S, & Friederici AD (2008). Children with specific language impairment also show impairment of music-syntactic processing. Journal of Cognitive Neuroscience, 20(11), 1940–1951. 10.1162/jocn.2008.20135 [DOI] [PubMed] [Google Scholar]
- Jones MR (1976). Time, our lost dimension: Toward a new theory of perception, attention, and memory. Psychological Review, 83, 323–355. 10.1037/0033-295X.83.5.323 [DOI] [PubMed] [Google Scholar]
- Jones MR (2016). Musical time In Oxford Library of Psychology. The Oxford handbook of music psychology (2nd ed., pp. 125–141). New York, NY: Oxford University Press. [Google Scholar]
- Jones MR (2019). Time will tell: A theory of dynamic attending. New York, NY: Oxford University Press; Retrieved from https://www.oxfordscholarship.com/view/10.1093/oso/9780190618216.001.0001/oso-9780190618216 [Google Scholar]
- Jones MR, & Boltz M (1989). Dynamic attending and responses to time. Psychological Review, 96(3), 459–491. 10.1037/0033-295X.96.3.459 [DOI] [PubMed] [Google Scholar]
- Jusczyk PW (1999). How infants begin to extract words from speech. Trends in Cognitive Sciences, 3(9), 323–328. 10.1016/S1364-6613(99)01363-7 [DOI] [PubMed] [Google Scholar]
- Jusczyk PW, Cutler A, & Redanz NJ (1993). Infants’ preference for the predominant stress patterns of English words. Child Development, 64(3), 675–687. 10.2307/1131210 [DOI] [PubMed] [Google Scholar]
- Kalashnikova M, Goswami U, & Burnham D (2019). Sensitivity to amplitude envelope rise time in infancy and vocabulary development at 3 years: A significant relationship. Developmental Science, 22(6), e12836 10.1111/desc.12836 [DOI] [PubMed] [Google Scholar]
- Kalashnikova M, Peter V, Di Liberto GM, Lalor EC, & Burnham D (2018). Infant-directed speech facilitates seven-month-old infants’ cortical tracking of speech. Scientific Reports, 8, 13745 10.1038/s41598-018-32150-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan BJ, Dewey DM, Crawford SG, & Wilson BN (2001). The term comorbidity is of questionable value in reference to developmental disorders. Journal of Learning Disabilities, 34(6), 555–565. 10.1177/002221940103400608 [DOI] [PubMed] [Google Scholar]
- Keitel C, Benwell CSY, Thut G, & Gross J (2018). No changes in parieto-occipital alpha during neural phase locking to visual quasi-periodic theta-, alpha-, and beta-band stimulation. European Journal of Neuroscience, 48(7), 2551–2565. 10.1111/ejn.13935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick RM, Legrand LN, Iacono WG, & McGue M (2011). A twin and adoption study of reading achievement: Exploration of shared-environmental and gene–environment-interaction effects. Learning and Individual Differences, 21(4), 368–375. 10.1016/j.lindif.2011.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klem M, Melby-Lervåg M, Hagtvet B, Lyster S-AH, Gustafsson J-E, & Hulme C (2015). Sentence repetition is a measure of children’s language skills rather than working memory limitations. Developmental Science, 18(1), 146–154. 10.1111/desc.12202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch S, Gunter TC, Wittfoth M, & Sammler D (2005). Interaction between syntax processing in language and in music: An ERP Study. Journal of Cognitive Neuroscience, 17(10), 1565–1577. 10.1162/089892905774597290 [DOI] [PubMed] [Google Scholar]
- Koelsch S, Vuust P, & Friston K (2019). Predictive processes and the peculiar case of music. Trends in Cognitive Sciences, 23(1), 63–77. 10.1016/j.tics.2018.10.006 [DOI] [PubMed] [Google Scholar]
- Kotz SA, & Gunter TC (2015). Can rhythmic auditory cuing remediate language-related deficits in Parkinson’s disease? Annals of the New York Academy of Sciences, 1337(1), 62–68. 10.1111/nyas.12657 [DOI] [PubMed] [Google Scholar]
- Kotz SA, Ravignani A, & Fitch WT (2018). Special Issue: Time in the brain the evolution of rhythm processing. Trends in Cognitive Sciences, 22(10), 896–910. 10.1016/j.tics.2018.08.002 [DOI] [PubMed] [Google Scholar]
- Kotz SA, Schwartze M, & Schmidt-Kassow M (2009). Non-motor basal ganglia functions: A review and proposal for a model of sensory predictability in auditory language perception. Cortex, 45(8), 982–990. 10.1016/j.cortex.2009.02.010 [DOI] [PubMed] [Google Scholar]
- Kovac I, Garabedian B, Du Souich C, & Palmour RM (2001). Attention deficit/hyperactivity in SLI children increases risk of speech/language disorders in first-degree relatives: A preliminary report. Journal of Communication Disorders, 34(4), 339–354. 10.1016/S0021-9924(01)00054-5 [DOI] [PubMed] [Google Scholar]
- Kujala T, Tervaniemi M, & Schröger E (2007). The mismatch negativity in cognitive and clinical neuroscience: Theoretical and methodological considerations. Biological Psychology, 74(1), 1–19. 10.1016/j.biopsycho.2006.06.001 [DOI] [PubMed] [Google Scholar]
- Kunert R, Willems RM, & Hagoort P (2016). Language influences music harmony perception: Effects of shared syntactic integration resources beyond attention. Royal Society Open Science, 3(2), 150685 10.1098/rsos.150685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladányi E, Lukács Á, Gervain J (submitted). Regular rhythm facilitates grammar processing in Hungarian-speaking children with and without specific language impairment.
- Lahey M, & Edwards J (1995). Specific language impairment: Preliminary investigation of factors associated with family history and with patterns of language performance. Journal of Speech and Hearing Research, 38(3), 643–657. 10.1044/jshr.3803.643 [DOI] [PubMed] [Google Scholar]
- Large EW, & Jones MR (1999). The dynamics of attending: How people track time-varying events. Psychological Review, 106(1), 119–159. 10.1037/0033-295X.106.1.119 [DOI] [Google Scholar]
- Lashley KS (1951). The problem of serial order in behavior. Cerebral Mechanisms in Behavior: The Hixon Symposium, 7, 112–135. 10.1016/j.humov.2007.04.001 [DOI] [Google Scholar]
- Law L, & Zentner M (2012). Assessing musical abilities objectively: Construction and validation of the profile of music perception skills. PLoS One, 7(12), e52508 10.1371/journal.pone.0052508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law J, Rush R, Schoon I, & Parsons S (2009). Modeling developmental language difficulties from school entry into adulthood: Literacy, mental health, and employment outcomes James. Hearing Research, 52(6), 1401–1417. 10.1044/1092-4388(2009/08-0142) [DOI] [PubMed] [Google Scholar]
- Lee H-Y, Sie Y-S, Chen S-C, & Cheng M-C (2015). The Music perception performance of children with and without dyslexia in Taiwan. Psychological Reports, 116(1), 13–22. 10.2466/15.28.PR0.116k15w8 [DOI] [PubMed] [Google Scholar]
- Leong V, & Goswami U (2014). Assessment of rhythmic entrainment at multiple timescales in dyslexia: Evidence for disruption to syllable timing. Hearing Research, 308, 141–161. 10.1016/j.heares.2013.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong V, Hämäläinen J, Soltész F, & Goswami U (2011). Rise time perception and detection of syllable stress in adults with developmental dyslexia. Journal of Memory and Language, 64(1), 59–73. 10.1016/j.jml.2010.09.003 [DOI] [Google Scholar]
- Leppänen PHT, Hämäläinen JA, Guttorm TK, Eklund KM, Salminen H, Tanskanen A, … Lyytinen H (2011). Infant brain responses associated with reading-related skills before school and at school age. Neurophysiologie Clinique, 42(1–2), 35–41. 10.1016/j.neucli.2011.08.005 [DOI] [PubMed] [Google Scholar]
- Leppänen PHT, Hämäläinen JA, Salminen HK, Eklund KM, Guttorm TK, Lohvansuu K, … Lyytinen H (2010). Newborn brain event-related potentials revealing atypical processing of sound frequency and the subsequent association with later literacy skills in children with familial dyslexia. Cortex, 46(10), 1362–1376. 10.1016/j.cortex.2010.06.003 [DOI] [PubMed] [Google Scholar]
- Leppanen PHT, Pihko E, Eklund KM, & Lyytinen H (1999). Cortical responses of infants with and without a genetic risk for dyslexia: II. Group effects. Neuroreport, 10(5), 969–973. 10.1097/00001756-199904060-00014 [DOI] [PubMed] [Google Scholar]
- Lerdahl F, & Jackendoff R (1983). A generative theory of tonal music. Cambridge, MA: MIT Press. [Google Scholar]
- Linnavalli T, Putkinen V, Lipsanen J, Huotilainen M, & Tervaniemi M (2018). Music playschool enhances children’s linguistic skills. Scientific Reports, 8(1), 8767 10.1038/s41598-018-27126-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wang J, Shao S, Luo X, Kong R, Zhang X, & Song R (2016). Descriptive epidemiology of prenatal and perinatal risk factors in a Chinese population with reading disorder. Scientific Reports, 6(June), 36697 10.1038/srep36697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizarazu M, Lallier M, Molinaro N, Bourguignon M, Paz-Alonso PM, Lerma-Usabiaga G, & Carreiras M (2015). Developmental evaluation of atypical auditory sampling in dyslexia: Functional and structural evidence. Human Brain Mapping, 36(12), 4986–5002. 10.1002/hbm.22986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohvansuu K, Hämäläinen JA, Ervast L, Lyytinen H, & Leppänen PHT (2018). Longitudinal interactions between brain and cognitive measures on reading development from 6 months to 14 years. Neuropsychologia, 108, 6–12. 10.1016/J.NEUROPSYCHOLOGIA.2017.11.018 [DOI] [PubMed] [Google Scholar]
- London J (2004). Hearing in Time: Psychological Aspects of Musical Meter. Oxford and New York: Oxford University Press. [Google Scholar]
- Magne C, Jordan DK, & Gordon RL (2016). Speech rhythm sensitivity and musical aptitude: ERPs and individual differences. Brain and Language, 153–154, 13–19. 10.1016/j.bandl.2016.01.001 [DOI] [PubMed] [Google Scholar]
- Mankel K, & Bidelman GM (2018). Inherent auditory skills rather than formal music training shape the neural encoding of speech. Proceedings of the National Academy of Sciences of the United States of America, 115(51), 13129–13134. 10.1073/pnas.1811793115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mareva S, & Holmes J (2019). Transdiagnostic associations across communication, cognitive, and behavioural problems in a developmentally at-risk population: A network approach. BMC Pediatrics, 19, 452 10.1186/s12887-019-1818-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie C, Magne C, & Besson M (2011). Musicians and the metric structure of words. Journal of Cognitive Neuroscience, 23(2), 294–305. 10.1162/jocn.2010.21413 [DOI] [PubMed] [Google Scholar]
- Mascheretti S, Andreola C, Scaini S, & Sulpizio S (2018). Beyond genes: A systematic review of environmental risk factors in specific reading disorder. Research in Developmental Disabilities, 82, 147–152. 10.1016/j.ridd.2018.03.005 [DOI] [PubMed] [Google Scholar]
- Mehler J, Jusczyk P, Lambertz G, Halsted N, Bertoncini J, & Amiel-Tison C (1988). A precursor of language acquisition in young infants. Cognition, 29(2), 143–178. 10.1016/0010-0277(88)90035-2 [DOI] [PubMed] [Google Scholar]
- Merchant H, & Bartolo R (2018). Primate beta oscillations and rhythmic behaviors. Journal of Neural Transmission, 125(3), 461–470. 10.1007/s00702-017-1716-9 [DOI] [PubMed] [Google Scholar]
- Merchant H, Grahn J, Trainor L, Rohrmeier M, & Fitch WT (2015). Finding the beat: A neural perspective across humans and non-human primates. Philosophical Transactions of the Royal Society, B: Biological Sciences, 370(1664), 20140093 10.1098/rstb.2014.0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabella G, Del Signore S, Lakens D, Averna R, Penge R, & Capozzi F (2017). Developmental Coordination Disorder affects the processing of action-related verbs. Frontiers in Human Neuroscience, 10, 661 10.3389/fnhum.2016.00661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinaro N, Lizarazu M, Lallier M, Bourguignon M, & Carreiras M (2016). Out-of-synchrony speech entrainment in developmental dyslexia. Human Brain Mapping, 37(8), 2767–2783. 10.1002/hbm.23206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz C, Yampolsky S, Papadelis G, Thomson JM, & Wolf M (2013). Links between early rhythm skills, musical training, and phonological awareness. Reading and Writing, 26(5), 739–769. 10.1007/s11145-012-9389-0 [DOI] [Google Scholar]
- Möttönen R, Dutton R, & Watkins KE (2013). Auditory-motor processing of speech sounds. Cerebral Cortex, 23(5), 1190–1197. 10.1093/cercor/bhs110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller KL, & Tomblin JB (2012). Examining the comorbidity of language impairment and attention-deficit/hyperactivity disorder. Topics in Language Disorders, 32(3), 228–246. 10.1097/TLD.0b013e318262010d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muneaux M, Ziegler JC, Truc C, Thomson J, & Goswami U (2004). Deficits in beat perception and dyslexia: Evidence from French. Neuroreport, 15(8), 1255–1259. 10.1097/01.wnr.0000127459.31232.c4 [DOI] [PubMed] [Google Scholar]
- Musacchia G, Sams M, Skoe E, & Kraus N (2007). Musicians have enhanced subcortical auditory and audiovisual processing of speech and music. Proceedings of the National Academy of Sciences of the United States of America, 104(40), 15894–15898. 10.1073/pnas.0701498104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers BR, Lense MD, & Gordon RL (2019). Pushing the Envelope: Developments in neural entrainment to speech and the biological underpinnings of prosody perception. Brain Sciences, 9, 70 10.3390/brainsci9030070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näätänen R, Tervaniemi M, Sussman E, Paavilainen P, & Winkler I (2001). “Primitive intelligence” in the auditory cortex. Trends in Neurosciences, 24(5), 283–288. 10.1016/s0166-2236(00)01790-2 [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. (2016). Speech and Language Disorders in Children: Implications for the Social Security Administration’s Supplemental Security Income Program. Washington, DC: National Academic Press; 10.17226/21872 [DOI] [PubMed] [Google Scholar]
- Nazzi T, Bertoncini J, & Mehler J (1998). Language discrimination by newborns: Toward an understanding of the role of rhythm. Journal of Experimental Psychology: Human Perception and Performance, 24(3), 756–766. 10.1037/0096-1523.24.3.756 [DOI] [PubMed] [Google Scholar]
- Niarchou M, Sathirapongsasuti JF, Jacoby N, Bell E, McArthur E, Straub P, … Gordon RL (2019). Unravelling the genetic architecture of rhythm. BioRxiv, 2019, 836197 10.1101/836197 [DOI] [Google Scholar]
- Noreika V, Falter CM, & Rubia K (2013). Timing deficits in attention-deficit/hyperactivity disorder (ADHD): Evidence from neurocognitive and neuroimaging studies. Neuropsychologia, 51(2), 235–266. 10.1016/j.neuropsychologia.2012.09.036 [DOI] [PubMed] [Google Scholar]
- Nozaradan S, Peretz I, Missal M, & Mouraux A (2011). Tagging the neuronal entrainment to beat and meter. Journal of Neuroscience, 31(28), 10234–10240. 10.1523/JNEUROSCI.0411-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntourou K, Conture EG, & Lipsey MW (2011). Language abilities of children who stutter: A meta-analytical review. American Journal of Speech-Language Pathology, 20(3), 163–179. 10.1044/1058-0360(2011/09-0102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Boyle DJ, Freeman JS, & Cody FW (1996). The accuracy and precision of timing of self-paced, repetitive movements in subjects with Parkinson’s disease. Brain, 119(1), 51–70. [DOI] [PubMed] [Google Scholar]
- Okbay A, Beauchamp JP, Fontana MA, Lee JJ, Pers TH, Rietveld CA, … Benjamin DJ (2016). Genome-wide association study identifies 74 loci associated with educational attainment. Nature, 533(7604), 539–542. 10.1038/nature17671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olander L, Smith A, & Zelaznik HN (2010). Evidence that a motor timing deficit is a factor in the development of stuttering. Journal of Speech, Language, and Hearing Research, 53(4), 876–886. 10.1044/1092-4388(2009/09-0007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overy K (2000). Dyslexia, temporal processing and music: The potential of music as an early learning aid for dyslexic children. Psychology of Music, 28(2), 218–229. 10.1177/0305735600282010 [DOI] [Google Scholar]
- Overy K, Nicolson RI, Fawcett AJ, & Clarke EF (2003). Dyslexia and music: Measuring musical timing skills. Dyslexia, 9(1), 18–36. 10.1002/dys.233 [DOI] [PubMed] [Google Scholar]
- Ozernov-Palchik O, & Gaab N (2016). Tackling the “dyslexia paradox”: Reading brain and behavior for early markers of developmental dyslexiax. Wiley Interdisciplinary Reviews: Cognitive Science, 7(2), 156–176. 10.1002/wcs.1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozernov-Palchik O, Wolf M, & Patel AD (2018). Relationships between early literacy and nonlinguistic rhythmic processes in kindergarteners. Journal of Experimental Child Psychology, 167, 354–368. 10.1016/j.jecp.2017.11.009 [DOI] [PubMed] [Google Scholar]
- Pasquini ES, Corriveau KH, & Goswami U (2007). Auditory processing of amplitude envelope rise time in adults diagnosed with developmental dyslexia. Scientific Studies of Reading, 11(3), 259–266. 10.1080/10888430701344280 [DOI] [Google Scholar]
- Patel AD (2003). Language, music, syntax and the brain. Nature Neuroscience, 6(7), 674–681. 10.1038/nn1082 [DOI] [PubMed] [Google Scholar]
- Patel AD (2008). Music, Language, and the Brain. New York: Oxford University Press. [Google Scholar]
- Patel AD (2011). Why would musical training benefit the neural encoding of speech? The OPERA Hypothesis. Frontiers in Psychology, 2, 142 10.3389/fpsyg.2011.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AD (2012). The OPERA hypothesis: Assumptions and clarifications. Annals of the New York Academy of Sciences, 1252(1), 124–128. 10.1111/j.1749-6632.2011.06426.x [DOI] [PubMed] [Google Scholar]
- Patscheke H, Degé F, & Schwarzer G (2016). The effects of training in music and phonological skills on phonological awareness in 4- to 6-year-old children of immigrant families. Frontiers in Psychology, 7, 1647 10.3389/fpsyg.2016.01647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, & Cohen DJ (1984). Outcomes of severe disorders of language acquisition. Journal of Autism and Developmental Disorders, 14(4), 405–421. 10.1007/BF02409831 [DOI] [PubMed] [Google Scholar]
- Peelle JE, & Davis MH (2012). Neural oscillations carry speech rhythm through to comprehension. Frontiers in Psychology, 3, 320 10.3389/fpsyg.2012.00320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz I, & Vuvan DT (2017). Prevalence of congenital amusia. European Journal of Human Genetics, 25(5), 625–630. 10.1038/ejhg.2017.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persici V, Stucchi N, & Arosio F (2019). Predicting the future in rhythm and language: The anticipation abilities of a group of Italian-speaking children In Guijarro-Fuentes P & Suárez-Gómez C (Eds.), Proceedings of GALA 2017: Language Acquisition and Development (pp. 451–468). Newcastle upon Tyne: Cambridge Scholar Publishers. [Google Scholar]
- Phillips-Silver J, & Trainor LJ (2007). Hearing what the body feels: Auditory encoding of rhythmic movement. Cognition, 105(3), 533–546. 10.1016/j.cognition.2006.11.006 [DOI] [PubMed] [Google Scholar]
- Plakas A, van Zuijen T, van Leeuwen T, Thomson JM, & van der Leij A (2013). Impaired non-speech auditory processing at a pre-reading age is a risk-factor for dyslexia but not a predictor: An ERP study. Cortex, 49(4), 1034–1045. 10.1016/j.cortex.2012.02.013 [DOI] [PubMed] [Google Scholar]
- Politimou N, Dalla Bella S, Farrugia N, & Franco F (2019). Born to speak and sing: Musical predictors of language development in preschoolers. Frontiers in Psychology, 10, 948 10.3389/fpsyg.2019.00948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power AJ, Colling LJ, Mead N, Barnes L, & Goswami U (2016). Neural encoding of the speech envelope by children with developmental dyslexia. Brain and Language, 160, 1–10. 10.1016/j.bandl.2016.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybylski L, Bedoin N, Krifi-Papoz S, Herbillon V, Roch D, Léculier L, … Tillmann B (2013). Rhythmic auditory stimulation influences syntactic processing in children with developmental language disorders. Neuropsychology, 27(1), 121–131. 10.1037/a0031277 [DOI] [PubMed] [Google Scholar]
- Puyjarinet F, Bégel V, Lopez R, Dellacherie D, & Dalla Bella S (2017). Children and adults with Attention-Deficit/Hyperactivity Disorder cannot move to the beat. Scientific Reports, 7(1), 11550 10.1038/s41598-017-11295-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan R, Camarata S, White K, Barbaresi W, Parish S, & Krahn G (2018). Population health in pediatric speech and language disorders: Available data sources and a research agenda for the field. Journal of Speech, Language, and Hearing Research, 61(5), 1279–1291. 10.1044/2018_JSLHR-L-16-0459 [DOI] [PubMed] [Google Scholar]
- Ramus F (2000). Language discrimination by human newborns and by cotton-top tamarin monkeys. Science, 288(5464), 349–351. 10.1126/science.288.5464.349 [DOI] [PubMed] [Google Scholar]
- Randell R, Somerville-Brown L, & Chen W (2018). How relevant is higher-order language deficit (HOLD) to children with complex presentations of attention-deficit hyperactivity disorder? Attention Deficit and Hyperactivity Disorders, 11(3), 325–332. 10.1007/s12402-018-0279-4 [DOI] [PubMed] [Google Scholar]
- Raschle NM, Stering PL, Meissner SN, & Gaab N (2014). Altered neuronal response during rapid auditory processing and its relation to phonological processing in prereading children at familial risk for dyslexia. Cerebral Cortex, 24(9), 2489–2501. 10.1093/cercor/bht104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautenberg I (2015). The effects of musical training on the decoding skills of German-speaking primary school children. Journal of Research in Reading, 38(1), 1–17. 10.1111/jrir.12010 [DOI] [Google Scholar]
- Redmond SM (2016). Language impairment in the attention-deficit/hyperactivity disorder context. Journal of Speech, Language, and Hearing Research, 59(1), 133–142. 10.1044/2015_JSLHR-L-15-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ML, Sell MA, & Hadley PA (1991). Social interactions of speech- and language-impaired children. Journal of Speech and Hearing Research, 34(6), 1299–1307. 10.1044/jshr.3406.1299 [DOI] [PubMed] [Google Scholar]
- Richards S, & Goswami U (2015). Auditory processing in Specific Language Impairment (SLI): Relations with the perception of lexical and phrasal stress. Journal of Speech, Language, and Hearing Research, 58(4), 1292–1305. 10.1044/2015_JSLHR-L-13-0306 [DOI] [PubMed] [Google Scholar]
- Richards S, & Goswami U (2019). Impaired recognition of metrical and syntactic boundaries in children with Developmental Language Disorders. Brain Sciences, 9(2), 33 10.3390/brainsci9020033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson U, Leppänen PHT, Leiwo M, & Lyytinen H (2003). Speech perception of infants with high familial risk for dyslexia differ at the age of 6 months. Developmental Neuropsychology, 23(3), 385–397. 10.1207/S15326942DN2303_5 [DOI] [PubMed] [Google Scholar]
- Roberts MY, & Kaiser AP (2015). Early intervention for toddlers with language delays: A randomized controlled trial. Pediatrics, 135(4),686–693. 10.1542/peds.2014-2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum S, & Regev N (2013). Timing abilities among children with developmental coordination disorders (DCD) in comparison to children with typical development. Research in Developmental Disabilities, 34(1), 218–227. 10.1016/j.ridd.2012.07.011 [DOI] [PubMed] [Google Scholar]
- Rudolph JM (2017). Case history risk factors for specific language impairment: A systematic review and meta-analysis. American Journal of Speech-Language Pathology, 26(3), 991–1010. 10.1044/2016_AJSLP-15-0181 [DOI] [PubMed] [Google Scholar]
- Ruiz-Narváez EA (2011). What is a functional locus? Understanding the genetic basis of complex phenotypic traits. Medical Hypotheses, 76(5), 638–642. 10.1016/j.mehy.2011.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabisch B, Hahne CA, Glass E, von Suchodoletz W, & Friederici AD (2009). Children with specific language impairment: The role of prosodic processes in explaining difficulties in processing syntactic information. Brain Research, 1261, 37–44. 10.1016/j.brainres.2009.01.012 [DOI] [PubMed] [Google Scholar]
- Sallat S, & Jentschke S (2015). Music perception influences language acquisition: Melodic and rhythmic-melodic perception in children with Specific Language Impairment. Behavioural Neurology, 2015, 1–10. 10.1155/2015/606470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson S, Finnström O, Flodmark O, Gäddlin PO, Leijon I, & Wadsby M (2006). A longitudinal study of reading skills among very-low-birthweight children: Is there a catch-up? Journal of Pediatric Psychology, 31(9), 967–977. 10.1093/jpepsy/jsj108 [DOI] [PubMed] [Google Scholar]
- Sansavini A, Bertoncini J, & Giovanelli G (1997). Newborns discriminate the rhythm of multisyllabic stressed words. Developmental Psychology, 33(1), 3–11. 10.1037/0012-1649.33.1.3 [DOI] [PubMed] [Google Scholar]
- Sares AG, Foster NEV, Allen K, & Hyde KL (2018). Pitch and time processing in speech and tones: The effects of musical training and attention. Journal of Speech, Language, and Hearing Research, 61(3), 496–509. 10.1044/2017_JSLHR-S-17-0207 [DOI] [PubMed] [Google Scholar]
- Sauer-Zavala S, Gutner CA, Farchione TJ, Boettcher HT, Bullis JR, & Barlow DH (2017). Current definitions of “trans-diagnostic” in treatment development: A search for consensus. Behavior Therapy, 48(1), 128–138. 10.1016/j.beth.2016.09.004 [DOI] [PubMed] [Google Scholar]
- Scabar A, Devescovi R, Blason L, Bravar L, & Carrozzi M (2006). Comorbidity of DCD and SLI: Significance of epileptiform activity during sleep. Child: Care, Health and Development, 32(6), 733–739. 10.1111/j.1365-2214.2006.00705.x [DOI] [PubMed] [Google Scholar]
- Schmidt-Kassow M, & Kotz SA (2009). Event-related brain potentials suggest a late interaction of meter and syntax in the P600. Journal of Cognitive Neuroscience, 21(9), 1693–1708. 10.1162/jocn.2008.21153 [DOI] [PubMed] [Google Scholar]
- Schön D, & Tillmann B (2015). Short-and long-term rhythmic interventions: Perspectives for language rehabilitation. Annals of the New York Academy of Sciences, 1337, 32–39. 10.1111/nyas.12635 [DOI] [PubMed] [Google Scholar]
- Selassie G, Jennische M, Kyllerman M, Viggedal G, & Hartelius L (2005). Comorbidity in severe developmental language disorders: Neuropediatric and psychological considerations. Acta Paediatrica, 94(4), 471–478. 10.1080/08035250410023692 [DOI] [PubMed] [Google Scholar]
- Sénéchal M, & LeFevre JA (2002). Parental involvement in the development of children’s reading skill: A five-year longitudinal study. Child Development, 73(2), 445–460. 10.1111/1467-8624.00417 [DOI] [PubMed] [Google Scholar]
- Slater JL, & Tate MC (2018). Timing deficits in ADHD: Insights from the neuroscience of musical rhythm. Frontiers in Computational Neuroscience, 12, 51 10.3389/fncom.2018.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slevc LR, Rosenberg JC, & Patel AD (2009). Making psycholinguistics musical: Self-paced reading time evidence for shared processing of linguistic and musical syntax. Psychonomic Bulletin & Review, 16(2), 374–381. 10.3758/16.2.374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, & Caplan DN (2018). Communication impairment in Parkinson’s disease: Impact of motor and cognitive symptoms on speech and language. Brain and Language, 185, 38–46. 10.1016/j.bandl.2018.08.002 [DOI] [PubMed] [Google Scholar]
- Smoller JW, Andreassen OA, Edenberg HJ, Faraone SV, Glatt SJ, & Kendler KS (2019). Psychiatric genetics and the structure of psychopathology. Molecular Psychiatry, 24(3), 409–420. 10.1038/s41380-017-0010-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow PC (2019). Speech-language pathology and the youth offender: Epidemiological overview and roadmap for future speech-language pathology research and scope of practice. Language, Speech, and Hearing Services in Schools, 50(2), 324–339. 10.1044/2018_LSHSS-CCJS-18-0027 [DOI] [PubMed] [Google Scholar]
- Snowling MJ (2013). Early identification and interventions for dyslexia: A contemporary view. Journal of Research in Special Educational Needs, 13(1), 7–14. 10.1111/j.1471-3802.2012.01262.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodini SM, Kemper KE, Wray NR, & Trzaskowski M (2018). Comparison of genotypic and phenotypic correlations: Cheverud’s conjecture in humans. Genetics, 209(3), 941–948. 10.1534/genetics.117.300630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovieff N, Cotsapas C, Lee PH, Purcell SM, & Smoller JW (2013). Pleiotropy in complex traits: Challenges and strategies. Nature Reviews Genetics, 14, 483–495. 10.1038/nrg3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltész F, Szűcs D, Leong V, White S, & Goswami U (2013). Differential entrainment of neuroelectric delta oscillations in Developmental Dyslexia. PLoS One, 8(10), e76608 10.1371/journal.pone.0076608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowiński J, & Dalla Bella S (2013). Poor synchronization to the beat may result from deficient auditory-motor mapping. Neuropsychologia, 51(10), 1952–1963. 10.1016/j.neuropsychologia.2013.06.027 [DOI] [PubMed] [Google Scholar]
- Steinbeis N, & Koelsch S (2008). Shared neural resources between music and language indicate semantic processing of musical tension-resolution patterns. Cerebral Cortex, 18(5), 1169–1178. 10.1093/cercor/bhm149 [DOI] [PubMed] [Google Scholar]
- Stephan MA, Lega C, & Penhune VB (2018). Auditory prediction cues motor preparation in the absence of movements. NeuroImage, 174, 288–296. 10.1016/j.neuroimage.2018.03.044 [DOI] [PubMed] [Google Scholar]
- Storch SA, & Whitehurst GJ (2001). The role of family and home in the literacy development of children from low-income backgrounds. New Directions for Child and Adolescent Development, 2001(92), 53–72. 10.1002/cd.15 [DOI] [PubMed] [Google Scholar]
- Strait DL, Hornickel J, & Kraus N (2011). Subcortical processing of speech regularities underlies reading and music aptitude in children. Behavioral and Brain Functions, 7(1), 44 10.1186/1744-9081-7-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromswold K (2006). Why aren’t identical twins linguistically identical? Genetic, prenatal and postnatal factors. Cognition, 101(2), 333–384. 10.1016/j.cognition.2006.04.007 [DOI] [PubMed] [Google Scholar]
- Sun L, Liu F, Zhou L, & Jiang C (2018). Musical training modulates the early but not the late stage of rhythmic syntactic processing. Psychophysiology, 55(2), 1–10. 10.1111/psyp.12983 [DOI] [PubMed] [Google Scholar]
- Sun Z, Zou L, Zhang J, Mo S, Shao S, Zhong R, … Song R (2013). Prevalence and associated risk factors of dyslexic children in a middle-sized city of China: A cross-sectional study. PLoS One, 8(2), e56688 10.1371/journal.pone.0056688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surányi Z, Csépe V, Richardson U, Thomson JM, Honbolygó F, & Goswami U (2009). Sensitivity to rhythmic parameters in dyslexic children: A comparison of Hungarian and English. Reading and Writing, 22(1), 41–56. 10.1007/s11145-007-9102-x [DOI] [Google Scholar]
- Tal I, Large EW, Rabinovitch E, Wei Y, Schroeder CE, Poeppel D, & Golumbic EZ (2017). Neural entrainment to the beat: The “missing-pulse” phenomenon. Journal of Neuroscience, 37(26), 6331–6341. 10.1523/JNEUROSCI.2500-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub GE, & Lazarus PJ (2012). The effects of training in timing and rhythm on reading achievement. Contemporary Issues in Education Research (CIER), 5(4), 343 10.19030/cier.v5i4.7598 [DOI] [Google Scholar]
- Thiede A, Virtala P, Ala-Kurikka I, Partanen E, Huotilainen M, Mikkola K, … Kujala T (2019). An extensive pattern of atypical neural speech-sound discrimination in newborns at risk of dyslexia. Clinical Neurophysiology, 130(5), 634–646. 10.1016/j.clinph.2019.01.019 [DOI] [PubMed] [Google Scholar]
- Thomson JM, Fryer B, Maltby J, & Goswami U (2006). Auditory and motor rhythm awareness in adults with dyslexia. Journal of Research in Reading, 29(3), 334–348. 10.1111/j.1467-9817.2006.00312.x [DOI] [Google Scholar]
- Thomson JM, & Goswami U (2008). Rhythmic processing in children with developmental dyslexia: Auditory and motor rhythms link to reading and spelling. Journal of Physiology, Paris, 102, 120–129. 10.1016/j.jphysparis.2008.03.007 [DOI] [PubMed] [Google Scholar]
- Thomson JM, Leong V, & Goswami U (2013). Auditory processing interventions and developmental dyslexia: A comparison of phonemic and rhythmic approaches. Reading and Writing, 26(2), 139–161. 10.1007/s11145-012-9359-6 [DOI] [Google Scholar]
- Tierney A, & Kraus N (2014). Auditory-motor entrainment and phonological skills: Precise auditory timing hypothesis (PATH). Frontiers in Human Neuroscience, 8, 949 10.3389/fnhum.2014.00949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin JB, Records NL, Buckwalter P, Zhang X, Smith E, & O’Brien M (1997). Prevalence of specific language impairment in kindergarten children. Journal of Speech, Language, and Hearing Research, 40(6), 1245–1260. 10.1044/jslhr.4006.1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torppa M, Eklund K, van Bergen E, & Lyytinen H (2015). Late-emerging and resolving dyslexia: A follow-up study from age 3 to 14. Journal of Abnormal Child Psychology, 43(7), 1389–1401. 10.1007/s10802-015-0003-1 [DOI] [PubMed] [Google Scholar]
- Torppa M, Poikkeus AM, Laakso ML, Eklund K, & Lyytinen H (2006). Predicting delayed letter knowledge development and its relation to grade 1 reading achievement among children with and without familial risk for dyslexia. Developmental Psychology, 42(6), 1128–1142. 10.1037/0012-1649.42.6.1128 [DOI] [PubMed] [Google Scholar]
- Toyomura A, Fujii T, & Kuriki S (2011). Effect of external auditory pacing on the neural activity of stuttering speakers. NeuroImage,57(4), 1507–1516. 10.1016/j.neuroimage.2011.05.039 [DOI] [PubMed] [Google Scholar]
- Trainor LJ, Chang A, Cairney J, & Li Y-C (2018). Is auditory perceptual timing a core deficit of developmental coordination disorder? Annals of the New York Academy of Sciences, 1423(1), 30–39. 10.1111/nyas.13701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley P, Walters RK, Maghzian O, Okbay A, Lee JJ, Fontana MA, … Benjamin DJ (2018). Multi-trait analysis of genome-wide association summary statistics using MTAG. Nature Genetics, 50(2), 229–237. 10.1038/s41588-017-0009-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylee DS, Sun J, Hess JL, Tahir MA, Sharma E, Malik R, … Glatt SJ (2018). Genetic correlations among psychiatric and immune-related phenotypes based on genome-wide association data. American Journal of Medical Genetics, Part B: Neuropsychiatric Genetics, 177(7), 641–657. 10.1002/ajmg.b.32652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullén F, Mosing MA, Holm L, Eriksson H, & Madison G (2014). Psychometric properties and heritability of a new online test for musicality, the Swedish Musical Discrimination Test. Personality and Individual Differences, 63, 87–93. 10.1016/j.paid.2014.01.057 [DOI] [Google Scholar]
- Ullman MT, & Pierpont EI (2005). Specific language impairment is not specific to language: The procedural deficit hypothesis. Cortex, 41, 399–433. 10.1016/s0010-9452(08)70276-4 [DOI] [PubMed] [Google Scholar]
- Valera EM, Spencer RMC, Zeffiro TA, Makris N, Spencer TJ, Faraone SV, … Seidman LJ (2010). Neural substrates of impaired sensorimotor timing in adult Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry, 68(4), 359–367. 10.1016/j.biopsych.2010.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Felsenfeld S, & Boomsma DI (2010). Bivariate genetic analyses of stuttering and nonfluency in a large sample of 5-year-old twins. Journal of Speech, Language, and Hearing Research, 53(3), 609–619. 10.1044/1092-4388(2009/08-0202) [DOI] [PubMed] [Google Scholar]
- van Bergen E, van der Leij A, & de Jong PF (2014). The intergenerational multiple deficit model and the case of dyslexia. Frontiers in Human Neuroscience, 8, 346 10.3389/fnhum.2014.00346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Herten M, Pasman J, van Leeuwen TH, Been PH, van der Leij A, Zwarts F, & Maassen B (2008). Differences in AERP responses and atypical hemispheric specialization in 17-month-old children at risk of dyslexia. Brain Research, 1201, 100–105. 10.1016/j.brainres.2008.01.060 [DOI] [PubMed] [Google Scholar]
- van Leeuwen T, Been P, Kuijpers C, Zwarts F, Maassen B, & van der Leij A (2006). Mismatch response is absent in 2-month-old infants at risk for dyslexia. Neuroreport, 17(4), 351–355. 10.1097/01.wnr.0000203624.02082.2d [DOI] [PubMed] [Google Scholar]
- van Zuijen TL, Plakas A, Maassen BAM, Been P, Maurits NM, Krikhaar E, … van der Leij A (2012). Temporal auditory processing at 17 months of age is associated with preliterate language comprehension and later word reading fluency: An ERP study. Neuroscience Letters, 528(1), 31–35. 10.1016/j.neulet.2012.08.058 [DOI] [PubMed] [Google Scholar]
- van Zuijen TL, Plakas A, Maassen BAM, Maurits NM, & van der Leij A (2013). Infant ERPs separate children at risk of dyslexia who become good readers from those who become poor readers. Developmental Science, 16(4), 554–563. 10.1111/desc.12049 [DOI] [PubMed] [Google Scholar]
- Verhoef E, Demontis D, Burgess S, Shapland CY, Dale PS, Okbay A, … Werge T (2019). Disentangling polygenic associations between attention-deficit/hyperactivity disorder, educational attainment, literacy and language. Translational Psychiatry, 9(1), 35 10.1038/s41398-018-0324-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viholainen H, Ahonen T, Cantell M, Lyytinen P, & Lyytinen H (2002). Development of early motor skills and language in children at risk for familial dyslexia. Developmental Medicine and Child Neurology, 44, 761–769. 10.1017/s0012162201002894 [DOI] [PubMed] [Google Scholar]
- Viholainen H, Ahonen T, Lyytinen P, Cantell M, Tolvanen A, & Lyytinen H (2006). Early motor development and later language and reading skills in children at risk of familial dyslexia. Developmental Medicine and Child Neurology, 48(05), 367 10.1017/S001216220600079X [DOI] [PubMed] [Google Scholar]
- Visscher PM, Hill WG, & Wray NR (2008). Heritability in the genomics era—Concepts and misconceptions. Nature Reviews Genetics,9(4), 255–266. 10.1038/nrg2322 [DOI] [PubMed] [Google Scholar]
- Vuolo J, Goffman L, & Zelaznik HN (2017). Deficits in coordinative bimanual timing precision in children with Specific Language Impairment. Journal of Speech, Language, and Hearing Research, 60(2), 393–405. 10.1044/2016_jslhr-l-15-0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan CY, Rüber T, Hohmann A, & Schlaug G (2010). The therapeutic effects of singing in neurological disorders. Music Perception, 27(4), 287–295. 10.1525/mp.2010.27.4.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-LS, Huss M, Hämäläinen JA, & Goswami U (2012). Basic auditory processing and developmental dyslexia in Chinese. Reading and Writing, 25(2), 509–536. 10.1007/s11145-010-9284-5 [DOI] [Google Scholar]
- Watanabe K, Stringer S, Frei O, Mirkov MU, de Leeuw C, Polderman TJC, … Posthuma D (2005). A global overview of pleiotropy and genetic architecture in complex traits. Nature Genetics, 51, 1339–1348. 10.1038/s41588-019-0481-0 [DOI] [PubMed] [Google Scholar]
- Weinert S (1992). Deficits in acquiring language structure: The importance of using prosodic cues. Applied Cognitive Psychology, 6(6),545–571. 10.1002/acp.2350060607 [DOI] [Google Scholar]
- Wells B, & Peppé S (2003). Intonation abilities of children with speech and language impairments. Journal of Speech, Language, and Hearing Research, 46(1), 5–20. 10.1044/1092-4388(2003/001) [DOI] [PubMed] [Google Scholar]
- Westerlund M, Bergkvist L, Lagerberg D, & Sundelin C (2002). Comorbidity in children with severe developmental language disability. Acta Paediatrica, 91(5), 529–534. 10.1111/j.1651-2227.2002.tb03272.x [DOI] [PubMed] [Google Scholar]
- Wieland EA, McAuley JD, Dilley LC, & Chang S-E (2015). Evidence for a rhythm perception deficit in children who stutter. Brain and Language, 144, 26–34. 10.1016/j.bandl.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Saygin AP, Sereno MI, & Iacoboni M (2004). Listening to speech activates motor areas involved in speech production. Nature Neuroscience, 7(7), 701–702. 10.1038/nn1263 [DOI] [PubMed] [Google Scholar]
- Winkler I, Haden GP, Ladinig O, Sziller I, & Honing H (2009). Newborn infants detect the beat in music. Proceedings of the National Academy of Sciences of the United States of America, 106(7), 2468–2471. 10.1073/pnas.0809035106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2010). International statistical classification of diseases and related health problems. Geneva: WHO; Retrieved from https://icd.who.int/browse10/2010/en#/F98.5 [Google Scholar]
- World Health Organization. (2018). International classification of diseases for mortality and morbidity statistics (11th Revision).Geneva: WHO. [Google Scholar]
- Woodruff Carr K, White-Schwoch T, Tierney AT, Strait DL, & Kraus N (2014). Beat synchronization predicts neural speech encoding and reading readiness in preschoolers. Proceedings of the National Academy of Sciences of the United States of America, 111(40), 14559–14564. 10.1073/pnas.1406219111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Lee SH, Mehta D, Vinkhuyzen AAE, Dudbridge F, & Middeldorp CM (2014). Research review: Polygenic methods and their application to psychiatric traits. Journal of Child Psychology and Psychiatry, 55(10), 1068–1087. 10.1111/jcpp.12295 [DOI] [PubMed] [Google Scholar]
- Yairi E, & Ambrose N (2013). Epidemiology of stuttering: 21st century advances. Journal of Fluency Disorders, 38(2), 66–87. 10.1016/j.jfludis.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Lee SH, Goddard ME, & Visscher PM (2011). GCTA: A Tool for genome-wide complex trait analysis. The American Journal of Human Genetics, 88(1), 76–82. 10.1016/j.ajhg.2010.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Large EW, Fuchs A, & Kelso JAS (2005). Gamma-band responses to perturbed auditory sequences: Evidence for synchronization of perceptual processes. Music Perception: An Interdisciplinary Journal, 22(3), 531–547. 10.1525/mp.2005.22.3.531 [DOI] [Google Scholar]
- Zelaznik HN, & Goffman L (2010). Generalized motor abilities and timing language impairment. Hearing Research, 53(2), 383–394. 10.1044/1092-4388(2009/08-0204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelaznik HN, Vaughn AJ, Green JT, Smith AL, Hoza B, & Linnea K (2012). Motor timing deficits in children with attention-deficit/hyperactivity disorder. Human Movement Science, 31(1), 255–265. 10.1016/j.humov.2011.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentner M, & Eerola T (2010). Rhythmic engagement with music in infancy. Proceedings of the National Academy of Sciences of the United States of America, 107(13), 5768–5773. 10.1073/pnas.1000121107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Zhang B, Chen Y, Zhou X, & Zuo P (2016). Environmental risk factors in Han and Uyghur children with dyslexia: A comparative study. PLoS One, 11(7), 1–15. 10.1371/journal.pone.0159042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao TC, & Kuhl PK (2016). Musical intervention enhances infants’ neural processing of temporal structure in music and speech. Proceedings of the National Academy of Sciences of the United States of America, 113(19), 5212–5217. 10.1073/pnas.1603984113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Zheng Z, Zhang F, Wu Y, Trzaskowski M, Maier R, … Yang J (2018). Causal associations between risk factors and common diseases inferred from GWAS summary data. Nature Communications, 9(1), 224 10.1038/s41467-017-02317-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk J, Bishop-Liebler P, Ozernov-Palchik O, Moore E, Overy K, Welch G, & Gaab N (2017). Revisiting the “enigma” of musicians with dyslexia: Auditory sequencing and speech abilities. Journal of Experimental Psychology: General, 146(4), 495–511. 10.1037/xge0000281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk J, Ozernov-Palchik O, Kim H, Lakshminarayanan K, Gabrieli JDE, Tallal P, & Gaab N (2013). Enhanced syllable discrimination thresholds in musicians. PLoS One, 8(12), 1–8. 10.1371/journal.pone.0080546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwicker JG, Missiuna C, & Boyd LA (2009). Neural correlates of developmental coordination disorder: A review of hypotheses. Journal of Child Neurology, 24(10), 1273–1281. 10.1177/0883073809333537 [DOI] [PubMed] [Google Scholar]


