Abstract
Cyclooxygenases (COX) -1 and -2 are key regulators of innate immune responses. We recently demonstrated that the expression of pro-inflammatory cytokines and chemokines is reduced in COX-1 null (−/−), and increased in COX-2−/− mice compared with their respective wild-type controls during lipopolysaccacharide (LPS)-induced innate immune activation. As chemokines are involved in leukocyte recruitment into the inflamed brain, we hypothesized that COX-1 and COX-2 deletion will differentially modulate blood-brain barrier (BBB) permeability in response to LPS. In the present study, using quantitative magnetic resonance imaging, we found that LPS-induced BBB disruption was exacerbated in COX-2−/− versus COX-2+/+ mice. In the hippocampus and cortex of LPS-treated mice, matrix metalloproteinase (MMP)-3 activity was significantly decreased in COX-1−/− mice, whereas in COX-2−/− mice the activity of both MMP-9 and MMP-3, known to mediate BBB breakdown, was increased. Brain mRNA expression of the leukocyte attracting chemokine Cxcl10, the intercellular interaction molecule Icam-1, the pan-leukocyte marker Cd45 was increased in COX-2−/− vs. COX-2+/+ mice, whereas Cxcl10 and Cd45 mRNA expression was decreased in COX-1−/− vs. COX-1+/+ mice after LPS. Altogether, these results indicate that COX-2 activity modulates MMP-9 and-3 activities and is necessary to maintain BBB integrity during toll-like receptor 4-dependent innate immune activation.
Keywords: cyclooxygenases, Blood-Brain Barrier, lipopolysaccharide, matrix metalloproteinases, MRI, neuroinflammation
Introduction
The blood-brain barrier (BBB) is a structural and functional barrier that is formed by the tight apposition of the brain microvascular endothelium, connected by intercellular tight junctions which limits the entry of plasma components, red blood cells and leukocytes into the brain (Zlokovic 2008). This highly specialized endothelium and the surrounding cells (pericytes, astrocytes and microglia) constitute the neurovascular unit. Disruption of the BBB integrity occurs during inflammatory conditions of the central nervous system (CNS), as observed in multiple sclerosis, bacterial meningitis, stroke and Parkinson’s disease (Weiss et al 2009). Alteration of the vascular endothelial permeability is thought to result from the release of cytokines, free radicals, matrix metalloproteinases (MMPs), nitric oxide, and products of arachidonic acid metabolism (Rosenberg 2002). MMPs have been shown to mediate BBB disruption and to contribute to the neuroinflammatory response by interacting with cytokines (Candelario-Jalil et al 2009; Rosenberg 2002).
Neuroinflammation, which involves production of cytokines, chemokines, and reactive oxygen species, can be modeled in vivo by intracerebroventricular (icv) injection of lipopolysaccharide (LPS) (Aid et al 2008; Choi et al 2008; Keene et al 2009; Rosenberg 2002). Pathophysiological events occurring in this experimental model include activation of MMP-9 (gelatinase B) and MMP-3 (stromelysin-1) and BBB breakdown (Gurney et al 2006; Mun-Bryce and Rosenberg 1998).
Prostaglandin endoperoxide synthases or cyclooxygenases (COX-1 and COX-2) are the pharmacological targets of non-steroidal anti-inflammatory drugs (NSAIDs), commonly used to treat pain and inflammation. COXs have a central role in the inflammatory cascade by converting arachidonic acid (AA), released from membrane phospholipids by a phospholipase A2 (PLA2), into bioactive prostanoids. Data on the role of each COX isoform in BBB permeability are limited and sometimes conflicting. For example, post treatment with COX inhibitors showed contrasting roles of the COX isoforms on BBB disruption in models of neuroinflammation and ischemic stroke, depending on the selectivity of the NSAIDs and the model used (Candelario-Jalil et al 2007a; Candelario-Jalil et al 2007b). The use of pharmacological inhibitors is complex, as one needs to consider the different ability of various NSAIDs to inhibit one COX isoform versus the other, possible off-target effects, as well as the route of administration, dosage, and duration of treatment. To this end, the use of knockout mice is advantageous in allowing full characterization of the specific roles of each COX isoform on BBB breakdown in response to LPS-induced neuroinflammation.
We demonstrated earlier that brain expressions of cytokines and chemokines were decreased in COX-1 deficient (COX-1−/−) mice and increased in COX-2 deficient (COX-2−/−) compared with their respective wild-type mice during LPS-induced innate immune activation (Aid et al 2008; Choi et al 2008). As chemokines are involved in the trafficking and the recruitment of leukocytes into the inflamed brain, and cytokines contribute to alter the neurovascular unit, we hypothesized that COX-1 and COX-2 will have distinct roles on the modulation of LPS-induced BBB disruption. To test this hypothesis, we used gadolinium-enhanced magnetic resonance imaging (MRI) to quantify LPS-induced BBB damage in the brain of COX-1 or COX-2 deficient mice. We also showed that LPS-induced BBB damage, as well as vascular and glial indicators of BBB integrity, correlated with MMP-9 and MMP-3 activities.
Material and methods
Animals housing
Four to six-month-old male COX-1+/+, COX-1−/−, COX-2+/+ and COX-2−/− mice on a C57BL/6–129/Ola genetic background were used. Mice were received at our animal facility at 10 weeks of age from a National institute of Environmental Health Sciences (NIEHS) colony maintained by Taconic Farms (Germantown, NY) with heterozygous by heterozygous breedings for greater than 35 generations. To prevent the inclusion of strain or genetic background confounders between COX null and wild type mice, all of the mice used in this study were progeny derived from heterozygous by heterozygous mating and therefore all contained the same strain and genetic background (Toscano et al 2007). Mice were housed at 25°C in our animal facility with a 12 h light/dark cycle with free access to food and water. All procedures were performed under NIH-approved animal protocols in accordance with NIH guidelines on the care and use of laboratory animals.
Stereotaxic surgery and intracerebroventricular injection of LPS
Mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg, i.p.) and positioned in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). Vehicle (sterile phosphate buffer saline, 5 µl) or LPS (LPS was TCA extracted from E. coli serotype 055:B5 (Sigma–Aldrich, St Louis, MO, USA); 5 µg in 5 µl of vehicle) was administered into the cerebral lateral ventricle using a 10 µl syringe with a 33 gauge needle (World Precision Instruments, Sarasota, FL) and a syringe pump (Stoelting, Wood Dale, IL) at a rate of 1 µl/min. This dose of LPS has been shown by us and by others to produce significant neuroinflammation 24 h after injection (Aid et al 2008; Choi et al 2008; Goralski et al 2005; Milatovic et al 2004). The coordinates for the stereotaxic injections were −2.3 mm dorsal/ventral, −1.0 mm lateral, and −0.5 mm anterior/posterior from the bregma (Paxinos and Franklin 2001). The needle was kept in this position for an additional 5 min after injection and then retrieved slowly from the brain.
Magnetic Resonance Imaging
Twenty-four hours after intracerebroventricular injection of LPS, mice were anesthetized by breathing freely oxygen-enriched air (FiO2 ~50%) containing isoflurane (5% induction followed by 1.5 to 2% maintenance. Rectal temperature was monitored and maintained at 37.5 ± 0.5 °C by means of a feedback-regulated MRI-compatible electric bed (Rapid Biomedical GmbH, Rimpar, Germany). Real-time monitoring of physiologic parameters (rectal temperature, pulse oximetry, end-tidal CO2, heart rate and respiratory rate) was performed during the entire duration of the study. A PE-10 catheter was placed into the lateral tail vein of each animal for injection of the contrast agent gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA). MRI with Gd-DTPA has been used both in rodents (Candelario-Jalil et al 2007b) and in humans to assess BBB disruption associated with stroke, brain injury and multiple sclerosis (Mikulis and Roberts 2007; Takanashi et al 2000).
MRI was performed on a 7T/30cm USR Bruker AVI MR scanner (Bruker Biospin, Billerica, MA), equipped with a 150 mm ID gradient set capable of 450 mT/m and slew rates of 3000 T/(m·s) (Resonance Research Inc., Billerica, MA). A transmit-only linear radio-frequency (RF) coil (i.d., 120 mm) was used for magnetic resonance excitation, and a dedicated 10 mm ID elliptical surface RF coil was used for signal acquisition. Initial localizer images were acquired using the following parameters: 2D RARE ; TR/TEeff, 12000/67 ms; matrix, 256 × 256(RARE factor 16); FOV, 2.56 cm; slice thickness, 0.5 mm; and one slice per orientation. After the localizer images were acquired, localized shimming over the entire brain was performed using the FASTMAP sequence (Gruetter 1993), and a new set of T2-weighted imaging was performed with the same parameters listed above over 24 consecutive coronal slices to serve as an additional reference scan and to identify the slice containing the LPS injection site. Once the LPS injection site was identified, 16 consecutive 0.5 mm-thick slices, running from approximately 4 mm anterior to 4 mm posterior to Bregma, were chosen for further data acquisition. High resolution spin-echo, T1-weighted MRI were acquired using the following parameters: TR/TE, 250/8.67 ms; matrix, 256 × 256; field of view (FOV), 2.56 cm; slice thickness, 0.5 mm; 16 slices; nominal spatial resolution, 100 × 100 × 500 µm3; total acquisition time 2min8s. Interleaved with the T1-weighted images, we also acquired quantitative T1 maps from the 8 innermost slices within the 16-slice set, using the following parameters: pulse sequence, spin-echo echo-planar imaging (SE-EPI); preparation, inversion-recovery; TR/TE, 10000/27 ms; matrix, 64 × 64; FOV, 2.56 cm; slice thickness, 0.5 mm; 8 slices; nominal spatial resolution, 400 × 400 × 500 µm3; total acquisition time 6min40s. For the T1-mapping, 20 inversion times (TI) were used, according to TI(n) = (50 + 500 × n) ms, where n = 0, 1, 2,… 19. The MR protocol for evaluating BBB permeability consisted of a reference baseline acquisition using both the T1-weighted MRI and the fast T1 mapping EPI sequence. Mice were then injected with 2.0 mmol/kg Gd-DTPA (molecular mass = 938 Da; Berlex, Montville, NJ) as a 0.1 ml bolus into the lateral tail vein via an indwelling catheter, followed by imaging with both the T1-weighted MRI and the fast T1 mapping EPI sequences, collecting 16 in an interleaved manner over 45 min.
The acquired MRI data were transferred to a dedicated computer workstation for post-processing. Post-processing of the raw data involved generating T1 maps from the raw data, reconstruction of permeability coefficient maps, and construction of the Patlak plots according to the methodology described in (Patlak et al 1983). All the data processing was performed using in-house software written in MATLAB (Mathworks, Natick, MA). Image analysis was performed using ImageJ (NIH, Bethesda, MD) and Stimulate software. Region of interest (ROI) analysis was performed by manually assisted segmentation of the hippocampus using routines written in MATLAB.
RNA extraction and Quantitative Real-Time PCR
Total RNA was extracted from the whole brain using RNeasy Lipid Tissue Midi Kit (Qiagen, Valencia, CA, USA). Briefly, 5 µg of total RNA were reverse transcribed using a High Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA). Quantitative PCR for tissue inhibitor of metalloproteinase-1 (Timp-1), intercellular adhesion molecule-1 (Icam-1), chemokine (C-X-C motif) ligand 10 (Cxcl10) and lymphocyte common antigen (ptprc or Cd45) was performed using specific FAM™ dye-labeled TaqMan gene expression assays (Applied Biosystems) with an ABI PRISM 7000 Sequence Detection System (Applied Biosystems). Data were analyzed using the comparative threshold cycle (ΔΔCt) method (Livak and Schmittgen 2001), and results were normalized with phosphoglycerate kinase 1 (Pgk1) as an endogenous control and expressed as fold difference from the vehicle-injected respective wild type mice, as reported earlier (Aid and Bosetti 2007; Aid et al 2008; Choi et al 2008).
Gelatin-Substrate Zymography
For zymography, tissues were homogenized in a lysis buffer containing 50 mM Tris-HCl pH 7.6, 150 mM NaCl, 5 mM CaCl2, 0.05% Brij-35, 1% Triton X-100, and 0.02% NaN3. Protein concentration in the homogenate was determined using the Micro BCA Protein Assay kit (Pierce, Rockford, IL). MMP-2 and MMP-9 in the homogenate were concentrated with Gelatin-Sepharose 4B beads (GE Healthcare Biosciences, Piscataway, NJ), and analyzed by gelatin zymography as previously described (Candelario-Jalil et al 2007b). Briefly, 800 µg of total protein in 500 µl was incubated with 80 µl of Gelatin-Sepharose 4B beads for 1 h at 4°C with gentle agitation. The beads were collected and the gelatinases were eluted by incubating with 80 µl of elution buffer (10% DMSO in phosphate buffered saline) for 30 min at 4°C with gentle shaking. Equal amount of samples (20 µl) were mixed in a loading buffer containing 80 mM Tris-HCl pH 6.8, 4% sodium dodecyl sulfate (SDS), 10% glycerol, and 0.01% bromophenol blue. Samples were electrophoretically separated on 10% SDS-polyacrylamide gels co-polymerized with 1 mg/ml gelatin (Sigma-Aldrich, St. Louis, MO) under non-reducing conditions. Gels were washed in 2.5% Triton X-100 and then incubated for 40 h at 37°C in a developing buffer containing 50 mM Tris pH 7.6, 5 mM CaCl2, 0.2 mM NaCl, and 0.02% Brij-35. After incubation, gels were stained with 0.125% Coomassie Brilliant Blue R-250 (Sigma-Aldrich, Saint Louis, MO) for 30 min in 10% acetic acid and 50% methanol. Gels were destained with a solution containing 10% acetic acid and 10% methanol until clear bands of gelatinolysis appeared on a dark blue background. To confirm that detected activities were zinc-dependent gelatinases, some zymogram gels were incubated with developing solution in the presence of 10 mM EDTA. Disappearance of lytic bands in these gels confirmed the metal dependence of gelatinolytic activity characteristic of MMPs. The gels were dried and densitometrically scanned (Alpha Imager™ 2200; Alpha Innotech, San Leandro, CA). Molecular weights were determined by both protein standards (Bio-Rad Laboratories, Hercules, CA), and conditioned media from HT1080 human fibrosarcoma cells, a well-known source of MMP-2 and -9. In addition, a mixture of active MMP-2 and -9 standards (Chemicon International, Temecula, CA) were used as gelatinase controls. Data were expressed as relative lysis units.
Fluorometric Assay of MMP-3 Enzymatic Activity
The activity of MMP-3 (stromelysin-1) was measured fluorometrically using a 5-carboxyfluorescein (5-FAM)/QXL520 fluorescence resonance energy transfer (FRET) peptide (AnaSpec Inc., San Jose, CA). In the intact FRET peptide [5-FAM-Arg-Pro-Lys-Pro-Val-Glu-Nva-Trp-Arg-Lys(QXL520)-NH2], the fluorescence of 5-FAM is quenched by QXL520. Upon cleavage into two separate fragments by the MMP-3 present in the sample, the fluorescence of 5-FAM is recovered and can be monitored at excitation/emission wavelengths of 490/520 nm. This peptide has been documented to be cleaved by only MMP-3 and MMP-12 (macrophage elastase) but not by other MMPs (Nagase et al 1994). Brain homogenates containing 100 µg of protein (aliquot from the lysate prepared for zymography) were mixed with assay buffer (50 mM Tris-HCl, pH 7.6, 200 mM NaCl, 5 mM CaCl2, 20 µM ZnSO4, and 0.05% Brij-35) containing the FRET peptide (1 µM final concentration) to a final volume of 200 µl. The change in fluorescence (expressed as relative fluorescence units) was monitored at 5-min intervals for 1 h at 37°C using a luminescence spectrometer (model LS55; PerkinElmer Instruments, Buckinghamshire, UK) attached to a workstation running FL WinLab software. For each sample, a linear regression was calculated using Sigma Plot for Windows, version 11.0 (Systat software, Inc, San Jose, CA, USA) and the mean velocity was calculated for each group.
Statistics
Data were expressed as mean ± SEM. Raw data obtained from MRI and MMP-9 activity were analyzed using Student’s t-test. MMP-3 activity was analyzed using two-way ANOVA followed by a posttest Bonferroni when p<0.05. For gene expression, log-transformed 2−ΔΔCt values were analyzed using two-way ANOVA (followed by a posttest Bonferroni). p values < 0.05 were considered statistically significant. Statistical analysis was performed using the program GraphPad Prism for Windows, version 4.0 (GraphPad Software Incorporated, San Diego, CA).
Results
LPS-induced blood-brain-barrier disruption is exacerbated in COX-2−/− mice
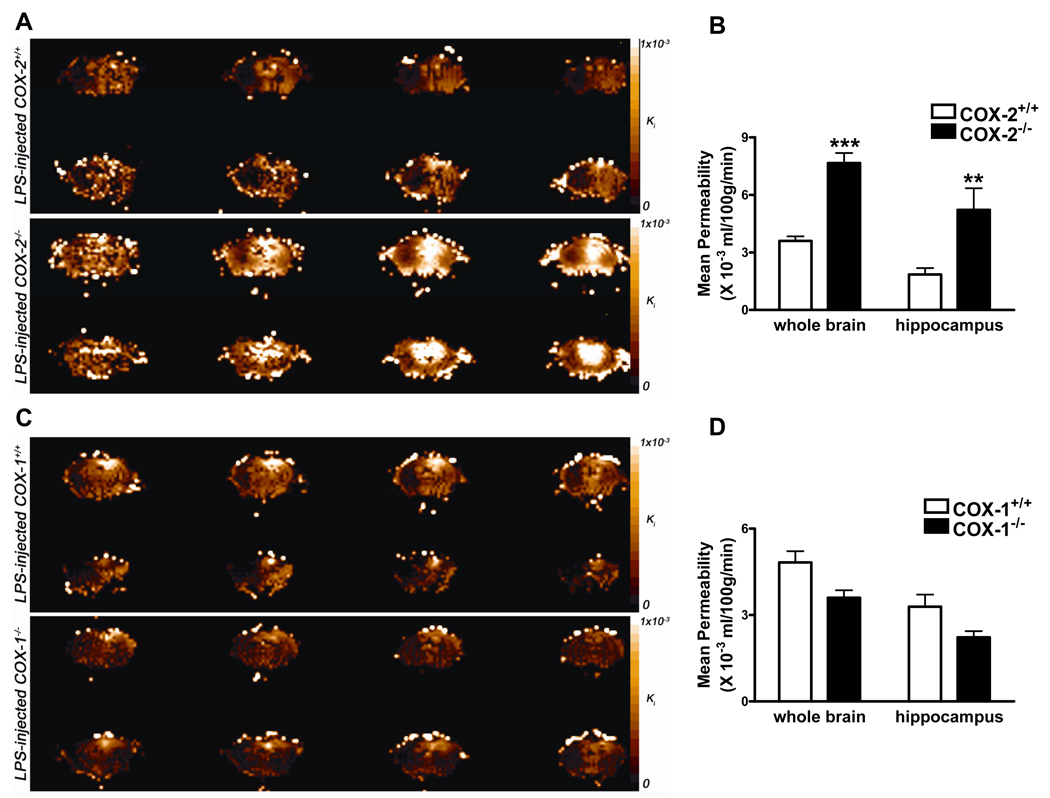
Using a quantitative MRI technique, we found that intracerebroventricular LPS induced an increased BBB permeability to Gd-DPTA at 24 h, which is in agreement with previous data obtained by others using biochemical techniques (Gurney et al 2006; Mun-Bryce and Rosenberg 1998). Figure 1A shows color-coded permeability coefficient (Ki) maps, expressed in mL/100g/min, for four representative mice. The top row shows the Ki maps for two LPS injected COX-2+/+ mice, whereas the bottom row shows Ki maps for two LPS injected COX-2−/− mice. Four consecutive 1 mm-thick coronal brain sections are shown from each mouse to follow the spatial extent of the gadolinium leakage into the brain. LPS injection into the right cerebral lateral ventricle causes a significant increase in BBB permeability to gadolinium compared with injection of vehicle (data not shown). Even though the increase in BBB permeability favors the right hemisphere of the brain, it spreads both posterior and anterior from the injection site. Therefore, whole brain analysis was performed to assess the effects of LPS on BBB breakdown in the different mice genotypes. The mean Ki was significantly higher in the whole brain of LPS-injected COX-2−/− mice compared with COX-2+/+ mice (Figure 1B). Previously, we have demonstrated that LPS causes neuronal damage and glial activation in the hippocampus (Aid et al 2008; Choi et al 2008). Therefore, we performed region-of-interest analysis to assess the effects of LPS on BBB disruption in the hippocampus. We found that the mean Ki was also higher in the hippocampus of COX-2−/− mice compared to COX-2+/+ mice after treatment with LPS (Figure 1B). Figure 1C shows the Ki maps for two LPS injected COX-1+/+ mice, while the bottom row shows Ki maps for two LPS injected COX-1−/− mice. Although the mean Ki was not significantly different in the whole brain of LPS-injected COX-1−/− mice compared to COX-1+/+ mice (Figure 1D), there was a trend to a decrease (p=0.06) in the hippocampus of COX-1−/− 24h after icv LPS (Figure 1D).
Figure 1. Effects of COX-2 and COX-1 deficiency on LPS-induced increase in BBB permeability, using gadolinium-enhanced magnetic resonance imaging.
A. Representative color-coded permeability coefficient map: LPS-injected COX-2+/+ mice (top two rows) and LPS-injected COX-2−/− mice (bottom two rows). Four consecutive 1 mm-thick coronal brain sections are shown for each mouse. B. Plot of mean permeability coefficient estimates Ki (mL/100g/min) in the whole brain and in the hippocampus obtained by Patlak Plot analysis in LPS-injected COX-2+/+ (white bars) and LPS-injected COX-2−/− mice (black bars). C. Representative color-coded permeability coefficient map: LPS-injected COX-1+/+ mice (top two rows) and LPS-injected COX-1−/− mice (bottom two rows). D. Plot of mean permeability coefficient estimates Ki (mL/100g/min) in the whole brain and in the hippocampus obtained by Patlak Plot analysis in LPS-injected COX-1+/+ (white bars) and LPS-injected COX-1−/− mice (black bars). Data are presented as mean ± SEM (n=5–6). ***p<0.001, **p<0.01 vs. LPS-injected respective wild-type mice.
MMP-9 gelatinolytic activity is increased in COX-2−/− mice after LPS
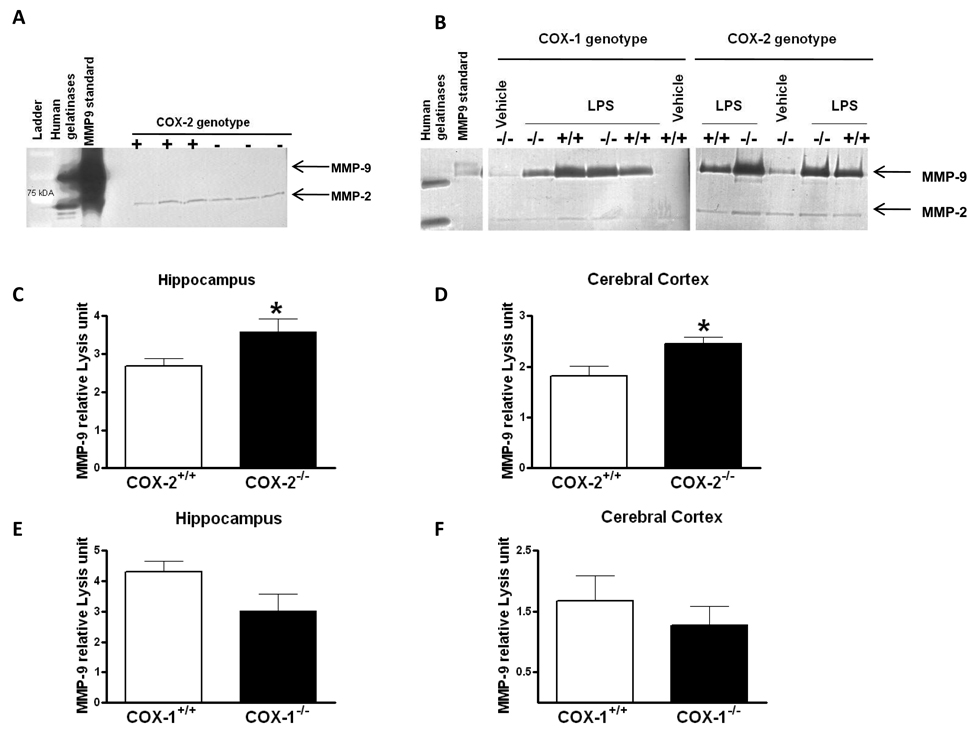
As gelatinase B (MMP-9) is involved in BBB disruption during LPS-induced neuroinflammation (Mun-Bryce and Rosenberg 1998), we investigated the effects of COX-1 or COX-2 deletion on MMP-9 activity in hippocampus and cerebral cortex, 24 h after icv injection of LPS. We used gelatin zymogram to assess the activity of MMP-9 as measured by the densitometric analysis of the 98 kDa band. Previous studies reported that only the full-length form of MMP-9 is detected in biological samples (active enzyme containing a propeptide domain) (Candelario-Jalil et al 2007b; Fridman et al 2003).
No MMP-9 band was detected in hippocampus or cerebral cortex of naïve COX-1 and COX-2 deficient and wild type mice, even after 48h incubation of the gels (Figure 2A), however, a band around 72kDa corresponding at the constitutive MMP-2 was detected in both brain areas and genotype (Figure 2A). Intracerebroventricular injection of LPS produced a significant increase in MMP-9 activity after 24 h compared with the vehicle-injected control, as revealed by gelatin zymography (Figure 2B). Deletion of COX-2 produced a significant increase in the gelatinolytic activity of MMP-9, both in hippocampus and cerebral cortex (Figure 2C–D). In contrast, COX-1 deletion did not affect significantly the gelatinolytic activity of MMP-9 in the hippocampus (Figure 2E, p=0.09) or in the cerebral cortex (Figure 2F; p=0.44). No significant change in MMP-2 activity was observed after LPS treatment or between the different genotypes (Figure 2B).
Figure 2. MMP-9 activity in hippocampus and cerebral cortex of COX-1+/+, COX-1−/−, COX-2+/+, and COX-2−/− mice 24 h after LPS.
A. Representative gelatin zymogram showing the absence of MMP-9 activity in naïve COX-2 deficient and wild type mice after 48h incubation of the gels. Only a band around 72kDa corresponding at MMP-2 was detected. B. Representative gelatin zymogram showing the effects of COX-1 and COX-2 deletion on MMP-9 activity. Mice were injected intracerebroventricularly with 5 µg LPS. C–F. Quantitative analysis of gelatinolytic activity of MMP-9 by zymography in hippocampus and cerebral cortex of LPS-injected COX-2+/+ (n=8), COX-2−/− (n=5), COX-1+/+ (n=5) and COX-1−/− (n=6) mice. Densitometric analysis of lytic zones at 72 kDa, corresponding to pro-MMP-2 activity, showed no significant change among treatments (data not shown). Data are presented as mean ± SEM of the 98-kDa band in arbitrary densitometric units. *p<0.05 vs. LPS-injected respective wild-type.
MMP-3 activity is increased in COX-2−/− mice but decreased in COX-1−/− mice 24 h after LPS
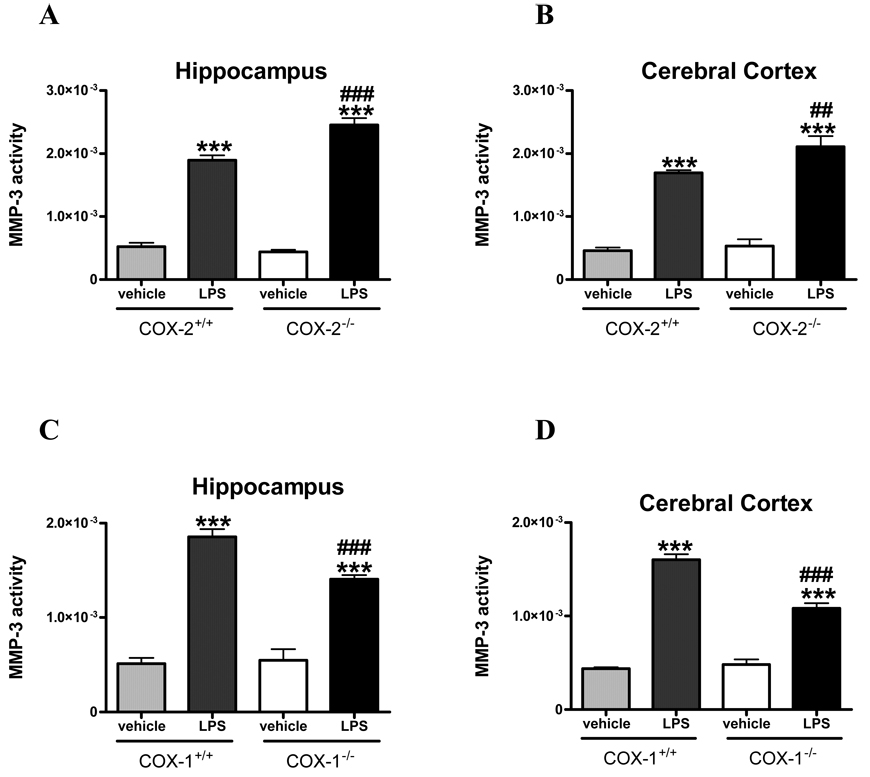
Stromelysin-1 (MMP-3) has been also shown to participate to LPS-induced BBB disruption (Gurney et al 2006); therefore we examined the effects of COX deletion on MMP-3 activity. In naïve mice, fluorescent enzymatic assay for MMP-3 activity was not different between COX deficient and their respective wild type mice (data not shown). LPS produced a significant increase (about 3–4 times) in MMP-3 activity after 24 h compared with vehicle treated mice in both hippocampus and cerebral cortex (Figure 3A–D). MMP-3 activity was significantly increased in LPS-injected COX-2−/− mice compared with COX-2+/+ mice in both hippocampus (Figure 3A, p < 0.001) and cerebral cortex (Figure 3B, p < 0.01). In contrast, MMP-3 activity was significantly decreased in LPS-injected COX-1−/− compared with COX-1+/+ mice, in both brain areas (Figure 3C–D, p <0.001), indicating that MMP-3 activity contributes to the differential effects of COX-1 and COX-2 deficiency on BBB disruption.
Figure 3. MMP-3 activity in hippocampus and cerebral cortex of COX-1+/+, COX-1−/−, COX-2+/+, and COX-2−/− mice 24 h after LPS.
Hippocampal and cortical MMP-3 activity were similar in vehicle injected mice for both COX genotype. LPS-induced increase in MMP-3 activity was higher in COX-2−/− mice (A, B) whereas it was reduced in COX-1−/− mice (C, D) as compared with their respective wild-type controls. Data are presented as mean velocity (relative fluorescence unit) ± SEM (Vehicle-injected mice n=3–4; LPS-injected mice, n=5–8). ***p<0.001 vs.vehicle-injected mice; ##p< 0.01, ###p < 0.001 vs. LPS-injected wild type mice.
Gene expression of Timp-1, Cxcl10, Cd45, and Icam-1 are differently regulated in the brain of COX-2−/− and COX-1−/− mice after LPS
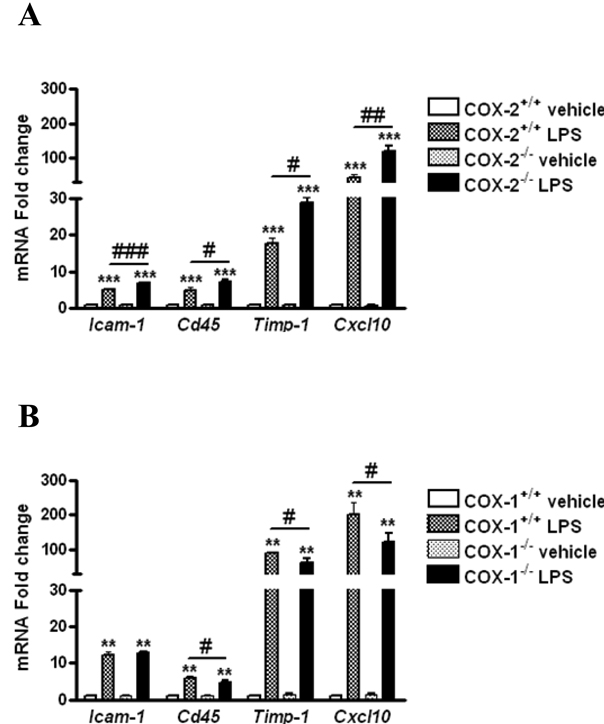
To assess the role of COX-2 deletion in worsening LPS-induced BBB disruption we examined the expression of several genes known to modulate BBB integrity, such as Timp-1, factor that mediates tissue penetration, the chemokine Cxcl10 implicated in the attraction of monocytes/macrophages, intercellular adhesion molecule-1 (Icam-1), which is an important endothelial adhesion molecule for the immune cells, and Cd45 a pan-leukocyte marker. LPS significantly increased the expression of Timp-1, Cxcl10, Cd45 and Icam-1 mRNA in all genotype (Figure 4A–B). However, the mRNA levels of these genes were higher in COX-2−/− compared with COX-2+/+ mice after LPS (Figure 4A). On the contrary, the mRNA levels of Timp-1, Cxcl10 and Cd45 were decreased in COX-1−/−compared with COX-1+/+ mice after LPS (Figure 4B), except for Icam-1 mRNA level.
Figure 4. Effects of COX-2 and COX-1 deficiency on LPS-induced expression of mediators of BBB disruption and regulators of brain infiltration by immune cells.
Quantitative real time-PCR analysis of whole brain Timp-1, Cxcl10, Cd45, Icam-1 for COX-2+/+, COX-2−/−, COX-1+/+ and COX-1−/− mice 24 h after intracerebroventricular injection of LPS or vehicle. Data are presented as mean ± SEM (n = 4–6). **p < 0.01 ***p< 0.001 vs. vehicle-injected mice; #p< 0.05, ##p< 0.01, ###p < 0.001 vs. LPS-injected wild-type mice.
Discussion
We have shown earlier that COX-1 and COX-2 play distinct roles during excitotoxic brain damage (Toscano et al 2008a; Toscano et al 2008b) and in LPS-induced acute neuroinflammation (Aid et al 2008; Choi et al 2008). In this study we used quantitative MRI combined with molecular techniques to investigate how COX-1 and COX-2 specifically modulate BBB integrity during LPS-induced innate immune activation. We showed that COX-2−/− mice exhibit an increase in LPS-induced BBB disruption in the whole brain and the hippocampus, mediated by an increase in MMP-9 and MMP-3 activities. We also showed that COX-1 deletion reduced the MMP-3 activity with no change in MMP-9 activity, although it did not significantly change the LPS-induced BBB permeability. These results further support the hypothesis that COX-1 and COX-2 mediate differential effects during the neuroinflammatory response, especially in maintaining the integrity of the neurovascular unit.
In physiological conditions, COX-1 and COX-2 have been shown to have distinct roles in cerebrovascular coupling. Specifically, COX-1 is important in the maintenance of resting cerebrovascular tone and in selected responses of the cerebral circulation, whereas COX-2 is involved in functional hyperemia (Niwa et al 2000; Niwa et al 2001; Stefanovic et al 2006). The present study provides new evidence for the specific action and the functional significance of COX-1 and COX-2 during neuroinflammation. The experimental model of isolated CNS inflammation we used in this study has been shown to result in pathophysiological changes characteristic of bacterial meningitis, including BBB breakdown, recruitment of neutrophils, and generation of meningeal inflammation (Mun-Bryce and Rosenberg 1998).
The integrity of the BBB during neuroinflammation is highly dependent on the endothelial status, the elaboration of cytokines and chemokines and the recruitment of circulating proinflammatory cells (Zlokovic 2008). In this study, we demonstrated that COX-2 deletion increases, whereas COX-1 deletion tends to reduces LPS-induced increase in BBB permeability and that MMP-3 activity contributes to this differential effect. Several studies agreed upon the increased expression of COX-2 in endothelial cells after central or systemic injection of endotoxin or cytokines (Cao et al 1998; Cao et al 1999; Laflamme et al 1999). Although LPS is a well known primary activator of microglia in the CNS, COX-2 expression induced by LPS seems restricted to endothelial cells, with little evidence of expression in microglia or macrophage cells (Cao et al 1999). There are conflicting data on the effect of COX inhibition on BBB permeability. In vitro studies showed that indomethacin, a COX-1 preferential inhibitor, and NS-398, a selective COX-2 inhibitor, both attenuated the cytokines-induced increase in transendothelial electrical resistance in endothelial cells (de Vries et al 1996; Mark et al 2001). Celecoxib, another COX-2 selective inhibitor, also has been shown to decrease the expression of tight junction proteins and of the vascular marker Icam in vitro (Germann et al 2008). However, in vivo acute post-administration of indomethacin, a preferential COX-1 inhibitor, but not nimesulide, a COX-2 selective inhibitor, attenuated TNF-α-induced BBB disruption (Candelario-Jalil et al 2007b). Explanation for this disparity may lie in the lack of complete inhibition of one isoform over the other by the NSAID used. For instance, nimesulide is not a “highly” selective COX-2 inhibitor, therefore depending on the dose and the dosing paradigm (acute vs. chronic), either not a sufficient inhibition of COX-2 is reached or COX-1 is also inhibited. The time of treatment may also have an important function. For instance, COX-2 selective inhibition has been linked to either attenuation or potentiation of kainate-induced excitotoxic damage depending on whether the treatment was given before or after kainate (Candelario-Jalil et al 2000; Kunz and Oliw 2001; Toscano et al 2008b). In agreement with our in vivo results, a recent study reported that chronic pretreatment with nimesulide precipitated the encephalopathy and exacerbated neuronal death in an experimental model of Wernicke’s encephalopathy (Gu et al 2008).
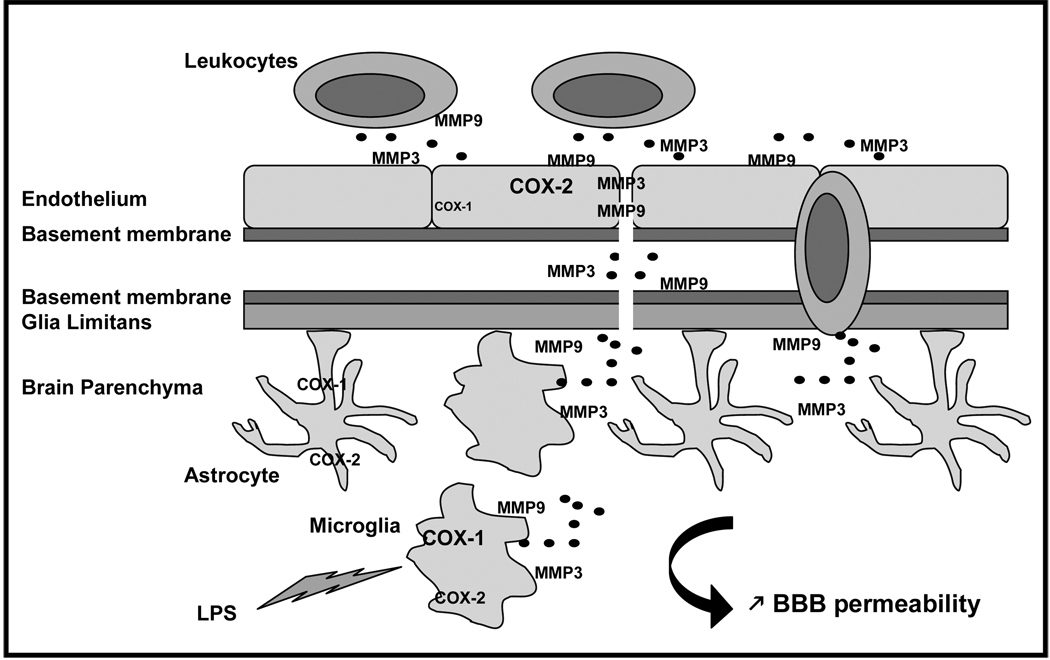
One possible explanation for the differential effects of COX-1 and COX-2 on BBB integrity may lie in MMP-3 (stromelysin-1) and MMP-9 (gelatinase B), which are two major inducible MMPs that have been recently identified during the neuroinflammatory response as causal mediators of BBB damage and infiltration of blood-borne cells (Candelario-Jalil et al 2009). We showed that the activity of MMP-9 and MMP-3 is increased after LPS in the COX-2−/− mice, and that their increase paralleled the LPS-induced BBB damage. Our data further confirm the role of MMP-9 and MMP-3 activities in mediating BBB permeability and also link those activities to a COX-dependent pathway. MMPs can be produced by all cell types in the brain including microglia, astrocytes, neurons and endothelial cells (Rosenberg 2002). However, a recent in vitro study showed that MMPs are major inflammatory mediators released by activated microglia (Woo et al 2008). As COX-2−/− mice display an exacerbated inflammatory response, with increases in microglia activation and in inflammatory mediators such as cytokines and chemokines (Aid et al 2008), MMPs produced by activated microglia are likely to mediate the increased LPS-induced BBB disruption. MMPs are also released by white blood cells to facilitate the passage of leukocytes across the BBB: activated microglia, through the release of chemokines can also further increase the number/and or the activation state of leukocytes recruited into the inflamed brain, contributing to the exacerbated inflammatory response and BBB damage in the LPS-injected COX-2−/− mice (Figure 5).
Figure 5. Distinct effects of COX-1 and-2 deletion on LPS-induced Blood-Brain Barrier disruption.
Lipopolysaccharide (LPS) activates primarily microglia, which releases cytokines, chemokines, MMP-9 and MMP-3. The neuroinflammatory response, through chemokines and cytokines, also propagates to the surrounding cells such as astrocytes and endothelial cells. Chemokines initiate signals that lead to leukocyte arrest, adhesion and extravasation through the endothelium and perivascular space. The action of MMP-9 and MMP-3, released by activated microglia and leukocytes, allow leukocyte entry into the brain parenchyma by attacking the basement membrane and glia limitans. Although present in all cell types, COX-1 is mainly found in microglia, and COX-2 is predominant in neurons and endothelial cells. COX-1 deletion decreases chemokine expression and MMP-3 activity which most likely will attenuate LPS-induced increase in BBB permeability. However, COX-2 deletion exacerbates LPS-induced BBB disruption, through an increase in chemokine release and MMP-9 and MMP-3 activities.
Interestingly, COX-1 deletion significantly decreases MMP-3 activity in both hippocampus and cerebral cortex, whereas MMP-9 activity was not significantly changed in the LPS-injected COX-1−/− mice. Although the discrepancy between MMPs may be ascribed to the differential sensitivity of the methods used to measure MMP-9 activity (gelatin zymography) and MMP-3 activity (fluorometric assay), it is possible that MMP-3 is the key player in mediating the differential effects of COX-1 and COX-2 deficiency on LPS-induced BBB disruption (Figure 5). Indeed, the reduced MMP-3 activity observed in the COX-1−/− mice are in agreement with the decrease in the expression of proinflammatory cytokines, chemokines, and oxidative stress markers, and with the reduction of glial activation and neuronal damage in response to LPS previously reported in the COX-1−/− mice (Choi et al 2008). Nevertheless, we could only detect a trend of a decrease in LPS-induced BBB breakdown in COX-1−/− compared with COX-1+/+ mice (P=0.06), suggesting that the change in cytokines/chemokines expression, glial activation, MMP-3 and MMP-9 activities are likely the causative events to the change in BBB permeability in response to LPS.
Cytokines such as IL-1β and TNF-α have been shown to mediate BBB disruption in vivo (Blamire et al 2000; Sibson et al 2002). We previously showed that brain levels of IL-1β, TNF-α and MIP-1α are downregulated in COX-1−/− and upregulated in COX-2−/− mice compared with their respective wild type, after intracerebroventricular LPS (Aid et al 2008; Choi et al 2008). In this study, we also show that the leukocyte attracting-chemokine Cxcl10, the pan leukocyte marker Cd45, and the adhesion molecule Icam-1 are upregulated in the COX-2−/−compared with COX-2+/+ mice after icv LPS. On the contrary, the gene expression of Cxcl10 and Cd45 are downregulated in the COX-1−/−compared with COX-1+/+ mice after intracerebroventricular LPS. IL-1β and TNF-α are particularly important for activation of the endothelium and leukocytes; chemokines control leukocytes extravasation and chemotaxis towards the affected tissues and the endothelial adhesion molecule Icam-1 is essential to leukocytes adhesion and transmigration into the inflamed neural tissue (Man et al 2007). As the expression of the above markers paralleled the LPS-induced BBB damage, they are likely to further contribute to the differential effect of COX-1 and COX-2 deficiency on the neuroinflammatory response by promoting leukocytes infiltration into the inflamed brain.
Conclusion
These findings prove in knockout models that COX-1 and COX-2 display unique roles in mediating BBB disruption during activation of the innate immune response. In particular, in this model COX-2 deletion increased the LPS-induced BBB disruption, through an increase in MMP-9 and MMP-3 activities, and in gene expression of chemokine (Cxcl10), leukocyte (Cd45) and endothelial (Icam-1) markers; whereas COX-1 deletion decreased MMP-3 activity and gene expression of Cxcl10 and Cd45. This study provides new evidence for the specific action and the functional significance of COX-1 and COX-2 during neuroinflammation. As alteration of BBB integrity or function seems to be a common event in neuropathologies with a marked inflammatory component, such as Parkinson’s disease, Alzheimer’s disease, multiple sclerosis and bacterial meningitis (Weiss et al 2009; Zlokovic 2008), our data suggest that the use of COX-1 preferential inhibitors should be preferred to the use of specific COX-2 inhibitors to preserve BBB integrity. This study also warrants new research into the mechanisms underlying the specific roles of COX-1 and COX-2 in the cross-talk between the blood-CNS barrier and the innate immune system.
Acknowledgements
We thank Dr. Robert Langenbach for providing COX-1−/−, COX-2−/− and wild type mice. We thank Xian Feng (Lisa) Zhang for her technical help in preparing the mice for MRI. We also thank Dr. Christopher D. Toscano for useful experimental suggestions and discussion and Dr. Alan B. Zonderman for statistical help. This work was supported by the NIH Intramural Research Program (NIA and NINDS) and by grants from the American Heart Association (0720160Z to ECJ) and the NIH (5R01NS04547 to GAR).
Abbreviations
- AD
Alzheimer’s disease
- ANOVA
analysis of variance
- BBB
blood-brain barrier
- COX
cyclooxygenase
- CNS
central nervous system
- Cxcl10
chemokine-C ligand 10
- Icam-1
intercellular adhesion molecule-1
- icv
intracerebroventricular
- IL-1β
interleukin1 beta
- LPS
lipopolysaccharide
- MIP-1α
macrophage inflammatory protein 1 alpha
- MMP
matrix metalloproteinase
- MRI
magnetic resonance imaging
- Timp-1
tissue inhibitor of metalloproteinase-1
- PBS
phosphate-buffered saline
- TNF-α
Tumor Necrosis Factor alpha
Footnotes
Competing interests
The authors declare that they have no competing interests.
REFERENCES
- Aid S, Bosetti F. Gene expression of cyclooxygenase-1 and Ca(2+)-independent phospholipase A(2) is altered in rat hippocampus during normal aging. Brain Res Bull. 2007;73:108–113. doi: 10.1016/j.brainresbull.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aid S, Langenbach R, Bosetti F. Neuroinflammatory response to lipopolysaccharide is exacerbated in mice genetically deficient in cyclooxygenase-2. Journal of neuroinflammation [electronic resource] 2008;5:17. doi: 10.1186/1742-2094-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blamire AM, Anthony DC, Rajagopalan B, Sibson NR, Perry VH, Styles P. Interleukin-1beta -induced changes in blood-brain barrier permeability, apparent diffusion coefficient, and cerebral blood volume in the rat brain: a magnetic resonance study. J Neurosci. 2000;20:8153–8159. doi: 10.1523/JNEUROSCI.20-21-08153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candelario-Jalil E, Ajamieh HH, Sam S, Martinez G, Leon Fernandez OS. Nimesulide limits kainate-induced oxidative damage in the rat hippocampus. Eur J Pharmacol. 2000;390:295–298. doi: 10.1016/s0014-2999(99)00908-5. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Gonzalez-Falcon A, Garcia-Cabrera M, Leon OS, Fiebich BL. Post-ischaemic treatment with the cyclooxygenase-2 inhibitor nimesulide reduces blood-brain barrier disruption and leukocyte infiltration following transient focal cerebral ischaemia in rats. J Neurochem. 2007a;100:1108–1120. doi: 10.1111/j.1471-4159.2006.04280.x. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Taheri S, Yang Y, Sood R, Grossetete M, Estrada EY, Fiebich BL, Rosenberg GA. Cyclooxygenase inhibition limits blood-brain barrier disruption following intracerebral injection of tumor necrosis factor-alpha in the rat. J Pharmacol Exp Ther. 2007b;323:488–498. doi: 10.1124/jpet.107.127035. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Matsumura K, Yamagata K, Watanabe Y. Cyclooxygenase-2 is induced in brain blood vessels during fever evoked by peripheral or central administration of tumor necrosis factor. Brain Res Mol Brain Res. 1998;56:45–56. doi: 10.1016/s0169-328x(98)00025-4. [DOI] [PubMed] [Google Scholar]
- Cao C, Matsumura K, Ozaki M, Watanabe Y. Lipopolysaccharide injected into the cerebral ventricle evokes fever through induction of cyclooxygenase-2 in brain endothelial cells. J Neurosci. 1999;19:716–725. doi: 10.1523/JNEUROSCI.19-02-00716.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Langenbach R, Bosetti F. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. FASEB J. 2008;22:1491–1501. doi: 10.1096/fj.07-9411com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries HE, Blom-Roosemalen MC, de Boer AG, van Berkel TJ, Breimer DD, Kuiper J. Effect of endotoxin on permeability of bovine cerebral endothelial cell layers in vitro. J Pharmacol Exp Ther. 1996;277:1418–1423. [PubMed] [Google Scholar]
- Fridman R, Toth M, Chvyrkova I, Meroueh SO, Mobashery S. Cell surface association of matrix metalloproteinase-9 (gelatinase B) Cancer Metastasis Rev. 2003;22:153–166. doi: 10.1023/a:1023091214123. [DOI] [PubMed] [Google Scholar]
- Germann B, Neuhaus W, Hofer-Warbinek R, Noe CR. Applying blood-brain barrier in vitro models to study the influence of drugs on endothelial cells--effects of selected COX-inhibitors. Pharmazie. 2008;63:303–307. [PubMed] [Google Scholar]
- Goralski KB, Abdulla D, Sinal CJ, Arsenault A, Renton KW. Toll-like receptor-4 regulation of hepatic Cyp3a11 metabolism in a mouse model of LPS-induced CNS inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;289:G434–G443. doi: 10.1152/ajpgi.00562.2004. [DOI] [PubMed] [Google Scholar]
- Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med. 1993;29:804–811. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- Gu B, Desjardins P, Butterworth RF. Selective increase of neuronal cyclooxygenase-2 (COX-2) expression in vulnerable brain regions of rats with experimental Wernicke's encephalopathy: effect of nimesulide. Metab Brain Dis. 2008;23:175–187. doi: 10.1007/s11011-008-9089-2. [DOI] [PubMed] [Google Scholar]
- Gurney KJ, Estrada EY, Rosenberg GA. Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol Dis. 2006;23:87–96. doi: 10.1016/j.nbd.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Keene CD, Chang R, Stephen C, Nivison M, Nutt SE, Look A, Breyer RM, Horner PJ, Hevner R, Montine TJ. Protection of hippocampal neurogenesis from toll-like receptor 4-dependent innate immune activation by ablation of prostaglandin E2 receptor subtype EP1 or EP2. Am J Pathol. 2009;174:2300–2309. doi: 10.2353/ajpath.2009.081153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz T, Oliw EH. Nimesulide aggravates kainic acid-induced seizures in the rat. Pharmacology & toxicology. 2001;88:271–276. doi: 10.1034/j.1600-0773.2001.d01-116.x. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Lacroix S, Rivest S. An essential role of interleukin-1beta in mediating NF-kappaB activity and COX-2 transcription in cells of the blood-brain barrier in response to a systemic and localized inflammation but not during endotoxemia. J Neurosci. 1999;19:10923–10930. doi: 10.1523/JNEUROSCI.19-24-10923.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Man S, Ubogu EE, Ransohoff RM. Inflammatory cell migration into the central nervous system: a few new twists on an old tale. Brain Pathol. 2007;17:243–250. doi: 10.1111/j.1750-3639.2007.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark KS, Trickler WJ, Miller DW. Tumor necrosis factor-alpha induces cyclooxygenase-2 expression and prostaglandin release in brain microvessel endothelial cells. J Pharmacol Exp Ther. 2001;297:1051–1058. [PubMed] [Google Scholar]
- Mikulis DJ, Roberts TP. Neuro MR: protocols. J Magn Reson Imaging. 2007;26:838–847. doi: 10.1002/jmri.21041. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Zaja-Milatovic S, Montine KS, Shie FS, Montine TJ. Neuronal oxidative damage and dendritic degeneration following activation of CD14-dependent innate immune response in vivo. Journal of neuroinflammation [electronic resource] 2004;1:20. doi: 10.1186/1742-2094-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun-Bryce S, Rosenberg GA. Gelatinase B modulates selective opening of the blood-brain barrier during inflammation. The American journal of physiology. 1998;274:R1203–R1211. doi: 10.1152/ajpregu.1998.274.5.R1203. [DOI] [PubMed] [Google Scholar]
- Nagase H, Fields CG, Fields GB. Design and characterization of a fluorogenic substrate selectively hydrolyzed by stromelysin 1 (matrix metalloproteinase-3) J Biol Chem. 1994;269:20952–20957. [PubMed] [Google Scholar]
- ANiwa K, Araki E, Morham SG, Ross ME, Iadecola C. Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J Neurosci. 2000;20:763–770. doi: 10.1523/JNEUROSCI.20-02-00763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa K, Haensel C, Ross ME, Iadecola C. Cyclooxygenase-1 participates in selected vasodilator responses of the cerebral circulation. Circ Res. 2001;88:600–608. doi: 10.1161/01.res.88.6.600. [DOI] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ, editors. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39:279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- Sibson NR, Blamire AM, Perry VH, Gauldie J, Styles P, Anthony DC. TNF-alpha reduces cerebral blood volume and disrupts tissue homeostasis via an endothelin- and TNFR2-dependent pathway. Brain. 2002;125:2446–2459. doi: 10.1093/brain/awf256. [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Bosetti F, Silva AC. Modulatory role of cyclooxygenase-2 in cerebrovascular coupling. Neuroimage. 2006;32:23–32. doi: 10.1016/j.neuroimage.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Takanashi Y, Shinonaga M, Naitoh M, Noguchi N. Magnetic resonance imaging with gadolinium DTPA enhancement in patients with acute head injury. J Neurotrauma. 2000;17:359–365. doi: 10.1089/neu.2000.17.359. [DOI] [PubMed] [Google Scholar]
- Toscano CD, Prabhu VV, Langenbach R, Becker KG, Bosetti F. Differential gene expression patterns in cyclooxygenase-1 and cyclooxygenase-2 deficient mouse brain. Genome biology. 2007;8:R14. doi: 10.1186/gb-2007-8-1-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano CD, Kingsley PJ, Marnett LJ, Bosetti F. NMDA-induced seizure intensity is enhanced in COX-2 deficient mice. Neurotoxicology. 2008a;29:1114–1120. doi: 10.1016/j.neuro.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano CD, Ueda Y, Tomita YA, Vicini S, Bosetti F. Altered GABAergic neurotransmission is associated with increased kainate-induced seizure in prostaglandin-endoperoxide synthase-2 deficient mice. Brain Res Bull. 2008b;75:598–609. doi: 10.1016/j.brainresbull.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss N, Miller F, Cazaubon S, Couraud PO. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta. 2009;1788:842–857. doi: 10.1016/j.bbamem.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Woo MS, Park JS, Choi IY, Kim WK, Kim HS. Inhibition of MMP-3 or -9 suppresses lipopolysaccharide-induced expression of proinflammatory cytokines and iNOS in microglia. J Neurochem. 2008;106:770–780. doi: 10.1111/j.1471-4159.2008.05430.x. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]