Abstract
Purpose of the Review:
Angiotensin-converting enzyme 2 (ACE2) is a key counter-regulatory component of the renin-angiotensin system. Here, we briefly review the mechanistic and target organ effects related to ACE2 activity, and the importance of ACE2 in SARS-CoV-2 infection.
Recent Findings:
ACE2 converts angiotensin (Ang) II to Ang-(1–7), which directly opposes the vasoconstrictive, proinflammatory, and prothrombotic effects of Ang II. ACE2 also facilitates SARS-CoV-2 viral entry into host cells. Drugs that interact with the renin-angiotensin system may impact ACE2 expression and COVID-19 pathogenesis, however the magnitude and direction of these effects are unknown at this time.
Summary:
Research is urgently needed to improve our understanding of how agents that act on the renin-angiotensin system impact ACE2 and COVID-19-related disease outcomes.
Keywords: Angiotensin-converting enzyme, renin-angiotensin system, angiotensin receptor blockers, angiotensin-converting enzyme inhibitors, hypertension, coronavirus infections
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for the coronavirus disease 2019 (COVID-19) pandemic, is associated with a high risk of acute respiratory distress syndrome and mortality [1–4]. Angiotensin-converting enzyme 2 (ACE2) facilitates SARS-CoV-2 entry into host cells in the respiratory tract, and altered ACE2 regulation is speculated to play a role in the pathogenesis of COVID-19 [5]. In the setting of the COVID-19 pandemic, there has been increasing interest in the physiologic and pathophysiologic function of ACE2 [6●]. Here, we briefly review the role of ACE2 in the renin-angiotensin system, the therapeutic potential of ACE2, as well as potential interactions of ACE2 with SARS-CoV-2 and its role in COVID-19 pathophysiology.
Counter-regulatory effects of ACE2 in the renin-angiotensin system
ACE2 is a mono-carboxypeptidase with a single enzymatic binding site that acts as a key counter-regulatory component of the renin-angiotensin system [7]. ACE2 is the only known homolog of ACE; it shares 42% of sequence identity with somatic ACE and 61% similarity in the area surrounding the active site [8, 9]. ACE2 is expressed on the surface of endothelial and epithelial cells in membrane-bound form as well as soluble form in several tissues throughout the body, including the kidneys, heart, gastrointestinal tract, and lungs [10, 11].
ACE converts angiotensin I (Ang I) to Ang II, which acts on the Ang II type 1 receptor (AT1R), resulting in vasoconstriction, sodium and fluid retention by the kidney, oxidative stress, inflammation, fibrosis, and impaired fibrinolysis [11, 12]. In direct opposition to the cascade of physiological effects of the ACE/Ang II pathway, the net effect of the ACE2/Ang-(1–7) pathway is vasodilation and anti-inflammation. ACE2 hydrolyzes Ang II, converting it to Ang-(1–7) (see Figure). The conversion of Ang II to Ang-(1–7) diminishes the availability of Ang II to bind AT1R, forestalling the vasoconstrictive, proinflammatory, and prothrombotic effects of AT1R activation [7, 10–13]. Additionally, Ang-(1–7) acts on the Mas receptor, causing the release of nitric oxide, prostaglandin E2, and bradykinin [11] resulting in vasodilation, natriuresis, and a reduction in oxidative stress and inflammation [14, 15]. ACE2 also cleaves several other peptides, including converting Ang I to Ang-(1–9), which is a less-bioactive peptide [16].
Figure. The counter-regulatory role of ACE2 in the renin-angiotensin system.
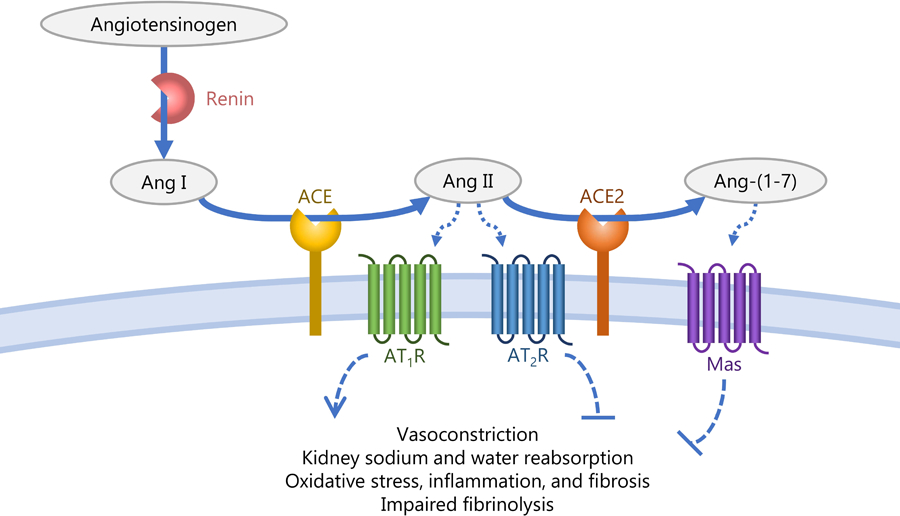
This figure demonstrates the conversion of angiotensinogen to Ang I by renin, Ang I to Ang II by ACE, and Ang II to Ang-(1–7) by ACE2. Ang II acts on the AT1R receptor to increase vasoconstriction, fluid and sodium retention by the kidney, and oxidative stress, resulting in increased blood pressure. Ang-(1–7) acts on the Mas receptor resulting in vasodilation, increased fluid and sodium excretion by the kidney, and a reduction in oxidative stress, resulting in reduced blood pressure.
Systemic effects of ACE2
ACE2 plays an important role in the development of several pathologic conditions, including hypertension, cardiac hypertrophy, and kidney disease. For example, in human kidney tissue, the ratio of ACE to ACE2 expression is higher in subjects with hypertension compared to those without hypertension [17]. These findings are consistent with more-pronounced vasoconstrictive and anti-natriuretic effects of ACE, compared to ACE2, activity in hypertensive individuals. Several experimental and human studies have also demonstrated a reduction in glomerular ACE2 expression in diabetic and non-diabetic kidney disease [8]. Correspondingly, experimental studies in mice show that pharmacologic inhibition of ACE2 promotes the development of microalbuminuria and diabetic nephropathy [18, 19]. Human data suggest that ACE2 may be upregulated in individuals with existing cardiovascular disease, which is speculated to be a compensatory response to counteract the deleterious effects of Ang II [20]. In SARS-CoV-2, while ACE2 facilitates viral entry in host cells, it may also have protective effects against severe acute lung injury [21].
ACE2 as a therapeutic target
Given its vasodilatory, natriuretic, anti-inflammatory, and anti-fibrotic effects via increased Ang-(1–7) and decreased Ang II, soluble ACE2 has been proposed as a potential therapeutic target in hypertension, chronic kidney disease, cardiovascular disease, and viral respiratory disease. In experimental studies, chronic administration of soluble ACE2 has been associated with degradation of Ang II and an increase in Ang-(1–7) [22–24]. Animal studies consistently demonstrate a reduction in blood pressure with recombinant ACE2 administration, and in mouse models recombinant ACE2 attenuated diabetic kidney injury [22] and myocardial remodeling (24). Additionally, recombinant ACE2 has been associated with a reduction in acute lung injury and acute respiratory distress syndrome in mice, and children infected with respiratory syncytial virus have higher plasma Ang II concentration compared to healthy controls [25].
ACE inhibitors block the conversion of Ang I to Ang II, while Ang II receptor blockers directly inhibit AT1R. Both of these medications decrease blood pressure and inflammation and mitigate fibrosis in hypertension, cardiovascular disease, and chronic kidney disease. In several animal models, these antihypertensive agents have been shown to increase ACE2 expression in the heart and kidneys [26–29]. However, in humans, ACE inhibitors and Ang II receptor blockers have not been associated with increased kidney ACE2 expression or circulating ACE2 activity [30, 31●].
The relationship between ACE2 and COVID-19
ACE2 is the binding site for the SARS-CoV-2 viral spike (S) protein and facilitates viral entry into the host cell. There has been recent speculation that ACE inhibitors and Ang II receptor blockers may increase the risk of development and severity of COVID-19 due to potential upregulation of ACE2 by these medications [32] However, SARS-CoV-2 facilitates ACE2 endocytosis, downregulates ACE2 expression, and promotes ACE2 shedding from the cell surface, leading to an increase in Ang II concentration and a decrease in Ang-(1–7). This is likely important in COVID-19 pathophysiology due to the proinflammatory effects of Ang II with corresponding loss of Ang-(1–7)-mediated counter-regulation [33]. The prothrombotic effects of excess Ang II could underly COVID-19 hypercoagulability [34, 35●]. Upregulation of ACE/Ang II with downregulation of ACE2/Ang-(1–7) in the vascular endothelium could promote COVID-19-associated vasculopathy [36, 37]. Extrapolating from SARS-CoV animal models, increased Ang II in patients with COVID-19 due to loss of ACE2 could mediate acute lung injury and acute respiratory distress syndrome [38]. In experimental models, Ang II administration induces AT1R-mediated ACE2 internalization and degradation [39]. ACE inhibitors and Ang II receptor blockers may have a therapeutic benefit in COVID-19 by reducing Ang II concentration and AT1R activation. Thus, it remains unclear if ACE inhibitors or Ang II receptor blockers have a beneficial or harmful effect in SARS-CoV-2 infection and COVID-19.
Conclusions
ACE2 has important counter-regulatory effects on the renin-angiotensin system and has been implicated in COVID-19 pathogenesis. Medications that act on the renin-angiotensin system, including ACE inhibitors and Ang II receptor blockers, may impact COVID-19 infection and severity via potential interactions with ACE2; however, the direction and magnitude of these effects are unknown at this time. Several international societies have released statements discouraging discontinuation of ACE inhibitors and Ang II receptor blockers in patients who are treated with these medications for hypertension, heart failure, and chronic kidney disease amidst the COVID-19 pandemic [6●]. Given the clear benefits of these agents in hypertension, chronic kidney disease, coronary heart disease, and diabetes, there is an urgent need for further research to improve our understanding of the relationship between agents that act on the renin-angiotensin system and outcomes among individuals with COVID-19, with several studies currently underway [6●].
Acknowledgments
Funding:
JBC is supported by K23-HL133843 from the National Heart, Lung, and Blood Institute
AMS is supported by R01-HL146818 from the National Heart, Lung, and Blood Institute; UC4DK108173 from the National Institute of Diabetes and Digestive and Kidney Diseases; and a Loan Repayment Program Award from the National Heart, Lung, and Blood Institute
APB is supported by 1K01HL133468 from the National Heart, Lung, and Blood Institute
REFERENCES
- 1.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020. [DOI] [PubMed]
- 2.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed]
- 3.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020. [DOI] [PMC free article] [PubMed]
- 4.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020. [DOI] [PMC free article] [PubMed]
- 5.Sparks MA, South A, Welling P, Luther JM, Cohen J, Byrd JB, et al. Sound Science before Quick Judgement Regarding RAS Blockade in COVID-19. Clin J Am Soc Nephrol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ●6.Sparks MA, Hiremath S, South A, Welling P, Luther JM, Cohen JB, et al. “The Coronavirus Conundrum: ACE2 and Hypertension Edition” NephJC http://www.nephjc.com/news/covidace2. Accessed 13 Apr 2020 2020.This is a multidiscplinary free online medical education document by several physician scientists whose research focuses on the renin-angiotensin system, which provides a comprehensive overview of research related to ACE2 and COVID-19, including live updates as new research emerges. The site had >260,000 page views within its first three weeks of launching.
- 7.South AM, Shaltout HA, Washburn LK, Hendricks AS, Diz DI, Chappell MC. Fetal programming and the angiotensin-(1–7) axis: a review of the experimental and clinical data. Clin Sci (Lond). 2019;133(1):55–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soler MJ, Wysocki J, Batlle D. ACE2 alterations in kidney disease. Nephrol Dial Transplant. 2013;28(11):2687–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guy JL, Jackson RM, Acharya KR, Sturrock ED, Hooper NM, Turner AJ. Angiotensin-converting enzyme-2 (ACE2): comparative modeling of the active site, specificity requirements, and chloride dependence. Biochemistry. 2003;42(45):13185–92. [DOI] [PubMed] [Google Scholar]
- 10.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87(5):E1–9. [DOI] [PubMed] [Google Scholar]
- 11.Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM. Classical Renin-Angiotensin system in kidney physiology. Compr Physiol. 2014;4(3):1201–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaughan DE. Angiotensin and vascular fibrinolytic balance. Am J Hypertens. 2002;15(1 Pt 2):3S–8S. [DOI] [PubMed] [Google Scholar]
- 13.Gromotowicz-Poplawska A, Stankiewicz A, Kramkowski K, Gradzka A, Wojewodzka-Zelezniakowicz M, Dzieciol J, et al. The acute prothrombotic effect of aldosterone in rats is partially mediated via angiotensin II receptor type 1. Thromb Res. 2016;138:114–20. [DOI] [PubMed] [Google Scholar]
- 14.Lelis DF, Freitas DF, Machado AS, Crespo TS, Santos SHS. Angiotensin-(1–7), Adipokines and Inflammation. Metabolism. 2019;95:36–45. [DOI] [PubMed] [Google Scholar]
- 15.Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/Angiotensin 1–7 Axis of the Renin-Angiotensin System in Heart Failure. Circ Res. 2016;118(8):1313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flores-Munoz M, Work LM, Douglas K, Denby L, Dominiczak AF, Graham D, et al. Angiotensin-(1–9) attenuates cardiac fibrosis in the stroke-prone spontaneously hypertensive rat via the angiotensin type 2 receptor. Hypertension. 2012;59(2):300–7. [DOI] [PubMed] [Google Scholar]
- 17.Wakahara S, Konoshita T, Mizuno S, Motomura M, Aoyama C, Makino Y, et al. Synergistic expression of angiotensin-converting enzyme (ACE) and ACE2 in human renal tissue and confounding effects of hypertension on the ACE to ACE2 ratio. Endocrinology. 2007;148(5):2453–7. [DOI] [PubMed] [Google Scholar]
- 18.Soler MJ, Wysocki J, Ye M, Lloveras J, Kanwar Y, Batlle D. ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int. 2007;72(5):614–23. [DOI] [PubMed] [Google Scholar]
- 19.Wong DW, Oudit GY, Reich H, Kassiri Z, Zhou J, Liu QC, et al. Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol. 2007;171(2):438–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice GI, Jones AL, Grant PJ, Carter AM, Turner AJ, Hooper NM. Circulating activities of angiotensin-converting enzyme, its homolog, angiotensin-converting enzyme 2, and neprilysin in a family study. Hypertension. 2006;48(5):914–20. [DOI] [PubMed] [Google Scholar]
- 21.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oudit GY, Liu GC, Zhong J, Basu R, Chow FL, Zhou J, et al. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes. 2010;59(2):529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wysocki J, Ye M, Rodriguez E, Gonzalez-Pacheco FR, Barrios C, Evora K, et al. Targeting the degradation of angiotensin II with recombinant angiotensin-converting enzyme 2: prevention of angiotensin II-dependent hypertension. Hypertension. 2010;55(1):90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong J, Basu R, Guo D, Chow FL, Byrns S, Schuster M, et al. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010;122(7):717–28, 18 p following 28. [DOI] [PubMed] [Google Scholar]
- 25.Gu H, Xie Z, Li T, Zhang S, Lai C, Zhu P, et al. Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus. Sci Rep. 2016;6:19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–10. [DOI] [PubMed] [Google Scholar]
- 27.Ocaranza MP, Godoy I, Jalil JE, Varas M, Collantes P, Pinto M, et al. Enalapril attenuates downregulation of Angiotensin-converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension. 2006;48(4):572–8. [DOI] [PubMed] [Google Scholar]
- 28.Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43(5):970–6. [DOI] [PubMed] [Google Scholar]
- 29.Soler MJ, Ye M, Wysocki J, William J, Lloveras J, Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol. 2009;296(2):F398–405. [DOI] [PubMed] [Google Scholar]
- 30.Walters TE, Kalman JM, Patel SK, Mearns M, Velkoska E, Burrell LM. Angiotensin converting enzyme 2 activity and human atrial fibrillation: increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. Europace. 2017;19(8):1280–7. [DOI] [PubMed] [Google Scholar]
- ●31.Ramchand J, Patel SK, Srivastava PM, Farouque O, Burrell LM. Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease. PLoS One. 2018;13(6):e0198144.This prospective observational study of 79 patients with obstructive coronary artery disease demonstrated that elevated ACE2 activity was independently associated with major adverse cardiovascular events over a median follow up of 10.5 years. The authors also observed that the frequency of elevated ACE2 activity was similar across users and non-users of ACE inhibitors and Ang II receptor blockers.
- 32.Roth M Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet. 2020;8:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318(5):H1084–H90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown NJ, Vaughan DE. Prothrombotic effects of angiotensin. Adv Intern Med. 2000;45:419–29. [PubMed] [Google Scholar]
- ●35.Senchenkova EY, Russell J, Yildirim A, Granger DN, Gavins FNE. Novel Role of T Cells and IL-6 (Interleukin-6) in Angiotensin II-Induced Microvascular Dysfunction. Hypertension. 2019;73(4):829–38.Using several mouse models, this study demonstrated that T cell IL-6 mediates the thrombotic abnormalities found in hypertension via the ACE/Ang II pathway. Additionally, using human samples, the authors found that IL-6 promotes platelet aggregation, leading to increased risk of thrombosis
- 36.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020. [DOI] [PubMed]
- 37.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292(1):C82–97. [DOI] [PubMed] [Google Scholar]
- 38.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deshotels MR, Xia H, Sriramula S, Lazartigues E, Filipeanu CM. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension. 2014;64(6):1368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]


