Abstract
Background
The COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) presents with a spectrum of clinical manifestations from asymptomatic or mild, self-limited constitutional symptoms to a hyperinflammatory state (“cytokine storm”) followed by acute respiratory distress syndrome (ARDS) and death.
Objective
To provide an evidence-based review of the associated pathways and potential treatment of the hyperinflammatory state associated with SARS-CoV-2 infection.
Recent findings
Dysregulated immune responses have been reported to occur in a smaller subset of those infected with SARS-CoV-2 leading to clinical deterioration 7 to 10 days following initial presentation. A hyperinflammatory state referred to as “cytokine storm” in its severest form has been marked by elevation of IL-6, IL-10, TNF-alpha and other cytokines and severe CD4+ and CD8+ T-cell lymphopenia and coagulopathy. Recognition of at-risk patients could permit early institution of aggressive intensive care, antiviral and immune treatment to reduce the complications related to this pro-inflammatory state. Several reports and ongoing clinical trials provide hope that available immunomodulatory therapies could have therapeutic potential in these severe cases.
Conclusion
This review highlights our current state of knowledge of immune mechanisms and targeted immunomodulatory treatment options for the current COVID-19 pandemic.
Keywords: interleukin −6, sepsis, cytokine storm, cytokines, COVID-19, SARS-CoV-2, tumor necrosis factor-alpha, JAK, STING, proinflammatory, hyperinflammatory, hemophagocytic lymphohistiocytosis
INTRODUCTION
The COVID-19 pandemic caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) presents an enormous challenge for public health and clinicians globally. Increased understanding of the immunopathogenesis of SARS-CoV-2 infection as well as ongoing clinical trials of host target drugs such as hydroxychloroquine, direct antivirals, convalescent plasma and other immunomodulatory agents hold promise for future evidence-based and targeted therapies to reduce the morbidity and mortality of the most vulnerable populations.
While infection is often asymptomatic or associated with mild to moderate self-limiting symptoms such as fever, dry cough, myalgia and fatigue (1–6), a subset of patients with severe SARS-CoV-2 infection develop a clinically severe hyperinflammatory state or cytokine storm (CS) for which pulmonary involvement such as acute respiratory distress syndrome (ARDS) is a cardinal feature (1, 7). Further, a subgroup of previously healthy children has been diagnosed with a multi-system inflammatory syndrome associated with acute SARS-CoV-2 infection that appears distinct from the adult CS (8).
While the individual components of CS are varied, interleukin-6 (IL-6) has emerged of particular interest in the context of SARS-CoV-2 infection after being identified as the most significant predictor of mortality in recent retrospective studies of patient survival in COVID-19 (1). Herein, we review the current understanding of the origin and mechanisms of cytokine storm associated with SARS-CoV-2 infection with focus on the identification and implication of IL-6 and other proinflammatory cytokines and pathways in CS-driven ARDS and discuss the potential utility of anti-IL-6 and other cytokine targeting immunomodulatory biologics for the treatment of this critically ill population.
Search strategy and selection criteria
We searched PubMed for peer-reviewed articles published between Jan 1st 2000 and April 18th 2020 (date of last search) with the terms (“IL-6” OR “interleukin-6” OR “cytokine”) AND (“sepsis” OR “SIRS” OR “systemic inflammatory response syndrome” OR “non-infectious systemic inflammatory response syndrome” OR “ARDS” OR “acute respiratory distress syndrome” OR “cytokine storm” OR “inflammatory response” OR “septic shock” OR “critically ill” OR “organ dysfunction” OR “infection”) AND (“ICU” OR “intensive care unit” OR “ED” OR “emergency department”). A second search was oriented on treatment with the terms (“IL-6” OR “interleukin-6” OR “IL-1” OR “interleukin-1” OR “TNF” OR “tumor necrosis factor” OR “interferon gamma” OR “STING” OR “interferon pathway”) AND (“sepsis” OR “SIRS” OR “systemic inflammatory response syndrome” OR “ARDS” OR “acute respiratory distress syndrome” OR “cytokine storm” OR “inflammatory response” OR “septic shock” OR “critically ill” OR “organ dysfunction” OR “infection”) AND (“IL-6 inhibitor” OR “interleukin-6 inhibitor” OR “JAK-STAT” OR “tocilizumab” OR “humanized IL-6R antibody” OR “anakinra” OR “IL-1 inhibitor”). Please refer to Supplement Figure 1 for details concerning the number of articles entered in PubMed with the “cytokine storm” keywords.
All recent articles on COVID-19/SARS-CoV-2 were reviewed including pre-prints from bioRxiv and medRxiv as a more real-time resources, but realizing the lack of peer review limitation. In order to carefully include the proposed trials for COVID-19, we researched the ClinicalTrials.gov/trials website.
Articles published in English were selected and reviewed. There was a focus on clinical trials, meta-analysis, randomized controlled trials and systematic reviews as well as novel and significant studies. Finally, we also identified several new references from those listed in the reviewed articles. Please note that although there is increasing information about the SARS-CoV-2 virus and its immune consequences, the majority of the literature available on COVID-19 disease and SARS-CoV-2 infection originates from the onset of the pandemic, in China, with various publications from disease phenotype to immunopathogenesis and follow-up.
OVERVIEW OF SEPSIS AND IMMUNE DYSREGULATION
The release of large quantities of proinflammatory cytokines is termed cytokine storm (CS) and is associated with a variety of infective precipitants and other hyperinflammatory states (9, 10). Virally associated causes of particular relevance are the 2003 severe acute respiratory syndrome (SARS) coronavirus Infection (SARS-CoV) that infected over 8000 globally, primarily in Asia and Canada (Toronto) with an 11% mortality rate (11–14), and the 2012 Middle East respiratory syndrome coronavirus (MERS- CoV) with a reported case fatality rate of 35% (15–17). While SARS-CoV-2 belongs to the same Betacoronavirus genus as SARS-CoV and MERS, the case fatality associated with both SARS-CoV and MERS significantly exceeds that of SARS-CoV-2 and the numbers of cases worldwide associated with SARS-CoV and MERS are much lower (18). Genomic evidence suggests that SARS-CoV and SARS-CoV-2 share the same human cell receptor for host entry, the angiotensin-converting enzyme 2 (ACE2) (19). SARS-CoV-2 binds with increased affinity to the ACE2 receptor compared to SARS-CoV, a possible explanation for the widely community transmission from asymptomatic hosts (18).
The clinical correlates of increased inflammation or CS are persistent hypotension, hypo or hyperthermia and end-organ dysfunction while the laboratory features include haematological anomalies (leukocytosis or leukopenia, thrombocytopenia and disseminated intravascular coagulation with high fibrinogen, triglycerides and ferritin), elevated IL-2R (CD25), elevated IL-6 and general markers of end-organ dysfunction (such as elevated creatinine, deranged liver function tests, etc.) (10). The CS clinical phenotype is variable and regroups systemic signs of inflammation, multi-organ failure and mortality (20).
The hyperinflammatory state associated with severe viral infection shares significant features with cytokine storm and genetic factors associated with hemophagocytic lymphohistiocytosis (HLH) have been identified suggesting these conditions may in fact be part of the same spectrum (21). HLH is caused by an abnormal regulation of activated macrophages and lymphocytes leading to a hyperinflammatory state (21). Interferon-gamma (IFN-γ) is an important cytokine responsible for the organ damage leading to mortality in HLH (20). Other cytokines such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), IL-1 and IL-18 are also increased during HLH (22). Several genes that play a role in the pathway of natural killer (NK) cells and CD8+ T-cells have been described as causal for the development of the familial form of HLH. Some examples include the LYST mutation described in the Chediak-Higashi syndrome, the RAB27A mutation in the Griscelli syndrome as well as other mutations in perforin (PRF1). The main clinical and laboratory manifestations of HLH are fever, cytopenia, coagulopathy, liver dysfunction, hyperferritinemia, decreased or absent NK cells function, increased soluble CD25 (IL-2R) and presence of hemophagocytosis on bone marrow biopsy or other organs (21). The secondary form of HLH has been described in influenza during the 2009 H1N1 pandemic, the largest cohort describing 9 cases with a mortality rate of 89% (8/9 cases) (23). The macrophage activation syndrome (MAS) has similar clinical features to HLH and is considered a related disorder that occurs primarily in patients with rheumatologic diseases (24). The excessive inflammatory response described in MAS is characterized by an increased IL-1β with successful reports on the use of anakinra, an IL-1 receptor antagonist (25, 26). In a cohort of 19 adult patients with secondary HLH/MAS, 63.1 % (12/19) had systemic infections as precipitating causes (27). This group used anakinra in 12/19 patients and reported 4 deaths among those patients treated with anakinra (27).
The concept of shared immunological features between HLH, MAS and CS has been entertained with extensive discussion that these diseases could be part of an overlapping immunopathology with shared but also distinctive triggers and genetic features (20). To assess the possible genetic predisposition of individuals with no past medical history to develop HLH secondary to severe H1N1 influenza, Schulert and al. performed whole-exome sequencing (WES) in 14 patients, 13 of whom had evidence of hemophagocytosis at autopsy, and detected 5 heterozygous variants in LYST (two of whom also had a heterozygous PRF1 mutation) (27).
Pediatric multisystem inflammatory syndrome
Children appear protected from SARS-CoV-2 infection and are more likely to be asymptomatic or have less severe infection compared to their adult counterparts (28, 29). However, more recently, a pediatric multi-system inflammatory syndrome has been described that appears distinct from the adult CS associated with acute SARS-CoV-2 infection and may follow mild or asymptomatic SARS-CoV-2 infection in previously healthy children. This illness has been reported in children aged from early childhood to adolescence, has Kawasaki-like features, and has been reported in areas with a high SARS-CoV-2 prevalence in children exposed to positive COVID-19 family members. In multiple cases, the child had positive SARS-CoV-2 serology but negative SARS-CoV-2 PCR. Deaths of at least three children associated with this syndrome have been reported in the United States and United Kingdom (8). Children with this syndrome have presented with similar features to Kawasaki disease (29, 30) with high fever, rash, conjunctivitis, diarrhea and shown evidence of myocardial involvement with elevated troponins, arrhythmia, shock, abnormal echocardiographic vascular findings and coronary artery aneurysms. They have shown evidence of a hyperinflammatory state with elevated ferritin, triglycerides and D-dimers. Treatments have included high dose intravenous immunoglobulin and aspirin as suggested for Kawasaki’s disease (30). Currently, the specific immunopathogenic link to SARS-CoV-2 infection and the role of adjunctive immunomodulatory and anti-cytokine therapy in this syndrome is unknown.
The immunopathogenesis of cytokine storm in the setting of a viral respiratory tract infection
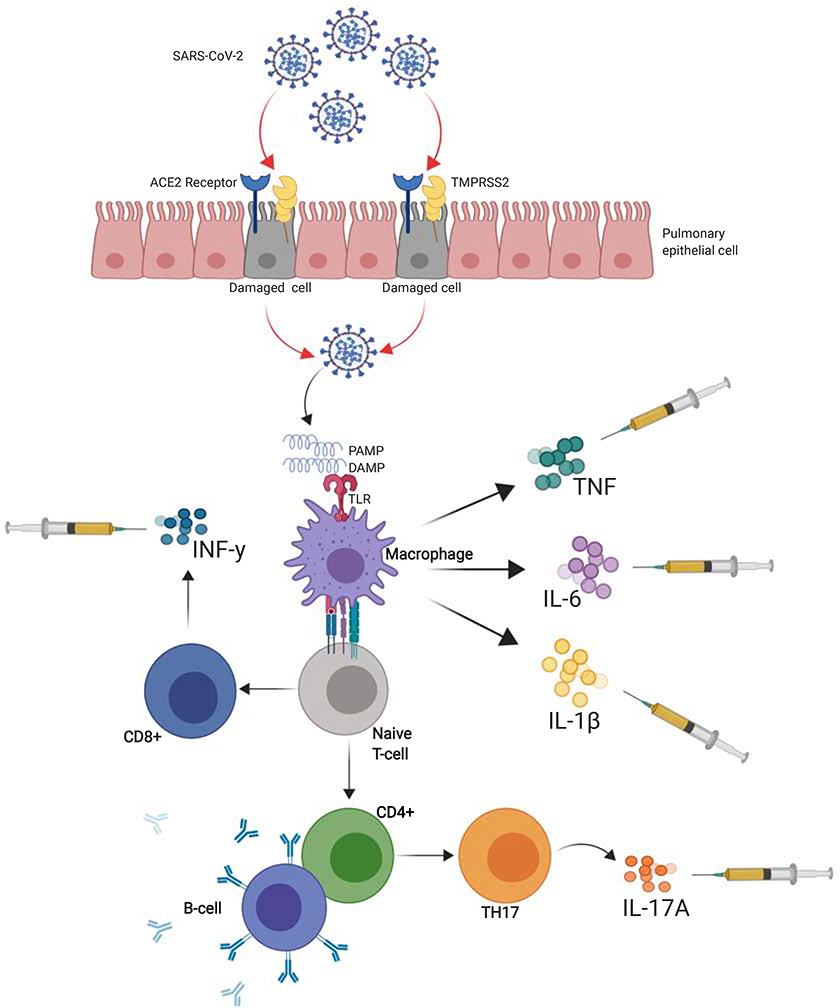
The airway epithelium is part of the first line of defense in presence of an airborne viral pathogen recognized as a pathogen-associated molecular pattern (PAMPs) and/or damage-associated molecular pattern (DAMPs) that bind to pattern recognition receptors (PRRs) such as toll-like receptors (TLRs) on the surface of macrophages (9). The resident activated alveolar macrophages, after several intracellular signaling cascades, generate tumor necrosis factor (TNF), interleukin-1β (IL-β) and IL-6 and trigger a systemic inflammatory response (Figure 1) (31). This simultaneously prompts a well-coordinated local innate response composed of specific enzymes (defensins, mucins, lysozymes), nitric oxide (NO), reactive oxygen species (ROS), platelet activating factor and other cytokines (32). Other key components of the innate immunity against viral infection are the type I interferons (IFNs). In contrast with findings on the influenza virus, severe COVID-19 patients have minimal peripheral quantities of type 1 IFNs but increased IFNs and IFNs genes in the bronchoalveolar environment, a discovery associated with cytokine storm development in a mouse model of SARS-CoV infection (33). Further, in vitro, SARS-CoV-2 failed to produce interferon expression in infected cells (34) indicating a dampened early innate immune response (35).
Figure 1: COVID-19 Immunological mechanisms for cytokine storm and possible role of biologics.
When SARS-CoV-2 pathogen-associated molecular pattern (PAMPs) and/or Damage-associated molecular patterns (DAMPs) bind to toll-like receptors (TLRs) on the surface of resident alveolar macrophages, they become activated and secrete tumor necrosis factor (TNF), interleukin-1β (IL-1β) and IL-6. In increased levels, these cytokines will be the hallmark of the cytokine storm responsible for the acute respiratory distress syndrome (ARDS) and cytokine storm (CS) in COVID-19. The different targets of biologics are illustrated in the figure. Specifically, the downstream effect IL-6 can be blocked with tocilizumab, sarilumab or siltuximab and the effects of IL-1β with anakinra or canakinumab.
CD8+ T-cells produce IFN-γ causing direct tissue damage, while activated CD4+ T-cells, in the presence of transforming growth factor β (TGF-β) and IL-6, will differentiate into the T helper 17 (Th 17) cell subset, responsible for secreting IL-17A and IL-17F who, among numerous roles, target macrophages, dendritic cells, endothelial cell and fibroblasts to increase the production of IL-1, IL-6 and TNF.
ACE2, Angiotensin converting enzyme 2; ARDS, DAMP, damage-associated molecular patterns; PAMP, pathogen-associated molecular pattern; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNF, tumour necrosis factor; TMPRSS2, transmembrane protease, serine 2.
Monocytes and macrophages play a central role and a disruption in the mononuclear phagocyte compartment is considered to increase the COVID-19 related hyperinflammation (36, 37). Also, an increase in the CD14+CD16+ monocytes producing IL-6 has been noted in the peripheral blood of COVID-19 critically-ill patients (38, 39). Bruton’s tyrosine kinase (BTK), an intracellular kinase, also appears to have a role in monocytes and macrophage activation and specifically in infection clearance by macrophages (40).
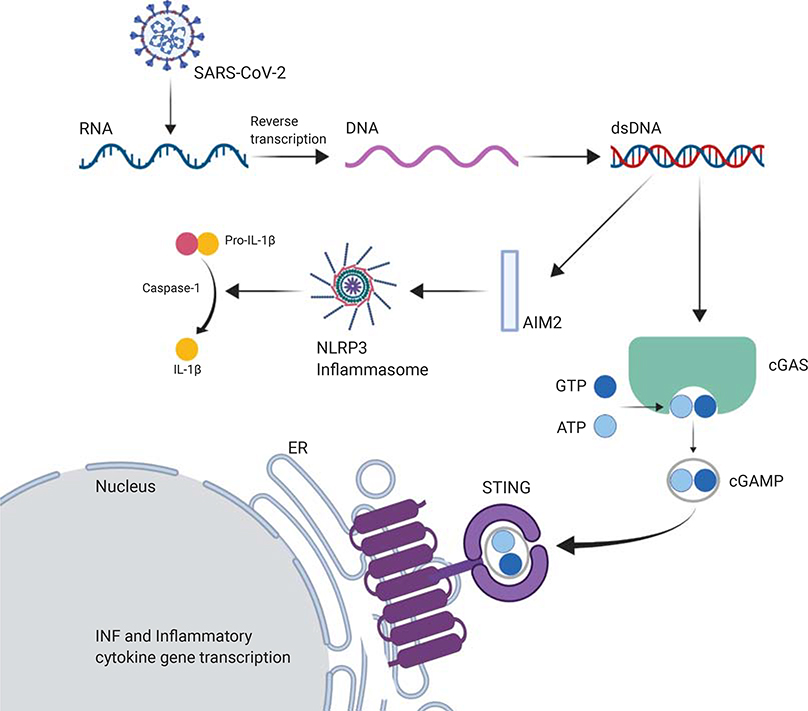
IL-1β is produced after the inflammasome (especially NLRP3), activated by AIM2 sensing foreign DNA, induces the formation of caspase-1 that cleaves pro-IL-1β into IL-1β (Figure 2). In a study using single-cell RNA sequencing in the peripheral blood mononuclear cells (PBMCs) of 10 COVID-19 patients compared with 5 healthy controls, the authors reported an increased quantity of CD14++ monocytes with inflammatory gene expression and CD14++ IL-1β+ monocytes in the early recovery stages of SARS-CoV-2 (41). IL-1β is also implicated in the activity of nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-κB) inducing the synthesis of various inflammatory genes of mediators such as IL-6 (9). Thus, a reduction in IL-1β activity would reduce IL-6 production (42).
Figure 2: The implications of the STING pathway in coronavirus.
The cyclic guanosine monophosphate (GMP)-adenosine monophosphate (AMP) synthetase (cGAS) stimulator of interferon (IFN) genes (STING) pathway is activated by sensing foreign cytosolic DNA (obtained after reverse transcription from the SARS-CoV-2). cGAS catalyzes the generation of cyclic GMP-AMP (cGAMP) that binds and activates STING in the ER (endoplasmic reticulum) leading to the expression of IFNs and other cytokines. IL-1β is produced after the NLRP3 inflammasome, activated by AIM2 sensing foreign DNA, induces the formation of caspase-1 that will cleave pro-IL-1β into IL-1β.
AMP, adenosine monophosphate; ATP, Adenosine triphosphate; cGAMP, cyclic GMP-AMP; DNA, deoxyribonucleic acid; GMP, guanosine monophosphate; GTP, Guanosine triphosphate; cGAS, cyclic GMP-AMP synthetase; ER, endoplasmic reticulum; IFN, interferon; RNA, ribonucleic acid; STING, stimulator of interferon.
Following initial escape of the innate response, recognition of virus promotes the migration of pulmonary dendritic cells to the lymph nodes for presentation of antigen to passing T-cells for the development of more robust antigen- specific T and B-cell adaptive response. During this response, soluble mediators play a role in cellular function and signal transduction by binding to specific receptors on the surface of target cells. For example, CD8+ T-cells produce excessive amounts of TNF-α and IFN-γ causing direct tissue damage, while activated CD4+ T-cells, in the presence of transforming growth factor β (TGF-β) and IL-6, will differentiate into the T helper 17 (Th 17) cell subset, important for extracellular pathogen elimination and auto-immunity (Figure 1) (43). The defining cytokines secreted by Th17 cells are IL-17A and IL-17F that primarily target macrophages, dendritic cells, endothelial cell and fibroblasts to increase the production of IL-1, IL-6 and TNF-α (43). In this particular setting, IL-6 will also inhibit the TGF-β dependent development of CD4+ regulatory T-cells (Treg), a critical mediator of immune tolerance with a major role in regulating the effector T-cell response (44).
In the setting of CS syndromes, over-activation of effector CD4+ and CD8+ T-cells and production of cytokines and chemokines generate an uncontrolled hyperinflammatory injury at the tissue level resulting in local and distant injury (13). Increased inflammation is associated with peripheral blood lymphopenia, a significant drop in the lymphocyte to neutrophil ratio and CD4+ T-cell dysfunction in observational studies but the mechanisms for these changes are unclear (38, 45, 46). In one of the first studies describing the post-mortem pathological findings in one COVID-19 patient, peripheral flow cytometry indicated a reduced CD4+ and CD8+ cell count but an increased proportion of activation markers such as HLA-DR and CD38 as well as an increased concentration of Th17 cells (47).
THE PLEIOTROPIC ROLE OF INTERLEUKIN-6 (IL-6) IN INFLAMMATORY AND VIRAL RESPONSES
IL-6 is secreted by a plethora of immune and stromal cells including monocytes, macrophages, endothelial cells, B and T-cells, hepatocytes, keratinocytes, adipocytes, dendritic cells and fibroblasts. IL-6 exerts effects on a similarly broad array of cellular targets expressing the functional IL-6 receptor (IL-6R) such as T-cells, B-cells, vascular endothelial cells, monocytes, and hepatocytes (44, 48). As may be expected, such diversity of targets translates into functional pleiotropy including the synthesis of acute phase proteins in the liver, such as C-reactive protein (CRP) which is a surrogate for IL-6; the decreased production of proteins such as albumin; the differentiation of B-cells into plasma cells; hematopoiesis and other metabolic and neurologic processes (44, 48). CRP is an acute phase reactant that binds the phospholipid component of microorganisms and damaged cells that is frequently used as a screening marker of infection and/or inflammation (31).
IL-6 affects cellular immunity, with both pro- and anti-inflammatory functions. IL-6 genetic knockout mice present with varied impairments of inflammatory response including a well-documented increased susceptibility to microbial infection, while humans expressing defective IL-6 receptors experience a hyperimmunoglobulin E syndrome (HIES)-like disorder which clinically manifests as dermatitis and recurrent (staphylococcal and mycotic) infections highlighting the important role that IL-6 likely plays in the diverse pathways of IgE mediated allergy and microbial defence (49).
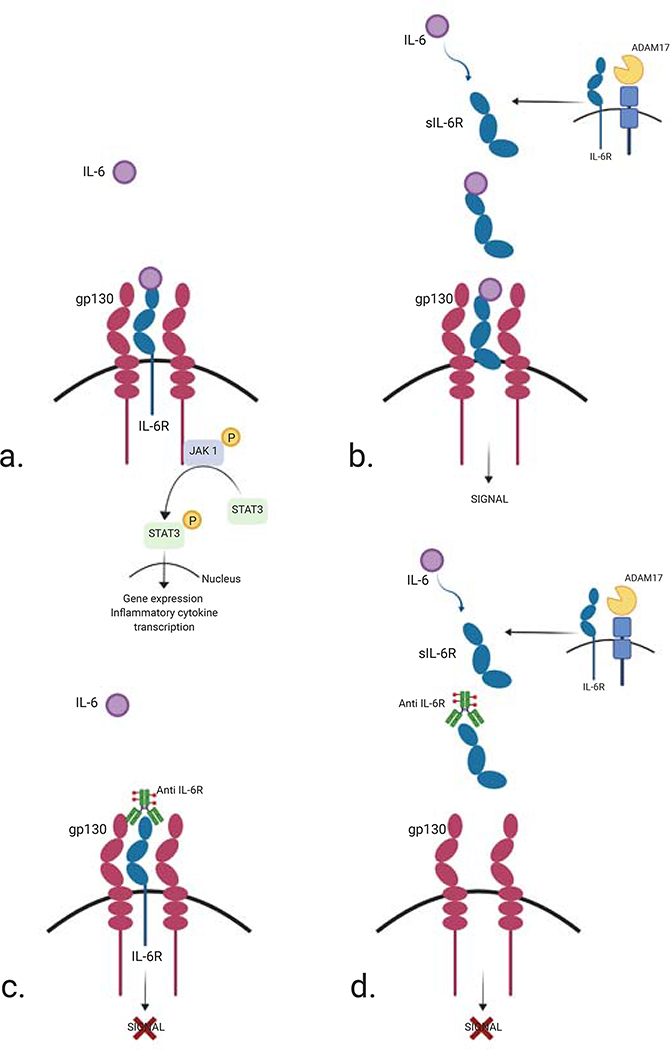
Contrasting inflammatory functions of IL-6 are mediated through its modality of receptor binding. Classical binding of IL-6 to the membrane-bound IL-6 receptor (IL-6R) leads to glycoprotein 130 (gp130) dimerization, Janus kinase (JAK) 1 signalling and activation, among others, of the classical RAS/RAF/MAPK pathways leading to anti-inflammatory responses (Figure 3) (50). While all human cells display preformed, inactive gp130 receptors on their cell surface, this receptor remains inactive without the presence of IL-6R which is only expressed on certain cell types (51). However, pro-inflammatory functions have been found to be mediated through binding of soluble IL-6R, termed trans-signalling, with important ramifications for potential therapeutic targeting (52). It has been shown that an important source of soluble IL-6R is shredded from cells undergoing ADAM17-mediated apoptosis which controls mononuclear phagocyte recruitment, leading to amplified inflammatory response (53). The pro-inflammatory responses of IL-6 are mediated by trans-signaling while the anti-inflammatory functions are probably realized by classic signaling (Figure 3) (50). Selective blockage of this trans-signaling pathway is likely to have the beneficial effect of blocking inflammation without the undesirable off-target effects of broad immune suppression.
Figure 3: Classic and trans-signaling IL-6R.
a. and b. Different signaling pathways stimulated by IL-6. Binding of IL-6 to the membrane-bound or soluble IL-6 receptor (IL-6R) leads to gp130 dimerization and Janus kinase 1 (JAK1) - STAT 3 signalling and activation leading to gene expression of inflammatory cytokines. This pathway is only represented in a. and replaced by the word “SIGNAL” in b.
a. Classic signaling, which is restricted to several cell types, is initiated through binding of IL-6 to the membrane IL-6R and forms a complex with gp130.
b. Trans-signaling is driven by IL- 6 in all gp130-expressing cells. Pro-inflammatory functions have been found to be mediated through binding of soluble IL-6R shredded from cells undergoing ADAM17-mediated apoptosis.
c. and d. IL-6 blockade therapy using a humanized anti-IL-6 receptor monoclonal antibody
A humanized anti-IL-6R antibody blocks IL-6-mediated signaling pathway by inhibiting IL-6 binding to the membrane (c.) and soluble (d.) receptors.
gp130, glycoprotein 130; IL, Interleukin; IL-6R, IL-6 receptor; sIL-6R, soluble IL-6R; JAK, Janus Associated Kinase; STAT, signal transducer and activator of transcription.
The role of IL-6 and other mediators in the response to SARS-CoV-2 infection
A multitude of markers for COVID-19 disease severity have been proposed such as CD4+ and CD8+ T-cell lymphopenia (3, 5, 54) as well as global lymphopenia. Homing of lymphocytes to the lungs is significantly increased in non-survivors compared to survivors (1). A number of publications now highlight that an increase of IL-6 correlates with severe response to SARS-CoV-2; defined as the development of sepsis, ARDS, requirement for mechanical ventilation and death (1, 3, 5, 7, 46, 54–56). The clinical and immunological parameters associated with in-hospital mortality in COVID-19 patients are reported in Table 1. The structural N protein of coronaviruses performs an essential role during host cell entry as well as virus particle assembly and release (11). The N protein from the SARS-CoV activates the transcription factor NF-κB and, subsequently, the expression of IL-6 in human airway epithelial cells, providing a biologically plausible explanation for the role of IL-6 in SARS-CoV-2 infection immune pathogenesis (11).
Table 1 –
Clinical and Immunological Parameters associated with in-hospital mortality in COVID-19
| Studies | Zhou et al. 2020 (1) | Ruan et al. 2020 (7) | Wu et al. 2020 (3) | Wang et al. 2020 (6) | Li et al. 2020 (85) |
|---|---|---|---|---|---|
| Country | China | China | China | China | China |
| Type of study | Retrospective cohort | Retrospective cohort | Retrospective cohort | Retrospective cohort | Retrospective cohort |
| Patients | 191 | 150 | 201 | 33 | 548 |
| Comorbidities* | Age > 69 years HTN CAD Diabetes |
Age > 68 years HTN CAD |
Age > 65 years HTN |
n/a | Age > 65 years HTN Male |
| Clinical* | ↑ Sofa score£> 4.5 | Dyspnea Respiratory failure, ARDS AKI Other infection |
Dyspnea | n/a | n/a |
| Laboratory* | Lymphopenia < 0.6 × 109 /L Leucopenia < 4 × 109 /L ↑ Procalcitonin < 0.1 ng/ml ↑ Creatinine > 133 μmol/L ↑ D-dimer >1 μg/ml ↑ ALT> 40 U/L ↑ LDH > 245 U/L ↑ Troponin I > 28 pg/mL ↑ CK > 185 U/L ↑ Ferritin> 300 μg/L |
Lymphopenia < 0.6 × 109 /L Leucocytosis > 10.6 × 109 /L ↑ CRP > 126.6 mg/L ↑ Creatinine > 91.2 μmol/L ↑ urea> 8.6 μmol/L ↑ Troponin I > 30 pg/mL ↑ Myoglobin > 258.9 ng/mL |
Lymphopenia < 0.6 × 109 /L ↑ urea > 7.4 μmol/L ↑ D-dimer >3.95 μg/ml ↑ LDH > 484 U/L |
Lymphopenia < 1.1 × 109 /L Neutrophilia > 6 × 109 /L ↑ creatinine> 100 μmol/L ↑ urea > 7.5 μmol/L ↑ D-dimer >500 μg/L |
Leucocytosis ↑ LDH > 445 U/L |
| IL-6 |
Non-survivors (N=54) 11 (7.5 – 14.4) pg/mL Survivors (N=137) 6.3 (5.0 – 7.9) pg/mL |
Non-survivors
¶ (N=68) 11.4 ± 8.5 ng/mL Survivors ¶ 6.8 ± 3.6 ng/mL (N=82) |
Non-survivors
¶ (N = 44) 10.1 (7.4 – 14.8) pg/L Survivors ¶ (N = 117) 6.3 (5.4 – 7.8) pg/L |
n/a | n/a |
Abbreviations: AKI, Acute kidney injury; ARDS, acute respiratory distress syndrome; CAD, coronary artery disease; HTN, hypertension; n/a, non-available;
Values expressed as means ± standard deviation or mean [interquartile range (IQR)].
Only variables statistically significant are presented (p < 0.05).
SOFA score: Sequential Organ Failure Assessment. This score includes multiples parameters such as assessment of respiratory status (partial pressure of oxygen, fraction of inspired oxygen and oxygen saturation), coagulation parameters (platelets), liver function (bilirubin), hypotension, central nervous assessment with Glasgow coma score and renal function (creatinine) (109).
The IL-6 units reported in these studies do not compare with the units generally presented. Unfortunately, the method used for measuring IL-6 was not provided.
In a cohort of 60 hospitalised SARS-CoV-2 patients, IL-6 concentrations were 163 ± 153 pg/mL for the group with mild symptoms and 517 ± 796 pg/mL for the patients with severe presentation (ICU admitted) (57). A summary of the observations from studies of IL-6 in hospitalised COVID-19 patients is presented in Table 2. IL-6 may be both a biomarker and a potential therapeutic target for hospitalised patients with COVID-19 which is an attractive concept in the absence of alternative direct acting antiviral strategies.
Table 2 –
Review of hospital admission IL-6 values in COVID-19 patients
| Reference | Setting Country | N∇ | Control * (pg/mL) | Cut-off (pg/mL) | Critically ill patients □ (pg/mL) | Predictor of Complications♦ (pg/mL) | Predictor of Mortality ♦ (pg/mL) | Method for IL-6 monitoring |
|---|---|---|---|---|---|---|---|---|
| (4) | Hospital Germany | 40 | 19.6 [0 – 76.5] | 80 | n/a | 121.0 [19.2 – 430.0] | n/a | n/a |
| N = 27 | N=13 | |||||||
| (3) ¶ | Hospital China | 201 | 6.3 [5.4 – 7.8] pg/L | n/a | 6.1 (5.1– 6.7] pg/L | 7.4 [5.6 – 10.9] pg/L | 10.1 [7.4 – 14.8] pg/L | n/a |
| N=117 | N=40 | N=84 | N=44 | |||||
| (7) ¶ | Hospital China | 150 | 6.8 ± 3.6 ng/mL | n/a | n/a | n/a | 11.4 ± 8.5 ng/mL | n/a |
| N = 82 | N = 68 | |||||||
| (1) | Hospital China | 191 | 6.3 [5.0 – 7.9] | n/a | n/a | n/a | 11.0 [7.5 – 14.4] | n/a |
| N = 137 | N = 54 | |||||||
| (55) | Hospital China | 48 | 10.4 [3.8 – 31.0] | 100 | 64 [25.6–111.9] | n/a | n/a | ECLIA (Roche Ltd) |
| N =21 | N = 17 | |||||||
| (56) | Hospital China | 43 | 10.6 [5.1 – 24.2] | 24.3 | n/a | 36.1 [23.0 – 59.2] | n/a | ECLIA (Rochecobase601) |
| N = 28 | N = 17 | |||||||
| (110) | Hospital China | 43 | 6.7 [4.4 – 12.4] | n/a | n/a | 51.7 [34.3 – 161.7] | n/a | n/a |
| N = 36 | N = 7 | |||||||
| (54) | Hospital China | 53 | 13.4 ± 1.8 | n/a | 37.8 ± 7.8 | n/a | n/a | FMBA (Qingdao Raisecare Biotechnology Co.) |
| N = 45 | N =18 |
Abbreviations: n/a, non-available; ARDS, acute respiratory distress syndrome; ECLIA, electrochemiluminescence method; FMBA, flow cytometer microsphere-based assay.
Values expressed as means ± standard deviation or mean [interquartile range (IQR)]
Number of patients included in each study
The “Control” IL-6 value represents the COVID-19 diagnosed patients with mild symptoms included in the studies (no healthy controls included).
Some studies included IL-6 levels dosed after hospital admission and during disease progression.
This value was recorded upon hospital admission and predicted either sepsis or mortality.
The IL-6 units reported in these studies do not match the units generally presented. Unfortunately, the method used for measuring IL-6 was not provided.
Importantly, proposed clinical cut-off values for IL-6 in this setting have started to emerge with > 80 pg/ml determined to predict respiratory failure in a study with 40 COVID-19 patients in which 13 required medical ventilation (4) and > 100 pg/ml in patients with detectable serum SARS-CoV-2 nucleic acid was found to correlate with mortality (N=17/48) (55). However, other authors have reported specificity at much lower concentrations with a retrospective study of 43 COVID-19 patients from China indicating that severe cases could be predicted using an IL-6 value greater than 24.3 pg/mL (sensitivity of 73.3% and a specificity of 89.3%) (56). While studies with larger sample sizes are urgently required to determine the true IL-6 cut-off associated with severe disease, ICU admission and mortality, these initial results are promising. The presented data is limited by its retrospective nature and uncertainty if IL-6 levels can be ascertained by clinical laboratories in an expedited fashion to guide therapy in real-time.
Lessons from IL-6 and other proinflammatory states including sepsis
IL-6 levels are considered to be undetectable or below 10 pg/mL (with some inter-test variability) in heathy controls (12, 58, 59). Conversely, mean IL-6 levels at presentation appear highest in severe sepsis (51.4 pg/mL) compared to patients that do not develop severe sepsis (36.5 pg/mL; p < 0.03) in a study of community-acquired pneumonia (60). This response is highly specific for severe disease and some studies indicate a role in disease progression, demonstrating up to a four-fold decrease in IL-6 three days after initial diagnosis. Moreover, IL-6 values appear to drop abruptly in survivors while remaining higher in non-survivor groups (59–61). Nonetheless, IL-6 currently represents one of the best characterised markers of disease severity and an early rise in IL-6 is associated with sepsis, organ failure and death (62–64).
Assigning discrete cut-off values for IL-6 to enable its use as a clinical diagnostic tool has remained ill-defined due to variations in the literature. Song et al. demonstrated in 142 patients that an IL-6 cut-off value of 52.60 pg/mL and 348.9 pg/mL was associated with a diagnostic and prognostic value, respectively, in patients with SIRS (65). In contrast, in another SIRS cohort (N=177), a cut-off of 75 pg/mL for sepsis and 145 pg/mL for septic shock was defined (61). Thus, even if further clarification is required, the literature demonstrates that elevated IL-6 values are associated with sepsis or septic shock development (59, 66–68). This is also supported by a 2016 meta-analysis of 2680 critically-ill patients from 22 studies - the use of IL-6 was of moderate diagnostic capacity and relatively high specificity in defining sepsis from other SIRS (69) and, thus, IL-6 may be of utility to confirm infectious causation in patients with complex presentation while considering the limitation in terms of availability of IL-6 levels. The specific IL-6 values from critically ill patients are represented in Table 3.
Table 3 –
Literature review of hospital admission IL-6 values in critically ill patients
| Reference | Population IL-6 dosage technique | Setting Country | Study Design | N∇ | Control * (pg/mL) | Cut-off ¥ (pg/mL) | Sepsis ϖ (pg/mL) | Predictor of Sepsis♦ (pg/mL) | Predictor of Mortality♦ (pg/mL) |
|---|---|---|---|---|---|---|---|---|---|
| (12) | SARS-CoV patients Detection level n/a (CBA) | Hospital Taiwan | MCRC | 88 | 7.5 ± 30.4 | n/a | 245.7 ± 770.2 | n/a | 387.2 ± 911.82 |
| (57) | SARS-CoV patients Detection >10 pg/ml (ELISA) | Hospital China | SCPC | 228 | 61.0 ± 10.1 | n/a | n/a | 163 ± 153 517 ± 796 (severe) |
n/a |
| (65) | SIRS, sepsis (S) and septic shock (C) patients Detection level n/a (ELISA) |
ED Korea | SCPC | 142 | 23.6 [11.2–43.5] | 52.60 (S) 348.9 (C) |
n/a | 89.9 [45.2–272.6] 1378.6 [256.411,062.1] (S) |
≥ 348.92 7609.5 [4526.0–12,208.4] (28 days) |
| (67) | Critically ill patients with organ dysfunction Detection level n/a (RT) |
ICU Japan | SCPC | 100 | 104 [46–152] | 152 | n/a | 720 [183–7,656] | n/a |
| (59) | Severe sepsis patients Detection level n/a (ELISA) |
ED Taiwan | SCPC | 76 | 32.9 [0–663.5] | n/a | n/a | 223.4 [3.1–979.1] septic shock | 196.3 [0.5–979.1] |
| (111) | Sepsis patients Detection level n/a (ELISA) |
ICU Finland | MCPC | 61 | 426 [234–1000] | n/a | n/a | n/a | 1000 [269–2000] |
| (112) | SIRS patients Detection >9.7pg/mL (ELISA) |
ICU Malaysia | SCPC | 239 | 183 [61–358] | 238 [86–3159] | 1127 [218–8643] (30 days) | ||
| (61) | SIRS patients Detection level n/a (ECLIA) |
ED Korea | SCPC | 177 | 55.3 ± 100.9 | 75 (sepsis) 145(shock) |
n/a | 900.1 ± 1643.4 | 1018.8 |
| (66) | Infection suspicion patients Detection level n/a (ECLIA) |
ED Finland | SCPC | 539 | 15.3 [1.5– 653] | n/a | n/a | 93.5 [1.5–43 790] | n/a |
| (68) | Infection and SIRS patients Detection level n/a (ECLIA) |
ICU Switzerland | SCPC | 78 | 44.2 | 200 | n/a | n/a | 1000 |
| (113) | Major trauma patients (female vs male) (ELISA) | ED Germany | SCPC | 343 | 163.7 ± 25.98 | n/a | n/a | 363.9 ± 72.58 | n/a |
| (58) | Major trauma patients Detection >7.8pg/ml (ELISA) |
ICU France | SCPC | 100 | 55.7 [45.9– 83.8] | n/a | n/a | 95.1 [71.3–210.3] | n/a |
| (62) | Major trauma patients Detection level n/a (ELISA) |
Trauma unit Switzerland | SCRC | 1032 | 282.1 ± 39.8 | n/a | n/a | 551.6 ± 124.1 | n/a |
| (60) | CAP patients Detection >5.9pg/mL (ECLIA) |
ED US | MCPC | 1426 | 38.7 | n/a | 98.7 | 51.4 | 109.4 (90 days) |
Abbreviations: CBA, Human Th1/Th2 Cytokine or Chemokine Bead Array Kit; CAP, community acquired pneumonia, ED, emergency department; ECLIA, electrochemiluminescence method; ELISA, enzyme-linked immunosorbent assay; ICU, intensive care unit; n/a, non-available; RT, routine testing; SARS-CoV, severe acute respiratory syndrome coronavirus; SIRS, Systemic inflammatory response syndrome; US, United States.
Types of study design: SCRC: single-center retrospective cohort; SCPC: single-center prospective cohort; MCRC: multi-center retrospective cohort; MCPC: multi-center prospective cohort.
Values expressed as means ± standard deviation or mean [interquartile range (IQR)].
Number of patients included in each study
The control group does not include any healthy controls.
Some studies included IL-6 levels dosed after sepsis diagnostic.
This initial value was recorded upon admission to the hospital (ED) or ICU depending on the study and predicted either sepsis or mortality.
The authors calculated a cut-off value that could predict sepsis.
Lessons from IL-6 response in CAR T-cell associated cytokine release syndrome
There are similarities between the immunopathology of sepsis-associated cytokine storm and the cytokine release syndrome (CRS), a well described complication of chimeric antigen receptor T-cell (CAR T-cell) therapy or hematopoietic cell transplantation (HCT). While these terms should not be used interchangeably, CRS was described as part of the cytokine storm syndromes [14]. The CRS is a cytokine-mediated systemic inflammatory disease that groups signs and symptoms of multiple organ damage ranging from mild constitutional symptoms (grade 1) to end-organ damage (grade 4) [23, 24]. Multiple grading systems for CRS have been provided in the literature and a commonly used one is presented in Table 4. In the case of CRS, the cytokines are released directly by the infused CAR T-cells or by other immune cells such as macrophages in response to the cytokines produced by the CAR T-cells [25]. In some series of CRS, serum IL-6 levels correlated with the activation of potent T lymphocytes and CAR T-cell expansion, predicting subsequent therapeutic response and tumour control (70, 71). Humanized IL-6R inhibitors such as tocilizumab have been integrated into CAR T-cell treatment protocols to pre-emptively manage CRS.
Table 4 –
Cytokine Release Storm - Grades and treatment (114)
| Cytokine release storm: A multi-organ clinical diagnostic involving constitutional, cardiovascular, hematological, gastrointestinal, cutaneous and neurological manifestations. | ||
|---|---|---|
| Grades | Clinical Manifestations | Recommended treatment |
| 1 - Mild | Patients require symptomatic treatment only Fever +/− other constitutional symptoms (no organ dysfunction) |
Supportive care (fluids, antipyretics, analgesics as needed) |
| 2 - Moderate | Symptoms respond to moderate intervention Hypoxia (oxygen requirement <40% Fi02) OR Hypotension (responsive to IV fluids or low dose vasopressors) Grade 2 organ toxicity* |
Supportive care Cardiac and other organ function monitoring If co-morbidities or older age, consider treatment as per for Grade 3 |
| 3 - Severe | Symptoms respond to aggressive intervention Hypoxia (oxygen requirement ≥40% Fi02) OR Hypotension requiring high dose or multiple vasopressors Grade 3 organ toxicity** or Grade 4 transaminitis*** |
Tocilizumab∇ Adults: 4 mg/kg in adults Children: 8 mg/kg in children Repeat the dose if clinical improvement does not occur within 24 to 48 hours. +/− Low dose corticosteroids ¥ |
| 4 - Life threatening | Requirement for mechanical ventilation OR Grade 4 organ toxicity*** (excluding transaminitis) | Tocilizumab +/− Low dose corticosteroids ¥ |
Common Terminology Criteria for Adverse Events (CTCAE) - Grade 2 - Moderate; minimal, local or non-invasive intervention indicated; limiting age-appropriate instrumental Activities of Daily Living (ADL).
CTCAE - Grade 3 - Severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care.
CTCAE - Grade 4 - Life-threatening consequences; urgent intervention indicated.
Dose of tocilizumab approved for adults and children with rheumatoid arthritis
Data concerning the use of steroids in COVID-19 is limited. Please refer to the National Institutes of Health (NIH) treatment guidelines (115).
ROLE OF BIOLOGICAL IMMUNOTHERAPIES IN SARS-CoV-2
Targeting IL-6
The use of biomarkers such as IL-6 and downstream CRP to recognise early the hyperinflammatory state of SARS-CoV-2 infection has been proposed as a trigger point for employing immunological therapies. Importantly, many such immunotherapies are already available for different treatment indications including those that target the IL-6 and IL-6R. Tocilizumab is a humanized anti-IL-6R antibody engineered by grafting the complementarily determining regions (CDR) of a mouse anti-human IL-6R antibody into a human IgGk to create a human antibody with a human IL-6R binding site. Critically for the opposing pro- and anti-inflammatory functions previously discussed, tocilizumab binds to both membrane-bound and soluble IL-6R for total inhibition of IL-6 signal transduction (Figure 3). The main side effects of completely blocking IL-6 signaling are neutropenia, thrombocytopenia and liver enzyme abnormalities (72). Serious infections have been reported in patients treated long term with tocilizumab so caution should be used (73). Nonetheless, tocilizumab is FDA approved for not only rheumatoid arthritis for which it was originally developed and provides beneficial relief of this largely Th17-driven disease, but, more recently, for severe or life-threatening (grade 3 or 4) CRS associated with CAR T-cell therapy (Table 4) with a dramatic reversal of the clinical manifestations (20). For CRS, initial studies dosed patients at 8 mg/kg and 12 mg/kg infused intravenously over 60 minutes with up to three additional doses if needed (minimum 8 hours between consecutive doses) (74). Responders were defined as patients with symptom resolution within 14 days.
Because of the proposed benefits of using tocilizumab in patients with CAR T-cell induced CRS and the described similarities between CRS and CS following infection, randomized trials are recruiting in COVID-19. In certain centres, tocilizumab has been employed in a compassionate access fashion in critically severe COVID-19 patients. A retrospective study from China (N=21) which used tocilizumab 400 mg intravenous drip (single dose) with or without lopinavir/ritonavir and methylprednisolone demonstrated improvement in fever, hypoxemia, CRP levels and pulmonary CT imaging, without adverse events (75). The mean CRP levels before the drug were 75.06 ± 66.80 mg/L and decreased to 38.13 ± 54.21 mg/L at day one, 10.61 ± 13.79 mg/L at day three and 2.72 ± 3.60 mg/L at day five (75). The mean IL-6 level prior to the first dose of tocilizumab was 132.38 ± 278.54 pg/ml (75). Although follow-up IL-6 levels were not subsequently ascertained in this study, the pre-treatment IL-6 concentration aligns with severe disease cut-offs in those studies mentioned earlier. Of immense importance for monitoring, increased serum IL-6 may be expected after initial treatment with tocilizumab (76). Indeed, it is considered that the usual IL-6R mediated consumption of IL-6 is altered by the bound between tocilizumab and IL-6R and that the IL-6 level during tocilizumab treatment probably reflects disease activity (76). Furthermore, in this study, IL-6 was also significantly increased in 20 healthy volunteers 7 days after a single dose of tocilizumab (3.0 +/− 0.6 pg/mL at baseline and 9.3 +/−1.0 pg/mL at day 7) (76). Therefore, it is proposed that post tocilizumab use, monitoring of CRP may be a more appropriate assay for monitoring inflammation (44, 76). A French center has also shared their experience with tocilizumab 8 mg/kg (up to two doses) in 30 severe SARS-CoV-2 patients defined as requiring more than 6 L/min oxygen therapy with rapid changes in oxygen needs (increase of more than 3 L/min in 12 hours) and having a more than 5 days disease diagnosis (77). The authors found that, when compared with a matched control group, the drug decreased the need for mechanical ventilation and ICU admission (23/30) (77). Finally, in an observational study from the United States, 153 patients with severe COVID-19 (defined as patients requiring supplemental oxygen and critical disease) were treated with an 8 mg/kg intravenous tocilizumab dose (maximum 800 mg). When compared to the non-severe group, survival rates were similar (p=0.11) (78).
In light of these promising results, the FDA has approved a randomized, double-blinded, placebo-controlled phase III clinical trial (COVACTA) with 55 locations in North America and Europe. This trial aims to test the efficacy and safety of intravenous tocilizumab in patients with severe SARS-CoV-2 infection (NCT04320615). Similarly, a multicenter, randomized controlled trial was started in China to test the efficacy and safety of tocilizumab in the treatment of patients with COVID-19 pneumonia and elevated IL-6 levels (ChiCTR2000029765). The Italian Regulatory Drug Agency (AIFA) has approved a multicenter, single-arm, open-label, phase 2 study (TOCIVID-19) where all the patients will be treated with tocilizumab 8 mg/kg intravenously (up to a maximum of 800 mg per dose), the primary goal being to assess the mortality rate after the first month (EudraCT: 2020–001110-38). There are currently more than 20 registered COVID-19 associated tocilizumab trials (Table 5). A study registered in Greece proposes to individualise immunomodulatory treatment including tocilizumab or anakinra in COVID-19 depending on their cytokine profile (NCT04339712).
Table 5 –
Potential therapies for COVID-19 ARDS and CS
| Name | Commercial name | Target | Role | FDA indications | Trials Country | Planned Clinical Trials |
|---|---|---|---|---|---|---|
| Tocilizumab | Actemra® RoActemra® |
membrane or soluble IL-6R | Inhibits IL-6 signal transduction | Cytokine Release Syndrome Rheumatoid arthritis Giant Cell Arteritis Juvenile Idiopathic Arthritis |
COVACTA -United States Italy Spain France Belgium Greece Switzerland Denmark Malaysia China |
NCT04320615 NCT04356937 NCT04331795 NCT04346355 NCT04332913 NCT04317092 NCT04315480 NCT04335305 NCT04332094 NCT04331808 NCT04330638 NCT04339712 NCT04335071 NCT04322773 NCT04345445 NCT04310228 NCT04306705 |
| Sarilumab | Kevzara® | membrane or soluble IL-6R | Inhibits IL-6 signal transduction | Rheumatoid arthritis | International United States Canada France Spain Denmark |
NCT04327388 NCT04315298 NCT04321993 NCT04324073 NCT04357808 NCT04357860 NCT04322773 NCT04345289 |
| Siltuximab | Sylvant® | IL-6 | Inhibits IL-6 signal transduction | Multicentric Castleman’s disease | Italy Spain Belgium |
NCT04322188 NCT04329650 NCT04330638 |
| Anakinra | Kineret® | type 1 IL-1 receptor | Inhibits IL-1α and IL-1β signal transduction. | Rheumatoid arthritis NOMID |
United States Italy Greece France |
NCT04362111 NCT04324021 NCT04339712 NCT04357366 NCT04341584 |
| Canakinumab | Ilaris® | IL-1β | blocking IL-1β interaction with IL-1 receptors | Periodic Fever Syndromes Juvenile Idiopathic Arthritis |
Italy | NCT04348448 |
| Ruxolitinib | Jakafi® Jakavi® |
JAK1, JAK2 inhibitor | Inhibits cytokine-induced STAT phosphorylation | Myelofibrosis Polycythemia Vera Acute Graft Versus Host Disease |
United States Canada Mexico Germany Spain |
NCT04354714 NCT04348071 NCT04331665 NCT04334044 NCT04359290 NCT04348695 |
| Tofacitinib | Xeljanz® | JAK1, JAK2, JAK3, TYK2 inhibitor | Rheumatoid Arthritis Psoriatic Arthritis Ulcerative Colitis |
Italy | NCT04332042 | |
| Baricitinib | Olumiant® | JAK2 (JAK 1/3, TYK2), AAK1 inhibitor | Rheumatoid arthritis | United States Canada Italy |
NCT04340232 NCT04321993 NCT04320277 |
|
| Fedratinib | Inrebic® | JAK2, FLT3 and BRD4 inhibitor | Myelofibrosis | None | None | |
| Acalabrutinib | Calquence® | BTK | Inhibits BTK signaling/ B-cell activation | Mantle cell lymphoma Chronic lymphocytic leukemia Small lymphocytic lymphoma |
United States Europe |
NCT04380688 NCT04346199 |
| Eculizumab | Soliris® | complement protein C5 | inhibits C5 cleavage to C5a and C5b (prevents formation of C5b-9) | Paroxysmal nocturnal hemoglobinuria (PNH) | United States France |
NCT04288713 NCT04346797 NCT04355494 |
| Ravulizumab | Ultomiris® | United States |
NCT04369469 NCT04390464 |
|||
| Emapalumab | Gamifant® | IFN-γ | binds to and neutralizes IFN-γ | Primary HLH | Italy | NCT04324021 |
| Adalimumab | Humira® | TNFα | inhibits TNF-α signal transduction | Rheumatoid Arthritis, Psoriatic Arthritis Ankylosing Spondylitis Crohn’s Disease Ulcerative Colitis Plaque Psoriasis Hidradenitis Suppurativa |
China | ChiCTR2000030089 |
| Secukinumab | Cosentyx® | IL-17A | Inhibits IL-17A signal transduction (via IL-17 receptor) | Psoriatic Arthritis Ankylosing Spondylitis Plaque Psoriasis |
None | None |
Abbreviations: AAK1, AP2-associated protein kinase 1; BRD4, Bromodomain-containing protein 4; BTK, Bruton’s tyrosine kinase: FLT3, fms-like tyrosine kinase 3; HLH, hemophagocytic lymphohistiocytosis; IFN, Interferon; IL, Interleukin; JAK, Janus Associated Kinase; NOMID, neonatal-onset multisystem inflammatory; STAT, Signal transducer and activator of transcription; TYK2, tyrosine kinase 2; TNF, Tumour Necrosis Factor.
Sarilumab, a monoclonal antibody to IL-6 receptor, is also being investigated in COVID-19 trials. A French multicenter randomized controlled trial (CORIMUNO-SARI) aiming to assess the efficacity and safety of sarilumab versus standard of care is ongoing (NCT04324073). Two additional industry driven clinical trials (NCT04315298, NCT04327388) aiming to assess the efficacy and safety of sarilumab in COVID-19 hospitalized patients are recruiting. While the clinical outcome data for sarilumab is lacking, the comparative response with tocilizumab will be of interest given the longer half-life of sarilumab and greater affinity for the IL-6R (72).
Siltuximab, a chimeric monoclonal antibody targeting IL-6 directly and preventing binding to both soluble and membrane bound IL-6 receptors, is FDA approved for the multicentric Castleman’s disease (79). There are currently 3 European trials recruiting COVID-19 diagnosed patients (NCT04322188, NCT04329650, NCT04330638).
Targeting IL-1β
IL-1β leads to an increase in body temperature, lung inflammation and fibrosis (36). Increased levels of IL-1β were noted in patients diagnosed with SARS-CoV (80) and similar to IL-6, were associated with increased mortality in sepsis (9). Anakinra is a non-glycosylated human decoy interleukin-1 receptor antagonist (IL-1Ra) that binds to the type 1 IL-1 receptor and inhibits IL-1α and IL-1β signal transduction (81). This drug is FDA approved for rheumatoid arthritis and neonatal-onset multisystem inflammatory disease (NOMID) (82) and suggested in the treatment algorithm for secondary HLH/MAS (83).
A recent study found that the serum I L-1β levels were undetectable in 100% (N=17) of the patients with severe or moderate SARS-CoV-2 infection (5), an expected result considering the mechanism of action of this exocrine cytokine. Anakinra was used in a cohort from Italy to treat 29 adult patients diagnosed with COVID-19 related moderate-to-severe ARDS and hyperinflammation (defined as serum CRP ≥100 mg/L, ferritin ≥900 ng/mL, or both) (84). Survival was 90% compared to 56% in a standard treatment group (N=16) (p=0.009) (84). Other improvements included a reduction in CRP and a decrease in mechanical ventilation use. Post-treatment inflammatory relapse was not reported and the treatment was well tolerated (84).
Further, the post-hoc analysis of a phase III randomized control trial studying the use of anakinra in severe sepsis indicated a significant improvement in survival of septic patients with features of macrophage activation syndrome (MAS) in the absence of any severe adverse reactions (26). CORIMUNO-ANA is a trial that aims to determine the efficacy of anakinra in SARS-CoV-2 infected patients (NCT04341584). Anakinra will be administered twice daily as decreasing doses of intravenous infusions (400 mg on day one, two and three; 200 mg on day four and 100 mg on day five). Canakinumab is a human anti-IL-1β monoclonal antibody that blocks IL-1β interaction with the IL-1 receptor for which there is currently one registered observational study (NCT04348448).
Targeting TNF-α and Interferon-γ
Similar to IL-1β, TNF-α has a direct role in acute systemic inflammation and is increased in patients with severe SARS-CoV-2 infection (2, 5, 85). However, this finding is not consistent among the different studies (54). Besides the observational reports that indicate an increase in the levels of this cytokine, a direct pathogenic mechanism of cellular viral entry involving the shedding of the coronavirus’ functional receptor, the angiotensin-converting enzyme 2 (ACE2), was studied. This process of binding and shedding of the ACE2 is coupled with TNF-α production and the production of a TNF-α-converting enzyme (TACE) (86). Thus, it has been suggested that an anti-TNF drug could not only inhibit TNF-α directly but also down-regulate the expression and shedding of the ACE2. Also, some studies showed a decrease in sepsis related mortality with anti-TNF treatment (9).
There are multiple commercialised anti-TNF biologics. Adalimumab is a recombinant human IgG1 monoclonal antibody that specifically binds to human tumor necrosis factor alpha (TNF-α) and blocks its interaction with the p55 and p75 cell surface TNF receptors. This drug could be potentially useful in managing severe COVID-19 manifestations. To analyze the benefits of an anti-TNF-α treatment in COVID-19, a randomized-controlled trial of adalimumab injection in severe COVID-19 patients has been registered (ChiCTR2000030089).
Similar to TNF, the major pro-inflammatory cytokine IFN-γ is also increased in the cytokine storm associated with COVID-19. IFN-γ was particularly well described in SARS-CoV patients (12) and may be targeted by emapalumab for which a comparative multicentre randomized clinical trial is also underway in combination with anakinra (NCT04324021).
Targeting IL-17
Another cytokine that could have a role in the cytokine storm caused by COVID-19 is IL-17. This was not a cytokine of interest in the recent SARS-CoV-2 studies and the only study that characterized IL-17 in COVID-19 found normal levels using a flow cytometry method (54).
As described in this review, IL-17 stimulates the production of pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α. Secukinumab is a human IgG1K monoclonal antibody that binds to IL-17A (inhibits the interaction with the IL-17 receptor) and is currently used for plaque psoriasis and several rheumatological conditions (87). The further rational for inhibiting IL-17 is that it is a proximal target to IL-1 and IL-6 and, hence, could reduce neutrophil recruitment to the lungs and prevent organ dysfunction in ARDS. To our knowledge, there are no ongoing trials involving this drug.
Targeting JAK
Targeting the Th17 pathway, research on murine models showed promising results with the use of fedratinib, a JAK2 inhibitor. In this study, the drug decreased the expression of IL-17. As IL-6 and IL-23 are signals for Th17 cell initial differentiation and effector function through the JAK2-STAT3 pathway, the use of this inhibitor could decrease the pro-inflammatory function of Th17 (88). This drug is currently FDA approved for myelofibrosis (89). To our knowledge, there are no current registered trials involving this drug.
As mentioned, the cell-surface ACE2 receptor is needed for coronavirus endocytosis and one of the regulators of this process is the AP2-associated protein kinase 1 (AAK1), part of the numb-associated kinase (NAK) family (90). AAK1 inhibitors have been shown to prevent virus infections by disrupting viral cell invasion. Baricitinib is an oral JAK inhibitor (JAK1/JAK2, JAK1/JAK3, JAK1/tyrosine kinase 2 (TYK2) and JAK2/TYK2) but also an AAK1 inhibitor, having direct antiviral activity, that is currently FDA approved for rheumatoid arthritis resistant to anti-TNF drugs (91). Several trials are ongoing to confirm its safety and efficacy and it is also being investigated in combination therapy with remdesivir (NCT04340232, NCT04321993, NCT04320277). Remdesivir, an adenosine analogue with demonstrated antiviral activity against a broad range of RNA virus families, has been used in a randomized, placebo-controlled trial showing a decrease in time to recovery (15 versus 11 days) and a trend towards decrease in mortality (92). This drug gained an FDA approval for use in children and adults with severe COVID-19.
Ruxolitinib is a JAK1 and JAK2 inhibitor that mediates the signaling of numerous cytokines such as IL-6, IFN-γ and growth factors with essential roles in immune function and hematopoiesis. This drug is FDA approved for myelofibrosis, hydroxyurea resistant polycythemia vera and steroid-refractory acute graft-versus-host disease (93). A multicenter, single-blinded, randomized trial (1:1) of 44 patients with COVID-19 showed a tendency (not statistically significant) towards improvement in clinical outcomes in the ruxolitinib group (94). Several larger clinical trials from North American and Europe are ongoing (Table 5).
Another attractive drug is tofacitinib that has been shown to inhibit the in vitro activity of JAK1/JAK2, JAK1/JAK3 and JAK2/JAK2 and thus decrease the related cytokines. According to the FDA, it can be used for rheumatoid arthritis, psoriatic arthritis and ulcerative colitis (73). There is one planned Italian trial that aims to assess the advantage of early administration of tofacitinib in SARS-COv2 related interstitial pneumonia (NCT04332042).
Serious bacterial, mycobacterial, fungal and viral infections have been reported with the use of JAK inhibitors. This potential off-target effect of these drugs combined with the decreased interferon innate response can lead to severe complications and caution should be used in the SARS-CoV-2 context with theoretical benefit for the anti-JAK molecules that have more specific targets.
Other targeted immunomodulatory therapies and combination therapies
As described, the production of cytokines and chemokines by macrophages is regulated by the BTK. Thus, inhibition of this protein could be a promising strategy for reducing COVID-19 related complications with therapeutic inhibition of BTK in patients with lymphoid malignancies resulting in decreased proinflammatory cytokines.
Company sponsored trials with acalabrutinib, a small-molecule inhibitor of BTK enzymatic activity, that aim to study its efficacy and safety compared to best supportive care in hospitalized patients with COVID-19 are currently listed and will begin recruitment shorty in the United States and Europe (Table 5).
By sensing self or pathogenic cytosolic double-stranded DNA (dsDNA), the cyclic guanosine monophosphate (GMP)-adenosine monophosphate (AMP) synthetase (cGAS) stimulator of interferon (IFN) genes (STING) plays an important role in innate immunity and tumor development (95). STING is expressed in T-cells, monocytes, NK cells, and dermal fibroblasts and cGAS-STING signaling promotes the production of IL-6 and the downstream activation of STAT3 (95). The STING-IFN-β pathway is triggered by the binding of cGAS to STING that leads to IFN regulatory factor 3 (IRF-3) phosphorylation and subsequent transcription of the gene encoding interferon-β (IFNB) (96). The JAK receptors and their specific pathways are activated by the IFN-β binding to its receptor. The regulation of STING and other proinflammatory cytokine genes is also achieved with the synthesis and release of interferons. Thus, this pro-inflammatory loop can be obstructed by JAK inhibition (96).
Combination therapy with lopinavir-ritonavir, ribavirin and IFN-p-1b compared with lopinavir-ritonavir monotherapy was evaluated in an intention to treat multicenter, randomized phase 2 clinical trial from China. The primary endpoint was the time before a negative nasopharyngeal swab (RT-PCR) in SARS-CoV-2 patients with the median time reported for the combination group (N=86) being 7 days and the time in control group (N=41) was 12 days (p=0.0010) (97). Anti-Coronavirus Therapies (ACT) COVID-19 is a clinical trial that aims to evaluate the combination of chloroquine and azithromycin with subcutaneous injection of IFN-β1b for SARS-CoV-2 prevention by assessing admission to intensive care, mechanical ventilation and/or death (NCT04324463).
The complement system is also a potential therapeutic target in SARS-CoV-2 infection. Complement is key to the innate immune response to all viruses and complement inhibition is a potential treatment for severe SARS-CoV-2 infection by reducing the severity and end-organ consequences of the innate immune response (98, 99). A recent mouse model suggested that complement activation through C3 exacerbates SARS-CoV associated ARDS and that C3-deficient mice infected with SARS-CoV showed less respiratory decline (100). Lung biopsy samples from patients with SARS-CoV-2 associated ARDS showed evidence of complement activation with C3 fragment deposition and associated increased serum 5a levels (98). However, there is little clinical data on the potential role of complement activation and its role in ARDS associated with SARS-CoV-2. There are now several proposed and ongoing studies examining the role of C5 inhibitors such as eculizumab and ravulizumab (Table 5).
Convalescent plasma
Given the lack of evidence-based treatment and the novelty of this disease, convalescent plasma (CP) has reemerged as an emergency intervention passive immunization strategy aiming to decrease morbidity and mortality in critically ill COVID-19 patients (101, 102). This treatment has been shown favourable during the SARS-CoV infection with a decrease in hospital stays and mortality compared to controls (103, 104). Also, a recent systematic review, while acknowledging the limited data, indicated that CP is safe, clinically effective and can play a role in reducing mortality (105). The described mechanisms of action are direct neutralization of the virus aimed at the spike (S) viral protein (106, 107) as well as other immunomodulatory and anti-inflammatory functions such as neutralization of cytokines, complement and autoantibodies (101). A clinical report on the use of CP in critically ill SARS-CoV-2 patients showed a hypothetical benefit with decrease of body temperature, increase in respiratory function and ARDS resolution in 4 of the 5 patients included (107). In an open-label, multicenter, randomized clinical trial from China, adding convalescent plasma to the treatment plan did not result in increased clinical recovery (108). Several questions remain unanswered regarding CP and there is a need for larger randomized controlled trials to answer these questions but emerging successful reports related with its use in severe COVID-19 highlights the intense inflammatory response that accompanies this infection.
CONCLUSION
Severe SARS-CoV-2 infection is associated with cytokine storm producing a hyperinflammatory state and a clinical and laboratory picture similar to hemophagocytic lymphohistiocytosis that typically occurs 7–10 days following the onset of acute illness. In this setting, IL-6 levels correlate with respiratory failure, poor outcomes and mortality. Blocking this and other appealing cytokines and signaling pathways at an early stage shows promise to target specific and undesirable immune responses in the setting of acute SARS-CoV-2 infection. Currently, studies examining the combination of direct antiviral agents with immunomodulatory therapy are ongoing and will be important in the quest to prevent acute respiratory deterioration, ventilation use, morbidity and mortality from SARS-CoV-2 infection.
Supplementary Material
Inflammatory cytokines and chemokines, including IL-6, IL-1β and TNF-α, are significantly elevated in patients with severe SARS-CoV-2 infection suggesting that cytokine storm may play a role in the SARS-CoV-2 severity, morbidity and mortality.
IL-6 has major effects on cellular immunity, with both pro- and anti-inflammatory functions.
Several recent publications have shown that an increase in the pro-inflammatory cytokine IL-6 correlates with disease severity, defined as sepsis, ARDS or mechanical ventilation and mortality in SARS-CoV-2.
As with the development of any novel diagnosis and as increased levels of IL-6 are associated with sepsis and septic shock, clinical cut-offs must be defined.
Clinical trials are urgently warranted to evaluate a therapeutic strategy targeting upstream and downstream pathways in SARS-CoV-2. The effective dose and the ideal administration timing of the immunomodulatory drugs remain under investigation.
Acknowledgements
Al Chorfi (graphic design work)
Austin Health Sciences Library
Funding sources: none to declare
Abbreviations
- AAK1
AP2-associated protein kinase 1
- ACE2
angiotensin-converting enzyme 2
- AIFA
Italian Regulatory Drug Agency
- AIM2
Absent in melanoma 2
- AMP
adenosine monophosphate
- ARDS
Acute respiratory distress syndrome
- BRD4
Bromodomain-containing protein 4
- BTK
Bruton’s tyrosine kinase
- CAR
Chimeric antigen receptor
- CP
convalescent plasma
- COVID-19
Coronavirus disease 2019
- CDR
Complementarily determining regions
- cGAS
cyclic guanosine monophosphate-adenosine monophosphate synthetase
- CRP
C-reactive protein
- CRS
Cytokine release syndrome
- CS
Cytokine storm
- CTCAE
Common Terminology Criteria for Adverse Events
- DAMP
Damage-associated molecular pattern
- DNA
deoxyribonucleic acid
- dsDNA
double-stranded DNA
- ED
Emergency department
- ELISA
Enzyme-linked immunosorbent assay
- gp130
glycoprotein 130
- FDA
Food and Drug Administration
- FLT3
fms-like tyrosine kinase 3
- GMP
guanosine monophosphate
- HCT
Hematopoietic cell transplantation
- HIES
Hyper Immunoglobulin E syndrome
- HLH
hemophagocytic lymphohistiocytosis
- ICU
Intensive care unit
- IL
Interleukin
- IFN
Interferon
- IQR
Interquartile range
- IRF-3
interferon regulatory factor 3
- JAK
Janus Associated Kinase
- LRR
leucine-rich repeats
- MAPK
Mitogen-activated protein kinase
- MAS
macrophage activation syndrome
- MERS
Middle East respiratory syndrome
- NAK
numb-associated kinase
- NF-kB
nuclear factor kappa-light-chain-enhancer of activated B-cells
- NK
natural killer
- NLRP3
NOD-, LRR- and pyrin domain-containing protein 3
- NOD
Nucleotide-binding and oligomerization domain
- NO
Nitric oxide
- NOMID
neonatal-onset multisystem inflammatory
- PAMP
Pathogen-associated molecular pattern
- PBMC
peripheral blood mononuclear cells
- pg/ml
picogram/milliliter
- PRRs
pattern recognition receptors
- RNA
Ribonucleic acid
- ROS
Reactive oxygen species
- SARS
Severe acute respiratory syndrome
- SARS-CoV-1
SARS-associated coronavirus 1
- SIRS
Systemic inflammatory response syndrome
- STAT
signal transducer and activator of transcription
- STING
stimulator of interferon genes
- TACE
TNF-α-converting enzyme
- TGF-β
transforming growth factor β
- Th 17
T helper 17
- TLRs
Toll-like receptors
- TNF
Tumour Necrosis Factor
- Treg
regulatory T-cells
- TYK2
tyrosine kinase 2
- WES
whole-exome sequencing
Footnotes
Conflicts of Interest: none to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herold T, Jurinovic V, Arnreich C, Hellmuth J, von Bergwelt-Baildon M, Klein M, et al. Level of IL-6 predicts respiratory failure in hospitalized symptomatic COVID-19 patients. medRxiv Preprint server for health sciences,. 2020. [Google Scholar]
- 5.Chen G, Wu D, Guo WZ, Cao Y, Huang D, Wang H, et al. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. The Journal of Clinical Investigation,. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39(5):517–28. [DOI] [PubMed] [Google Scholar]
- 10.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Wu K, Wang D, Yue X, Song D, Zhu Y, et al. Nucleocapsid protein of SARS-CoV activates interleukin-6 expression through cellular transcription factor NF-kappaB. Virology. 2007;365(2):324–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang KJ, Su IJ, Theron M, Wu YC, Lai SK, Liu CC, et al. An interferon-gamma-related cytokine storm in SARS patients. J Med Virol. 2005;75(2):185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12(10):1203–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi WS, Kang CI, Kim Y, Choi JP, Joh JS, Shin HS, et al. Clinical Presentation and Outcomes of Middle East Respiratory Syndrome in the Republic of Korea. Infect Chemother. 2016;48(2):118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saad M, Omrani AS, Baig K, Bahloul A, Elzein F, Matin MA, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azhar EI, Hui DSC, Memish ZA, Drosten C, Zumla A. The Middle East Respiratory Syndrome (MERS). Infect Dis Clin North Am. 2019;33(4):891–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020;26(6):729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020;94(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Behrens EM, Koretzky GA. Review: Cytokine Storm Syndrome: Looking Toward the Precision Medicine Era. Arthritis Rheumatol. 2017;69(6):1135–43. [DOI] [PubMed] [Google Scholar]
- 21.Filipovich A, McClain K, Grom A. Histiocytic disorders: recent insights into pathophysiology and practical guidelines. Biol Blood Marrow Transplant. 2010;16(1 Suppl):S82–9. [DOI] [PubMed] [Google Scholar]
- 22.Schulert GS, Grom AA. Macrophage activation syndrome and cytokine-directed therapies. Best Pract Res Clin Rheumatol. 2014;28(2):277–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beutel G, Wiesner O, Eder M, Hafer C, Schneider AS, Kielstein JT, et al. Virus-associated hemophagocytic syndrome as a major contributor to death in patients with 2009 influenza A (H1N1) infection. Crit Care. 2011;15(2):R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulert GS, Grom AA. Pathogenesis of macrophage activation syndrome and potential for cytokine-directed therapies. Annu Rev Med. 2015;66:145–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miettunen PM, Narendran A, Jayanthan A, Behrens EM, Cron RQ. Successful treatment of severe paediatric rheumatic disease-associated macrophage activation syndrome with interleukin-1 inhibition following conventional immunosuppressive therapy: case series with 12 patients. Rheumatology (Oxford). 2011;50(2):417–9. [DOI] [PubMed] [Google Scholar]
- 26.Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, et al. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit Care Med. 2016;44(2):275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulert GS, Zhang M, Fall N, Husami A, Kissell D, Hanosh A, et al. Whole-Exome Sequencing Reveals Mutations in Genes Linked to Hemophagocytic Lymphohistiocytosis and Macrophage Activation Syndrome in Fatal Cases of H1N1 Influenza. J Infect Dis. 2016;213(7):1180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gudbjartsson DF, Helgason A, Jonsson H, Magnusson OT, Melsted P, Norddahl GL, et al. Spread of SARS-CoV-2 in the Icelandic Population. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deza Leon MP, Redzepi A, McGrath E, Abdel-Haq N, Shawaqfeh A, Sethuraman U, et al. COVID-19 Associated Pediatric Multi-System Inflammatory Syndrome. J Pediatric Infect Dis Soc. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones VG, Mills M, Suarez D, Hogan CA, Yeh D, Segal JB, et al. COVID-19 and Kawasaki Disease: Novel Virus and Novel Case. Hosp Pediatr. 2020;10(6):537–40. [DOI] [PubMed] [Google Scholar]
- 31.Faix JD. Biomarkers of sepsis. Crit Rev Clin Lab Sci. 2013;50(1):23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chalmers S, Khawaja A, Wieruszewski PM, Gajic O, Odeyemi Y. Diagnosis and treatment of acute pulmonary inflammation in critically ill patients: The role of inflammatory biomarkers. World J Crit Care Med. 2019;8(5):59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acharya D, Liu G, Gack MU. Dysregulation of type I interferon responses in COVID-19. Nat Rev Immunol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu H, Chan JF, Wang Y, Yuen TT, Chai Y, Hou Y, et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Brien TR, Thomas DL, Jackson SS, Prokunina-Olsson L, Donnelly RP, Hartmann R. Weak Induction of Interferon Expression by SARS-CoV-2 Supports Clinical Trials of Interferon Lambda to Treat Early COVID-19. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID 19 patients. Perspective Immunology,. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang D, Guo R, Lei L, Liu H, Wang Y, Wang Y, et al. COVID-19 infection induces readily detectable morphological and inflammation-related phenotypic changes in peripheral blood monocytes, the severity of which correlate with patient outcome. medRxiv Preprint server for health sciences,. 2020. [Google Scholar]
- 40.Weber ANR, Bittner Z, Liu X, Dang TM, Radsak MP, Brunner C. Bruton’s Tyrosine Kinase: An Emerging Key Player in Innate Immunity. Front Immunol. 2017;8:1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wen W, Su W, Tang H, Le W, Zhang X, Zheng Y, et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117(14):3720–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361(9):888–98. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka T, Kishimoto T. The biology and medical implications of interleukin-6. Cancer Immunol Res. 2014;2(4):288–94. [DOI] [PubMed] [Google Scholar]
- 45.Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). medRxiv Preprint server for health sciences, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uciechowski P, Dempke WCM. Interleukin-6: A Masterplayer in the Cytokine Network. Oncology. 2020;98(3):131–7. [DOI] [PubMed] [Google Scholar]
- 49.Spencer S, Kostel Bal S, Egner W, Lango Allen H, Raza SI, Ma CA, et al. Loss of the interleukin-6 receptor causes immunodeficiency, atopy, and abnormal inflammatory responses. J Exp Med. 2019;216(9):1986–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–88. [DOI] [PubMed] [Google Scholar]
- 51.Adam N, Rabe B, Suthaus J, Grotzinger J, Rose-John S, Scheller J. Unraveling viral interleukin-6 binding to gp130 and activation of STAT-signaling pathways independently of the interleukin-6 receptor. J Virol. 2009;83(10):5117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scheller J, Ohnesorge N, Rose-John S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand J Immunol. 2006;63(5):321–9. [DOI] [PubMed] [Google Scholar]
- 53.Schumacher N, Rose-John S. ADAM17 Activity and IL-6 Trans-Signaling in Inflammation and Cancer. Cancers (Basel). 2019;11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wan S, Yi Q, Fan S, Lv J, Zhang X, Guo L, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv Preprint server for health sciences,. 2020. [Google Scholar]
- 55.Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. medRxiv Preprint server for health sciences,. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao Y, Li T, Han M, Li X, Wu D, Xu Y, et al. Diagnostic Utility of Clinical Laboratory Data Determinations for Patients with the Severe COVID-19. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Li J, Zhan Y, Wu L, Yu X, Zhang W, et al. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect Immun. 2004;72(8):4410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gouel-Cheron A, Allaouchiche B, Guignant C, Davin F, Floccard B, Monneret G, et al. Early interleukin-6 and slope of monocyte human leukocyte antigen-DR: a powerful association to predict the development of sepsis after major trauma. PLoS One. 2012;7(3):e33095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu HP, Chen CK, Chung K, Tseng JC, Hua CC, Liu YC, et al. Serial cytokine levels in patients with severe sepsis. Inflamm Res. 2009;58(7):385–93. [DOI] [PubMed] [Google Scholar]
- 60.Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167(15):1655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jekarl DW, Lee SY, Lee J, Park YJ, Kim Y, Park JH, et al. Procalcitonin as a diagnostic marker and IL-6 as a prognostic marker for sepsis. Diagn Microbiol Infect Dis. 2013;75(4):342–7. [DOI] [PubMed] [Google Scholar]
- 62.Billeter A, Turina M, Seifert B, Mica L, Stocker R, Keel M. Early serum procalcitonin, interleukin-6, and 24-hour lactate clearance: useful indicators of septic infections in severely traumatized patients. World J Surg. 2009;33(3):558–66. [DOI] [PubMed] [Google Scholar]
- 63.Stensballe J, Christiansen M, Tonnesen E, Espersen K, Lippert FK, Rasmussen LS. The early IL-6 and IL-10 response in trauma is correlated with injury severity and mortality. Acta Anaesthesiol Scand. 2009;53(4):515–21. [DOI] [PubMed] [Google Scholar]
- 64.Maier B, Lefering R, Lehnert M, Laurer HL, Steudel WI, Neugebauer EA, et al. Early versus late onset of multiple organ failure is associated with differing patterns of plasma cytokine biomarker expression and outcome after severe trauma. Shock. 2007;28(6):668–74. [PubMed] [Google Scholar]
- 65.Song J, Park DW, Moon S, Cho HJ, Park JH, Seok H, et al. Diagnostic and prognostic value of interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: a prospective controlled study according to the Sepsis-3 definitions. BMC Infect Dis. 2019;19(1):968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uusitalo-Seppala R, Koskinen P, Leino A, Peuravuori H, Vahlberg T, Rintala EM. Early detection of severe sepsis in the emergency room: diagnostic value of plasma C-reactive protein, procalcitonin, and interleukin-6. Scand J Infect Dis. 2011;43(11–12):883–90. [DOI] [PubMed] [Google Scholar]
- 67.Takahashi W, Nakada TA, Yazaki M, Oda S. Interleukin-6 Levels Act as a Diagnostic Marker for Infection and a Prognostic Marker in Patients with Organ Dysfunction in Intensive Care Units. Shock. 2016;46(3):254–60. [DOI] [PubMed] [Google Scholar]
- 68.Harbarth S, Holeckova K, Froidevaux C, Pittet D, Ricou B, Grau GE, et al. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med. 2001;164(3):396–402. [DOI] [PubMed] [Google Scholar]
- 69.Ma L, Zhang H, Yin YL, Guo WZ, Ma YQ, Wang YB, et al. Role of interleukin-6 to differentiate sepsis from non-infectious systemic inflammatory response syndrome. Cytokine. 2016;88:126–35. [DOI] [PubMed] [Google Scholar]
- 70.Klaver Y, van Steenbergen SCL, Sleijfer S, Debets R, Lamers CHJ. Plasma IFN-γamma and IL-6 levels correlate with peripheral T-cell numbers but not toxicity in RCC patients treated with CAR T-cells. Clin Immunol. 2016;169:107–13. [DOI] [PubMed] [Google Scholar]
- 71.Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24(5):563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ogata A, Kato Y, Higa S, Yoshizaki K. IL-6 inhibitor for the treatment of rheumatoid arthritis: A comprehensive review. Mod Rheumatol. 2019;29(2):258–67. [DOI] [PubMed] [Google Scholar]
- 73.FDA. XELJANZ® (tofacitinib) 2018. [Available from: https://www.fda.gov/Drugs.
- 74.Le RQ, Li L, Yuan W, Shord SS, Nie L, Habtemariam BA, et al. FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell-Induced Severe or Life-Threatening Cytokine Release Syndrome. Oncologist. 2018;23(8):943–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective Treatment of Severe COVID-19 Patients with Tocilizumab. wwwchinaxivorg,. 2020. [DOI] [PMC free article] [PubMed]
- 76.Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N, Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112(10):3959–64. [DOI] [PubMed] [Google Scholar]
- 77.Roumier M, Paule R, Groh M, Vallée A, Ackermann F. Interleukin-6 blockade for severe COVID-19. medRxiv Preprint server for health sciences,. 2020. [Google Scholar]
- 78.Price CC, Altice FL, Shyr Y, Koff A, Pischel L, Goshua G, et al. Tocilizumab treatment for Cytokine Release Syndrome in hospitalized COVID-19 patients: survival and clinical outcomes. Chest. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.FDA. SYLVANT® (siltuximab) 2014. [Available from: https://www.fda.gov/Drugs.
- 80.Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, Chan IH, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136(1):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cohen SB. The use of anakinra, an interleukin-1 receptor antagonist, in the treatment of rheumatoid arthritis. Rheum Dis Clin North Am. 2004;30(2):365–80, vii. [DOI] [PubMed] [Google Scholar]
- 82.FDA. Kineret® (anakinra) 2012. [Available from: https://www.fda.gov/Drugs.
- 83.Kumar B, Aleem S, Saleh H, Petts J, Ballas ZK. A Personalized Diagnostic and Treatment Approach for Macrophage Activation Syndrome and Secondary Hemophagocytic Lymphohistiocytosis in Adults. J Clin Immunol. 2017;37(7):638–43. [DOI] [PubMed] [Google Scholar]
- 84.Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatology. 2020;2:e325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haga S, Yamamoto N, Nakai-Murakami C, Osawa Y, Tokunaga K, Sata T, et al. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc Natl Acad Sci U S A. 2008;105(22):7809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fala L Cosentyx (Secukinumab): First IL-17A Antagonist Receives FDA Approval for Moderate-to-Severe Plaque Psoriasis. Am Health Drug Benefits. 2016;9(Spec Feature):60–3. [PMC free article] [PubMed] [Google Scholar]
- 88.Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.FDA. INREBIC® (fedratinib) 2019. [Available from: https://www.fda.gov/Drugs.
- 90.Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.FDA. OLUMIANT® (baricitinib) 2018. [Available from: https://www.fda.gov/Drugs.
- 92.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19 - Preliminary Report. N Engl J Med. 2020. [DOI] [PubMed] [Google Scholar]
- 93.FDA. JAKAFI® (ruxolitinib) 2019. [Available from: https://www.fda.gov/Drugs.
- 94.Cao Y, Wei J, Zou L, Jiang T, Wang G, Chen L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pei J, Zhang Y, Luo Q, Zheng W, Li W, Zeng X, et al. STAT3 inhibition enhances CDN-induced STING signaling and antitumor immunity. Cancer Lett. 2019;450:110–22. [DOI] [PubMed] [Google Scholar]
- 96.Liu Y, Jesus AA, Marrero B, Yang D, Ramsey SE, Sanchez GAM, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med. 2014;371(6):507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hung IF, Lung KC, Tso EY, Liu R, Chung TW, Chu MY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Risitano AM, Mastellos DC, Huber-Lang M, Yancopoulou D, Garlanda C, Ciceri F, et al. Complement as a target in COVID-19? Nat Rev Immunol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Campbell CM, Kahwash R. Will Complement Inhibition be the New Target in Treating COVID-19 Related Systemic Thrombosis? Circulation. 2020. [DOI] [PubMed] [Google Scholar]
- 100.Gralinski LE, Sheahan TP, Morrison TE, Menachery VD, Jensen K, Leist SR, et al. Complement Activation Contributes to Severe Acute Respiratory Syndrome Coronavirus Pathogenesis. mBio. 2018;9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rojas M, Rodriguez Y, Monsalve DM, Acosta-Ampudia Y, Camacho B, Gallo JE, et al. Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun Rev. 2020:102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Soo YO, Cheng Y, Wong R, Hui DS, Lee CK, Tsang KK, et al. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10(7):676–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rajendran K, Krishnasamy N, Rangarajan J, Rathinam J, Natarajan M, Ramachandran A. Convalescent plasma transfusion for the treatment of COVID-19: Systematic review. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20(4):398–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jones AE, Trzeciak S, Kline JA. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med. 2009;37(5):1649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pettila V, Hynninen M, Takkunen O, Kuusela P, Valtonen M. Predictive value of procalcitonin and interleukin 6 in critically ill patients with suspected sepsis. Intensive Care Med. 2002;28(9):1220–5. [DOI] [PubMed] [Google Scholar]
- 112.Mat-Nor MB, Md Ralib A, Abdulah NZ, Pickering JW. The diagnostic ability of procalcitonin and interleukin-6 to differentiate infectious from noninfectious systemic inflammatory response syndrome and to predict mortality. J Crit Care. 2016;33:245–51. [DOI] [PubMed] [Google Scholar]
- 113.Mors K, Braun O, Wagner N, Auner B, Voth M, Stormann P, et al. Influence of gender on systemic IL-6 levels, complication rates and outcome after major trauma. Immunobiology. 2016;221(8):904–10. [DOI] [PubMed] [Google Scholar]
- 114.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.National Institutes of Health. The National Institutes of Health COVID-19 Treatment Guidelines Panel Provides Recommendations for Dexamethasone in Patients with COVID-19 2020. [Available from: https://www.covid19treatmentguidelines.nih.gov/dexamethasone/ on 30/06/2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.