Abstract
Aortic stiffness is associated with an increased risk of cardiovascular disease and mortality and may increase risk of dementia. The aim of the present study is to examine the association between arterial stiffness and cognitive decline in a large prospective cohort study with three repeated cognitive assessment over 7 years of follow-up. Aortic pulse wave velocity (PWV) was measured among 4,300 participants (mean±standard deviation age 65.1±5.2 years) in 2007–09 and categorized based on the tertiles: (lowest third: <7.41 m/s), (middle third: 7.41–8.91 m/s), and (highest third: >8.91 m/s). Cognitive function was assessed in 2007–09, 2012–13, and 2015–16. Standardized global cognitive score in highest third versus lowest third of PWV category at baseline (−0.12, 95% CI −0.18, −0.06). Accelerated 7-year cognitive decline was observed among individuals with the highest PWV [difference in 7-year cognitive change for highest third vs. lowest third PWV: −0.06, 95% CI −0.11, −0.01, P <0.01]. Longitudinal trajectories of global cognitive score differed across PWV distribution (P <0.01). Higher aortic stiffness was associated with faster cognitive decline. Clinicians may be able to use arterial stiffness severity as an indicator to administer prompt treatments to prevent or delay the onset of dementia. Future studies need to determine if early intervention of vascular stiffness may be effective in delaying cognitive decline.
Keywords: Ageing, Arterial stiffness, Cognitive decline, Pulse-wave velocity
Introduction
The substantial expansion in life expectancy and population ageing is a major achievement but also one of the 21st century public health challenges. Ageing is associated with exponential increases in number of people living with cognitive impairment and dementia, both of which reduce quality of life [1, 2]. The number of people with dementia is projected to increase from 0.7M in 2016 to 1.2M by 2040 in England and Wales [3]. Arterial stiffness, due to loss of elasticity, increases with age and is associated with higher systolic, pulse pressure and atherosclerosis [4], which are in turn important predictors of cognitive decline [5]. If such vascular pathophysiological alterations are better understood, it may be possible to prevent or delay cognitive decline and improve quality of life in older people.
Aortic pulse wave velocity (PWV) is the non-invasive gold standard to determine arterial stiffness [6]. Increased arterial stiffness is significantly and independently associated with higher risk for cardiovascular morbidity and mortality [4]. The link between cardiovascular disorders and cognitive decline and dementia remains to be understood. Small-scale observational studies [7–19] have assessed the association of arterial stiffness with cognitive impairment, but their results are inconsistent. It was suggested that arterial stiffness may influence specific cognitive domains such as executive function [19, 7], memory [11, 16, 17], and processing speed [7]. The assessment of the effect of arterial stiffness on cognitive decline has methodological challenges as longitudinal cognitive test data spanning a decade are rare and the selective attrition (younger persons with a better cardiovascular risk profile are remaining) are the main limitation of these studies. Further, cognitive function was mainly assessed with the Mini-Mental State Examination (MMSE), a global test with low sensitivity for detection of small changes in cognitive status [20]. Our study is the first longitudinal study with the largest sample size and high response rate for all three points measures of cognitive function that examine whether arterial stiffness is associated with deficits and changes in specific cognitive domains in our large study.
We hypothesized that higher PWV would be associated with both concurrently assessed cognitive performance and subsequent cognitive decline. We examined a global measure of cognitive function, and the cognitive domains of memory, executive function and verbal fluency using data on 4,300 individuals.
Methods
Study population
The Whitehall II study is a longitudinal cohort study, initially of 10,308 (67% men, aged 35–55 years) London-based British civil servants, recruited in 1985. Since then, follow-up questionnaire surveys and clinical examinations have taken place every 2–3 years and 5 years, respectively. Ethical approval for each study phase was obtained from the NHS National Research Ethics Service and the local Research Ethics Committee. All participants provided written consent prior to each study phase.
The 2007–2009 clinical examination was attended by 6,225 participants. Of these, 4,300 participants provided data on both PWV and cognitive function, forming the analytic sample to examine the association between PWV and cognitive trajectories (Analysis 1) (Fig 1). The association between PWV and “pronounced cognitive decline” (Analysis 2) was assessed in analysis based on 3,828 participants.
Fig 1.
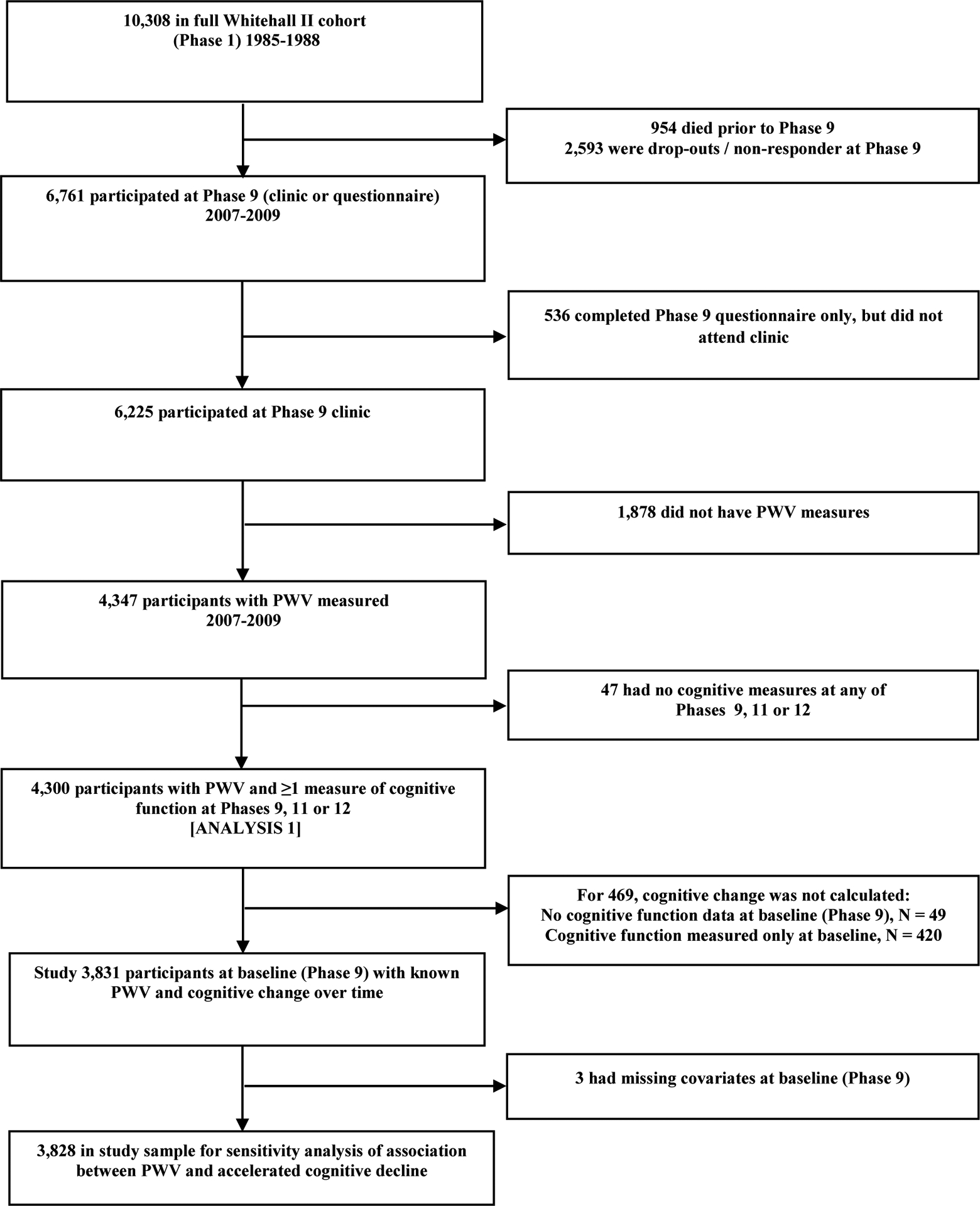
Study Flowchart
Aortic PWV
PWV was assessed between the carotid and femoral sites using applanation tonometry (SphygmoCor, Atcor Medical, Australia), a validated method of measuring PWV. Path length was determined with a tape measure by subtracting the carotid-sternal notch distance from the femoral-sternal notch distance. PWV was measured twice for each participants and a third measurement was taken when the difference between the two measurements were larger than 0.5 m/s. The average of all the measurements was used in the analysis. PWV measurements were repeated within 60 days for 125 participants to assess short-term repeatability. Median difference of the repeats were 0.83 m/s (interquartile range 0.43–1.40). The PWV distribution showed slight right skewness which was normalized by using the logged PWV values before standardizing the distribution to mean zero and standard deviation one.
Cognitive function
The cognitive test battery was administered at the clinical examinations in 2007–09, 2012–2013, and 2015–16. Test-retest reliability was assessed in 556 participants within three months and showed excellent reliability (range 0.6–0.9). The cognitive tests were as follows. Memory: participants were presented with a 20-word list of one or two syllable words at two second intervals, with 2 minutes time to write down as many words as they can recall, regardless of word order. Reasoning: participants had 10 minutes to complete the AH4-I (Alice Heim 4-I), a series of 65 verbal and mathematical reasoning items of increasing difficulty. Verbal Fluency: phonemic fluency was assessed via the “S” words and semantic fluency via “animal” words tests. One minute was allowed for each test. The 30-item Mini-Mental-State-Examination (MMSE) was also used to assess global cognitive status.
Covariates
Sociodemographic variables included age, sex, ethnicity (white, others), socioeconomic status using employment grade (three categories: high, intermediate, and low representing income and status at work), and education (five categories: less than primary school (up to age 11), lower secondary school (up to age 16), higher secondary school (up to age 18), university, and higher university degree).
Health behaviors were assessed by questionnaire and included smoking (current smokers, ex-smokers, and never smokers) and, alcohol consumption (number of alcoholic drinks consumed in the previous days, converted to units of alcohol consumed in a week and categorized as “no/occasional alcohol consumption,” “moderate alcohol consumption” (1–14 units/week), and “heavy alcohol consumption” (≥14 units/week)).
Health related covariates included body mass index assessed at the clinical examination (categorized as <20, 20–24.9, 25–29.9, and ≥30 kg/m2), hypertension (systolic/diastolic blood pressure ≥140/90 mm Hg or use of antihypertensive drugs), self-reported use of drugs, prevalent diabetes mellitus (determined by fasting glucose ≥7.0 mmol/L, reported diabetes diagnosed by a doctor, or use of diabetes drugs), and cardiovascular diseases (CVD) (including coronary heart disease and stroke (identified using linkage to national hospital records)).
Statistical analysis
Analysis 1:
Linear mixed models were used to estimate both the cross-sectional and the longitudinal association of PWV with the global cognitive score. These methods use all available cognitive data over the three measures and take into account the fact that repeated measures on the same individual are correlated with each other. The intercept and the slope were fitted as random effects, allowing individuals to have different cognitive scores at baseline and different rates of cognitive decline over the follow-up. The PWV distribution were categorized into the tertiles: (lowest third: <7.41 m/s), (middle third: 7.41–8.91 m/s), and (highest third: >8.91 m/s)
We then fitted the mixed models using the tertiles categories of PWV with global cognitive score and also for each cognitive domain as the dependent variable. Since the mean follow-up (time between the first and last cognitive measure) was 7.3 years, we expressed the changes in the cognitive measures per 7 years. The PWV×time×sex interactions suggested no sex differences in the PWV associations with cognitive function or with cognitive decline (all P >0.14) and, therefore, all analyses were conducted with men and women combined. We also used MMSE<27 and MMSE<26 as two ways to define those with cognitive decline and mixed logistic models with these two outcomes were fitted. The analyses were adjusted for sex, age, ethnicity and employment grade by fitting these terms and their interactions with the linear follow-up time component.
Analysis 2:
In order to examine PWV as a predictor of the most pronounced cognitive decline, a linear mixed model was used to estimate the decline in global cognitive score across the three waves of data collection for each participant. A binary outcome variable was created to indicate the 20% of participants with the fastest cognitive declines. Logistic regression models were fitted and odds ratios (ORs) and corresponding 95% confidence intervals (95% CIs) calculated to estimate the association between PWV and the fastest rate of cognitive decline. These models were adjusted for sex, age, ethnicity and employment grade. A sensitivity analysis was conducted to assess the robustness of the estimates to using a different range of threshold (10–25%) for the proportion of participants with the fastest cognitive decline. Statistical significance was inferred as a two-tailed P-value <0.05.
The analyses using splines and the global cognitive score trajectories with age were conducted in StataSE 15.1 with all other models fitted using the Proc Mixed procedure from the SAS software version 9.4 (SAS Institute, Cary, NC, USA).
Results
Fig 1 shows the number eligible participants included in the analysis. Of the 10,308 participants at study inception (1985–88), 954 had died, 2,593 had withdrawn from the study, and 536 did not attend clinic in 2007–09. Our first analysis was based on 4,300 of the 6,225 individuals still in the study. Those included in the analyses were more educated (40% vs. 32% had a university degree, P <0.001), were younger (65 years vs. 67 years, P <0.001) and generally had a healthier risk factor profile than those excluded (Supplementary Table 1). Demographic and clinical characteristics at baseline among those included in the analyses are shown in Table 1 according to the tertiles of PWV. Participants in the highest third were older and had a more adverse risk factor profile than those in the lowest third.
Table 1.
Baseline characteristics of 4300 participants in the study sample according to the thirds of pulse wave velocity
| [Mean (SD) or %] | P value for heterogeneity | |||
|---|---|---|---|---|
| Thirds of baseline pulse wave velocity (m/s) | ||||
| Lowest third (< 7.41 ) | Middle third (7.41 – 8.91) | Highest third (>8.91) | ||
| Number | 1436 | 1438 | 1426 | |
| Age, y | 63.1 (4.9) | 64.8 (5.5) | 68.0 (5.6) | <0.001 |
| Female, % | 28.9 | 25.1 | 21.7 | <0.001 |
| Non-white ethnicity, % | 5.6 | 6.9 | 10.4 | <0.001 |
| Body Mass Index, kg/m2 | <0.001 | |||
| - Underweight (<18.5) | 1.1 | 0.6 | 0.9 | |
| - Normal weight (18.5 – 24.9) | 50.1 | 37.1 | 32.7 | |
| - Overweight (25.0 – 29.9) | 37.7 | 47.3 | 48.2 | |
| - Obese (≥ 30.0) | 10.4 | 15.0 | 18.2 | |
| Systolic Blood Pressure (mmHg) | 117.6 (13.4) | 124.0 (13.9) | 132.0 (15.5) | <0.001 |
| Diastolic Blood Pressure (mmHg) | 67.5 (9.4) | 71.0 (9.5) | 73.5 (10.3) | <0.001 |
| Anti-hypertension medication, % | 23.6 | 32.9 | 41.6 | <0.001 |
| Serum cholesterol , mmol/L | 5.28 (1.03) | 5.19 (1.04) | 5.17 (1.09) | 0.009 |
| Lipid lowering medication, % | 23.3 | 32.0 | 36.6 | <0.001 |
| Education | <0.001 | |||
| - ≤ Lower secondary | 26.4 | 32.7 | 36.9 | |
| - Higher secondary | 29.5 | 28.1 | 26.6 | |
| - ≥ Degree | 44.1 | 39.2 | 36.5 | |
| Employment grade | 0.04 | |||
| - High | 51.9 | 49.3 | 48.2 | |
| - Intermediate | 40.7 | 42.5 | 41.5 | |
| - Low | 7.4 | 8.2 | 10.3 | |
| Smoking habit | 0.03 | |||
| - Never | 51.4 | 47.3 | 48.6 | |
| - Ex-smoker | 43.4 | 47.0 | 47.5 | |
| - Current | 5.2 | 5.8 | 3.9 | |
| Alcohol consumption | <0.001 | |||
| - No alcohol | 14.5 | 17.1 | 17.6 | |
| - Moderate alcohol | 71.7 | 65.3 | 64.2 | |
| - Heavy alcohol | 13.8 | 17.6 | 18.1 | |
| Moderate or vigorous physical activity | 0.01 | |||
| - < 1 hr/wk | 19.3 | 23.4 | 23.2 | |
| - 1 – 6.9 hrs/wk | 61.7 | 59.7 | 61.3 | |
| - ≥ 7 hrs/wk | 19.0 | 17.0 | 15.5 | |
| Diabetes, % | 5.9 | 9.5 | 18.5 | <0.001 |
| History of cardiovascular disease, % | 9.6 | 10.8 | 14.7 | <0.001 |
Cross-sectional and longitudinal associations between PWV and global cognitive score and individual cognitive domains, are shown in Table 2. In the cross-sectional analysis, global cognitive score was significantly lower in the middle third and the highest third of PWV (>8.91 & 7.41–8.91 m/s) compared to the lowest third (<7.41 m/s). A cross-sectional association was seen for the highest third with all cognitive domains (P <0.05), compared to the lowest third. In the longitudinal analysis, global cognitive function declined significantly faster in the highest third compared to the lowest third after adjustment for age, sex, ethnicity and employment grade [difference in 7-year change in score for >8.91 m/s vs. <7.41 m/s = −0.06 (−0.11 to −0.01), P=0.01]. Similar effects were seen for some of the cognitive domains but only with phonemic fluency the association was statistically significant (P=0.03). The declines in global cognitive score with time, after adjustment for sex, ethnicity and birth cohort, are shown for the three PWV categories in Fig 2. The global cognitive scores decreasing fastest in the highest and the middle third categories of PWV. We assessed the association between MMSE and PWV, both cross-sectionally and longitudinally and the results were not significant (Supplementary Table 2).
Table 2.
Cross-sectional and longitudinal association between the thirds of pulse wave velocity and standardized scores of global cognitive score and individual cognitive domains
| Cognitive domain outcome | Pulse wave velocity | Standardized cognitive score at baseline | Change in standardized cognitive score (per 7 years) | ||
|---|---|---|---|---|---|
| Difference* (95% CI) | P value | Difference* (95% CI) | P value | ||
| Global cognitive score | |||||
| Lowest third | 1.0 (Ref) | - | 1.0 (Ref) | - | |
| Middle third | −0.06 (−0.12, −0.01) | 0.03 | −0.02 (−0.06, 0.03) | 0.48 | |
| Highest third | −0.12 (−0.18, −0.06) | <0.001 | −0.06 (−0.11, −0.01) | 0.01 | |
| Memory | |||||
| Lowest third | 1.0 (Ref) | - | 1.0 (Ref) | - | |
| Middle third | −0.04 (−0.10, 0.03) | 0.28 | 0.04 (−0.03, 0.11) | 0.23 | |
| Highest third | −0.12 (−0.19, −0.05) | <0.001 | −0.01 (−0.08, 0.07) | 0.84 | |
| AH4-I | |||||
| Lowest third | 1.0 (Ref) | - | 1.0 (Ref) | - | |
| Middle third | −0.06 (−0.12, 0.00) | 0.03 | 0.01 (−0.02, 0.05) | 0.52 | |
| Highest third | −0.08 (−0.13, −0.02) | 0.01 | −0.03 (−0.07, 0.01) | 0.09 | |
| Phonemic fluency | |||||
| Lowest third | 1.0 (Ref) | - | 1.0 (Ref) | - | |
| Middle third | −0.08 (−0.15, −0.01) | 0.02 | −0.03 (−0.09, 0.04) | 0.45 | |
| Highest third | −0.10 (−0.17, −0.03) | 0.007 | −0.08 (−0.15, −0.01) | 0.03 | |
| Semantic fluency | |||||
| Lowest third | 1.0 (Ref) | - | 1.0 (Ref) | - | |
| Middle third | −0.02 (−0.09, 0.04) | 0.45 | −0.05 (−0.11, 0.00) | 0.06 | |
| Highest third | −0.08 (−0.14, −0.01) | 0.03 | −0.06 (−0.13, 0.00) | 0.06 | |
Differences are adjusted for age and sex and their interactions with time and time squared and for ethnicity and employment grade and their interactions with time.
Fig 2.
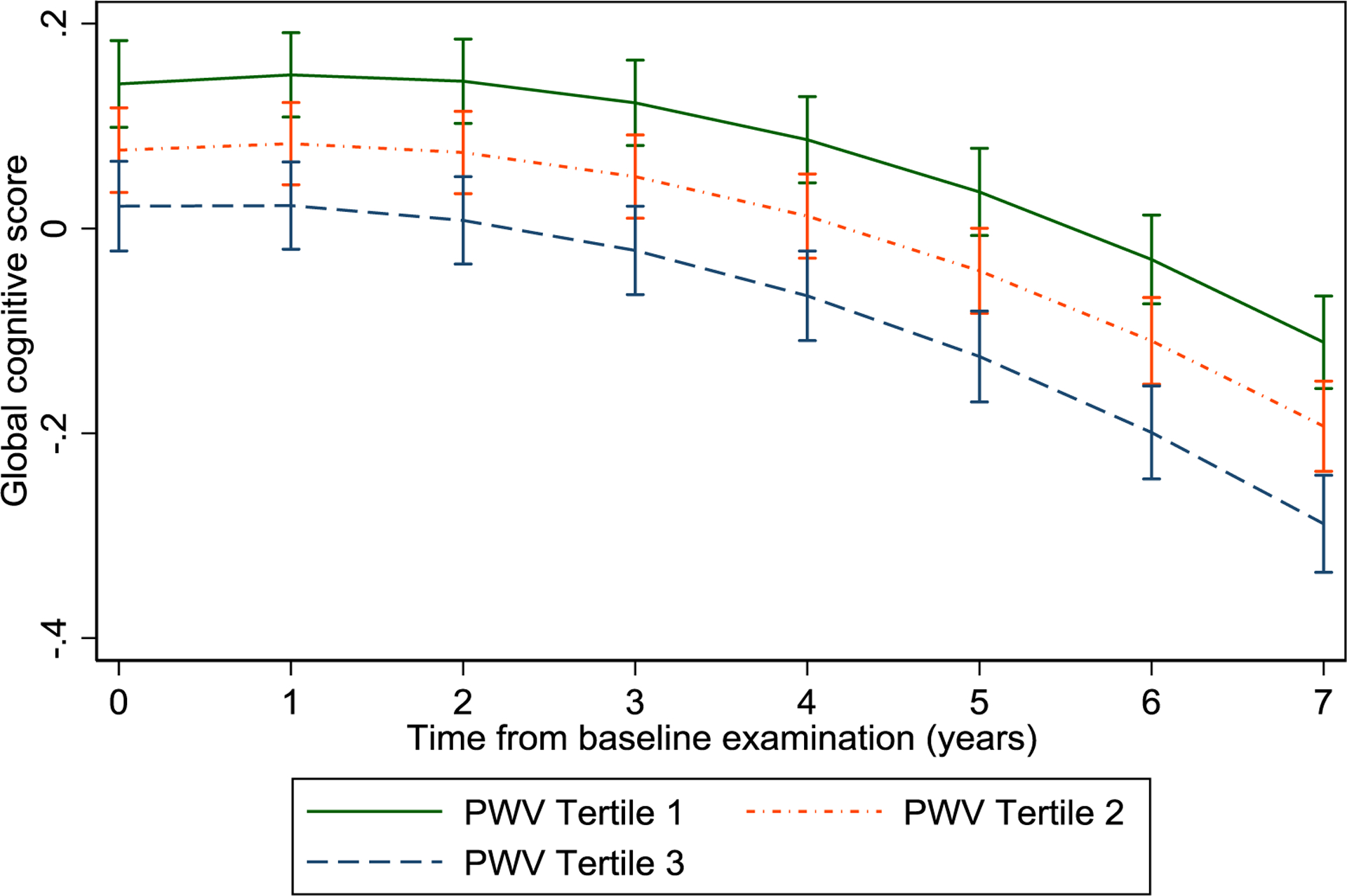
Trajectoriesa of global cognitive score with follow-up time from baseline examination according to tertiles of pulse wave velocity.
a The trajectories of global cognitive score are the predicted values that come from the mixed model that was used to estimate the effects of pulse wave velocity on global cognitive function shown in Table 2.
In the analyses to predict those with the fastest cognitive declines as the outcome (binary variable of top 20% vs. rest), individuals with the in the highest category of PWV were 70% more likely to exhibit rapid declines in cognitive function during prospective follow-up (OR 1.70, 95% CI: 1.31, 2.20), following adjustment for age, sex, ethnicity and employment grade (Table 3). Sensitivity analyses assessing different percentages (10, 15, 20, and 25%) of participants with the fastest cognitive declines were broadly consistent with main findings (Supplementary Table 3).
Table 3.
Association between pulse wave velocity and accelerated global cognitive decline during follow-up
| The 20%a of participants with the greatest cognitive decline over the follow-up, N (%) | ||||
|---|---|---|---|---|
| Pulse wave velocity | No. participants | Odds ratiob (95% CI) | P value | |
| Lowest third | 1316 | 136 (10.3%) | 1.0 (Ref) | |
| Middle third | 1300 | 227 (17.5%) | 1.49 (1.16, 1.93) | 0.002 |
| Highest third | 1212 | 326 (26.9%) | 1.70 (1.31, 2.20) | <0.001 |
| Effect per 1 SD increase | 3828 | 689 (18.0%) | 1.19 (1.08, 1.30) | <0.001 |
The cut-point for those with the greatest global cognitive decline is based upon 5303 participants who had global cognitive score assessed at Phase 9 and at least one other subsequent assessment at phases 11 or 12, irrespective of whether PWV was also measured
Odds ratios of being in the group with the greatest cognitive decline are adjusted for age, sex, ethnicity and employment grade
Discussion
Aortic stiffness was associated with faster global cognitive decline over seven years of follow-up. Individuals with in the highest third category of PWV had 70% increased odds of rapid cognitive decline. We did not see a robust association between arterial stiffness and cognitive decline in all cognitive domains. Decline in phonemic fluency was most clearly associated with aortic stiffness, while decline in reasoning capacity assessed by the AH4-I was linked with aortic stiffness only marginally.
Although several studies [7–9, 12–15, 17, 16, 19] have identified an inverse association between arterial stiffness and cognition, a few studies [10, 11, 18] did not find an association. Our large longitudinal study adds to the evidence that aortic stiffness predicts cognitive decline. Several proposed pathways link arterial stiffness to cognitive decline. First, the stiff aorta loses its cushioning capacity and promotes transmission of potentially deleterious central pressure pulses to the fragile small vessels of target organs such as the brain. Increased pulsatile flow in the brain may accelerate intracranial stenosis, and dysfunction in the cerebral microcirculation [21, 22]. Second, chronic cerebral hypoperfusion may induce cerebral white matter injury or lead to toxic metabolic byproducts accumulation within the brain and blood vessels [23]. Moreover, thrombosis and microinfarcts that can be caused as a result of thickening of the cerebrovascular endothelium and associated endothelial dysfunction are also apparent in malignant hypertension [24]. Third, arterial stiffness is associated with increased risk of CVD events such as stroke, which can cause cognitive impairment [25].
Previously, a study reported concurrent decline in both phonemic and sematic fluency in a group of people with mild cognitive impairment (MCI) [26], while another study [27] showed that individuals with MCI performed worse on phonemic fluency than sematic fluency. While the underlying mechanisms remained largely unknown, evidence shows that multiple brain regions involves during fluency tasks, phonemic fluency tasks are proposed to rely mainly upon executive functioning and pre-frontal lobe processes, whereas sematic fluency tasks are proposed to rely on temporal lobe process [28]. The findings of present study support the hypothesis that aortic stiffness is linked to cognitive decline mediated by frontal lobe atrophy as a result of small vessel disease.”
Our findings were in accordance with those from the Health, Aging and Body Composition (Health ABC) study (N=2,488) [15], which found associations between higher arterial stiffness, as measured by PWV, and more rapid cognitive decline, as measured by MMSE over nine years of follow-up. Additionally, previous results from a sub sample (N=552) [16] of older adults in Health ABC found an association between higher PWV and greater cognitive decline on the psychomotor speed only but not on tests of memory and global cognitive function. Furthermore, findings from Baltimore Longitudinal Study of Aging (BLSA) (N=582) [17] showed significant associations between higher PWV and more rapid cognitive decline on the Blessed Information Memory Concentration Test (working memory test). However, results from the BLSA study did not provide evidence for an association between PWV and tests of global cognitive function [17]. In contrast to our findings, PWV did not predict cognitive decline between two time points over an average of five years in the Rotterdam Study [18].
The inconsistency of results across studies may due to differences in aspects of study design, including the specific neuropsychological tests used, sample size of the study, and differences in participant’s characteristics. Many studies used MMSE to assess cognitive impairments in adults. It has been suggested that MMSE is not a suitable measure for cognitive function among healthy participants due to the ceiling effect. Moreover, MMSE is insensitive to small changes in cognitive function [20]. The sample size is crucial to provide adequate statistical power. Previous studies of the association of arterial stiffness measured by PWV with cognitive decline had fewer than 500 participants.
Our study is the first population-based study to examine the association between arterial stiffness and cognitive decline in a large prospective cohort study of British civil servants who underwent three repeated cognitive assessment over 7 years of follow-up. The PWV is regarded as the gold standard for measuring arterial stiffness and thus provides a great strength to our study. We examined the influence of arterial stiffness on different aspects of cognitive functioning rather than assessing general impairment with a widely used tool for assessing cognitive mental status such as MMSE. A battery of cognitive tests used in this study is a better measure for capturing variability in cognitive scores. Our study also has several limitations, the data derived from white-collar civil servants and may not be the representative of general populations. Given the longitudinal nature of the study, mortality or other competing risks may have resulted in an attenuation of our results. Our study has limitations. Cognitive data derived from generally healthy, retired white-collar civil servants may not be broadly representative. Cognitive decline may be slower than among the general population, and this phenomenon may help to explain why we did not observe a clear link between aortic stiffness and change in AH4-I score.
In conclusion, our results indicate that high arterial stiffness is associated with accelerated decline in global cognitive score. Among the domains, decline in phonemic fluency was most clearly linked to stiffness of the aorta. We showed that executive function could be at risk of impairment due to reduced aortic compliance and loss of elasticity. Future studies need to determine if the interventions on reducing or preventing arterial stiffness can be effective in delaying cognitive decline.
Supplementary Material
Acknowledgements
The Whitehall II study has been supported by grants K013351, R024227, and S011676 from the UK Medical Research Council (MRC); grants PG/11/63/29011 and RG/13/2/30098 from the British Heart Foundation; funding from the British Health and Safety Executive; the British Department of Health; grant R01HL036310 from the National Heart, Lung, and Blood Institute; grants R01AG056477 and R01AG034454 from the National Institute on Aging; and grant ES/J023299/1 from the Economic and Social Research Council. MK was supported by NordFork, the MRC, the Academy of Finland (311492) and Helsinki Institute of Life Science.
Reference
- 1.Rizzi L, Rosset I, Roriz-Cruz M. Global epidemiology of dementia: Alzheimer’s and vascular types. Biomed Res Int. 2014;2014:908915. doi: 10.1155/2014/908915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guzman-Castillo M, Ahmadi-Abhari S, Bandosz P, Capewell S, Steptoe A, Singh-Manoux A et al. Forecasted trends in disability and life expectancy in England and Wales up to 2025: a modelling study. Lancet Public Health. 2017;2(7):e307–e13. doi: 10.1016/S2468-2667(17)30091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmadi-Abhari S, Guzman-Castillo M, Bandosz P, Shipley MJ, Muniz-Terrera G, Singh-Manoux A et al. Temporal trend in dementia incidence since 2002 and projections for prevalence in England and Wales to 2040: modelling study. Bmj-Brit Med J. 2017;358. doi:ARTNj2856 10.1136/bmj.j2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palombo C, Kozakova M. Arterial stiffness, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. Vascul Pharmacol. 2016;77:1–7. doi: 10.1016/j.vph.2015.11.083. [DOI] [PubMed] [Google Scholar]
- 5.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 7.Lim SL, Gao Q, Nyunt MS, Gong L, Lunaria JB, Lim ML et al. Vascular Health Indices and Cognitive Domain Function: Singapore Longitudinal Ageing Studies. J Alzheimers Dis. 2016;50(1):27–40. doi: 10.3233/JAD-150516. [DOI] [PubMed] [Google Scholar]
- 8.Pase MP, Himali JJ, Mitchell GF, Beiser A, Maillard P, Tsao C et al. Association of Aortic Stiffness With Cognition and Brain Aging in Young and Middle-Aged Adults: The Framingham Third Generation Cohort Study. Hypertension. 2016;67(3):513–9. doi: 10.1161/HYPERTENSIONAHA.115.06610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riba-Llena I, Nafria C, Filomena J, Tovar JL, Vinyoles E, Mundet X et al. High daytime and nighttime ambulatory pulse pressure predict poor cognitive function and mild cognitive impairment in hypertensive individuals. J Cereb Blood Flow Metab. 2016;36(1):253–63. doi: 10.1038/jcbfm.2015.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustavsson AM, Stomrud E, Abul-Kasim K, Minthon L, Nilsson PM, Hansson O et al. Cerebral Microbleeds and White Matter Hyperintensities in Cognitively Healthy Elderly: A Cross-Sectional Cohort Study Evaluating the Effect of Arterial Stiffness. Cerebrovasc Dis Extra. 2015;5(2):41–51. doi: 10.1159/000377710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer J, Trollor JN, Crawford J, O’Rourke MF, Baune BT, Brodaty H et al. The Association between Pulse Wave Velocity and Cognitive Function: The Sydney Memory and Ageing Study. Plos One. 2013;8(4). doi:ARTNe61855 10.1371/journal.pone.0061855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugawara N, Yasui-Furukori N, Umeda T, Kaneda A, Sato Y, Takahashi I et al. Comparison of ankle-brachial pressure index and pulse wave velocity as markers of cognitive function in a community-dwelling population. Bmc Psychiatry. 2010;10. doi:Artn46 10.1186/1471-244x-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pase MP, Beiser A, Himali JJ, Tsao C, Satizabal CL, Vasan RS et al. Aortic Stiffness and the Risk of Incident Mild Cognitive Impairment and Dementia. Stroke. 2016;47(9):2256–61. doi: 10.1161/STROKEAHA.116.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taniguchi Y, Fujiwara Y, Nofuji Y, Nishi M, Murayama H, Seino S et al. Prospective Study of Arterial Stiffness and Subsequent Cognitive Decline Among Community-Dwelling Older Japanese. J Epidemiol. 2015;25(9):592–9. doi: 10.2188/jea.JE20140250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeki Al Hazzouri A, Newman AB, Simonsick E, Sink KM, Sutton Tyrrell K, Watson N et al. Pulse wave velocity and cognitive decline in elders: the Health, Aging, and Body Composition study. Stroke. 2013;44(2):388–93. doi: 10.1161/STROKEAHA.112.673533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson NL, Sutton-Tyrrell K, Rosano C, Boudreau RM, Hardy SE, Simonsick EM et al. Arterial stiffness and cognitive decline in well-functioning older adults. J Gerontol A Biol Sci Med Sci. 2011;66(12):1336–42. doi: 10.1093/gerona/glr119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51(1):99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- 18.Poels MM, van Oijen M, Mattace-Raso FU, Hofman A, Koudstaal PJ, Witteman JC et al. Arterial stiffness, cognitive decline, and risk of dementia: the Rotterdam study. Stroke. 2007;38(3):888–92. doi: 10.1161/01.STR.0000257998.33768.87. [DOI] [PubMed] [Google Scholar]
- 19.Meyer ML, Palta P, Tanaka H, Deal JA, Wright J, Knopman DS et al. Association of Central Arterial Stiffness and Pressure Pulsatility with Mild Cognitive Impairment and Dementia: The Atherosclerosis Risk in Communities Study-Neurocognitive Study (ARIC-NCS). J Alzheimers Dis. 2017;57(1):195–204. doi: 10.3233/JAD-161041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell AJ. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res. 2009;43(4):411–31. doi: 10.1016/j.jpsychires.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol (1985). 2008;105(5):1652–60. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henskens LHG, Kroon AA, van Oostenbrugge RJ, Gronenschild EHBM, Fuss-Lejeune MMJJ, Hofman PAM et al. Increased Aortic Pulse Wave Velocity Is Associated With Silent Cerebral Small-Vessel Disease in Hypertensive Patients. Hypertension. 2008;52(6):1120–U72. doi: 10.1161/Hypertensionaha.108.119024. [DOI] [PubMed] [Google Scholar]
- 23.Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke. 2009;40(3 Suppl):S40–4. doi: 10.1161/STROKEAHA.108.533638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nichols WW, Denardo SJ, Davidson JB, Huo TY, Merz CNB, Pepine CJ. Association of aortic stiffness and wave reflections with coronary flow reserve in women without obstructive coronary artery disease: An ancillary study from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE). Am Heart J. 2015;170(6):1243–54. doi: 10.1016/j.ahj.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mok VC, Wong A, Lam WW, Fan YH, Tang WK, Kwok T et al. Cognitive impairment and functional outcome after stroke associated with small vessel disease. J Neurol Neurosurg Psychiatry. 2004;75(4):560–6. doi: 10.1136/jnnp.2003.015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nutter-Upham KE, Saykin AJ, Rabin LA, Roth RM, Wishart HA, Pare N et al. Verbal fluency performance in amnestic MCI and older adults with cognitive complaints. Arch Clin Neuropsychol. 2008;23(3):229–41. doi: 10.1016/j.acn.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rinehardt E, Eichstaedt K, Schinka JA, Loewenstein DA, Mattingly M, Fils J et al. Verbal fluency patterns in mild cognitive impairment and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2014;38(1–2):1–9. doi: 10.1159/000355558. [DOI] [PubMed] [Google Scholar]
- 28.Newman LM, Trivedi MA, Bendlin BB, Ries ML, Johnson SC. The Relationship Between Gray Matter Morphometry and Neuropsychological Performance in a Large Sample of Cognitively Healthy Adults. Brain Imaging Behav. 2007;1(1–2):3–10. doi: 10.1007/s11682-007-9000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


