Abstract
Background:
Up to 50% of women with non-optimal vaginal microbial community state type (CST) have Bacterial vaginosis (BV). Little is known about what distinguishes women with and without BV diagnosis within non-optimal CST. We identified features of women and their male sex partners associated with BV among women with non-optimal vaginal CST.
Methods:
In this prospective study, 252 heterosexual couples were observed at 1-, 6-, and 12- months following baseline. Microbiomes were characterized in cervicovaginal lavage and penile meatal swabs through high-throughput 16s ribosomal RNA gene amplicon sequencing. Non-optimal CST was defined as CST-IV. BV was defined as Nugent score 7–10. Generalized estimating equation (GEE) analysis estimated adjusted odds ratios (aOR) for BV among women with non-optimal CST.
Results:
At baseline, women with non-optimal CST were median age 22 years, 44% with BV, 16% with HIV, and 66% with HSV-2. Male partners were median age 27 years, 12% with HIV, 48% with HSV-2, and 55% circumcised. Within non-optimal CST, S. sanguinegens, Prevotella spp., P. amnii, and Clostridiales BV-associated bacteria-2 (BVAB-2) were statistically significantly enriched in observations with BV. In multivariable GEE controlling for CST, HIV and HSV-2, BV was increased among women with CST-IVA (aOR=1.91; p=0.087), HIV (aOR=2.30; p=0.051), HSV-2 (aOR=1.75; p=0.065), and enrichment of male partner penile taxa: Dialister (aOR=1.16; p=0.034), Megasphaera (aOR=1.22; p=0.001), Brevibacterium (aOR=1.13; p=0.019).
Conclusions:
These results provide insight on factors differentiating women with BV among those with non-optimal vaginal CST. Interrupting the sexual exchange of penile and vaginal taxa may be beneficial for preventing pathologic state of vaginal microbiome.
Keywords: Molecular BV, Bacterial vaginosis, CST-IV, Kenya, HIV, HSV-2, penile microbiome, vaginal microbiome, non-optimal vaginal microbiome, CST
Brief Summary:
Within non-optimal vaginal microbial community state type, bacterial composition varied by Nugent BV status, and Nugent BV was associated with specific penile taxa, controlling for vaginal CST, HSV-2, and HIV.
Introduction
Bacterial vaginosis (BV) is a common condition affecting up to 20% of women worldwide [1] and 20–50% of women in sub-Saharan Africa [2]. BV is associated with increased risk of HIV acquisition [3] and other sexually transmitted infections (STI) [4]. The cost of BV is substantial, stemming from increased risk of adverse outcomes in pregnancy, including preterm birth and premature rupture of membranes [1, 5]. BV represents a polymicrobial shift in the vaginal microbiome, often from a Lactobacillus dominant community to one that is diverse, with several species of anaerobic bacteria [6]. For women presenting with symptoms, meeting at least 3 of 4 Amsel’s criteria [7] or having a Nugent score of 7–10 [8] are indications for treatment, primarily with topical or oral antibiotics [9–10]. Despite the clinical and public health significance of BV, there are no guidelines or recommendations for screening for BV among asymptomatic women. This stems from limited evidence regarding to what extent asymptomatic BV represents a pathologic condition.
As characterized by 16s rRNA gene sequencing, quantitative polymerase chain reaction (qPCR), and metaproteomics, McKinnon et al. categorize vaginal CST-IV (“depleted of lactobacilli with abundant anaerobes”) as “molecular BV”, based on its consistent association with increased risk of HIV and mucosal inflammation in an expansive review of the literature [11]. CSTs –I (L. crispatus dominated), -II (L. gasseri dominated), -III (L. iners dominated), and –V (L. jensenii dominated) are not considered molecular BV and have not been consistently associated with adverse outcomes (e.g., HIV or STI risk, adverse outcomes in pregnancy, mucosal inflammation) [11]. While women with CST-IV are much more likely to have BV, as many as 50% do not have BV [11–12]. Investigators call for studies characterizing which women among those with non-optimal vaginal CST-IV/molecular BV have clinical BV, because this may be beneficial in understanding and typifying pathologic states of non-optimal vaginal microbiome. The objective of this study was to identify demographic and behavioral factors, symptoms, clinical findings, and genital microbiome composition in women and their male sex partners that differentiate women with and without clinical BV among those with non-optimal CST-IV.
Methods
This study was approved by the Ethical Review Committee of Maseno University (Kisumu, Kenya), and the Institutional Review Board of the University of Illinois at Chicago (USA).
Study Design and Participants
Subjects in this analysis were enrolled in Afya Jozi, Afya Jamii (Kiswahili for “Healthy Pair, Healthy Community”), a prospective cohort study of heterosexual couples in Kisumu, Kenya. Recruitment and eligibility criteria have been published [13]. Eligible members of couples independently confirmed they had been in a sexual relationship for at least 6 months duration, and agreed to attend all study visits together. Eligible men were aged 18–35 years and their female partners aged 16 years and older. Couples in which one or both members had taken antibiotics within the past 30 days were not enrolled until 30 days had passed since completion of antibiotics. Research clinicians obtained written informed consent and conducted study procedures, including interviews, in the participant’s preferred language (English, Dho Luo, Kiswahili). Each member of the couple received 400 Kenyan shillings (~$4 USD) at each completed study visit. Couples were scheduled for follow-up at 1 month, 6 months, and 12 months after baseline. Couples were enrolled from April 1, 2014, through June 22, 2016 and 12-month follow-up was completed June 21, 2017.
Data and Specimen Collection
At each visit, participants underwent a standardized medical history and physical examination and personal interview to obtain socio-demographic information and information on sexual behavior; men and women underwent study procedures separately in private examination rooms. At baseline and each follow-up visit, penile meatal swabs and cervicovaginal lavage (CVL) were obtained for microbiome characterization. To obtain the meatal swab, the clinician applied light pressure and twirled a pre-moistened mini-tip flocked swab (Copan Diagnostics, Inc., Corona, California, USA) at the meatal depression for 3–5 rotations (the swab was not inserted into the urethra). CVL specimens were immediately aliquoted to 2.5mL cryovials, and along with penile swabs, stored at −80° C until shipment. Prior to CVL collection, the clinician collected three vaginal swabs for assessment of BV, whiff test, and wet mount microscopy. Slides for BV and wet mount microscopy were taken immediately to the on-site lab. If trichomonads were observed, this was recorded in the lab findings. After Gram staining, BV was evaluated according to Nugent’s criteria, in which a score of 7–10 is defined as BV [8]. Treatment of BV was provided at point of care based on Amsel’s criteria [7]; women were treated with either 2g oral tinidazole for 2 days, or with 400mg oral metronidazole for 7 days [9]. HIV (parallel rapid assays) and HSV-2 (HSV-2 IgG ELISA, Kalon Biological Limited, Aldershot, United Kingdom) were measured at baseline, 6 months, and 12 months, as previously detailed [13].
Vaginal and Penile Microbiome Characterization
DNA extraction was performed using EZ1 instrument, implementing the EZ1 DNA tissue protocol (Qiagen, Hilden, Germany). gDNA was used as template for PCR amplification of the V3-V4 variable region of bacterial 16S rRNA genes employing a two-stage PCR protocol with primers CS1_341F and CS2_806R [14]. After pooling, amplicons were sequenced on an Illumina MiSeq instrument, implementing V3 chemistry (600 cycles). DNA extraction, library preparation and sequencing were performed at the UIC Sequencing Core. Quality control and taxonomic annotation were conducted by University of Maryland Institute for Genomic Science following previously a published protocol [15]. Meatal microbiome data were filtered to retain taxa that contributed at least 0.01% of the total sequence reads. This resulted in selection of 57 taxa. Raw sequence data files are available in the Sequence Read Archive (National Center for Biotechnology Information; BioProject identifier PRJNA516684).
Definition of Analytic Sample
Among 252 women, there were 732 observations at which both the vaginal microbiome and BV were measured. For analysis we defined clinical BV according to Nugent criteria due to superior reproducibility and accuracy over Amsel’s criteria [16–17]. Each vaginal microbiome observation was assigned to a CST based on its distance from the centroid of CSTs defined in a reference database of over 13,000 vaginal communities characterized by 16s rRNA sequencing [18]. This analysis was carried out by Institute for Genomic Medicine at University of Maryland and methods are described in Brown et al [19]. Overall, non-optimal vaginal microbiome/molecular BV was present at 43.9% of the 732 observations: CST-IVA (n=46) and CST-IVB (n=275). Inferential analyses are restricted to these 321 CST-IV observations, as the goal of this analysis was to identify factors associated with clinical BV among women with non-optimal vaginal microbiome/molecular BV. Penile microbiome measure was available for 307 of the 321 observations with non-optimal vaginal microbiome.
Statistical Analysis
Descriptive Analyses.
We visualized differences in community composition by vaginal CST and Nugent BV status through heatmaps. For the penile microbiome, stacked bar plots summarize the relative abundance of the top 10 most common taxa (accounting for 68.2% of sequence reads), stratified by male circumcision status and female partner BV status. Among observations with non-optimal CST-IV, analysis of similarity (ANOSIM) was used for global test of significance for comparison of bacterial communities by Nugent BV status. These analyses were conducted in Primer 7.0 [20]. We compared clinical characteristics and other lab findings by Nugent BV status but did not enter these variables in statistical modeling, because these features are more likely to be a result of or representative of BV rather than explanatory to BV. Similarly, alpha diversity measures (i.e., measures of bacterial community variation) are presented descriptively (vegan package, implemented in R [21]).
Inferential Analyses.
To estimate the odds ratio (OR) of Nugent BV among women with non-optimal CST/molecular BV, we applied generalized estimating equation (GEE) analysis which incorporated the within-subject correlation among repeated measures, assuming binomial distributions with logit link. We compared demographic (age, educational attainment), behavioral (e.g., days since last sex, multiple sex partners, condom use), and health factors (e.g., HIV status, HSV-2 serostatus, contraceptive use) of women and male sex partners by BV status. All explanatory variables, except for age, educational attainment, and previous pregnancy, were assessed as time varying covariates. Baseline values of HIV, HSV-2, number of sex partners in the past 6 months, and contraceptive use were carried forward to the one-month observation, and were time-updated with the 6- and 12- month response values. Because male sex partner circumcision status and penile microbiome are associated with BV [22–23], we examined the association of Nugent BV with circumcision status and penile microbiome composition. To identify meatal taxa associated with Nugent BV, we first applied stability selection for feature selection (stabs package, implemented in R [24]). In this approach, we applied Lasso regression to 250 randomly generated subsets of the penile microbiome data and used a cutoff of p<0.20 in combination with detection of a specific taxa in at least 50% of subsets. Lasso regression is a machine learning algorithm used to identify taxa which relate to the target variable (BV). Stability selection strengthens our confidence in the selected taxa and reduces likelihood of false positive selections by identifying which taxa are important in a majority of sampled versions of the data – i.e., the selected taxa have stable importance. Prior to feature selection, data were center log ratio transformed following geometric Bayesian multiplicative prior imputation of zeros (zCompositions package, implemented in R [25]), to address sparsity and while maintaining the same total number of reads [26].
We first built a covariates only model (Model 1), and then a second model incorporating penile taxa (Model 2) to enable direct interpretation of how covariates may change in the presence of penile taxa. For each model, variables statistically significant at the p<0.20 level in univariate analyses were entered into multivariable analyses [27], with p<0.10 for retention of variables in the multivariable model, following stepwise backwards variable selection. Standard errors were obtained using an exchangeable correlation structure with robust estimation. Time was treated as a categorical variable. Sensitivity analyses excluding observations with intermediate Nugent score (4–6) are presented in supplemental files. GEE modeling was conducted using Stata/SE 15.2 for Windows (Stata Corp., College Station, TX).
Results
Among 732 observations (Table S1), we identified six CSTs: CST-I (L. crispatus dominant; n=79, 10.8% of observations), CST-II (L. gasseri dominant, n=7, 0.96% of observations), CST-III (L. iners dominant, n=297, 40.6% of observations), CST-IVA (BVAB dominant, n=46, 6.3% of observations), CST-IVB (G. vaginalis dominant, n=275, 37.6% of observations), CST-IVC (S. amnii dominant, n=19, 2.6% of observations), and CST-V (L. jensenii dominant, n=9, 1.2% of observations). Figure 1 is a heatmap representing relative abundance of the most abundant taxa by CST and BV status; CST-II, CST-IVC, and CST-V are excluded due to sparsity and difficulty visualizing. Overall, Nugent BV was detected in 184 observations, 89% of which occurred in CST-IVA and CST-IVB (Table S1).
Figure 1. Heatmap summarizing relative abundance for the 10 most abundant vaginal taxa by community state type (CST) and Nugent BV status.
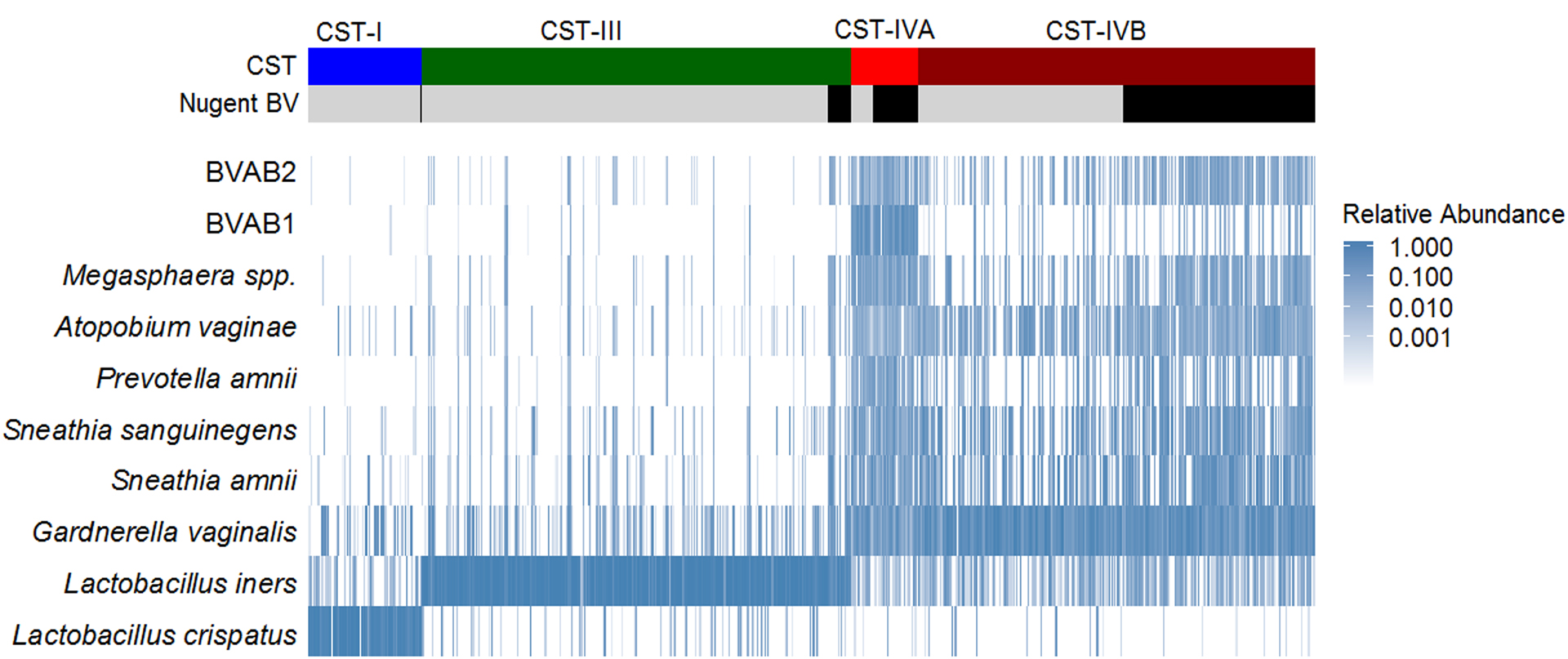
The heatmap represents the relative abundance of the ten taxa with highest mean relative abundance, shown for each observation, clustered by CST and Nugent BV status. Greater relative abundance is shaded with increasing intensity of blue color. Observations with Nugent BV (Nugent score 7–10) are indicated with black coloring.
Non-optimal vaginal CST composition varied by Nugent BV status
CST-IVA (relative abundance) was comprised of: Clostridiales BV-associated bacteria-1 (BVAB-1) (20.5%), G. vaginalis (12.8%), S. amnii (9.6%), and S. sanguinegens (7.9%), while CST-IVB was comprised of G. vaginalis (30.7%), S. amnii (11.7%), and S. sanguinegens (9.1%). Within CST-IVB, there were substantial differences in several taxa (ANOSIM, p=0.001). S. amnii, S. sanguinegens, Megasphaera spp., BVAB-2, and P. amnii were enriched for observations with BV compared to observations without BV; these 5 taxa contributed to 17% of the overall differences in community composition by women’s BV status within CST-IVB (Table 1 and Figure 2). Among numerous other differential taxa, enriched taxa included Prevotella spp. (i.e., Prevotella identified at the genus level, but species was not identified) and several other Prevotella identified at the species level, along with L. iners, Atopobium vaginae, and species of Dialister. Alpha diversity indices (Shannon, Simpson, Richness, Evenness) were greater for observations in CST-IVA than CST-IVB, and within CST-IVB were greater for observations with BV than without (Table 2). Within CST-IVA, the microbiome composition did not differ by Nugent BV status (ANOSIM, p=0.120; Table S2; Figure S1).
Table 1.
Results of similarity of percentages analysis: Taxa contributing to 70% dissimilarity between women with and without Bacterial vaginosis within CST-IVB
| Taxon id: Taxa | Ave. Abundance1 | Ave. Diss2 | Diss/ SD3 | Contrib%4 | Cum.%5 | |
|---|---|---|---|---|---|---|
| BV Negative | BV Positive | |||||
| Species: Sneathia amnii | 4.32 | 6.24 | 1.92 | 1.17 | 3.49 | 3.49 |
| Species: Sneathia sanguinegens | 4.28 | 5.90 | 1.87 | 1.19 | 3.40 | 6.89 |
| Genus: Megasphaera | 2.40 | 5.00 | 1.85 | 1.29 | 3.36 | 10.25 |
| Species: BVAB-2 | 2.28 | 5.07 | 1.82 | 1.30 | 3.31 | 13.55 |
| Species: Prevotella amnii | 2.15 | 3.98 | 1.71 | 1.11 | 3.10 | 16.65 |
| Species: Prevotella timonensis | 3.47 | 5.00 | 1.57 | 1.24 | 2.86 | 19.51 |
| Genus: Prevotella | 2.04 | 4.24 | 1.56 | 1.27 | 2.84 | 22.35 |
| Species: Dialister succinatiphilus | 2.39 | 4.64 | 1.50 | 1.31 | 2.73 | 25.07 |
| Species: Lactobacillus iners | 3.91 | 4.30 | 1.50 | 1.28 | 2.71 | 27.79 |
| Species: Prevotella bivia | 2.56 | 2.26 | 1.42 | 1.01 | 2.58 | 30.37 |
| Species: Atopobium vaginae | 4.96 | 6.06 | 1.37 | 0.98 | 2.49 | 32.86 |
| Genus: Anaerococcus | 3.26 | 3.12 | 1.35 | 1.19 | 2.45 | 35.32 |
| Species: Aerococcus christensenii | 3.14 | 3.06 | 1.28 | 1.24 | 2.33 | 37.65 |
| Family: Coriobacteriaceae | 1.65 | 3.55 | 1.26 | 1.33 | 2.28 | 39.93 |
| Genus: Firmicutes bacterium, unclassified | 2.37 | 2.13 | 1.24 | 1.08 | 2.25 | 42.18 |
| Species: Gemella haemolysans | 2.23 | 2.73 | 1.21 | 1.18 | 2.19 | 44.36 |
| Species: Ralstonia pickettii | 2.83 | 1.89 | 1.18 | 1.17 | 2.15 | 46.51 |
| Species: Peptoniphilus gorbachii | 2.56 | 3.46 | 1.17 | 1.24 | 2.13 | 48.64 |
| Family: Ruminococcaceae | 1.04 | 2.78 | 1.16 | 1.20 | 2.11 | 50.75 |
| Species: Sediminibacterium salmoneum | 2.64 | 1.35 | 1.15 | 1.10 | 2.08 | 52.83 |
| Species: Finegoldia magna | 3.88 | 4.89 | 1.14 | 1.12 | 2.07 | 54.90 |
| Species: Ureaplasma urealyticum | 2.17 | 0.87 | 1.06 | 0.93 | 1.93 | 56.83 |
| Species: Peptoniphilus lacrimalis | 1.44 | 2.21 | 1.00 | 1.10 | 1.82 | 58.65 |
| Species: Dialister micraerophilus | 2.92 | 3.24 | 0.99 | 1.14 | 1.80 | 60.45 |
| Species: Mycoplasma hominis | 1.70 | 1.72 | 0.98 | 0.99 | 1.79 | 62.23 |
| Species: Porphyromonas asaccharolytica | 1.49 | 1.81 | 0.96 | 0.98 | 1.74 | 63.98 |
| Species: Peptostreptococcus anaerobius | 1.61 | 1.58 | 0.95 | 0.96 | 1.73 | 65.71 |
| Family: Veillonellaceae | 0.56 | 1.86 | 0.91 | 0.75 | 1.65 | 67.36 |
| Species: BVAB-1 | 0.83 | 1.70 | 0.91 | 0.72 | 1.65 | 69.02 |
| Species: Prevotella disiens | 1.17 | 1.67 | 0.89 | 0.89 | 1.62 | 70.63 |
Average abundance as presented in table is natural log transformed sequence counts averaged across subjects.
Ave Diss = Average Bray Curtis dissimilarity
Diss/SD = Dissimilarity divided by Standard Deviation
Contrib % = Percent contribution to dissimilarity between BV negative and BV positive communities
Cum % = Cumulative percent contribution to dissimilarity between BV negative and BV positive communities
Figure 2. Non-metric multi-dimensional scaling plot showing the similarity of vaginal microbiome community of women with and without Bacterial vaginosis (Nugent score 7–10 vs. 0–6), within community state type (CST) IVB.
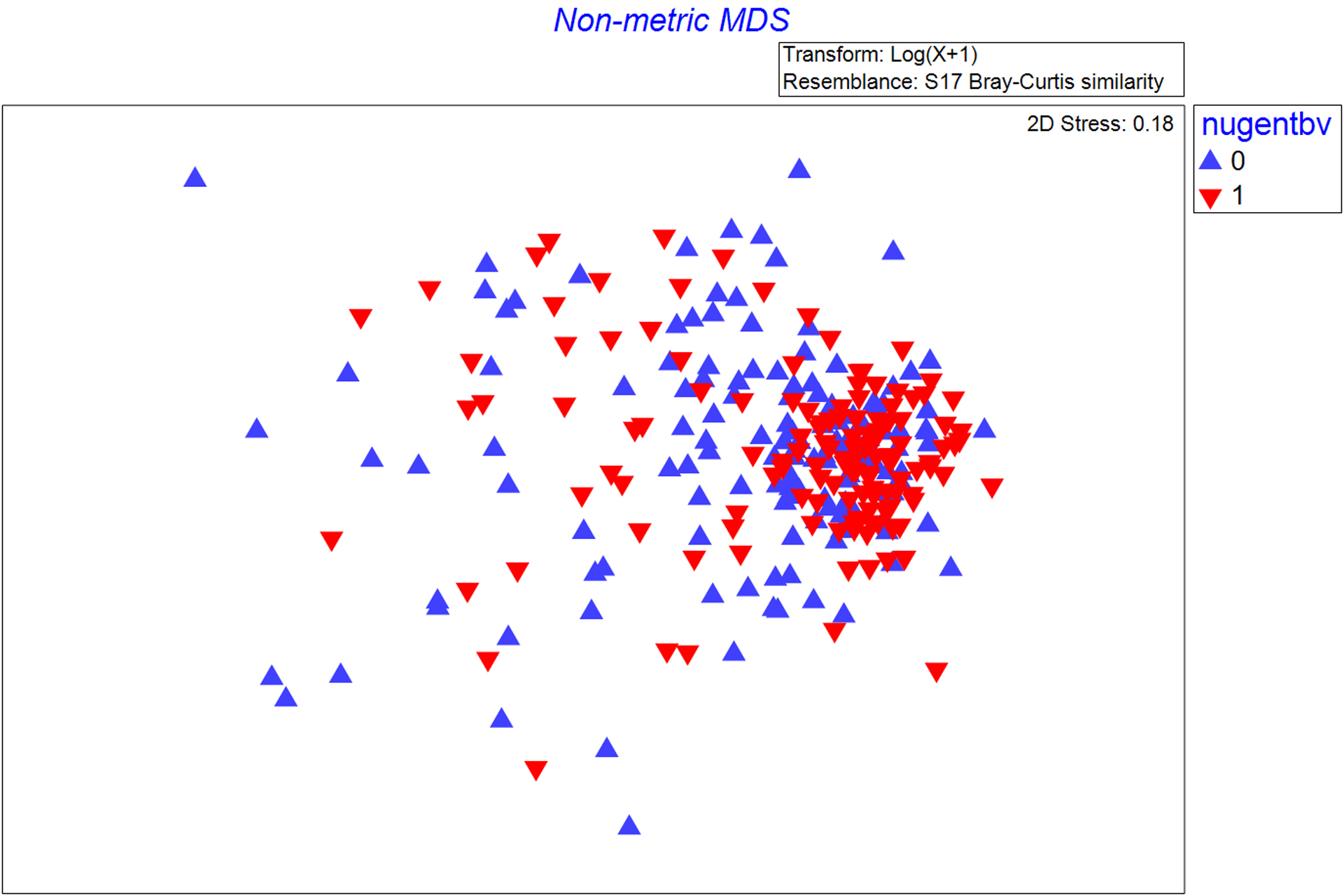
The non-metric multidimensional scaling plot (MDS) represents the pairwise Bray-Curtis dissimilarity between observations. Red triangles represent observations in which Nugent BV is detected (Nugent score 7–10) and blue triangles represent observations in which Nugent BV is absent (Nugent score 0–6).
Table 2.
Distribution of clinical and laboratory factors by Nugent Bacterial vaginosis (BV) status among observations in which the vaginal microbiome is CST-IVA and CST-IVB.
| CST-IVA, N=46 | CST-IVB, N=275 | |||
|---|---|---|---|---|
| No BV, N=15 n (%) |
BV, N=31 n (%) |
No BV, N=142 n (%) |
BV, N=133 n (%) |
|
| Reported vaginal discharge | 2 (13) | 9 (29) | 19 (3) | 24 (18) |
| Clinician detected vaginal discharge | 8 (53) | 19 (61) | 31 (22) | 75 (56) |
| Vaginal pH >4.5 | 8 (53) | 22 (71) | 59 (42) | 106 (80) |
| Clue cells detected on wet mount | 9 (60) | 31 (100) | 43 (30) | 129 (97) |
| Whiff test positive | 8 (53) | 23 (74) | 32 (23) | 102 (77) |
| BV by Amsel’s criteria (≥3 criteria met) | 8 (53) | 23 (74) | 23 (16) | 100 (76) |
| Documented antimicrobial treatment for BV | 9 (60) | 27 (87) | 53 (37) | 120 (90) |
| 7 – 10 | 31 (100) | 133 (100) | ||
| Evenness | 0.69 (0.57 – 0.73) | 0.70 (0.63 – 0.75) | 0.55 (0.44 – 0.63) | 0.63 (0.55 – 0.68) |
IQR = Interquartile range
Note: P-values are not estimated because the purpose of this analysis is descriptive rather than inferential, and because the sample size (N) represents observations rather than individuals, and thus there are repeated observations within individual.
CST-IVA and CST-IVB were relatively stable over time. Among women with any CST-IVA or CST-IVB observation at any time point, CST-III was the most likely alternative state, but the majority remained in CST-IVA or CST-IVB (Table S3, Figure S2). Among 97 women with CST-IVB at baseline, 44.3% remained free of BV throughout observation, 24.7% had one observation of BV, and 30.9% had two or more observations with BV. Among 14 women with CST-IVA at baseline, only 3 (21%) remained free of BV throughout follow-up, while 4 (28.6%) had one observation of BV and 7 (50%) had BV at 2 or more observations.
The penile microbiome differed by men’s circumcision status and female partner BV status
Ten penile taxa with the highest relative abundance accounted for 68% of all sequence reads (Table 3). The penile taxa with the highest relative abundance were Corynebacterium (12.6%), Anaerococcus (8.3%), Finegoldia (7.3%), S. sanguinegens (6.9%) and Streptococcus (6.8%), and differed substantially by men’s circumcision status (Figure 3). Stability selection resulted in selection of 7 penile taxa (fraction of times selected): Dialister (0.54), Aerococcus (0.56), Fastidiosipila (0.62), Fusobacterium (0.65), Brevibacterium (0.76), Ureaplasma (0.87), Megasphaera (0.97) (Table 3). The presence and relative abundance of the taxa identified by stability selection had minor absolute differences in relative abundance though some had two- to three-fold relative differences (Table 3).
Table 3.
Presence and mean relative abundance by circumcision and bacterial vaginosis status for the 10 most abundant meatal taxa and those identified by stability selection.
| Most abundant taxa | Circumcised | Uncircumcised | ||
|---|---|---|---|---|
| BV Negative (Nugent 0–6) N=92 % present1 (% mean RA)2 | BV Positive (Nugent 7–10) N=74 % present1
(% mean RA)2 |
BV Negative (Nugent 0–6) N=63 % present1
(% mean RA)2 |
BV Positive (Nugent 7–10) N=78 % present1 (% mean RA)2 | |
| Corynebacterium | 98.9 (17.9) | 100 (15.8) | 93.7 (8.74) | 91.0 (9.60) |
| Anaerococcus | 95.7 (7.51) | 96.0 (8.49) | 100 (11.7) | 97.4 (8.00) |
| Streptococcus | 83.7 (8.05) | 83.8 (12.4) | 74.6 (6.29) | 69.2 (7.92) |
| L. iners | 47.8 (10.2) | 41.9 (11.7) | 50.8 (11.1) | 50.0 (10.6) |
| Staphylococcus | 95.7 (7.87) | 98.7 (4.60) | 71.4 (2.14) | 76.9 (2.87) |
| Finegoldia | 91.3 (3.09) | 93.2 (4.96) | 100 (12.7) | 96.2 (11.8) |
| S. sanguinegens | 56.5 (14.3) | 59.5 (14.1) | 55.6 (10.4) | 60.3 (8.54) |
| Peptoniphilus | 84.5 (3.66) | 93.2 (3.95) | 100 (9.03) | 93.6 (9.62) |
| Ezakiella | 75.0 (2.50) | 71.6 (2.46) | 85.7 (8.91) | 85.9 (8.25) |
| Ralstonia | 54.4 (13.9) | 68.9 (5.29) | 73.0 (3.24) | 70.5 (3.22) |
| Taxa identified by stability selection | ||||
| Dialister | 50.0 (0.63) | 66.2 (0.92) | 84.1 (1.25) | 80.8 (1.58) |
| Aerococcus | 54.4 (0.61) | 50.0 (0.82) | 52.4 (0.33) | 44.9 (0.31) |
| Fastidiosipila | 47.0 (1.10) | 55.4 (0.55) | 47.6 (0.41) | 56.4 (0.79) |
| Fusobacterium | 33.7 (1.70) | 36.5 (3.79) | 27.0 (2.02) | 21.8 (1.23) |
| Brevibacterium | 62.0 (0.27) | 71.6 (0.58) | 34.9 (0.22) | 46.2 (0.46) |
| Ureaplasma | 66.3 (3.46) | 68.9 (1.03) | 61.9 (0.92) | 50.0 (1.27) |
| Megasphaera | 10.9 (0.86) | 31.1 (0.36) | 15.9 (0.57) | 30.8 (0.38) |
“% present” is the proportion of observations in which there is any amount of the specified taxa.
“% mean RA” is the percent mean relative abundance of the specified taxa in the observations in which it is present.
N=307; penile microbiome measure missing for 14 observations among the 321 observations with non-optimal vaginal microbiome.
Figure 3. Stacked bar charts summarizing mean relative abundance of 10 meatal taxa with highest relative abundance by circumcision status and female partner Bacterial vaginosis (BV) status.
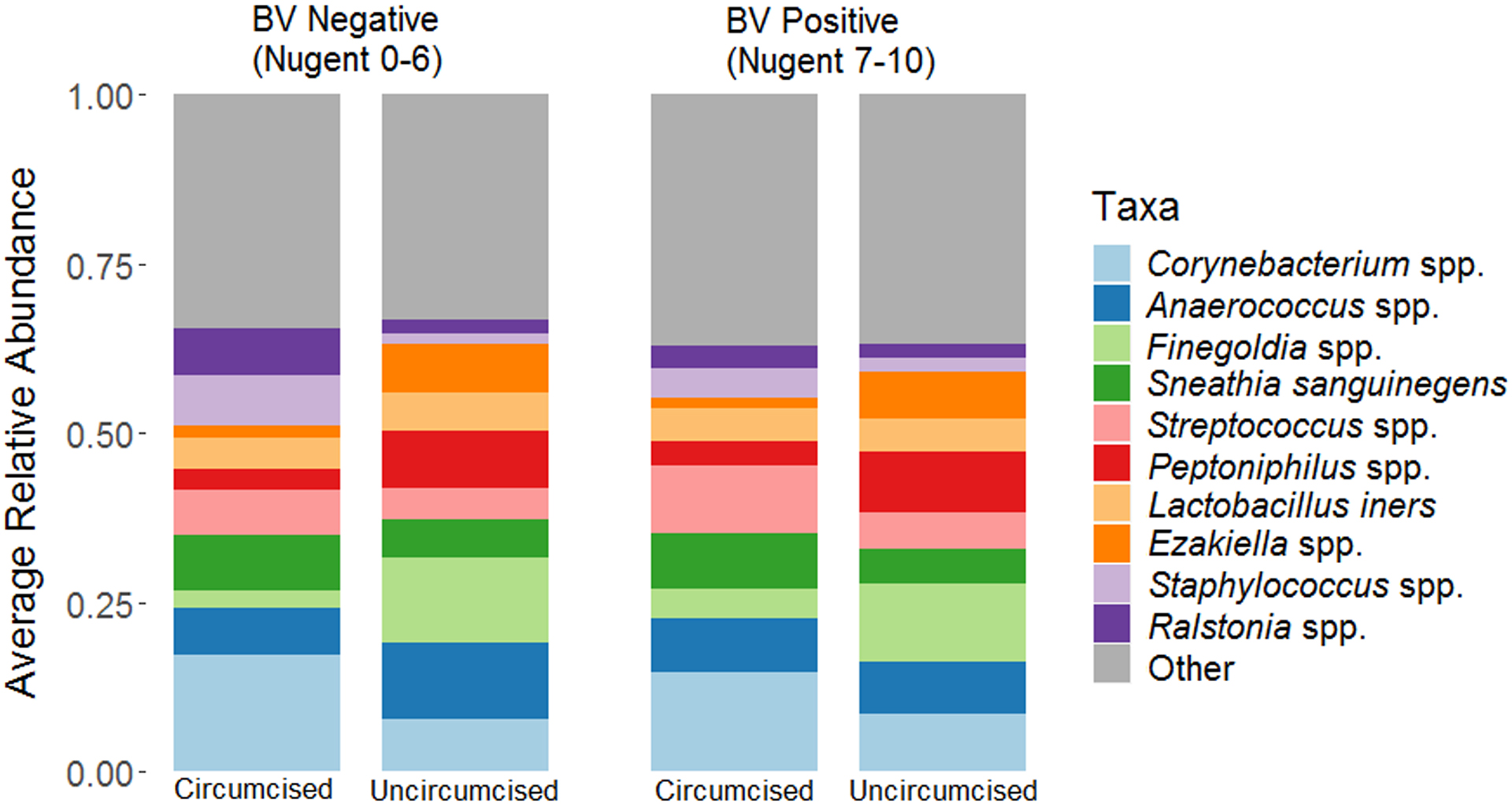
These stacked bar charts summarize the mean relative abundance (y-axis) of ten penile taxa with highest mean relative abundance, stratified by Nugent BV status and man’s circumcision status.
Within non-optimal CST, clinical and laboratory features differed by Nugent BV status
Overall, only 16.8% of women with CST-IV reported vaginal discharge, though 41.4% of women had vaginal discharge detected on examination (Table 2). A greater proportion of observations with Nugent BV had clinician-detected vaginal discharge, elevated vaginal pH (>4.5), whiff test positive samples, and clue cells detected on wet mount. Among observations in which BV was not diagnosed, a greater proportion of observations had Nugent score 0–3 for CST-IVB (63%) than CST-IVA (33%), though observations for CST-IVA without BV were infrequent. For observations in which BV was not diagnosed, compared to CST-IVA, observations classified as CST-IVB were less likely to have reported vaginal discharge, clinician detected vaginal discharge, elevated vaginal pH, detection of clue cells, and positive whiff test (and accordingly, BV by Amsel’s criteria). Alpha diversity measures were greater for observations in CST-IVA than CST-IVB, similar by BV status within CST-IVA, and modestly higher among women with BV within CST-IVB.
Within non-optimal CST, women with CST-IVA, HIV, and HSV-2 were more likely to have Nugent BV
At baseline, women were median age 22 years, 16% with HIV infection, and 66% were HSV-2 seropositive (Table 4). The median age of male sex partners was 27 years, 12% were HIV positive, 48% HSV-2 seropositive, and 55% were circumcised. In univariate analysis, women with Nugent BV were more likely to be in CST-IVA and more likely to be HIV positive, HSV-2 seropositive, and with T. vaginalis detected on wet mount (Table 4). The male sex partners of women with Nugent BV were less likely to be circumcised, and more likely to be HIV positive and to report using condoms at the last sexual intercourse. In multivariable adjusted analyses, the odds of BV were increased for women with CST-IVA (vs. CST-IVB) (adjusted odds ratio (aOR) = 1.90, 95% CI: 0.97 – 3.73, p=0.063), HSV-2 (aOR = 1.62, 95% CI: 0.92 – 2.85, p=0.095), or HIV (aOR = 2.48, 95% CI: 1.16 – 5.34, p=0.020) (Table 5, Model 1). In a sensitivity analysis excluding observations with intermediate Nugent score (4–6), the associations of women’s covariates (CST, HSV-2, and HIV status) remained of similar magnitude, though the protective association of circumcision status was strengthened and statistically significant (aOR=0.45, 95% CI: 0.24 – 0.84; Table S4, Model 1).
Table 4.
Distribution of Demographic, Behavioral, and Health Status Factors by Nugent’s BV Status at Baseline and Follow-Up Visits, and Results of Time-Adjusted Univariate Generalized Estimating Equation Analysis.
| Study Visit | Baseline | 1 Month | 6 Month | 12 Month | Time-adjusted Univariate Odds Ratio [95% CI] |
||||
|---|---|---|---|---|---|---|---|---|---|
| No BV, N=59 n (%) |
BV, N=47 n (%) |
No BV, N=38 n (%) |
BV, N=39 n (%) |
No BV, N=30 n (%) |
BV, N=42 n (%) |
No BV, N=30 n (%) |
BV, N=36 n (%) |
||
| IV-B | 54 (91) | 39 (83) | 34 (89) | 35 (90) | 27 (90) | 30 (71) | 27 (90) | 29 (81) | reference |
| Demographic and Behavioral Factors | |||||||||
| Women | |||||||||
| Median age in years (IQR) (fixed) | 22 (20–25) | 22 (20–25) | 21 (20–27) | 23 (21–26) | 21 (20–23) | 24 (21–26) | 23.5 (21–28) | 22.5 (21–26) | 1.02 (0.96 – 1.08) |
| Post-secondary | 7 (12) | 5 (11) | 7 (18) | 3 (8) | 3 (10)_ | 5 (12) | 6 (20) | 5 (14) | 0.65 (0.30 – 1.40) |
| Two or more sex partners past 6 months | 1 (1.8) | 2 (4.4) | 1 (3.1) | 2 (5.9) | 0 (0) | 1 (2.4) | 1 (3.7) | 2 (5.7) | 1.86 (0.46 – 7.49) |
| 7 or more | 17 (29) | 8 (17) | 16 (42) | 13 (33) | 8 (27) | 18 (43) | 13 (43) | 20 (56) | 1.12 (0.66 – 1.90) |
| How soon clean vagina after sex (< 1 hour vs. 1 hour or more) | 35 (59) | 34 (72) | 23 (68) | 24 (67) | 21 (70) | 28 (67) | 23 (77) | 28 (78) | 0.85 (0.51 – 1.42) |
| Men | |||||||||
| Median age in years (IQR) | 26 (24–30) | 27 (23–30) | 27 (24–31) | 27 (23–31) | 26 (24–30) | 27 (24–32) | 27 (25–30) | 27 (24–32) | 1.00 (0.94 – 1.06) |
| Post-secondary | 11 (19) | 5 (11) | 5 (13) | 5 (13) | 6 (20) | 5 (12) | 6 (20) | 6 (17) | 0.70 (0.32 – 1.52) |
| Two or more sex partners past 6 months | 18 (31) | 15 (33) | 13 (34) | 13 (34) | 4 (13) | 6 (16) | 5 (19) | 9 (32) | 1.13 (0.65 – 1.96) |
| Condom used at last sexual intercourse | 5 (8.5) | 9 (19) | 5 (13.5) | 7 (18) | 3 (10) | 8 (21) | 4 (14) | 6 (21) | 1.85 (0.98 – 3.49)^ |
| Clinical Factors | |||||||||
| Women | |||||||||
| HSV-2 seropositive | 35 (59) | 35 (74) | 20 (53) | 31 (79) | 19 (63) | 28 (67) | 23 (77) | 24 (67) | 1.63 (0.94 – 2.83)^ |
| HIV positive | 6 (10) | 11 (23) | 3 (8.1) | 7 (18) | 1 (3.3) | 11 (27) | 5 (17) | 7 (20) | 2.47 (1.21 – 5.03)* |
| T. vaginalis detected on wet mount | 4 (6.8) | 2 (4.3) | 1 (2.6) | 1 (2.6) | 0 (0) | 6 (14) | 1 (3.3) | 2 (5.6) | 2.41 (0.92 – 6.32)^ |
| Previous pregnancy (fixed) | 50 (85) | 40 (85) | 32 (84) | 33 (85) | 25 (83) | 39 (93) | 28 (93) | 31 (86) | 1.02 (0.53 – 1.95) |
| Pregnant (urine HCG positive) | 5 (8.5) | 3 (6.4) | 3 (8.8) | 1 (2.8) | 4 (14) | 1 (2.4) | 0 (0) | 4 (11) | 0.70 (0.15 – 3.38) |
| Men | |||||||||
| HSV-2 seropositive | 28 (48) | 22 (47) | 17 (46) | 19 (49) | 18 (60) | 21 (54) | 18 (62) | 18 (62) | 0.94 (0.57 – 1.55) |
| HIV positive | 5 (8.8) | 7 (15) | 3 (8.3) | 8 (21) | 2 (6.7) | 7 (18) | 3 (10) | 6 (21) | 2.18 (0.99 – 4.80)^ |
| Circumcised | 34 (58) | 24 (51) | 25 (68) | 19 (49) | 18 (60) | 15 (41) | 15 (52) | 15 (52) | 0.66 (0.39 – 1.10) |
p<0.05;
0.05<p-value<0.10
This category of contraception includes 39 observations with no contraception, 2 observations with IUD, 11 observations with condoms as main method of contraception, 1 observation of lactation amenorrhea, 1 observation of tubal ligation, and 1 observation emergency pills.
“Fixed” refers to variables that do not change after baseline.
Table 5.
Results of Crude and Multivariable Adjusted Generalized Estimating Equation Analysis: Factors associated with Nugent’s Bacterial vaginosis (BV) among women with community state types CST-IVA or CST-IVB.
| Time-Adjusted Univariate Odds Ratio (95% CI), p-value | Multivariable Adjusted Model 11 Covariates Only N=303 Adjusted Odds Ratio (95% CI), p-value | Multivariable Adjusted Model 21 Covariates and Meatal Taxa N=296 Adjusted Odds Ratio (95% CI), p-value | |
|---|---|---|---|
| CST-IVA vs. CST-IVB | 1.90 (1.07 – 3.35), p=0.027 | 1.90 (0.97 – 3.73), p=0.063 | 1.91 (0.91 – 4.00), p=0.087 |
| Woman is HSV-2 seropositive | 1.63 (0.94 – 2.83), p=0.083 | 1.62 (0.92 – 2.85), p=0.095 | 1.75 (0.97 – 3.17), p=0.065 |
| Woman is HIV positive | 2.47 (1.21 – 5.03), p=0.013 | 2.48 (1.16 – 5.34), p=0.020 | 2.30 (1.00 – 5.32), p=0.051 |
| T. vaginalis detected on wet mount | 2.41 (0.92 – 6.32), p=0.074 | ||
| Male partner is HIV positive | 2.18 (0.99 – 4.80), p=0.052 | ||
| Male partner is circumcised | 0.66 (0.39 – 1.10), p=0.109 | 0.73 (0.43 – 1.24), p=0.243 | 0.79 (0.45 – 1.41), p=0.427 |
| Male partner reported using condom at last sex | 1.85 (0.98 – 3.49), p=0.058 | ||
| Penile relative abundance2: Dialister spp. | 1.16 (1.01 – 1.34), p=0.034 | ||
| Penile relative abundance2: Megasphaera spp. | 1.22 (1.09 – 1.37), p=0.001 | ||
| Penile relative abundance2: Brevibacterium spp. | 1.13 (1.02 – 1.25), p=0.019 | ||
| 12 months | 1.41 (0.80 – 2.48), p=0.234 | 1.05 (0.55 – 1.98), p=0.890 | 1.17 (0.60 – 2.28), p=0.653 |
Model 1 and Model 2 are simultaneously adjusted for all variables presented under each.
Penile relative abundance data are center log ratio transformed prior to analysis
Penile bacteria were associated with increased odds of Nugent BV among women with non-optimal CST
In multivariable modeling including covariates from Model 1, increasing penile relative abundances of Dialister spp., Megasphaera spp. and Brevibacterium spp. remained statistically significantly associated with increased odds of BV in the female partner (Table 5, Model 2). Women with CST-IVA, HSV-2, and HIV had increased odds of BV with similar coefficients and confidence intervals as in Model 1. Controlling for penile taxa, male partner circumcision status was not associated with BV (aOR = 0.79; 95% CI: 0.45 – 1.41). In a sensitivity analysis excluding observations with intermediate Nugent score (4–6), the coefficients of female covariates and penile taxa remained similar, though the protective association of circumcision status was strengthened and remained statistically significant even when controlling for penile taxa (Table S4, Model 2).
Discussion
Main Findings
(1) Though 89% of Nugent BV cases occurred among women with non-optimal vaginal CST-IV, 49% of CST-IV observations did not have Nugent BV. (2) Among women with non-optimal CST-IV, the vaginal microbiome was not homogenous, with enrichment of S. amnii, S. sanguinegens, Megasphaera, BVAB-2, and P. amnii among women with BV. (3) Among women with non-optimal CST-IV, Nugent BV was more likely for observations with CST-IVA, HIV, or HSV-2, and male sex partner enrichment of specific penile bacteria.
Interpretation
Our findings are in keeping with other studies that demonstrate a substantial proportion of women have persistently low or moderate relative abundance of vaginal lactobacilli and absence of BV; this is generally more common among African American and African women [1]. It is possible that these bacterial communities are functioning in ways that replicate the protective mechanisms of lactobacilli (e.g., lactic acid production, preventing biofilm formation), or there may be host-mediated mechanisms that contribute to or prevent a pathological state in non-lactobacillus dominant communities. For example, the amount of glycogen or α-amylase production, or variation in Toll-like receptors could explain variability in Nugent BV among women with non-optimal vaginal microbiome [28–29]. If stable, low to moderate vaginal lactobacillus and diverse CST is a homeostatic state, then antimicrobial, live biotherapeutic, or other interventions to alter CST may have adverse effects [30]. On the other hand, studies have demonstrated that women with CST-IV have increased mucosal inflammation and epithelial barrier disruption, and subsequent risk of HIV acquisition, regardless of BV status, symptoms, or other STIs [31–33].
-
Women with CST-IVA (BVAB-1 dominant) vaginal community were more likely to have Nugent BV and symptoms, signs, or microscopy findings associated with BV. A review by Marrazzo summarizes mechanisms for increased risk of BV with several BVAB species: contribution to biofilm formation, potential antibiotic resistance, potential penile reservoir, ability to establish dominance in the vaginal community [34]. G. vaginalis is highly prevalent in women across many studies; Muzny and Schwebke indicate G. vaginalis is likely co-pathogenic, requiring other bacteria or other factors to initiate the conversion to BV [35]. These differences in pathogenesis may explain why women with the CST-IVA sub-type had greater likelihood of BV. Comprehensive modeling of temporal dynamics is outside the scope of the current analysis; however, for the majority of women in our sample who ever had CST-IVA or CST-IVB, CST-IV was persistent, despite nearly all women with BV having documented treatment. Longitudinal studies evaluating host factors and bacterial function among women with persistent, non-optimal CST may identify potential protective mechanisms against BV, and factors preceding shifts from non-optimal CST without BV, to non-optimal CST with BV.
Meta-analysis demonstrates increased risk of BV among women with HSV-2 [36]; Esber et al. suggest host response and HSV-2 viral expression lead to a vaginal environment that inhibits healthy vaginal flora. Our analysis shows the association between HSV-2 and BV holds true even within non-optimal CST. Many HSV-2 viral genes encode for products that are toxic to epithelial cells, and HSV-2 engages CD8+ cell activity [37], which may contribute to perturbation of the vaginal microbiome during reactivations. BV increases the risk of HIV acquisition [3] and transmission [38], and given the chronic, recurrent nature of BV, they are more likely to be co-detected. Currently, guidelines do not recommend screening and treatment of BV in asymptomatic women. For women with HIV or HSV-2, screening and treatment for BV in the absence of symptoms may have benefits related to reduced frequency of HSV-2 outbreaks, mucosal inflammation, or HSV-2 or HIV shedding. We are unaware of published studies evaluating the potential utility of BV screening and treatment among asymptomatic women with HSV-2 and HIV in relation to these outcomes.
As defined by Verstraehlen et al., BV is a “sexually enhanced” condition [39], with substantial epidemiologic and microbiologic evidence demonstrating increased risk with increasing sexual exposures [40]. Female sex partners of Ugandan men undergoing voluntary medical male circumcision (VMMC) had 40% lower prevalence of BV at one-year post-circumcision [22]. Subsequent study showed BV among female partners was associated with greater penile enrichment of BV associated anaerobic bacteria at the coronal sulcus [23] among uncircumcised compared to circumcised men [41]. We found that enrichment of penile Dialister spp., Megasphaera spp., and Brevibacterium spp. were positively associated with BV (Megasphaera spp. and Dialister spp. were also enriched in the vaginal microbiomes of women with BV, though Brevibacterium was not). The protective effect of male partner circumcision on BV likely extends to women with non-optimal vaginal communities, CST-IVA or CST-IVB. When we excluded from analysis women with intermediate Nugent scores (Table S4), the protective effect of having a circumcised male partner was strengthened; this makes sense as women with intermediate Nugent scores may represent misclassified observations of BV or observations that are more likely to progress to BV. Alternatively, these bacteria may not be residents of the penile microbiome and instead may be mechanically transferred back and forth; thus the penile microbiome serves as a reflective pool rather than a potential reservoir. However, BV-related bacteria are also found in men’s urine and semen. As reported by Nelson et al., the microbiota of men’s urine contained high abundances of bacteria that are also found in the vagina [42], and Mändar et al. found a high concordance of microbiota between semen and vaginal samples, supporting their hypothesis that “semen serves as a medium for the transmission of microorganisms between men and women” [43]. Given the high prevalence and high relative abundances of these BV-related bacteria recovered from sites throughout the male genitourinary system (semen, urethra, urine, glans, coronal sulcus) in our studies and those of others [23, 41–43], it is likely that microbiologic paradigms of the penis should consider the possibility that these bacteria reside there.
Strengths and Limitations
While there may have been misclassification, the distribution of BV by CST and sub-type (Table S1) indicates that specificity was very high, with only 3 of 184 BV cases in the entire cohort occurring in CST-I or CST-II. Therefore, misclassification likely represents under-estimation of BV, as suggested by our sensitivity analysis. Only 3 cases of Nugent BV were classified within 19 observations of CST-IVC and we therefore excluded CST-IVC from analyses due to sparsity and inability to draw inference on the small number of cases in this CST. We did not measure other STIs such as chlamydia or gonorrhea, and these can also influence the composition of the vaginal microbiome [44]. We did not measure bacterial load of any taxa, and this would have provided improved insight on relation of penile microbiota to BV and symptoms in female partners, because absolute abundance more accurately represents changes in taxa (as it does not suffer the statistical constraint of compositionality) [45]. A limitation inherent in amplicon sequencing is annotation of bacteria. While a standardized and replicable approach was applied, this algorithm has not been optimized for the penile microbiome. Several associations did not reach statistical significance at the p<0.05 level. While sample size affected precision of estimates, the magnitude of coefficients were stable across different modeling conditions, and results are in keeping with known findings of biological relevance. This study adds to the literature regarding epidemiologic and clinical understanding of molecular BV with several strengths: prospective and multiple sampling of cervicovaginal microbiota paired with male sex partner penile microbiota, measurement of HIV and HSV-2 status, standardized assessment of clinical symptoms and signs, and behavioral practices. An advantage of this study is that we recruited couples from the community, rather than amongst women seeking clinical care related to BV. This more likely represents the average associations with BV in a setting of high HIV and HSV-2 prevalence. However, loss to follow-up of may affect generalizability of results.
In this cohort of community-recruited Kenyan women with non-optimal CST-IV/molecular BV, the vaginal microbiome composition was not homogeneous and was enriched with S. amnii, S. sanguinegens, Megasphaera, BVAB-2, and P. amnii among observations with clinical BV. For women with clinical BV, the penile microbiome of sex partners was enriched with taxa that are also associated with BV in women. Evaluation of the potential utility of BV screening among asymptomatic women with HIV and HSV-2 is warranted, given the increased rates of Nugent BV for these women. Interventions to modify the penile microbiome, or to interrupt sexual exchange of BV associated bacteria within partnerships, may reduce BV in female partners.
Supplementary Material
Figure S2. Sankey diagram visualizing community state type (CST) pathways over time for women who had CST IV at any point in time. CSTs I, II, III, IV, and V are represented by the numbers 1, 2, 3, 4, 5, respectively. Undefined is indicated where microbiome is unobserved (i.e., missing data). In this directional flow chart, the width of “flows” is proportional to the number of women in the flow between CSTs over time. The height of the “nodes” (vertical bars representing each CST at each time point) is proportional to the number of women in the CST. Of note, the majority of observations with CST-IV were observed at baseline, though a substantial proportion of women with CST-III transitioned to CST-IV (e.g., light blue connecting to orange) and vice versa (orange connecting to light blue). Very few women started in CST-I (green) or CST-II (peach) and transitioned to CST-IV over time, and conversely, very few women transitioned from CST-IV to CST-I (orange to green) or CST-IV to CST-II (orange to peach).
Figure S1. Non-metric multi-dimensional scaling plot showing the similarity of vaginal microbiome community of women with and without Bacterial vaginosis (Nugent score 7–10 vs. 0–6), within community state type (CST) IVA The non-metric multidimensional scaling plot (MDS) represents the pairwise Bray-Curtis dissimilarity between observations. Red triangles represent observations in which Nugent BV is detected (Nugent score 7–10) and blue triangles represent observations in which Nugent BV is absent (Nugent score 0–6).
Funding Source and Role:
This study was supported by grant number R01-AI110369 from the National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of Microbiology. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosures of interest: No conflicts of interest have been declared.
Details of Ethics Approval
This study was approved by the Ethical Review Committee of Maseno University (Kisumu, Kenya; MSU/DRPC/MUERC/00054/13; January 13, 2014), and the Institutional Review Board of the University of Illinois at Chicago (USA; 2013–0511; February 12, 2014).
References
- 1.Peebles K, Velloza J, Balkus JE, et al. High global burden and costs of Bacterial vaginosis: a systematic review and meta-analysis. Sex Transm Dis 2019; 46:304–311. [DOI] [PubMed] [Google Scholar]
- 2.Torrone EA, Morrison CS, Chen PL, et al. Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: An individual participant data meta-analysis of 18 HIV prevention studies. PLoS Med 2018; 15:e1002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atashili J, Poole C, Ndumbe PM, et al. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS 2008;22:1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis FM, Bernstein KT, Aral SO. Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet Gynecol 2017;129:643–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brocklehurst P, Gordon A, Heatley E, Milan SJ. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev 2013;1:CD000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med 2005;353:1899–911. [DOI] [PubMed] [Google Scholar]
- 7.Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983;74:14–22. [DOI] [PubMed] [Google Scholar]
- 8.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991;29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines, 2015. MMWR 2015;64:69–72. [DOI] [PubMed] [Google Scholar]
- 10.Clinical Effectiveness Group, British Association of Sexual Health and HIV. UK National Guideline for the management of Bacterial Vaginosis 2012. Available from: https://www.bashhguidelines.org/media/1041/bv-2012.pdf. Last accessed September 12, 2019.
- 11.McKinnon LR, Achilles SL, Bradshaw CS, et al. The evolving facets of Bacterial vaginosis: Implications for HIV transmission. AIDS Res Hum Retroviruses 2019;35:219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravel J, Brotman RM. Translating the vaginal microbiome: gaps and challenges. Genome Med 2016;8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta SD, Nordgren RK, Agingu W, et al. Sexual quality of life and association with HIV and STIs among a cohort of heterosexual couples in Kenya. J Sex Med 2018;15:1446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naqib A, Poggi S, Wang W, et al. Making and sequencing heavily multiplexed, high-throughput 16S ribosomal RNA gene amplicon libraries using a flexible, two-stage PCR protocol In Gene Expression Analysis (pp. 149–169). Humana Press, New York, NY, 2018. [DOI] [PubMed] [Google Scholar]
- 15.Holm JB, Humphrys MS, Robinson CK, et al. Ultrahigh-throughput multiplexing and sequencing of >500-base-pair amplicon regions on the Illumina HiSeq 2500 platform. mSystems 2019;4:e00029–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sha BE, Chen HY, Wang QJ, et al. Utility of Amsel criteria, Nugent score, and quantitative PCR for Gardnerella vaginalis, Mycoplasma hominis, and Lactobacillus spp. for diagnosis of bacterial vaginosis in human immunodeficiency virus-infected women. J Clin Microbiol 2005;43:4607–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwebke JR, Hillier SL, Sobel JD, et al. Validity of the vaginal gram stain for the diagnosis of bacterial vaginosis. Obstet Gynecol 1996;88:573–76. [DOI] [PubMed] [Google Scholar]
- 18.France M, Ma B, Gajer P, et al. VALENCIA: A nearest centroid classification method for vaginal microbial communities based on composition (Preprint) doi: 10.21203/rs.2.24139/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown SE, Schwartz JA, Robinson CK, et al. The vaginal microbiota and behavioral factors associated with genital Candida albicans detection in reproductive-age women. Sex Transm Dis 2019;46:753–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke KR, Gorley RN. Getting started with PRIMER v7. PRIMER-E: Plymouth, Plymouth Marine Laboratory. 2015. [Google Scholar]
- 21.Oksanen J, Blanchet FG, Friendly M, et al. vegan: Community Ecology Package. 2019 R package version 2.5–4 https://CRAN.R-project.org/package=vegan. [Google Scholar]
- 22.Gray RH, Kigozi G, Serwadda D, et al. The effects of male circumcision on female partners’ genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. Am J Obstet Gynecol 2009;200:42.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu CM, Hungate BA, Tobian AAR, et al. Penile microbiota and female partner bacterial vaginosis in Rakai, Uganda. MBio 2015;6(3):e00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meinshausen N, Bühlmann P. Stability selection. J R Stat Soc B, Stat Methodology 2010; 72:417–73. [Google Scholar]
- 25.Palarea-Albaladejo J, Martin-Fernandez JA. zCompositions – R package for multivariate imputation of left-censored data under a compositional approach. Chemometr Intell Lab Syst 2015;143:85–96. [Google Scholar]
- 26.Aitchison J The statistical analysis of compositional data. J R Stat Soc B, Stat Methodology 1982;44(2):139–77. [Google Scholar]
- 27.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd edition John Wiley & Sons, New York: 2000: p. 95. [Google Scholar]
- 28.Spear GT, French AL, Gilbert D, et al. Human α-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus. J Infect Dis 2014;210:1019–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor BD, Darville T, Ferrell RE, et al. Cross-sectional analysis of Toll-like receptor variants and bacterial vaginosis in African American women with pelvic inflammatory disease. Sex Transm Infect 2014;90:563–66. [DOI] [PubMed] [Google Scholar]
- 30.Brotman RM, Shardell MD, Gajer P, et al. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause 2014;21:450–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S2. Sankey diagram visualizing community state type (CST) pathways over time for women who had CST IV at any point in time. CSTs I, II, III, IV, and V are represented by the numbers 1, 2, 3, 4, 5, respectively. Undefined is indicated where microbiome is unobserved (i.e., missing data). In this directional flow chart, the width of “flows” is proportional to the number of women in the flow between CSTs over time. The height of the “nodes” (vertical bars representing each CST at each time point) is proportional to the number of women in the CST. Of note, the majority of observations with CST-IV were observed at baseline, though a substantial proportion of women with CST-III transitioned to CST-IV (e.g., light blue connecting to orange) and vice versa (orange connecting to light blue). Very few women started in CST-I (green) or CST-II (peach) and transitioned to CST-IV over time, and conversely, very few women transitioned from CST-IV to CST-I (orange to green) or CST-IV to CST-II (orange to peach).
Figure S1. Non-metric multi-dimensional scaling plot showing the similarity of vaginal microbiome community of women with and without Bacterial vaginosis (Nugent score 7–10 vs. 0–6), within community state type (CST) IVA The non-metric multidimensional scaling plot (MDS) represents the pairwise Bray-Curtis dissimilarity between observations. Red triangles represent observations in which Nugent BV is detected (Nugent score 7–10) and blue triangles represent observations in which Nugent BV is absent (Nugent score 0–6).


