Abstract
Treatment for head and neck cancer precipitates a myriad of distressing symptoms, Patients may be isolated both physically and socially and may lack the self-efficacy to report problems and participate as partners in their care. The goal of this project was to design a telehealth intervention to address such isolation, develop patient self-efficacy, and improve symptom management during the treatment experience. Participatory action research and a review of the literature were used to develop electronically administered symptom management algorithms addressing all major symptoms experienced by patients undergoing treatment for head and neck cancers, Daily questions and related messages were then programmed into an easy-to-use telehealth messaging device, the Health Buddy®. Clinician and patient acceptance, feasibility, and technology issues were measured. Using participatory action research is an effective means for developing electronic algorithms acceptable to both clinicians and patients. The use of a simple tele-messaging device as an adjunct to symptom management is feasible, affordable, and acceptable to patients. This telehealth intervention provides support and education to patients undergoing treatment for head and neck cancers.
Keywords: head and neck cancer, telehealth, symptom management, palliative care
Introduction
Patients receiving treatment for head and neck cancers require complex multidisciplinary care.1 Healthcare teams caring for such patients often include physicians, nurses, dieticians, psychologists, social workers, dentists, and speech pathologists. Physical symptoms include pain that can be chronic, severe, and persistent,2 fatigue, impaired eating, xerostomia, cough, nasal discharge, malodorous secretions, bleeding, hoarseness, and insomnia.3 Those undergoing surgical interventions must often cope with altered facial appearance,4 disfigurement,5 and communication dysfunctions.6,7
Symptoms creating psychological distress include changes in body image, functional alterations in speech and eating, alcohol and tobacco dependence and related efforts to abstain, and myriad coping issues related to life-threatening illness and pain.8 Drug abuse, suicidal behavior, strong characterological disorders, noncompliance with treatment regimens, and poor adjustment in general can complicate treatment.5 Patients may experience anxiety related to problems with airway clearance and breathing disruption,6 and inadequate pain management may result in irritability, sleeplessness, and depression. Numerous studies have identified high levels of depression and psychological distress in this patient population.9–11
In addition to this substantial symptom burden, patients with head and neck cancer also have significant care access and communication barriers. These characteristics make a disease group high priority for telemedicine interventions and research.12 While telehealth modalities have been most often used by patients and clinicians who are geographically isolated,13–19 it is also important to include patients who are isolated because of their medical condition, physical or psychological debilitation, or social limitations.18 Social and/or physical isolation can seriously impact adherence to treatment regimens and, therefore, negatively impact recovery and survival.
Information technology has been frequently touted as a means for improving healthcare quality, including better management of the processes of care; protection of patient safety20; development of patient-centered, timely, efficient care; and reduction in the burdens of illness and disability.21 Distance medicine technology also enables greater continuity of care because it improves access and supports the coordination of activities.22 Social and physical isolation can be thwarted as the technology and related interventions are available to the patient within their own living environment. These potential benefits of telehealth address many of the care needs particular to the patient being treated for head and neck cancer. Telehealth technologies have been used with good results in areas of preventive care and management of certain chronic diseases including osteoarthritis, heart disease, and diabetes.21 Telephone-linked care using computer-controlled digital human speech has been used to monitor chronic diseases (including asthma, hypertension, congestive heart failure, and high-risk pregnancy), counsel patients on health behaviors, and provide information and support to caregivers23; and similar technology has been used to monitor chemotherapy side effects and symptoms.24 Computerized surveys have been used to report the symptoms and quality of life of patients with advanced lung cancer25 and cancer pain.26 Mobile phone technology has been used to assess and manage the symptoms of patients receiving chemotherapy.23,27 To date, studies have proven the feasibility and acceptability of such technology and have demonstrated the satisfaction of involved patients and clinicians, but none has analyzed the interventions in terms of cost effectiveness and cost avoidance related to healthcare utilization.
Led by our hypothesis that a telehealth intervention could educate and empower patients, facilitate communication with their healthcare providers, and improve symptom management during treatment, our research team developed symptom management algorithms to be communicated via a simple telehealth messaging device.
Materials and Methods
The study was divided into three phases. In phase I, the aim was to utilize current best practices and participatory action research methods to develop a telehealth algorithm for self-monitoring of symptoms experienced by head and neck cancer patients. Once developed, the algorithm was programmed into a simple telemessaging device to be used daily by patients during their active treatment for head and neck cancer. Phase I, completed in June 2006, and findings related to feasibility, use, and acceptance of the intervention will be presented in this paper.
In phases II and III, a randomized clinical trial comparing the telehealth intervention to a standard of care control condition is being conducted and evaluated. It was hypothesized that participants in the experimental condition would experience significantly less symptom distress, improved quality of life, increased self-efficacy, and improved satisfaction with symptom management when compared to the control condition.
Prior to the initiation of study activities, the study protocol, including the informed consent process, was reviewed and approved by both the University of Louisville Human Subjects Protection Program and the James Graham Brown Cancer Center's Clinical Science Review Committee.
Setting
The development of the algorithm involved input solicited from past patients and current clinicians of the Multidisciplinary Head and Neck Cancer Team of the James Graham Brown Cancer Center (JGBCC) located in Louisville, Kentucky and affiliated with the University of Louisville School of Medicine, This team consists of surgical, medical, and radiation oncologists as well as representatives from nursing, social work, psychology, speech therapy, and nutrition therapy. The team meets weekly and works as a coordinated unit to plan and administer both physical and psychosocial care to patients. Approximately 150 patients diagnosed with head and neck cancer are treated by this team annually.
Phase I: Development of the Algorithms
Development of the telehealth algorithms consisted of the following seven steps:
Survey of patients who had completed treatment for a diagnosis of head and neck cancer to solicit their opinions related to problematic symptoms and their treatment experience.
Survey of members of the multidisciplinary treatment team providing care to ascertain their opinions related to significant symptoms experienced by their patients.
Review of best practices related to the treatment experience of patients with head and neck cancer.
Draft writing of the algorithms related to the symptoms to be targeted.
Review and revision of the draft algorithm with key team members.
Determination of the content of each daily session and the desired repetition patterns.
Programming of the algorithms into the telehealth system.
Specific aspects of these steps are further described below.
Patient Feedback
Surveys were mailed to patients who had received treatment for head and neck cancers at the JGBCC during 2005. The survey packet included the Memorial Symptom Assessment Scale, a patient-rated, multidimensional instrument that evaluates the intensity, frequency, and distress associated with 32 physical and psychological symptoms.28 Patients were asked to complete this instrument based upon symptoms experienced during their cancer care and to complete a satisfaction survey.
Of the 79 surveys that were mailed, 39 (49%) were returned. Results are displayed in Table 1. The majority of the patients reported satisfaction with the care received for their head and neck cancer.
Table 1.
Symptom Ratings Head and Neck Patient- Treatment Completed (N=39)
| SYMPTOM | HAD SX | FREQUENCY | SEVERITY | AMOUNT BOTHERED | ||||
|---|---|---|---|---|---|---|---|---|
| YES | NO | FREQ | CONSTANT | SEVERE | VERY SEVERE | QUITE A BIT | VERY MUCH | |
| Difficulty Swallowing | 39 | 0 | 17 (44%) | 11 (28%) | 14 (36%) | 15 (39%) | 12 (33%) | 16 (41%) |
| Sore mouth | 35 | 4 | 11 (31%) | 8 (23%) | 7 (20%) | 8 (23%) | 10 (29%) | 10 (29%) |
| Pain | 39 | 0 | 24 (62%) | 9 (23%) | 15 (39%) | 10 (26%) | 13 (33%) | 15 (39%) |
| Cough | 31 | 8 | 5 (13%) | 0 | 1 (3%) | 2 (7%) | 6 (19%) | 2 (7%) |
| Problems with teeth | 17 | 22 | 5 (30%) | 1 (6%) | 1 (6%) | 4 (24%) | 1 (6%) | 4 (24%) |
| Dry mouth | 39 | 0 | 17 (44%) | 13 (33%) | 6 (15%) | 16 (41%) | 9 (23%) | 15 (39%) |
| Nausea | 30 | 9 | 7 (23%) | 1 (3%) | 3 (10%) | 4 (10%) | 1 (3%) | 7 (23%) |
| Difficulty chewing | 35 | 4 | 12 (34%) | 9 (26%) | 8 (23%) | 8 (23%) | 7 (20%) | 8 (23%) |
| Problem opening mouth | 32 | 7 | 6 (19%) | 5 (16%) | 6 (19%) | 3 (9%) | 5 (16%) | 3 (9%) |
| Difficulty sleeping | 31 | 8 | 14 (45%) | 4 (13%) | 9 (29%) | 4 (13%) | 8 (26%) | 6 (19%) |
| Skin soreness or sensitivity | 34 | 5 | 14 (41%) | 6 (18%) | 5 (15%) | 9 (27%) | 6 (18%) | 10 (29%) |
| Difficulty with speech | 34 | 5 | 7 (21%) | 14 (36%) | 9 (27%) | 9 (27%) | 9 (27%) | 10 (29%) |
| Felt ill | 38 | 1 | 9 (24%) | 12 (32%) | 9 (24%) | 7 (18%) | 6 (16%) | 9 (24%) |
| Lack of energy | 39 | 0 | 11 (28%) | 21 (54%) | 9 (23%) | 17 (44%) | 10 (26%) | 17 (44%) |
| Weight loss | 35 | 4 | 10 (29%) | 15 (43%) | 7 (20%) | 10 (29%) | 9 (26%) | 11 (31%) |
| Vomiting | 28 | 11 | 8 (29%) | 2 (7%) | 3 (11%) | 3 (11%) | 3 (11%) | 3 (11%) |
| Shortness of breath | 29 | 10 | 6 (21%) | 2 (7%) | 3 (10%) | 2 (7%) | 8 (28%) | 1 (3%) |
| Sticky ,thick saliva | 36 | 3 | 12 (33%) | 22 (61%) | 9 (23%) | 19 (53%) | 10 (28%) | 20 (56%) |
| Feeling sad | 33 | 6 | 10 (30%) | 6 (18%) | 2 (6%) | 8 (24%) | 10 (64%) | 9 (27%) |
| Change in smell | 30 | 9 | 9 (30%) | 9 (30%) | 5 (17%) | 6 (20%) | 5 (17%) | 5 (17%) |
| Worrying | 36 | 3 | 9 (25%) | 11 (31%) | 9 (25%) | 6 (17%) | 6 (17%) | 10 (28%) |
| Problems with sexual Interest/activity | 33 | 6 | 4 (12%) | 17 (52%) | 5 (15%) | 12 (36%) | 6 (18%) | 10 (30%) |
| Weak voice | 34 | 5 | 8 (24%) | 19 (56%) | 7 (21%) | 10 (29%) | 10 (29%) | 9 (27%) |
| Lack of appetite | 36 | 3 | 9 (25%) | 15 (42%) | 8 (22%) | 12 (33%) | 13 (36%) | 10 (28%) |
| Difficulty with social relationships | 29 | 10 | 9.(31%) | 5 (17%) | 5 (17%) | 4 (14%) | 5 (17%) | 6 (21%) |
| Feeling irritable | 35 | 4 | 10 (29%) | 9 (26%) | 7 (20%) | 6 (17%) | 8 (23%) | 7 (20%) |
| Change in way food tastes | 37 | 2 | 6 (16%) | 26 (70%) | 11 (30%) | 15 (41%) | 11 (30%) | 15 (41%) |
| Constipation | 28 | 11 | 11 (40%) | 5 (18%) | 6 (21%) | 5 (18%) | 4 (14%) | 5 (18%) |
| Difficulty stop smoking | 20 | 19 | 3 (15%) | 8 (21%) | 1 (5%) | 9 (45%) | 4 (20%) | 8 (40%) |
| Concern over appearance | 30 | 9 | 8 (27%) | 6 (17%) | 1 (3%) | 5 (17%) | 4 (13%) | 4 (13%) |
| Difficulty breathing | 24 | 15 | 7 (29%) | 1 (4%) | 6 (26%) | 0 | 7 (29%) | 2 (8%) |
| Hoarseness | 34 | 5 | 12 (35%) | 10 (29%) | 8 (24%) | 9 (27%) | 8 (24%) | 8 (24%) |
| Choking | 32 | 7 | 14 (44%) | 2 (6%) | 10 (31%) | 2 (6%) | 6 (19%) | 5 (16%) |
| Loss of appetite | 35 | 4 | 13 (37%) | 11 (31%) | 9 (26%) | 12 (34%) | 9 (26%) | 11 (31%) |
| Financial problems | 27 | 12 | 12 (44%) | 6 (22%) | 2 (7%) | 5 (19%) | 2 (7%) | 6 (22%) |
| Problems getting supplies/meds | 21 | 18 | 1 (5%) | 1 (5%) | 0 | 1 (5%) | 0 | 1 (5%) |
| Trouble getting to appointments | 11 | 28 | 2 (18%) | 0 | 2 (18%) | 0 | 1 (9%) | 0 |
| Employment problems | 14 | 25 | 2 (14%) | 3 (21%) | 2 (14%) | 4 (29%) | 3 (21%) | 3 (21%) |
| Caregiver problems | 10 | 29 | 0 | 0 | 0 | 0 | 0 | 0 |
| Difficulty taking care of myself | 26 | 13 | 6 (23%) | 2 (8%) | 3 (12%) | 5 (19%) | 2 (8%) | 6 (23%) |
| Problems with tracheostomy care | 12 | 27 | 1 (8%) | 2 (17%) | 3 (25%) | 1 (8%) | 0 | 4 (33%) |
| Runny nose | 28 | 11 | 8 (29%) | 4 (14%) | 6 (21%) | 3 (8%) | 7 (25%) | 1 (4%) |
| Alcohol related problems | 10 | 29 | 0 | 0 | 0 | 0 | 0 | 0 |
Clinician Feedback
Members of the Multidisciplinary Head and Neck Cancer Team at the JGBCC were asked to name and rate the top three symptoms experienced by patients according to frequency, distress, and management difficulty. Results of the feedback from the 14 clinicians who responded are depicted in Table 2.
Table 2.
Telehealth Symptom Management in Head and Neck Cancer: Results of Staff Survey (N=14)
| SYMPTOM | NUMBER OF TIMES NAMED AS ONE OF 3 MOST FREQUENT SYMPTOMS |
NUMBER OF TIMES LISTED AS ONE OF 3 MOST DISTRESSFUL SYMPTOMS |
NUMBER OF TIMES LISTED AS ONE OF 3 SYMPTOMS MOST DIFFICULT TO MANAGE |
|---|---|---|---|
| Dysphagia/sore throat | 9 | 9 | 7 |
| Loss of appetite /taste | 7 | 1 | 4 |
| Pain | 6 | 10 | 5 |
| Dry mouth | 6 | 7 | 5 |
| Mucositis | 4 | 2 | 3 |
| Skin irritation | 3 | 1 | 0 |
| Dehydration | 2 | 0 | 0 |
| Nausea | 1 | 2 | 1 |
| Difficulty breathing | 1 | 0 | 0 |
| Distress | 1 | 0 | 1 |
| Thick copious secretions |
1 | 0 | 2 |
| Trismus | 1 | 1 | 1 |
| Weakness | 1 | 2 | 1 |
| G-tube/feeding complications |
1 | 1 | 2 |
| Dysphonia | 0 | 1 | 1 |
| Weight loss | 0 | 1 | 2 |
| Difficulty chewing | 1 | 1 | 2 |
| Oral condition/Care | 0 | 1 | 2 |
| Top Three Nonphysical Problems | |||
| PROBLEM | TIMES MENTIONED IN TOP 3 | ||
| Depression/emotional problems | 10 | ||
| Smoking | 10 | ||
| Resource needs | 9 | ||
| Alcohol | 3 | ||
| Poor social support | 2 | ||
| Anxiety/fear of disease | 2 | ||
| Nutritional needs | 3 | ||
| Home health needs | 1 | ||
Literature Review
A thorough literature search of symptom management in head and neck cancer was conducted. Relevant papers, oncology nursing textbooks, and patient education materials were reviewed to identify important symptoms and related best practice interventions to be included in the algorithm.
The literature review preparatory to this research revealed one previous use of a telemedicine support system for head and neck cancer patients in the Netherlands, Between 1999 and 2002, investigators at the Erasmus Medical Center in Rotterdam, The Netherlands, conducted a controlled clinical trial evaluating the impact of a telemedicine application on the quality of life of patients undergoing surgery for head and neck cancer.29–31 Patients in the experimental group were able to access an electronic health information support system for 6 weeks after hospital discharge. These patients used laptop computers to communicate, gamer information, interact with other patients of the same diagnosis, and be monitored via electronic questionnaires. This research group willingly translated and shared the protocols reflected in their electronic questionnaires with our research team, giving us a starting point for constructing our own algorithms. Symptoms addressed in the Amsterdam protocol are listed in Table 332 Two symptoms–runny nose and deafness–identified by the Dutch research team were omitted from the final telehealth algorithm as they were not identified as problematic by clinicians or patients treated at the JGBCC.
Table 3.
Problems Addressed in Amsterdam Protocol
| Fatigue | Swelling in the neck |
| Food moves down poorly/gets stuck | Runny nose |
| Food comes out the nose | Skin feels numb |
| Pain when swallowing | Deafness |
| Shortness of breath without coughing | Dry mouth |
| Cannula no longer fits | Need for more information |
| Viscous mucus in mouth or throat | Tension anxiety, agitation |
| Coughing | Despair about the future |
| Pain in the mouth when wearing dentures | Dejection, worrying |
| Unable to speak clearly | Having difficulty falling asleep |
| Cannot make self understood | Feeling others don't understand |
| Loss of taste | Difficulty accepting self |
| Pain | Problems with appearance |
| Problems related to care providers | Reluctance to go out |
Incorporation of Feedback into Algorithms
The overall intervention allows for 120 daily messaging sessions spread over a 4-month period. This makes it feasible to include all symptoms identified as significant by past patients and the clinical team on the previously described surveys and the literature review with fairly frequent repetition of algorithms addressing significant symptoms. Priority symptom algorithms such as pain are asked with increased frequency (Le., twice weekly), whereas problems related to a specific treatment (i.e., surgery recovery, tracheotomy care) are asked only of participants receiving such an intervention. Such message tailoring helps to individualize the intervention and target specific issues in segments of the population.
Study Population
Intended users of the telehealth application are patients newly diagnosed with head and neck cancers including cancers of the oral cavity, salivary gland, paranasal sinuses, nasal cavity, pharynx, and larynx.
Patients receive the intervention at the onset of their treatment. Surgery patients begin the intervention upon their return home from surgery. Those receiving chemotherapy and/or radiation begin using the telehealth device on the first day of their treatment Patients continue to use the device for approximately 2 weeks past the end of their treatment as symptoms persist for several weeks after the completion of such therapy.
Patients in both the treatment and control groups continue to receive routine care from the multidisciplinary team. Messaging via the telehealth system encourages the patient to discuss unresolved symptoms and serious issues with the healthcare providers during these visits or to call them if they do not have an appointment scheduled. The telehealth intervention as implemented in this study is an adjunct to routine care and is intended to encourage rather than replace the interface between patients and their providers.
For this specific intervention, the telehealth monitoring device requires that a land phone line be available to the participant daily. For this initial trial, the algorithm was written in English only. Therefore, participants must be capable of reading, comprehending, and responding in English with at least an eighth-grade reading level. Other inclusion criteria for the purpose of this study include adequate cognitive functioning and the capacity to consent to participation.
Participant Involvement
Participants were expected to respond to the telehealth device daily unless hospitalized for treatment. Daily response time was approximately 5–10 minutes. Because many of the patients treated at the participating cancer center were elderly and/or of lower socioeconomic status, using a mechanism not requiring a personal computer and Internet access and/or computer literacy was an important consideration. The use of simple technology design was an intentional effort to avoid possible lack of acceptance by the intended population. In the Amsterdam study, 9 of the 20 patients refusing participation in the intervention group cited computer-related issues as their reason for nonparticipation.31
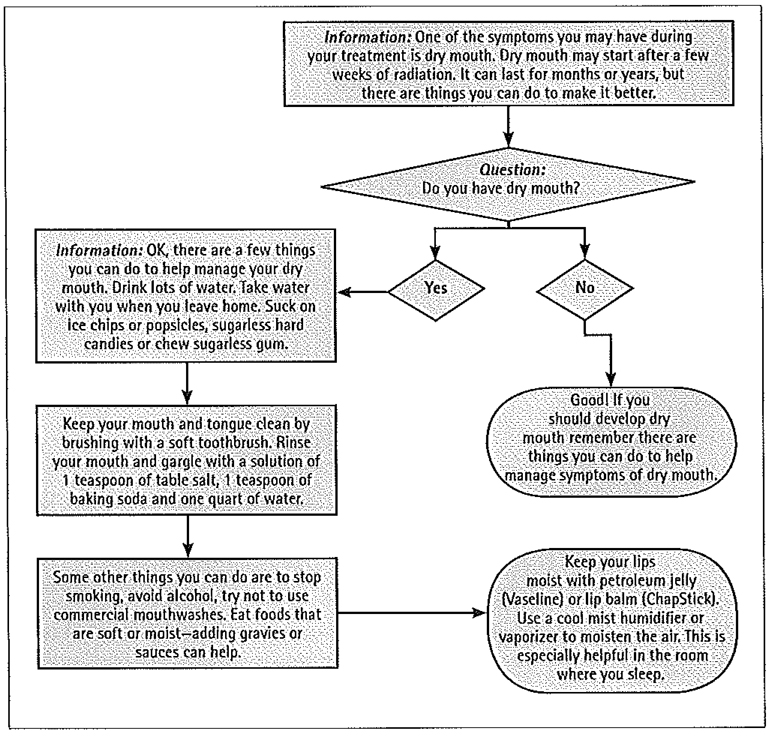
Many telehealth interventions expect healthcare professionals to closely monitor the transmitted information and intervene when issues are identified. In this intervention, we focused on encouraging the patient to take action on identified issues. Messages sent to the patient encouraged self-management behaviors as well as directions to contact healthcare providers when indicated. Programming of the algorithm provides feedback based upon the participant’s answer to the previous question. For instance, a patient responding that he/she did not have mouth sores receives a different message back than a patient indicating that mouth sores were present. Therefore, patients receive needed information on a just-in-time basis. Figure 1 depicts a sample algorithm for dry mouth. A registered nurse reviews responses daily, and, if a patient has an unresolved or escalating issue, the nurse calls and encourages the recommended action by the patient and/or contacts the clinical care team.
Fig. l.
Sample symptom management algorithm: dry mouth.
In order to provide another level of protocol specificity, the algorithm can be programmed to address certain patient conditions including whether the patient is a current smoker (in which case, smoking cessation messages would be programmed into their daily protocols), whether the patient is recovering from surgery (as opposed to receiving chemotherapy and/or radiation alone), and whether the patient has a tracheostomy. It is routine practice at this particular clinic to insert a percutaneous gastrostomy feeding tube and a vascular access port prior to treatment; therefore, questions and information related to care of these devices are included for every patient. Each daily session ends on a positive note with an inspiring statement or famous quotation.
System Description
Health Buddy®, a product of the Health Hero Network (Palo Alto, CA), was the appliance chosen to communicate the intervention algorithms. The device is approximately 9 × 6 inches in size and plugs into a telephone line and an electrical outlet. A light on the appliance blinks when the questions and information for the daily session are available. When the participant presses one of the buttons, the screen of the Health Buddy® lights up and is ready to deliver the questions and related information for the day’s session. The screen size allows for large, easy-to-read print Responses are registered by pressing one of four buttons below the screen. When the session is complete, the screen darkens. The appliance silently and automatically dials a toll-free number to send the participant entered information to Health Buddy® System’s Health Insurance Portability and Accountability Act (HIPAA) compliant secure Internet site where it becomes immediately available to the provider for review on the Health Buddy® Desktop. The appliance uses very little power and never disrupts an incoming or outgoing call. The telehealth messaging device is shown in Figure 2.
Fig. 2.
The Health Buddy® appliance.
The Health Hero Network estimates the cost to a provider for a patient to use an existing program on the system for 1 month to be $45.00 (Wojtek D, Health Hero Network, personal communication). If the provider develops their own algorithms to be programmed into the Health Buddy®, the cost is more, depending on the time and expertise provided by the Network staff.
Security Safeguards
Health Hero Network provides data security and privacy via a security architecture that involves four areas of protection: application layer security, network level security, server level security, and physical site security. Systems reside in a SAS70 portion of the data center under tight physical and administrative controls. This data center is protected by intrusion alarms, biometric devices, surveillance cameras, fire detection and suppression systems, and around-the-clock security personnel.33
The Health Hero Network platform ensures authenticity of users and the integrity and confidentiality of patient records during transmission by employing a combination of private phone lines and an encrypted hypertext transmission protocol (HTTP) using secure sockets layer technology for relaying information over the Internet or other Wide Area Network. Patient information is transferred from the telecommunication servers to the database servers over secure Virtual Private Network Internet tunnels using the HTTPS protocol for security. Network perimeter access is controlled by inspection firewalls. Accessible ports are tightly controlled limiting external access to HTTP/HTTPS traffic. Intrusion detection systems are employed to detect and mitigate attacks on the network.33 The Network’s server infrastructure uses highly secure Solaris and Linux operating systems.33 Client data are stored and logged in a central, secure database that is backed up nightly.
Results
To date, 75 patients have consented to study participation: 42 of these have participated in the intervention group. Of these participants, 98% reported they had no problems setting up the appliance and 86% reported it took 10 minutes or less to set it tip in their home. Although approximately one fourth have low reading and educational levels (high school or below) and many have been elderly (the mean age of the total study population thus far is 60; 15% [14] have been over 70 years of age and 3 were over 80 years), only 8% felt the questions were difficult to answer, 85% stated the appliance was very easy to use, while the remaining 15% stated it was easy to use. Sixty-five percent reported being more satisfied with the communication with their doctor or nurse as a result of the intervention.
A content satisfaction questionnaire asking the participant to evaluate the impact of the intervention on knowledge and management of their disease is administered every 90 days via the appliance. Participants in this study responded as follows:
84% agreed or strongly agreed with the statement “my experience with questions that repeat on the Health Buddy® reinforce my knowledge, and help me understand more about my condition.”
84% stated that their understanding of their medical condition was much better or somewhat better since using the Health Buddy®.
96% stated they were able to manage their medical condition much better or somewhat better since they started responding to the Health Buddy®.
92% stated they were willing to recommend the Health Buddy® device to others.
The mean number of days patients had the Health Buddy® available in their home was 99, and the average number of days they responded during that period was 83, placing the response rate at 84%. This level of response is indicative of excellent compliance since patients may have had days they were away from home due to hospitalization for chemotherapy or days when their symptoms resulted in the need for total rest or other limitations in activity or energy.
Qualitative feedback from exit interviews with those completing the intervention has been favorable. Out of those patients who have completed the intervention, only one denied that having the Health Buddy® available during treatment was helpful and that person felt it to have a neutral rather than adverse effect.
When asked how they were helped by having the Health Buddy®, those completing the intervention offered the following statements:
It told me what to expect of my illness and the concerns of my healthcare providers.
It gave me good directions so I could take care of my symptoms and didn’t have to ask about everything at the Cancer Center.
It taught me some things about symptom management I wasn’t told elsewhere; I didn’t have to search for answers.
It somewhat helped my depression through acknowledging it as a normal reaction.
It had valuable information concerning symptoms and reactions to radiation that kept me aware of what I needed to do in order to make the period easier.
It reminded me to take my medications and exercise.
It helped my thinking–gave me confidence to report symptoms–told me what to report.
Several patients living alone have utilized the Health Buddy® and reported that the intervention was extremely supportive to them. Statements from these patients include the following: “It was very reassuring,” “I liked the idea that someone was monitoring me,” and “It was like having someone to talk to.” One patient summarized his feelings saying “the buddy was very helpful and informative. I found myself looking forward to the next session. I especially liked the daily quotes at the end of each session. It became part of my daily routine and I miss it already!”
During exit interviews, participants are providing feedback that will assist in identifying over-repetition related to specific symptoms, information that was not helpful, issues not addressed and information, or questions not presented well. This input will enable the researchers to refine the protocols and overall sequencing of questions and Information. The fact that the telehealth system will be able to provide reports on the timing, frequency, and intensity of specific symptoms and related resolution will also be a great benefit in refining the intervention program.
An example of a patient-identified improvement in the algorithm relates to the symptom of depression and how to ask about related issues without unduly upsetting the participant. In the current algorithm, participants are asked if they are considering hurting themselves. While it is known that this patient group has a propensity toward depression and suicide attempts are not uncommon, several participants have noted that this question may be suggestive of such a possibility and that it upset them to even consider such an option. In revising the algorithm, this is an area (suicide assessment) that will need to be re-evaluated.
Conclusions
Thus far, this telehealth intervention has proven to be a feasible approach readily accepted and used effectively by patients, physicians, and members of the multidisciplinary cancer team. Both clinicians and patients have found the algorithms to be appropriate and inclusive. Patients completing the intervention report that most symptoms are addressed adequately without needless repetition.
This simple telehealth messaging device appears to provide a feasible interface for both the patient and the provider by offering daily support and information on an as-needed basis. It is a helpful adjunct to traditional care when the treatment regimen is complicated and laden with distressing symptoms and when patients may be overwhelmed with the amount of information provided at the onset of treatment.
Initial reactions by patients receiving the telehealth intervention have been favorable, and it appears to be a beneficial adjunct to routine care. Refinement of the algorithms related to appropriate timing and repetition of questions/information is indicated. Also, the wording and approach used in addressing sensitive issues such as depression and suicide potential need modification based upon participant feedback.
With changing technology such as the prevalence of cell phones (and the resulting absence of land phone lines in many homes) and the availability of personal computer access, the need for a separate messaging device may dissipate, but for the elderly and those of lower socioeconomic status, this appliance provides a feasible alternative. The algorithms could be programmed into a Web site to be accessed by those with personal computers. One disadvantage of this approach is that the participant would not see the daily reminder of a flashing light and the presence of an obvious reminder to participate daily.
Analysis of patient measures at the end of the randomized clinical trial now under way will provide further evaluation of the intervention’s impact on symptoms, self-efficacy, and treatment satisfaction. Further evaluation of the impact of such an intervention related to cost savings via avoidance of escalating symptoms, emergency room visits, and inpatient hospitalizations would also be beneficial.
Acknowledgments
This project was partially funded by the National Cancer Institute, National Institutes of Health.
Footnotes
Disclosure Statement
No competing financial interests exist for any of the authors.
REFERENCES
- 1.Birchall M, Richardson A, Lee L. Eliciting views of patients with head and neck cancer and carers on professionally derived standards for care. Br Med J. 2002;324:516–519. doi: 10.1136/bmj.324.7336.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chua K, Reddy S, Lee M, Patt R. Pain and loss of function in head and neck cancer survivors. J Pain Symptom Manage. 1999;18:193–202. doi: 10.1016/s0885-3924(99)00070-6. [DOI] [PubMed] [Google Scholar]
- 3.Dropkin MJ. Literature review on quality of life following head and neck cancer: 1996–1997. Head Neck Nurs. 1998;16:22–23. [PubMed] [Google Scholar]
- 4.De Leeuw J, De Graeff A, Ros W, Horduk G, Blijham G, Winnubst J. Negative and positive influences of social support on depression in patients with head and neck cancer: A prospective study. Psycho-Oncology. 2000;9:20–28. doi: 10.1002/(sici)1099-1611(200001/02)9:1<20::aid-pon425>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 5.Petrucci RJ, Harwick RD. Role of the psychologist on a radical head and neck surgical service team. Professional Psychol Res Pract. 1984;15:538–543. [Google Scholar]
- 6.Moadel Ab, Ostroff JS, Schantz SP. Head and neck cancer. In: Holland JC, editor. Psycho-oncology. New York: Oxford University Press; 1998. pp. 414–323. [Google Scholar]
- 7.Katz M, Irish J, Devins G, Rodin G, Gullane P. Psychosocial adjustment in head and neck cancer: The impact of disfigurement, gender, and social support. Head Neck. 2003;25:103–112. doi: 10.1002/hed.10174. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro P, Kornfeld D. Psychiatric aspects of head and neck cancer surgery. Psychiotr Clin North Am. 1987;10:87–100. [PubMed] [Google Scholar]
- 9.D’Antonio L, Long S, Zimmerman G, Peterman A, Petti G, Chonkich G. Relationship between quality of life and depression in patients with head and neck cancer. Laryngoscope. 1998;108:806–811. doi: 10.1097/00005537-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Kugaya A, Akechi T, Okamura H, Mikami I, Uchitomi Y. Correlates of depressed mood in ambulatory head and neck cancer patients. psycho-Oncology. 1999;8:494–499. doi: 10.1002/(sici)1099-1611(199911/12)8:6<494::aid-pon403>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 11.Esple C, Freelander E, Campsie L, Soutar D, Robertson A. Psychological distress at follow-up after major surgery for intra-oral cancer. J Psychosom Res. 1989;33:441–448. doi: 10.1016/0022-3999(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 12.Hersh WR. Telemedicine for the Medicare population. Portland, OR: Oregon Health Sciences University; 2001. Jul, Report no. 24. [Google Scholar]
- 13.Edwards M, Patel A. Telemedicine in the state of Maine; a model for growth driven by rural needs. Telemed J E-Health. 2003;9:25–39. doi: 10.1089/153056203763317620. [DOI] [PubMed] [Google Scholar]
- 14.LeBlanc A. Louisiana rural health access program. J Louisiana State Med Soc. 2000;152:89–93. [PubMed] [Google Scholar]
- 15.Savard L, Borstad A, Tkachuck J, Lauderdale D, Conroy B. Telerehabilitation consultations for clients with neurologic diagnoses; Cases from rural Minnesota and American Samoa. NeuroRehabilitation. 2003;18:93–102. [PubMed] [Google Scholar]
- 16.Buckwalter K, Davis L, Wakefield B, Kienzle M, Murray MA. Telehealth for elders and their Caregivers in rural communities. Community Health. 2002;25:31–40. doi: 10.1097/00003727-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Saysell E, Routley C. Telemedicine in community-based palliative care; evaluation of a videolink teleconference project. Int J Palliative Nurs. 2003;9:489–494. doi: 10.12968/ijpn.2003.9.11.11874. [DOI] [PubMed] [Google Scholar]
- 18.Coyle N, Khojainova N, Francavilla JM, Gonzales G. Audio-visual communication and its use in palliative care. J Pain Symptom Manage. 2002;23:171–175. doi: 10.1016/s0885-3924(01)00402-x. [DOI] [PubMed] [Google Scholar]
- 19.Whitten P, Doolittle G, Mackert M. Telehealth in Michigan: Use and patient acceptance. Am J Hospice Palliative Med. 2004;21:191–195. doi: 10.1177/104990910402100307. [DOI] [PubMed] [Google Scholar]
- 20.Committee on Quality of Healthcare in America. To err is human: Building a safer health system. Washington, DC: National Academy Press; 1999. [Google Scholar]
- 21.Committee on Quality of Health Care in America; Institute of Medicine. Crossing the quality chasm: A new health system for the 21st century. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 22.Balas EA, Jeffrey F, Kiperman GJ, et al. Electronic communication with patients: Evaluation of distance medicine technology. JAMA. 1997;278:152–159. [PubMed] [Google Scholar]
- 23.Kearney N, Kidd L, Miller M, et al. Utilizing handheld computers to monitor and support patients receiving chemotherapy: Results of a UK-based feasibility study. Supportive Care Cancer. 2006;14:742–752. doi: 10.1007/s00520-005-0002-9. [DOI] [PubMed] [Google Scholar]
- 24.Mooney KH, Beck SL, Friedman RH, Farzanfar R. Telephone-linked care for cancer symptom monitoring. Cancer practice. 2002;10:147–154. doi: 10.1046/j.1523-5394.2002.103006.x. [DOI] [PubMed] [Google Scholar]
- 25.Davis K, Yount S, Del Ciello K, et al. An innovative symptom monitoring tool for people with advanced lung cancer; A pilot demonstration. Supportive Oncol. 2007;5:381–387. [PubMed] [Google Scholar]
- 26.Wilkie DJ, Judge MK, Berry DL, Dell J, Zong S, Gilespie R. Usability of a computerized PAINReportlt in the general public with pain and people with cancer pain. J Pain Symptom Manage. 2003;25:213–224. doi: 10.1016/s0885-3924(02)00638-3. [DOI] [PubMed] [Google Scholar]
- 27.Weaver A, Young AM, Rowntree J, et al. Application of mobile phone technology for managing. chemotherapy-associated side-effects. Ann Oncol. 2007;18:1887–1892. doi: 10.1093/annonc/mdm354. [DOI] [PubMed] [Google Scholar]
- 28.Portenoy R, Thaler H, Kornblith A, et al. The Memorial Symptom Assessment Scale: An instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A:1326–1336. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 29.van den Brink JL, Moorman PW, de Boer MF, van Bemmel JH, Pruyn JFA, Verwoerd CDA. An information system to support the care for head and neck cancer patients. Supportive Care Cancer. 2003;11:452–459. doi: 10.1007/s00520-002-0425-5. [DOI] [PubMed] [Google Scholar]
- 30.van den Brink JL, Moorman PW, de Boer MF, Pruyn JF, Verwoerd CD, van Bemmel JH. Involving the patient: A prospective study on use, appreciation and effectiveness of an information system in head and neck cancer care. Int J Med Inform. 2005;74:839–849. doi: 10.1016/j.ijmedinf.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 31.van den Brink JL, Moorman PW, de Boer MF, et al. Impact on quality of life of a telemedicine system supporting head and neck cancer patients: A controlled trial during the postoperative period at home. J Am Med Inform Assoc. 2007;14:198–205. doi: 10.1197/jamia.M2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Boer M, van den Brink J, Moorman P, Pruyn J. The Erasmus Medical Center monitoring questionnaire for head and neck cancer patients. Rotterdam, The Netherlands: Erasmus Medical Center; 2004. [Google Scholar]
- 33.Health Hero Network. Health Hero Network security architecture. Redwood City, CA: Health Hero Network; 2006. [Google Scholar]