Abstract
The likelihood of development of degenerative joint disease (DJD) of the temporomandibular joint (TMJ) is related to the integrity of the TMJ disc. Predilection for mechanical failure of the TMJ disc may reflect inter-individual differences in TMJ loads. Nine females and eight males in each of normal TMJ disc position and bilateral disc displacement diagnostic groups consented to participate in our study. Disc position was determined by bilateral magnetic resonance images of the joints. Three-dimensional (3D) anatomical geometry of each subject was used in a validated computer-assisted numerical model to calculate ipsilateral and contralateral TMJ loads for a range of biting positions (incisor, canine, molar) and angles (1–13). Each TMJ load was a resultant vector at the anterosuperi or-most mediolateral midpoint the condyle and characterized in terms of magnitude and 3D orientation. Analysis of variance (ANOVA) was used to test for effects of biting position and angle on TMJ loads. Mean TMJ loads in subjects with disc displacement were 9.5–69% higher than in subjects with normal disc position. During canine biting, TMJ loads in subjects with disc displacement were 43% (ipsilateral condyle, p=0.029) and 49% (contralateral condyle, p=0.015) higher on average than in subjects with normal disc position. Biting angle effects showed that laterally directed forces on the dentition produced ipsilateral joint loads, which on average were 69% higher (p=0.002) compared to individuals with normal TMJ disc position. The data reported here describe large differences in TMJ loads between individuals with disc displacement and normal disc position. The results support future investigations of inter-individual differences in joint mechanics as a variable in the development of DJD of the TMJ.
Keywords: modeling, computer, force, temporomandibular joint, biting
Introduction
Degenerative changes of the human temporomandibular joint (TMJ) are evident in 3–29% of the population aged 19–40 years.1 Although degenerative joint disease (DJD) is common in the human TMJ, the pathomechanics are poorly understood. The mean age of onset of DJD of the human TMJ is between 25 and 35 years1–9 and approximately 20 years earlier than reported for the hip.10–12 Therapeutic interventions to ameliorate the effects of DJD in the TMJ, like other synovial joints, have not been predictably successful.13 The intra-articular disc is the main mechanism of load distribution and lubrication in the TMJ.14–18 Although the high prevalence of disc displacement in otherwise asymptomatic adults has led some researchers to propose that it is a non-pathological variation of anatomy,19,20 disc displacement is absent mostly in the young, increases with time through adolescence and early adulthood, and thus precedes the precocious time-line of DJD in the TMJ.21–23 It has been postulated that the propensity to develop DJD of the TMJ depends on the health of the disc,22 which is anisotropic with respect to mechanical fatigue.24
The objective of our study was to use a numerical modeling approach25–27 to test the hypothesis that TMJ loads during static biting are larger in subjects with TMJ disc displacement compared to subjects with normal disc position.
Materials and Methods
Thirty-four subjects gave informed consent to participate. The study protocol was approved by Institutional Review Boards. Subjects had generally intact dentitions, and did not report or exhibit postcranial DJD, orofacial pain, gross asymmetries in craniomandibular anatomy as determined by examination, and were not pregnant as determined by medical history. Diagnostic classification was established by a clinical examiner using research diagnostic criteria for temporomandibular disorders28 and a radiologist using magnetic resonance imaging and three-dimensional (3D) computed tomography.29 The subjects, 18 females and 16 males, were divided evenly into two diagnostic groups (Table 1). Mean ages (SD) were 35 (14) and 34 (15) years for disc displacement and normal disc position groups, respectively.
Table 1.
Subjects in two diagnostic groups.
| Gender | Number of subjects with normal disc position bilaterally | Number of subjects with disc displacement (II) according to RDC/TMD categories (a, b, c): | ||
|---|---|---|---|---|
| IIa | IIb | IIc | ||
| Female | 9 | 6 | 1 | 2 |
| Male | 8 | 6 | 1 | 1 |
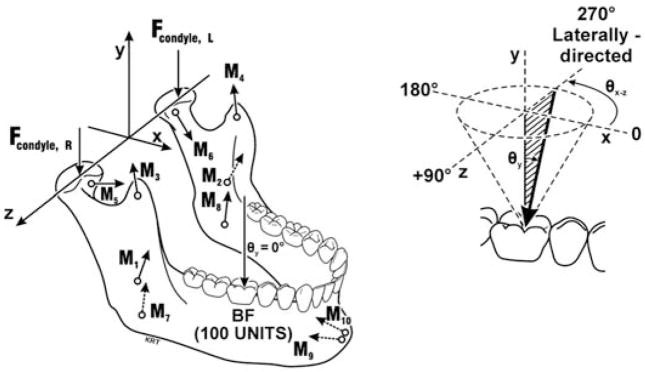
A geometry file was created for each subject that described positions of the mandibular condyles, teeth, and five pairs of masticatory muscles (masseter, anterior temporalis, medial pterygoid, lateral pterygoid, anterior digastric), determined from standardized lateral and pos-teroanterior cephalographs according to a 3D coordinate system25,27 (Figure 1). Geometry files were used in a previously described numerical model,30 first to validate the accuracy of the model in predicting data in each subject, and then to investigate inter-group differences in magnitudes of TMJ loads. Model-predicted ipsi-lateral and contralateral TMJ loads for a given static mandibular loading situation were resultant vectors at the anterosuperior-most mediolateral midpoint on the corresponding condyle and characterized in terms of magnitude and 3D orientation.
Figure 1.
Force vectors involved in numerical models of isometric biting in humans. Forces of biting (BF, 100 units), at the joints (Fcondyle), and representing five muscle pairs (M1,2=masseter, M3,4=anterior temporalis, M5,6= lateral pterygoid, M7, 8= medial pterygoid, M9,10=anterior digastric muscles) are illustrated. The axis system used to characterize the relative positions of the condyles, teeth, and muscle vectors, based on an individual’s anatomy, is shown also. Force magnitudes were expressed as percentages of BF. Enlargement (upper right) shows the azimuth angle (θxz°), measured parallel to the occlusal plane, which varies between 0 and 359°, and the vertical angle (θy°) where θy=0° is normal to the occlusal plane. For example, laterally-directed molar BFs had θxz=270° and θy=20°, 40°, and were biting angles 2, 3, respectively. (Modified from previous work.26)
Model validation was determined by the ability to predict right and/or left sagittal plane projections of the TMJ stress-field trajectory in each subject31 during symmetrical protrusion and retrusion of the mandible. That is, model-predicted orientations of TMJ loads were used as described previously and compared to individual-specific jaw tracking data measured in vivo.25,27,32,33 Accuracy between model-predicted and measured data was deemed to be acceptable based on average errors of 16% (Iwasaki et al., personal communication). Then the validated model was used to predict magnitudes of TMJ forces per unit biting force (BF) using an objective function of minimization of muscle effort (MME).26,34 The MME model calculated joint forces for biting on incisor, canine, and molar teeth, at a variety of angles (Tables 2, 3). Data were pooled and averaged by group. Analysis of variance (ANOVA) was used to test for significant differences between groups for magnitudes of TMJ loads during biting on incisors, canines, and molars at 13 angles.
Table 2.
Mean (SD) TMJ loads for three biting positions in two diagnostic groups.
| Displaced TMJ disc group | Normal TMJ disc position group | |||
|---|---|---|---|---|
| Biting position | Ipsilateral TMJ | Contralateral TMJ | Ipsilateral TMJ | Contralateral TMJ |
| Load (% BF) | Load (% BF) | Load (% BF) | Load (% BF) | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Canines | 124a (14) | 138b (14) | 81a (13) | 89b (14) |
| Incisors | 119 (14) | 118 (14) | 94 (13) | 92 (14) |
| Molars | 76 (14) | 87 (14) | 52 (13) | 67 (14) |
Where similar superscript letters indicate significant differences, p<0.05.
Table 3.
| Table 3a. Mean (SE) ipsilateral TMJ loads for thirteen biting angles in two diagnostic groups. | |||||
|---|---|---|---|---|---|
| Unilateral right biting angle (Description) | BF directions (°) | Mean ipsilateral TMJ load (% of BF) | p | ||
| θxz | θy | ||||
| Displaced TMJ disc group (SE = 15) | Normal TMJ disc position group (SE = 15) | ||||
| 1 (Vertical) | 90 | 0 | 116 | 69 | 0.028 |
| 2 (Laterally-directed) | 270 | 20 | 91 | 42 | 0.023 |
| 3 (Laterally-directed) | 270 | 40 | 113 | 44 | 0.002 |
| 4 (Medially-directed) | 90 | 20 | 115 | 100 | 0.469 |
| 5 (Medially-directed) | 90 | 40 | 131 | 121 | 0.655 |
| 6 (Posterolaterally-directed) | 355 | 20 | 103 | 77 | 0.237 |
| 7 (Posterolaterally-directed) | 355 | 40 | 107 | 85 | 0.293 |
| 8 (Posteromedially-directed) | 5 | 20 | 104 | 81 | 0.273 |
| 9 (Posteromedially-directed) | 5 | 40 | 107 | 89 | 0.400 |
| 10 (Anteromedially-directed) | 175 | 20 | 94 | 70 | 0.268 |
| 11 (Anteromedially-directed) | 175 | 40 | 94 | 73 | 0.314 |
| 12 (Anterolaterally-directed) | 185 | 20 | 108 | 66 | 0.049 |
| 13 (Anterolaterally-directed) | 185 | 40 | 101 | 65 | 0.096 |
| Table 3b. Mean (SE) contralateral TMJ loads for thirteen biting angles in two diagnostic groups. | |||||
|---|---|---|---|---|---|
| Unilateral right biting angle (Description) | BF directions (°) | Mean contralateral TMJ load (% of BF) | p | ||
| θxz | θy | ||||
| Displaced TMJ disc group (SE = 16) | Normal TMJ disc position group (SE = 15) | ||||
| 1 (Vertical) | 90 | 0 | 127 | 76 | 0.023 |
| 2 (Laterally-directed) | 270 | 20 | 150 | 105 | 0.047 |
| 3 (Laterally-directed) | 270 | 40 | 160 | 121 | 0.074 |
| 4 (Medially-directed) | 90 | 20 | 86 | 54 | 0.144 |
| 5 (Medially-directed) | 90 | 40 | 84 | 50 | 0.120 |
| 6 (Posterolaterally-directed) | 355 | 20 | 122 | 100 | 0.319 |
| 7 (Posterolaterally-directed) | 355 | 40 | 127 | 114 | 0.545 |
| 8 (Posteromedially-directed) | 5 | 20 | 119 | 97 | 0.309 |
| 9 (Posteromedially-directed) | 5 | 40 | 122 | 108 | 0.500 |
| 10 (Anteromedially-directed) | 175 | 20 | 91 | 64 | 0.224 |
| 11 (Anteromedially-directed) | 175 | 40 | 80 | 54 | 0.242 |
| 12 (Anterolaterally-directed) | 185 | 20 | 114 | 69 | 0.043 |
| 13 (Anterolaterally-directed) | 185 | 40 | 101 | 62 | 0.079 |
Results
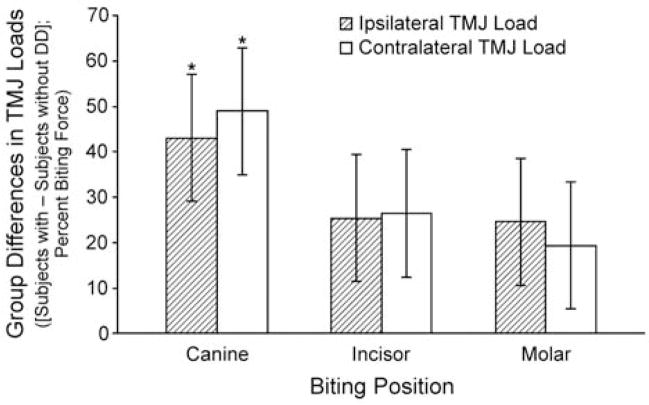
Among all biting positions and angles, mean predicted TMJ loads were 9.5–69% higher in subjects with disc displacement compared to subjects with normal disc position. The highest mean predicted TMJ loads occurred during canine biting in subjects with disc displacement (Table 2), where mean contralateral joint loads were 138% of the BF. During canine biting, between-group differences in mean TMJ loads of 43% (ipsilateral, p=0.029) and 49% (contralateral, p=0.015) were statistically significant (Figure 2).
Figure 2.
Between-group differences (disc displacement group - normal disc position group) in mean ipsilateral and contralateral TMJ loads for three biting positions. Mean differences between disc displacement (DD, n=17) and normal disc position (n=17) groups in ipsilateral and contralateral TMJ loads are plotted on the vertical axis for three biting positions (canines, incisors, molars) along the horizontal axis. Differences in TMJ loads were expressed as a percentage of the applied bite force. For all biting positions, subjects with TMJ disc displacement had higher joint loads. *indicates p<0.05.
With respect to the effects of biting angle, vertical and laterally-directed BF produced significant differences in joint loads between the two diagnostic groups. In all cases, ipsilateral and contralateral joint loads in the subjects with disc displacement were higher, with statistically significant differences (all p<0.05) occurring during vertical (biting angle 1), laterally-directed (biting angles 2, 3), and anterolaterally-directed biting (biting angle 12; Figure 3; Table 3a,b).
Figure 3.
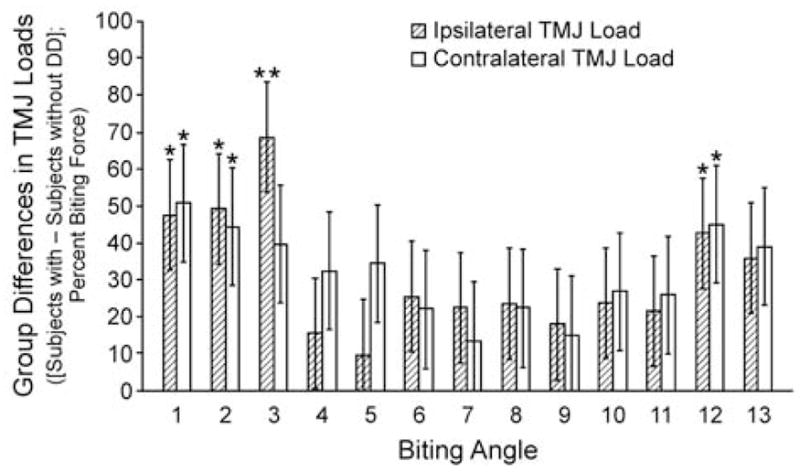
Between-group differences (disc displacement group – normal disc position group) in mean ipsilateral and contralateral TMJ loads for 13 biting angles. Mean differences between disc displacement (DD, n= 17) and normal disc position (n= 17) groups in ipsilateral and contralateral TMJ loads are plotted on the vertical axis for 13 biting angles (see Table 2 for descriptions) along the horizontal axis. Differences in TMJ loads were expressed as a percentage of the applied bite force. For all biting angles, subjects with TMJ disc displacement had higher joint loads. *indicates p<0.05, **indicates p<0.005.
Discussion
The data presented here are the first to imply that subjects with TMJ disc displacement have higher joint loads compared to subjects with normal disc position. During daily activities, subjects with disc displacement were capable of producing TMJ loads >60% higher than control subjects. TMJ loads predicted by validated computer modeling showed intergroup differences and suggest that inter-individual differences in joint mechanics are potential variables in DJD of the TMJ.
The numerical modeling approach has limitations that have been discussed in detail previously.28 One of these limitations is the simplified representation of joint and muscle forces. The model predicts magnitudes of these forces and also the direction of joint forces based on individual-specific anatomical data that include the position and direction of masticatory muscle forces. The area of joint loading in the individual is an important variable and should be investigated in future studies; for example, through dynamic stereometry.31 Furthermore, the muscles of mastication are multi-pennate muscles and, theoretically, each anatomical portion of a muscle can be represented by a vector. These vectors can be summed to produce a single unit vector. Variation in the direction of this unit vector depends on whether or not discrete areas of a muscle can be differentially activated. Contrary to reported conclusions, documented data for the masseter and temporalis muscles fail to show discrete differential activation of portions of muscles except with cortical feedback. That is, for the masseter muscle no single portion was solely active for a variety of tasks performed35 and near absence of differential activation was shown for most biting loads.36 The posterior deep portion behaved most differently from the rest of the masseter muscle, but behaved similarly to the neighboring temporalis muscle,37 which may indicate cross-talk from the latter muscle. Similarly all portions of the temporalis muscle were active during biting tasks and extremes of unit vector orientation were relatively inconsequential (<10°).37 These findings support the use of a unit vector as a reasonable first approximation of the force vector. Another limitation is the 2D validation currently used; however, capabilities for 3D validation using dynamic stereometry should be possible in future. In addition, further validation of muscle force magnitudes during modeled jaw tasks using EMG data recorded in vivo during similar tasks should be carried out in future.
Nevertheless, currently there are no other acceptable means known, besides the validated numerical modeling approach, to determine individual-specific TMJ forces during static or dynamic loading of the jaws. Additional future work should focus on diagnostic group differences in intracapsular mechanics and frequency of loading as variables associated with fatigue failure of the TMJ articulating tissues.
Acknowledgments
This work was supported by NIDCR 5R01 DE16417-2, and was based, in part, on a Master of Science thesis by M.J. Crosby at the University of Nebraska Medical Center (UNMC), Omaha NE. We gratefully acknowledge Mr. Kim Theesen, Graphic Artist, UNMC College of Dentistry, for helping with the figures, and Ms. Theresa Speers, RN, University at Buffalo, for her research assistance.
Footnotes
Contributions: JN PI of this NIH-sponsored study; YG diagnosis and recruitment of subjects for the project, obtaining informed consent; WDMcC, RO writing of data acquisition programs; JN, LI recording data from subjects, analyzing numerical modeling data; MC analyzing numerical modeling data; DM statistical analyses; DM, JN, LI, MC, RO, WDMcC, YG preparation and final approval of the manuscript.
Conflict of interest: the authors report no conflicts of interest.
This work is licensed under a Creative Commons Attribution 3.0 License (by-nc 3.0).
References
- 1.Pullinger AG, Seligman DA, Solberg WK. Temporomandibular disorders. Part I: Functional status, dentomorphologic features, and sex differences in a nonpatient population. J Prosthet Dent. 1988;59:228–35. doi: 10.1016/0022-3913(88)90019-4. [DOI] [PubMed] [Google Scholar]
- 2.Heloe B, Heloe LA. Characteristics of a group of patients with temporomandibular joint disorders. Community Dent Oral Epidemiol. 1975;3:72–9. doi: 10.1111/j.1600-0528.1975.tb00284.x. [DOI] [PubMed] [Google Scholar]
- 3.Israel HA, Ramamurthy NS, Greenwald R, et al. The potential role of doxycycline in the treatment of osteoarthritis of the temporomandibular joint. Adv Dent Res. 1998;12:51–5. doi: 10.1177/08959374980120012001. [DOI] [PubMed] [Google Scholar]
- 4.Luder HU. Factors affecting degeneration in human temporomandibular joints as assessed histologically. Eur J Oral Sci. 2002;110:106–13. doi: 10.1034/j.1600-0722.2002.11212.x. [DOI] [PubMed] [Google Scholar]
- 5.Murakami KI, Shibata T, Kubota E, et al. Intra-articular levels of prostaglandin E2, hyaluronic acid, and chondroitin-4 and -6 sulfates in the temporomandibular joint synovial fluid of patients with internal derangement. J Oral Maxillofac Surg. 1998;56:199–203. doi: 10.1016/s0278-2391(98)90869-2. [DOI] [PubMed] [Google Scholar]
- 6.Nilner M. Prevalence of functional disturbances and diseases of the stomatognathic system in 15–18 year olds. Swed Dent J. 1981;5:189–97. [PubMed] [Google Scholar]
- 7.Ong TK, Franklin CD. A clinical and histopathological study of osteoarthrosis of the temporomandibular joint. Br J Oral Maxillofac Surg. 1996;34:186–92. doi: 10.1016/s0266-4356(96)90375-7. [DOI] [PubMed] [Google Scholar]
- 8.Solberg WK, Woo MW, Houston JB. Prevalence of mandibular dysfunction in young adults. J Am Dent Assoc. 1979;98:25–34. doi: 10.14219/jada.archive.1979.0008. [DOI] [PubMed] [Google Scholar]
- 9.Stegenga B, De Bont LG, Boering G. A proposed classification of temporomandibular disorders based on synovial joint pathology. Cranio. 1989;7:107–18. doi: 10.1080/08869634.1989.11678273. [DOI] [PubMed] [Google Scholar]
- 10.Felson DT, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40:728–33. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence RC, Hochberg MC, Kelsey JL, et al. Estimates of the prevalence of selected arthritic and musculoskeletal diseases in the United States. J Rheumatol. 1989;16:427–41. [PubMed] [Google Scholar]
- 12.Vingard E, Alfredsson L, Malchau H. Osteoarthrosis of the hip in women and its relation to physical load at work and in the home. Ann Rheum Dis. 1997;56:293–8. doi: 10.1136/ard.56.5.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandt KD, Dieppe P, Radin EL. Etio-pathogenesis of osteoarthritis. Rheum Dis Clin North Am. 2008;34:531–59. doi: 10.1016/j.rdc.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Beek M, Koolstra JH, van Eijden TM. Human temporomandibular joint disc cartilage as a poroelastic material. Clin Biomech (Bristol, Avon) 2003;18:69–76. doi: 10.1016/s0268-0033(02)00135-3. [DOI] [PubMed] [Google Scholar]
- 15.Nickel JC, Iwasaki LR, Beatty MW, et al. Static and dynamic loading effects on temporomandibular joint disc tractionalforces. J Dent Res. 2006;85:809–13. doi: 10.1177/154405910608500906. [DOI] [PubMed] [Google Scholar]
- 16.Nickel JC, McLachlan KR. In vitro measurement of the stress-distribution properties of the pig temporomandibular joint disc. Arch Oral Biol. 1994;39:439–48. doi: 10.1016/0003-9969(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 17.Nickel JC, McLachlan KR. In vitro measurement of the frictional properties of the temporomandibular joint disc. Arch Oral Biol. 1994;39:323–31. doi: 10.1016/0003-9969(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 18.Spilker RL, Nickel JC, Iwasaki LR. A biphasic finite element model of in vitro plowing tests of the temporomandibular joint disc. Ann Biomed Eng. 2009;37:1152–64. doi: 10.1007/s10439-009-9685-2. [DOI] [PubMed] [Google Scholar]
- 19.Hellsing G, Holmlund A. Development of anterior disk displacement in the temporomandibular joint: an autopsy study. J Prosthet Dent. 1985;53:397–401. doi: 10.1016/0022-3913(85)90521-9. [DOI] [PubMed] [Google Scholar]
- 20.Stegenga B. Osteoarthritis of the temporomandibular joint organ and its relationship to disc displacement. J Orofac Pain. 2001;15:193–205. [PubMed] [Google Scholar]
- 21.Kurita H, Uehara S, Yokochi M, et al. A long-term follow-up study of radiographically evident degenerative changes in the temporomandibular joint with different conditions of disk displacement. Int J Oral Maxillofac Surg. 2006;35:49–54. doi: 10.1016/j.ijom.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Larheim TA. Role of magnetic resonance imaging in the clinical diagnosis of the temporomandibular joint. Cells Tissues Organs. 2005;180:6–21. doi: 10.1159/000086194. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka E, Detamore MS, Mercuri LG. Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. J Dent Res. 2008;87:296–307. doi: 10.1177/154405910808700406. [DOI] [PubMed] [Google Scholar]
- 24.Beatty MW, Bruno MJ, Iwasaki LR, et al. Strain rate dependent orthotropic properties of pristine and impulsively loaded porcine temporomandibular joint disk. J Biomed Mater Res. 2001;57:25–34. doi: 10.1002/1097-4636(200110)57:1<25::aid-jbm1137>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki LR, Petsche PE, McCall WD, Jr, et al. Neuromuscular objectives of the human masticatory apparatus during static biting. Arch Oral Biol. 2003;48:767–77. doi: 10.1016/s0003-9969(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 26.Iwasaki LR, Thornton BR, McCall WD, Jr, et al. Individual variations in numerically modeled human muscle and temporomandibular joint forces during static biting. J Orofac Pain. 2004;18:235–45. [PubMed] [Google Scholar]
- 27.Nickel JC, Iwasaki LR, Walker RD, et al. Human masticatory muscle forces during static biting. J Dent Res. 2003;82:212–7. doi: 10.1177/154405910308200312. [DOI] [PubMed] [Google Scholar]
- 28.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992;6:301–55. [PubMed] [Google Scholar]
- 29.Mansur A, Hollender L, Anderson Q, et al. Research diagnostic criteria for temporomandibular disorders (RDC/TMD): development of image analysis criteria and examiner reliability for image analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:844–60. doi: 10.1016/j.tripleo.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trainor PG, McLachlan KR, McCall WD. Modelling of forces in the human masticatory system with optimization of the angulations of the joint loads. J Biomech. 1995;28:829–43. doi: 10.1016/0021-9290(94)00128-q. [DOI] [PubMed] [Google Scholar]
- 31.Gallo LM, Chiaravalloti G, Iwasaki LR, et al. Mechanical work during stress-field translation in the human. J Dent Res. 2006;85:1006–10. doi: 10.1177/154405910608501106. [DOI] [PubMed] [Google Scholar]
- 32.Iwasaki LR, Baird BW, McCall WD, Jr, et al. Muscle and temporomandibular joint forces associated with chincup loading predicted by numerical modeling. Am J Orthod Dentofacial Orthop. 2003;124:530–40. doi: 10.1016/s0889-5406(03)00575-4. [DOI] [PubMed] [Google Scholar]
- 33.Nickel JC, Yao P, Spalding PM, et al. Validated numerical modeling of the effects of combined orthodontic and orthognathic surgical treatment on TMJ loads and muscle forces. Am J Orthod Dento-facial Orthop. 2002;121:73–83. doi: 10.1067/mod.2002.120138. [DOI] [PubMed] [Google Scholar]
- 34.Nickel JC, Iwasaki LR. In vivo tests of TMJ morphology and masticatory muscle forces predicted by computer-assisted models. In: Davidovitch Z, Mah J, editors. Biological mechanisms of tooth movement and craniofacial adaptation. Boston: Harvard Society for the Advancement of Orthodontics; 2004. pp. 59–70. [Google Scholar]
- 35.McMillan AS, Hannam AG. Task dependence of human masseter motor unit reflex behaviour. Exp Brain Res. 1992;88:443–6. doi: 10.1007/BF02259119. [DOI] [PubMed] [Google Scholar]
- 36.Blanksma NG, Van Eijden TM, Weijs WA. Electromyographic heterogeneity in the human masseter muscle. J Dent Res. 1992;71:47–52. doi: 10.1177/00220345920710010801. [DOI] [PubMed] [Google Scholar]
- 37.Blanksma NG, Van Eijden TM. Electromyographic heterogeneity in the human temporalis muscle. J Dent Res. 1990;69:1686–90. doi: 10.1177/00220345900690101101. [DOI] [PubMed] [Google Scholar]