Abstract
Myocarditis is an inflammatory disease of the heart that may occur because of infections, immune system activation, or exposure to drugs. The diagnosis of myocarditis has changed due to the introduction of cardiac magnetic resonance imaging. We present an expert consensus document aimed to summarize the common terminology related to myocarditis meanwhile highlighting some areas of controversies and/or uncertainties and the unmet clinical needs. In fact, controversies persist regarding mechanisms that determine the transition from the initial trigger to myocardial inflammation and from acute myocardial damage to chronic ventricular dysfunction. It is still uncertain which viruses (besides enteroviruses) cause direct tissue damage, act as triggers for immune-mediated damage, or both. Regarding terminology, myocarditis can be characterized according to etiology, phase and severity of the disease, predominant symptoms, and pathological findings. Clinically, acute myocarditis (AM) implies a short time elapsed from the onset of symptoms and diagnosis (generally less than one month). On the other hand, chronic inflammatory cardiomyopathy (infl-CMP) indicates myocardial inflammation with established dilated cardiomyopathy or hypokinetic non-dilated phenotype, that in the advanced stages evolves into fibrosis without detectable inflammation. Suggested diagnostic and treatment recommendations for AM and chronic infl-CMP are mainly based on expert opinion given the lack of well-designed contemporary clinical studies in the field. We will provide a shared and practical approach to patient diagnosis and management, underlying differences between European and United Stated scientific statements on this topic. We explain the role of histology that defines subtypes of myocarditis and its prognostic and therapeutic implications.
Keywords: acute myocarditis, chronic inflammatory cardiomyopathy, endomyocardial biopsy, cardiac magnetic resonance imaging, cardiac sarcoidosis
DEFINITIONS, EPIDEMIOLOGY, AND PATHOPHYSIOLOGY
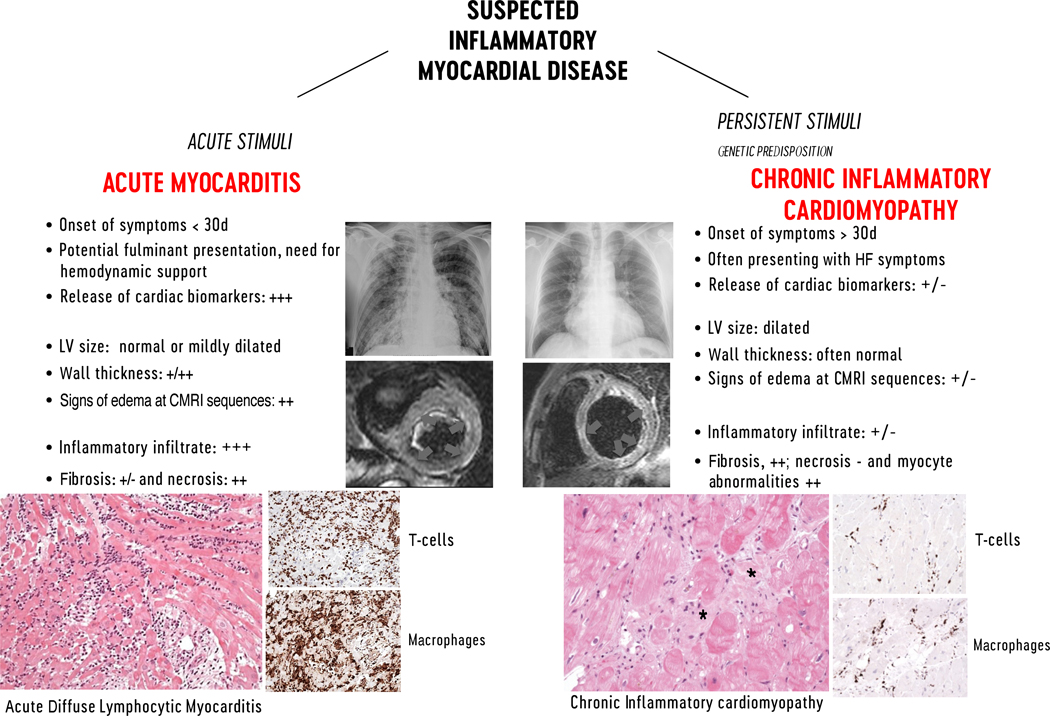
Myocarditis is an inflammatory disease of the heart that may occur as a consequence of infections, exposure to toxic substances, and immune system activation,1, 2 and is included among secondary cardiomyopathies in the 1996 World Health Organization classification.3 Myocarditis has a wide spectrum of clinical presentations and trajectories, with most cases resolving spontaneously. It is also a relatively common cause of sudden cardiac death (SCD) in young people (from 6 to 10% in autopsy-based series, Supplemental Table I).4, 5 Furthermore, in some patients inflammation may cause extensive scarring that triggers left ventricular (LV) remodeling leading eventually to dilated cardiomyopathy (DCM);6 or alternatively to a predominant hypokinetic nondilated phenotype of cardiomyopathy. Myocarditis can be characterized according to etiology, phase and severity of the disease, predominant symptoms, and pathological findings. Clinically, acute myocarditis (AM) implies a short time elapsed from the onset of symptoms and diagnosis (generally less than 1 month), while chronic inflammatory cardiomyopathy (infl-CMP) indicates myocardial inflammation with established DCM or hypokinetic non-dilated phenotype generally with a longer duration of symptoms (longer than 1 month) (Figure 1). Based on the cell types infiltrating, myocarditis can be classified as eosinophilic, lymphocytic, giant cells or granulomatous (Figure 2). Chronic myocarditis can represent an intermediate stage between AM and chronic infl-CMP in patients with persisting myocardial inflammation (Supplemental Figure I). Due to evolving diagnostic criteria and differences in the conceptual view and interpretation of myocarditis within the medical community, definitions associated with myocarditis have changed over the last decades. A list of definitions used in this document is presented in Table 1.
FIGURE 1. Characteristics features of lymphocytic acute myocarditis and chronic inflammatory cardiomyopathy. On the left imaging features of acute myocarditis:
Chest X-ray of a patient admitted for chest pain and suspected acute myocarditis with no enlargement of the cardiac silhouette and cardiac magnetic resonance imaging (CMRI) showing normal left ventricular (LV) volume and significantly increased cardiac mass with diffuse high signal in T2-weighted images (arrows) suggesting diffuse edema. Histology shows acute lymphocytic myocarditis with myocyte necrosis and diffuse mononuclear cell infiltrates by hematoxylin-eosin and immunohistological stain on CD3+T cells and CD68+ macrophages, compatible with an active myocarditis based on Dallas criteria (magnitude x 200). On the right imaging features of chronic lymphocytic cardiomyopathy: Chest X-ray of a patient admitted with heart failure symptoms, showing enlargement of cardiac silhouette; at CMRI the LV is dilated, with normal thickness and focal areas of high signal intensity at T2-weighted images suggesting localized edema (arrows). At histology chronic inflammatory cardiomyopathy typically presents fibrosis (*) within areas with inflammatory cellular infiltrates and myocyte abnormalities (magnitude x200). D indicates days; HF, heart failure.
FIGURE 2. Different patterns of myocardial inflammation demonstrated by histological and immunohistological stainings on endomyocardial biopsy.
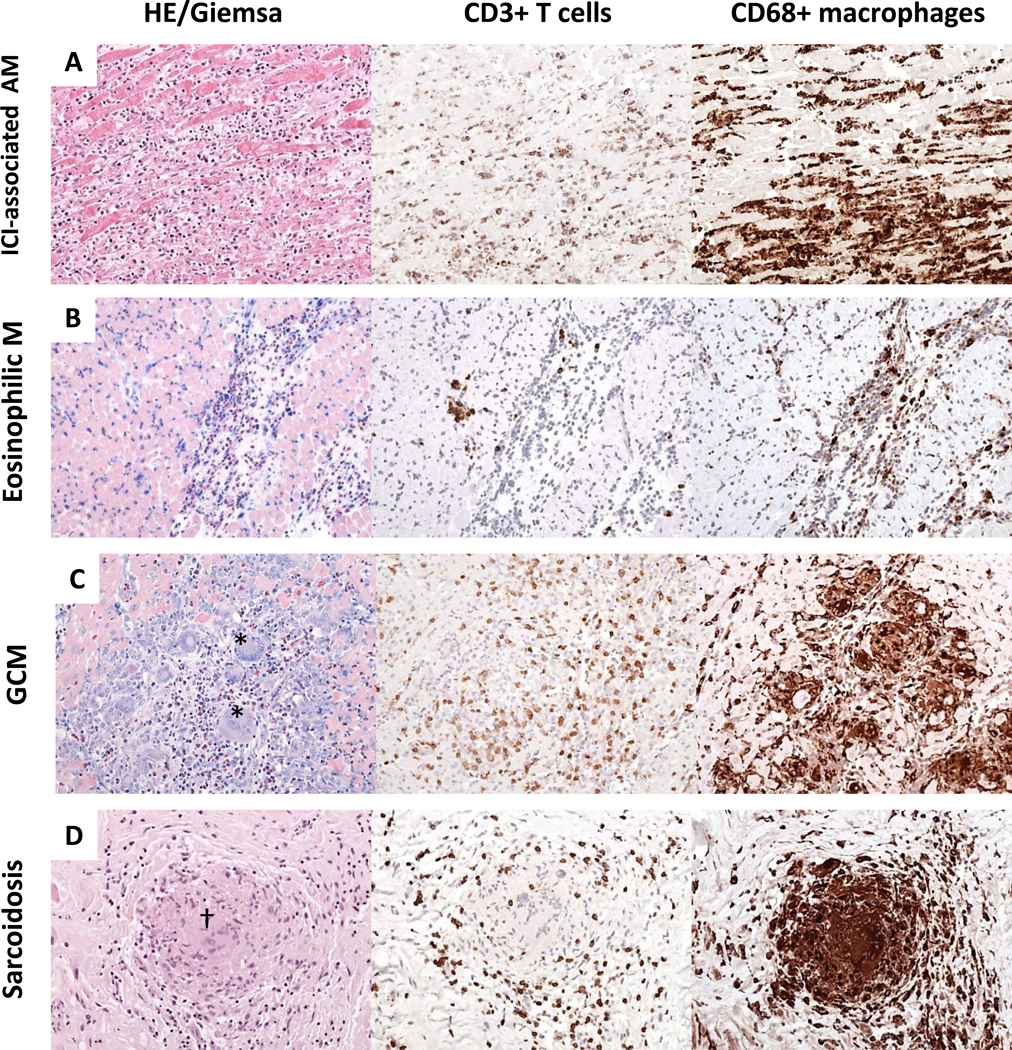
A) Immune checkpoint inhibitors (ICI) associated myocarditis frequently reveal diffuse mononuclear infiltrates composed of CD3+T cells and CD68+ macrophages. Based on hematoxylin-eosin (HE), and immunohistological stainings, ICI-associated myocarditis resembles a diffuse lymphocytic myocarditis. (B) In eosinophilic myocarditis (EM) prominent inflammatory cells are eosinophilic granulocytes (Giemsa) and macrophages. (C) Giant cell myocarditis (GCM) is characterized by large mononucleated infiltrates with presence of giant cells (*) and eosinophils (Giemsa); (D) Cardiac sarcoidosis can be differentiated from GCM by the presence of granuloma (†) and absence of necrotic myocytes. (In all pictures magnitude x200). AM indicates acute myocarditis; M, myocarditis.
TABLE 1.
Glossary of the terms used in this document regarding myocarditis.
| Terms (in alphabetical order) | Domain(s) | Definition | |||
|---|---|---|---|---|---|
| Clinical Presentation | Time | Etiology, Patho-physiology | Pathology | ||
| Active Myocarditis | X | On the basis of Dallas criteria, active myocarditis (versus borderline myocarditis or no myocarditis) indicates the presence of infiltrating inflammatory mononucleated cells and myocyte necrosis, with or without fibrosis, at routine light microscopy evaluation of EMB.54 | |||
| Acute Myocarditis | X | X | Myocarditis with symptoms of recent onset (on average within ~ 1 month), generally with increased levels of high-sensitivity troponins, and evidence of edema on CMRI if performed within 4 weeks, or alternatively positive cardiac FDG-PET imaging (not suggested as routine diagnostic tool). Histologically is characterized by an active myocarditis. We proposed the acute presentation when medical attention occurs within 1 month from the symptoms’ onset compared with previous 3-month interval reported in previous ESC and AHA scientific statements. The term subacute myocarditis could be used to describe the interval between 1- to 3-month interval from the symptoms’ onset. The general idea is that the earlier are performed the diagnostic investigations (i.e. EMB) and potential intervention (immunosuppression) are started and the higher is the likelihood to reach the diagnosis of myocarditis and to have benefit from immunosuppression. | ||
| Borderline Myocarditis | X | On the basis of Dallas criteria, borderline myocarditis (versus active myocarditis or no myocarditis) indicates the presence of inflammatory mononucleated cell infiltrate, in the absence of myocytolysis, at routine light microscopy evaluation of EMB.54 This term has been abandoned due to ambiguity and poor consistency among pathologists. | |||
| Clinically suspected myocarditis | X | X | Proposed definition in the ESC position statement2 is presence of (1) ≥1 clinical presentation (acute chest pain, or new onset dyspnea [days up to 3 months], or in subacute/chronic dyspnea [>3 months], or palpitations/unexplained arrhythmia symptoms, or unexplained cardiogenic shock and (2) ≥1 diagnostic criteria from different categories (electrocardiographic features of cardiac injury, elevated markers of myocardial necrosis, functional/structural abnormalities on echocardiogram/angiogram or CMRI, tissue characterization by CMRI), in the absence of: (a) angiographically detectable coronary artery disease (coronary stenosis ≥ 50%); (b) known pre-existing cardiovascular disease or extra-cardiac causes that could explain the syndrome (e.g. valve disease, congenital heart disease, hyperthyroidism, etc.). Suspicion is higher with higher number of fulfilled criteria. If the patient is asymptomatic ≥2 diagnostic criteria should be met. The limitation of this overarching definition is that, for example dyspnea associated to mild increase of troponin plus evidence of new evidence of electrocardiographic or echocardiographic changes can be enough for the suspect of myocarditis. Findings based on CMRI are probably more accurate than other diagnostic findings, even if it does not emerge from this definition. |
||
| Chronic inflammatory cardiomyopathy | X | X | X | Indicates a persistent/chronic myocardial inflammatory condition (symptoms’ onset >1 month) with clinical phenotype of hypokinetic either dilated or non-dilated cardiomyopathy, that can be associated with arrhythmogenic substrate. Histologically it is generally characterized by myocyte abnormalities (e.g. variations of myocyte diameter), focal and/or diffuse fibrosis with inflammatory infiltrates. | |
| Chronic Myocarditis | X | X | X | Defines an ongoing inflammatory process with fibrosis but without myocyte necrosis or myocyte abnormalities. Chronic myocarditis can represent an intermediate stage between acute myocarditis and chronic inflammatory cardiomyopathy in patients with persisting myocardial inflammation. This phenotype can be observed in non-dilated or mild dilated arrhythmogenic cardiomyopathy or in the setting of an autoimmune disease or syndrome. There is some overlapping with subacute myocarditis. |
|
| Complicated acute Myocarditis | X | A working term aimed to identify high-risk patients, i.e. those presenting with one or more of the followings: left ventricular dysfunction (LVEF<50% on first echocardiogram), sustained ventricular arrhythmias, advanced heart block, HF, low cardiac output syndrome, cardiogenic shock. Uncomplicated myocarditis defines a myocarditis without the above manifestations. |
|||
| Drug-induced myocarditis | X | Myocarditis caused by direct cytotoxic effect of the drug (i.e cocaine) | |||
| Eosinophilic Myocarditis | X | Myocarditis characterized by eosinophilic infiltrate at EMB. Peripheral eosinophilia at differential WBC count is suggestive, but it is not always present. | |||
| Fulminant Myocarditis | X | X | X | A working term indicating severe forms of acute myocarditis, with fast evolution and hemodynamic compromise (low-output syndrome or cardiogenic shock) requiring inotropes and/or mechanical circulatory support.64 It is a form of acute myocarditis complicated by cardiogenic shock. When performed, EMB often (but not always) shows diffuse inflammatory infiltrates. | |
| Giant-cell Myocarditis | X | Myocarditis characterized by large multinuclear cells infiltrating the heart on histology (see text for details) in the absence of well-formed granuloma. It is usually associated with heart dysfunction and is often clinically fulminant. | |||
| Healing myocarditis | X | A subacute myocarditis can be also defined as a healing myocarditis if there is evidence of a previous active myocarditis. In case of follow-up biopsies, the term healing myocarditis defines a partial resolution of a previous active myocarditis. It can be used as synonymous of subacute myocarditis. | |||
| Hypersensitivity Myocarditis (or allergic myocarditis) | X | Indicates that myocardial damage is caused by an abnormal reaction or overreaction with drugs (i.e. clozapine) acting as stimuli/triggers. When performed, EMB demonstrates eosinophilic infiltrates. It is also called allergic myocarditis. | |||
| Immune-checkpoints associated myocarditis | X | It is a specific form of Immune-mediated myocarditis associated with the use of immune-checkpoints inhibitors anticancer drugs. They can be also termed immune-checkpoints induced myocarditis. | |||
| Immune-mediated Myocarditis | X | Myocarditis caused by immune mechanisms (autoimmunity in most of the cases, although heart transplant rejection represents an example of myocarditis mediated by alloimmunity). | |||
| Infarct-like myocarditis | X | Myocarditis presenting with chest pain and diffuse ST-elevation on the ECG, that represents about 45.8% of admitted cases of AM based on a contemporary registry. The tern is misreading since this presentation can be associated with both normal or reduced LVEF, thus without a real prognostic utility. In fact, contrasting results are reported about the outcome of patients with infarct-like myocarditis. Instead, the term uncomplicated myocarditis is preferred to refer to patients with acute myocarditis presenting with chest pain and normal LVEF. | |||
| Infective myocarditis | X | It refers to myocarditis definitely caused by infection targeting the heart, and should be limited to viruses, protozoans and bacteria that cause direct pathogen-mediated injury. Although the list of potential agents is long, a few of such cases are currently observed in immunocompetent subjects in Western countries or in infants (e.g. Enterovirus). The term can be misleading to define all types of virus-induced myocarditis or all virus-positive myocarditis/infl-CMP. | |||
| Lymphocytic Myocarditis | X | Myocarditis characterized by small mononuclear cells (CD3+T lymphocytes) infiltrating the heart. It is the most frequent histologic pattern and may or may not be associated with heart dysfunction. It is the histological subtype more often associated with virus-induced myocarditis and immune checkpoints-associated myocarditis. | |||
| Myopericarditis | X | Inflammatory process of the heart involving both the pericardium and the myocardium, without systolic dysfunction. This term and perimyocarditis (see below) are frequently used as synonyms, although “myocarditis with evidence of pericardial involvement” would be preferable in both cases. | |||
| Myocarditis with pericardial involvement | X | Inflammatory process of the heart involving both the pericardium and the myocardium, with or without systolic dysfunction. This term focuses the attention on the myocardium, as pericardium is often involved due to continuity, thus it is preferable to the terms of peri-myocarditis/myopericarditis. | |||
| Perimyocarditis | X | Inflammatory process of the heart involving both the pericardium and the myocardium, with evidence of systolic dysfunction (see above). | |||
| Post-viral myocarditis | X | X | X | Myocarditis that occurs shortly after an episode of possible/proven viral infection (e.g. common cold, flu-like syndrome) – see also Viral myocarditis. It is often used in patients with prodromal symptoms without isolation of a specific virus. | |
| Probable AM | X | X | A clinical syndrome, including HF of <3 months’ duration, associated with an otherwise unexplained elevation in troponin or electrocardiographic features of cardiac injury. New wall motion abnormalities, a pericardial effusion on echocardiography, or characteristic tissue features on CMRI strengthen the diagnosis. This definition has been proposed by the AHA Scientific Statement on specific DCM.65 The term is similar, to clinically suspected myocarditis proposed by the ESC position statement,2 even if it appears too generic. Furthermore, proposed AM term is limited to acute forms compared to clinically suspected myocarditis that includes also chronic forms. The term probable AM has a proposed timeframe of 3 months for acuity compared with the current proposal of 1 month for AM in this document. | ||
| Sarcoidotic myocarditis | X | X | Patients presenting with an acute myocarditis associated with known or new systemic sarcoidosis. It can also be the clinical presentation of an isolated cardiac sarcoidosis. Sarcoidotic myocarditis is characterized by infiltration by activated macrophages, which in some cases can lead to chronic inflammation and fibrotic replacement with non-necrotizing granulomas. | ||
| Subacute Myocarditis | X | X | X | Persistent/ongoing myocardial damage due to persistent or recurrent stimulus for inflammation. There is some overlapping with chronic myocarditis, since time threshold have not been defined. A subacute myocarditis can be also defined as a healing myocarditis if there is evidence of a previous active myocarditis. The term can be also used to describe a myocarditis with symptoms’ onset between 1- to 3-month interval before diagnosis. | |
| Viral Myocarditis | X | Myocarditis that occurs during the course of an episode of possible/proven viral infection or in patients with prodromal symptoms (e.g. common cold, flu-like syndrome) – It is often used as synonymous of post-viral myocarditis in clinical practice. | |||
| Virus-induced myocarditis | X | X | Myocarditis that is definitely or very probably related to a viral infection. This term should be preferred over infective or viral myocarditis when referring to both virus-mediated and virus-triggered myocarditis (see below) | ||
| Virus-mediated Myocarditis | X | X | Myocarditis that is related to a viral infection via direct viral cytotoxicity at myocardial level (e.g. coxsackie virus myocarditis). Demonstration of the virus in the myocardium is required. | ||
| Virus-positive (vs. virus-negative) Myocarditis/chronic infl-CMP | X | Biopsy-proven (more often lymphocytic) myocarditis or infl-CMP, with demonstration of the presence of viral genome in the myocardium by means of real-time PCR.2 Note that type of viruses, number of viral genome copies, and techniques for their identification and measurements are not standardized. | |||
| Virus-triggered myocarditis | X | X | Immune-mediated lymphocytic myocarditis that are triggered by common viruses (such as influenza and coronaviruses) in the absence of viral genome in the myocardium. Viral PCR on pharyngeal swabs in these patients can support the association between viral exposure and the onset of acute myocarditis. This myocarditis could be also termed post-viral or viral myocarditis. | ||
Terms that are suggested are highlighted in bold characters. Terms non-highlighted in bold are not suggested or have been abandoned due to ambiguity.
AM indicates acute myocarditis; CMRI, cardiac magnetic resonance imaging; EMB, endomyocardial biopsy; HF, heart failure; Infl-CMP, inflammatory; cardiomyopathy; LVEF, left ventricular ejection fraction; PCR, polymerase chain reaction. WBC, whole blood cell.
The disease burden of myocarditis is difficult to define. Based on hospital discharge forms between 1990 and 2013, an incidence of 22 cases out of 100,000 patients annually was estimated by the Global Burden of Disease Study.7 However, this report did not distinguish between AM or chronic infl-CMP and other cardiomyopathies, with possible overestimation of myocarditis. Among patients presenting to the emergency department, AM was the second most common cardiac cause of chest pain (3%) in a French registry.8 Furthermore, nearly 33% of the patients initially labeled as myocardial infarction with non-obstructed coronary arteries are later diagnosed as AM.9 According to contemporary registries, AM is a cardiac condition affecting relatively young patients (median age of onset ranges between 30 and 45 years in most of series), and males more than females (male prevalence ranges between 60 to 80%)(Table 2).10–17 The absolute prevalence and relative proportion of different etiologies may vary over time and according to endemic diseases. For example, immune checkpoints inhibitors (ICI) associated myocarditis is a recently recognized entity, whose rate of diagnosis has increased due to larger awareness and to the larger population of cancer patients eligible for treatment with ICI.18 On the other hand, AM and chronic infl-CMP may have a different incidence in specific geographic areas according to local epidemiology (such as Chagas disease in South America). Controversies still exist regarding the mechanisms that determine the transition from the initial trigger to myocardial inflammation, and from acute myocardial injury to chronic dysfunction. So far it is not known which viruses, other than enteroviruses may cause direct tissue damage in humans, or act mainly as triggers for autoimmunity-mediated damage, or both.19, 20 It must be considered that the experimental evidences on murine models of viral myocarditis are based on infections with Coxsackie B viruses, whereas for the most common agent in virus-positive myocarditis patients, parvovirus (PV)-B19,21, 22 no animal models are available. A possible association between genetic abnormalities and susceptibility to inflammation has been suggested. In particular, patients with mutations responsible for arrhythmogenic cardiomyopathy may be at risk for AM and/or share clinical and pathological aspects with chronic infl-CMP,23, 24 although further studies are required to elucidate this association and understand its mechanistic underpinnings.
TABLE 2.
Principal studies that evaluated the long-term outcome of adult patients with myocarditis based on histology or cardiac magnetic resonance findings or the combination of both published since 1995.
| First Author | Years | Type of study and country | N | Age; Male sex (%) | Time since the onset of symptoms | Histology | CMRI | Viral search in the heart | LVEF (%) and; LV dim. at admission | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Grogan et al.35 1995 | 1979–1988 | Retrospective monocentric US | 27 | 47y; 59% | Median Symptoms′ duration 3.5 months | All based on EMB (all positive Dallas criteria: borderline or lympho.) | None | None | LVEF 38%; LV dim.: not reported |
At 5 years: Survival 56% |
| Mason et al.36 1995 | 1986–1994 | Randomized trial, multicenter (N=31), US (treated with immunosuppression) (all with LVEF<45%) | 64 | 43y 58%; | 43% with symptoms duration <30 days; 57% with symptoms above 30 days | All based on EMB (61% with positive Dallas criteria; all lympho.) | None | None | LVEF 24%; LV dim.: EDD: 64 mm |
At 4.3 years: Survival 44% Independent predictors: reduced LVEF, extent of CD2+ cells in the myocardium |
| Mason et al.36 1995 | 1986–1994 | Randomized trial, multicenter (N=31), US (control group) (all with LVEF<45%) | 47 | 41y; 32% | 51% with symptoms duration <30 days; 49% with symptoms above 30 days | All based on EMB (67% with positive Dallas criteria; all lympho.) | None | None | LVEF 24%; LV dim.: EDD: 64 mm |
At 4.3 years: Survival 44% Independent predictors: reduced LVEF, extent of CD2+ cells in the myocardium |
| McMarthy et al.37 2000 | 1984–1997 | Retrospective, Monocentric, US (only fulminant myocarditis) | 15 | 35y; 73% | Symptoms duration <1 year | All, based on EMB (only lympho.) | None | None | LVEF: Not reported LV dim.: Not reported |
Median follow up 5.3 years: Survival free of HTx 93% |
| McMarthy et al.37 2000 | 1984–1997 | Retrospective, Monocentric, US (only acute nonfulminant myocarditis) | 132 | 43y; 64% | Symptoms duration <1 year | All based on EMB (only lympho.) | None | None | LVEF: Not reported LV dim.: Not reported |
Median follow up 5.7 years: Survival free of HTx 45% |
| Magnani et al.38 2006 | 1978–2003 | Retrospective monocentric (on inotrope 51%) US | 112 | 47y; 60% | Symptoms duration not specified, retrospective analysis based on EMB. | All based on EMB performed at the discretion of each patient’s attending physician (Lympho. 55%, granulomatous 10% GCM 6%, eosinoph. 6%, borderline 22%) | None | None | LVEF: 37%; LV dim.: Not reported |
At 5 years: Survival free of HTx 56% Independent predictors PCWP>15 mmHg, type of histology (lymphocytic/ granulomatous/ GCM vs. others) |
| Caforio et al.,39 2007 | 1992–2005 | Not specified if prospective, monocentric Italy | 174 | 36y; 63% | Symptoms duration between 0 and 6 months | All, based on EMB (positive Dallas criteria), including all histologies (i.e. GCM) | None | Viral PCR in the myocardium for all cardiotropic viruses (most frequently reported Enterovirus, 12.5%, and adenovirus, 5%) | LVEF: 43%: LV dim.: LVEDVi: 83 ml/m2 |
At 2 years: Estimated Survival free of HTx 87%. Independent predictors: sign and symptoms of LV and RV failure). Lost at follow up: 14% |
| Kindermann et al.,40 2008 | 1994–2007 | Prospective, monocentric (excluded patients presenting with cardiogenic shock) Germany | 181 | 42y; 67% | Symptoms duration not specified | All, based on EMB (38% with positive and 62% negative for Dallas criteria). Immuno- histological signs of inflammation 50%. Not specified type of histology | Unknown the number of CMRI. Data not reported |
Viral PCR detected in 44%: PVB19: 29% HHV6: 11% Enterovirus 6% |
LVEF: 38% LV dim.: LVEDDi: 36 mm/m2, reported LV dilation in 51% |
Mean follow up approx. 5 years: Survival free of HTx 78% Independent predictors: NYHA III-IV, signs of inflammation at histology, lack of B-blocker therapy. Lost at follow up: 8% |
| Grun et al.16 2012 | 2002–2008 | Not specified if prospective Monocentric Germany | 203 | 52y; 69% | Symptoms duration not specified | All, based on EMB (diagnosis: CD3+/CD68+ infiltration + myocardial damage or fibrosis + HLA-class II +) | All, within 5 days from admission | Viral PCR for PVB19, HHV6 and EBV. Detected in 81%: PVB19: 56% HHV6: 24% EBV 1% | LVEF: 45% on CMRI LV dim.: LVEDV: 165 mL |
Median follow up 4.7 years: Survival: 80%. Best independent predictor: LGE presence |
| Anzini et al.17 2013 | 1981–2009 | Not specified if prospective, Monocentric, Italy | 82 | 38y; 70% | Median duration of symptoms 8 days | All based on EMB (positive Dallas criteria for active myocarditis), including all histologies (lympho. 91%, eosinophilic 6%, GCM 1%) | None | Viral PCR in the myocardium for all cardiotropic viruses (results not reported) |
LVEF 32%; of whom 59% with LVEF<50% LV dim.: LVEDDi: 35 mm/m |
At 9 years: Estimated Survival free of HTx 64% Independent predictors: LVEF<50%, enlargement of the left atrium |
| Sanguineti et al.15 2015 | 2008–2011 | Prospective, Monocentric, France | 203 | 43y; 76% | Mean duration of symptoms 8 days | None | All (all with presence of LGE) | None | LVEF: 57% on CMRI LV dim.: LVEDVi: 73 ml/m2 |
Median follow up 19 months: Survival: 100%. |
| Inaba et al.41 2017 | 2007–2009 | Retrospective, multicenter, Japan Tokyo CCU Network: they compared fulminant (inhospital death or need for MCS, N=42) vs. non-fulminat myocarditis (survivors without MCS, N=96) Cases younger than 15 years were excluded | 138 | 42y; 57% | Not reported | In 21% (n=29) of cases EMB was performed. 21% lympho., 3% GCM, 76% non-specific findings | In 20% (n=28) of cases. LGE detected in 64% of them. | None | In FM: LVEF: 31% at echo LV dim.: LVEDD: 46 mm In NFM: LVEF: 49% at echo LV dim.: LVEDD: 49 mm |
In-hospital mortality 14% At multivariate analysis low systolic blood pressure and QRS>120 ms were associated with death or need for MCS |
| Ammirati et al.42 2017 | 2001–2016 | Retrospective, Multicenter (N=2), Italy (only fulminant myocarditis) | 55 | 33y; 49% | Symptoms duration<30 days | In 71% of cases. Based on EMB, autopsy or explanted heart (positive active and borderline Dallas criteria). All histologies | In 45% of cases | None | LVEF: 22% at echo LV dim.: LVEDD: 48 mm |
At 9 years: Estimated survival free of HTx 65% |
| Ammirati et al.42 2017 | 2001–2016 | Retrospective, Multicenter (N=2), Italy (only acute nonfulminant myocarditis) | 132 | 33y; 88% | Symptoms duration<30 days | In 8% of cases. Based on EMB (positive active and borderline Dallas criteria) All histologies | In 94% of cases | None | LVEF: 55% at echo LV dim.: LVEDD: 49 mm |
At 9 years: Estimated survival free of HTx 100% |
| Grani et al.14 2017 | 2002–2015 | Not specified if prospective, monocentric, US (not ALL admitted as inpatients; only 38% cases were admitted) | 670 | 48y; 59% | 52% with symptoms onset <2 weeks; 48% with symptoms onset above 2 weeks (not specified the median duration) | None | All | None | LVEF: 50% on CMRI, of whom 30% with LVEF<40% LV dim.: LVEDVi: 98 ml/m2 LVEDV 189 mL |
Median follow up 4.7 years: Survival: 95.7%. 0.3% lost at follow up. Main predictors: age, presence of LGE, LVEF<40%, reduced RVEF |
| Aquaro et al.11 2017 | 2006– 2013 | Not specified if prospective, multicenter (N=10), Italy | 374 | 35y; 77% | Symptoms duration not specified | 18 EMB (5%). Results not reported | All (presence of 2 Lake Louise criteria was used) | None | All with preserved LVEF 61% on CMRI LV dim.: LVEDVi: 83 ml/m2 |
Median follow up 4.3 years: Survival: 1.1%. Independent predictor: Presence of antero-septal LGE |
| Ammirati et al.10 2018 | 2001–2017 | Retrospective, Multicenter (N=19), Italy Per protocol all patients hospitalized |
443 | 34 y; 81% | Symptoms duration<30 days | In 14% cases. Based on EMB, autopsy or explanted heart (positive active and borderline Dallas criteria). All histologies | In 94% of cases (all with presence of 2 Lake Louise criteria) | Occasionally. Not reported | LVEF: 55% at echo LV dim.: LVEDD: 49 mm |
At 5 years: Estimated survival free of HTx 96% Markers of unfavorable prognosis: presence of LVEF<50%, SVT or low cardiac output syndrome at presentation Lost at follow up: 1% |
| Imazio et al.43 2018 | 2010–2016 | Retrospective, monocentric, Italy | 71 | 47 y, 75% | Symptoms duration<30 days (median time from symptoms to CMRI 11 days) | None | All (according with Lake Louise criteria was used) | None | Mean LVEF: 52% on CMRI Not reported mean LV dim. | At a mean follow up of 5 years: Estimated survival free of HTx 100% |
| Berg et al.44 2019 | 2010–2017 | Retrospective, monocentric, Switzerland | 45 | 34 y, 87% | Symptoms duration <11 days | None | All (according with Lake Louise criteria was used) | None | Mean LVEF: 56% on CMRI | At a 1 year: Survival free of HTx 100% |
| Ammirati et al.26 2019 | 2001–2018 | Retrospective, Multicenter (N=16), USA, Europe, Japan Per protocol all patients hospitalized with LVEF<50% |
220 | 42 y, 54% | Symptoms duration<30 days | All based on EMB, autopsy or explanted heart (positive active and borderline Dallas criteria). All histologies (lympho. 73%, GCM 14%, eosinophilic 11%, sarcoidosis 2%) | Not reported | Viral PCR search in the myocardium in 29% (n=63) of cases (FM: 20%; NFM 55%) Positive in 19% (n=12) of cases. PVB19 (n=8; 67%) was the most frequent reported. Other viruses: 2 EBV, 1 HHV6, 1 unspecified. |
In FM: LVEF: 22% at echo LV dim.: LVEDD: 49 mm In NFM: LVEF: 33% at echo LV dim.: LVEDD: 56 mm |
At 60 days: Estimated survival free of HTx in FM: 72%; in NFM: 98% At 7 years: Estimated survival free of HTx in FM: 52%; in NFM: 90% Markers of unfavorable prognosis at muntivariate analysis: - fulminant presentation, - GCM on histology, - QRS>120 ms on ECG Lost at follow up: 1.8% were lost after discharge |
| Ammirati et al.26 2019 | 2001–2018 | Subanalysis of the Retrospective, Multicenter (N=16), USA, Europe, Japan Per protocol all lymphocytic myocarditis, LVEF<50%, older that 16 years | 146 | 40 y, 52% | Symptoms duration<30 days | All based on EMB, autopsy or explanted heart (positive active and borderline Dallas criteria). All lympho. histologies (lympho.) | Not reported | Viral PCR search in the myocardium in 36% (n=52). Positive in 15% (n=8) of cases. PVB19 (n=6; 75%) was the most frequent reported. Other viruses: 1 HHV6, and 1 EBV. |
In FM: LVEF: 21% at echo LV dim.: LVEDD: 49 mm In NFM: LVEF: 30% at echo LV dim.: LVEDD: 56 mm |
At 60 days: Estimated survival free of HTx in FM: 80%; in NFM: 100% At 7 years: Estimated survival free of HTx in FM: 59%; in NFM: 97% Markers of unfavorable prognosis at muntivariate analysis: - fulminant presentation |
| White et al.13 2019 | 2009–2014 | Retrospective, monocentric, Canada | 100 | 40y, 82% | Symptoms duration<10 days | None | All. LGE detected in 72% of patients. | None | Mean LVEF: 57% on CMRI LV dim.: LVEDVi: 84 ml/m2 |
At a 1 year: Survival free of HTx 100% |
| Younis et al.12 2019 | 2005–2017 | Retrospective, monocentric, Israel | 322 | 37y, 84% | Symptoms duration<30 days | In 3% cases (Lympho. 25%, GCM 13%, eosinoph. 6%, borderline/ negative 56%) | In 73% of cases (83% with LGE, not specified presence of edema) | None | Mean LVEF: 58% on CMRI LV dim.: LVEDD: 50 mm |
In-hospital mortality: 0 |
N indicates numbers; CMRI cardiac magnetic resonance imaging, LVEF, left ventricular ejection fraction; LV vol, left ventricular dim., left ventricular dimension; EMB, endomyocardial biopsy; lympho., lymphocytic histology; EDD, end-diastolic diameter; HTx, heart transplant; eosinoph, eosinophilic histology; GCM, giant cell myocarditis, PCR, polymerase chain reaction; PCWP, pulmonary capillary wedge pressure; LVEDVi, indexed-left ventricular end-diastolic volume; RV, right ventricular; PVB19, parvovirus B19; HHV6, human herpes virus-6; EBV, Ebstein virus; LVEDDi: indexed-left ventricular end-diastolic diameter; LGE, late gadolinium enhancement; SVT, sustained ventricular tachycardia; MCS, mechanical circulatory support.
This review will try to summarize a shared and practical approach to patients presenting with AM or chronic infl-CMP, meanwhile pointing out the areas of controversies or uncertainties, and the unmet clinical needs. Specific conditions such as pediatric myocarditis, including rheumatic carditis, Chagas disease, and HIV cardiomyopathy deserve separate discussion, and are not addressed in this document.
DIAGNOSTIC APPROACH TO AM AND INFL-CMP
AM - Symptoms and signs
Patients with suspected AM are generally evaluated in the emergency room due to chest pain, dyspnea, fatigue, palpitations, or syncope.1 Based on large registries, chest pain is the most frequent symptom (85–95% of cases),10–13, 15 followed by dyspnea (19–49% of cases),10, 13, 14 whereas syncope occurs in about 6%.10 Fever is common (about 65%),10, 12 while other prodromal manifestations, such as flu-like symptoms, gastrointestinal disorders, sore throat, or respiratory tract infections may have preceded the acute phase by a few days or weeks, with a prevalence ranging from 18 to 80%.10, 11, 15
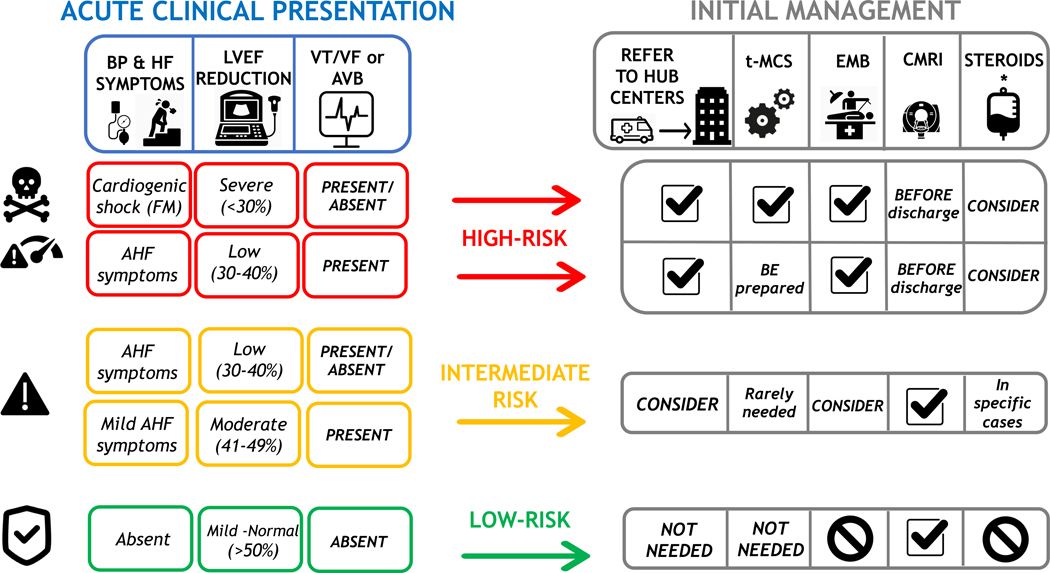
In a recent retrospective registry of 443 AM, 26.6% had a presentation complicated by left ventricular systolic dysfunction, ventricular arrhythmias or cardiogenic shock (i.e. fulminant myocarditis [FM] that accounted for 8.6% of total cases). On the other hand, the majority of AM (73.4%) had no such complications (uncomplicated AM), and presented chest pain in 97% of cases, and ST-elevation on ECG in 62.3% of cases and they had no deaths or heart transplantation (HTx) at 5 years.10 When collecting patient history, attention should focus on specific causes including recent exposure to drugs (e.g. antibiotics, clozapine, ICI) or toxic substances (e.g. cocaine or amphetamine),2 or to infectious agents (e.g. ingestion of raw meat suggesting a helminthic infections,25 travels to areas where viruses associated with AM, such as Dengue, are endemic). A proposed approach to AM is summarized in Figure 3.
FIGURE 3. Proposed risk-based approach to acute myocarditis.
In the left panel, clinical features that characterize high (red boxes), intermediate (orange box), or low (green boxes) risk are summarized, according to the presence of low blood pressure (BP) and severity of acute heart failure (AHF), initial left ventricular ejection fraction (LVEF) on first echocardiogram, and ECG (presence of ventricular tachycardia or ventricular fibrillation [VT/VF] or advanced atrioventricular block [AVB]). The right panel indicates how these risk features may influence patient management in terms of referral to expert centers, temporary mechanical circulatory support (t-MCS), need for endomyocardial biopsy (EMB) and/or cardiac magnetic resonance imaging (CMRI), and consideration for steroid treatment. Symbols and abbreviations: Tag sign indicates recommended actions. No symbol indicates not recommended. (*) immunosuppression with intravenous steroids may be considered and quite often used in patients with fulminant myocarditis (FM), however clinical studies that demonstrate their efficacy are lacking. HF indicates heart failure.
AM - Electrocardiogram (ECG)
The ECG is abnormal in about 85% of cases,10, 12 ST-segment elevation mimicking acute myocardial infarctions is the most frequent abnormality;10, 12 inferior and lateral leads are commonly involved. QRS width greater than 120 milliseconds, atrioventricular (AV) block, symptomatic bradycardia, or tachycardia and ventricular arrhythmias should increase the suspicion of AM and suggest high-risk forms.26 Second or 3rd degree AV block is rarely observed in patients with normal LV ejection fraction (EF) >50%, except in cardiac sarcoidosis (CS), Lyme carditis, as well as ICI-associated myocarditis.27
AM - Laboratory tests
Recommended laboratory test for identification of patients with suspected AM are myocardial necrosis biomarkers (high sensitivity [HS] troponins, creatinine kinase-MB). Trends in troponin levels after presentation may suggest a waxing and waning clinical course, but only a weak correlation exists between troponin release and the severity of cardiac dysfunction.28 Other laboratory tests routinely requested include markers of inflammation such as C-reactive protein that is positive in 80 to 95% in recent series.10, 11 Erythrocyte sedimentation rate (ESR) is also commonly increased, but it is generally not available in the emergency department. A persistently increased ESR can suggest an associated autoimmune disorder. Furthermore, differential white blood count can show eosinophilia, suggesting the presence of eosinophilic myocarditis (EM).25 Finally, peripheral blood serologic and virologic tests are rarely informative,2 with some exceptions (e.g. HIV and Borrelia burgdorferi antibodies). A search for viral genomes with polymerase chain reaction (PCR) in aerial tract fluids and pharyngeal swabs can identify viruses of the respiratory tract, such as influenza, and severe acute respiratory syndrome (SARS) coronavirus (CoV)-2, that can trigger an AM.29, 30 Autoantibodies (e.g. antinuclear antibody test) and other tests may be indicated in patients with known or possible history of autoimmune disorders.2
AM - Echocardiography
Echocardiography is part of the standard evaluation of patients with a suspected acute cardiac condition and may show a broad spectrum of findings. Even when LVEF is normal, the presence of increased wall thickness, mild segmental hypokinesia, in particular in the inferior and inferolateral walls, diastolic dysfunction, abnormal tissue Doppler imaging, mild right ventricular (RV) dysfunction, pericardial effusion, and abnormal myocardial echogenicity may suggest AM. In the early phase, LV dimensions are generally normal even when LVEF is low or very low,26 a condition that may result in severe stroke volume reduction and tachycardia. LVEF on admission is a powerful prognostic marker.10, 12, 26 Furthermore, cardiac function may evolve rapidly during AM, either spontaneously or after treatment.10, 12
Suggested indications for cardiac magnetic resonance imaging (CMRI) in AM and chronic infl-CMP
CMRI has emerged as a powerful noninvasive diagnostic tool for tissue characterization, including recognition and quantification of inflammation and replacement fibrosis in the setting of AM, and infl-CMP.31, 32 Furthermore, CMRI is the gold-standard for the quantification of bi-ventricular volumes, EF and cardiac mass (Supplemental Table II).31 CMRI is recommended in patients with clinically suspected AM or in patients with chest pain, normal coronaries, and raised troponin, for the differential diagnosis of ischemic vs. non ischemic origin,33 with the exception of those in critical condition or with usual contraindication for this diagnostic tool.2, 31 CMRI should be performed in patients that initially presented with fulminant forms to assess the presence, extent and localization of residual inflammation and replacement fibrosis when they are hemodynamically stable (Table 3). Unless recurrent flares occur, edema tends to decline 4 weeks after disease onset.34 Therefore, to rule in or rule out myocardial inflammation reliably, CMRI should be performed within 2–3 weeks from the onset of symptoms, although accuracy may be lower during the very first days. The availability of HS troponins and CMRI have improved the accuracy of non-invasive diagnosis of AM,31 which resulted in the identification of more low-risk patients than before, when diagnosis was mainly based on endomyocardial biopsy (EMB), that was performed more often in sicker patients. Thus, observational studies reported a more favorable prognosis of AM over the last decades (Table 2).10–17, 26, 35–44 In fact, in 5 out of 6 studies with EBM-based diagnosis, mean echocardiographic LVEF was <40%.17, 35, 36, 38–40 In 2009, a consensus group published the original Lake Louise Criteria, that identified three hallmarks of myocardial inflammation with corresponding CMRI markers:32 1) hyperemia, i.e. intense signal in early gadolinium enhancement images, 2) tissue edema, i.e. increased myocardial T2 relaxation time or an increased signal intensity in T2-weighted images; and 3) necrosis /fibrosis based on late gadolinium enhancement (LGE) images. If two out of these three criteria are positive, AM can be diagnosed with 74% sensitivity and 86% specificity.45 With mounting evidence that CMRI mapping increases the overall diagnostic accuracy, the Lake Louise Criteria have been recently updated (Supplemental Figure II).31 The updated criteria include T2 mapping for edema and native T1 as well as extracellular volume for inflammatory injury.31 A study has confirmed an increased sensitivity of the updated criteria (87.5%) while keeping a high specificity in AM (96.2%).46 A single positive criterion can support diagnosis of myocardial inflammation if clinical suspicion is strong.31 CMRI cannot identify specific cause of myocardial inflammation, and the histological subtypes, although regional distribution of inflammatory changes in the tissue provide diagnostic clues (e.g. basal septal involvement in CS). In patients with de novo DCM or unexplained ventricular arrhythmias, CMRI can suggest previous myocardial inflammation based on the regional distribution of LGE, however, its sensitivity is not high in chronic forms.47 The presence, and location in the mid layer of the septum (mid-wall strip) of LGE and low LVEF at baseline appear to be the strongest negative predictors of outcome.14, 48 CMRI is useful also in the follow-up of AM and is generally performed 6 to 12 months after the index event (Table 2). Disappearance of edema is frequent at follow-up (up to 84% of cases), while LGE generally persists (in up to 89%), although its extent is reduced from 6.2 to 4.1% of LV mass after 6 months in the ITAMY registry including 187 cases.49 This finding is in keeping with other studies that analyzed the changes in LGE and edema at follow-up.13, 50 The extent of LGE is a dynamic process in AM, mainly related to tissue edema in the acute phase that progressively vanishes over time, while in the late phase LGE mainly reflects post-inflammatory replacement fibrosis.50 Persistence of LGE and disappearance of edema are markers of unfavorable prognosis compared with complete resolution or persistence of both LGE and edema.49 A potential explanation is that persistent edema can suggest a still active process with some residual chance of recovery,49 further stressing the role of CMRI also in monitoring patients with AM and infl-CMP over time.
TABLE 3.
Comparison of suggested indications for endomyocardial biopsy, viral search and cardiac magnetic resonance imaging among previous US and European scientific statements and current document.
| Document | AHA/ACC/ESC Scientific Statement (Cooper et al62) | ESC Position Statement (Caforio et al2) | AHA Scientific Statement (Bozkurt et al65) | AHA Scientific Statement (Kociol et al60) | Expert consensus document (Ammirati et al) |
|---|---|---|---|---|---|
| Year | 2007 | 2013 | 2016 | 2020 | 2020 |
| Specific disease | Role of EMB in the management of cardiovascular disease | Myocarditis | Specific dilated cardiomyopahy | Fulminant myocarditis (FM) | Acute myocarditis (AM) and chronic inflammatory cardiomyopathy (inf-CMP) |
| Standard diagnostic tools | ECG, echocardiogram, measurements of blood markers of myocardial necrosis and inflammation (e.g. CRP, WBC), and invasive or CT coronary angiography | ||||
| Suggested Indication for EMB | Specific indications in case of: - New-onset HF of 2 weeks’ duration associated with a normal-sized or dilated LV and hemodynamic compromise (recommendation I, level of evidence B) - New-onset HF of 2 weeks’ to 3 months’ duration associated with a dilated LV and new ventricular arrhythmias, second- or third-degree AVB, or failure to respond to usual care within 1 to 2 weeks (I, B) - HF of >3 months’ duration associated with a dilated LV and new ventricular arrhythmias, second- or third-degree AVB, or failure to respond to usual care within 1 to 2 weeks (IIa, C) - HF associated with a DCM of any duration associated with suspected allergic reaction and/or eosinophilia (IIa, C) ____ New-onset HF of 2 weeks’ to 3 months’ duration associated with a dilated LV, without new ventricular arrhythmias or II- or III-degree AVB, that responds to usual care within 1 to 2 weeks (IIb, B - The writing group did not recommend performing EMB for the routine evaluation of this clinical scenario) HF of > 3 months′ duration associated with a dilated LV, without new ventricular arrhythmias or II- or III-degree AVB, that responds to usual care within 1 to 2 weeks (IIb, C- The writing group recognized that divergent evidence existed with regards to the utility of EMB in this clinical scenario) |
-All patients with clinically suspected myocarditis should be considered for EMB and coronary angiography -EMB may be repeated if necessary to monitor response to etiology-directed therapy, or if a sampling error is suspected in a patient with unexplained progression of HF. -Follow-up EMB may be required to guide the intensity and the length of immunosuppression |
-Myocarditis: - Unexplained acute cardiomyopathy requiring inotropic or MCS - Unexplained acute cardiomyopathy with Mobitz type 2 second degree or higher AVB - Unexplained acute cardiomyopathy With sustained or symptomatic ventricular tachycardia - Unexplained acute cardiomyopathy with failure to respond to guideline based medical management within 1–2 weeks (for all recommendation I, level of evidence B) - Hypereosinophilic syndrome (presence of eosinophils >1500/uL for a 6-month duration) If suspected eosinophilic myocarditis is suspected, EMB is reasonable (level of evidence C) -Autoimmune cardiomyopathy EMB can be useful to confirm hydroxychloroquine-mediated HF (level of evidence C) Routine use of EMB is not recommended in patients with cardiomyopathy caused by suspected autoimmune, rheumatologic, or collagen vascular disease (level of evidence C) -Cardiac sarcoidosis EMB can be useful to confirm cardiac sarcoidosis when pathology yields evidence of non-ca seating granulomas, but absence does not rule out the possibility of cardiac sarcoidosis (level of evidence C) |
Unexplained acute cardiomyopathy: -Same indications as reported in the 2016 AHA Scientific Statement by Bozkurt et al.65) |
-Specific indications In case of: -AM presenting with cardiogenic shock (i.e. FM) /acute HF, ventricular arrhythmias or high-degree AVB, especially in case of non-/mildly dilated LV and recent onset of symptoms -Myocarditis in the setting of ICI where appropriate diagnosis has implications for patient receiving additional cancer therapy and accuracy of CMR for diagnosis is not known -AM or chronic inf-CMP associated with peripheral eosinophilia -AM or DCM suspected for chronic INFL-CMP with persistent/relapsing release of myocardial necrosis markers; especially if associate to a suspected/known autoimmune disorders or ventricular arrhythmias or II-III-degree AVB for therapeutic implications |
| RELEVANT POINTS for EMB | TIMING OF ONSET (in most of cases < 3 months from symptoms’ onset) LV DIMENSION: non-dilation together with hemodynamic compromise and recent symptoms′ onset are recognized strong indications for EMB PRESENCE OF VENTRICULAR ARRHYTHMIAS AND/OR II-III-degree AVB are modifiers of the indication for EMB, leading to include also patients with subacute presentation and LV dilation EOSINOPHILIA as marker of specific form of myocarditis that can be specifically treated |
-EXTENSIVE INDICATION FOR EMB independently of points that AHA’s scientific statements recognized as clues that increase the likelihood of a diagnostic EMB or when EMB can change management | NEEDS OF INOTROPES or MCS in the setting of acute unexplained setting -ACUTE SETTING of unexplained cardiomyopathy presenting with ventricular arrhythmias and/or II-III-degree AVB -CONFIRMATION OF THE INDICATION IN CASE OF EOSINOPHILIA compared with the 2007 AHA scientific statement |
-CONFIRMATION OF THE INDICATIONS REPORTED in 2016 AHA Scientific Statement | -DIFFERENTATION OF INDICATIONS in AM (generally with normal/mildly increased LV and symptoms’ duration <1 month) from new-onset unexplained DCM suspected for chronic INFL-CMP (dilated LV with symptoms’ duration >1 month) even in the acute setting of presentation -In the setting of AM indications IN CASE OF COMPLICATED PRESENTATION by acute HF (or FM), or in presence of ventricular arrhythmias or II—III-degree AVB, while uncomplicated cases have no indication due to observed good prognosis -RELEVANCE OF PERSISTENT TROPONIN RELEASE especially in case of chronic INFL-CMP -RECOGNITION OF NEW AM/chronic INFL-CMP RELATED TO ICI: relevance to perform an accurate diagnosis due to clinical implication of withdrawing life-saving treatments -RECOGNITION OF REACHING ACCURATE DIAGNOSIS IN AUTOIMMUNE-related AM/DCM suspected for chronic INFL-CMP: as the inflammatory involvement of the heart has a prognostic impact on several autoimmune disorders (including inflammatory disorders like sarcoidosis) that can lead to changes in the treatments. CONFIRMATION OF THE AHA SCIENTIFIC STATEMENTS REGARDING indications in case of VENTRICUALR ARRHYTHMIAS/II-III DEGREE AVB and EOSINOPHILIA |
| Suggested Indication viral search in the myocardium | Because of uncertainties in the methods (for instance sampling errors and false negative results), and interpretation at centers not experience in these techniques, the consensus is that routine testing for viral genomes in EMB specimen is not recommended at this time outside of centers with extensive experience. | -Suggested in all cases to differentiate virus-positive (infective) from virus-negative myocarditis (on heart tissue and a blood sample) | -Myocarditis: The role of viral genome analysis of EMB tissue to guide management remains uncertain. | -Further precision may be achieved by the use of viral genome analysis when diagnostic uncertainty exists despite histology |
-We report different opinions among the experts of this document. Specifically, German Authors recommends viral search in all cases. For other Italian and US Authors there is not enough evidence to routinely perform viral search as no clear therapeutic or prognostic benefit is demonstrated especially in the setting of AM. Presence of enteroviruses has a prognostic implication in particular in newborn and infants. They generally have poor prognosis and it is believed that immunosuppression can be harmful based on animal studies. Presence of enteroviruses is currently considered rare in particular in adults. Presence of PVB19, that is the most common virus found in the myocardium has no clear utility to further guide treatment thus some experts do not suggest systematic search for viruses. In immunosuppressed (i.e. HIV patients) subjects search for CMV could be relevant, in particular if suggestive signs of cytolysis are found on H&E. |
| Suggested indication for CMRI | Not reported | -CMRI may be considered in clinically stable patients prior to EMB -CMRI does not replace EMB in diagnosis of myocarditis and should not delay EMB in life-threatening presentations |
-Myocarditis: CMRI is reasonable for the diagnosis of myocarditis in clinically stable patients with clinically suspected myocarditis (recommendation II, level of evidence C) -Autoimmune cardiomyopathy CMRI or FDG-PET imaging can be useful to identify patients at risk for HF and to identify the degree of fibrosis (level of evidence B) -Cardiac sarcoidosis: CMRI or FDG-PET imaging can be useful to diagnose cardiac sarcoidosis or follow response to therapy (level of evidence B) |
-Myocarditis: CMRI is reasonable for the diagnosis of myocarditis in clinically stable patients with clinically suspected myocarditis, thus it is rarely indicated in the early diagnosis of FM (recommendation II, level of evidence C) |
-CMRI is the preferred diagnostic tool for AM without complications on presentation (preserved/mildly reduce LVEF and no ventricular arrhythmias) -CMRI should be avoided in hemodinamically unstable patients (i.e. FM), and EMB must be performed in particular to rule out GCM or other specific histology -CMRI should be performed even in FM when they are hemodynamically stable to assess the presence/extent and localization of LGE -It is recognized that CMRI’s diagnostic accuracy for detecting chronic INFL-CMP is reduced in case of ventricular arrhythmias or frequent PVCs -CMRI is suggested at 3–6 months of follow up to demonstrate resolution of edema (to modulate immunosuppression if any, or to resume intense physical activities), and define the final LGE extent |
| Suggested indication for immune-suppression | Not reported | -Immunosuppression should be started only after ruling out active infection on EMB by PCR -The rational for routinely use of immunosuppression in virus-negative myocarditis is that the ESC task group considers “infection-negative” (negative viral search in the myocardium) as an autoimmune form of myocarditis. - Steroid therapy is indicated in cardiac sarcoidosis in the presence of ventricular dysfunction and/or arrhythmia -Steroid therapy is indicated in some forms of infection-negative eosinophilic or toxic myocarditis with HF and/or arrhythmia |
-Myocarditis: Do not generally recommend empirical, upfront, immunomodulatory agents before diagnosis for myocarditis -Autoimmune cardiomyopathy IV steroids, systemic immunosuppressants, or immunomodulatory agents can be useful for biopsy-proven myocarditis believed to be caused by SLE, RA, and PAN -Cardiac sarcoidosis: -Corticosteroids are recommended to treat patients with cardiac sarcoidosis (level of evidence B) - Other immunosuppressive therapies (eg, MTX, AZA, MMF, cyclophosphamide, pentoxifylline, and thalidomide) are reasonable in patients who cannot tolerate corticosteroids and in patients who continue to worsen clinically despite treatment with corticosteroids (level of evidence C) - In collaboration with a pulmonologist or rheumatologist, immune-modulating therapy can be useful to treat sarcoidosis (level of evidence C) |
-Myocarditis: Compared with 2016 AHA Scientific statement, this document introduces this new concept: “If a high suspicion for immune-mediated FM exists (e.g. GCM), 1 g solumedrol is often administered urgently, before biopsy-confirmed diagnosis or further diagnostic testing. Steroids will not obscure the results of the biopsy if given before this diagnostic test. If the diagnosis is GCM, other immunosuppressing agents will need to be added to obtain effective treatment.” |
-AM: In case of FM or complicated AM by acute HF or ventricular arrhythmias or high-degree AVB, use of empirical IV corticosteroids can be considered. Maintenance of immunosuppression is based on the results of EMB. -Specifically, maintenance of immunosuppression is useful in case of eosinophilic myocarditis, GCM or sarcoidotic myocarditis or in case of demonstrated new diagnosis of a systemic autoimmune disorder. -Viral search in the myocardium can identify patients in whom to withdraw immunosuppression in case of positive results, especially for enterovirus, CMV and adenovirus. -Maintenance of immunosuppression in case of positive PVB19 and HHV6 can depend on (1) observed initial response to immunosuppression (significant reduction of troponin or recovery of LVEF); or (2) low viral load. In all cases of ICI-associated AM immunosuppression is suggested, with IV corticosteroids as first line of therapy. -Chronic infl-CMP Immunosuppression can be started in case of eosinophilic myocarditis, GCM or cardiac sarcoidosis or in case of associated systemic autoimmune disorder. In isolated lymphocytic forms search for viral genomes is suggested to exclude presence of enterovirus, CMV or adenovirus that can contraindicate immunosuppression Immunosuppression can be considered in lymphocytic forms without evidence of viral genomes, or in patients with PVB19. Clear demonstration of a survival benefit is lacking, while some studies suggest LVEF improvement. Rational to wait for viral genomes in chronic infl-CMP is that delays in the initiation of therapy is not expected to affect the prognosis as it could happen in FM. |
ACC indicates American College of Cardiology; AHA, American Heart association; AVB, atrioventricular block; AZA, azathioprine; CMP, cardiomyopathy; CMV, cytomegalovirus; CRP, C-reactive protein; CT, computed tomography; DCM, dilated cardiomyopathy; ECG, electrocardiogram; EMB, endomyocardial biopsy; ESC, European Society of Cardiology; FDG-PET, fluorodeoxyglucose position emission tomography; GCM, giant cell myocarditis; H&E, hematoxylin and eosin; HHV6, human herpes virus-6; HIV, human immunodeficiency virus; ICI, immune checkpoint inhibitors; HF; heart failure; IV, intravenous; LGE, late gadolinium enhancement; LV, left ventricle; LVEF, left ventricular ejection fraction; MCS, mechanical circulatory supports, MTX, methotrexate; MMF, mycophenolate mofetil; CMRI, cardiac magnetic resonance imaging; PAN, Polyarteritis Nodosa; PCR, polymerase chain reaction; PVB19, parvovirus B19; PVCs, premature ventricular contractions; RA, rheumatoid arthritis; SLE, Systemic Lupus Erythematosus; WBC, whole blood count
Suggested indications for positron emission tomography (PET) in AM and chronic infl-CMP
Although PET is not usually employed in the setting of AM or chronic infl-CMP, it can be considered as an alternative noninvasive diagnostic tool in stable patients with contraindication to CMRI, or in patients with suspected systemic autoimmune disease where other organs could be involved by the inflammatory process.51 PET is especially useful for the diagnosis and monitoring of CS.52, 53 T cells, macrophages or granulocytes that infiltrate the myocardium, either as a nonspecific response to cell injury or as primary lesion in CS, are characterized by an enhanced glucose metabolism that can be detected by the focal uptake of the glucose analogue 18F-fluorodeoxyglucose. PET can reveal hypermetabolic mediastinal and hilar lymph nodes differentiating CS from other autoimmune disease with cardiac involvement (e.g. vasculitis). This technique provides a tool to monitor the progression of damage and its regression in response to immunosuppressive therapy.52 Recent development of additional “immuno-PET” tracers in oncology has dramatically expanded the usefulness of PET imaging to detect endogenous immune cells and may provide novel diagnostic and prognostication strategies in the myocarditis patients.
Suggested indication for EMB in AM and chronic infl-CMP
EMB is considered the reference standard for the diagnosis of myocarditis,2, 54 however it is an invasive procedure that portends some risks. Cardiac complications have been reported in 1–2% of the patients at expert centers but in up to 8.9% at low-volume centers.55, 56 The sensitivity of EMB is relatively low when evaluated with standard hematoxylin–eosin staining,57 since sampling sites do not always correspond to the distribution of inflammation. Sensitivity may be increased by increasing the number of collected specimens over the minimum recommended number (from 4 to 6 specimens).2 Immunohistochemistry-specific antibodies for leukocytes (CD45), macrophages (CD68), T cells (CD3) and their main subtypes, helper (CD4) and cytotoxic (CD8), and B cells (CD19/CD20) can also increase the sensitivity of EMB.2 Quantitative criteria to improve the diagnostic yield of EMB in myocarditis include the Marburg’s criteria, based on the presence of >14 mononuclear leukocytes/mm2 on bioptic samples,58 with the presence of >7 T-lymphocytes per mm2.59 These criteria were adopted in a position statement by the European Society of Cardiology (ESC) experts.2 Despite relatively low sensitivity, the information derived from EMB is fundamental for identifying the mechanisms and deciding therapy in specific clinical scenarios both in AM and chronic infl-CMP (Table 3):
AM presenting with severe heart failure (HF) or cardiogenic shock (i.e. FM);60
AM complicated by severe myocardial dysfunction, acute HF, ventricular arrhythmias or high-degree AV block;
AM or suspected chronic infl-CMP associated with peripheral eosinophilia;
AM or chronic infl-CMP with persistent or relapsing release of biomarkers of myocardial necrosis, particularly if associated to a suspected/known autoimmune disorder or ventricular arrhythmias or high-degree AV block;
Myocarditis in the setting of ICI, where appropriate diagnosis has implications for patient receiving additional cancer therapy.61
Most of these recommendations were first released in the 2007 American Heart Association (AHA)/American College of Cardiology (ACC)/ESC scientific statement on the role of EMB in the management of cardiovascular disease,62 which were validated by a retrospective analysis on 851 patients with unexplained HF that underwent EMB.55 The diagnostic yield of EMB for myocarditis in the setting of AM is considered to be higher if performed within 2 weeks since symptoms’ onset and in case of normal sized or mildly dilated LV or in presence of markers (ventricular arrhythmias or high-degree AV blocks) of specific subsets such as giant cell myocarditis (GCM) or CS. AM is often a self-limiting disease and can be managed non-invasively in low-risk patients.56 However, at present, EMB in largely underutilized also in the recommended settings, as shown by some reports on the use of temporary mechanical circulatory supports (MCS) in FM.63 Thus far, a relationship between the extent of inflammatory infiltrates with prognosis, and its therapeutic implications have not been consistently found across different settings of inflammatory cardiac disorders. In a retrospective study, patients with acute lymphocytic myocarditis who received a MCS or died during hospitalization, had more inflammatory infiltrates compared with patients who survived without MCS.42
Differential diagnosis
Invasive coronary arteriography or computed tomography (CT) angiography are often necessary to rule out an acute coronary syndrome.2 Furthermore, patients with AM and acute pericarditis can complain of similar symptoms. Elevation of HS-troponin can steer the diagnosis towards AM. AM can be also associated with signs of pericarditis (i.e., pericardial effusion on echocardiogram or CMRI; evidence of inflammation of pericardial layers on CMRI). FM should be differentiated from other conditions that may cause hypotension,60 acute myocardial dysfunction and cardiac shock (such as septic shock, Shoshin beriberi syndrome, systemic capillary leak syndrome and pheochromocytoma).
Chronic Inflammatory cardiomyopathy
Chronic infl-CMP can be found in patients at their first evaluation for new-onset HF symptoms, or in patients with subacute/chronic HF and/or DCM or hypokinetic-non-dilated phenotype. Chronic infl-CMP may represent the evolution of one or more AM episodes that, either diagnosed or missed in the acute phase, caused myocardial damage and systolic dysfunction. A mild elevation of troponin out of proportion compared to LVEF impairment, associated with a dilated LV with normal or mildly increased wall thickness, can suggest a chronic infl-CMP over AM.64 Accordingly, CMRI and EMB may show less florid inflammation, and replacement fibrosis may prevail. Furthermore, cardiomyocytes with morphological abnormalities may be found at histology (Figure 1 and Supplemental Figure I). Due to progressive LV remodeling, patients presenting with an chronic infl-CMP have chronic HF symptoms (generally >1 month), but may be hemodynamically stable, and are treated as patients with DCM.65 Anamnestic clues, subtle electrocardiographic alterations (such as low voltage or fragmentation of QRS in peripheral leads, minor conduction disturbances, and non-specific ST-T abnormalities), low-grade, persistent elevation of troponin, and failure to respond to standard HF treatment should promote the search for an inflammatory cause.65 In chronic infl-CMP the presence of high number of T lymphocytes or macrophages on EMB has a unique value in predicting an increased risk of mortality or transplantation over the next decade.66 The extent of myocardial fibrosis should be reported, as it could be related to the likelihood of recovery. Finally, the additional analysis to search for cardiotropic viruses (e.g. RNA enteroviruses and DNA adenoviruses), bacteria and parasites, in biopsy specimens using quantitative real time PCR is recommended by ESC experts to guide immunosuppressive therapy in the setting of chronic infl-CMP.2, 67 Conversely, AHA experts do not recommend routine viral genome analysis outside of centers with experience,65 even if they consider it as additional option when diagnostic uncertainty exists.60
VIRUS-INDUCED AND IMMUNE-MEDIATED LYMPHOCYTIC AM AND INFL-CMP
Lymphocytic AM and chronic infl-CMP have been attributed to a variety of pathogens, mainly viruses (by direct virus-mediated or indirect immune-mediated myocardial injury), toxic effect of drugs or radiation, and autoimmune injury in the setting of systemic inflammatory disorders.
Virus-induced AM can refer to both virus-mediated myocarditis and virus-triggered myocarditis. Enteroviral (coxsackievirus) myocarditis are examples of virus-mediated AM, as viral replication can cause direct cardiomyocyte injury.68 The cases of enterovirus-mediated AM have been mainly reported in newborns and infants in recent years.69 Respiratory viruses, such as influenza and coronaviruses, are examples of common viruses that can trigger an immune-mediated lymphocytic myocarditis in the absence of viral genome in the myocardium.29, 70 In virus-triggered AM, molecular mimicry between viral and cardiac antigens, which can result in autoreactive T-cell infiltration in the myocardium in predisposed individuals, is suspected to be the underlying mechanism of myocardial injury.19 The resolution of the viral syndrome with the extinction of viral antigens, could explain the frequent self-resolving natural history of most AM. Of note, PVB19 appears to cause both virus-mediated and virus-triggered myocarditis. In children in particular, PVB19 may cause a systemic infection associated with AM where PVB19 can be detected both in plasma and myocardium. In adults PVB19 has been associated with both AM and chronic infl-CMP,71 and the viral genome has been detected with different titers in the myocardium of these subjects, while it is not generally detected in the bloodstream. PVB19 has been the only virus found in patients with lymphocytic FM in an international registry.22, 26 A preliminary report showing a benefit from immunosuppression in chronic infl-CMP with PVB19 presence in the myocardium seems to support the hypothesis that the immune response plays a role in the development of myocardial inflammation after viral infection.72 Alternatively, low copy number of PVB19 DNA may reflect latent infection, and should be interpreted as a bystander, since they can be found also in normal myocardium.73 These findings may suggest that immunosuppression is not contraindicated in all virus-positive myocarditis, but the involved virus (for example, it may be considered with PVB19 but not with coxsackievirus), the host (e.g. infants vs. adults or immunodeficient vs. immunocompetent individuals) and the setting (non-complicated vs. FM) should be considered in the decision to start immunosuppressive drugs. According to several researchers, high viral loads and replicating (versus non-replicating) viruses could stand against the use of immunosuppression, and possibly in favor of treatment with antiviral drugs or with agents that reinforce native immune response, e.g. interferon-beta,74 even if clinical data from trials are substantially lacking in the setting of AM. Similarly to PVB19, human herpes virus-6 has been occasionally found in the myocardium of patients with AM and chronic infl-CMP, but its pathogenetic role is unclear.16
Immune-mediated AM and chronic infl-CMP.
AM or chronic infl-CMP can be associated with systemic autoimmune disorders (e.g. systemic lupus erythematosus or dermatomyositis), or organ/system-specific autoimmune/inflammatory diseases (e.g. inflammatory bowel disorders). The immune system activation stimulated by an intercurrent infection could favor a flare of the underlying immune disorder involving the heart. The identification of the myocarditis-associated condition is relevant for the specific treatments, given that not infrequently AM is the first manifestation of a systemic inflammatory/autoimmune disease.25 The Lombardy registry of AM reported that 7.2% of patients had an associated autoimmune or systemic disorders, and this condition was more frequent in patients with complicated presentation (15.4%).10
ICI ASSOCIATED MYOCARDITIS
ICI have transformed cancer treatment, with regulatory approval in nearly 20 different cancer types. The percentage of patients with cancer that were eligible for ICI increased from 1.5% in 2011 to nearly 50% in 2020.75 These agents include monoclonal antibodies which block immune brakes or regulators, termed cytotoxic T lymphocyte antigen-4 (CTLA-4), programmed death receptor-1 (PD-1), and its ligand (PD-L1) that, when stimulated, can dampen the immune response to an immunologic stimulus. By blocking these checkpoints from binding with their partner proteins, ICI inhibit the “off” signal, activating T cells and promoting killing of cancer cells. By activating the immune system, ICI can also lead to immune mediated adverse events (such as colitis, dermatitis and pneumonitis).76
Etiology and Pathogenesis.
In 2016, Johnson et al.77 described two cases of fatal FM after treatment with ICI. These patients presented with refractory electrophysiological disturbances and concomitant myositis, with pathology indicating T-cell and macrophage-dependent myocardial infiltration. Other case series of ICI-associated AM reported an incidence between 1 and 2% when ICI are used in combination.18, 78 Pre-clinical data suggest a critical role for CTLA-4 and PD-1 in the cross-talk between the cardiovascular and immune systems. Inhibition of CTLA-4 and PD-1, either genetically or pharmacologically, were found to contrast ICI-associated myocarditis and other cardiovascular toxicities in mice.79
Diagnosis.
The largest case series of 122 patients with ICI-associated myocarditis had an early onset of symptoms (median of 30 days after initial exposure to ICI), and up to 50% died.27 The increasing number of reports in the past few years is consistent with the growing awareness of this new clinical syndrome, as well as with the more widespread use of ICI. Patients on combination ICI treatment (e.g., ipilimumab and nivolumab) should have an ECG and troponin assay at baseline. Once started on therapy, troponin should be checked weekly during the first six weeks. In addition, given concomitant myositis in a substantial number of cases of ICI-associated myocarditis, a defined work-up for myositis (including checking for CK and possibly skeletal muscle biopsy) is recommended in suspected cases.
Treatment.
High-dose intravenous corticosteroids associated with withdrawal of ICI are considered the first line therapy, although mortality remains high. Alemtuzumab (anti-CD52 antibody), anti-thymocyte globulin (anti-CD3 antibody), and abatacept (a CTLA-4 agonist) have been proposed as second-line therapy (Table 3). A better mechanistic understanding of ICI-associated cardiovascular toxicity by using pre-clinical models could help for defining preventive and treatment strategies in patients.
EOSINOPHILIC MYOCARDITIS
EM is relatively uncommon, but it is often unrecognized, thus its incidence may be underestimated. The rate of death or HTx in patients with EM and a fulminant presentation was over 26% at 60 days after admission.26
Etiology and Pathogenesis.
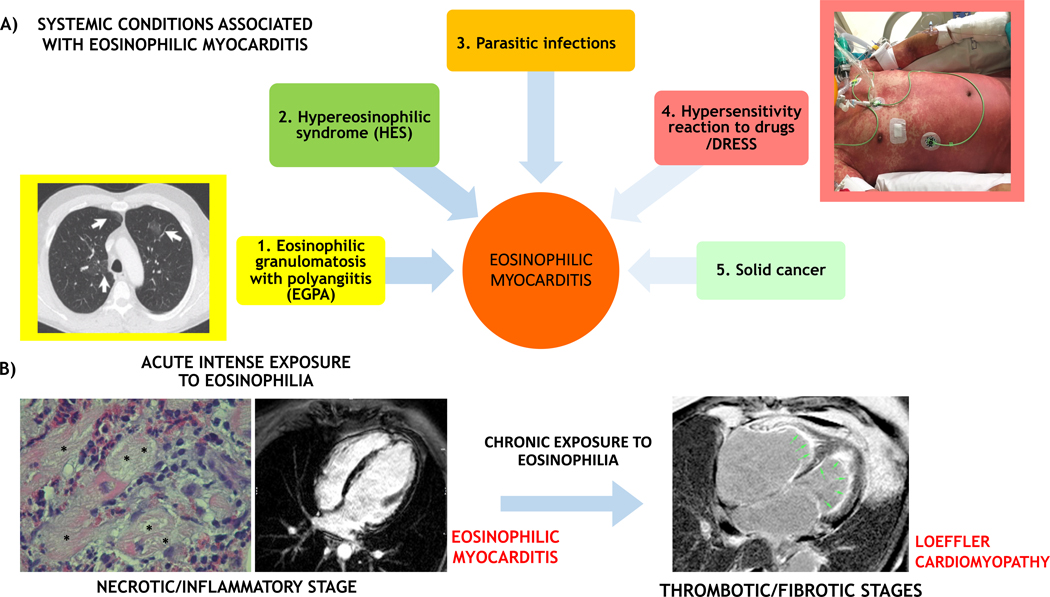
EM is generally associated with hypersensitivity reactions to chemicals (in particular, clozapine, carbamazepine, minocycline and β-lactam antibiotics, and occasionally vaccination), or with systemic conditions such as eosinophilic granulomatosis with polyangiitis (EGPA, former Churg Strauss syndrome), or hypereosinophilic syndrome (HES; idiopathic or clonal), or with a parasitic infection, mainly due to Toxocara canis transmitted by raw meat.25 In rare circumstances, EM can be associated with solid organ malignancy as a paraneoplastic event (i.e., lung cancer). A 3-phase process of the eosinophilic injury has been proposed: an initial inflammatory/necrotic phase (observed during AM), followed by thrombotic and fibrotic remodeling of the endo-myocardium (typical of Loeffler cardiomyopathy; Figure 4).
FIGURE 4. Eosinophilic myocardial injury: associated conditions and transition from acute myocarditis to inflammatory cardiomyopathy.
(A) Eosinophilic myocarditis can be idiopathic or associated with a systemic disorder. The associated conditions can be 1) eosinophilic granulomatosis with polyangiitis (EGPA), that is often associated with asthma, pulmonary non-fixed infiltrates (arrows on a chest computed tomography scan image in the yellow inset) and paranasal sinus abnormalities; 2) hypereosinophilic syndromes characterized by persistent peripheral eosinophilia (≥1.5×109/L for over 6 months), that can be a complex idiopathic form or a myeloproliferative variant like the clonal form associated with FIP1L1/PDGFRA mutation; 3) parasitic infections; 4) hypersensitivity reactions to drugs and drug reaction with eosinophilia and systemic symptoms (DRESS) that are generally characterized by fever and diffuse skin rush (like in the patient with DRESS showed in the rose inlet), with frequent delay onset after drug initiation (up to 2–6 weeks); and rarely 5) solid tumors. While in the acute phase the eosinophilic myocarditis is the main determinant of prognosis, the associated conditions can be the major determinants of prognosis in the mid and long-term. (B) An acute intense exposure to eosinophilia can cause an acute eosinophilic myocarditis (on the left), that in some case can be described as necrotizing due to extensive areas of the cardiomyocyte necrosis (*) caused by diffuse eosinophilic infiltrates on endomyocardial biopsy histology. The acute inflammatory phase can cause subendocardial and transmural injuries, identified by late gadolinium enhancement on cardiac magnetic resonance imaging (CMRI). If the eosinophilic exposure persists, eosinophilic injury evolves to a thrombotic and fibrotic stage, with diffuse subendocardial fibrosis with apical thrombi (green arrows), as identified by CMRI; the latter are characteristic features of Loeffler cardiomyopathy (on the right).
Diagnosis.
EM may affect middle-aged individuals, with similar prevalence in both sexes, presenting mainly with chest pain and dyspnea, with evidence of LV systolic dysfunction. The diversity of possible underlying causes may be responsible of the variety of clinical scenarios; specifically, fever and skin rash are more common in hypersensitivity-related EM, and asthma in EGPA-related EM.25 Eosinophilia can be evident in the course of the disease, but it is absent in about 25% of the patients at admission.80 Echocardiography and CMRI provide information on cardiac function and may detect intracardiac thrombosis (particularly at the LV apex), mostly described in HES-related EM (up to 29% of cases) and EGPA-related EM (up to 19% of cases).25 In contrast with the typical sub-epicardial LGE pattern observed in other forms of myocarditis, EM is generally associated with subendocardial LGE.25
Treatment.
A meta-analysis of 179 case series has shown a lower incidence of in-hospital mortality with the use of corticosteroids, although randomized trials is lacking.25 Identification and treatment of the underlying causes should be promptly considered. In particular, immediate withdrawal of the offending substance in combination with corticosteroid administration is recommended in hypersensitivity-related EM. Albendazole and corticosteroids should be given in EM associated with Toxocara canis infection,25 and imatinib is utilized in myeloproliferative variants of HES. Combined immunosuppressive therapy, including corticosteroids and cyclophosphamide, azathioprine or methotrexate, may be considered in EM associated with EGPA and HES.25 The rate of recurrence is not known, but fatal recurrences have been reported.25 Patients with HES and EGPA are at increased risk of late recurrence, in particular if immunosuppressive agents are withdrawn.
GCM
GCM is a form of rapidly progressing necrotizing myocarditis with a poor prognosis including an approximately 85% rate of death or HTx at 3 years.26, 81 GCM is responsible for approximately 1 in 200 cases of myocarditis, and approximately 10% of all FM.
Etiology and Pathogenesis.
GCM is characterized by myocardial destruction mediated by a large numbers of cytotoxic T cells, macrophages, giant cells, and eosinophils. This leads to LV dysfunction and ventricular arrhythmias. Associated autoimmune disorders, in particular inflammatory bowel diseases and thyroid disorders have been reported in approximately 20% of cases.81
Diagnosis.
GCM affects equally males and females. Median age at onset is between 43 and 53 years, higher than observed in lymphocytic myocarditis.26, 81 GCM frequently presents as acute HF or cardiogenic shock, and with ventricular tachycardia (VT) or complete AV block.82 EMB is generally the first diagnostic tool. GCM shares some histologic features with CS, therefore the differential diagnosis can be challenging.82
Treatment.
Immunosuppressive therapy should be initiated promptly. Treatment with anti-T-lymphocyte based (i.e. anti-thymocyte globulin) and calcineurin inhibitor therapy can lead to clinical remission in up two-thirds of patients, in particular in those not requiring MCS.83 The initial approach may vary based on the clinical presentation. In case of FM, anti-thymocyte globulin associated with pulse high dose of corticosteroids are preferred, and cyclosporine is titrated to trough levels of 150–250 ng/L a few days after the administration of anti-thymocyte globulin (Table 4).84 There is a variable rate of LVEF recovery without transplant, among published series.26, 81, 83, 85 Dosage of oral corticosteroids after the acute phase is 1 mg/kg in the first months with subsequent slow tapering over one year, while cyclosporine is generally maintained more than 2 years, with a target plasma through level of 80–100 ng/L. Azathioprine at 1–2mg/kg/die divided in two daily doses or mycophenolate mofetil (500–1000 mg b.i.d.) can be added. There are anecdotal but consistent data suggesting that discontinuation of immunosuppression after one year of treatment may be followed by relapse and death.84 GCM patients have a high risk of VT, and placement of an implantable cardiac defibrillator (ICD) is generally recommended in all patients including those with full recovery of LVEF.86 Compared with historical results, current combined immunosuppressive treatment suggests an improvement in transplant-free survival from 11% to 55% at 1 year.83, 85 HTx is an effective therapy, with similar post-transplant survival in patients with GCM as in those with other causes. Nevertheless, recurrence of GCM on the transplanted hearts and a higher rate of early cellular rejection have been reported.87
TABLE 4.
Immunosuppressive treatment used for fulminant myocarditis or acute myocarditis complicated by severe heart failure not supported by evidences from clinical trials.
| Lymphocytic Fulminant Myocarditis | ICI-associated Myocarditis | Eosinophilic Myocarditis | Giant-Cell Myocarditis | Cardiac Sarcoidosis | |
|---|---|---|---|---|---|
| 1st line | I.v. Methylprednisolone, 7–14 mg/kg, pulsed doses for three days, then 1 mg/kg/die and subsequent tapering 42,70, 101 | ||||
| Alternative/ additional | Alternative: IVIG (2g/kg),102, 103 single continuous infusion in 24–48 hrs, or divided in 4 days; -or/and- Plasmapheresis, 3–5 sessions in 5–10 days | Withdraw ICI therapy |
If EGPA: I.v. cyclophosphamide, 600 mg/m2 (BSA) at days 1, 15 and 30 25, 109
If HES, myeloproliferative variant: Imatinib 100 to 400 mg daily 4–28 days (up to normalization of eosinophilic count)110 If Helmintic infection: Albendazolo 200 mg od to 400 mg bi.d. for 2 to 7 weeks111 If hypersensitivity reaction Withdraw medication suspected for the allergic reaction |
± ATG, 1 mg/Kg, usually single-dose 60, 84, 113−115 -or (alternative)- I.v. Alemtuzumab (anti-CD52 antibody) single dose of 30 mg116 + oral CyA, b.i.d, target through level 150–250 ng/ml 84 If hemodynamically stable patients: Only oral CyA, b.i.d, target through level 150–250 ng/ml 83 |
|
| If associated systemic autoimmune disorders (e.g. SLE, APS): | |||||
| Second line treatment | Add aggressive treatment of associated conditions104, 105 | ATG, 1 mg/kg, usually single dose106
- or - I.v. Alemtuzumab (anti-CD52 antibody), 30 mg, single dose107 - or - I.v. Abatacept (a CTLA-4 agonist), 500 mg every 2 weeks, for a total of five doses108 |
If HES, myeloproliferative variant I.v. Alemtuzumab (anti-CD52 antibody) 3 mg, 10 mg, 30 mg on consecutive days; then 30 mg 3 times a week for a total of 12 doses)112 |
See above, alternative - or- i.v. Rituximab 375mg*m2 (BSA) mg (once a week for 4 weeks, and then every 4 months as maintenance therapy) |
+ S.c. Methotrexate 15–20 mg/week90,92,117 - or- I.v. Infliximab 5mg/kg (up to 500 mg) at time 0, and after 2 and 4 weeks90,118 |
Immunosuppression is not routinely recommended for acute lymphocytic myocarditis, even when presenting with hemodynamic compromise and/or with severe heart dysfunction. Recommendations for this and other forms of acute, complicated myocarditis, are not supported by evidences from randomized clinical trials, but are derived from case series and pathophysiological considerations. Drugs and dosages are based on published clinical cases and revised by the Authors, that are reported in the references or in the text.
APS indicates antiphospholipid syndrome; ATG, anti-thymocyte globulin; BSA, body surface area; CyA: cyclosporine; EGPA, eosinophilic granulomatosis with polyangiitis; HES, hypereosinophilic syndrome; hrs, hours; ICI, immune checkpoint inhibitors; i.v. intravenous; IVIG, intravenous immunoglobulins; S.c., subcutaneous; SLE, systemic lupus erythematosus.
SARCOIDOTIC MYOCARDITIS
Sarcoidosis is a worldwide disease with a prevalence of about 4.7–64 in 100,000; the highest rates are reported in Northern European and African American individuals, particularly in females.52
Etiology and Pathogenesis.
Sarcoidosis is a multisystem, granulomatous disease of unknown etiology. Accumulating evidence suggests an immunological response to an unidentified antigenic trigger in genetically susceptible individuals. Organ involvement is variable, but most patients have pulmonary and lymph node involvement.52 Clinically manifest cardiac involvement occurs in about 5% of patients with pulmonary/systemic sarcoidosis.52 Sarcoidotic myocarditis is characterized by infiltration by activated macrophages, which in some cases can lead to chronic inflammation and fibrotic replacement with non-necrotizing granulomas. Eosinophils and necrosis are rare or absent.82 The macrophages within sarcoid granulomas tend to become epithelioid and form multinucleated giant cells.
Diagnosis.
Most cases occur in patients 25–60 years old. The three principal manifestations of CS are conduction abnormalities, ventricular arrhythmias, and HF.52 There is a growing awareness that CS can be the first manifestation of sarcoidosis in any organ.88 For example, between 16–35% of patients presenting with complete AV block (<60 years of age) or VT of unknown etiology had previously undiagnosed CS as the underlying etiology.88 The ventricular septum and LV basal free wall are most commonly affected. EMB has only 20% to 30% sensitivity, 52 if not imaging-guided.89 Experts’ position statements propose criteria to reach diagnosis of CS that are mainly based on positive histology in the heart or extracardiac histologic evidence of sarcoidosis plus demonstration of cardiac involvement based on imaging (Supplemental Table III).52
Treatment.
Corticosteroids therapy is advocated for the treatment of CS by most experts. It is unknown whether all patients with CS should be treated, or only those with clinical manifestations of the disease.52 Optimal doses of corticosteroids, and how best to assess response to therapy, is unknown. Methotrexate is often used as a second-line agent in refractory cases and/or if there are significant steroid side effects. Other therapies that have been used in CS include azathioprine, cyclophosphamide, infliximab,52 and rarely rituximab (Table 4).90
Patients with CS are at risk of SCD, and there are limited data to help with risk stratification (Supplemental Table 3). In a recent Finnish nationwide study, 10-year survival was 92.5% in 102 patients.91 Notably, CS can recur in transplanted hearts.92 Key unresolved questions related to treatment are if we should treat clinically silent CS and which drugs should be first- and second-line therapy for CS.
SPECIFIC TREATMENTS
Patients with AM or chronic infl-CMP associated with autoimmune disorders are treated according to indications regarding the systemic condition. Corticosteroids are generally the cornerstone of therapy, frequently in combination with another agent. During the acute phase, drugs with a rapid onset of action such as intravenous immunoglobulin, cyclophosphamide and rituximab may be preferred, while for maintenance therapy, mycophenolate mofetil, methotrexate, and azathioprine may be used to allow tapering of corticosteroids over time. Plasmapheresis is occasionally used in the acute setting, for instance in AM associated with antiphospholipid syndromes. Excluding AM associated with systemic inflammatory conditions, no specific evidence-based treatments are available for lymphocytic AM. Only one trial is currently recruiting patients with AM (clinicaltrial.gov NCT03018834) testing the efficacy of anakinra. AM is often a self-limiting disease, and spontaneous recovery of myocardial dysfunction may occur. There is a rationale for using immunosuppressive treatments in high-risk AM, but no trial has tested this hypothesis in the very acute phase. Thus, there are no specific recommendations for therapy in the acute phase beyond standard therapy for LV dysfunction and acute HF. The only study that assessed the efficacy of immunosuppression in AM, the Myocarditis Treatment Trial (MTT), reported no benefit from immunosuppression.36 However, the initiation of treatment was delayed, since patients were enrolled between 2 weeks and 1 year from symptoms’ onset. Almost all studies with corticosteroids focused on chronic infl-CMP with 6-month history of HF symptoms. An improvement of cardiac function has been observed, but most studies were inadequately powered,67 and there was no improvement in survival.93 The single-center TIMIC trial that randomized 85 patients with virus-negative chronic infl-CMP at a 6-month course of prednisone plus azathioprine or standard HF medications only showed a significant improvement of symptoms and echocardiographic parameters (median LVEF from 27% to 46% after 6 months) in the prednisone and azathioprine group. Given these findings, a large randomized trial is needed to assess the benefit and risk of long-term immunosuppression. Furthermore, few data exist supporting treatments for patients with virus-positive chronic infl-CMP. Usual HF-treatments are recommended in those patients with chronic inf-CMP or AM with reduced LVEF and stable hemodynamics. Beta blockers are often used after an AM also in patients with uncomplicated presentation (53.8% based on the Lombardy registry of AM),10 probably due to the perceived protection against arrhythmic events. Finally concerning the prevention of SCD at discharge, patients with infl-CMP follow the general indication for ICD, with the above-mentioned exceptions concerning GCM and CS. In patients with AM, a multiparametric stratification of risk is reasonable for decision on ICD implantation. It may include family history of SCD or arrhythmogenic cardiomyopathy, VT on presentation,94 presence and septal localization of LGE on CMRI,11, 14 and histology compatible with CS or GCM. Currently, ICD is rarely implanted after an AM with preserved LVEF (2% in the Lombardy registry,10 and 1.6% in the ITAMY registry). The cumulative percentage of SCD, resuscitated cardiac arrest, and appropriate ICD shock was 2.1% at 4.3 years of follow up in the ITAMY registry.11
MCS AND HTx
Patients with AM complicated by refractory HF or cardiogenic shock require inotropic and/or MCS.60 Myocarditis is often a reversible condition, thus temporary devices such as intra-aortic balloon pumps (IABP), veno-arterial extracorporeal membrane oxygenation (VA-ECMO), rotary pumps, or intra-aortic axial pumps (Impella®), should be considered first. Observational studies and multicenter registries report a short-term transplant-free survival of 55–80% in patients with FM who received temporary MCS.95, 96 An analysis of trends in myocarditis incidence and management in the United States between 2005 and 2014,97 has reported a growing rate of use of any temporary MCS, from 4.5% to 8.6%, with a significant trend for all devices except IABP, that anyway was the most frequently used support (3.8% overall). In theory, the use of devices that reduce LV afterload, such as centrifugal pumps or intra-aortic axial pumps, alone or in combination with VA-ECMO, could favour myocardial recovery more than VA-ECMO alone, through both hemodynamic and anti-inflammatory mechanisms.98 Nonetheless, a multicenter registry on Impella use for FM (34 patients from 2009 to 2016) showed a survival to discharge of 62%,99 not different from the 61% discharge rate reported among 185 patients supported with VA-ECMO in Taiwan from 2001 to 2011.100 if there is no weaning from MCS after 2–3 weeks, long-term left ventricular assist device or urgent HTx may be considered.
KNOWLEDGE GAPS AND PERSPECTIVE
Critical knowledge gaps exist regarding diagnosis, prognostication and treatment of AM and chronic infl-CMP which need to be addressed. Though EMB is the gold reference for diagnosis, it is not available or is underperformed in most hospitals,56 and has a relatively low sensitivity using conventional histology. Hence, novel sensitive and specific biomarkers and/or imaging modalities are needed. The advent of novel technologies developed in the immuno-oncology space (e.g., single cell RNA sequencing, mass cytometry, high-frequency and deeper T-cell receptor sequencing, multiplex immunofluorescence and other technologies) should become novel research strategies and further advance the usefulness of tissue analysis. Prospective large interventional trial or registries in the field of AM and chronic infl-CMP could help standardize the diagnostic and therapeutic approaches, which currently vary widely. Prospective registries aimed at identifying low versus high-risk patients at the time of hospitalization, and to refine and characterize the risk for specific events beyond death or HTx (e.g. recurrence, evolution to DCM, and arrhythmias) at discharge and during follow-up are needed. Finally, a common terminology to describe cases of AM and infl-CMP, and shared clinical pathways for patient management, could increase our knowledge on this condition, potentially improving patient outcome.
Supplementary Material
ACKNOWLEDGEMENTS
EA and MF acknowledge The Fondazione Centro Cardiologia e Cardiochirurgia A. De Gasperis, Niguarda Hospital, Milano, Italy, for its research support. CB is supported by the Registry of Cardio-Cerebral-Vascular Pathology, Veneto Region, Italy. CT acknowledges the Federal Ministry of Education and Research (BMBF), Germany for the CaPACITY-program (Cortisone in PArvovirus inflammatory CardIomyopaThY) and the German Centre for Cardiovascular Research (DZHK) for the Voltage-mapping- and MRI-guided endomyocardial biopsy in myocarditis and DCMi study.
Footnotes
DISCLOSURES
EDA is a consultant for Abbott, Abiomed, AstraZenica, Endotronix, Ionis, Medtronic, Novartis, and is on the board of directors of Genstem Therapeutics and shareholder of Rocket Pharmaceuticals.
MB is an employee at Ionis Pharmaceuticals. JJM has served on advisory boards for Pfizer, Novartis, Bristol-Myers Squibb, Deciphera, Audentes Pharmaceuticals, Nektar, Takeda, Ipsen, Myokardia, AstraZeneca, GlaxoSmithKline, Intrexon, and Regeneron and has been supported by National Institutes of Health grants R56 HL141466 and R01 HL141466. CT is a consultant of Cardiotropic Labs, Miami, USA. PGC is consultant for Servier.
All the other Authors declared no conflicts of interest.
REFERENCES
- 1.Cooper LT Jr. Myocarditis. N Engl J Med. 2009;360:1526–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, et al. European Society of Cardiology Working Group on M and Pericardial D. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–48, 2648a-2648d. [DOI] [PubMed] [Google Scholar]
- 3.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996;93:841–842. [DOI] [PubMed] [Google Scholar]
- 4.Corrado D, Basso C and Thiene G. Sudden cardiac death in young people with apparently normal heart. Cardiovasc Res. 2001;50:399–408. [DOI] [PubMed] [Google Scholar]
- 5.Harmon KG, Drezner JA, Maleszewski JJ, Lopez-Anderson M, Owens D, Prutkin JM, Asif IM, Klossner D and Ackerman MJ. Pathogeneses of sudden cardiac death in national collegiate athletic association athletes. Circ Arrhythm Electrophysiol. 2014;7:198–204. [DOI] [PubMed] [Google Scholar]
- 6.Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, Messere J, Cox GF, Lurie PR, Hsu D, Canter C, Wilkinson JD and Lipshultz SE. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867–1876. [DOI] [PubMed] [Google Scholar]
- 7.Global Burden of Disease Study C. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charpentier S, Beaune S, Joly LM, Khoury A, Duchateau FX, Briot R, Renaud B, Ageron FX and Network IRU. Management of chest pain in the French emergency healthcare system: the prospective observational EPIDOULTHO study. Eur J Emerg Med. 2018;25:404–410. [DOI] [PubMed] [Google Scholar]
- 9.Pasupathy S, Air T, Dreyer RP, Tavella R and Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861–870. [DOI] [PubMed] [Google Scholar]
- 10.Ammirati E, Cipriani M, Moro C, Raineri C, Pini D, Sormani P, Mantovani R, Varrenti M, Pedrotti P, Conca C, et al. and Registro Lombardo delle Miocarditi. Clinical Presentation and Outcome in a Contemporary Cohort of Patients With Acute Myocarditis. Circulation. 2018;138:1088–1099. [DOI] [PubMed] [Google Scholar]
- 11.Aquaro GD, Perfetti M, Camastra G, Monti L, Dellegrottaglie S, Moro C, Pepe A, Todiere G, Lanzillo C, Scatteia A, et al. and Cardiac Magnetic Resonance Working Group of the Italian Society of C. Cardiac MR With Late Gadolinium Enhancement in Acute Myocarditis With Preserved Systolic Function: ITAMY Study. Am Coll Cardiol. 2017;70:1977–1987. [DOI] [PubMed] [Google Scholar]
- 12.Younis A, Matetzky S, Mulla W, Masalha E, Afel Y, Chernomordik F, Fardman A, Goitein O, Ben-Zekry S, Peled Y, et al. Epidemiology characteristics and outcome of patients with clinically diagnosed acute myocarditis. Am J Med. 2020;133:492–499.. [DOI] [PubMed] [Google Scholar]
- 13.White JA, Hansen R, Abdelhaleem A, Mikami Y, Peng M, Rivest S, Satriano A, Dykstra S, Flewitt J, Heydari B, et al. Natural History of Myocardial Injury and Chamber Remodeling in Acute Myocarditis. Circ Cardiovasc Imaging. 2019;12:e008614. [DOI] [PubMed] [Google Scholar]
- 14.Grani C, Eichhorn C, Biere L, Murthy VL, Agarwal V, Kaneko K, Cuddy S, Aghayev A, Steigner M, Blankstein R, et al. Prognostic Value of Cardiac Magnetic Resonance Tissue Characterization in Risk Stratifying Patients With Suspected Myocarditis. J Am Coll Cardiol. 2017;70:1964–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanguineti F, Garot P, Mana M, O’H-Ici D, Hovasse T, Unterseeh T, Louvard Y, Troussier X, Morice MC and Garot J. Cardiovascular magnetic resonance predictors of clinical outcome in patients with suspected acute myocarditis. J Cardiovasc Magn Reson. 2015;17:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grun S, Schumm J, Greulich S, Wagner A, Schneider S, Bruder O, Kispert EM, Hill S, Ong P, Klingel K, et al. Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol. 2012;59:1604–1615. [DOI] [PubMed] [Google Scholar]
- 17.Anzini M, Merlo M, Sabbadini G, Barbati G, Finocchiaro G, Pinamonti B, Salvi A, Perkan A, Di Lenarda A, Bussani R, et al. Long-term evolution and prognostic stratification of biopsy-proven active myocarditis. Circulation. 2013;128:2384–2394. [DOI] [PubMed] [Google Scholar]
- 18.Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B and Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391:933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rose NR. Learning from myocarditis: mimicry, chaos and black holes. F1000Prime Rep. 2014;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trachtenberg BH and Hare JM. Inflammatory Cardiomyopathic Syndromes. Circ Res. 2017;121:803–818. [DOI] [PubMed] [Google Scholar]
- 21.Fung G, Luo H, Qiu Y, Yang D and McManus B. Myocarditis. Circ Res. 2016;118:496–514. [DOI] [PubMed] [Google Scholar]
- 22.Veronese G, Ammirati E, Brambatti M, Merlo M, Cipriani M, Potena L, Sormani P, Aoki T, Sugimura K, Sawamura A, et al. Viral genome search in myocardium of patients with fulminant myocarditis. Eur J Heart Fail. 2020. Online ahead of print. doi: 10.1002/ejhf.1738. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Ayala JM, Pastor-Quirante F, Gonzalez-Carrillo J, Lopez-Cuenca D, Sanchez-Munoz JJ, Oliva-Sandoval MJ and Gimeno JR. Genetics of myocarditis in arrhythmogenic right ventricular dysplasia. Heart rhythm. 2015;12:766–773. [DOI] [PubMed] [Google Scholar]
- 24.Belkaya S, Kontorovich AR, Byun M, Mulero-Navarro S, Bajolle F, Cobat A, Josowitz R, Itan Y, Quint R, Lorenzo L, et al. Autosomal Recessive Cardiomyopathy Presenting as Acute Myocarditis. J Am Coll Cardiol. 2017;69:1653–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brambatti M, Matassini MV, Adler ED, Klingel K, Camici PG and Ammirati E. Eosinophilic Myocarditis: Characteristics, Treatment, and Outcomes. J Am Coll Cardiol. 2017;70:2363–2375. [DOI] [PubMed] [Google Scholar]
- 26.Ammirati E, Veronese G, Brambatti M, Merlo M, Cipriani M, Potena L, Sormani P, Aoki T, Sugimura K, Sawamura A, et al. Fulminant Versus Acute Nonfulminant Myocarditis in Patients With Left Ventricular Systolic Dysfunction. J Am Coll Cardiol. 2019;74:299–311. [DOI] [PubMed] [Google Scholar]
- 27.Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, Gobert A, Spano JP, Balko JM, Bonaca MP, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilotra NA, Minkove N, Bennett MK, Tedford RJ, Steenbergen C, Judge DP, Halushka MK and Russell SD. Lack of Relationship Between Serum Cardiac Troponin I Level and Giant Cell Myocarditis Diagnosis and Outcomes. J Card Fail. 2016;22:583–585. [DOI] [PubMed] [Google Scholar]
- 29.Bratincsak A, El-Said HG, Bradley JS, Shayan K, Grossfeld PD and Cannavino CR. Fulminant myocarditis associated with pandemic H1N1 influenza A virus in children. J Am Coll Cardiol. 2010;55:928–929. [DOI] [PubMed] [Google Scholar]
- 30.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J Am Coll Cardiol. 2018;72:3158–3176. [DOI] [PubMed] [Google Scholar]
- 32.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P and International Consensus Group on Cardiovascular Magnetic Resonance in M. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53:1475–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhatia S, Anstine C, Jaffe AS, Gersh BJ, Chandrasekaran K, Foley TA, Hodge D and Anavekar NS. Cardiac magnetic resonance in patients with elevated troponin and normal coronary angiography. Heart. 2019;105:1231–1236. [DOI] [PubMed] [Google Scholar]
- 34.Luetkens JA, Homsi R, Dabir D, Kuetting DL, Marx C, Doerner J, Schlesinger-Irsch U, Andrie R, Sprinkart AM, Schmeel FC, et al. Comprehensive Cardiac Magnetic Resonance for Short-Term Follow-Up in Acute Myocarditis. J American Heart Assoc. 2016;5:e003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grogan M, Redfield MM, Bailey KR, Reeder GS, Gersh BJ, Edwards WD and Rodeheffer RJ. Long-term outcome of patients with biopsy-proved myocarditis: comparison with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1995;26:80–84. [DOI] [PubMed] [Google Scholar]
- 36.Mason JW, O’Connell JB, Herskowitz A, Rose NR, McManus BM, Billingham ME and Moon TE. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N Engl J Med. 1995;333:269–275. [DOI] [PubMed] [Google Scholar]
- 37.McCarthy RE 3rd, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Hare JM and Baughman KL. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Medicine. 2000;342:690–695. [DOI] [PubMed] [Google Scholar]
- 38.Magnani JW, Danik HJ, Dec GW Jr. and DiSalvo TG. Survival in biopsy-proven myocarditis: a long-term retrospective analysis of the histopathologic, clinical, and hemodynamic predictors. Am Heart J. 2006;151:463–470. [DOI] [PubMed] [Google Scholar]
- 39.Caforio AL, Calabrese F, Angelini A, Tona F, Vinci A, Bottaro S, Ramondo A, Carturan E, Iliceto S, Thiene G et al. A prospective study of biopsy-proven myocarditis: prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur Heart J. 2007;28:1326–1333. [DOI] [PubMed] [Google Scholar]
- 40.Kindermann I, Kindermann M, Kandolf R, Klingel K, Bultmann B, Muller T, Lindinger A and Bohm M. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118:639–648. [DOI] [PubMed] [Google Scholar]
- 41.Inaba O, Satoh Y, Isobe M, Yamamoto T, Nagao K and Takayama M. Factors and values at admission that predict a fulminant course of acute myocarditis: data from Tokyo CCU network database. Heart Vessels. 2017;32:952–959. [DOI] [PubMed] [Google Scholar]
- 42.Ammirati E, Cipriani M, Lilliu M, Sormani P, Varrenti M, Raineri C, Petrella D, Garascia A, Pedrotti P, Roghi A, et al. Survival and Left Ventricular Function Changes in Fulminant Versus Nonfulminant Acute Myocarditis. Circulation. 2017;136:529–545. [DOI] [PubMed] [Google Scholar]
- 43.Imazio M, Angelico G, Andriani M, Lobetti-Bodoni L, Davini O, Giustetto C and Rinaldi M. Prevalence and Prognostic Impact of Septal Late Gadolinium Enhancement in Acute Myocarditis With or Without Preserved Left Ventricular Function. Am J Cardiol. 2018;122:1955–1958. [DOI] [PubMed] [Google Scholar]
- 44.Berg J, Lovrinovic M, Baltensperger N, Kissel CK, Kottwitz J, Manka R, Patriki D, Scherff F, Schmied C, Landmesser U, et al. Non-steroidal anti-inflammatory drug use in acute myopericarditis: 12-month clinical follow-up. Open Heart. 2019;6:e000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan JA, Lee YJ and Salerno M. Diagnostic Performance of Extracellular Volume, Native T1, and T2 Mapping Versus Lake Louise Criteria by Cardiac Magnetic Resonance for Detection of Acute Myocarditis: A Meta-Analysis. Circ Cardiovasc Imaging. 2018;11:e007598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luetkens JA, Faron A, Isaak A, Dabir D, Kuetting D, Feisst A, Schmeel FC, Sprinkart AM and Thomas D. Comparison of Original and 2018 Lake Louise Criteria for Diagnosis of Acute Myocarditis: Results of a Validation Cohort. Radiology: Cardiothoracic Imaging. 2019;1:e190010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lurz P, Eitel I, Adam J, Steiner J, Grothoff M, Desch S, Fuernau G, de Waha S, Sareban M, Luecke C, et al. Diagnostic performance of CMR imaging compared with EMB in patients with suspected myocarditis. JACC Cardiovascr Imaging. 2012;5:513–524. [DOI] [PubMed] [Google Scholar]
- 48.Blissett S, Chocron Y, Kovacina B and Afilalo J. Diagnostic and prognostic value of cardiac magnetic resonance in acute myocarditis: a systematic review and meta-analysis. Int J Cardiovasc Imaging. 2019;35:2221–2229. [DOI] [PubMed] [Google Scholar]
- 49.Aquaro GD, Ghebru Habtemicael Y, Camastra G, Monti L, Dellegrottaglie S, Moro C, Lanzillo C, Scatteia A, Di Roma M, Pontone G, et al. and Cardiac Magnetic Resonance” Working Group of the Italian Society of C. Prognostic Value of Repeating Cardiac Magnetic Resonance in Patients With Acute Myocarditis. J Am Coll Cardiol. 2019;74:2439–2448. [DOI] [PubMed] [Google Scholar]
- 50.Ammirati E, Moroni F, Sormani P, Peritore A, Milazzo A, Quattrocchi G, Cipriani M, Oliva F, Giannattasio C, Frigerio M, et al. Quantitative changes in late gadolinium enhancement at cardiac magnetic resonance in the early phase of acute myocarditis. Int J Cardio. 2017;231:216–221. [DOI] [PubMed] [Google Scholar]
- 51.Perel-Winkler A, Bokhari S, Perez-Recio T, Zartoshti A, Askanase A and Geraldino-Pardilla L. Myocarditis in systemic lupus erythematosus diagnosed by (18)F-fluorodeoxyglucose positron emission tomography. Lupus Sci Med. 2018;5:e000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Birnie DH, Nery PB, Ha AC and Beanlands RS. Cardiac Sarcoidosis. J Am Coll Cardiol. 2016;68:411–421. [DOI] [PubMed] [Google Scholar]
- 53.Chareonthaitawee P, Beanlands RS, Chen W, Dorbala S, Miller EJ, Murthy VL, Birnie DH, Chen ES, Cooper LT, Tung RH, et al. Joint SNMMI-ASNC expert consensus document on the role of (18)F-FDG PET/CT in cardiac sarcoid detection and therapy monitoring. J Nucl Cardiol. 2017;24:1741–1758. [DOI] [PubMed] [Google Scholar]
- 54.Aretz HT. Myocarditis: the Dallas criteria. Hum Pathol. 1987;18:619–624. [DOI] [PubMed] [Google Scholar]
- 55.Bennett MK, Gilotra NA, Harrington C, Rao S, Dunn JM, Freitag TB, Halushka MK and Russell SD. Evaluation of the role of endomyocardial biopsy in 851 patients with unexplained heart failure from 2000–2009. Circ Heart Fail. 2013;6:676–684. [DOI] [PubMed] [Google Scholar]
- 56.Singh V, Mendirichaga R, Savani GT, Rodriguez A, Blumer V, Elmariah S, Inglessis-Azuaje I and Palacios I. Comparison of Utilization Trends, Indications, and Complications of Endomyocardial Biopsy in Native Versus Donor Hearts (from the Nationwide Inpatient Sample 2002 to 2014). Am J Cardiol. 2018;121:356–363. [DOI] [PubMed] [Google Scholar]
- 57.Chow LH, Radio SJ, Sears TD and McManus BM. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J Am Coll Cardiol. 1989;14:915–920. [DOI] [PubMed] [Google Scholar]
- 58.Maisch B, Portig I, Ristic A, Hufnagel G and Pankuweit S. Definition of inflammatory cardiomyopathy (myocarditis): on the way to consensus. A status report. Herz. 2000;25:200–209. [DOI] [PubMed] [Google Scholar]
- 59.Kuhl U, Noutsias M, Seeberg B and Schultheiss HP. Immunohistological evidence for a chronic intramyocardial inflammatory process in dilated cardiomyopathy. Heart. 1996;75:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kociol RD, Cooper LT, Fang JC, Moslehi JJ, Pang PS, Sabe MA, Shah RV, Sims DB, Thiene G, Vardeny O, American Heart Association Heart F and Transplantation Committee of the Council on Clinical C. Recognition and Initial Management of Fulminant Myocarditis: A Scientific Statement From the American Heart Association. Circulation. 2020:CIR0000000000000745. [DOI] [PubMed] [Google Scholar]
- 61.Hu JR, Florido R, Lipson EJ, Naidoo J, Ardehali R, Tocchetti CG, Lyon AR, Padera RF, Johnson DB and Moslehi J. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res. 2019;115:854–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Starling RC, Towbin J, et al. , American Heart A, American College of C and European Society of C. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116:2216–33. [DOI] [PubMed] [Google Scholar]
- 63.Lorusso R, Centofanti P, Gelsomino S, Barili F, Di Mauro M, Orlando P, Botta L, Milazzo F, Actis Dato G, Casabona R, et al. and Investigators G. Venoarterial Extracorporeal Membrane Oxygenation for Acute Fulminant Myocarditis in Adult Patients: A 5-Year Multi-Institutional Experience. Ann Thorac Surg. 2016;101:919–926. [DOI] [PubMed] [Google Scholar]
- 64.Ammirati E, Veronese G, Cipriani M, Moroni F, Garascia A, Brambatti M, Adler ED and Frigerio M. Acute and Fulminant Myocarditis: a Pragmatic Clinical Approach to Diagnosis and Treatment. Curr Cardiol Rep. 2018;20:114. [DOI] [PubMed] [Google Scholar]
- 65.Bozkurt B, Colvin M, Cook J, Cooper LT, Deswal A, Fonarow GC, Francis GS, Lenihan D, Lewis EF, McNamara DM, et al. , American Heart Association Committee on Heart F, Transplantation of the Council on Clinical C, Council on Cardiovascular Disease in the Y, Council on C, Stroke N, Council on E, Prevention, Council on Quality of C and Outcomes R. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement From the American Heart Association. Circulation. 2016;134:e579–e646. [DOI] [PubMed] [Google Scholar]
- 66.Nakayama T, Sugano Y, Yokokawa T, Nagai T, Matsuyama TA, Ohta-Ogo K, Ikeda Y, Ishibashi-Ueda H, Nakatani T, Ohte N, et al. Clinical impact of the presence of macrophages in endomyocardial biopsies of patients with dilated cardiomyopathy. Eur J Heart Fail. 2017;19:490–498. [DOI] [PubMed] [Google Scholar]
- 67.Frustaci A, Russo MA and Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J. 2009;30:1995–2002. [DOI] [PubMed] [Google Scholar]
- 68.Klingel K, Selinka HC, Huber M, Sauter M, Leube M and Kandolf R. Molecular pathology and structural features of enteroviral replication. Toward understanding the pathogenesis of viral heart disease. Herz. 2000;25:216–220. [DOI] [PubMed] [Google Scholar]
- 69.Matsuura H, Ichida F, Saji T, Ogawa S, Waki K, Kaneko M, Tahara M, Soga T, Ono Y and Yasukochi S. Clinical Features of Acute and Fulminant Myocarditis in Children- 2nd Nationwide Survey by Japanese Society of Pediatric Cardiology and Cardiac Surgery. Circ J. 2016;80:2362–2368. [DOI] [PubMed] [Google Scholar]
- 70.Veronese G, Cipriani M, Bottiroli M, Garascia A, Mondino M, Pedrotti P, Pini D, Cozzi O, Messina A, Droandi G, et al. Fulminant myocarditis triggered by OC43 subtype coronavirus: a disease deserving evidence-based care bundles. J Cardiovasc Med. 2020;21:529–531. [DOI] [PubMed] [Google Scholar]
- 71.Bock CT, Klingel K and Kandolf R. Human parvovirus B19-associated myocarditis. N Engl J Med. 2010;362:1248–1249. [DOI] [PubMed] [Google Scholar]
- 72.Tschope C, Elsanhoury A, Schlieker S, Van Linthout S and Kuhl U. Immunosuppression in inflammatory cardiomyopathy and parvovirus B19 persistence. Eur J Heart Fail. 2019;21:1468–1469. [DOI] [PubMed] [Google Scholar]
- 73.Verdonschot J, Hazebroek M, Merken J, Debing Y, Dennert R, Brunner-La Rocca HP and Heymans S. Relevance of cardiac parvovirus B19 in myocarditis and dilated cardiomyopathy: review of the literature. Eur J Heart Fail. 2016;18:1430–1441. [DOI] [PubMed] [Google Scholar]
- 74.Schultheiss HP, Piper C, Sowade O, Waagstein F, Kapp JF, Wegscheider K, Groetzbach G, Pauschinger M, Escher F, Arbustini E, et al. Betaferon in chronic viral cardiomyopathy (BICC) trial: Effects of interferon-beta treatment in patients with chronic viral cardiomyopathy. Clin Res Cardiol. 2016; 105: 763–773. [DOI] [PubMed] [Google Scholar]
- 75.Haslam A and Prasad V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw Open. 2019;2:e192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bonaca MP, Olenchock BA, Salem JE, Wiviott SD, Ederhy S, Cohen A, Stewart GC, Choueiri TK, Di Carli M, Allenbach Y, Kumbhani DJ, et al. Myocarditis in the Setting of Cancer Therapeutics: Proposed Case Definitions for Emerging Clinical Syndromes in Cardio-Oncology. Circulation. 2019;140:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med. 2016;375:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Col Cardiol. 2018;71:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grabie N, Gotsman I, DaCosta R, Pang H, Stavrakis G, Butte MJ, Keir ME, Freeman GJ, Sharpe AH and Lichtman AH. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation. 2007;116:2062–2071. [DOI] [PubMed] [Google Scholar]
- 80.Morimoto S, Kubo N, Hiramitsu S, Uemura A, Ohtsuki M, Kato S, Kato Y, Sugiura A, Miyagishima K, Mori N, et al. Changes in the peripheral eosinophil count in patients with acute eosinophilic myocarditis. HeartVessels. 2003;18:193–196. [DOI] [PubMed] [Google Scholar]
- 81.Cooper LT Jr., Berry GJ and Shabetai R. Idiopathic giant-cell myocarditis--natural history and treatment. Multicenter Giant Cell Myocarditis Study Group Investigators. N Engl J Med. 1997;336:1860–1866. [DOI] [PubMed] [Google Scholar]
- 82.Okura Y, Dec GW, Hare JM, Kodama M, Berry GJ, Tazelaar HD, Bailey KR and Cooper LT. A clinical and histopathologic comparison of cardiac sarcoidosis and idiopathic giant cell myocarditis. J Am Coll Cardiol. 2003;41:322–329. [DOI] [PubMed] [Google Scholar]
- 83.Kandolin R, Lehtonen J, Salmenkivi K, Raisanen-Sokolowski A, Lommi J and Kupari M. Diagnosis, treatment, and outcome of giant-cell myocarditis in the era of combined immunosuppression. Circ Heart Fail. 2013;6:15–22. [DOI] [PubMed] [Google Scholar]
- 84.Cooper LT Jr., Hare JM, Tazelaar HD, Edwards WD, Starling RC, Deng MC, Menon S, Mullen GM, Jaski B, Bailey KR, et al. and Giant Cell Myocarditis Treatment Trial I. Usefulness of immunosuppression for giant cell myocarditis. Am J Cardiol. 2008;102:1535–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ekstrom K, Lehtonen J, Kandolin R, Raisanen-Sokolowski A, Salmenkivi K and Kupari M. Long-term outcome and its predictors in giant cell myocarditis. E J Heart Fail. 2016;18:1452–1458. [DOI] [PubMed] [Google Scholar]
- 86.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Circulation. 2018;138:e272–e391. [DOI] [PubMed] [Google Scholar]
- 87.Elamm CA, Al-Kindi SG, Bianco CM, Dhakal BP and Oliveira GH. Heart Transplantation in Giant Cell Myocarditis: Analysis of the United Network for Organ Sharing Registry. J Card Fail. 2017;23:566–569. [DOI] [PubMed] [Google Scholar]
- 88.Kandolin R, Lehtonen J and Kupari M. Cardiac Sarcoidosis and Giant Cell Myocarditis as Causes of Atrioventricular Block in Young and Middle-Aged Adults. Circ Arrhythm Electrophysiol. 2011;4:303–309. [DOI] [PubMed] [Google Scholar]
- 89.Liang JJ, Hebl VB, DeSimone CV, Madhavan M, Nanda S, Kapa S, Maleszewski JJ, Edwards WD, Reeder G, Cooper LT et al. Electrogram guidance: a method to increase the precision and diagnostic yield of endomyocardial biopsy for suspected cardiac sarcoidosis and myocarditis. JACC Heart Fail. 2014;2:466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fussner LA, Karlstedt E, Hodge DO, Fine NM, Kalra S, Carmona EM, Utz JP, Isaac DL and Cooper LT. Management and outcomes of cardiac sarcoidosis: a 20-year experience in two tertiary care centres. Eur J Heart Fail. 2018;20:1713–1720. [DOI] [PubMed] [Google Scholar]
- 91.Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K, Kaikkonen K, Tuohinen S, Haataja P, Kerola T, et al. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131:624–632. [DOI] [PubMed] [Google Scholar]
- 92.Veronese G, Cipriani M, Petrella D, Geniere Nigra S, Pedrotti P, Garascia A, Masciocco G, Bramerio MA, Klingel K, Frigerio M et al. Recurrent cardiac sarcoidosis after heart transplantation. Clin Res Cardiol. 2019; 108:1171–1173 [DOI] [PubMed] [Google Scholar]
- 93.Chen HS, Wang W, Wu SN and Liu JP. Corticosteroids for viral myocarditis. Cochrane Database Syst Revi. 2013:CD004471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peretto G, Sala S, Rizzo S, Palmisano A, Esposito A, De Cobelli F, Campochiaro C, De Luca G, Foppoli L, Dagna L, et al. Ventricular Arrhythmias in Myocarditis: Characterization and Relationships With Myocardial Inflammation. J Am Coll Cardiol. 2020;75:1046–1057. [DOI] [PubMed] [Google Scholar]
- 95.Saito S, Toda K, Miyagawa S, Yoshikawa Y, Hata H, Yoshioka D, Domae K, Tsukamoto Y, Sakata Y and Sawa Y. Diagnosis, medical treatment, and stepwise mechanical circulatory support for fulminat myocarditis. J Artif Organs. 2018;21:172–179. [DOI] [PubMed] [Google Scholar]
- 96.Li S, Xu S, Li C, Ran X, Cui G, He M, Miao K, Zhao C, Yan J, Hui R, et al. A life support-based comprehensive treatment regimen dramatically lowers the in-hospital mortality of patients with fulminant myocarditis: a multiple center study. Sci China Life Sci. 2019;62:369–380. [DOI] [PubMed] [Google Scholar]
- 97.Pahuja M, Adegbala O, Mishra T, Akintoye E, Chehab O, Mony S, Singh M, Ando T, Abubaker H, Yassin A, et al. Trends in the Incidence of In-Hospital Mortality, Cardiogenic Shock, and Utilization of Mechanical Circulatory Support Devices in Myocarditis (Analysis of National Inpatient Sample Data, 2005–2014). J Cardiac Fail. 2019;25:457–467. [DOI] [PubMed] [Google Scholar]
- 98.Tschope C, Van Linthout S, Klein O, Mairinger T, Krackhardt F, Potapov EV, Schmidt G, Burkhoff D, Pieske B and Spillmann F. Mechanical Unloading by Fulminant Myocarditis: LV-IMPELLA, ECMELLA, BI-PELLA, and PROPELLA Concepts. J Cardiovasc Transl Res. 2019;12:116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Annamalai SK, Esposito ML, Jorde L, Schreiber T, S AH, O’Neill WW and Kapur NK. The Impella Microaxial Flow Catheter Is Safe and Effective for Treatment of Myocarditis Complicated by Cardiogenic Shock: An Analysis From the Global cVAD Registry. J Card Fail. 2018;24:706–710. [DOI] [PubMed] [Google Scholar]
- 100.Chang JJ, Lin MS, Chen TH, Chen DY, Chen SW, Hsu JT, Wang PC and Lin YS . Heart Failure and Mortality of Adult Survivors from Acute Myocarditis Requiring Intensive Care Treatment - A Nationwide Cohort Study. Int J Med Sci. 2017;14:1241–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reiff C, Missov E. A Case of Fulminant Lymphocytic Myocarditis Responsive to Immunosuppression. Am J Med 2018;131:e465–e466. [DOI] [PubMed] [Google Scholar]
- 102.Drucker NA, Colan SD, Lewis AB et al. Gamma-globulin treatment of acute myocarditis in the pediatric population. Circulation 1994;89:252–257. [DOI] [PubMed] [Google Scholar]
- 103.Chou HW, Wang CH, Lin LY, Chi NH, Chou NK, Yu HY, Chen YS. Prognostic Factors for Heart Recovery in Adult Patients With Acute Fulminant Myocarditis and Cardiogenic Shock Supported With Extracorporeal Membrane Oxygenation. J Crit Care. 2020;57:214–219. [DOI] [PubMed] [Google Scholar]
- 104.Berman H, Rodriguez-Pinto I, Cervera R et al. Rituximab use in the catastrophic antiphospholipid syndrome: descriptive analysis of the CAPS registry patients receiving rituximab. Autoimmun Rev 2013;12:1085–1090. [DOI] [PubMed] [Google Scholar]
- 105.Castellanos-Moreira R, Rodriguez-Garcia S, Lopez-Sobrino T, Capdevila A, Prieto-Gonzalez S, Espinosa G. Successful Extracorporeal Membrane Oxygenation in a Patient With Fulminant Lupus Myocarditis. Rev Esp Cardiol (Engl Ed) 2017;70:1013–1014. [DOI] [PubMed] [Google Scholar]
- 106.Jain V, Mohebtash M, Rodrigo ME, Ruiz G, Atkins MB, Barac A. Autoimmune Myocarditis Caused by Immune Checkpoint Inhibitors Treated With Antithymocyte Globulin. J Immunother 2018;41:332–335. [DOI] [PubMed] [Google Scholar]
- 107.Esfahani K, Buhlaiga N, Thebault P, Lapointe R, Johnson NA, Miller WH Jr. Alemtuzumab for Immune-Related Myocarditis Due to PD-1 Therapy. N Engl Med. 2019;380:2375–2376. [DOI] [PubMed] [Google Scholar]
- 108.Salem JE, Allenbach Y, Vozy A, Brechot N, Johnson DB, Moslehi JJ and Kerneis M. Abatacept for Severe Immune Checkpoint Inhibitor-Associated Myocarditis. N Engl Med. 2019;380:2377–2379. [DOI] [PubMed] [Google Scholar]
- 109.Courand PY, Croisille P, Khouatra C, Cottin V, Kirkorian G, Bonnefoy E. Churg-Strauss syndrome presenting with acute myocarditis and cardiogenic shock. Heart Lung Circ. 2012;21:178–181. [DOI] [PubMed] [Google Scholar]
- 110.Gotlib J, Cools J, Malone JM 3rd, Schrier SL, Gilliland DG, Coutre SE. The FIP1L1-PDGFRalpha fusion tyrosine kinase in hypereosinophilic syndrome and chronic eosinophilic leukemia: implications for diagnosis, classification, and management. Blood 2004;103:2879–2891. [DOI] [PubMed] [Google Scholar]
- 111.Kuenzli E, Neumayr A, Chaney M, Blum J. Toxocariasis-associated cardiac diseases—A systematic review of the literature. Acta Trop 2016;154:107–120. [DOI] [PubMed] [Google Scholar]
- 112.Syed FF, Bleeker JS, Glockner J, Pardanani A, Cooper LT, Jr. Response to alemtuzumab in FIP1L1/PDGFRA-negative hypereosinophilic myocarditis on serial cardiac magnetic resonance imaging. J Am Coll Cardiol. 2012;59:430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.