Abstract
Desensitization has enabled incompatible living donor kidney transplantation (ILDKT) across HLA/ABO barriers, but added immunomodulation might put patients at increased risk of infections. We studied 475 recipients from our center from 2010-2015, categorized by desensitization intensity: none/compatible (n=260), low (0-4 plasmaphereses,n=47), moderate (5-9,n=74), and high (≥10,n=94). The 1-year cumulative incidence of infection was 50.1%, 49.8%, 66.0%, and 73.5% for recipients who received none, low, moderate, and high intensity desensitization (p<0.001). The most common infections were UTI (33.5% of ILDKT vs 21.5% compatible), opportunistic (21.9% vs 10.8%), and bloodstream (19.1% vs 5.4%) (p<0.001). In weighted models, a trend towards increased risk was seen in low (wIRR=0.771.402.56,p=0.3) and moderately (wIRR=0.881.352.06,p=0.2) desensitized recipients, with a statistically significant 2.22-fold (wIRR=1.332.223.72,p=0.002) increased risk in highly desensitized recipients. Recipients with ≥4 infections were at higher risk of prolonged hospitalization (wIRR=2.623.574.88, p<0.001) and death-censored graft loss (wHR=1.154.0113.95,p=0.03). Post-KT infections are more common in desensitized ILDKT recipients. A subset of highly desensitized patients is at ultra-high risk for infections. Strategies should be designed to protect patients from the morbidity of recurrent infections, and to extend the survival benefit of ILDKT across the spectrum of recipients.
INTRODUCTION
HLA- and/or ABO-incompatible living donor kidney transplantation (ILDKT) confers a survival benefit for kidney transplant candidates who have a willing, but incompatible donor (1–3). Alternatives include waiting as many as 10 years on the deceased donor waitlist for a compatible deceased donor transplant, or entering a kidney paired donation (KPD) program (1), though those with high panel reactive antibody are less likely to find a donor through KPD (4–5). Since ILDKT recipients are at risk of antibody-mediated rejection (AMR), they undergo modulation of the B cell compartment for desensitization, classically with either plasmapheresis plus low-dose intravenous immunoglobulin (IVIg), or high-dose IVIg (1–3, 6–7). Anti-B cell agents such as rituximab (7), and other agents such as bortezomib (8), eculizumab (9), and C1 esterase inhibitor (10) have also been used, as well as rescue splenectomy in occasional patients with refractory AMR (9).
Since these interventions are administered to ILDKT recipients in addition to standard post-transplant immunosuppressive medications, there is potentially an increased risk for infectious complications after ILDKT. Moreover, the incidence and severity of infections may differ within the ILDKT group itself, since some desensitization protocols are more intensive than others. Such information is not only essential for risk prediction and informed consent, but also as a foundation for devising more effective strategies for infection prevention in this vulnerable population. Unfortunately, transplant registry data lacks details of ILDKT treatment regimens and post-transplant infections, so studying this has been thus far challenging.
To investigate this further, we collected detailed treatment, infection, and outcomes data on our ILDKT recipients to understand the landscape and impact of infections after ILDKT. Specifically, we sought to (i) quantify the post-KT incidence of a variety of infection types by desensitization intensity, (ii) estimate the adverse impact of infections on post-KT hospitalization, acute cellular rejection, death-censored graft failure (DCGF), and mortality by number of infections developed during the first year post-KT, (iii) determine whether ILDKT confers additional risk compared with compatible living donor kidney transplantation (CLDKT), and (iv) characterize the outcomes of an ultra-high-risk group of ILDKT recipients who had high numbers of recurrent infections during the first year post-KT.
METHODS
Data sources
Data collection from electronic medical record
Using our center’s electronic medical record, for each individual patient, we collected induction immunosuppression, number of pre-transplant plasmapheresis treatments, number of post-transplant plasmapheresis treatments over the first 6 months post-KT and any use of rituximab, bortezomib, eculizumab, C1 esterase inhibitor, and splenectomy. Episodes of biopsy-proven cellular rejection were recorded, including histologic grade and treatment. Other outcomes recorded included hospitalization days during the first-year post-KT, and the last date of follow-up at this center. This study was approved by the Johns Hopkins University Institutional Review Board.
OPTN/SRTR
Additional risk factors and outcomes, including graft failure and death, augmented through linkage to the Centers for Medicare and Medicaid Services and the Social Security Death Master File, were ascertained through linkage with the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere (11). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
Study population
In this retrospective study, we identified all adult (≥18 years or ≥17 years and followed by our adult team) who underwent CLDKT and ILDKT at Johns Hopkins Hospital between 1/1/2010 and 12/31/2015. ILDKT recipients were defined as patients who had detectable donor-specific antibody of any strength (single-bead, flow cytometric crossmatch, or cytotoxic crossmatch) that was deemed to require perioperative desensitization therapy to receive a kidney from an HLA- or ABO-incompatible living donor (2–3). At our institution, desensitized recipients underwent plasmapheresis with low-dose IVIg, (except one who received high-dose IVIg), with or without rituximab, as previously described elsewhere (2–3). ILDKT recipients who did not receive any desensitization (n=6, 1.2%) and CLDKT recipients who received plasmapheresis for reasons other than desensitization (n=13, 2.6%) were excluded from the main analyses.
ILDKT intensity
In the present study, we categorized ILDKT recipients into 3 groups according to total intensity of desensitization: low (total plasmaphereses: 0-4; median pre vs. post-transplant treatments: 1 vs. 2), moderate (total: 5-9; median: 2 vs. 4), and high (total: ≥10; median: 4 vs. 11.5). The number of plasmaphereses was determined according to the strength of the initial donor anti-HLA antibody, response to initial plasmapheresis treatments, real-time measurements of post-transplant donor anti-HLA antibody levels, and occurrence of post-transplant antibody-mediated rejection (2, 8–9). Additional interventions such as rituximab, bortezomib, eculizumab, and rescue splenectomy were per transplant clinician choice, guided by the above considerations (8–9), and administration of C1 esterase inhibitor was in the setting of a clinical trial for antibody-mediated rejection (10).
Infection prophylaxis
All kidney transplant recipients received Pneumocystis prophylaxis for 6 months post-KT (trimethoprim-sulfamethoxazole unless allergic, otherwise atovaquone or dapsone), and antiviral prophylaxis according to CMV status (valganciclovir for 6 months for CMV donor seropositive/recipient seronegative, valganciclovir for 3 months for recipient seropositive, and acyclovir for donor seronegative/recipient seronegative patients). All patients received nystatin oral suspension post-KT and no routine systemic antifungal prophylaxis.
Patients who received eculizumab had received prior meningococcal vaccination, and received prophylactic amoxicillin until 1 month after completion of eculizumab. Patients who received rescue splenectomy generally had received pneumococcal, meningococcal, and Hemophilus influenzae vaccines prior to transplant (as they were judged antecedently high-risk, so the possibility of splenectomy was foreseen and these immunizations administered pre-transplant), or received these vaccinations post-transplant if not received prior to transplant.
In addition, it has been part of our standard of care for all transplant candidates to receive pneumococcal vaccination pre-transplant (most recently, both pneumococcal polysaccharide vaccine and conjugated pneumococcal vaccine).
Infection ascertainment and definitions
Using our center’s electronic medical record, for each individual patient, we collected data on all infections of clinical significance occurring within the first 3 years post-KT, or until the date of death or the last date of follow-up at this center (if either of these events occurred earlier than 3 years post-transplant). All infection data were reviewed by a transplant infectious disease physician. We used a rigorous system of infection data collection that incorporates published consensus definitions of specific infections (12–18), and includes microbiologic, syndromic, and histopathologic data. We reviewed all hospital discharge summaries for the first 3 post-KT years; all pathology biopsy results, and all CMV PCR and BKV PCR results as well as other microbiologic results and clinical notes as needed for additional details on particular infections.
Infections of clinical significance included: i) all infections which required a hospital admission, ii) all infections which occurred during a hospital admission, iii) all instances of cytomegalovirus (CMV) infection, and iv) all instances of BK virus (BKV) infection. For each infection, the infection type (e.g. pneumonia), date of diagnosis, and organism (if known) were recorded. Infections were categorized according to standard definitions (12–16). CMV infection episodes were classified as CMV viremia (DNAemia) versus tissue-invasive CMV disease (end-organ disease), according to standard definitions (17). BK virus infection episodes were classified as BKV viremia (DNAemia) versus BK virus allograft nephropathy (BKVAN), according to standard definitions (18). Mild infections in outpatients such as upper respiratory tract infections, sinusitis, bronchitis, asymptomatic bacteriuria, mildly symptomatic urinary tract infections, and minor skin/soft tissue infections were not considered to be clinically significant infections for purposes of this study.
For purposes of this analysis, opportunistic infections were classified as those infections that would be unlikely to develop in immunocompetent patients, including invasive fungal infections, Pneumocystis jiroveci pneumonia, nocardiosis, disseminated zoster, disseminated adenovirus infection, severe chronic norovirus infection, BKVAN, and tissue-invasive CMV disease.
BKV viremia (DNAemia) was defined as any positive quantitative BKV PCR above the lab-specific lower limit of quantitation of the assay. “Recurrence” of BKV or CMV was defined as a reappearance of BKV or CMV viremia/DNAemia after at least 2 consecutive undetectable PCR results obtained at least 1 week apart.
Time to first post-KT infection
We first used the Kaplan-Meier method to compare the cumulative incidence of infection one-year post-KT by intensity of desensitization. As ILDKT is carefully considered for a specific recipient population, they may differ considerably across baseline characteristics (e.g. calculated panel reactive antibody (cPRA), sex, years on dialysis, etc.) compared to their CLDKT counterparts. Taken with our smaller sample size, estimating the impact of ILDKT, and thus intensity of desensitization, on infection risk may not be meaningful if we were to use conventional regression adjustment methodology. In an effort to correctly quantify this association, we used doubly robust Cox regression (19). Briefly, this two-step approach combines the use of inverse probability weights (IPWs) with regression adjustment to obtain unbiased estimates of risk (20). To start, we generated the IPWs. We used multinomial logistic regression to obtain the propensity score which estimated the likelihood of receiving no, low, moderate, or high levels of desensitization after adjusting for age, sex, race, blood group, cPRA, number of previous kidney transplants, diabetes status, time on dialysis, percentage of time with a functioning kidney transplant during renal-replacement therapy, and year of transplant. We took the reciprocal of the predicted probabilities generated by the propensity score to calculate the IPWs. Weights were stabilized to limit the influence of outliers. We quantified the standardized mean differences in measured covariates to compare balance, and those that remained unbalanced were included in the final model (19). We followed recipients until date of first infection, death, last day evaluated at Johns Hopkins Hospital, or administrative censorship one year after transplant.
Incidence rate of infection
We first used negative binomial regression to calculate the crude incidence rate of the number of infections developed during the first year post-KT by intensity of desensitization. We then used doubly robust negative binomial regression to obtain the incidence rate ratio (IRR) of infection comparing recipients who were desensitized to those who did not receive any desensitization. Model specification for doubly robust estimation of the IRR was the same as that used to evaluate time to first post-KT infection analysis. Person-time was calculated from date of transplant until death, last day evaluated at Johns Hopkins Hospital, or administrative censorship one year after transplant.
Adverse impact of infections
Number of infections developed within 1-year post-KT
We used regression to estimate whether the number of infections developed within the first year of transplant was associated with adverse post-transplant outcomes, including number of hospitalization days within the first year post-KT, biopsy-proven acute cellular rejection, death-censored graft survival (DCGF), and death. Specifically, we used doubly robust negative binomial regression for the outcome of hospitalization days, logistic regression for the outcome of acute cellular rejection, and Cox regression for the outcomes of DCGF and death. For our analysis of DCGF and death, we started following recipients one-year post-KT given the time-varying nature of our exposure.
To define our exposure, we created clinically meaningful categories that were sufficient for well-powered comparisons by dividing the number of infections during the first post-transplant year into the following strata: 0, 1-3, and ≥4 infections. We then used the same two-step approach as previously described to create the IPWs. We used multinomial logistic regression to derive the propensity score which estimated the likelihood of developing 0, 1-3, or ≥4 infections during the first post-KT year, adjusting for the aforementioned characteristics in addition to intensity of desensitization. Weights were stabilized to limit the influence of outliers. We quantified the standardized mean differences in measured covariates to compare balance, and those that remained unbalanced were included in the final model.
ILDKT vs. CLDKT
We included an interaction term in each model to test whether ILDKT recipients had the same risk of the aforementioned post-transplant outcomes as CLDKT recipients with the same number of infections post-KT. For these models, we used adjusted negative binomial regression for the outcome of hospitalization days, logistic regression for the outcome of acute cellular rejection, and Cox regression for the outcomes of DCGF and death. Models were adjusted for the same characteristics as those in prior analyses.
Statistical analysis
Baseline characteristics of ILDKT and CLDKT recipients were compared using ANOVA for normally distributed continuous variables, Kruskal-Wallis rank sum test for non-normally distributed continuous variables, or the chi-squared test for binary or categorical variables. For all weighted analyses, a robust, sandwich estimator was used to prevent overestimation of the variance given that the weights were estimated (20–21). Confidence intervals are reported as per the method of Louis and Zeger (22). All analyses were performed using Stata 16.0/MP for Linux (College Station, Texas).
RESULTS
Study population
We studied 475 LDKT recipients; 215 were incompatible, of whom 47 received low intensity, 74 received moderate intensity, and 94 received high intensity desensitization. Compared to CLDKT (i.e. no desensitization) recipients, ILDKT recipients spent longer time on dialysis prior to transplant (median none= 0.4 years, low=1.1, moderate=2.3, high=2.6, p<0.001), were more likely to have had two or more previous transplants (none=1.2%, low=10.7%, moderate=13.5%, high=12.8%, p<0.001), were more likely to have a cPRA≥80% (none=0.8%, low=29.8%, moderate=32.4%, high=45.7%, p<0.001), and were less likely to receive a kidney from a related donor (none=47.3%, low=36.2%, moderate=29.7%, high=25.5%, p=0.001) (Table 1). There were no differences in CMV donor/recipient serostatus for ILDKT and CLDKT recipients (p=0.2).
Table 1.
Characteristics of living donor kidney transplant recipients, by desensitization intensity (%).
| CLDKT | ILDKT* | ||||
|---|---|---|---|---|---|
| Characteristics | None (n=260) | Low (n=47) | Moderate (n=74) | High (n=94) | p-value |
| Recipient |
|||||
| Type of ILDKT | <0.001 | ||||
| ABOi | 10.6 | 12.1 | 20.2 | ||
| HLAi | - | 89.4 | 75.7 | 56.4 | |
| ABOi & HLAi | 0.0 | 12.2 | 23.4 | ||
| Age (years), mean (SD) | 48.0 (15.3) | 49.8 (14.4) | 46.7 (15.8) | 45.5 (14.1) | 0.4 |
| Female Sex | 41.9 | 46.8 | 50.0 | 57.4 | 0.07 |
| Race/ethnicity | 1.0 | ||||
| White | 70.4 | 76.6 | 73.0 | 70.2 | |
| Black | 22.7 | 17.0 | 20.2 | 21.3 | |
| Other | 6.9 | 6.4 | 6.8 | 8.5 | |
| BMI (kg/m2), mean (SD) | 27.2 (6.2) | 27.1 (5.3) | 26.9 (5.4) | 26.9 (6.4) | 1.0 |
| Cause of ESRD | 0.3 | ||||
| Glomerular Diseases | 21.9 | 23.4 | 29.7 | 23.4 | |
| FSGS | 10.8 | 6.4 | 12.2 | 14.9 | |
| Diabetes | 12.3 | 8.5 | 8.1 | 12.8 | |
| Hypertension | 24.6 | 25.5 | 17.6 | 21.3 | |
| Polycystic Kidney Disease | 13.5 | 6.4 | 5.4 | 8.5 | |
| Other | 16.9 | 29.8 | 27.0 | 19.1 | |
| Years on Dialysis, median (IQR) | 0.4 (0.0, 1.6) | 1.1 (0.0, 3.7) | 2.3 (0.2, 4.4) | 2.6 (1.0, 5.0) | <0.001 |
| Number of Previous Transplants | <0.001 | ||||
| 0 | 91.1 | 48.9 | 47.3 | 54.2 | |
| 1 | 7.7 | 40.4 | 39.2 | 33.0 | |
| ≥2 | 1.2 | 10.7 | 13.5 | 12.8 | |
| cPRA (%) | <0.001 | ||||
| 0 | 90.3 | 40.4 | 41.9 | 29.8 | |
| 1-20 | 3.1 | 4.3 | 2.7 | 5.3 | |
| 21-79 | 5.8 | 25.5 | 23.0 | 19.2 | |
| ≥80 | 0.8 | 29.8 | 32.4 | 45.7 | |
| CMV | 0.2 | ||||
| D+/R− | 14.6 | 14.9 | 13.5 | 10.6 | |
| D+/R+ | 29.2 | 38.3 | 40.5 | 43.6 | |
| Other |
56.2 | 46.8 | 46.0 | 45.8 | |
| Donor |
|||||
| Age, mean (SD) | 44.0 (12.6) | 43.9 (14.0) | 44.7 (11.2) | 44.9 (13.1) | 0.9 |
| Race/ethnicity | 0.2 | ||||
| White | 76.5 | 78.7 | 68.9 | 66.0 | |
| Black | 16.2 | 17.0 | 16.2 | 23.4 | |
| Other | 7.3 | 4.3 | 14.9 | 10.6 | |
| Related donor |
47.3 | 36.2 | 29.7 | 25.5 | 0.001 |
| Immunosuppression |
|||||
| Desensitization Regimen | |||||
| Rituximab | - | 44.7 | 58.1 | 84.0 | <0.001 |
| Eculizumab | - | 10.6 | 6.8 | 21.3 | 0.02 |
| Bortezomib | - | 0.0 | 0.0 | 4.3 | 0.2 |
| C1 Esterase Inhibitor | - | 0.0 | 0.0 | 9.6 | 0.002 |
| Splenectomy | 0.002 | ||||
| Yes | - | 0.0 | 0.0 | 10.6 | |
| Previous History | - | 0.0 | 1.4 | 2.1 | |
| High-Dose IVIg | - | 0.0 | 0.0 | 1.1 | 1.0 |
| Number of desensitization agents received | <0.001 | ||||
| 0 | - | 44.7 | 39.2 | 10.7 | |
| 1 | - | 55.3 | 55.4 | 58.5 | |
| 2 | - | 0.0 | 5.4 | 20.2 | |
| 3 | - | 0.0 | 0.0 | 8.5 | |
| 4 | - | 0.0 | 0.0 | 2.1 | |
| Induction Immunosuppression | <0.001 | ||||
| No induction | 3.5 | 0.0 | 0.0 | 0.0 | |
| Thymoglobulin only | 91.9 | 87.2 | 86.5 | 81.9 | |
| Basiliximab only | 4.6 | 12.8 | 10.8 | 8.5 | |
| Both | 0.0 | 0.0 | 2.7 | 9.6 | |
Low (0-4 plasmaphereses), Moderate (5-9 plasmaphereses), and High (≥10 plasmaphereses)
BMI, body mass index; CLDKT, compatible living donor kidney transplantation; cPRA, calculated panel reactive antibody; CMV; cytomegalovirus; D, donor; ESRD, end-stage renal disease; FSGS; focal segmental glomerulosclerosis; IQR, interquartile range; ILDKT, incompatible living donor kidney transplantation; LDKT living donor kidney transplantation; R, recipient; SD, standard deviation.
Desensitization and induction immunosuppression regimens
Among ILDKT recipients, the number of desensitization agents received increased with increasing intensity of desensitization (Table 1). Recipients who received high intensity desensitization were more likely to receive rituximab (low=44.7%, moderate=58.1%, high=84.0%, p<0.001), eculizumab (low=10.6%, moderate=6.8%, high=21.3%, p=0.02), C1 esterase inhibitor (low=0.0%, moderate=0.0%, high=9.6%, p=0.002), and rescue splenectomy (low=0.0%, moderate=0.0%, high=10.6%, p=0.002) (Table 1). The only use of bortezomib, C1 esterase inhibitor, and rescue splenectomy was in the high-intensity desensitization group. ILDKT recipients were less likely to receive thymoglobulin alone for induction compared to CLDKT recipients (none=91.9%, low=87.2%, moderate=86.5%, high=81.9%, p<0.001).
Time to first post-kidney transplant infection
At 1 month, the post-KT incidence of infection was 19.2%, 25.5%, 28.4%, and 45.9% for recipients who received no desensitization (i.e. CLDKT), low, moderate, and high intensity desensitization (p<0.001) (Figure 1). At 1 year, the cumulative incidence of a post-KT infection rose to 50.1%, 49.8%, 66.0%, and 73.5%, respectively (p<0.001). In weighted models, a trend towards increased risk was seen in patients who received low (weighted hazard ratio [wHR]= 0.551.112.23, p=0.8) and moderate intensity desensitization (wHR=0.901.482.44, p=0.1), and a statistically significant 1.71-fold increased risk of developing an infection during the first year post-KT was seen for those who received high intensity desensitization (wHR=1.101.712.66, p=0.02) (Table 2).
Figure 1.

Time to first infection within 1-year post-transplant, by intensity of desensitization.
Compared to recipients who received no desensitization (i.e. CLDKT), ILDKT recipients had a faster time to infection. At 1 month, the post-KT incidence was 19.2%, 25.5%, 28.4%, and 45.9% for recipients who received none, low, moderate, and high levels of desensitization, respectively (p<0.001). At 6 months, the post-KT incidence was 37.2%, 43.0%, 54.6%, and 70.1% for recipients who received none, low, moderate, and high levels of desensitization (p<0.001). At 1 year, the post-KT incidence was 50.1%, 49.8%, 66.0%, and 73.5% for recipients who received none, low, moderate, and high levels of desensitization (p<0.001).
Table 2.
Incidence of infection at 1-year post-transplant, by intensity of desensitization (%).
| CLDKT | ILDKT | |||
|---|---|---|---|---|
| None | Low | Moderate | High | |
| Time-to-first infection, wHR | ref | 0.551.112.23 | 0.901.482.44 | 1.101.712.66 |
| Rate of infection, wIRR | ref | 0.771.402.56 | 0.881.352.06 | 1.332.223.72 |
Low (0-4 plasmaphereses), Moderate (5-9 plasmaphereses), and High (≥10 plasmaphereses)
Bold indicates p<0.05
wHR; weighted hazard ratio; wIRR, weighted incidence rate ratio; CLDKT, compatible living donor kidney transplantation; ILDKT, incompatible living donor kidney transplantation.
Incidence rate of infection
At 1 year, the crude post-KT infection rate was 96.2 per 100 person-years, 141.0 per 100 person-years, 142.1 per 100 person-years, and 292.9 per 100 person-years for those who received no desensitization (i.e. CLDKT), low, moderate, and high intensity desensitization. In weighted models, a trend towards increased risk was seen in recipients who received low (weighted incidence rate ratio [wIRR]=0.771.402.56, p=0.3) or moderate (wIRR=0.881.352.06, p=0.2) intensity desensitization, and a statistically significant 2.22-fold (wIRR=1.332.223.72, p=0.002) increase in the number of infection cases was seen for those who received high intensity desensitization, compared to recipients who received none during the first year post-KT (Table 2).
Types of infection
There was wide variation in the types of infections experienced by ILDKT and CLDKT recipients in the first-year post-transplant (Table 3) (Figure 2). The most common infections overall were urinary tract infections (UTI) (27.0%), opportunistic infections (15.8%), and BK viremia (15.8%) (Figure 2) (Table 3).
Table 3.
Incidence of infection at 1-year post-transplant, by intensity of desensitization (%).
| CLDKT | ILDKT* | ||||||
|---|---|---|---|---|---|---|---|
| Infection | None (n=260) | p-valuea | Overall (n=215) | Low (n=47) | Moderate (n=74) | High (n=94) | p-valueb |
| Median Number, (IQR) |
1 (0, 2) | <0.001 | 1 (0, 3) | 1 (0, 3) | 1 (1, 3) | 2 (1, 4) | <0.001 |
| Type |
|||||||
| Urinary Tract | 21.5 | 0.004 | 33.5 | 31.9 | 25.7 | 40.4 | 0.005 |
| Opportunistic | 10.8 | <0.001 | 21.9 | 21.3 | 20.3 | 23.4 | 0.008 |
| BK Virus | |||||||
| Viremia | 18.5 | 0.1 | 12.6 | 14.9 | 14.9 | 9.6 | 0.2 |
| BKVAN | 3.1 | 0.005 | 9.3 | 10.6 | 9.5 | 8.5 | 0.02 |
| Bloodstream | 5.4 | <0.001 | 19.1 | 14.9 | 14.9 | 24.5 | <0.001 |
| CMV | |||||||
| Viremia | 10.0 | 0.6 | 12.1 | 6.4 | 8.1 | 18.1 | 0.1 |
| Tissue-Invasive | 0.8 | 0.3 | 2.3 | 0.0 | 2.7 | 3.2 | 0.2 |
| Pneumonia | 4.6 | 0.002 | 12.1 | 8.5 | 10.8 | 16.0 | 0.005 |
| C. difficile | 3.8 | <0.001 | 13.5 | 8.5 | 9.5 | 19.1 | <0.001 |
| Other Viral | 3.5 | 0.007 | 9.8 | 6.4 | 10.8 | 10.6 | 0.02 |
| Surgical-Site | 3.8 | 0.2 | 6.5 | 0.0 | 5.4 | 10.6 | 0.03 |
| Gastroenteritis | 4.6 | 0.8 | 5.1 | 8.5 | 1.4 | 6.4 | 0.2 |
| Invasive Fungal | 2.3 | 0.02 | 7.0 | 2.1 | 2.7 | 12.8 | 0.001 |
| Skin/Soft Tissue | 2.3 | 0.1 | 5.1 | 2.1 | 4.1 | 7.4 | 0.1 |
Low (0-4 plasmaphereses), Moderate (5-9 plasmaphereses), and High (≥10 plasmaphereses)
p-value tests difference between overall ILDKT vs. CLDKT
p-value tests difference between intensity of desensitization vs. CLDKT
BKVAN; BK virus-associated nephropathy; C. difficile; clostridium difficile; CMV, cytomegalovirus; IQR, interquartile range.
Figure 2.

Incidence of infection at 1-year post-transplant, by transplant type.
BKVAN; BK virus associated nephropathy; CMV, cytomegalovirus; C. diff; Clostridium difficile; UTI, urinary tract infection.
There was wide variation in the types of infections developed between ILDKT and CLDKT recipients during the first-year post-transplant. The most common infections were urinary tract infections (UTI) (overall: 27.0%; ILDKT: 15.2%), opportunistic infections (overall: 15.8%; ILDKT: 9.9%), and BK viremia (overall: 15.8%; ILDKT: 5.8%). The least common infections were gastroenteritis (overall: 4.8%; ILDKT: 2.3%), invasive fungal (overall: 4.5%; ILDKT: 3.2%), skin/soft tissue infections (overall: 3.6%; ILDKT: 2.3%), and tissue invasive CMV (overall: 1.5%; ILDKT: 1.1%).
Risks for specific types of infections within the first year differed by intensity of desensitization, generally with highest risks in those who received highest-intensity desensitization (Table 3). Specific infections that followed this pattern included urinary tract infections, opportunistic infections, bloodstream infections, pneumonias, C. difficile diarrhea, surgical site infections, and invasive fungal infections (Table 3). Regarding viral infections, there was no significant difference in risk for tissue-invasive CMV between desensitization groups, but BKVAN and other viral infections were more likely to occur in ILDKT compared to CLDKT recipients (Table 3).
Infection recurrence after the first post-KT year
In the second year post-KT, 23.2% of recipients developed an infection; of these, 66.4% were recurrent infections. ILDKT recipients were more likely to develop recurrent infections compared to CLDKT recipients (none=12.7%, low=19.1%, moderate=13.5%, high=22.3%) (Figure 3). In the third year post-KT, 16.6% of recipients developed an infection; of these, 84.8% were recurrent infections. ILDKT recipients were more likely to develop recurrent infections compared to CLDKT recipients (none=12.7%, low=10.6%, moderate=14.9%, high=19.1%) (Figure 3).
Figure 3.
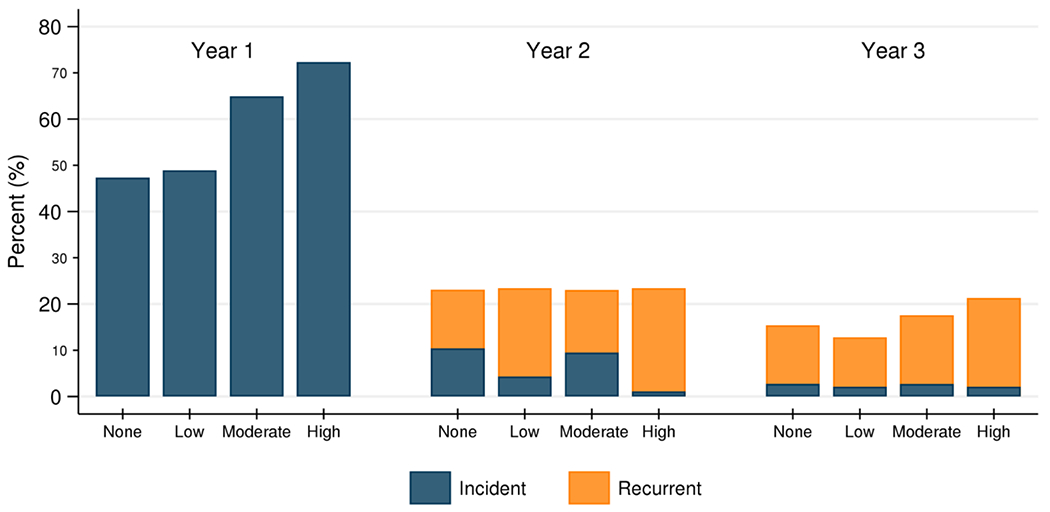
Frequency of incident and recurrent infections from years 1-3 post-transplant, by intensity of desensitization.
ILDKT recipients were more likely to develop both incident and recurrent infections, particularly those who were highly desensitized. In the second year post-KT, 23.2% of recipients developed an infection; of these, 66.4% were recurrent infections (none=12.7%, low=19.1%, moderate=13.5%, high=22.3%). In the third year post-KT, 16.6% of recipients developed an infection; of these, 84.8% were recurrent infections (none=12.7%, low=10.6%, moderate=14.9%, high=19.1%).
Adverse impact of infections
Adverse impact of number of infections within the first year post-KT
Recipients who developed between 1-3 infections within the first year post-KT had a higher rate of hospitalization days (wIRR=1.051.321.66, p=0.02), but no difference in the risk of acute cellular rejection, DCGF, and mortality compared to those who did not develop any infection (Table 4). Recipients who developed ≥4 infections within the first-year post-KT had a substantially higher rate of hospitalization days (wIRR=2.623.574.88, p<0.001), and increased risk of DCGF (wHR=1.154.0113.95, p=0.03), but no difference in the risk of acute cellular rejection and mortality compared to those who did not develop any infection (Table 4).
Table 4.
Adverse impact of infections, according to number of infections developed within 1-year post-KT and transplant type.
| Overalla | CLDKTb | ILDKTb | p for interactionc | |
|---|---|---|---|---|
| Hospitalization days, IRR |
||||
| 0 infections | ref | ref | ref | - |
| 1-3 infections | 1.051.321.66 | 1.091.331.63 | 1.141.441.80 | 0.6 |
| ≥4 infections |
2.623.574.88 | 2.663.905.71 | 2.934.005.44 | 0.9 |
| Acute cellular rejection, OR |
||||
| 0 infections | ref | ref | ref | - |
| 1-3 infections | 0.530.941.67 | 0.621.674.50 | 0.380.761.52 | 0.2 |
| ≥4 infections |
0.230.661.92 | 0.121.1010.01 | 0.300.932.94 | 0.9 |
| Mortality, HR |
||||
| 0 infections | ref | ref | ref | - |
| 1-3 infections | 0.460.931.88 | 0.822.135.49 | 0.340.811.91 | 0.1 |
| ≥4 infections |
0.431.153.03 | 0.291.497.63 | 0.862.255.89 | 0.7 |
| Death-censored graft failure, HRd |
||||
| 0 infections | ref | |||
| 1-3 infections | 0.661.825.05 | - | - | - |
| ≥4 infections | 1.154.0113.95 |
Bold indicates p<0.05
Overall: weighted analyses (i.e. wIRR, wOR, wHR)
CLDKT/ILDKT: adjusted analyses (i.e. aIRR, aOR, aHR)
p for interaction compares CLDKT vs. ILDKT
Did not achieve convergence for CLDKT and ILDKT estimates
HR; hazard ratio; IRR, incidence rate ratio; OR, odds ratio; CLDKT, compatible living donor kidney transplantation; ILDKT, incompatible living donor kidney transplantation.
Recurrent Infections Ultra-High-Risk Group
There were 10 recipients (including 7 who had received high-intensity desensitization) who developed 7–9 infections, and 7 recipients (all of whom had received high-intensity desensitization) who developed ≥10 infections within the first-year post-KT. For those who developed 7–9 infections: 4 recipients received thymoglobulin (2 of whom did not receive plasmapheresis [i.e. CLDKT]); 2 recipients received thymoglobulin and rituximab; 2 recipients received thymoglobulin, basiliximab, and rituximab; 1 recipient received thymoglobulin, basiliximab, and splenectomy; 1 recipient received thymoglobulin, rituximab, eculizumab, splenectomy, and bortezomib. Similarly, for those who developed ≥10 infections: 4 recipients received thymoglobulin and rituximab; 1 recipient received thymoglobulin, rituximab, and eculizumab; 1 recipient received basiliximab, rituximab, and eculizumab; 1 recipient received thymoglobulin, rituximab, eculizumab, and splenectomy.
Although these numbers were too small for stable statistical analysis, the group who developed 7–9 infections had a median of 106 hospitalization days (IQR 98, 117) and mortality of 60%, and the group who developed ≥10 infections had a median of 117 hospitalization days (IQR (95, 224) and mortality of 43%.
Effect modification of adverse impact of infections: ILDKT vs CLDKT
To determine if ILDKT status was associated with additional risk beyond that associated with number of infections post-KT, we included an interaction term in each model to test whether ILDKT recipients had the same risk of adverse post-transplant outcomes as CLDKT recipients with the same number of infections post-KT. The risk of hospitalization, acute cellular rejection, or mortality was no higher for ILDKT than for CLDKT recipients, irrespective of the number of infections developed (p for interactions ≥0.1) (Table 4).
DISCUSSION
In this single-center study using systematic, granular ascertainment of post-transplant infections in 475 living donor kidney recipients, ILDKT recipients were at higher risk than CLDKT recipients for many infectious complications during the first year post-KT. At 1 year, the cumulative incidence of a post-KT infection ranged from 50.1% in those who received no desensitization to 73.5% for recipients who received high intensity desensitization. We found a comparable incidence rate of infection for recipients who received low or moderate intensity desensitization, but a 2.2-fold higher incidence rate of infection for those who received high intensity desensitization compared to recipients who received none. A higher incidence of urinary tract infections, bloodstream infections, pneumonias, Clostridium difficile, surgical-site infections, invasive fungal infections, and opportunistic infections was associated with increasing intensity of desensitization.
Additionally, recipients who developed infections had significantly more hospitalization days than those with no infections during the first post-KT year. Specifically, recipients with 1-3 infections during the first post-KT year had a 1.3-fold increase, and those with ≥4 infections had a 3.6-fold increase in the rate of hospitalization day. Further, recipients with ≥4 infections had a 4.0 –fold higher risk of death-censored graft failure. A small group of patients, most of whom had undergone high-intensity desensitization, had extremely high numbers of infections during the first post-transplant year (10 had 7 – 9 infections, and 7 had ≥10 infections); these patients had a median of 106 and 117 hospitalization days, respectively; 60% of those with 7 - 9 infections and 43% of those with ≥10 infections died. There is clearly much to be learned about the heterogeneity within the ILDKT group, and not all of this can be explained by intensity of desensitization, as only 14/94 (15%) of those who had received high-intensity desensitization were in this ultra-high-risk group with 7 or more infections.
Previous studies that have reported infection data in HLAi ILDKT recipients have reported divergent results (23–27). Kahwaji et al. found no significant differences in infection risk between ILDKT who received rituximab plus IVIg for desensitization, compared with a non-sensitized, ABO-compatible group (23). By contrast, Kamar et al. reported increased risk for fungal, and decreased risk for viral infections, with use of rituximab for any indication in KT recipients (24). The Korean national registry study reported infections in terms of causes of death, but did not include data on incidence of infections or specific infections (27). Reasons for these heterogeneous results could include differences in patient populations, immunosuppression and desensitization protocols, and infection definitions and ascertainment. This heterogeneity underscores the need for more research to delineate differential risks between ILDKT and CLDKT groups.
Our study has some limitations worthy of mention. First, as with any single-center investigation, there are always limitations to generalizability. However, our findings are likely generalizable to other centers using PP/IVIG based protocols, which represents the majority of HLAi centers worldwide. Additionally, our findings are likely generalizable to other protocols, given that we found a “dose-response” with intensity of desensitizationSecond, infections resulting in admission to other medical centers may not have been reported in our transplant center’s electronic medical record; however, particularly in the first post-KT year, our center is vigilant about obtaining outside medical records on our recipients, and most patients hospitalized at outside centers with serious infections are transferred to our center for further management. Therefore, we feel that focusing only on the most serious infections, and on the first post-transplant year, has minimized the likelihood of missing substantial numbers of these infections.
In conclusion, ILDKT recipients are at increased risk for recurrent post-transplant infection, particularly those undergoing high-intensity of desensitization (≥10 plasmapheresis treatments, +/− rituximab, bortezomib, or other agents). These post-KT infections have a substantial impact on outcomes: development of ≥4 infections in the first year was associated with increased hospitalization days and death-censored graft failure. With these adverse outcomes in mind, optimizing infection prevention is crucial in this population. Further study is required before firm recommendations can be made, but interventions might include more frequent monitoring of microbiologic and immunologic parameters in high-risk patients, and/or individualized antimicrobial prophylaxis programs based on each patient’s previous patterns of infection. A better understanding of the high-risk phenotype for infections will enable development of improved protocols for infection prevention in the most vulnerable patients.
ACKNOWLEDGMENTS
This work was supported by grant numbers F32DK113719 (Jackson), K01DK101677 (Massie), RO1DK098431 (Segev), K24AI144954 (Segev), and K23DK115908 (Garonzik-Wang) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). This work was also supported by grant number K24AI085118 (Marr) from the National Institute of Allergy and Infectious Diseases (NIAID). Dr. Garonzik-Wang is supported by a Clinician Scientist Development Award from the Doris Duke Charitable Research Foundation. The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation.
R Avery: Study grant support from Aicuris, Astellas, Chimerix, Merck, Oxford Immunotec, Qiagen, Takeda/Shire.
R. A. Montgomery: Study grant support from Hansa Medical AB; Consulting income from Immucor kSORT, Biologix LLC, RMEI, Shire, and Vitaeris/CSL Behring.
K.A. Marr: Consulting / advisory board income from Amplyx, Chimerix, Cidara, Merck, Scynexis, and Sfunga. Equity / licensing review from MycoMed Technologies.
D. Segev: Consulting/speaking honoraria from Sanofi, Novartis, CSL Behring, and Veloxis.
Abbreviations:
- ABOi
ABO blood group incompatible
- aHR
adjusted hazard ratio
- AMR
antibody-mediated rejection
- aOR
adjusted odds ratio
- BKV
BK virus
- BKVAN
BK virus allograft nephropathy
- BMI
body mass index
- CLDKT
compatible living donor kidney transplant
- cPRA
calculated panel reactive antibody
- CMV
cytomegalovirus
- DSA
donor-specific antibody
- ESRD
end-stage renal disease
- HLAi
human leukocyte antigen incompatible
- HRSA
Health Resources and Services Administration
- ILDKT
incompatible living donor kidney transplant
- IRR
incidence rate ratio
- IPWs
inverse probability weights
- IVIg
intravenous immunoglobulin
- KPD
kidney paired donation
- KT
kidney transplant
- LDKT
living donor kidney transplant
- OPTN
Organ Procurement and Transplantation Network
- PCR
polymerase chain reaction
- SRTR
Scientific Registry of Transplant Recipients
- UTI
urinary tract infection
Footnotes
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Segev DL, Gentry SE, Warren DS, Reeb B, Montgomery RA et al. Kidney paired donation and optimizing the use of live donor organs. JAMA 2005; 293:1883–90. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery RA, Lonze BE, King KE et al. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med 2011; 365: 381–26. [DOI] [PubMed] [Google Scholar]
- 3.Orandi BJ, Luo X, Massie AB, et al. Survival benefit with kidney transplants from HLA-incompatible live donors. N Engl J Med 2016; 374: 940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holscher CM, Jackson K, Chow EKH, et al. Kidney exchange match rates in a large multicenter clearinghouse. Am J Transplant 2018; 18:1510–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holscher CM, Jackson KR, Segev DL. Transplanting the untransplantable. Am J Kidney Dis 2020; 75:114–123. [DOI] [PubMed] [Google Scholar]
- 6.Wongsaroj P, Kahwaji J, Vo A, Jordan S. Modern approaches to incompatible kidney transplantation. World J Nephrol 2015; 4(3): 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vo AA, Lukovsky M, Toyoda M, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation N Engl J Med; 2008; 359: 242–251.. [DOI] [PubMed] [Google Scholar]
- 8.Lonze BE, Dagher NN, Simpkins CE, et al. The fate of anti-HLA antibody among renal transplantation recipients treated with bortezomib. Clin Transplant 2009; 377–384. [PubMed] [Google Scholar]
- 9.Orandi BJ, Zachary AA, Dagher NN, et al. Eculizumab and splenectomy as salvage therapy for severe antibody-mediated rejection after HLA-incompatible kidney transplantation. Transplantation 2014; 98: 857–63. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery RA, Orandi BJ, Racusen L, et al. Plasma-derived C1 esterase inhibitor for acute antibody-mediated rejection following kidney transplantation: results of a randomized double-blind placebo-controlled pilot study. Am J Transplant 2016; 16:3468–3478. [DOI] [PubMed] [Google Scholar]
- 11.Scientific Registry of Transplant Recipients home page (http://www.srtr.org/)
- 12.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSB) Consensus Group. Clin Infect Dis 2008; 46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63:e61–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomkin JS. Evaluating evidence and grading recommendations: the SIS/IDSA guidelines for the treatment of complicated intra-abdominal infections. Surg Infect (Larchmt) 2010: 11: 269–74. [DOI] [PubMed] [Google Scholar]
- 15.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49:1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parasuruman Julian K. Urinary tract infections after transplantation. Am J Transplant 2013; 13(s4): 327–336. [DOI] [PubMed] [Google Scholar]
- 17.Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis 2017; 64:87–91. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch HH, Randhawa P. BK polyomavirus in solid organ transplantation. Am J Transplant 2013; 13 Suppl 4: 179–88. [DOI] [PubMed] [Google Scholar]
- 19.Funk MK, Westreich D, Wiesen C, Sturmer T, Brookhart MA, Davidian M. Doubly robust estimation of causal effects. Am J Epidemiol 2011; 173: 761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Statist Med 2015; 34:3661–3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joffe MM, Ten Have TR, Feldman HI, Kimmel SE. Model selection, confounder control, and marginal structural models. Am Statistician 2004; 58:272–279. [Google Scholar]
- 22.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics 2009; (1): 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahwaji J, Sinha A, Toyoda M, et al. Infectious complications in kidney-transplant recipients desensitized with rituximab and intravenous immunoglobulin. Clin J Am Soc Nephrol 2011; 6:2894–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamar N, Milioto O, Puissant-Lubrano B, et al. Incidence and predictive factors for infectious disease after rituximab therapy in kidney-transplant patients. Am J Transplant 2010; 10:89–98. [DOI] [PubMed] [Google Scholar]
- 25.Couzi L, Manook M, Perera R, et al. Difference in outcomes after antibody-mediated rejection between ABO-incompatible and positive cross-match transplantations. Transpl International 2015; 1205–1215. [DOI] [PubMed] [Google Scholar]
- 26.Sharif A, Alachkar N, Bagnasco S, et al. Incidence and outcomes of BK virus allograft nephropathy among ABO- and HLA-incompatible kidney transplant recipients. Clin J Am Soc Nephrol 2012; 7:1320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ko EJ, Yu JH, Yang CW, Chung BH, and the Korean Organ Transplantation Registry Study Group. Clinical outcomes of ABO- and HLA-incompatible kidney transplantation: a nationwide cohort study. Transpl Int 2017; 30: 1215–1225. [DOI] [PubMed] [Google Scholar]


