Abstract
Objectives
We sought to determine whether unhealthy behaviors play a stress-buffering role in observed racial disparities in physical and mental health.
Methods
We conducted logistic regressions by race on data from the first 2 waves of the Americans’ Changing Lives Survey to determine whether unhealthy behaviors had buffering effects on the relationship between major stressors and chronic health conditions, and on the relationship between major stressors and meeting the criteria for major depression.
Results
Among Whites, unhealthy behaviors strengthened the relationship between stressors and meeting major-depression criteria. Among Blacks, however, the relationship between stressors and meeting major-depression criteria was stronger among those who had not engaged in unhealthy behaviors than among those who had. Among both race groups there was a positive association between stressors and chronic health conditions. Among Blacks there was an additional positive association between number of unhealthy behaviors and number of chronic conditions.
Conclusions
Those who live in chronically stressful environments often cope with stressors by engaging in unhealthy behaviors that may have protective mental-health effects. However, such unhealthy behaviors can combine with negative environmental conditions to eventually contribute to morbidity and mortality disparities among social groups.
The strain of living under inhospitable environmental conditions is hypothesized to result in physical health disparities among racial groups.1–3 In addition, the inequities associated with inhospitable environments—inequalities in employment, income, and educational opportunities that favor non-Hispanic Whites over Blacks—are hypothesized to cause not only poorer physical health but also worse mental health among Blacks. However, epidemiological and clinical data show that in comparison with non-Hispanic Whites, Blacks suffer the same or lower rates of most major mental disorders, even while suffering higher rates of psychological distress.4–7 These apparently contradictory disparities in physical and mental health statuses raise questions about the presumed relationships among negative life conditions and stressors on the one hand and poor physical health and mental disorders on the other.1,3
RACIAL AND ETHNIC HEALTH DISPARITIES
Compared with Americans of European descent, Black Americans have greater physical health morbidity and mortality at every age.8 For example, Black women are twice as likely as White women to die of hypertensive cardiovascular disease. In addition, Blacks have a lower average life expectancy (70 years) than Whites do (77 years), with Black men having a life expectancy of only 66 years. Although the causes of these differences are debated, what is notable is how consistently these physical health disparities favor non-Hispanic Whites over Blacks.9
Conversely, psychiatric epidemiological surveys find that Blacks in noninstitutionalized populations have lower-than-expected rates of most major mental disorders. For example, the Epidemiological Catchment Area study found roughly comparable rates of mental disorders for Blacks and Whites10; age-adjusted analyses by gender and study site did not suggest higher levels of lifetime or 6-month prevalence of major depression among Blacks.11 Even more striking, results from the National Comorbidity Study and the recently concluded Collaborative Studies of Psychiatric Epidemiology revealed that rates of mental disorders, especially mood disorders, were consistently lower for Black Americans than they were for White Americans.6,12
We theorize that, over the life course, coping strategies that are effective in “preserving” the mental health of Blacks may work in concert with social, economic, and environmental inequalities to produce physical health disparities in middle age and late life.13 Exposures to stress-inducing events are more accessible to one’s consciousness than the biological degenerations (e.g., growth in tumors, atherosclerosis, and so on) that eventuate in physical health ailments and chronic health conditions (e.g., heart disease, cancer). Thus, we hypothesize that when individuals are chronically confronted with stressful conditions in daily life (e.g., poverty, crime, poor housing), they will engage in unhealthy behaviors (e.g., smoking, alcohol use and abuse, drug use, and overeating, especially of comfort foods) that help to alleviate the resulting symptoms of stress.7,14 However, these same behaviors silently contribute to physical health morbidities and early mortality. Thus, we hypothesize that engaging in unhealthy behaviors alleviates the symptoms of stress and the possible biological cascade to mental disorders while simultaneously combining with the effects of poor living conditions to contribute to the development of physical health ailments and chronic physical health disorders later in life.13
We believe that these unhealthy behaviors may either block the neurologic cascade or mask the physiological and psychological experiences of poor mental health by acting on the hypothalamic-pituitary-adrenalcortical (HPA) axis and related biological systems.15 These unhealthy behaviors may have salubrious effects by helping stave off mental disorders among some race groups, but the direct effects of stressful living16 combine with the direct effects of the unhealthy behaviors themselves to create large physical health disparities that are unfavorable to Blacks.4
THE HYPOTHALAMIC–PITUITARY–ADRENALCORITCAL AXIS
The physiological “stress response” likely evolved to deal with acute stressors (usually short-term, life-threatening stressors) by mobilizing energy for immediate use and suppressing nonessential systems.17 Although the stress response is well-adapted to deal with acute stressors, chronic activation of the system—as is often the case for those with poor living conditions and psychological stressors—results in poor psychological and physical health outcomes.18 Several biological systems are activated by stress, but we focus on the HPA axis and the implications this system has for negative health behaviors that may buffer the effects of stress on mental disorders.
When an organism experiences stress, the HPA axis response begins with the release of corticotropin-releasing factor (CRF) from the hypothalamus, stimulating the release of adrenocorticotropic hormone (ACTH) from the pituitary gland. ACTH travels through the bloodstream to stimulate the release of cortisol from the adrenal cortex. Via a negative feedback loop, cortisol then acts on the hypothalamus and pituitary gland to shut down the release of both CRF and ACTH.
Recent research from Dallman et al.19,20 suggests that consumption of foods that are high in fats and carbohydrates reduces anxiety via feedback to the HPA axis. During chronic stress, the negative feedback loop through which cortisol regulates further release of CRF breaks down as glucocorticoid receptors are downregulated and the release of CRF continues. Continued release of CRF is associated with feelings of anxiety as CRF mRNA expression in the amygdala is increased20; consuming comfort foods aids in the “shutdown” of the stress response by regulating the release of CRF. Abdominal fat deposits resulting from comfort food consumption signal increased metabolic energy stores, which in turn decrease the expression of CRF mRNA in the hypothalamus via the inhibition of catecholamine production in the nucleus of the tractus solitarius. Put more succinctly, eating comfort food reduces anxiety by inhibiting the release of CRF.19,20
High rates of obesity are observed in Black populations, particularly among women,21 and it is believed that consuming large amounts of comfort foods may contribute to this condition. Consuming comfort foods may be a socially accepted, gender-appropriate way of dealing with chronic stress among this population.3 Also, sources of comfort foods may be more prominent in poorer and Black communities because of the proliferation of fast-food outlets and convenience stores in these areas. However, this stress-reduction technique imposes a cost. Chronic activation of the HPA axis has been linked to type II diabetes via promotion of insulin resistance in fat cells. In addition, consumption of high-fat, high-carbohydrate foods is related to stroke, cardiovascular disease, and other disorders.
Alcohol intake is believed to reduce anxiety and relieve tension.22 Human studies reveal that there is a positive relationship between stress levels and negative psychological states on the one hand and alcohol consumption on the other.23,24 We believe that alcohol’s simultaneous elevation of dopamine and β-endorphin levels in the brain results in a feeling of relaxation and subjective release from stress.25 Thus, alcohol consumption activates the HPA axis, increases release of dopamine and β-endorphins, and likely reduces feelings of stress.26
Smoking and nicotine ingestion are often reported to result in mild euphoria, increased energy, suppressed appetite, and a sense of well-being.27 Nicotine is thought to reduce stress-related anxiety,28 and researchers have focused on how the HPA axis is affected by tobacco use. Research in both humans and animals has found evidence that nicotine increases levels of stress hormones,29,30 suggesting that nicotine has an anxiogenic effect. Nicotine has other neurologic effects, however, that may explain the anxiolytic effects reported by individuals who use tobacco. Paradoxically, the release of stress hormones in response to nicotine actually mediates the response of the mesolimbic dopamine system, giving rise to feelings of relaxation, reduced anxiety, and calm.31
The same pattern of physiological responses is also found following the use of illegal stimulants.32 In addition, these drugs also activate the HPA axis, which may increase the allostatic load of the individual.18 Thus, although individuals may be protected from the psychological effects of stress, they are not protected from its physical effects.
METHODS
We analyzed data from the first 2 waves (collected in1986 and 1989) of the Americans’ Changing Lives study, conducted by the Survey Research Center, Institute for Social Research, University of Michigan. The Americans’ Changing Lives study is a multistage, stratified, area-probability sample of noninstitutionalized people aged 25 years and older residing in the 48 contiguous United States. Data were collected in face-to-face interviews in wave 1 and largely by telephone in wave 2. In the initial pool of respondents, there was an over-sampling of Blacks and people aged 60 years or older. The full wave 1 sample included 3617 respondents, for a 68% response rate; the full wave 2 sample included 2867 individuals, 83% of wave 1 respondents who were still alive in 1989. For the current analyses, our starting sample included Blacks (n = 874) and Whites (n = 1906) who responded at both waves.33
Measures
Because we conceptualized our model as a series of processes that unfold over time, we used predictor measures from variables collected at wave 1, and we used outcome measures from wave 2 assessments. The wave 2 major-depression measure represented an algorithm that includes survey questions; the lead-in question was a modified version of the stem question from Diagnostic Interview Schedule version III-R and assessed whether the respondent has ever had a time in their life lasting an entire week when they felt sad, blue, or depressed most of the time, or when they lost interest in all things.34 Respondents who replied yes to this screening question were asked additional questions regarding duration of episodes, timing of episodes, and symptoms needed to ascertain whether a person met DSM-II-R criteria for major depression. The final measure incorporates all of these items and indicates whether the respondent had met the criteria for major depression since wave 1 data collection.35
The depression-assessment questions were not asked at wave 1, but we wanted to predict change in depression status, so we included as a control a dichotomous version of the Center for Epidemiological Studies–Depression Scale (CES-D) that was assessed at wave 1. The CES-D is a measure of depressive symptoms, an 11-item measure scaled to 20 by multiplying the sum by 1.818. A CES-D score of 16 or higher suggests that criteria for clinical depression have been met.36 Several studies have shown that Blacks have more depression-related symptoms than do Whites but do not meet criteria for major depression on instruments based on the Diagnostic Interview Schedule or the Composite International Diagnostic Interview; therefore, using wave 1 CES-D scores represents a conservative strategy for assessing new cases between waves 1 and 2.
The physical health measure represented a count of the total number of chronic health conditions that respondents reported experiencing within the prior year. The list presented to respondents included 10 conditions: arthritis/rheumatism, lung disease, hypertension, heart attack or heart trouble, diabetes, cancer or any malignant tumor, stroke, broken or fractured bone, foot problems, and urinary incontinence. Because of slight skewness of this count measure, we modeled a 2-level version of this variable that was split at the median (respondents at or below the median were coded as 0, and those above the median were coded as 1).
Sex and region were both 2-level variables, with 1 =males and 1 =residing in the South. Respondent’s age and highest level of education were measured in years. Employment status and occupation were 2-level variables, with 1 =employed and 1 =blue-collar job. Poverty was a size-adjusted measure of household income, calculated by dividing total household income by the official US poverty threshold37 corresponding to the size of the household. Values above 1.0 represented households where income exceeds officially defined needs.
Respondents were asked if they had experienced any of 9 stressful events within given time frames: serious physical attack, life-threatening illness, or accidental injury at any point in one’s life; moved to a new residence, involuntarily lost job (excluding retirement), robbery or burglary, or other upsetting event within the prior 3 years; and providing care to a friend or family member who needs assistance or having an injury or other sudden crisis within the prior year. The stressors measure represented the total count of these 9 events that the respondent had experienced.
Unhealthy behavior represented a count of negative health behaviors, including smoking cigarettes (currently or ever), drinking alcohol (ever), and being obese, defined by having a body mass index ([BMI; defined as weight in kilograms divided by height in meters squared]) of 30 kg/m2 or more.
Analyses
The χ2 test and t test were conducted to assess differences between Blacks and Whites on all measures included in the analyses. Parallel logistic regression analyses were run within race groups to examine key segments of the framework. Specifically, the analyses examined the direct and moderating effects of stressors and unhealthy behaviors on meeting DSM-III criteria for depression, and on being in the higher of a 2-category measure of chronic conditions experienced within the prior 12 months. Moderating effects were assessed by including an interaction term for stressors × unhealthy behavior. The interaction terms included a mean-centered version of the stressors variable; when using a continuous variable as an interaction term, a mean-centered construction of the variable reduces collinearity between the interaction term and the main effect.38 A Wald test of difference was calculated to confirm the differential magnitude of the stressors × unhealthy behavior interaction in predicting depression.
RESULTS
Table 1 presents a comparative overview of the study variables for Blacks and Whites. The results suggest that there were no race differences in the percentage of respondents meeting DSM-III criteria for depression (13.0% of Blacks versus 12.5% of Whites); however, there was a greater percentage of Blacks than Whites with a high level of chronic conditions (46.6% of Blacks versus 36.4% of Whites). There were a number of race differences in the control and socioeconomic measures, and in the stressors and unhealthy behaviors measures. Overall, the data reveal that compared with Whites, Blacks lived in more precarious socioeconomic circumstances but had experienced fewer major life stressors (1.4 versus 1.8) and had engaged in slightly fewer unhealthy behaviors (1.3 versus 1.3, with rounding).
TABLE 1.
Sample Characteristics, by Race/Ethnicity: Americans’ Changing Lives Survey, 1986 and 1989
| Black | White | t or χ2 | |
|---|---|---|---|
| Dependent measures (wave 2) | |||
| Meets DSM-III depression criteria, % (no.) | 13.0 (869) | 12.5 (1896) | 0.68 |
| Has 2-level chronic conditions, % | 46.6 | 36.4 | 26.1* |
| Control measures (wave 1) | |||
| Meets CES-D depression criteria, % (no.) | 35.0 (871) | 21.4 (1900) | 58.3* |
| Male, % | 32.2 | 37.8 | 8.2* |
| Age, y | 1.9* | ||
| Mean (SD) | 52.4 (16.8) | 53.7 (17.2) | - |
| Range | 24–96 | 25–91 | |
| Residence in South, % | 56.1 | 32.5 | 139.0* |
| Socioeconomic measures (wave 1) | |||
| Education, no. | 872 | 1904 | 12.8* |
| Mean y (SD) | 10.4 (3.7) | 12.3 (3.0) | - |
| Range, y | 0–17 | 0–17 | |
| Poverty ratioc | −16.7* | ||
| Mean (SD) | 1.6 (1.8) | 3.0 (2.5) | |
| Range | 0.1–15.9 | 0.10–17.0 | |
| Employed,d % | 52.2 | 54.4 | 1.2 |
| Blue-collar occupation, % | 31.7 | 18.6 | 58.3* |
| Stressor and unhealthy behavior measures (wave 1) | |||
| Stressors | −6.8* | ||
| Mean (SD) | 1.4 (1.3) | 1.8 (1.4) | |
| Range | 0–6 | 0–8 | |
| Unhealthy behaviorsb | −2.0* | ||
| Mean (SD) | 1.3 (0.9) | 1.3 (0.8) | |
| Range | 0–3 | 0–3 | |
Note. CES-D = Center for Epidemiological Studies—Depression Scale; DSM-III = Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition. Unless otherwise noted, for Blacks, n = 874; for Whites, n = 1906. For differences in race ethnicity, the t test was used for continuous variables and the χ2 test for dichotomous variables.
An 11-item measure scaled to 20 by multiplying the sum by 1.818. A CES-D score of 16 or higher represents meeting the criteria for clinical depression.
Unhealthy behaviors include smoking (current or ever), drinking (ever), and being obese (defined as having a body mass index [weight in kilograms divided by height in meters squared] of 30 or higher). Reported difference in count of unhealthy behaviors reflects rounding; the mean for Blacks is 1.2746, and the mean for Whites is 1.3410.
Lower scores indicate more impoverished status.
For Whites, n = 1905.
P < .05.
Table 2 presents the logistic regression models predicting depression, run separately for Blacks and Whites. The models included demographic control measures, indicators of socioeconomic status, a count of stressful life events (stressors), and the interaction between stressors and unhealthy behaviors. The key finding here is that the direction of the interaction term among Blacks (odds ratio [OR]=0.81; 95% confidence interval [CI]=0.67, 0.97) is opposite the direction of the interaction term among Whites (OR=1.11; 95% CI=0.98, 1.25). The interaction term for Whites is only significant at the trend level (P<.10).
TABLE 2.
Results of Logistic Regressions Predicting DSM-III Depression at Wave 2 Among Blacks (n=864) and Whites (n=1887): Americans’ Changing Lives Survey, 1986 and 1989
| Blacks, OR (95% CI) | Whites, OR (95% CI) | |
|---|---|---|
| Control measures (wave 1) | ||
| Male | 0.90 (0.56, 1.46) | 0.51* (0.37, 0.72) |
| Age, y | 0.97* (0.96, 0.99) | 0.99* (0.98, 1.00) |
| Residence in South | 0.93 (0.60, 1.43) | 1.19 (0.87, 1.61) |
| Meets CES-D depression criteriaa | 2.63* (1.70, 4.08) | 2.40* (1.77, 3.27) |
| Socioeconomic measures (wave 1) | ||
| Education, y | 0.94 (0.86, 1.02) | 0.97 (0.92, 1.03) |
| Poverty ratiob | 0.99 (0.86, 1.14) | 0.93 (0.86, 1.00) |
| Employed | 1.80 (0.95, 3.41) | 1.30 (0.87, 1.94) |
| Blue-collar occupation | 0.56 (0.31, 1.02) | 1.08 (0.71, 1.65) |
| Stressor and unhealthy behavior measures (wave 1) | ||
| Stressorsc | 1.64* (1.22, 2.21) | 1.15 (0.94, 1.41) |
| Unhealthy behaviorsd | 0.90 (0.69, 1.15) | 0.97 (0.79, 1.18) |
| Stressors × unhealthy behaviors | 0.81* (0.67, 0.97) | 1.11 (0.98, 1.25) |
| Wald χ2 | 61.9* | 124.9* |
| Rescaled R2 | 0.14 | 0.13 |
Note. CES-D = Center for Epidemiological Studies—Depression Scale; CI = confidence interval; DSM-III = Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition; OR = odds ratio.
An 11-item measure scaled to 20 by multiplying the sum by 1.818. A CES-D score of 16 or higher represents meeting the criteria for clinical depression.
Lower scores indicate more impoverished status.
Stressors represent a count of stressful life events that respondents reported experiencing. The inventory of events included 9 events (e.g., loss of job, physical attack, serious injury, and so on).
Unhealthy behaviors include smoking (current or ever), drinking (ever), and being obese (defined as having a body mass index [weight in kilograms divided by height in meters squared] of 30 or higher). The Wald test of difference between Blacks and Whites for unhealthy behaviors is 7.70; P = .09; df=1.
P < .05.
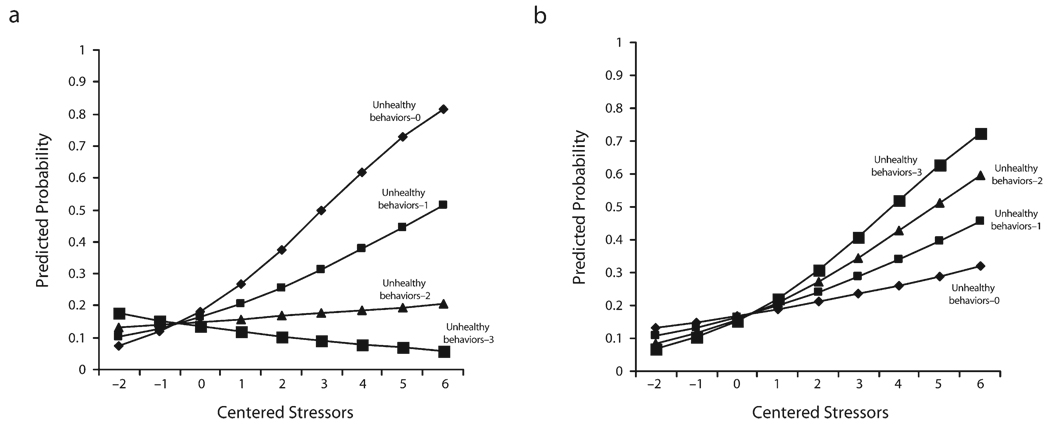
Figure 1 depicts the moderating effect of unhealthy behaviors for Blacks and Whites separately. The relationship between stressors and meeting criteria for depression varied by the level of unhealthy behaviors; however, the direction of the effect of poor health behavior was strikingly different for Blacks and Whites. Among Blacks, we found that the relationship between stressors and meeting criteria for depression was stronger among those who had engaged in none of the unhealthy behaviors than among those who had engaged in unhealthy behaviors (Figure 1a). In contrast, among Whites, there was an increasingly more positive association between stressors and meeting criteria for depression at higher levels of unhealthy behaviors, and unhealthy behavior exacerbated the relationship between stressors and depression (Figure 2b).
FIGURE 1.
Logistic regression model predicting DSM-III depression at different levels of unhealthy behaviors, by stressor, for (a) Blacks and (b) Whites: Americans’ Changing Lives survey, 1986 and 1989.
In Table 3 , we present 2 statistical models for both Blacks and Whites, predicting a 2-level measure of chronic conditions. Model 1 includes all measures, with the exception of the stressors × unhealthy behaviors interaction, and model 2 includes all measures. Those results suggest that among both Blacks and Whites there was a positive association between stressors and the higher of the 2 levels of chronic conditions. Among Blacks there was an additional positive and independent relationship between number of unhealthy behaviors and chronic conditions. Importantly, we did not find evidence of unhealthy behaviors moderating the effect of stressors on physical health among Blacks or Whites.
TABLE 3.
Results of Logistic Regressions Predicting 2 Levels of Chronic Health Conditions at Wave 2: Americans’ Changing Lives Survey, 1986 and 1989
| Blacks (n = 872) | Whites (n = 1903) | |||
|---|---|---|---|---|
| Model 1, OR (95% CI) | Model 2, OR (95% CI) | Model 1, OR (95% CI) | Model 2, OR (95% CI) | |
| Control measures (wave 1) | ||||
| Male | 0.47* (0.33, 0.68) | 0.47* (0.33, 0.68) | 0.61* (0.48, 0.78) | 0.61* (0.48, 0.77) |
| Age, y | 1.07* (1.06, 1.09) | 1.07* (1.06, 1.09) | 1.07* (1.06, 1.08) | 1.07* (1.06, 1.07) |
| Residence in South | 1.69* (1.21, 2.37) | 1.69* (1.21, 2.37) | 1.12 (0.89, 1.42) | 1.12 (0.89, 1.42) |
| Socioeconomic measures (wave 1) | ||||
| Education, y | 0.95* (0.89, 1.00) | 0.95* (0.89, 1.00) | 0.93* (0.89, 0.97) | 0.93* (0.89, 0.97) |
| Poverty ratioa | 0.96 (0.86, 1.08) | 0.96 (0.86, 1.07) | 0.99 (0.94, 1.03) | 0.99 (0.94, 1.03) |
| Employed | 0.63 (0.38, 1.06) | 0.63 (0.38, 1.07) | 0.80 (0.59, 1.06) | 0.79 (0.59, 1.06) |
| Blue-collar occupation | 1.40 (0.85, 2.30) | 1.41 (0.86, 2.31) | 1.15 (0.81, 1.63) | 1.15 (0.81, 1.63) |
| Stressor and unhealthy behavior measures (wave 1) | ||||
| Stressors | 1.27* (1.11, 1.45) | 1.41* (1.10, 1.81) | 1.38* (1.27, 1.50) | 1.43* (1.22, 1.69) |
| Unhealthy behaviorsb | 1.33* (1.09, 1.62) | 1.30* (1.06, 1.59) | 1.07 (0.93, 1.23) | 1.07 (0.93, 1.23) |
| Stressors × unhealthy behaviors | 0.93 (0.80, 1.08) | 0.98 (0.89, 1.08) | ||
| Wald χ2 | 195.66 | 195.86* | 366.30* | 366.22* |
| Rescaled R2 | 0.37 | 0.37 | 0.33 | 0.33 |
Note. CI = confidence interval; OR = odds ratio. Two-level chronic health conditions: less than or equal to median (0), and greater than median (1).
Lower scores indicate more impoverished status.
Unhealthy behaviors include smoking (current or ever), drinking (ever), and being obese (defined as having a body mass index [weight in kilograms divided by height in meters squared] of 30 or higher). The Wald test of difference between Blacks and Whites for unhealthy behaviors in model 1 is 3.2; P = .07; df=1.
P < .05.
DISCUSSION
Many Black Americans live in chronically precarious and difficult environments.1,3 These environments produce stressful living conditions, and often the most easily accessible options for addressing stress are various unhealthy behaviors (e.g., smoking, drinking, drug use, and so on). As we have noted, these behaviors may alleviate the symptoms of stress through the same mechanisms that are hypothesized to contribute to some mental disorders: the HPA axis.15 What is certain is that negative health behaviors, such as smoking, overeating (especially comfort foods), drinking alcohol, and drug use also have direct and debilitating effects on physical health. Thus, although these activities have the effect of alleviating or masking the ostensible symptoms of stress, they contribute—along with difficult living environments—to the disparities in mortality and physical health morbidity observed between Black and non-Hispanic White populations.13
Limitations
Our analysis was limited by the fact that it was based on data not collected to test the specific hypotheses. Thus, we have had to make compromises in operationalizing the variables in the statistical models. For example, our measure of stressors is a simple count of negative life events and does not include any assessment of perceived social or psychological stress. Another limitation is that BMI is not a direct assessment of overeating as conceptualized in the framework. A better test would include direct measures of overeating comfort foods, because a person could have a high body mass index for reasons not related to overeating comfort foods (genetic, hormonal, and so on).
Also, because we lacked the same measure of depression at waves 1 and 2, we had to use the CES-D with suggested cutoffs for depressive disorder, to feel more confident we were looking at “new” cases from wave 1 to wave 2. It would have been optimal if we had been able to use the same DSM measure at both points in time. Because the CES-D is based on a total symptom count, it tends to overstate the likelihood of meeting criteria for depression and is not based upon gateway symptoms, as is the case with DSM criteria for major depression. In addition, the analyses in this article are only relevant for noninstitutionalized populations. We do not know what the effects would be if we included, for example, incarcerated Blacks and Whites. Although these limitations may need to be addressed in future studies, their effect is to make our test of the hypothesis in this paper more conservative, because in all cases the limitations dampen the statistical assessment of the hypothesized relationships. Thus, we have more confidence that the observed statistical relationships reflect likely significant associations among the variables in the models.
Conclusions
A full understanding of racial disparities in physical and mental health requires a consideration of the life course, socioeconomic status, culture, and gender.1,3,4 Descriptively, health and mortality disparities are not constant over the life course; they are greatest at middle age and beyond.3,4 Analytically, shifts in an array of factors, such as health status and available resources, help to explain which coping strategies are chosen and the timing of these choices. At younger ages, Blacks are able to employ a variety of strategies that, when combined with the more robust physical health of youth, effectively mask the cascade to the negative health effects that appear during and after middle age.
However, decreases in the availability and effectiveness of support systems and the deterioration of health over the life course lead Blacks to cope with the conscious manifestations of stress behaviorally, often through unhealthy behaviors that work through the HPA axis and related hormonal and brain mechanisms. These behaviors are differentiated by a number of factors, including social and economic statuses, material environments, class, culture, and gender.7 For example, as we noted earlier, Black American women show heightened rates of obesity over the life course. Overeating is an effective, early, well-learned response to chronic environmental stressors that only strengthens over the life course. In contrast, for a variety of social and cultural reasons, Black American men’s coping choices and trajectories are different from that of Black women’s. Early in life, Black men tend to lead active, athletic lives, but in middle age the viability and effectiveness of this dopamine-producing coping strategy is reduced because of physical deterioration. It is at middle age that Black men begin to show increased rates of smoking, alcohol consumption, and illicit drug use (Black men do not show high prevalence rates of obesity at any age).7,20
This process is not inherently linked to racial group membership. On the contrary, a large part of the negative outcomes observed among the Black population is attributable to the disproportionate distribution among Blacks—a distribution that is quantitatively higher and qualitatively worse than among Whites—of chronic, negative environmental, social, and psychological stressors, as well as the greater availability of environmental sources of unhealthy behaviors. We believe that White Americans who lived under similar situations and facilitating structures would demonstrate the same processes and outcomes that we are suggesting occur among the Black American population.
Successful interventions to reduce the use of unhealthy coping behaviors over the life course among populations living under chronically stressful conditions depend upon the recognition that such behaviors may have adaptive, neurological effects that alleviate negative psychological and physiological states. For many individuals, especially among materially disadvantaged ethnic groups, the short-term benefits of reducing states such as anxiety, depression, and frustration may psychologically outweigh the risk of poor long-term physical health from behaviors such as overeating, consuming alcohol, using tobacco, and using over-the-counter or illicit drugs.9
The most effective methods of addressing what we suggest is an important source of physical health disparities in this country would be the reduction of environmentally produced and mediated stressors—both those that are related to race and those that are not—improving living conditions, creating good job opportunities, eliminating poverty, and reducing the poor quality of inner-city urban life.8 Paradoxically, the lack of attention to these macroenvironmental conditions contributes to the use of unhealthy coping behaviors by populations living under these conditions. Although these behaviors contribute to lower population rates of mental disorders, over the life course they play a significant role in leading to greater physical health morbidities and eventual earlier mortality than found in the general population.4,9
Acknowledgments
This work was supported by the National Institute of Mental Health (grant U01-MH57716), with supplemental support from the Office of Behavioral and Social Science Research, the National Institute on Aging (grant 5 R01 AG020282-04), the National Institute on Drug Abuse, the National Center for Minority Health Disparities (grant 1 P60 MD002249-01), and the University of Michigan. Preparation of this paper was also aided by the National Institute of Mental Health (grants 1P01 MH58565 and 1T32 MH67555).
We appreciate the assistance provided by the Program for Research on Black Americans (PRBA) faculty and by members of the PRBA and EXPORT Research Group: Jamie Abelson, MSW, MS, Deborah Coral, BA, Myriam Torres, MS, Briana Mezuk, PhD, Elizabeth Young, MD, Kiarri Kershaw, MPH, Sha Juan Colbert, MPH, and Darrell Hudson, MPH.
Footnotes
Contributors
J. S. Jackson conceptualized the study, supervised all aspects of its implementation, and coordinated the writing of the article. K. M. Knight and J. A. Rafferty completed the analyses and contributed to the writing of the article. All authors conceptualized ideas, interpreted findings, and reviewed drafts of the article.
Human Participant Protection
Data collection and subsequent analyses of the data for the Americans’ Changing Lives study were approved by the University of Michigan’s institutional review board.
Contributor Information
James S. Jackson, Department of Psychology, the Department of Health Behavior and Health Education, and the Institute for Social Research, all at the University of Michigan, Ann Arbor..
Katherine M. Knight, Department of Psychology and the Institute for Social Research, University of Michigan, Ann Arbor..
Jane A. Rafferty, Department of Sociology and the Institute for Social Research, University of Michigan, Ann Arbor..
References
- 1.Massey DS. Segregation and stratification: a biosocial perspective. Du Bois Rev. 2004;1(1):7–25. [Google Scholar]
- 2.Schnittker J, McLeod JD. The social psychology of health disparities. Annu Rev Sociol. 2005;31:75–103. [Google Scholar]
- 3.Geronimus AT, Thompson PJ. To denigrate, ignore or disrupt: racial inequality in health and the impact of a policy-induced breakdown of African American communities. Du Bois Rev. 2004;1(2):247–279. [Google Scholar]
- 4.Hayward MD, Miles TP, Crimmins EM, Yang Y. The significance of socioeconomic status in explaining the racial gap in chronic health conditions. Am Sociol Rev. 2000;65(6):910–930. [Google Scholar]
- 5.Breslau J, Aguilar S, Kendler K, Su M, Williams D, Kessler RC. Specific race-ethnic differences in risk for psychiatric disorder in a USA national sample. Psychol Med. 2006;36(1):57–68. doi: 10.1017/S0033291705006161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessler R, McGonagle K, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 7.Jackson JS. Health and mental health disparities among Black Americans. In: Hager M, editor. Modern Psychiatry: Challenges in Educating Health Professionals to Meet New Needs. New York, NY: Josiah Macy Jr. Foundation; 2002. pp. 246–254. [Google Scholar]
- 8.Jackson JS. African American experiences through the adult years. In: Kastenbaum R, editor. The Encyclopedia of Adult Development. Phoenix, AZ: Oryx Press; 1993. pp. 18–26. [Google Scholar]
- 9.Heisler M, Rust G, Patillo R, Dubous AM. Improving health, eliminating disparities: finding solutions for better health care for all. Ethn Dis. 2004;15(2):S2-1–S2-4. [PubMed] [Google Scholar]
- 10.Robins L, Regier D. Psychiatric Disorders in America: The Epidemiologic Catchment Area Study. New York, NY: Free Press; 1991. [Google Scholar]
- 11.Somervell PD, Leaf PK, Weissman MM, Blazer DG, Bruce ML. The prevalence of major depression in Black and White adults in five United States communities. Am J Epidemiol. 1989;130(4):725–735. doi: 10.1093/oxfordjournals.aje.a115394. [DOI] [PubMed] [Google Scholar]
- 12.Blazer DG, Kessler RC, McGonagle KA, Swartz MS. The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry. 1994;151(7):979–986. doi: 10.1176/ajp.151.7.979. [DOI] [PubMed] [Google Scholar]
- 13.Jackson JS, Knight KM. Race and self-regulatory behaviors: the role of the stress response and HPA axis in physical and mental health disparities. In: Carstensen LL, Schaie KW, editors. Social Structure, Aging and Self-Regulation in the Elderly. New York, NY: Springer; 2006. [Google Scholar]
- 14.Winkleby MA, Cubbin C. Racial/ethnic disparities in health behaviors: a challenge to current assumptions. In: Anderson NB, Bulatao RA, Cohen B, editors. Critical Perspectives on Racial and Ethnic Disparities in Health in Later Life. Washington, DC: National Research Council; 2004. pp. 171–226. [Google Scholar]
- 15.Barden N. Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. J Psychiatry Neurosci. 2004;29(3):185–193. [PMC free article] [PubMed] [Google Scholar]
- 16.Dohrenwend BP. The role of adversity and stress in psychopathology: some evidence and its implicatons for theory and research. J Health Soc Behav. 2000;41(1):1–19. [PubMed] [Google Scholar]
- 17.Sapolsky RM. Endocrinology of the stress-response. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral Endocrinology. Cambridge: MIT Press; 2002. pp. 409–450. [Google Scholar]
- 18.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 19.Dallman MF, Akana SF, Laugero KD, et al. A spoonful of sugar: feedback signals of energy stores and corticosterone regulate responses to chronic stress. Physiol Behav. 2003;79:3–12. doi: 10.1016/s0031-9384(03)00100-8. [DOI] [PubMed] [Google Scholar]
- 20.Dallman MF, Pecoraro N, Akana SF, et al. Chronic stress and obesity: a new view of “comfort food.”. Proc Natl Acad Sci USA. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahng SK, Dunkle R, Jackson JS. The relationship between the trajectory of body mass index and health trajectory among older adults: multilevel modeling analyses. Res Aging. 2004;26(1):31–61. [Google Scholar]
- 22.Lê AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking behavior by priming injections of alcohol and exposure to stress in rats. Psychopharmacology (Berl) 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- 23.Peele S, Brodsky A. Exploring psychological benefits associated with moderate alcohol use: a necessary corrective to assessments of drinking outcomes. Drug Alcohol Depend. 2000;60:221–247. doi: 10.1016/s0376-8716(00)00112-5. [DOI] [PubMed] [Google Scholar]
- 24.Akil H, Cicero TJ. Overview of the endogenous opioid systems: anatomical, biochemical and functional issues. In: Rodgers RJ, Cooper SJ, editors. Endorphins, Opiates and Behavioural Processes. Chichester, UK: Wiley; 1986. pp. 1–23. [Google Scholar]
- 25.Piazza PV, LeMoal ML. Pathophysiological basis of vulnerability to drug use: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- 26.Koob GF, Roberts AJ, Schultheis G, et al. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22(1):3–9. [PubMed] [Google Scholar]
- 27.Benowitz NL. Pharmacology of nicotine: addiction and therapeutics. Annu Rev Pharmacol Toxicol. 1996;36:597–613. doi: 10.1146/annurev.pa.36.040196.003121. [DOI] [PubMed] [Google Scholar]
- 28.Benowitz NL. Pharmacologic aspects of cigarette smoking and nicotine addiction. N Engl J Med. 1988;319:1318–1330. doi: 10.1056/NEJM198811173192005. [DOI] [PubMed] [Google Scholar]
- 29.Porcu P, Sogliano C, Cinus M, Purdy RH, Biggio G, Concas A. Nicotine-induced changes in cerebrocortical neuroactive steroids and plasma corticosterone concentrations in the rat. Pharmacol Biochem Behav. 2003;74(3):683–690. doi: 10.1016/s0091-3057(02)01065-1. [DOI] [PubMed] [Google Scholar]
- 30.Kirschbaum C, Wust S, Strasburger CJ. “Normal” cigarette smoking increases free cortisol in habitual smokers. Life Sci. 1992;50:435–442. doi: 10.1016/0024-3205(92)90378-3. [DOI] [PubMed] [Google Scholar]
- 31.Piazza PV, LeMoal ML. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- 32.Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- 33.House JS, Lepkowski JM, Kinney AM, Mero RP, Kessler RC, Herzog AR. The social stratification of aging and health. J Health Soc Behav. 1994;35:213–234. [PubMed] [Google Scholar]
- 34.Robbins LN, Helzer JE, Croughhan JL, Ratcliff KL. National Institute of Mental Health Diagnosis Interview Schedule: its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 35.Diagnostic and Statistical Manual of Mental Disorders. Revised Third Edition. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 36.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 37.Fisher GM. The development and history of the poverty thresholds. Social Security Bulletin. 1992;55(4):3–14. [PubMed] [Google Scholar]
- 38.Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]