Abstract
BACKGROUND:
Provider implicit bias can negatively affect clinician–patient communication. We measured implicit bias training among pediatric oncology providers and exposure to implicit-associations tests (IATs). We then assessed associations between IATs for race and socioeconomic status (SES) and recommendations for clinical trial enrollment.
METHODS:
We conducted a prospective multisite study to measure implicit bias among oncology providers at St. Jude Children’s Research Hospital (SJCRH) and affiliate clinics. We used an Implicit-Association Test (IAT) to assess bias in the domains of race and socioeconomic status (SES). We used case vignettes to associate bias with provider recommendation of trial enrollment. Data were analyzed by t-tests or Wilcoxon tests for comparisons and by Jonckheere-Terpstra tests for association.
RESULTS:
Of 105 total participants, 95 (90%) had not taken an IAT and 97 (92%) had no prior implicit bias training. We found a large effect for (bias towards) high SES (Cohen’s d 1.93) and European American race (Cohen’s d 0.96). Most participants (90%) had a vignette score of 3 or 4, indicating recommendation of trial enrollment for most or all vignettes. IAT and vignette scores did not significantly differ between providers at SJCRH or affiliate clinics. We found no association between IAT and vignette scores for race (P = .58) or SES (P = .82).
CONCLUSION:
We found a paucity of prior exposure to implicit bias self-assessments and training. Although these providers demonstrated preferences for high SES and European American race, this did not appear to affect clinical trial enrollment recommendations, as assessed by vignettes.
Keywords: pediatric oncology, clinical trials, implicit bias, enrollment, implicit association tests
Precis for use in the Table of Contents:
This multisite study measures implicit bias in the domains of race and socio-economic status among pediatric oncology providers in an academic center and community-based practices. The impact of implicit bias in clinical trial enrollment is assessed by case vignettes.
INTRODUCTION
Advancements in cancer treatments, due in part to well-designed clinical trials, have led to an overall 5-year survival for pediatric cancer patients of greater than 85%.1 Clinical trial participation is the standard approach for improving outcomes for childhood cancer in academic centers.2 Because of the structured nature of pediatric clinical trials, participants receive uniformly adjudicated treatment and have lower mortality and complication rates.1 Despite these benefits, enrollment rates are variable and range from under 20% to 86%.2-4 Known barriers to enrollment include structural challenges such as trial availability, clinical barriers like eligibility restrictions, patient-level obstacles including travel and financial concerns, and provider-level concerns including time constraints and implicit bias.3,5,6
Among the St. Jude Children’s Research Hospital (SJCRH) affiliate network, we noted a wide range of clinical trial enrollment rates. Similar to national data, average enrollment approximates50-60%, however enrollment from some affiliate sites is as low as 20%. In an effort to understand this variation we previously investigated physician-perceived barriers of clinical trial enrollment across the SJCRH affiliate network.7 We identified three major barriers: language discordance, transportation issues, and complex trial design. We also observed socio-economic status (SES) as a factor pediatric oncology providers occasionally considered when deciding whether to recommend a clinical trial (unpublished data).
Studies exploring the role of implicit bias in clinician decision-making have demonstrated variable associations between implicit bias and patient care.8 Maina et al., (2018) reviewed a decade of research on implicit bias in health care and found that implicit bias was associated with disparities in treatment in six of the fourteen studies that examined outcomes.9 Implicit race bias has been associated with poorer communication in race discordant clinical interactions,10,11 and research shows that children of a specific race may receive suboptimal care.1,12 For example, Sabin et al. demonstrated that pediatrician implicit pro-white biases were associated with a reduced likelihood of prescribing appropriate narcotic pain medication for an African American patient.12 Implicit bias is negatively associated with supportive communication and length of clinical interactions for adult patients with cancer.13 Pediatricians implicitly associate black patients with non-compliance, which may affect enrollment in clinical trials.14 However, the role of implicit bias in enrolling pediatric oncology patients into clinical trials has not been systematically or prospectively studied.
Maximizing clinical trial enrollment is a cornerstone of the mission of SJCRH. We hypothesized that implicit bias in the domains of race and SES influences recommendations by pediatric oncology providers for patient enrollment in clinical trials. To test this hypothesis, we measured implicit biases in race and SES via the Implicit-Association Test (IAT)15 and determined whether implicit bias scores were associated with provider recommendations for enrolling patients described in four case vignettes (CVs) into a clinical trial.
METHODS
This was a prospective nontherapeutic, noninterventional study that included pediatric oncology faculty and advanced practice providers (APPs) involved in direct patient care at SJCRH and its affiliate clinics. This study was approved by the SJCRH Institutional Review Board. The eight SJCRH affiliate clinics are located in Shreveport and Baton Rouge, Louisiana; Charlotte, North Carolina; Huntsville, Alabama; Johnson City, Tennessee; Peoria, Illinois; Springfield; Missouri; and Tulsa, Oklahoma. SJCRH is a tertiary specialized pediatric cancer center located in Memphis, TN, whereas the affiliate clinics are in community-based hospitals. The racial demographics of the catchment areas for SJCRH and the affiliate clinics vary. Three have a slight majority African American population. One has a heterogeneous population with no majority racial group. Four have a majority white population, and one has mixed racial demographics that include 10% American Indian. Patients in the affiliate network have access to pediatric cancer trials sponsored by SJCRH, and resources for clinical trial participation, including translated informed consent documents and patient advocacy training, are available at all sites.
Pediatric oncology providers at SJCRH and the affiliate clinics were recruited for participation via email. A reminder email was sent at 3 weeks and 1 week before the close of the study period of 6 weeks. Before starting IATs and CVs, we asked each participant their practice location, provider type (physician or APP), years of practice, number of IATs previously taken, and history of implicit bias training. No additional demographic or identifying information was collected from participants to preserve confidentiality and anonymity. APPs (i.e., nurse practitioners and physician assistants) were included in the study because they actively participate in discussions regarding clinical care and research and are partners to physicians in the decision-making process for clinical trial enrollment in pediatric oncology.
We conducted this study in collaboration with Project Implicit, a nonprofit organization that studies and measures implicit social cognition. The order of IATs and CVs was randomly assigned independently by Project Implicit staff.16 Testing was performed on a secure website.
Study Measures
We used race and SES IATs to measure how pediatric oncology providers associate specific traits with distinct social categories.15 IATs are timed cognitive tests, measuring the relative association strength between two pairs of concepts, including a target concept such as race (e.g., European American versus African American) and an evaluation concept (e.g., good versus bad). For the SES IAT, positive scores indicated an implicit preference for upper SES over lower SES. Positive scores on the race IAT indicated implicit preference for European Americans over African Americans. Negative scores indicated the opposite preferences. Both the race and SES IATs required approximately 10 minutes each to complete.
The CVs were written by senior physicians on the study team and had not been previously used (Supplemental Table 1). Each participant was given four CVs in two domains (high/low SES and white/black race). CVs are a validated strategy of data collection to unveil associations of race and social bias.17 All four CVs described a new pediatric cancer diagnosis with an option to enroll the patient in a phase II or III clinical trial. We chose four diagnoses for the CVs that were representative of four frontline SJCRH initiated clinical trials (Hodgkin’s lymphoma, acute lymphoid leukemia, acute myeloid leukemia and infant leukemia). A degree of uncertainty was incorporated into the CVs, as uncertainty may add to bias in real-life clinical decision-making. Socioeconomic descriptions embedded in CVs included employment type, education, housing, and insurance. CV responses were timed (90 seconds). Responses were either yes or no to recommend enrolling the patient in a clinical trial, with elaboration embedded in the answer choices or as an optional written response (Supplemental Table 1).
Analysis
IAT scores were calculated with a standard scoring algorithm. Scores ranged from −2 to +2, with 0 indicating no relative preference between conditions.18 CVs were scored 0 to 4 (0 = enrolling no CV patients and 4 = enrolling all four CV patients).
Random assignment resulted in six possible orders of IAT and CV tests. We randomized study elements to remove potential effects of participants viewing the IAT prior to CV or vice versa. To assess IAT effect sizes, we used the Cohen’s d statistic, a standardized effect size measure. Cohen’s d values are interpreted as follows: d = 0.2, small effect; d = 0.5, medium effect; and d = 0.80, large effect.19 We calculated Cohen’s d scores to measure the standardized distance from the mean IAT scores to an ideal score of 0. We also calculated Cohen’s d values to measure the mean difference in IAT scores between providers from SJCRH and the affiliate clinics by using pooled within-group standard deviations.
Summary statistics included mean, standard deviation, median, and range for continuous variables and count and frequencies for categorical variables. Two-sample t-tests or Wilcoxon rank sum tests were used for continuous variables and χ2 or Fisher exact tests were used for categorical variables. One-sample t-tests or Wilcoxon signed-rank tests were used to determine whether the sample means, or medians were different from 0. The nonparametric Spearman rank correlation (Spearman correlation coefficient ρ) and Jonckheere–Terpstra tests were used to ascertain correlations between race and SES IAT and CV scores. All P values were two-sided, and P < .05 was considered significant. Analyses were performed with SAS 9.420 or R v3.6.1.21
RESULTS
Of the 251 providers invited, 105 (42%) completed all three components of the study (race IAT, SES IAT, and CVs). Four additional participants were excluded because they did not complete all three components. The participants included 81 (77%) providers from SJCRH and 24 (23%) from the affiliate clinics. The overall distribution by provider type was 65 (62%) for APPs and 40 (38%) for physicians. The participants from SJCRH included 56 (69%) APPs and 25 (31%) physicians, whereas the affiliate network participants included 9 (38%) APPs and 15 (62%) physicians (P = .005). The total proportions of physicians and APPs who participated (from those invited) were similar by provider type (43% physicians and 41% APPs). Additional characteristics of the participants are shown in Table 1. Approximately half of the providers had practiced more than 10 years, with similar distributions between SJCRH and the affiliate clinics (P = .7). The majority of providers (90%) had not taken any IATs before the study nor had previous implicit bias training (92%). The proportion of APPs and physicians who had taken previous IATs (P = .18) or received previous training (P = .48) did not significantly differ. The proportion of participants from SJCRH or the affiliate clinics also did not significantly differ for previous IATs (P = 1.00) or training (P = .38).
TABLE 1.
Participant Characteristics by Site
| Characteristics | SJCRH, n = 81 (%) | Affiliate, n = 24 (%) | Total, n = 105 | P† |
|---|---|---|---|---|
| Type of provider | 0.005 | |||
| Physician | 25 (31) | 15 (62.5) | 40 | |
| APP | 56 (69) | 9 (37.5) | 65 | |
| Years of practice | 0.70 | |||
| < 10 years | 39 (48) | 10 (42) | 49 | |
| 10–20 years | 23 (28) | 9 (37) | 32 | |
| > 20 years | 19 (24) | 5 (21) | 24 | |
| Taken an IAT before | 1.00 | |||
| No | 73 (90) | 22 (92) | 95 | |
| Yes | 8 (10) | 2 (8) | 10 | |
| Previous formal implicit bias training | 0.38 | |||
| No | 76 (94) | 21 (88) | 97 | |
| Yes | 5 (6) | 3 (12) | 8 |
Abbreviations: APP, advanced practice provider; IAT, implicit-association test.
Fisher exact or Pearson χ2 tests were used to compare groups.
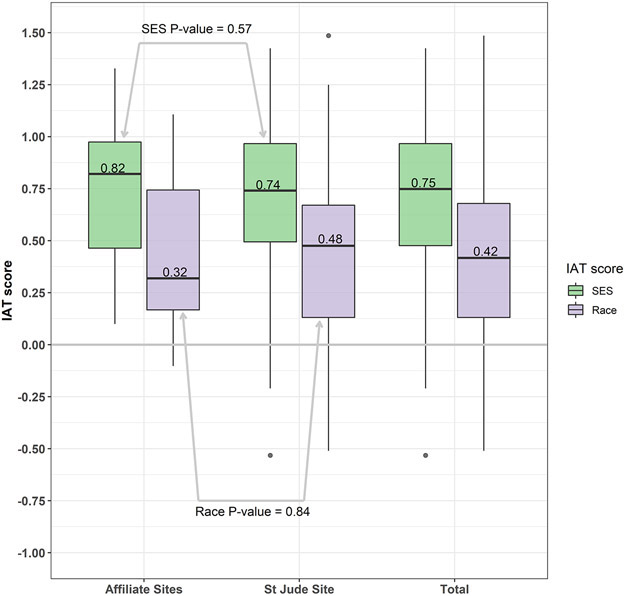
We report the ranges and medians, in addition to Cohen’s d values. Among all participants, we found a very strong preference for high SES over low SES. The overall, SJCRH, and affiliate clinic mean SES IAT scores were 0.71 (Cohen’s d = 1.93), 0.69 (Cohen’s d = 1.88), 0.76 (Cohen’s d = 2.09), respectively, which were all significantly different from zero (P < .001) (Table 2). The median SES IAT scores were 0.75 for overall, 0.74 for SJCRH, and 0.82 for the affiliate clinics, indicating a strong preference for high SES (Figure 1). Among all the participants for the Race IAT, we found a strong preference for European Americans over African Americans. The overall, SJCRH, and affiliate clinic mean race IAT scores were 0.39 (Cohen’s d = 0.96), 0.39 (Cohen’s d = 0.92), and 0.41 (Cohen’s d = 1.15), respectively, which all significantly differed from zero (P < .001) (Table 2). The median race IAT scores were 0.42 for overall, 0.48 for SJCRH, and 0.32 for the affiliate clinics, demonstrating a preference for European American race (Figure 1).
TABLE 2.
Implicit-Association Test Scores by Site
| SJCRH, n = 81 |
Affiliate, n = 24 |
Total, n = 105 |
SJCRH v Affiliate |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bias test | Mean (SD) | Cohen d* | P† | Mean (SD) | Cohen d* | P† | Mean (SD) | Cohen d* | P† | Cohen d‡ | P§ |
| SES IAT | 0.69 (0.37) | 1.88 | < .001 | 0.76 (0.36) | 2.09 | < .001 | 0.71 (0.37) | 1.93 | < .001 | 0.17 | .56 |
| Race IAT | 0.39 (0.42) | 0.92 | < .001 | 0.41 (0.35) | 1.15 | < .001 | 0.39 (0.41) | 0.96 | < .001 | 0.05 | .84 |
Abbreviations: IAT, implicit-association test; SD, standard deviation; SES, socioeconomic status.
Cohen’s d value calculated for SJCRH or affiliate clinic providers as an effect size to measure the standardized distance from the mean IAT scores to an ideal score of 0.
P values calculated with one-sample t-tests.
Cohen’s d calculated as effect size to measure the mean difference between SJCRH and affiliate clinic providers by using the pooled within-group SD.
P values calculated with two-sample t-tests.
FIG 1.
Box and whisker plot of IAT score distribution by site. The zero point indicates no preference in either direction. The median is represented by dark horizontal bars. For both race and socioeconomic status, the median values did not differ between providers at SJCRH (P = .57) and the affiliate clinics (P = .84).
Most participants (90%) had CV scores of 3 or 4, indicating they offered the clinical trial for most or all CVs (Supplemental Table 2). Participants’ qualitative descriptions for choosing to offer trial enrollment were informative. The participants stated that the trial was “the best medical treatment” or offered “what is best for the child.” In addition, addressing health disparities for underserved populations was cited as an explicit reason for offering a clinical trial: “African Americans are underrepresented in clinical research;” “Minority patients must participate in trials to determine if standard treatment is best for them.” Participants also cited financial factors that influenced their decision-making: “All patients should be offered trials, regardless of socioeconomic status;” including “good family support” or “[the trial is] affordable…with Medicaid.” Although limited, the qualitative descriptions for not offering participation in clinical trials included patient- or disease-specific factors, such as “curative leukemia” and “established treatment available.” Neither race nor SES was explicitly mentioned as reasons to not offer participation in a clinical trial. However, a few participants mentioned social considerations, such as “frequency of follow-up for the clinical trial may be difficult” or “family has insurance.”
When comparing providers from SJCRH or the affiliate clinics, we found the distribution of continuous IAT scores did not significantly differ for race (Cohen’s d = 0.05, P = .84) or SES (Cohen’s d = 0.17, P = .56) (Table 2). In addition, we found no difference among the different CVs (CV 1, P = .12; CV 2, P = .69; CV 3, P = .73; and CV 4, P = .55) (Supplemental Table 2). When we compared physician and APP participants, we found no difference for SES (P = .76), or race (P = .94) IAT scores (Supplemental Table 3).
No significant associations occurred between participant IAT and CV scores (Race: ρ = −0.06, P = .58; SES: ρ = −0.02, P = .82). Analysis of the randomization order demonstrated that participants who saw the CVs first had less dynamic IAT scores for SES (P = .007 for continuous scores), potentially indicating exposure influences. However, randomization order did not significantly affect race IAT scores (Table 3).
TABLE 3.
Continuous IAT scores for SES and Race Based on Randomization Order*
| Variable | Overall | CVs first, n = 29 | CVs not first, n = 76 | P† |
|---|---|---|---|---|
| SES IAT score | ||||
| Mean (SD) | 0.71 (0.37) | 0.52 (0.45) | 0.78 (0.30) | .007 |
| Median (range) | 0.75 (−0.53 to 1.42) | 0.53 (−0.53 to 1.34) | 0.81 (−0.01 to 1.42) | |
| Race IAT score | ||||
| Mean (SD) | 0.39 (0.41) | 0.37 (0.39) | 0.40 (0.42) | .78 |
| Median (range) | 0.42 (−0.51 to 1.49) | 0.38 (−0.33 to 1.49) | 0.47 (−0.51 to 1.25) |
Abbreviations: CVs, case vignettes; IAT, implicit-association test; SD, standard deviation; SES, socioeconomic status.
Randomization assignment order: CVs before IATs versus IATs before CVs.
P values determined by t-tests.
DISCUSSION
This study demonstrates a remarkable lack of formal training in implicit bias or prior exposure to IATs among pediatric oncology providers in our network (both physicians and APPs). While the initial goal of this study was to evaluate the impact of racial and SES bias on clinical trial enrollment recommendation, we were surprised to find that >90% of participants had no previous exposure to the concepts of implicit bias. This finding emphasizes the need for increased diversity and inclusion training for pediatric hematology–oncology providers in all practice settings. Awareness and education regarding implicit bias and its role in clinical settings is one of the first steps toward mitigating its potential effects.22
In addition, our results demonstrate provider bias favoring high SES and European American race, with a stronger bias for SES. This difference may reflect increased societal awareness surrounding the effect of race, as compared to SES, on health disparities. Alternatively, it may reflect a healthcare workforce that is more racially diverse than it is economically diverse. In general, it highlights the importance of SES bias as an under-recognized area of research that may affect clinical care.
Most providers offered clinical trials in all four CVs, and neither SES nor race bias significantly affected CV scores. This finding is unsurprising because pediatric oncology is a clinical trial-centric field, and most children with cancer in the US are offered trial enrollment at some point during cancer care or survivorship. SJCRH is a research hospital, and providers at SJCRH and its affiliate clinics are encouraged to enroll patients in clinical trials. The limited qualitative data in this study suggest that providers believe trial enrollment is beneficial. Notably, some providers directly indicated their consideration of social factors when deciding to offer clinical trial enrollment. The offer for trial enrollment in the CVs suggests the standard approach at large pediatric oncology centers to recommend clinical trial enrollment may supersede the potential effect of other factors, such as implicit bias.
Although the participant IAT scores did not affect CV scores, the randomization order was significant for SES. The participants who completed the CVs first showed less bias on the SES IAT. Therefore, the CVs may have functioned as a limited educational tool, personalizing patients and prompting awareness of the participants’ potential biases.
We did not collect demographic information of the participants (e.g., race, ethnicity, or sex) to protect anonymity and thus could not use this information to analyze potential differences in implicit bias. Although IATs are the most widely used instrument to identify bias, these tools have their own limitations and should be interpreted in context. IATs are sensitive to the context in which they are taken, as scores can change from one test to another.23 Despite these limitations, IATs capture attitudes that are distinct from those of self-reports. Although CVs are constrained in their ability to represent or convey nuances of real-life clinical care, they are a tool used to study medical decision-making and have been used in similar studies.12 Our vignettes were specifically designed for this study and thus were not previously used or pre-tested, which is a limitation of this study. Furthermore, each participant in this study was exposed to all four vignettes which may have impacted their responses. Although the participation rate for this study was similar to that of other surveys of health care providers, selection bias may have affected our results. However, the similar distribution of participant types across sites assuages that concern.
Our findings highlight a need for increased awareness and formal education on implicit bias, as well as interventions that support diversity and inclusion. Educational curricula may include awareness tools, such as IATs, didactics regarding social determinants of health, and interactive workshops exploring provider bias.24 Expert facilitation is essential to diffuse defensiveness in implicit bias education. Although strong implicit bias was not associated with enrollment recommendation in clinical trials as defined by theoretical CVs, recent evidence shows that implicit bias education can have a positive impact on providers.25 Diversity and inclusion education has been emphasized for trainees by formal organizations such as the Accreditation Council for Graduate Medical Education. Our data suggest this education should be offered to providers at all levels of their career continuum. In addition to formal training, diversity and inclusion efforts may be supported by offices dedicated to equity and supported by leadership, diversity officers, and human resource efforts focused on diversifying the healthcare workforce.
The lack of association between IAT and CV scores suggests that implicit bias does not influence the conscious decisions of providers to recommend pediatric cancer clinical trial participation. However, CVs are an insufficient substitute for real-time clinical decision-making, and further studies are needed to assess the effect of implicit bias on pediatric oncology patient-provider communication at the time of trial enrollment. Null results in implicit bias vignette studies may be the result of vignette’s inability to capture the high time pressure and cognitive demand of busy real-world clinical settings.26,27 Previous studies in fields such as surgery28 and adult medicine29 demonstrated that interventions, including consensus treatment guidelines and protocols, can mitigate the effects of implicit bias on clinical care. For example, in a real-world study of the association of provider implicit bias with hypertension treatment that showed null results the authors speculated that emphasis of guideline adherence in the organizations studied may mitigate the impact of implicit bias on treatment decisions.29 Specifically, checklists and protocols that discourage provider discretion are potentially protective against bias. The protocolized nature of pediatric oncology practice may be similarly protective. Additional interventions to address and mitigate implicit bias in medical settings and other industries include recruiting a diverse healthcare workforce, care checklists and algorithms, and patient navigators.22 Continued investigation and adaptation of these tools and training methods for pediatric oncology is warranted to further understand implicit bias and its potential effect on patient care, including clinical trial enrollment.
Supplementary Material
Acknowledgements:
We greatly appreciate the editorial skills of Dr. Nisha Badders and the contribution of the study participants.
Funding support: This work was supported by the National Cancer Institute [St. Jude Cancer Center Support (CORE) under Grant P30 CA21765] and the American Lebanese Syrian Associated Charities (ALSAC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest disclosure: None
REFERENCES
- 1.Caldwell PH, Murphy SB, Butow PN, Craig JC. Clinical trials in children. Lancet. 2004;364(9436):803–811. doi: 10.1016/S0140-6736(04)16942-0 [DOI] [PubMed] [Google Scholar]
- 2.Faulk KE, Anderson-Mellies A, Cockburn M, Green AL. Assessment of enrollment characteristics for Children’s Oncology Group (COG) upfront therapeutic clinical trials 2004–2015 Tiao G, ed. PLoS One. 2020;15(4):e0230824. doi: 10.1371/journal.pone.0230824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unger JM, Cook E, Tai E, Bleyer A. The Role of Clinical Trial Participation in Cancer Research: Barriers, Evidence, and Strategies. Am Soc Clin Oncol Educ B. 2016;(36):185–198. doi: 10.1200/edbk_156686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aristizabal P, Singer J, Cooper R, et al. Participation in pediatric oncology research protocols: Racial/ethnic, language and age-based disparities. Pediatr Blood Cancer. 2015;62(8):1337–1344. doi: 10.1002/pbc.25472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aristizabal P Diverse populations and enrollment in pediatric cancer clinical trials: Challenges and opportunities. Pediatr Blood Cancer. April 2020. doi: 10.1002/pbc.28296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickens DS, Roth ME, Pollock BH, Langevin A-M. Understanding the Barriers to Pediatric Oncologist Engagement and Accrual to Clinical Trials in National Cancer Institute–Designated Community Oncology Research Programs. JCO Oncol Pract. May 2020:JOP.19.00707. doi: 10.1200/jop.19.00707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russo C, Stout L, House T, Santana VM. Barriers and facilitators of clinical trial enrollment in a network of community‐based pediatric oncology clinics. Pediatr Blood Cancer. 2020;67(4). doi: 10.1002/pbc.28023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall WJ, Chapman MV., Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: A systematic review. Am J Public Health. 2015;105(12):e60–e76. doi: 10.2105/AJPH.2015.302903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maina IW, Belton TD, Ginzberg S, Singh A, Johnson TJ. A decade of studying implicit racial/ethnic bias in healthcare providers using the implicit association test. Soc Sci Med. 2018;199:219–229. doi: 10.1016/j.socscimed.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 10.Cooper LA, Roter DL, Carson KA, et al. The Associations of Clinicians’ Implicit Attitudes About Race With Medical Visit Communication and Patient Ratings of Interpersonal Care. doi: 10.2105/AJPH.2011.300558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penner LA, Dovidio JF, West T V, et al. Aversive Racism and Medical Interactions with Black Patients: A Field Study. J Exp Soc Psychol. 2010;46(2):436–440. doi: 10.1016/j.jesp.2009.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabin JA, Greenwald AG. The influence of implicit bias on treatment recommendations for 4 common pediatric conditions: pain, urinary tract infection, attention deficit hyperactivity disorder, and asthma. Am J Public Health. 2012;102(5):988–995. doi: 10.2105/AJPH.2011.300621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penner LA, Dovidio JF, Gonzalez R, et al. The effects of oncologist implicit racial bias in racially discordant oncology interactions. J Clin Oncol. 2016;34(24):2874–2880. doi: 10.1200/JCO.2015.66.3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabin JA, Rivara FP, Greenwald AG. Physician Implicit Attitudes and Stereotypes About Race and Quality of Medical Care.; 2008. https://implicit.harvard.edu. Accessed August 9, 2020. [DOI] [PubMed]
- 15.Greenwald AG, McGhee DE, Schwartz JLK. Measuring individual differences in implicit cognition: The implicit association test. J Pers Soc Psychol. 1998;74(6):1464–1480. doi: 10.1037/0022-3514.74.6.1464 [DOI] [PubMed] [Google Scholar]
- 16.ProjectImplicit. https://www.projectimplicit.net/.
- 17.Haider AH, Sexton J, Sriram N, et al. Association of unconscious race and social class bias with vignette-based clinical assessments by medical students. JAMA - J Am Med Assoc. 2011;306(9):942–951. doi: 10.1001/jama.2011.1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenwald AG, Nosek BA, Banaji MR. Understanding and Using the Implicit Association Test: I. An Improved Scoring Algorithm. J Pers Soc Psychol. 2003;85(2):197–216. doi: 10.1037/0022-3514.85.2.197 [DOI] [PubMed] [Google Scholar]
- 19.Cohen J Statistical Power Analysis for the Behavioral Sciences Second Edition. [Google Scholar]
- 20.SAS ® 9.4 Statements Reference SAS ® Documentation; 2013. [Google Scholar]
- 21.Team RC. R: A Language and Environment for Statistical Computing. 2019. http://www.r-project.org.
- 22.Tsai JW, Kesselheim JC. Addressing implicit bias in pediatric hematology‐oncology. Pediatr Blood Cancer. 2020;67(5). doi: 10.1002/pbc.28204 [DOI] [PubMed] [Google Scholar]
- 23.Oswald FL, Mitchell G, Blanton H, Jaccard J, Tetlock PE. Using the IAT to predict ethnic and racial discrimination: Small effect sizes of unknown societal significance. J Pers Soc Psychol. 2015;108(4):562–571. doi: 10.1037/pspa0000023 [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez CM, Garba RJ, Liguori A, Marantz PR, McKee MD, Lypson ML. How to Make or Break Implicit Bias Instruction: Implications for Curriculum Development. Acad Med. 2018;93(11S Association of American Medical Colleges Learn Serve Lead):S74–S81. doi: 10.1097/ACM.0000000000002386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitzgerald C, Martin A, Berner D, Hurst S. Interventions designed to reduce implicit prejudices and implicit stereotypes in real world contexts: A systematic review. BMC Psychol. 2019;7(1):1–12. doi: 10.1186/s40359-019-0299-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albarracin D Handbook of Attitudes, Volume 2: Applications. Routledge; 2018. doi: 10.4324/9781315178080 [DOI] [Google Scholar]
- 27.Samuels EA, Boatright D, Sanchez LD, et al. Clinical Vignettes Inadequate to Assess Impact of Implicit Bias: Concerning Limitations of a Systematic Review Burton JH, ed. Acad Emerg Med. 2017;24(12):1531–1532. doi: 10.1111/acem.13317 [DOI] [PubMed] [Google Scholar]
- 28.Haider AH, Schneider EB, Sriram N, et al. Unconscious race and social class bias among acute care surgical clinicians and clinical treatment decisions. JAMA Surg. 2015;150(5):457–464. doi: 10.1001/jamasurg.2014.4038 [DOI] [PubMed] [Google Scholar]
- 29.Blair I V, Steiner JF, Hanratty R, et al. An investigation of associations between clinicians’ ethnic or racial bias and hypertension treatment, medication adherence and blood pressure control. J Gen Intern Med. 2014;29(7):987–995. doi: 10.1007/s11606-014-2795-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.