Abstract
Background and Purpose
The application of volumetric techniques to preterm infants has revealed brain volume reductions. Such quantitative data are not available in routine neonatal radiological care. The objective of this study was to develop simple brain metrics to compare brain size in preterm and term infants, and correlate these metrics with brain volumes from volumetric MR techniques.
Methods
MR images from 189 preterm infants <30 weeks’ gestational age or <1250 g birthweight scanned at term-equivalent age and 36 term infants were studied. Fifteen tissue and fluid measures were systematically evaluated on 4 selected slices. The results were correlated with total brain, grey matter, white matter and CSF volumes.
Results
The mean bifrontal, biparietal and transverse cerebellar diameters were reduced (−11.6 %; CI= −13.8 to −9.3%; −12%, −14 to −9.8% and −8.7%, −10.5 to −7% respectively) and the mean left ventricle diameter was increased (+22.3%; 2.9 to 41.6%) in preterm infants (p<0.01). Strong correlations were found between the bifrontal and biparietal measures with total brain tissue volume, while the size of the ventricles and the interhemispheric measure correlated with CSF volume. Intra-observer reliability was high (ICC >0.7), while inter-observer agreement was acceptable for tissue measures (ICC >0.6) but lower for fluid measures (ICC <0.4)
Conclusions
Simple brain metrics at term-equivalent age showed smaller brain diameters and increased ventricle size in preterm infants compared with full term infants. These measures represent a reliable and easily applicable method to quantify brain growth and assess brain atrophy in this at-risk population.
Keywords: Newborn brain, MR imaging, Brain measures
Introduction
The survival rates for preterm infants have increased steadily over the two last decades1,2. Alongside increased survival rates, there is continuing recognition of the high prevalence of motor, cognitive and behavioral disabilities suffered by survivors of preterm birth3. The major neuropathologies recognized on cranial ultrasound in the preterm infant, such as intraventricular hemorrhage and cystic periventricular leukomalacia (PVL) have remained stable or declined in prevalence over this period4, without accompanying improvements in outcomes. It is now increasingly recognized that the “cerebral lesions” responsible for these broad range of disabilities may not be visible on cranial ultrasound and may involve altered cerebral development as well as injury.
Quantitative evaluation of cerebral structure in at risk preterm infants may assist in defining the impact of preterm birth on cerebral development. Previous approaches to this have included quantitative volumetric magnetic resonance imaging (MRI) techniques as well as cranial ultrasound measures. Cranial ultrasound has shown a decrease in the rate of growth of the corpus callosum in preterm infants, which has correlated with later outcomes5. MRI techniques have evaluated total and regional cerebral volumes by tissue segmentation and voxel base morphometry. These studies have strongly suggested a reduction in total cerebral volumes with regional variation that is apparent at term equivalent6 and persists into childhood7.
An alternative biometric approach to cerebral growth has been developed in fetal brain imaging by applying a set of measurements to the qualitative MR image, including the biparietal diameter or the transverse cerebellar diameter8. These measures showed a close correlation with maturity9. Thus, we adapted this methodology for a simple quantitative determinant of brain size in preterm infants approaching term age. The primary objectives of this study were (i) to develop a simple metric for the evaluation of brain size in preterm infants scanned at term corrected age and to compare the results obtained between preterm and full term infants, and (ii) to compare the results with the quantitative volumetric MRI data on the same cohort.
Material and Methods
Subjects
Our study was conducted on existing MRI data sets collected on a prospective longitudinal cohort study of preterm infants born <1250 g or <30 weeks, and term born control infants. The gestational age was based on maternal last menstrual date or dating by earliest ultrasound scan. The preterm infants were admitted between April 2001 and December 2003 to the Royal Women’s Hospital in Melbourne, Australia. Perinatal data (i.e. sociodemographic and obstetrical data, birth characteristics, neonatal therapies and morbidities) were obtained by chart review. For an early detection of brain lesions including intraventricular hemorrhage (IVH) cranial ultrasound scans were obtained serially throughout the neonatal intensive care course in all infants within the first 48 h and at ages 4–7 days and 4–6 weeks. During the study period, 348 eligible preterm infants were admitted and 236 (64%) were recruited. There were no significant differences in obstetrical or birth data between infants recruited compared with those not recruited (data not shown). Infants with known or suspected brain malformation or congenital abnormalities (n=4) were excluded. Infants were also excluded if there was insufficient or of suboptimal quality of MRI (n=39) or perinatal data (n=4). Thus, MR images were studied for 189 preterm infants. Fifty one term controls were also enrolled, 13 were excluded for technical issues related to acquisition of the MR images and two for proven neonatal sepsis. Thus MR images were analyzed for 36 term controls.
MRI scanning
All infants were scanned at term or term equivalent age without sedation. Infants were fed, swaddled, outfitted with earphones, and placed in a vacuum-fixation bean bag. Sleeping infants were scanned in a 1.5 Tesla General Electric Sigma System MR scanner with a pediatric quadrature head coil (Milwaukee, WI, USA), located at the Royal Children’s Hospital, Melbourne. Two different imaging modes were applied: 3D T1-weighted (T1W) spoiled gradient recalled (SPGR) [1.2 mm coronal slices; flip angle 45; repetition time (TR) 35 ms; echo time (TE) 9 ms; field of view (FOV) 21 · 15 cm2; matrix 256 · 192] and T2W dual echo (interleaved acquisition) fast recovery fast spin echo sequences (2 mm coronal; TR 4000 ms; TE 60/160 ms; FOV 22 16 cm2; matrix 256 192, interpolated 512 · 512). Coronal T2W slices were available for 189 preterm infants and 36 term controls, axial T2W slices were available for 50 preterm infants and 8 term controls, sagittal T1W slices were available for 74 preterm infants and 13 term controls. Due to the small number of axial T1W slices, only the coronal T2W MR and the sagittal T1W images were studied.
MRI analysis
Brain metrics
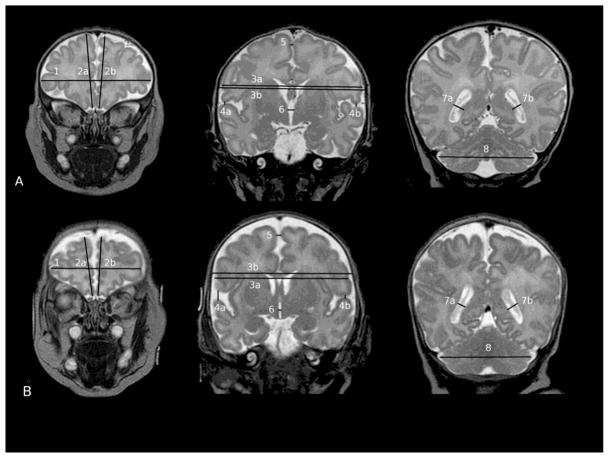
The MR images were displayed using a Dicom browser (DicomWorks®). Fifteen parameters divided into “tissue” measures (i.e. bifrontal diameter, left and right frontal height, brain and bone biparietal diameter, frontal-occipital diameter, length of corpus callosum, surface of the vermis, transverse cerebellar diameter) and “fluid” measures of the pericerebral space (interhemispheric distance, craniocaudal left and right interopercular distances,) and the intracerebral spaces (diameter of the left and right lateral ventricles, third ventricle diameter) were manually measured on four selected slices. Slices and landmarks are detailed in Table 1 and Fig. 1.
Table 1.
Brain metrics, the selected slices and the landmarks
| Measure (see landmarks in fig. 1) | Slice | Landmark |
|---|---|---|
| Tissues measures | ||
| 1 - Bifrontal diameter | Coronal T2W | Level of the olfactory sulci, 4 teeth apparent, maximal distance perpendicular to the interhemispheric fissure |
| 2a&2b - Frontal Lobe Height Left & Right | Coronal T2W | Idem to 1, Level of the gyrus rectus |
| 3a - Brain Biparietal Diameter | Coronal T2W | Level of the third ventricle, cochlea and basilar truncus apparent |
| 3b - Bone Biparietal Diameter | Coronal T2W | Level of the third ventricle, cochlea and basilar truncus apparent, inner limits of the skull |
| 8- Transverse Cerebellar Diameter | Coronal T2W | Level of the atria (plexus choroid apparent), maximal horizontal distance |
| 9 - Fronto-occipital diameter | Sagittal T1W | Midline, maximal distance from the anterior to the posterior edge of the brain |
| 10 - Length of the corpus callosum | Sagittal T1W | Midline from the genu to the posterior extremity of the splenium |
| 11 - Surface of the vermis | Sagittal T1W | Midline |
| Fluid measures | ||
| Extra-axial space | ||
| 4a&4b - Cranio-caudal interopercular distance Left & Right | Coronal T2W | Level of the third ventricle cf biparietal diameter |
| 5 - Interhemispheric distance | Coronal T2W | Level of the third ventricle |
| Intracerebral space | ||
| 6 - Third ventricle | Coronal T2W | Level of the third ventricle |
| 7a&7b - Right and Left Lateral ventricle atrial diameter | Coronal T2W | Level of the atria (plexus choroid apparent) on an axis perpendicular to that of the ventricle at mid height of the ventricle’s size (same as Transverse Cerebellar Diameter) |
Figure 1.
Selected slices and landmarks (see Table 1) used to calculate the coronal brain metrics with example of a full term infant (A) and a preterm infant (B)
The metrics on the coronal slices were tested for reliability. We calculated the interobserver correlation from repeating measurements on 7 scans by 3 different observers. The intraobserver correlation was calculated from 3 scans measured twice by each of the three observers. A screen copy of each slice selected for measurement was produced by the observers. They were checked in order to search for the source of variability relating to the slice selection.
The brain metrics were compared to the growth curves of normative data produced from fetal MRI brain assessment (biparietal diameter, fronto-occipital diameter and transverse cerebellar diameter).
Qualitative MRI analysis
MR scans were scored using a standardized scoring system10. White matter abnormality was graded according to five scales, which assessed (i) the nature and extent of white matter signal abnormality, (ii) loss of periventricular white matter, (iii) presence of cysts, (iv) degree of ventricular dilatation and (v) thinning of the corpus callosum. The scores from individual scales were then combined to give an overall white matter abnormality score categorized as normal, mild, moderate or severe white matter abnormality.
Quantitative volumetric MR analysis
A previous volumetric analysis using Sun Microsystems workstations (Palo Alto, CA) has been reported6. The parameters retained for analysis in the current study were the volumes of the total brain tissue, the cortical grey matter, the myelinated and unmyelinated white matter, the deep nuclear grey matter and the CSF.
Statistical analyses
Statistical analysis was performed using SPSS version 15 (SPSS, Chicago, IL). Study group characteristics were evaluated using t-tests for continuous variables and chi-square tests for categorical variables. Exploratory analysis indicated that brain tissue metrics were approximately normally distributed and that the distribution of fluid measures was left skewed. For univariate analysis, a t-test or a Kruskall-Wallis nonparametric test were used as appropriate to study the effect of the group (preterm vs. full tem) and the gender on brain metrics. The Pearson correlation coefficient was used to study the effects of gestational age at MRI on brain metrics. A general linear model approach was used to model the most relevant brain metrics as a function of the study group and gender, with gestational age at MRI as a covariate. The relationships between these brain metrics and brain volumes were studied by the Pearson correlation coefficient. The intraclass correlation coefficients (ICC) for consistency (two-way random model, single measure) were calculated to analyze the inter- and intra-observer agreement.
Results
Subjects
The characteristics of the cohort are presented in Table 2. The rate of multiple pregnancies was relatively high (42%). More than 80 % of MRIs were considered as qualitatively normal or mildly abnormal. The mean gestational age at MRI was 40.1 SD 1.3 [range 36–44] weeks although the preterm infants were younger than term infants at MRI (t=−2.4, p=0.02). The head circumference and the weight at MRI were significantly smaller in preterm infants, before and after adjustment for gestational age at MRI (t= −4.2, p<0.001 for weight, t=−2.4, p=0.016 for head circumference).
Table 2.
Characteristics of the cohort
| Preterm | Term | |
|---|---|---|
| n = 189 | n = 36 | |
| Gestational age at birth (weeks) mean (SD) | 27.5 (1.9) | 38.9 (1.2) |
| Gestational age at MRI (weeks) mean (SD) | 40 (1.3) | 40.3 (1.3) |
| Birth weight (g) mean (SD) | 969 (225) | 3277 (524) |
| Weight at MRI (g) mean (SD) | 3005 (551) | 3482 (482) |
| Head circumference at MRI (cm) mean (SD) 2 missing | 34.9 (2.1) | 36 (1.4) |
| Male n (%) | 97 (51) | 19 (53) |
| Multiple birth n infants (%) | 80 (42) | 2 (5.6) |
| Intra Uterine Growth Restriction n (%) | 21 (11) | 1 (3) |
| White matter injury n (%) | ||
| No | 71 (37.6) | 0 |
| Mild | 84 (44.4) | 0 |
| Moderate | 27 (14.3) | 0 |
| Severe | 7 (3.7) | 0 |
| Intraventricular hemorrhage grade 3 or 4 n (%) | 7 (3.7) | 0 |
Reproducibility
The intra-rater and inter-rater consistencies were high for all the tissue measures, with ICC coefficients above 0.8 (Table 3). For the fluid measures, the intra-rater consistency was acceptable but the inter-rater consistency was low due to some discrepancies in the choice between adjacent slices.
Table 3.
Intraclass correlation coefficients (ICC) for intra- and interobserver reliability
| Intraobserver ICC [CI] | Interobserver ICC [CI] | |
|---|---|---|
| Bifrontal diameter | 0.912 [0.660–0.979] | 0.831 [0.594–0.95] |
| Left Frontal height | 0.901 [0.623–0.977] | 0.828 [0.588–0.949] |
| Right Frontal Height | 0.892 [0.595–0.974] | 0.787 [0.51–0.936] |
| Brain biparietal diameter | 0.988 [0.946–0.974] | 0.976 [0.933–0.994] |
| Bone biparietal diameter | 0.998 [0.989–0.999] | 0.99 [0.972–0.997] |
| Transverse cerebellar diameter | 0.992 [0.967–0.998] | 0.899 [0.738–0.971] |
| Inter hemispheric fissure | 0.93 [0.723–0.984] | 0.282 [−0.093–0.702] |
| Third ventricle | 0.626 [−0.010–0.901] | 0.396 [0.004–0.769] |
| Left Lateral Ventricle | 0.774 [0.277–0.944] | 0.332 [−0.053–0.733] |
| Right Lateral Ventricle | 0.683 [0.091–0.919] | 0.011 [−0.275–0.485] |
Brain metrics
Results of brain metrics are presented in Table 4.
Table 4.
Comparison of brain metrics between preterm and term infants.
| Pretern | Term | preterm – term differences | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | (%) | 95% CI | p | |
| Bifrontal diameter (mm) | 189 | 64 | 4.6 | 36 | 72.5 | 4.7 | −11.6 | −13.8 to −9.3 | <0.001** |
| Left Frontal height (mm) | 189 | 47.5 | 5 | 36 | 49.3 | 6 | −3.65 | −7.45 to 0.16 | 0.06 |
| Right Frontal height (mm) | 189 | 47.7 | 5.1 | 36 | 49.8 | 6.3 | −4.4 | −8.2 to −0.5 | 0.026* |
| Brain biparietal diameter (mm) | 189 | 75.1 | 5 | 36 | 85.3 | 4.6 | −10.17 | −14 to −9.8 | <0.001** |
| Bone biparietal diameter (mm) | 189 | 79.5 | 5.5 | 36 | 89.3 | 4.3 | −11.1 | −13.2 to −8.9 | <0.001** |
| Transverse cerebellar diameter (mm) | 189 | 50.8 | 3.3 | 36 | 55.6 | 2.6 | −8.7 | −10.47 to −6.9 | <0.001** |
| Fronto occipital diameter (mm) | 73* | 108.97 | 7.2 | 13 | 109.3 | 4 | −0.3 | −2.9 to 2.3 | 0.87 |
| Surface of the vermis (mm2) | 74* | 428.2 | 71 | 13 | 428.4 | 48 | −0.07 | −9.7 to 9.5 | 0.989 |
| Length of corpus callosum (mm) | 72* | 42 | 4.2 | 13 | 42 | 3.8 | −0.4 | −5.852 to 5.042 | 0.98 |
| Inter hemispheric fissure (mm) | 189 | 3.4 | 1.6 | 36 | 2.7 | 1 | + 29.2 | 14.2 to 44.2 | 0.005* |
| Extra-axial space (biparietal bone diameter – biparietal brain diameter) (mm) | 189 | 4.28 | 1.7 | 36 | 3.99 | 2.4 | + 7.2 | −9.5 to 23.9 | 0.395 |
| Third ventricle (mm) | 189 | 2.9 | 0.9 | 36 | 3 | 0.8 | 2.5 | −9.3 to 14 | 0.675 |
| Interopercular distance Left (mm) | 189 | 1.96 | 0.8 | 36 | 1.91 | 0.7 | −4.1 | −18.5 to 10.3 | 0.726 |
| Interopercular distance Right (mm) | 189 | 1.7 | 0.6 | 36 | 1.9 | 0.9 | −10.75 | −25.2 to 3.7 | 0.176 |
| Left lateral ventricle (mm) | 189 | 7 | 3.3 | 36 | 5.7 | 1.5 | + 22.3 | 2.94 to 41.7 | 0.006* |
| Right lateral ventricle (mm) | 189 | 6.5 | 2.8 | 36 | 5.8 | 1.6 | + 11.7 | −4.68 to 28.1 | 0.146 |
Good quality sagittal slices were not available for the entire cohort
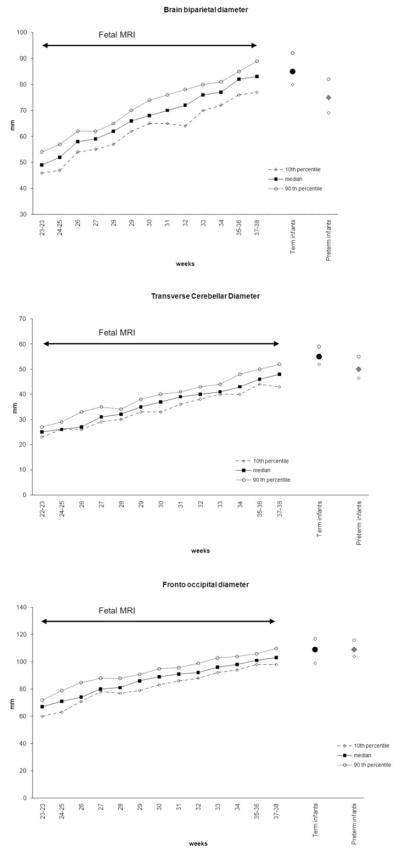
The measures obtained in the full term infants were compared visually to those previously published with the same methodology on fetal MRIs (Fig. 2) and were found to be in accordance with these values. The term infants seemed to display a slightly larger transverse cerebellar diameter than the prenatal cohort scanned at 37 to 38 weeks GA.
Figure 2.
Comparison (median, 10th–90th percentiles) of the fronto-occipital, biparietal and transverse cerebellar diameters measured in term infants (circle) and in the preterm infants (diamond) with the results obtained during pregnancy by measuring fetal brains (square) with the same methodology (adapted from Garel with permission).
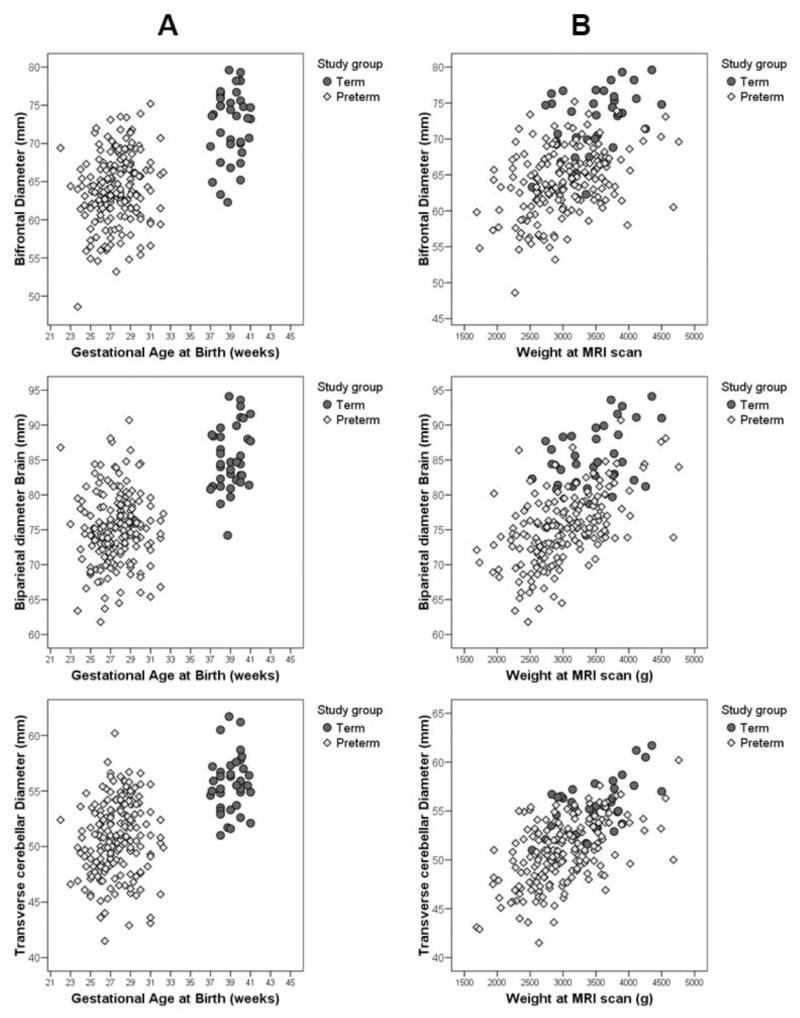
Three tissue measures were markedly decreased in preterm infants compared with full term infants (Fig. 3). These included a reduction in the bifrontal diameter, the brain and bone biparietal diameters, and the transverse cerebellar diameter. A smaller reduction of the frontal heights was also observed, significant only for the left side. The fronto-occipital diameter, the surface of the vermis and the length of the corpus callosum did not differ between the two groups. For fluid measures, the peri-cerebral space was larger in preterm infants in both the inter-hemispheric distance and extra axial space. Preterm infants also had larger lateral ventricles than term infants with the difference being significant only for the left side.
Figure 3.
Comparison of bifrontal, biparietal and transverse cerebellar diameters in preterm infants (diamond) and full term infants (circle) (A) by their gestational age at birth and (B) by their post menstrual age at time of MRI demonstrating the lower values obtained in the preterm cohort.
In both groups there was a significant correlation between gestational age at MRI and tissue brain metrics (Fig. 3B) with no interaction between this variable and the study group. There was no effect of the gestational age at MRI on the fluid measures.
Examination of gender differences demonstrated that the bifrontal and the biparietal diameters were larger in boys (+4.2%; 95%CI = 2 to 6.4%, p < 0.001 and +2.5 %, 0.43 to 4.68 %, p=0.019 respectively). Gender differences were not observed for the transverse cerebellar diameter and the frontal heights, or for any of the fluid measures.
A multivariate analysis by linear regression was undertaken for the tissue measures demonstrating differences in preterm infants with a relatively narrow confidence interval in the bifrontal diameter, the biparietal diameter and the transverse cerebellar diameter. The results are presented in Table 5 along with the same analysis for the fronto-occipital diameter and the length of corpus callosum. There was an effect of group, associated with the effects of gestational age and gender for the bifrontal and biparietal diameters with no interaction between these variables. The transverse cerebellar diameter showed equivalent effects of group and gestational age at MRI, but no effect of gender. For the fronto-occipital diameter and the length of the corpus callosum, only the gestational age at MRI had an effect (significant only for the fronto-occipital diameter).
Table 5.
Multivariate analysis by linear regression, studying the effects of the study group (full term infants as reference group), gender (female as reference group) and gestational age at MRI (GA at MRI) on brain metrics (unstandardised regression coefficients and 95 % confidence intervals)
| Brain measure | Preterm group | GA at MRI (per week) | Male | % variance explained |
|---|---|---|---|---|
| Bifrontal diameter | −7.7 [−9.2; −6.2]** | 1.1 [0.6;1.5]** | 2.4 [1.4;3.5]** | 42.5% |
| Biparietal diameter | −9.3 [−11.0; −7.7]** | 1.4 [0.9;1.9]** | 1.6 [0.4;2.8]** | 46.5 % |
| Transverse cerebellar diameter | −4.1 [−5.1; −3.1]** | 1.3 [1.0;1.6]** | 0.4 [−0.3;1.1] | 44.2 % |
| Fronto-occipital diameter | 0.2 [−0.4;0.4] | 1.6 [0.05;0.3]* | 0.16 [−0.1;0.4] | 7.9 % |
| Corpus callosum length | 0.01 [−0.22;0.23] | 0.06 [−0.002;0.13] | 0.05 [−0.12;0.21] | 1.7 % |
p<0.05
p<0.001
Correlations with brain volumes and head circumference
Quantitative volumetric data were available for 178 preterm infants and 29 term control infants. Correlations between brain metrics and volumes are reported in Table 6. The tissue measurements (i.e. bifrontal diameter, frontal heights, biparietal diameter and transverse cerebellar diameter) were well correlated with the brain tissues volumes (total brain tissue, cortical gray matter, unmyelinated white matter and deep nuclear gray matter) except for the myelinated white matter. The strongest relationships were seen between the transverse cerebellar, bi- frontal and bi-parietal diameters and the total brain tissue and cortical gray matter volumes.
Table 6.
Correlation coefficients between brain metrics head circumference and volumetric data
| Tissue measures | Fluid measures | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pearson correlation | Bifrontal Diameter | Left Frontal Height | Right Frontal Height | Brain Biparietal diameter | Transverse cerebellar Diameter | IHD | Right Ventricle Diameter | Left Ventricle Diameter | Third Ventricle |
| Total Tissue (noCSF) - | 0.663** | 0.357** | 0.331** | 0.626** | 0.707** | 0.027 | 0.079 | −0.034 | 0.234** |
| Cortical Gray Matter | 0.482** | 0.144* | 0.132 | 0.538** | 0.658** | 0.018 | 0.048 | −0.049 | 0.133 |
| Myelinated White Matter | 0.023 | −0.030 | −0.038 | 0.103 | 0.112 | −0.070 | 0.093 | 0.076 | 0.126 |
| Unmyelinated White Matter | 0.589** | 0.455** | 0.421** | 0.446** | 0.441** | 0.037 | 0.099 | 0.016 | 0.247** |
| Basal Ganglia | 0.413** | 0.325** | 0.319** | 0.371** | 0.427** | 0.006 | −0.204** | −0.247** | 0.053 |
| Cerebrospinal Fluid | 0.088 | 0.111 | 0.108 | 0.135 | 0.082 | 0.478** | 0.482** | 0.481** | 0.520** |
| Head Circumference | 0.549** | 0.279** | 0.239** | 0.465** | 0.558** | 0.216** | 0.168* | 0.09 | 0.312** |
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
IHD; Inter Hemispheric Distance
The fluid measures correlated well with the total volume of CSF (r>0.4, p<0.01). There was also a negative correlation between the lateral ventricles measures and the volume of the deep nuclear gray matter (Table 6).
Head circumference was measured at MRI for 188 preterm infants and 35 controls. Head circumference was significantly correlated with the brain metrics although the correlation coefficients were lower than those obtained between the brain metrics and the brain volumes (Table 6).
Discussion
This study demonstrates that measuring the diameters of the cerebral structures and the cerebral cavity on raw MRI images at term may represent a simple and reliable approach for the evaluation of brain size and hence brain growth in the at-risk infant. Our results show striking differences between preterm and full term infants. The bifrontal cerebral, biparietal cerebral and transverse cerebellar diameters were clearly reduced in preterm infants, without an increase in the fronto-occipital diameter. The ventricular size was larger in preterm infants, the difference being significant only for the left side. In preterm infants as in full term infants, the gestational age at MRI was correlated with the cerebral diameters which continued to increase over the last weeks of gestation. The ventricular size and the extra axial space dimensions were not affected by the gestational age at MRI. Gender influenced some parameters with males having larger bifrontal and biparietal diameters. Brain metrics, as one-dimensional measures on qualitative MR images, were well correlated to the 3-dimensional volumetric data previously calculated in this cohort and were also correlated at a lesser degree with the head circumference.
The measures obtained in our full term infants appear in accordance with those previously published with the same methodology on fetal MRIs9. The transverse cerebellar diameter observed in the term infants scanned at 40 weeks PMA was slightly larger than the fetal brain results obtained at 37–38 weeks GA. That is likely to be explained by the rapid growth rate of the cerebellum during the last trimester11. Few data exist about such MRI measures utilized postnatally. In one study comparing ultrasound and MRI measures in 26 preterm and 8 term infants scanned after 37 weeks, similar measures for the length of the corpus callosum and transverse cerebellum to those in our study were documented12. Mewes13 also observed a similar length for the corpus callosum, splenium of the corpus callosum, the bitemporal diameter and the fronto-occipital diameter in 20 term infants. This study then compared these measures with 24 low risk preterm infants at term after reconstruction of the sagittal and axial slices and alignment on the Talairach atlas. The authors found similar results to ours for the length of the corpus callosum (no difference) and the bitemporal diameter (decreased in preterm infants). But they observed a significant increase in the fronto-occipital diameter, which they hypothesized reflected the non-synostotic dolichocephaly commonly observed in preterm infants14 due to their sideway head position required by the respiratory support. In contrast in our study, we did not find any difference in the fronto-occipital diameter in our preterm infants. This may have differed as many of the infants in our cohort had water pillows, a method known to prevent head deformation15,16 and were nursed supine from the first days of life as early endotracheal extubation to nasal CPAP was the common practice in the NICU.
Smaller brain volumes in children and adolescents born preterm have been described in several studies, along with ventricular enlargement and an increased volume of CSF. Brain metrics, similar to those used in this study have been applied in evaluating adolescents born preterm with reductions noted in coronal and sagittal diameters and length of corpus callosum17. Using manual measurement in 66 preterm and 48 full term individuals scanned at 15 years, Nosarti7 also demonstrated a decreased whole brain volume (−6%), reduced cortical grey matter (−11.8%) and enlarged ventricles (+42 %) which persisted after controlling for brain volume. Semi-automated volumetric techniques have explored both regional and tissue specific alterations in brain structure in preterm adolescents. A recent large study demonstrated decreased grey matter volume in temporal, frontal and occipital cortices as well as in cerebellum, putamen and thalamus18. In this study, loss in white matter volume was observed in the brain stem, the internal capsule and the temporal and frontal lobes. Interestingly some cerebral regions demonstrated increased grey matter and white matter volume. These increases were most marked in adolescents who had suffered from major neonatal neuropathologies and suggest that a compensatory abnormal cytoarchitectural organization may have developed secondary to cerebral injury Consistent with the hypothesis of abnormal organization, alterations in orbito frontal sulcal formation has been shown in ex-preterm adolescents19.
Importantly, reduced volume and/or altered cerebral structure have been linked to suboptimal functional outcomes. For example, lower IQ has been shown to be associated with smaller corpus callosum in very preterm infants 20,21, bilateral reduction of the parieto-occipital volumes22, and reduced cerebellar volumes23.
Computational morphometric volumetric techniques have notable variation in the findings in preterm infants at term equivalent. There is a consensus across the studies documenting increased CSF and reduced deep gray matter volumes24,25. However, variation exists in the delineation of reductions in cerebellar volumes which are found universally in one study11, or only in the presence of injury in others 27,28. Variation also exists in relation to total brain tissue volumes. Previous data in this same cohort identified a reduction in total brain tissue volume6 in both low and high risk preterm infants compared with term infants in a similar fashion to another previous smaller cohort study28. However, other studies with a smaller number of subjects have not demonstrated this reduction in brain volume in preterm infants 29. In one larger study (89 preterm compared to 20 full term) Boardman30 described increased ventricular CSF volume with similar total brain volumes. In contrast to our study, the Boardman cohort consisted of more mature low risk preterm infants (mean GA at birth 29.9 weeks, mean birthweight 1290 g) whose head circumference did not differ from the control population. Such discrepancies had raised the question of the timing of the alterations in brain structure - either during the neonatal period or later in childhood. Our results using simple measurements confirm that impairment in brain growth or brain atrophy may be present at term-equivalent age. This emphasizes the need for a better understanding of the factors influencing brain growth during the stay within the NICU. The apparent discordant results within this field may be related to differences in population characteristics as large variations in prenatal and postnatal standard of cares between countries have been demonstrated31.
There are limitations to our approach. The imaging protocol and sequence acquisition varied throughout the study period due to considerations regarding volumetric analyses. The small size of the control cohort further weakened the statistical analysis. In particular, the small number of control infants with sagittal slices lowered the statistical power for the group comparisons for the fronto-occiptal diameter and the length of corpus callosum. The positions of the landmarks were not precisely defined as the images were not realigned in AC-PC space. As the principal aim was to develop a simple and widely applicable method for standard clinical practice, we choose to use the raw MR images, displayed with a Dicom Browser without reconstructions. This choice led us to eliminate some MRIs from the study because of an inaccurate orientation of the slices. However, the landmarks chosen allowed good reproducibility, with the exception of ventricular size.
It should also be noted that there was a high rate of multiple pregnancies (42%) in our preterm cohort. Twin pregnancies are associated with lower birthweight32 and higher risk of neurological impairment33. Even though the proportion of IUGR (11%) in our population was the range of the rates previously published in similar cohorts34,35 these factors - associated with an enhanced role of genetic factors on brain size - may affect our results.
We did not adjust the brain metrics for head circumference because we considered that the skull growth in our population may reflect directly the cerebral tissue growth or CSF expansion, and not external factors. We chose to study the whole cohort including the infants with cerebral injury, including intraventricular hemorrhage. The number of infants with severe cerebral injury (cystic PVL and IVH grade 4) was low representing less than 10 % of the population. Moreover, it appears that such neuropathologies may represent only the visible component of a much more diffuse lesion that could be detected by brain metrics.
Conclusions
This study has defined a reliable reproducible set of brain metrics that can be easily applied in the clinical setting. These measures have good comparability to that of brain volumes. Finally, the measures identify alterations in brain structure in preterm infants in three key brain diameters – bifrontal, biparietal and transverse cerebellum. Further investigations are in progress to identify the relationships between brain metrics with neonatal factors and neurodevelopmental outcome. These measures may assist in both the research and clinical settings to better delineate the impact of preterm birth on brain development.
Acknowledgments
We would like to acknowledge funding support from the National Health and Medical Research Council of Australia (237117), the hard work of Merilyn Bear and the Vibes research team and the wonderful ongoing support of the families and children of the Vibes cohort. We would also like to acknowledge support from NIH K23 HD053212.
Footnotes
Presented in part at the Pediatric American Society annual meeting, Hawaii, 2008
References
- 1.Horbar JD, Badger GJ, Carpenter JH, et al. Trends in mortality and morbidity for very low birth weight infants, 1991–1999. Pediatrics. 2002;110:143–51. doi: 10.1542/peds.110.1.143. [DOI] [PubMed] [Google Scholar]
- 2.Wilson-Costello D, Friedman H, Minich N, et al. Improved neurodevelopmental outcomes for extremely low birth weight infants in 2000–2002. Pediatrics. 2007;119:37–45. doi: 10.1542/peds.2006-1416. [DOI] [PubMed] [Google Scholar]
- 3.Larroque B, Ancel PY, Marret S, et al. Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. Lancet. 2008;371:813–20. doi: 10.1016/S0140-6736(08)60380-3. [DOI] [PubMed] [Google Scholar]
- 4.Hamrick SE, Miller SP, Leonard C, et al. Trends in severe brain injury and neurodevelopmental outcome in premature newborn infants: the role of cystic periventricular leukomalacia. J Pediatr. 2004;145:593–9. doi: 10.1016/j.jpeds.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 5.Anderson NG, Laurent I, Cook N, et al. Growth rate of corpus callosum in very premature infants. AJNR Am J Neuroradiol. 2005;26:2685–90. [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson DK, Warfield SK, Carlin JB, et al. Perinatal risk factors altering regional brain structure in the preterm infant. Brain. 2007;130:667–77. doi: 10.1093/brain/awl277. [DOI] [PubMed] [Google Scholar]
- 7.Nosarti C, Al-Asady MH, Frangou S, et al. Adolescents who were born very preterm have decreased brain volumes. Brain. 2002;125:1616–23. doi: 10.1093/brain/awf157. [DOI] [PubMed] [Google Scholar]
- 8.Garel C. The role of MRI in the evaluation of the fetal brain with an emphasis on biometry, gyration and parenchyma. Pediatr Radiol. 2004;34:694–9. doi: 10.1007/s00247-004-1249-x. [DOI] [PubMed] [Google Scholar]
- 9.Garel C. MRI of the fetal brain Berlin. New York: Springer; 2004. [Google Scholar]
- 10.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–94. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 11.Limperopoulos C, Soul JS, Gauvreau K, et al. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics. 2005;115:688–95. doi: 10.1542/peds.2004-1169. [DOI] [PubMed] [Google Scholar]
- 12.Leijser LM, Srinivasan L, Rutherford MA, et al. Structural linear measurements in the newborn brain: accuracy of cranial ultrasound compared to MRI. Pediatr Radiol. 2007;37:640–8. doi: 10.1007/s00247-007-0485-2. [DOI] [PubMed] [Google Scholar]
- 13.Mewes AU, Zöllei L, Hüppi PS, et al. Displacement of brain regions in preterm infants with non-synostotic dolichocephaly investigated by MRI. Neuroimage. 2007;36:1074–85. doi: 10.1016/j.neuroimage.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchison BL, Thompson JM, Mitchell EA. Determinants of nonsynostotic plagiocephaly: a case-control study. Pediatrics. 2003;112:e316. doi: 10.1542/peds.112.4.e316. [DOI] [PubMed] [Google Scholar]
- 15.Marsden DJ. Reduction of head flattening in preterm infants. Dev Med Child Neurol. 1980;22:507–9. doi: 10.1111/j.1469-8749.1980.tb04356.x. [DOI] [PubMed] [Google Scholar]
- 16.Schwirian PM, Eesley T, Cuellar L. Use of water pillows in reducing head shape distortion in preterm infants. Res Nurs Health. 1986;9:203–7. doi: 10.1002/nur.4770090304. [DOI] [PubMed] [Google Scholar]
- 17.Cooke RW, Abernethy LJ. Cranial magnetic resonance imaging and school performance in very low birth weight infants in adolescence. Arch Dis Child Fetal Neonatal Ed. 1999;81:F116–21. doi: 10.1136/fn.81.2.f116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nosarti C, Giouroukou E, Healy E, et al. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain. 2008;131:205–17. doi: 10.1093/brain/awm282. [DOI] [PubMed] [Google Scholar]
- 19.Giménez M, Junqué C, Vendrell P, et al. Abnormal orbitofrontal development due to prematurity. Neurology. 2006;28(67):1818–22. doi: 10.1212/01.wnl.0000244485.51898.93. [DOI] [PubMed] [Google Scholar]
- 20.Nosarti C, Rushe TM, Woodruff PW, et al. Corpus callosum size and very preterm birth: relationship to neuropsychological outcome. Brain. 2004;127:2080–9. doi: 10.1093/brain/awh230. [DOI] [PubMed] [Google Scholar]
- 21.Narberhaus A, Segarra D, Caldú X, et al. Corpus callosum and prefrontal functions in adolescents with history of very preterm birth. Neuropsychologia. 2008;46:111–6. doi: 10.1016/j.neuropsychologia.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. Pediatrics. 2003;111:939–48. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 23.Allin MP, Salaria S, Nosarti C, et al. Vermis and lateral lobes of the cerebellum in adolescents born very preterm. Neuroreport. 2005;16:1821–4. doi: 10.1097/01.wnr.0000185014.36939.84. [DOI] [PubMed] [Google Scholar]
- 24.Boardman JP, Counsell SJ, Rueckert D, et al. Abnormal deep grey matter development following preterm birth detected using deformation-based morphometry. Neuroimage. 2006;32:70–8. doi: 10.1016/j.neuroimage.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 25.Srinivasan L, Dutta R, Counsell SJ, et al. Quantification of deep gray matter in preterm infants at term-equivalent age using manual volumetry of 3-tesla magnetic resonance images. Pediatrics. 2007;119:759–65. doi: 10.1542/peds.2006-2508. [DOI] [PubMed] [Google Scholar]
- 26.Shah DK, Anderson PJ, Carlin JB, et al. Reduction in cerebellar volumes in preterm infants: relationship to white matter injury and neurodevelopment at two years of age. Pediatr Res. 2006;60:97–102. doi: 10.1203/01.pdr.0000220324.27597.f0. [DOI] [PubMed] [Google Scholar]
- 27.Srinivasan L, Allsop J, Counsell SJ, et al. Smaller cerebellar volumes in very preterm infants at term-equivalent age are associated with the presence of supratentorial lesions. AJNR Am J Neuroradiol. 2006;27:573–9. [PMC free article] [PubMed] [Google Scholar]
- 28.Inder TE, Warfield SK, Wang H, et al. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–94. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- 29.Zacharia A, Zimine S, Lovblad KO, et al. Early assessment of brain maturation by MR imaging segmentation in neonates and premature infants. AJNR Am J Neuroradiol. 2006;27:972–7. [PMC free article] [PubMed] [Google Scholar]
- 30.Boardman JP, Counsell SJ, Rueckert D, et al. Early growth in brain volume is preserved in the majority of preterm infants. Ann Neurol. 2007;62:185–92. doi: 10.1002/ana.21171. [DOI] [PubMed] [Google Scholar]
- 31.Zeitlin J, Draper ES, Kollée L, et al. MOSAIC research group. Differences in rates and short-term outcome of live births before 32 weeks of gestation in Europe in 2003: results from the MOSAIC cohort. Pediatrics. 2008;121:e936–44. doi: 10.1542/peds.2007-1620. [DOI] [PubMed] [Google Scholar]
- 32.Sebire NJ, Snijders RJ, Hughes K, et al. The hidden mortality of monochorionic twin pregnancies. Br J Obstet Gynaecol. 1997;104:1203–7. doi: 10.1111/j.1471-0528.1997.tb10948.x. [DOI] [PubMed] [Google Scholar]
- 33.Pharoah PO. Neurological outcome in twins. Semin Neonatol. 2002;7:223–30. doi: 10.1053/siny.2002.0109. [DOI] [PubMed] [Google Scholar]
- 34.Anderson PJ, Doyle LW Victorian Infant Collaborative Study Group. Executive functioning in school-aged children who were born very preterm or with extremely low birth weight in the 1990s. Pediatrics. 2004;114:50–7. doi: 10.1542/peds.114.1.50. [DOI] [PubMed] [Google Scholar]
- 35.Stoelhorst GM, Rijken M, Martens SE, et al. Changes in neonatology: comparison of two cohorts of very preterm infants (gestational age <32 weeks): the Project On Preterm and Small for Gestational Age Infants 1983 and the Leiden Follow-Up Project on Prematurity 1996–1997. Pediatrics. 2005;115:396–405. doi: 10.1542/peds.2004-1497. [DOI] [PubMed] [Google Scholar]