Abstract
An epidemic of acute respiratory syndrome (Covid-19) started in humans in Wuhan in 2019, and became a pandemic. Groups from China Identified and sequenced the virus responsible for COVID-19, named SARS-CoV-2, and determined that it was a novel coronavirus (CoV) that shared high sequence identity with bat- and pangolin-derived SARS-like CoVs, suggesting a zoonotic origin. SARS-CoV-2 is a member of Coronaviridae, a family of enveloped, positive-sense, single-stranded RNA viruses that infect a broad range of vertebrates. The rapid release of the sequence of the virus has allowed the development of diagnostic tools (e.g., RT-PCR). Additionally, serological tests can allow identification of persons who have been infected. In humans, CoVs tend to cause mild to moderate upper respiratory tract infections. The fatality rate is around 1–3% for infected persons. An acute respiratory distress syndrome (ARDS) likely due to an uncontrolled immune activation (“cytokine storm”) occurs in patients with severe disease and poor prognosis. Risk factors for mortality include: advanced age, obesity, diabetes, hypertension and other comorbidities. Drug repurposing has been used to rapidly identify potential treatment for COVID-19, which could move quickly to phase-3. Better knowledge of the virus, its enzymes, will be mandatory to develop more potent and specific direct-acting antiviral agents (DAA). In the long term, a vaccine to prevent infection would be crucial; however even if successful it might not be available before 2021–22. To date, with the exception of intravenous Remdesivir and dexamethasone, which have modest effects in moderate to severe COVID-19, no strong clinical evidence supports the efficacy and safety of any other drugs against SARS-CoV-2. The aim of this review is to provide insights on the discovery of SARS-CoV-2, its virology, the diagnostic tools, and the ongoing drug discovery effort.
Keywords: SARS-CoV-2, coronavirus, pathogenesis, drug repurposing, remdesivir
Introduction
The World Health Organization (WHO) announced on March 11th 2020, that the outbreak of “COronaVIrus Disease 2019” (COVID-19), which initially started in Asia, has become pandemic. On September 4th 2020 the etiologic agent “Severe Acute Respiratory Syndrome (SARS)-CoV-2 has spread all over the world with a global estimation of around 26 million confirmed cases and around 865,000 deaths [1]. The rapid availability of the virus RNA genome sequence was instrumental in the development of diagnostic tools and for the identification of experimental treatments. In this review, we will clarify aspects related to the discovery of SARS-CoV-2, virological features, diagnostic tools, complex pathogenesis, including a focus on the liver and gastro-intestinal lesions, and of course drug discovery.
Concise overview of SARS-CoV2 virology
The causative agent of COVID-19 is a novel coronavirus (CoV) officially named SARS-CoV-2. It was named after SARS-CoV-1, because of a genomic homology [2]. Coronaviruses are enveloped, large positive-sense single-stranded RNA viruses (+ssRNA), gathered in the Coronaviridae family. CoVs can infect a broad range of vertebrates, including bats, birds, pangolins, snakes, mice, and humans. Due to sequence similarities with RaTG13 bat and pangolin CoV strains, it is currently thought that SARS-CoV2 has a zoonotic origin and has secondary acquired human-to-human spreading capacity [3]. In particular, the acquisition of i) mutations in the receptor binding area, ii) a polybasic furin cleavage site (RRRAR) at the junction of subdomain 1 and 2 of the spike protein and iii) a site of O-linked glycosylation in the same area, has enabled the virus to efficiently interact with high affinity (via its spike protein) with its bona fide cellular receptor (angiotensin-converting enzyme 2 (ACE-2))[4], to become more virulent and pathogenic, while potentially evading immune response by O-glycan epitope masking [3].
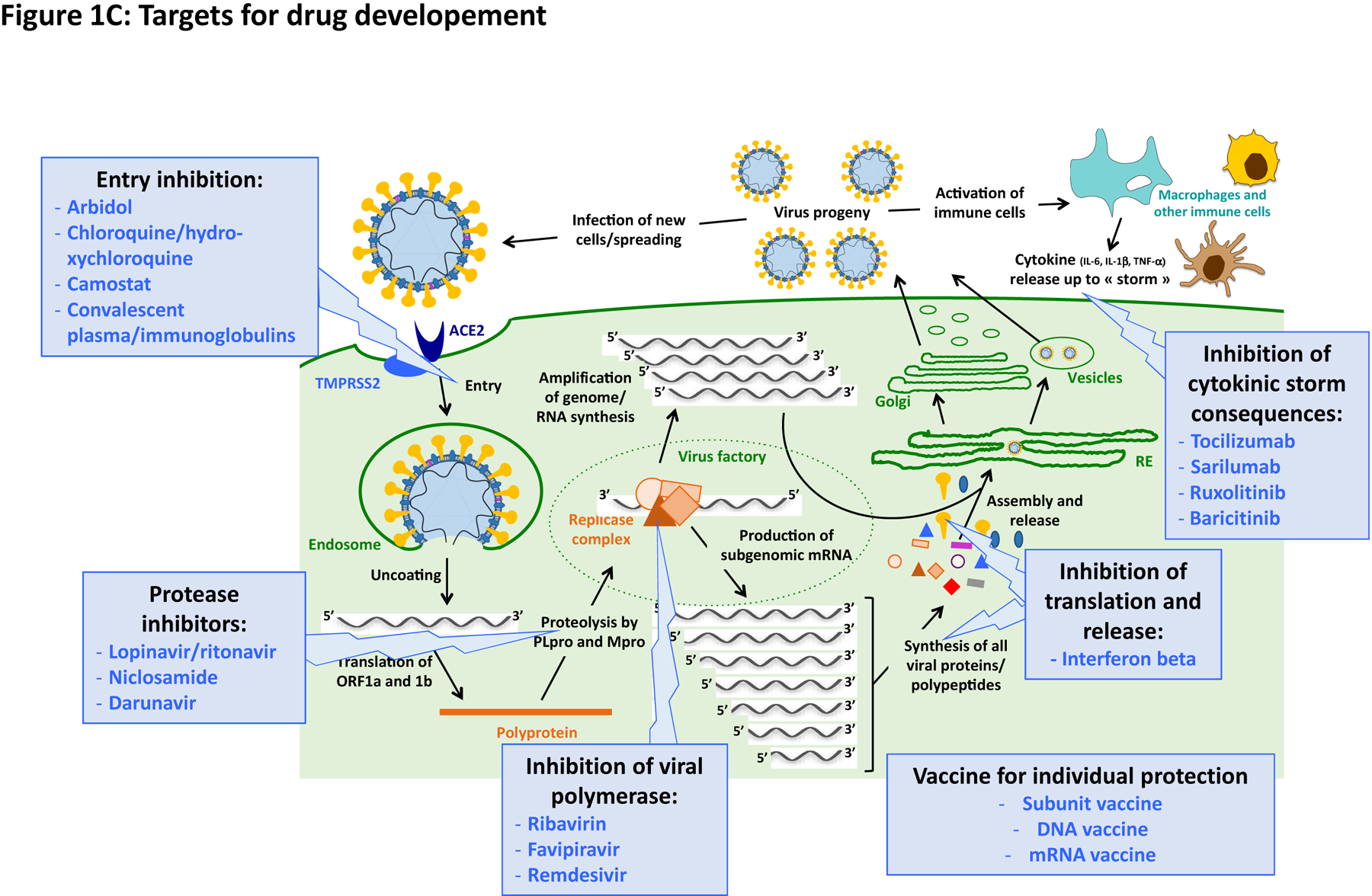
Figure 1 gathers general information on virologic aspects and replication cycle of SARS-CoV2, as well as a schematic representation of targets for drug development.
Figure 1. Virology, replication cycle and targets for drug development.

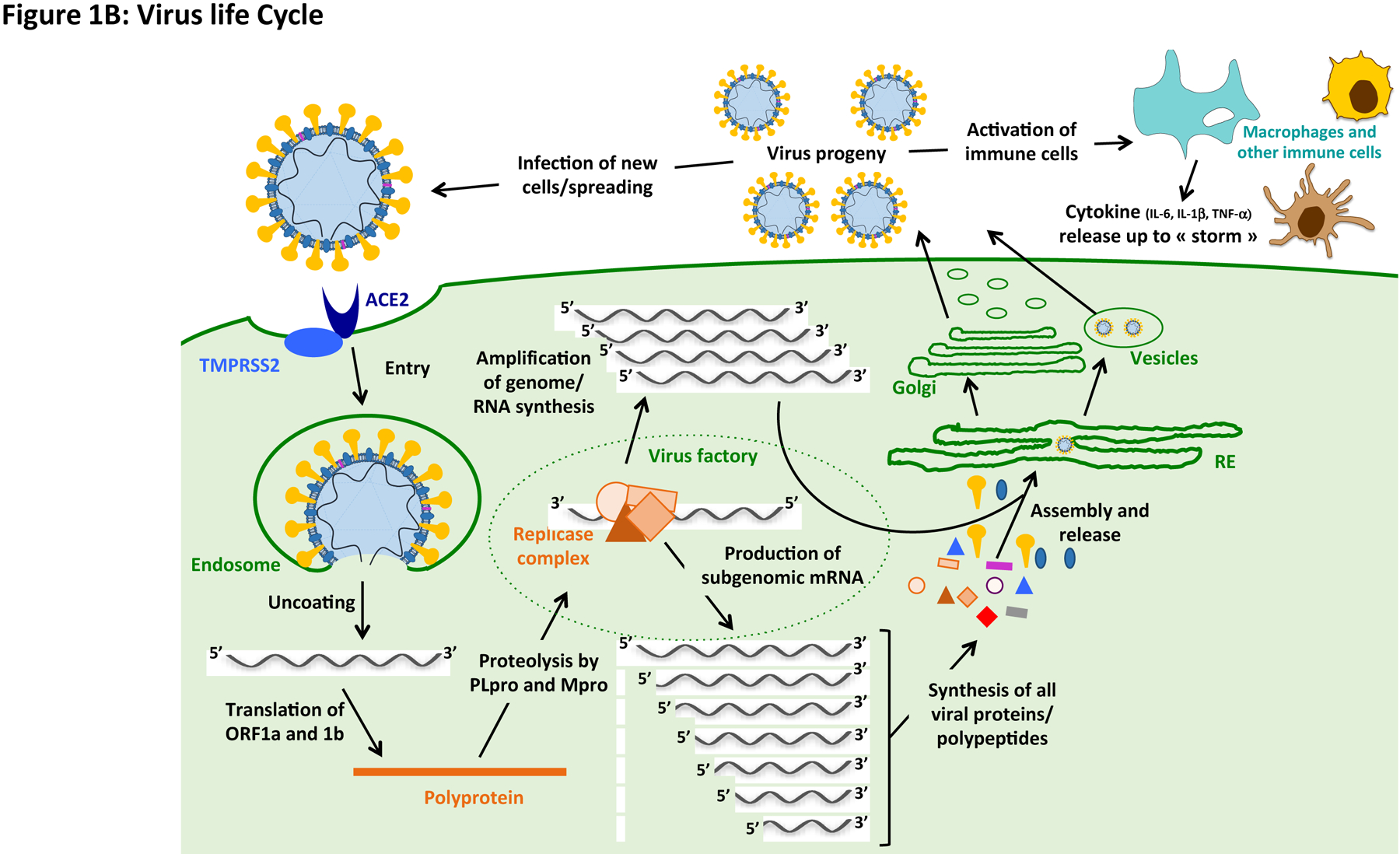
(A) CoVs have a long, capped and poly-adenylated RNA genome, which contains between 8 to 10 open-reading-frames (ORFs), allowing structural, non-structural and accessory viral protein synthesis [87]. SARS-CoV2 is 29,903 base-long and contains 6 majors ORFs, as well as additional accessory genes; the reference sequence is registered in GenBank with ID: MN908947.3 [1]. (A and B) Up to 28 different polypeptides are potentially produced in fine from the different ORFs and after polyprotein processing by viro-encoded proteases [87]. If the RNA genome contained in virions can already serve, after cell entry, as a template for the synthesis of non-structural proteins, which are involved in the early phase of virus replication (mainly by forming the replicase complex), subgenomic messenger RNAs (sgmRNAs) are also produced in the late phase of the cycle to allow the synthesis of structural proteins (e.g. spike (S), envelope (E), membrane (M) and nucleocapsid (N) proteins), as well as other accessory polypeptides. Another main replication intermediate is the complementary minus-sens RNA, which is used by the viro-encoded RNA-dependent RNA-polymerase (RdRp), within replicase complex, to amplify the full-length genome, which is then capped and polyadenylated by both viral and host enzymes before being incorporated into virus progeny. (B) After entry into ACE2-positive (entry receptor) and TMPRSS2-positive (co-factor for entry) cells, and the membrane fusion (i.e. uncoating process), a full-length genome is released in the cytoplasm of cells. This full-length polycistronic RNA is directly used to efficiently encode a polyprotein from the first ORFs present on the molecule, starting from 5’ extremity, i.e. ORF1a and ORF1b; the latter is read after a frame-shift from ribosomal scanning of ORF1a. (A and B) The polyprotein is then processed by two viro-encoded proteases, a papain-like cysteine protease (PLpro/Nsp3) and a chemotrypsin-like protease (3CLpro/Nsp5; also known as main protease (Mpro)), into 16 proteins/polypeptides (Nsp1 to 16). (B) These non-structural proteins/polypeptides are important for the early stage of infection, as they allow in particular the formation of the replicase complex around the RdRp enzymatic activity, which is involved in the synthesis of negative-sense full-length RNA, as well as sgmRNAs by a discontinuous transcription strategy [87]. The latters enable an efficient and stochiometric synthesis of all other viral proteins/polypeptides, which are important for virus assembly and release of progeny virions.
Where does the virus replicate?
Following replication and sub-genomic RNA synthesis, the viral structural proteins, are translated and inserted into the endoplasmic reticulum (ER). These proteins move along the secretory pathway into the ER-Golgi intermediate compartment. In infected cells, the CoV RNA-synthesizing machinery associates with modified ER membranes that are transformed into the viral replication organelle; double-membrane vesicles appear to be the central hub for viral RNA synthesis [5]. Otherwise, the duration of SARS-CoV-2 is significantly longer in stool samples than in respiratory samples [6].
Mechanisms of virus-induced toxicity.
The virus may be cytotoxic during the first days. In biopsy or autopsy studies, pulmonary pathology for COVID-19 patients showed diffuse alveolar damage with the formation of hyaline membranes, mononuclear cells, and macrophages infiltrating air spaces, and a diffuse thickening of the alveolar wall [7–8]. The lungs from patients with Covid-19 also showed severe endothelial injury associated with the presence of intracellular virus and disrupted cell membrane [9]. Viral particles were observed in the epithelial cells by electron microscopy suggesting that these lesions might be partially caused by a direct cytotoxicity.
Future directions for basic research and target identification
A better understanding of the functions/roles of viral proteins, as well as of the virus replication cycle, with a particular attention on host-cell/virus interactions, will allow the identification of novel or a better characterization of targets for antiviral agent development. The success of drug development for hepatitis C virus (HCV) has inspired scientists to achieve the same for other viruses [10].
Entry process.
Many cell types could express ACE2 and transmembrane serine protease 2 (TMPRSS2), the two cellular factors important for virus entry [11], including nasal and lower airway epithelial cells (pneumocytes), lung resident immune cells, endothelial cells, as well as neurons, enterocytes, cardiomyocytes, hepatocytes, kidney cells [12–14]. But the presence of mRNA in these cell-types is not sufficient. Study of the expression at protein levels of these entry factors, as well as the demonstration of bona fide viral entry and active replication in all these cell-types is yet missing. Interestingly, it was very recently shown that ACE2 is an interferon-stimulated-gene (ISG) [15], meaning that the presence of IFNs in the microenvironment of the virus replication site could further enhance the spreading of the virus. The molecular details of the entry process, involving the spike protein and host receptor/coreceptor, have already been studied [16]. Polybasic furin cleavage site at the junction of subdomain 1 and 2 of the spike protein, may explain the large number of cell types that may be infected by the virus with the consequent diverse organ manifestations, including, possibly the thrombotic complications as a result of possible endothelial cell infection. This research will facilitate the identification of neutralizing antibodies or small molecules, which could target this step of the life cycle.
Viral enzymes.
CoVs encode several enzymes that are crucial for the replication of the virus and ideal target for antiviral development, amongst which two proteases/proteinases (PLpro and 3CLpro), the RdRp, a helicase, an mRNA-cap-methyltransferase, and an exoribonuclease. These enzymes have been well studied for SARS-CoV, and thanks to the high homology between the two SARS CoVs, we could expect functional similarities and the possible repurposing of drugs [3–4, 11].
The RdRp (also identified as Nsp12) bears the main enzymatic activity of the replicase complex. Recent advance in antiviral research against HCV [10, 17], has confirmed that RdRp are major target for very specific antiviral discovery. Similarly to HCV, SARS-CoV2 genome is characterized by a positive-sense single-strand RNA and share a similar replication cycle requiring a RdRp. This polymerase displays resembling catalytic mechanisms and key conserved amino acids in the active site. The RdRp 3D structure has been readily characterized [18–19]. Interestingly it has a large N-terminal extension containing a kinase-like fold. The polymerase domain, like HCV, is composed of three subdomains; a fingers subdomain, a palm subdomain, and a thumb subdomain. Moreover, also the 3-chymotrypsin-like protease (3CLpro) is vital to virus replication and the 3CLpro cleavage sites are highly conserved, so it could be a promising drug target [18].
PLpro and 3CLpro/Mpro are essential enzymes for the proteolytic processing of CoV replicase polyprotein; their activities are needed very early in the infection process to step-by-step release other viral enzymatic activities. They are also attractive targets for specific antiviral discovery. SARS-CoV1 and SARS-CoV2 share high amino-acid sequence identities for these two proteases and 3D structures are also available. Moreover biochemical assays are also available for functional testing, at least for the SARS-CoV proteins [20]. PLpro is a cysteine protease, encoded by Nsp3, and involved in the release of Nsp1 to 3, as well as in the regulation of host innate immunity functions to allow viral escape [20]. Although the similarity between PLpro of SARS-CoVs is not very high, the catalytic domain, around the triad Cyst-His-Asp, is well conserved; therefore drug already in the pipeline for SARS-CoV1 might be repurposed.
3CTpro/Mpro is encoded by Nsp 5, forms a functional homodimer, utilizes a catalytic dyad Cys-His, and is involved in the release of Nsp4 to 16 from polyprotein. Its activity is key for CoV replication-cycle and its inhibition very efficient at stopping viral replication. Due to the dimeric nature of this protease, not only catalytic inhibitors, but also allosteric ones can be developed increasing possibilities of success. Moreover the very high similarity of 3CTpro/Mpro SARS-CoVs allow repurposing of drugs [20]. Some specific antiviral screenings have been promptly started on 3CTpro of SARS-CoV-2 and several drug candidates already identified [21–22].
Exacerbated innate immune functions
Beside virologic aspects of COVID-19, it is also important to better understand immunologic ones, as well as their mutual amplification responsible for an increased pathogenesis in patients. If the virus can be studied in cell culture model, immunological aspects of COVID-19 can only be studied either in relevant animal model or during clinical studies, using patient samples. It is now rather well established that in patients with poor outcome there is an uncontrolled “cytokine storm”, featuring a local and systemic production of pro-inflammatory cytokines such as IL-6, TNF-α and IL-1β [23–27]. Recently, it has been reported that ACE2 is a human interferon-stimulated gene (ISG) in vitro using airway epithelial cells and extend the findings to in vivo viral infections. these data suggest that SARS-CoV-2 could exploit species-specific interferon-driven upregulation of ACE2, a tissue-protective mediator during lung injury, to enhance infection [28]. More studies are needed to clarify the origin of this massive and uncontrolled cytokine production.
Diagnostic tools for Covid-19
COVID-19 tests can be grouped as nucleic acid, serological, antigen, and ancillary tests, all of which play distinct roles in hospital, point-of-care, or large-scale population testing [29].
Methods for the detection of viral nucleic acid.
PCR tests for SARS-CoV2 have been available since January 2020. RT-qPCR-based assays performed on respiratory specimens has emerged as the cornerstone of COVID-19 diagnostic testing. The USA Centers for Disease Control and prevention (CDC) has developed a widely used SARS-CoV2 RT-qPCR assay [30]. The kit contains PCR primer-probe sets for two regions of the viral nucleocapsid gene (N1 and N2), and for the human RNase-P gene to ensure the RNA extraction was successful. This assay differs from the WHO’s one, which target SARS-CoV2 RdRP and E genes [31]. To avoid potential cross-reaction with other endemic CoVs, as well as potential genetic drift, at least two molecular targets should be included in the assay. Evolution and potential mutations in SARS-CoV-2 genome strengthens the need to continue optimizing the oligonucleotides by global updated sharing of SARS-CoV-2 genomes [32]. Theoretical specificity of most RT-qPCR assays is 100% because the primer design is specific to the SARS-CoV-2 genome. Occasional false positive results may occur due to technical errors or reagent contamination [33]. A cycle threshold (Ct) value of RT-qPCR less than 40 is generally interpreted as positive when results are interpreted as qualitative [34–35]. Quantitative interpretation of Ct as indicators of the copy number of SARS-CoV-2 RNA in specimens needs an appropriate standard curve with adequate limit of detection [36]. A rigorous assessment of the diagnostic accuracy of the many newly introduced SARS-CoV-2 assays is hampered [37–38]. The sensitivity of viral RNA testing varies with timing of testing relative to exposure. A false positive result erroneously labels a person as infected, with consequences including unnecessary quarantine and contact tracing [39]. False negative results are more consequential, because infected persons may not be isolated and can infect others. One modeling study estimated that the probability of a false-negative result in an affected patient decreases from 100% on day 1 to 67% on day 4 [40]. On the day of symptom onset, the median false-negative rate estimation was 38%. Sample pooling strategy was suggested to offer a viable alternative to detect community transmission at a time when tests are in short supply globally [41–43]. One potential limitation of pool testing is that the false-negative rate may increase owing to dilution of positive samples. Point-of-care PCR kits can shorten the turnaround time for screening and diagnosing patients with suspected SARS-CoV-2. These rapid tests typically have lower throughput and are generally more expensive than other tests. Time efficient methods that do not require thermal cycling have been designed and are evaluated [44]. CRISPR-Cas12/Cas13-based assay are also currently in development for point-of-care use [45–46].
Nature of samples tested.
The current diagnostic strategy to identify patients with COVID-19 is to test samples taken from the respiratory tract to assess for the presence of SARS-CoV-2 specific nucleic acid targets [47]. A nasopharyngeal specimen is the preferred choice for testing, but oropharyngeal, mid-turbinate, or anterior nares samples are also acceptable [48]. A pharyngeal virus shedding was shown to be very high during the first week of symptoms [49]. Infectious virus was readily isolated from throat- and lung-samples, but not from stool ones. Serum and urine were usually negative for the presence of viral nucleic acid [50–51]. The viral load in nasopharyngeal samples peaks within the first few days after symptom onset, before declining [48, 51–52]. For nasopharyngeal specimen, samples should be obtained by using a flocked swab to enhance the collection and release of cellular material [53–54]. Samples taken from sputum, endotracheal aspirates, and bronchoalveolar may have greater sensitivity than upper respiratory tract specimens [50]. Inadequate sample collection may result in a false-negative test. Bronchoalveolar lavage fluid specimens had the highest positive rates of SARS-CoV2 RT-qPCR assay [50]. A single nasopharyngeal swab has become the preferred swab, as it is well tolerated and safe. Saliva may also be an alternative specimen source that requires less personal protective equipment and fewer swabs, but requires further validation [55–56].
Serologic testing.
If RT-qPCR-based molecular assays for detecting SARS CoV-2 in respiratory specimens remain the current reference standard for diagnosis, point-of care technologies, and serologic immunoassays have also rapidly emerged [57–59]. Serologic tests that identify antibodies to SARS-CoV-2 from clinical specimens may be less complex than molecular tests [60]. As antibody responses to infection take days to weeks to be reliably detected [60], their utility for diagnosing acute infections is limited [48]. Rapid antigen detection tests have recently entered the diagnostic market. Compared with RT-PCR, they are cheaper, and easy to use with faster turn-around times. The widespread and frequent use of such tests has recently been proposed but antigen rapid antigen detection tests’s differ greatly in their ability to detect infectious cases, therefore requiring careful validation before routine application. Serologic assays might be more relevant in surveying for asymptomatic infection or in scenarios in which patients present to medical care with late complications of disease, when RT-qPCR may be falsely negative [55, 61].
Seroconversion in most cases of SARS occurs during the second week of symptoms [49]. For SARS-COV-2 infection, timing of seroconversion appears similar to or slightly earlier than in SARS-CoV-1 infection [62]. In a study of 285 patients with COVID-19, 100% of patients were tested positive for antiviral IgG within 19 days after symptom onset, with seroconversion for IgG and IgM occurring simultaneously or sequentially [61]. Negative results would not exclude COVID-19 infection, particularly among those with recent exposure to the virus. The viral spike protein is perceived as the clear candidate for inclusion in an immunoassay that detects whether antibodies are present [58; 63]. The other protein that appears to be important antigen for the development of serological assays is the N protein (structural component of the nucleocapsid). Indeed, antibodies to this protein are frequently detected in COVID-19 patient [64–65], suggesting that N protein may be one of the immunodominant antigens in the early diagnosis of COVID-19 [60, 66–67]. It is now established that SARS-CoV-2 pre-existing immune reactivity can exist in the general population. Serum samples from patients with COVID-19 showed some cross-reactivity for the SARS-CoV-1 nucleocapsid antigens [61, 68–69]. A recent study detected SARS-CoV-2-reactive CD4+ T cells in 50% of unexposed individuals, suggesting cross reactive T cell recognition between circulating “common cold” coronaviruses and SARS-CoV-2 [66]. T cell reactivity was highest against proteins other than the coronavirus spike protein, but T cell reactivity was also detected against spike. Several monoclonal antibodies have been described that target the S glycoprotein of SARS-CoV-2 from memory B cells of an individual who was infected with SARS-CoV-1 in 2003 [68]. One antibody (S309) potently neutralizes SARS-CoV-2 by engaging the receptor-binding domain of the S glycoprotein.
Enzyme-linked immunosorbent assays (ELISA) and CLIA are common laboratory platforms that can measure antibody titers (IgG and IgM). A variation of these tests can use magnetic, protein-coated microparticles, known as a chemiluminescent microparticle immunoassay. Being able to quantify antibodies will be important for identifying convalescent plasma donors with abundant titers and studying how the immune system responds to the virus. Neutralizing antibodies (NAbs) play important roles in virus clearance and have been considered as a key immune product for protection or treatment against viral diseases. In COVID-19, transfusion of convalescent plasma or serum from recovered patients was also considered as a promising therapy [71]. The neutralization assay is a laboratory-based test that uses live virus and cell culture methods to determine if patient antibodies can prevent viral infection in vitro [72].
Because immunofluorescence assay is a labor-intensive method, a substantial number of the new commercial COVID-19 antibody tests developed as screening tests are not ELISA-based. They are lateral flow immunoassays (LFIA), which provide no quantitative information. These qualitative LFIA represent typically small, portable rapid diagnostic tests (RDT), and can be used at point of care.
Conclusions on serologic testing.
Antibody testing is ramping up quickly, with a growing list of commercial kits and test protocols from academic researchers [57]. Many questions will have to be answered. The first and most urgent is the validation of serologic tests. A recent meta-analysis showed wide range sensitivities from 66% in LFIAs to 98% in the CLIAs [73]; sensitivities were higher with increased time after symptom onset. The specificities are excellent (99%). Assays must be optimized further, independently validated, and used in an algorithm format to achieve the highest possible accuracy for decision making [74–75]. Second, there is insufficient data of the magnitude and duration of antibody responses after infections. Although data suggest that neutralizing titers correlate with severity of infection [61], it remains elusive, whether this effect is caused by ongoing somatic hypermutation or ongoing production of highly potent antibodies that were initially generated. Moreover, any documentation that limits individual freedoms on the basis of biology risks becoming a platform for restricting human rights [76].
Physiopathology, Clinical Characteristics and Management of COVID-19
Physiopathology.
Several potential pathogenic mechanisms may be involved including coagulopathy, endothelial dysfunction, and excessive release of pro-inflammatory cytokines. The endothelial dysfunction caused by infection activates an excessive thrombin generation and inhibits fibrinolysis, which designates hypercoagulability [77]. Lymphopenia is frequent in patients with COVID-19 [78]. The cytokine release syndrome could have a major role in patients with severe COVID-19 as in acute respiratory distress syndrome (ARDS) [79]. The pathological features of COVID-19 related ARDS are diffuse alveolar damage with hyaline membrane formation with fibrin deposition and a few multinucleated enlarged cells [7–8]. In patients who died from Covid-19-associated respiratory failure, the histologic pattern in the peripheral lung was diffuse alveolar damage with perivascular T-cell infiltration [9]. The lungs also showed distinctive vascular features, consisting of severe endothelial injury, but also widespread thrombosis with microangiopathy. Alveolar capillary microthrombi were frequent, with a high amount of new vessel growth (intussusceptive angiogenesis).
Transmission by asymptomatic carriers.
Several findings are consistent with person-to-person transmission of this novel coronavirus in hospital and family settings [47,80]. A case of SARS-CoV-2 infection acquired outside Asia in which transmission appears to have occurred during the incubation period [81]. Furthermore, in a previously reported family cluster, some of the family members had positive RT-qPCR results without any symptoms [47].
Clinical Characteristics.
Among 1,099 patients from China with laboratory-confirmed COVID-19, 5.0% were admitted to the intensive care units (ICU), 2.3% underwent invasive mechanical ventilation, and 1.4% died [78]. The most common symptoms were fever and cough. The median incubation period was 4 days. In another study including 191 patients, with 54 who died in hospital, half of the patients had a comorbidity, with hypertension being the most common, followed by diabetes and coronary heart disease [52]. In-hospital death was associated with older age, higher Sequential Organ Failure Assessment (SOFA) score, and D-dimer greater than 1 μg/mL on admission. In another study of the 1,591 patients infected with SARS-CoV-2 admitted to ICU in Italy, the median age was 63 years and 82% were male [82]. Among 1,300 patients with available respiratory support data, 99% needed respiratory support, including 88% who received mechanical ventilation and 11% who received non-invasive ventilation. Finally, in this case series of critically ill patients admitted to ICUs, the majority were older men and ICU mortality was 26%.
Moreover, data from previous coronavirus infections such as severe acute respiratory syndrome and Middle East respiratory syndrome, as well as emerging data from the COVID-19 pandemic, suggest there could be substantial fibrotic consequences following SARS-CoV-2 infection [83].
Imaging findings.
The hallmarks of COVID-19 were bilateral and peripheral ground-glass and consolidative pulmonary opacities [84]. Notably, 56% of patients with early disease had a normal CT. With a longer time after the onset of symptoms, CT findings were more frequent, including consolidation, bilateral and peripheral disease, greater total lung involvement, linear opacities, “crazy-paving” pattern and the “reverse halo” sign. Bilateral lung involvement was observed in 28% in early phase and 88% in late phase of the disease. CT scans at the time of symptoms may increase diagnosis rate since RT-PCR sensitivity may be as low as 60% [85]. Also, chest x-ray findings in COVID-19 patients frequently showed bilateral lower zone consolidation [86].
Extra-Pulmonary manifestations
Coagulopathy disorders.
SARS-COVID-19-induced infection can be associated with a coagulopathy, findings consistent with infection-induced inflammatory changes as observed in patients with disseminated intravascular coagulopathy (DIC) [87]. The initial coagulopathy of COVID-19 presents with elevation of D-dimer and fibrin/fibrinogen-degradation products. COVID-19-associated coagulopathy should be managed as it would be for any critically ill patient, using thromboembolic prophylaxis and standard supportive care measures for those with sepsis-induced coagulopathy or DIC. Current data do not suggest the use of high anticoagulation doses [87].
Among all the numerous clinical manifestations associated with COVID-19 infection, we can recall cardiological lesions with acute myocardial injury [88]; neurological lesions with encephalitis and myalgia [89–90], cutaneous manifestations with rash and urticaria [91], and acute kidney injury [92]. (Figure 3).
Figure 3.
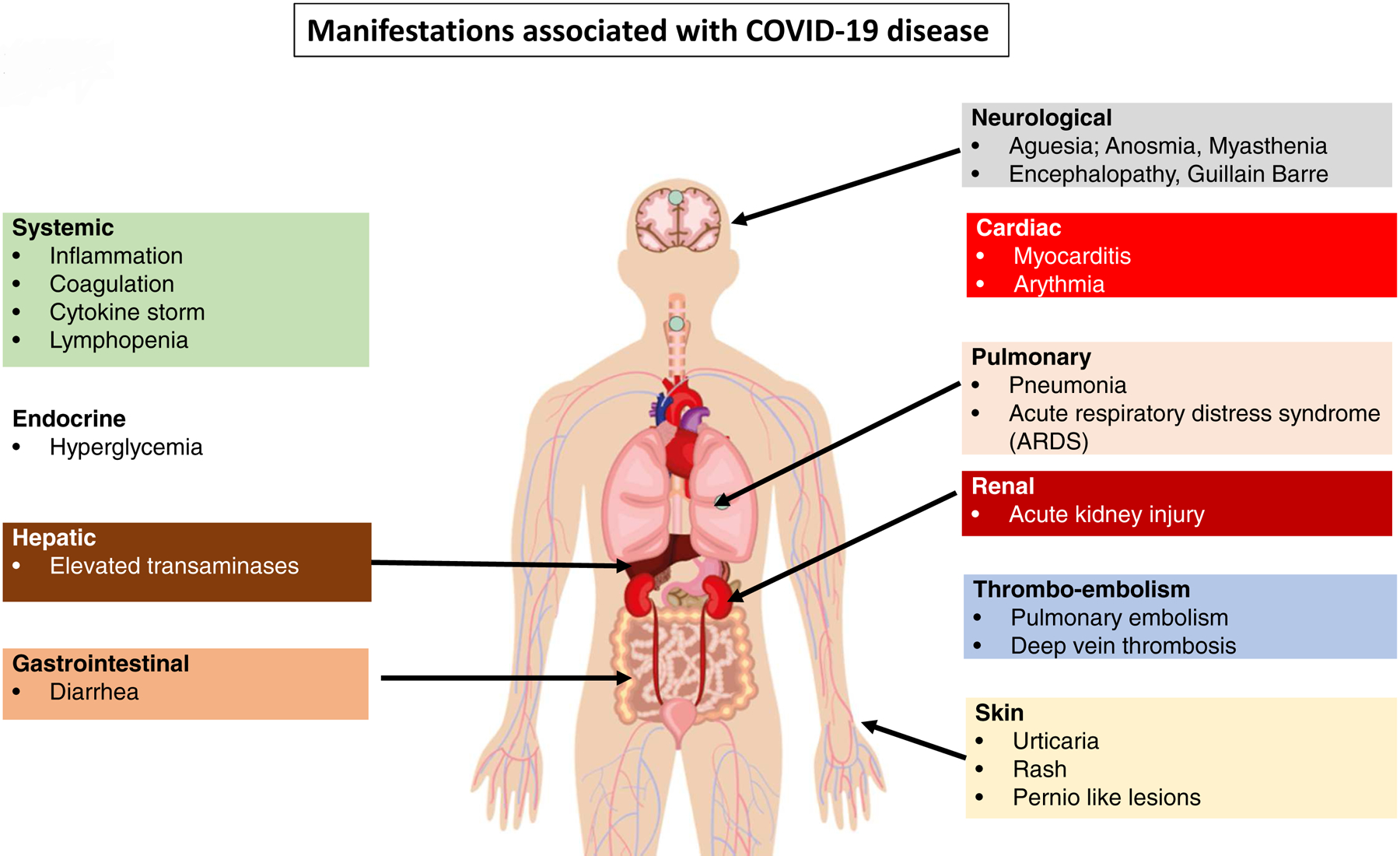
Systemic manifestations of Covid-19
COVID-19 and Liver.
Elevation of liver enzyme occurs in 5 to 50 % of patients. The pattern of liver injury is mainly at hepatocellular rather than cholestatic level [93–94], with hepatocyte degeneration, focal necrosis, capillary bile duct cholestasis and inflammation in the portal area, but interestingly SARS-CoV-2 cannot be detected in samples [95]. Frequently, the severity of liver injury had been correlated to the severity of COVID-19. The presence of underlying chronic liver diseases could render COVID-19 patients at higher risk of severe liver injury, such as acute-on-chronic liver failure [96], with data suggesting that NAFLD/MADLD could be an independent risk factors for severe COVID-19 [97–98].
The virus was found in stool samples in around 50% of patients with COVID-19, with around 18% of them complaining of abdominal pain and diarrhea [99]. It was demonstrated that SARS-CoV2 is capable to productively replicate in ACE2-positive enterocytes [12]. Due to the abundance of the virus in the small intestine, liver cell exposure through the hepatic reticular system is expected. Hepatic default immune status might play a critical role in COVID-19 infection. Indeed, it has been shown that in patients with MAFLD, the polarization status of macrophage might be skewed due to metabolic stimuli such as fatty acids and thus affecting host-inflammatory response to signals generated from the gut-liver axis [97]. In COVID-19, the “cytokine storm” bears resemblance to SARS caused by the SARS-CoV-1, where cytokine storm has been associated with disease [100–102].
On the other hand, direct cytopathic damage by SARS-CoV-2 is also possible as there are entry receptors ACE-2 in cholangiocytes [103]. Also, learning from SARS experience, the use of antibiotics, antivirals, together with possible secondary bacterial infection, might lead to liver injury in COVID-19 patients [104]. Moreover, tocilizumab is evaluated for the treatment of COVID-19 patients with serious lung damage and accompanying elevated blood levels of IL-6 [105]. Prophylactic nucleoside analogs against hepatitis B virus had been recommended for those hepatitis B surface antigen positive COVID-19 patients planned for immunosuppressive therapy [102]. Liver damage, which lead to drug withdrawal, has been reported in patients treated with Remdesivir. Accordingly, it is not recommended for those COVID-19 patients with ALT> 5 times ULN or with liver decompensation to receive Remdesivir [106]. Lastly, hypoxia and shock induced by COVID-19-related complications may also cause hepatic ischemia [107]. To manage liver injury related to COVID-19, several guidelines have been issued [100–102, 108].
Gastro-intestinal manifestations.
Clinically, approximately 10% of the patients with COVID-19 suffered from gastrointestinal symptoms such as nausea or vomiting, diarrhea and anorexia [109], with similar incidence among adults and children [110]. Patients with gastrointestinal symptoms may require longer duration of hospitalization [78–79, 111]. In some patients, gastrointestinal without respiratory symptoms might be the presenting clinical features [112–113]. The underlying mechanism may be related to the abundant expression of ACE2 mRNA and receptor protein in the enterocytes [112–113]. Histological changes with the presence of plasma cell and lymphocytes infiltration in patients’ lamina propria of enterocytes suggested an immune-mediated response [114]. The capability of SARS-CoV2 to infect enterocytes has also been demonstrated in human intestinal organoids [12]. One of the major concerns of enteric infection is whether fecal source can lead to fomite transmission, especially when infective aerosols are generated from the toilet plume. Indeed, cluster of cases infected with COVID-19, in analogy to “Amoy Garden” during the SARS in 2003, has recently been suggested in Hong Kong [115]. In accordance to the surface stability study on plastic and different materials, SARS-CoV-2 could remain viable up to 72 hours [116]. In one study, in 20% of patients, SARS-C0V2 RNA remained positive in feces despite clearance in the respiratory specimen [114]. Taking together, it is of great importance that the presence of SARS-CoV-2 in the stool need to be determined for the epidemiology control of COVID-19.
Co-infections.
There is a major concern with the potential concomitant infection of SARS-CoV-2 with influenza or other respiratory diseases such a respiratory syncytial virus, or tuberculosis or even bacterial infections or mycoplasma. Co-infection with SARS-CoV-2 and influenza A virus in a patient with pneumonia has been reported in China [117]. COVID-19 might be underdiagnosed because of false-negative tests for upper respiratory specimens or co-infection with other respiratory viruses.
Treatment Strategies
Prevention and transmission control measures.
Washing hands frequently, using mask and social distancing are important. China banned travel to and from Wuhan city on 23 January 2020, and this shutdown was associated with the delayed arrival of COVID-19 in other cities by approximately 3 days [118]. Suspending intra-city public transport, closing entertainment venues and banning public gatherings were associated with reductions in case incidence. Early on, the spatial distribution of COVID-19 cases in China was explained well by human mobility data [119]. Following the implementation of control measures, this correlation dropped and growth rates became negative in most locations. A contact-tracing application, which builds a memory of proximity contacts and immediately notifies contacts of positive cases could achieve epidemic control if used by enough people [120].
Timing of treatment.
Much like with influenza, antiviral drugs to be effective likely need to be started early in infection course. In turn, this represents a burden to identify drugs that are indeed effective against the virus in clinical trials. Patients with early disease may benefit from antiviral agents to reduce viral load, patients with severe and late disease may benefit from anti-inflammatory drugs. Furthermore, in the beginning of the disease, anti-inflammatory drugs might be harmful by increasing viral load.
Drug repurposing.
Drug repurposing (also called drug repositioning or reprofiling) is a strategy for identifying new uses for approved or investigational drugs that are outside the scope of the original medical indication [121]. This strategy offers various advantages over developing an entirely new drug, with a reduction risk of failure because safety has already been evaluated. But also the time frame and the cost can be reduced, because most of the preclinical testing and safety assessment have been done. An extensive repositioning activity of approved drugs has been embarked for the COVID pandemic. A selection of drugs being tested for COVID-19 are represented in Table 1. For example of large randomized ongoing trial the design of “Solidarity”, is provided in Table 2.
Table 1 :
Drugs evaluated in clinical trials for Treatment of COVID-19 (not exhaustive)
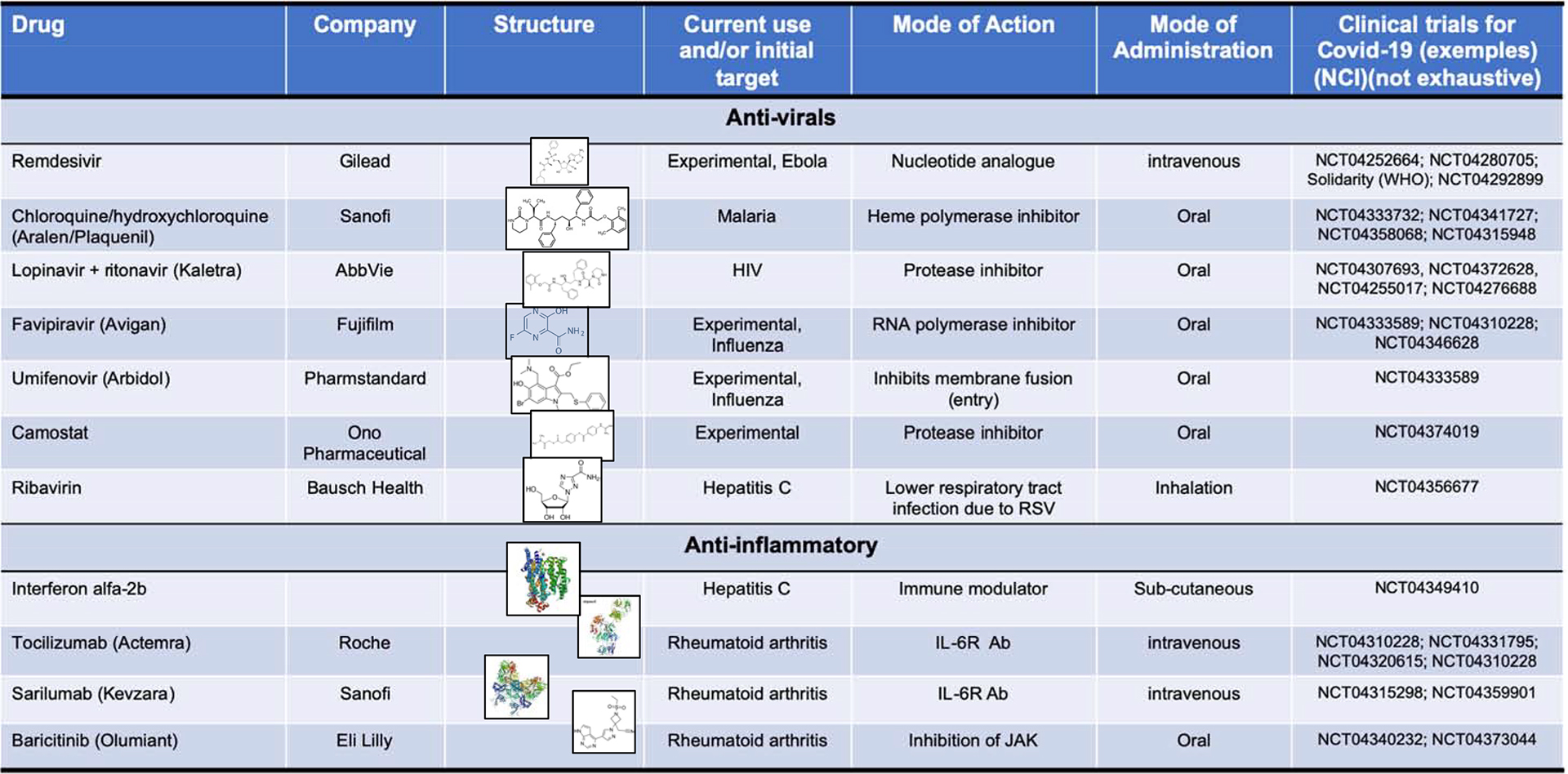
|
Refer to: https://clinicaltrials.gov/ and WHO for complete trial information.
Abbreviations: IL-6R, interleukin 6 receptor; Ab, antibodies; JAK, Janus Kinases; RSV, respiratory syncytial virus.
Table 2 :
WHO Master Protocol: Solidarity Trial
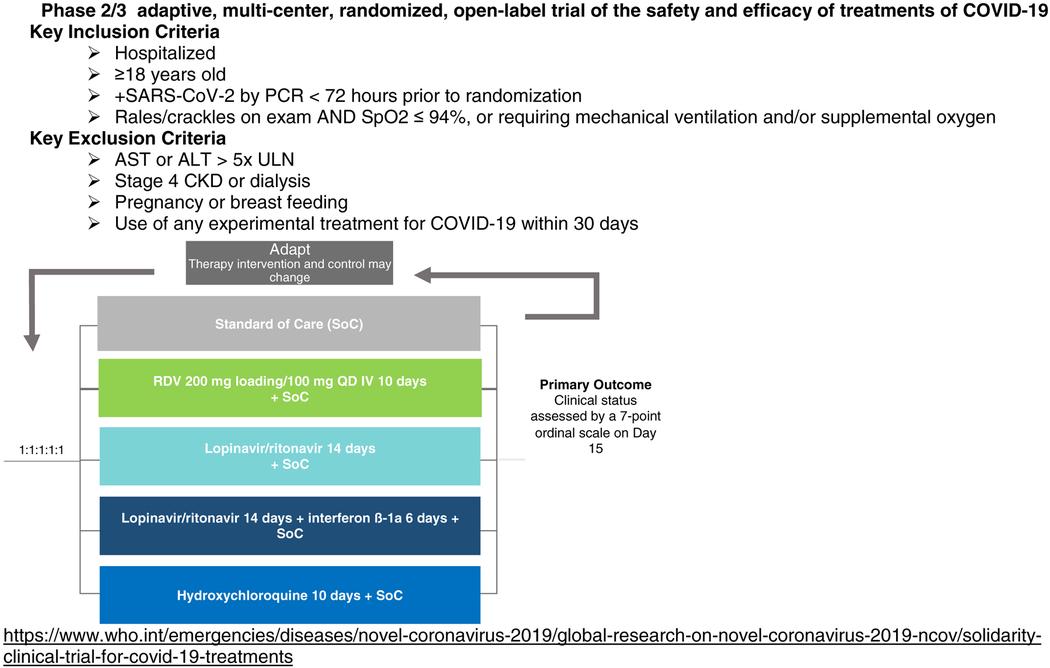
|
Existing antiviral medicines targeting the virus.
Hydroxychloroquine (HCQ) is a medication used to prevent and treat lupus and malaria. HCQ has also been combined with azithromycin, an antibiotic. HCQ would inhibit SARS-Cov-2 entry into cells. Few data are coming from reports and small studies [122]. A systematic review on the efficacy and safety of HCQ for the treatment of COVID-19 concluded that there is currently no evidence from RCTs to inform on HCQ efficacy [123]. In a multicenter, open label, randomized controlled trial, 150 patients admitted to hospital with laboratory confirmed covid-19 were included in the intention to treat analysis (75 patients assigned to HCQ plus standard of care (SOC), 75 to SOC [124]. There was no difference in term of efficacy between the 2 arms. Adverse events were higher in HCQ recipients than in non-recipients.
Lopinavir is an antiretroviral protease inhibitor used in combination with ritonavir in HIV therapy, which has shown some antiviral activity against SARS-CoV [125]. A randomized, controlled, open-label trial involving hospitalized adult patients with confirmed SARS-CoV-2 infection and severe respiratory illness COVID-19 was performed [126]. Patients were randomly assigned to receive either lopinavir-ritonavir, in addition to SOC, or SOC alone. There were no differences between groups (virologic aspects, duration of disease, mortality), indicating that there is no benefit in hospitalized adult patients with severe COVID-19. Cell culture data suggest that this compound demonstrates activity with an EC50 of 26.6 μM [127]. One wonders why it was selected for clinical trials with such a weak activity. Evaluating in humans repurposed drugs that are essentially ineffective in culture against SARS-CoV-2 is being repeated over and over again wasting time and resources.
Remdesivir is a prodrug of a nucleotide analog that is intracellularly metabolized to an analogue of adenosine triphosphate that inhibits viral RNA polymerases. Remdesivir has broad-spectrum activity against members of several virus families, including filoviruses (e.g., Ebola) and coronaviruses (e.g., SARS-CoV and MERS-CoV [128]. Six large studies are ongoing (Table 3). Unfortunately remdesivir must be given intravenously for at least 5 days, although an aerosol formulation is being developed.
Table 3 :
Clinical Trials of Remdesivir for Treatment of COVID-19
| Study ID | Study Design | Location | Sponsor | Study size (randomization) | Primary endpoint/outcome |
|---|---|---|---|---|---|
| Completed Status | |||||
| NCT04257656 (terminated) | Double-blind, placebo-controlled (Severe) | Beijing, China | Capital Medical University, China | N=453 (2:1) 10d RDV:Placebo |
Time to clinical improvement by Day 28 |
| NCT04252664 (suspended) | Double-blind, placebo-controlled (Mild/Moderate) | Wuhan, China | Capital Medical University, China | N=308 (1:1) 10d RDV:Placebo |
Time to clinical recovery by Day 28 |
| NCT04292899 | Open-label (Severe) | Global | Gilead | Part A N=400 (1:1) 10d RDV:5d RDV |
Endpoint: Clinical status at Day 14 on 7-point ordinal scale |
| NCT04292730 | Open-label (Moderate) | Global | Gilead | Part A N=600 (1:1:1) 10d RDV:5d RDV:SoC |
Endpoint: Clinical status at Day 11 on 7-point ordinal scale |
| NCT04280705 | Adaptive, double-blind, placebo-controlled | Global | NIAID | N=572 (1:1) 10d RDV:Placebo |
Outcome: Time to recovery [Time Frame: Day 1 through Day 29] |
| Recruiting Status | |||||
| NCT04315948 | Adaptive, open-label | Europe | WHO/Institut National de la Santé Et de la Recherche Médicale, France | N=3100 (1:1:1:1:1) 10d RDV:LPV/r: LPV/r+IFN: Hydroxychloroquine:SoC |
Outcome: Percentage of subjects reporting each severity rating on a 7-point ordinal scale [Time Frame: Day 15] |
| Solidarity Master Protocol | Adaptive, double-blind, placebo-controlled | Global | WHO | (1:1:1:1:1) 10d RDV:LPV/r: LPV/r+IFN: Hydroxychloroquine:SoC |
Outcome: Clinical status assessed by a 7-point ordinal scale on Day 15 |
Refer to https://clinicaltrials.gov/ and WHO for complete trial information
Results of remdesivir based on a compassionate-use for patients hospitalized with severe Covid-19 were reported [129]. From the 53 patients whose data were analyzed, clinical improvement was observed in 36/53 patients (68%). A randomized, double-blind, placebo-controlled, multicenter trial was performed in China [106]. Mortality at day 28 was similar between the two groups (14% died in the remdesivir group vs 13% in the placebo group). There was no difference in the two groups regarding clinical improvement and viral load decreased. This trial did not attain the predetermined sample size because the outbreak of COVID-19 was brought under control in China, therefore, it is difficult to reach a definitive conclusion.
Gilead is conducting two randomized, open-label, multicenter, phase 3 clinical studies to evaluate the safety and efficacy of two dosing durations - 5 days and 10 days - of remdesivir in adults diagnosed with COVID-19 (The Simple studies). The first SIMPLE study is involving hospitalized patients with confirmed SARS-CoV-2 infection, oxygen saturation of 94% or less while they were breathing ambient air, and radiologic evidence of pneumonia [130]. In the second Simple study patients were randomized to receive open-label remdesivir for 5 or 10 days or SOC alone. At Day 11, a higher proportion of patients in the 5-day treatment group achieved improvement in clinical status versus the SOC group, achieving statistical significance for a ≥ 1-point improvement in ordinal scale (p=0.026)(gilead press release). However, most clinician would have preferred to see a decrease in mortality on treatment. Clearly another controlled study will have to be performed soon.
Among other antiviral drugs being tested for COVID-19 we can quote arbidol [131–132], favipiravir [133], famotidine [134–135], and camostat (TMPRSS2 inhibitor) [11].
Existing antiviral medicines targeting the inflammation.
Dexamethasone.
Glucocorticoids may modulate inflammation-mediated lung injury and thereby reduce progression to respiratory failure and death. In a controlled, open-label trial of patients hospitalized with Covid-19, patients were randomly assigned to receive oral or intravenous dexamethasone (6 mg once daily) for up to 10 days or to receive SOC alone [136]. In the dexamethasone group, the incidence of death was lower than that in the SOC group among patients receiving invasive mechanical ventilation (29.3% vs 41.4%) and among those receiving oxygen without invasive mechanical ventilation (23.3% vs 26.2%) but not among those who were receiving no respiratory support at randomization (17.8% vs 14.0%).
In a recent trial involving patients with ARDS who were undergoing mechanical ventilation, mortality at 60 days was 15 percentage points lower among those receiving dexamethasone than among those receiving SOC [137]. In the early phase of the infection, anti-inflammatory drugs may not be efficient (maybe harm-full) increasing viral load. Viral shedding in SARS-CoV-2 appears to be higher early in the illness and declines thereafter [49, 54, 138]. The greater mortality benefit of dexamethasone in patients with Covid-19 who are receiving respiratory support and among those recruited after the first week of their illness suggests that at that stage the disease may be dominated by inflammation, with active viral replication playing a secondary role. Clearly a trial of the combination remdesivir and dexamethasone may yield interesting results.
Interferon beta-1b
Early triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin was safe and superior to lopinavir-ritonavir alone in alleviating symptoms and shortening the duration of viral shedding and hospital stay in patients with mild to moderate COVID-19. Future clinical study with interferon beta-1b as a backbone is warranted [139].
Tocilizumab (Actemra), also known as Atlizumab, and Sarilumab (Kevzara) are both immunosuppressive drugs, mainly for the treatment of rheumatoid arthritis. They are both humanized monoclonal against the interleukin-6 receptor (IL-6R), and are given by injection. Clinical trials are ongoing. Moreover other monoclonal antibodies or agents targeting other inflammatory cytokines (TNF-α, IL-1β…) should be soon tested.
Kinase inhibitors.
The Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway has been implicated as a key driver in many inflammatory diseases. With the development of small molecule inhibitors that can selectively and specifically target key JAKs involved in controlling downstream inflammation, exploration of their utility across a broad range of diseases has become a rapidly expanding field [140,141], including for other viral infections (e.g. HIV; [142–144]). Baricitinib and ruxolitinib are 2 known JAK inhibitors. Recently, artificial intelligence enabled the identification of a group of approved drugs that could inhibit clathrin-mediated endocytosis and thereby inhibit viral infection of cells [145, 146]. The drug targets are members of the numb-associated kinase (NAK) family. Baricitinib was identified as a NAK inhibitor, with a particularly high affinity for AAK1, a pivotal regulator of clathrin-mediated endocytosis. This drug is also known to target JAK and could have a dual action against the virus and inflammation [147]. The NIH/NIAID sponsored ACTT-2 study is still ongoing and compares remdesivir to remdesivir plus baricitinib in patients with moderate to severe COVID-19. In a small uncontrolled cohort of Veterans Affairs patients with moderate-severe COVID-19, treatment with baricitinib plus hydroxychloroquine was associated with recovery in 11 of 15 patients [148].
Two other kinase inhibitors, namely imatinib mesylate and dasatinib, could also be envisaged to treat COVID-19 [149]. Furthermore, ruxolitinib (another JAK inhibitor-Incyte) is being evaluated in a multicentre phase II clinical trial [150].
Therapeutic antibodies are becoming increasingly attractive for the treatment of SARS-CoV-2, as they can be designed to specifically target viral antigens. REGN-COV-2 is a dual-antibody cocktail that contains two potent, non-competing and virus-neutralizing antibodies (Regeneron, press release). The two antibodies of REGN-COV2 bind non-competitively to critical portions of the receptor binding domain of the virus’ spike protein. The treatment could also help prevent infection by blocking the ability of the spike protein to bind to target host cells and facilitate viral entry. In addition to Regeneron, Eli Lilly, AbCellera and other companies also began testing their antibody treatment in humans.
Convalescent plasma.
The immediate use of convalescent plasma provides a promising treatment. In a preliminary uncontrolled case series of 5 critically ill patients with COVID-19 and ARDS, administration of convalescent plasma containing neutralizing antibody was followed by improvement in their clinical status [71]. The limited sample size of this study precludes a definitive statement about the efficacy of this treatment.
Vaccines are the most effective strategy for preventing infectious disease since they reduce morbidity and mortality, and they are more cost-effective than treatment. Despite previous coronaviruses epidemics, there is still no approved vaccine for human coronaviruses.
We will have to improve our understanding and knowledge regarding immune response to SARS-Cov2. Interestingly, in rhesus macaques, comparing the humoral and cellular immunity between primary infection and re-challenge revealed notably enhanced neutralizing antibody and immune responses [151]. These results suggest that primary SARS-CoV-2 exposure protects against subsequent reinfection in rhesus macaques. In human, in a large study of the Icelandic population observed that humoral response did not decline within 4 months after infection, that 44% of persons who had been infected had not been diagnosed with PCR, and that the fatality rate was 0.3% [152]. We have also to recall that few cases of SARS-CoV-2 re-infection were reported. Epidemiological, clinical, serological and genomic analyses confirmed that the patient had re-infection instead of persistent viral shedding from first infection [153]. This case lead to several open questions: How frequent is reinfection? Are reinfections less severe than the first? Will vaccine protect against reinfections? These results suggest SARS-CoV-2 may continue to circulate among the human populations despite herd immunity due to natural infection or vaccination. Further studies of patients with re-infection will shed light on protective correlates important for vaccine design. In the past two decades, the world has seen three coronaviruses emerge and cause outbreaks that have caused considerable global health consternation [154] with no vaccine available up to now. Regarding vaccine development, among the different strategies, we can recall the use of recombinant subunit vaccine, DNA or mRNA vaccine. Subunit vaccines are believed to be highly safe because they are expected to induce the immune system without introducing infectious viruses [155]. A better knowledge of SARS-CoV-2 spike and/or N protein organisations will be required to develop such vaccines. The SARS-CoV-2 spike glycoprotein mediates host cell attachment and is required for viral entry; it is the primary vaccine target for many candidate SARS-CoV-2 vaccines. DNA vaccines are based on direct injection of plasmids encoding the desired viral antigens, which induce a large range of immune responses. mRNA-based vaccines contain mRNAs encoding the antigens, which are translated at the host cellular machinery by vaccination [156]. mRNA vaccines have advantages over conventional vaccines, including the absence of genome integration, the improved immune responses, their rapid development, and the production of multimeric antigens [156, 157]. A preliminary report on an mRNA vaccine against SARS-CoV-2 has been published [158]. The candidate vaccine mRNA-1273 (Moderna) is a lipid nanoparticle–encapsulated, nucleoside-modified mRNA–based vaccine that encodes the SARS-CoV-2 spike glycoprotein stabilised in its prefusion conformation. A phase I, dose-escalation, open-label trial was conducted including 45 healthy adults, who received 2 vaccinations, 28 days apart, with mRNA-1273. After the second vaccination, serum-neutralising activity was detected in all participants evaluated. The pseudovirus neutralising activity was low before the second vaccination, which supports the need for a 2-dose vaccination schedule. Finally, the mRNA-1273 vaccine-induced anti-SARS-CoV-2 immune responses in all participants, with no limiting safety concerns. The significance of SARS-Cov-2 binding and neutralising antibody titres and their capacity to prevent infection will have to be determined. Humoral and cell-mediated immune responses have been associated with vaccine-induced protection against challenge or subsequent re-challenge after SARS-CoV-2 infection in a rhesus macaque model [159]. Long-term assessment will be relevant given that natural history studies suggest that SARS-CoV may not generate long-lived antibody responses [160]. Furthermore, safety evaluations are mandatory since there have been concerns about the potential for vaccine-associated enhanced respiratory disease. Of the 3 doses evaluated, the 100 μg dose elicits high neutralisation responses and Th1-skewed CD4 T cell responses, coupled with a reactogenicity profile that is more favourable than that of the higher dose.
In addition, we want to mention the results of 2 early phase COVID-19 vaccine trials, one at Oxford University (UK), with support from AstraZeneca [161] and the second supported by CanSino Biologics in China [162]. Both groups used an adenoviral vector, and both report the vaccine achieving humoral responses against the SARSCoV-2 spike glycoprotein receptor-binding domain by day 28, as well as T cell responses.
Although these preliminary data are encouraging SARS-CoV-2 is a novel pathogen in humans, and many of the technologies being used to build vaccines are relatively untested. There is still a long way to go and phase 3 trials of these vaccines will require thousands of subjects in order to confirm efficacy and safety.
Conclusions
The rapid sequencing of the virus has allowed the development of diagnostic tools. Table 4 summarize future directions. A Test and Trace programs will be essential. Later, “Test, Trace and Treat (T3)” programs will become mandatory once effective drugs would have been identified and safe therapies developed (Figure 4). Several issues will be important to understand. It will be important to precise how transmissible and pathogenic is SARS-CoV-2 in the ongoing and future epidemic. Furthermore, it will be important to improve diagnostic tools. Ideally a single or combined test that provides virological and serological output would be ideal. In many countries, at the end of containment, strict recommended measures will be important to avoid new waves of contamination. However, few innovative treatment modalities have been discovered since the bulk of the effort to date has been focused on a vaccine. Vaccines might not be enough to quell this pandemic.
Table 4,
COVID-19: Perspectives
|
Figure 4. Milestones for SARS-CoV-2 elimination.
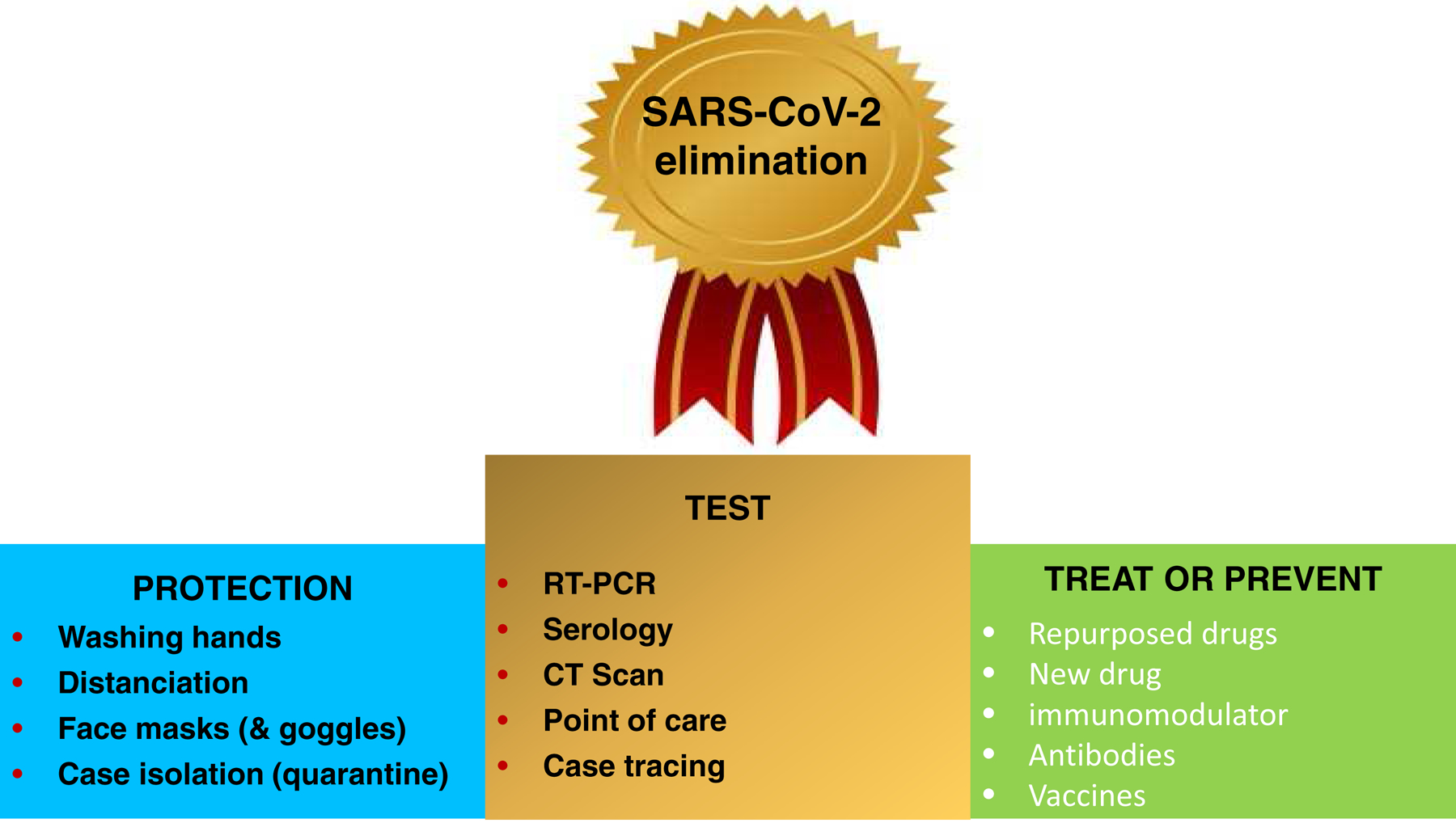
To achieve SARS-CoV-2 elimination there will be a need to improve protection, testing, treating and preventing strategies. A Test and Trace programs will be essential. Later, Test, Trace and Treat (T3) programs will become mandatory once effective and safe therapies are developed
Although a large number of repurposed drug trials are being evaluated, many are redundant and lack a strong rationale to warrant clinical development. There is a small chance that some trials could grind to a halt, simply because the pandemic has been so well controlled by lockdowns and other measures. However, the risks of epidemics of coronavirus remain clear and present and it is imperative that the work continues to develop vaccines and effective drugs for coronaviruses to prevent future social and economic hardships globally.
Figure 2.
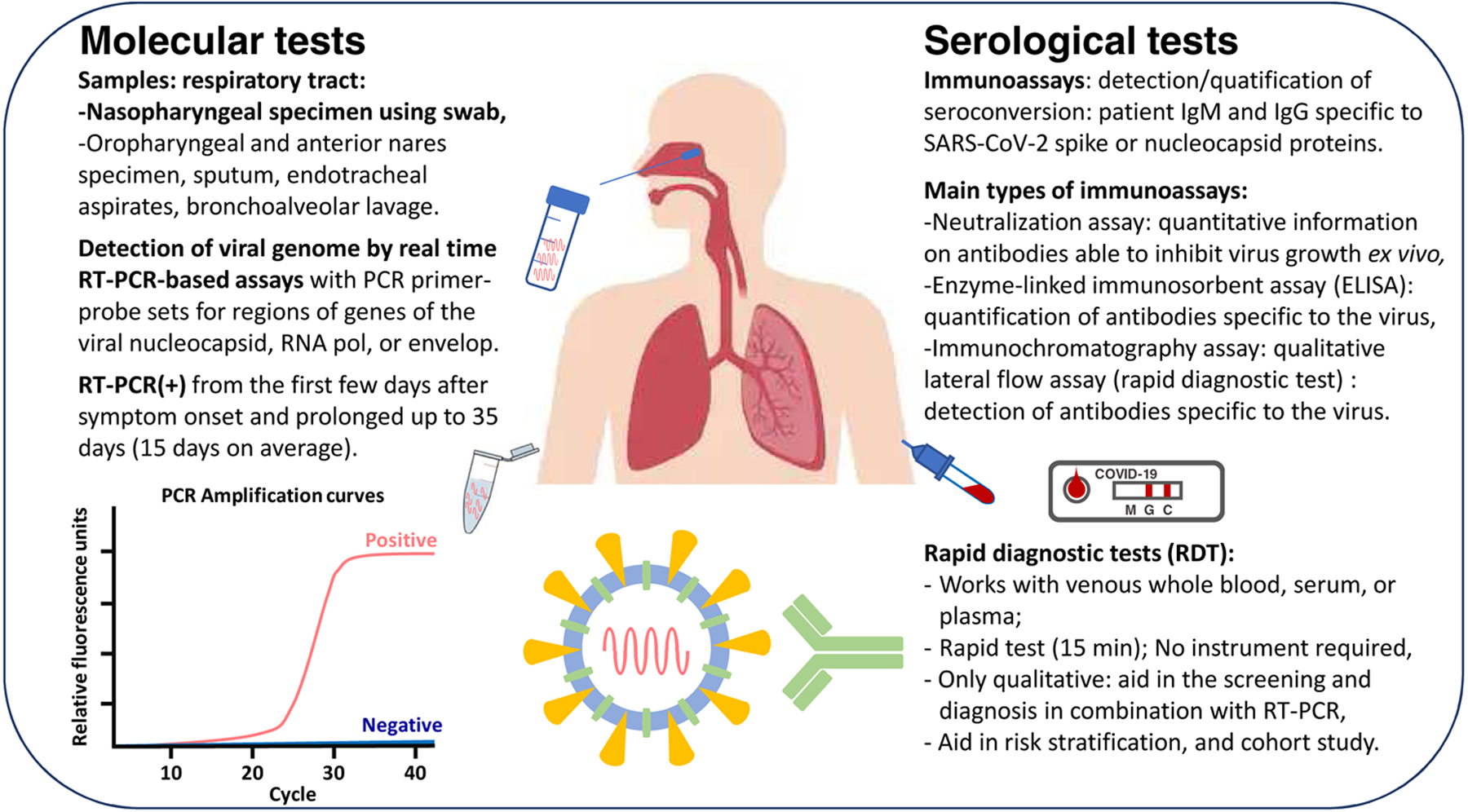
Diagnostic tools.
Key points.
An epidemic of acute respiratory syndrome (Covid-19) started in humans in Wuhan in 2019, and became a pandemic.
Groups from China identified and sequenced the virus responsible for COVID-19, named SARS-CoV-2, and determined that it was a novel coronavirus (CoV) that shared high sequence identity with bat-derived SARS-like CoV, suggesting it had originated in bats.
SARS-CoV-2 is a member of Coronaviridae, a family of enveloped, positive-sense, single-stranded RNA viruses that infect a broad range of vertebrates.
The sequencing of the virus has allowed the development of diagnostic tools (e.g., RT-PCR). Additionally, serological tests can allow the identification of persons who have been infected.
Testing and tracing programs will be essential. Later, testing, tracing and treating (T3) programs will become mandatory, once effective and safe therapies are developed.
Early strong social distancing efforts are needed to stop transmission of the virus, and are important measures to reduce case incidence. In addition, the use of masks, soaps, and disinfectants are critical to reduce or eliminate virus spread. Case isolation and contact-tracing has proven also efficacy to reduce epidemic.
Drug repurposing is a strategy for identifying new uses for approved or investigational drugs that are outside the scope of the original medical indication. This strategy has been used to rapidly identify treatment for the COVID-19 infection that could move quickly to phase-3.
To date, with the exception of intravenous Remdesivir or dexamethasone which have a modest effect, but significant on severe Covid-19, no strong clinical evidence supports the efficacy and safety of any drug against SARS-CoV-2.
Better knowledge of the virus, its enzymes, and immune response will be mandatory to develop direct-acting antiviral agents and effective vaccines.
A vaccine to prevent infection would be crucial to obtain, however even if 50% effective or more, the immunological protection might not persist and it may not be available before 2021 if at all.
Acknowledgements.
Supported in part by NIH grant R01-AI-141327, the Center for AIDS Research/NIH grant P30-AI-050409 and National Science Foundation grant 2032273 (to RFS).
Abbreviations
- aa
amino acid
- ACE-2
Angiotensin-Converting Enzyme 2
- ARDS
Acute respiratory distress syndrome
- CT
computed tomography
- CoVs
coronavirus
- COVID-19
coronavirus disease 19
- ELISA
Enzyme-linked immunosorbent assays
- ER
endoplasmic reticulum
- FDA
Food and Drug Administration
- EMA
European Medicines Agency
- HCQ
Hydroxychloroquine (HCQ)
- IFN-α
interferon alpha
- IL
Interleukin
- R0
the reproduction number
- SARS
severe acute respiratory syndrome
- SOC
standard of care
- TMPRSS2
Transmembrane serine protease 2
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
Tarik Asselah has acted as a speaker and investigator for AbbVie, Janssen, Gilead, Roche, and Merck. David Durantel, Eric Pasmant and George Lau have nothing to declare.
Raymond Schinazi was an unpaid consultant for Lilly and holds equity in Lilly and Gilead.
References
- [1].WHO Covid-19 situation report 181 (https://www.who.int/).
- [2].Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nature medicine 2020; 26:450–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003; 426:450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Snijder EJ, Limpens RWAL, de Wilde AH, de Jong AWM, Zevenhoven-Dobbe JC, Maier HJ, et al. A unifying structural and functional model of the coronavirus replication organelle: Tracking down RNA synthesis. PLoS Biol. 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020; 8(4):420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020; 382(8):727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020; 383(2):120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Asselah T, Marcellin P, Schinazi RF. Treatment of hepatitis C virus infection with direct-acting antiviral agents: 100% cure? Liver Int. 2018;38 S1:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lamers MM, Beumer J, Van der Vaart J, Knoops K, Puschhof J, Breugem TI, et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020;369(6499):50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Magrone T, Magrone M, Jirillo E. Focus on receptors for coronaviruses with special reference to angiotensin-converting enzyme 2 as a potential drug target - A perspective. Endocrine, metabolic & immune disorders drug targets 2020; 20(6):807–811. [DOI] [PubMed] [Google Scholar]
- [14].Sungnak W, Huang N, Becavin C, Berg M, Queen R, Litvinukova M, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nature medicine 2020; 26(5):681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 2020. 28;181(5):1016–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020; 581(7807):221–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Baumert TF, Berg T, Lim JK, Nelson DR. Status of direct-acting antiviral therapy for hepatitis C virus infection and remaining challenges. Gastroenterology 2019; 156:431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gao Y, Yan L, Huang Y, Liu F, Zhao Y, Cao L, et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 2020, ;368(6492):779–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kirchdoerfer RN, Ward AB. Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nature communications 2019; 10:2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ghosh AK, Brindisi M, Shahabi D, Chapman ME, Mesecar AD. Drug development and medicinal chemistry efforts toward SARS-Coronavirus and Covid-19 therapeutics. ChemMedChem 2020; 15(11):907–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dai W, Zhang B, Su H, Li J, Zhao Y, Xie X, et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science 2020; 368(6497):1331–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jin Z, Du X, Xu Y, Deng Y, Liu M, Zhao Y, et al. Structure of M(pro) from COVID-19 virus and discovery of its inhibitors. Nature 2020; 582(7811):289–293. [DOI] [PubMed] [Google Scholar]
- [23].Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. The Journal of clinical investigation 2020; 130:2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ledford H. How does COVID-19 kill? Uncertainty is hampering doctors’ ability to choose treatments. Nature 2020; 580:311–312. [DOI] [PubMed] [Google Scholar]
- [25].Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. The Journal of clinical investigation 2020; 130:2202–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang F, Hou H, Luo Y, Tang G, Wu S, Huang M, et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI insight 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell. 2020; 181(5):1016–1035.e [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Weissleder R, Lee H, Ko J, Pittet MJ. COVID-19 diagnostics in context; Sci Transl Med. 2020;12(546):eabc1931. [DOI] [PubMed] [Google Scholar]
- [30].Tang YW, Schmitz JE, Persing DH, Stratton CW. The laboratory diagnosis of COVID-19 infection: Current issues and challenges. Journal of clinical microbiology 2020; 58(6):e00512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin 2020;25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Osório NS, Correia-Neves M. Implication of SARS-CoV-2 evolution in the sensitivity of RT-qPCR diagnostic assays. Lancet Infect Dis 2020;S1473–3099(20)30435–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sethuraman N, Jeremiah SS, Ryo A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA 2020, in press. [DOI] [PubMed] [Google Scholar]
- [34].Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA 2020; 323(18):1843–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vogels CBF, Brito AF, Wyllie AL, Fauver JR, Ott IM, Kalinich CC, et al. Analytical sensitivity and efficiency comparisons of SARS-CoV-2 RT-qPCR primer-probe sets. Nat Microbiol 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Han MS, Byun JH, Cho Y, Rim JH. RT-PCR for SARS-CoV-2: quantitative versus qualitative. Lancet Infect Dis 2020; S1473–3099(20)30424–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Liu Y, Yan LM, Wan L, Xiang TX, Le A, Liu JM, et al. Viral dynamics in mild and severe cases of COVID-19. The Lancet Infectious diseases 2020; 20(6):656–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cheng MP, Papenburg J, Desjardins M, Kanjilal S, Quach C, Libman M, et al. Diagnostic testing for severe acute respiratory syndrome-related coronavirus-2: A narrative review. Ann Intern Med 2020: 172(11):726–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Woloshin S, Patel N, Kesselheim AS. False Negative Tests for SARS-CoV-2 Infection - Challenges and ImplicationsN Engl J Med. 2020, in press. [DOI] [PubMed] [Google Scholar]
- [40].Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction-Based SARS-CoV-2 Tests by Time Since Exposure. Ann Intern Med 2020; M20–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hogan CA, Sahoo MK, Pinsky BA. Sample Pooling as a Strategy to Detect Community Transmission of SARS-CoV-2. JAMA 2020; 323(19):1967–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lohse S, Pfuhl T, Berkó-Göttel B, Rissland J, Geißler T, Gärtner B, et al. Pooling of samples for testing for SARS-CoV-2 in asymptomatic people. Lancet Infect Dis 2020; S1473–3099(20)30362–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mallapaty S. The mathematical strategy that could transform coronavirus testing. Nature 2020. doi: 10.1038/d41586-020-02053-6. [DOI] [PubMed] [Google Scholar]
- [44].Park GS, Ku K, Baek SH, Kim SJ, Kim SI, Kim BT, Maeng JS. Development of Reverse Transcription Loop-Mediated Isothermal Amplification Assays Targeting Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). J Mol Diagn 2020; 22(6):729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Broughton JP, Deng X, Yu G, Fasching CL, Servellita V, Singh J, et al. CRISPR-Cas12-based detection of SARS-CoV-2. Nat Biotechnol 2020; 38(7):870–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ackerman CM, Myhrvold C, Thakku SG, Freije CA, Metsky HC, Yang DK, Ye SH, et al. Massively multiplexed nucleic acid detection with Cas13. Nature 2020;582(7811):277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. The New England journal of medicine 2020;382(22):2081–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. The New England journal of medicine 2020; 382:1177–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wolfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Muller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581(7809):465–469. [DOI] [PubMed] [Google Scholar]
- [50].Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in different Types of Clinical Specimens. Jama 2020; 323(18):1843–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, et al. Epidemiologic features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. Jama 2020; 323(15):1488–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Vermeiren C, Marchand-Senecal X, Sheldrake E, Bulir D, Smieja M, Chong S, et al. Comparison of Copan Eswab and FLOQswab for COVID-19 PCR diagnosis: working around a supply shortage. Journal of clinical microbiology 2020; 58(6):e00669–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Marty FM, Chen K, Verrill KA. How to Obtain a Nasopharyngeal Swab Specimen. N Engl J Med 2020; 382(22):e76. [DOI] [PubMed] [Google Scholar]
- [55].To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020; 20(5):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Williams E, Bond K, Zhang B, Putland M, Williamson DA. Saliva as a non-invasive specimen for detection of SARS-CoV-2. J Clin Microbiol 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Abbasi J. The promise and Peril of Antibody Testing for COVID-19. Jama 2020, in press. [DOI] [PubMed] [Google Scholar]
- [58].Petherick A. Developing antibody tests for SARS-CoV-2. Lancet 2020; 395:1101–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Amanat F, Stadlbauer D, Strohmeier S, Nguyen THO, Chromikova V, McMahon M, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020; 26(7):1033–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Guo L, Ren L, Yang S, Xiao M, Chang, Yang F, et al. Profiling early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin Infect Dis. 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nature medicine 2020; 26(6):845–848. [DOI] [PubMed] [Google Scholar]
- [62].Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 2003. ;361:1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zost SJ, Gilchuk P, Case JB, Binshtein E, Chen RE, Nkolola JP, Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 2020; 584(7821):443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Liu Y, Eggo RM, Kucharski AJ. Secondary attack rate and superspreading events for SARS-CoV-2. Lancet 2020; 395:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Liu L, Wang P, Nair MS, Yu J, Rapp M, Wang Q, et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature 2020; 584(7821):450–456. [DOI] [PubMed] [Google Scholar]
- [66].Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, Rawlings SA. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020; 181(7):1489–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ju B, Zhang Q, Ge J, Wang R, Sun J, Ge X, et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020, in press. [DOI] [PubMed] [Google Scholar]
- [68].Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nature communications 2020;11:1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sette A, Crotty S. Pre-existing immunity to SARS-CoV-2: the knowns and unknowns. Nat Rev Immunol 2020;1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Pinto D, Park YJ, Beltramello M, Walls AC, Tortorici MA, Bianchi S, et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 2020; 583(7815):290–295. [DOI] [PubMed] [Google Scholar]
- [71].Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically Ill Patients With COVID-19 With Convalescent Plasma. Jama 2020; 323(16):1582–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Rogers TF, Zhao F, Huang D, Beutler N, Burns A, He WT, et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 2020; 369(6506):956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lisboa Bastos M, Tavaziva G, Abidi SK, Campbell JR, Haraoui LP, Johnston JC, et al. Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Spijker R, Taylor-Phillips S, Adriano A, Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev 2020. ;6:CD013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].GeurtsvanKessel CH, Okba NMA, Igloi Z, Bogers S, Embregts CWE, Laksono BM, Leijten L. An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat Commun 2020;11(1):3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kofler N, Baylis F. Ten reasons why immunity passports are a bad idea. Nature 2020; 581(7809): 379–381. [DOI] [PubMed] [Google Scholar]
- [77].Gupta N, Zhao YY, Evans CE. The stimulation of thrombosis by hypoxiaThromb Res. 2019; 181:77–83. [DOI] [PubMed] [Google Scholar]
- [78].Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Guan W-j, Ni Z-y, Hu Y, Liang W-h, Ou C-q, He J-x, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. New England Journal of Medicine 2020; 382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020; 395:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med 2020;382(10):970–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. Jama 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, et al. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kanne JP, Little BP, Chung JH, Elicker BM, Ketai LH. Essentials for Radiologists on COVID-19: An Update-Radiology Scientific Expert Panel. Radiology 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Wong HYF, Lam HYS, Fong AH, Leung ST, Chin TW, Lo CSY, et al. Frequency and distribution of Chest Radiographic Findings in COVID-19 Positive Patients. Radiology 2019: 201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation Blood. 2020;135(23):2033–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system Nat Rev Cardiol. 2020; 17(5):259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med 2020; 382(23):2268–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Guidon AC, Amato AA. COVID-19 and neuromuscular disorders. Neurology 2020;94(22):959–969. [DOI] [PubMed] [Google Scholar]
- [91].Wollina U, Karadağ AS, Rowland-Payne C, Chiriac A, Lotti T. Cutaneous signs in COVID-19 patients: A review; Dermatol Ther 2020;e13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Gabarre P, Dumas G, Dupont T, Darmon M, Azoulay E, Zafrani L. Acute kidney injury in critically ill patients with COVID-19; Intensive Care Med. 2020; 46(7):1339–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, et al. COVID-19: Abnormal liver function tests. Journal of hepatology 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver international : 2020; 40:998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory medicine 2020;8:420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ji D, Zhang D, Yang TN, Mu JS, Zhao P, Xu J, et al. Effect of COVID-19 on patients with compensated chronic liver diseases. Hepatol Intl In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. Journal of hepatology 2020; 73(2):451–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Zhou YJ, Zheng KI, Wang XB, Yan HD, Sun QF, Pan KH, et al. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: A multicenter preliminary analysis. Journal of hepatology 2020; ;S0168–8278(20)30271–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Cheung KS, Hung IF, Chan PP, Lung KC, Tso E, Liu R, et al. Gastrointestinal manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples from the Hong Kong Cohort and Systematic Review and Meta-analysis. Gastroenterology 2020; 159(1):81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].https://http://www.aasld.org/sites/default/files/2020-04/AASLD-COVID19-ClinicalInsights-4.07.2020-Final.pdf Ag. Insights for hepatology and liver transplant providers during the COVID-19 pandemic. 2020.
- [101].https://easl.eu/wp-content/uploads/2020/04/EASL-ESCMID-Position-Paper-on-COVID-19-and-the-liver-2-April-2020.pdf E-EPP. Care of patients with liver disease during the COVID-19 pandemic. J Hepatol 2020; in press. [DOI] [PMC free article] [PubMed]
- [102].force AEPCRbACT. Clinical practice Guidance for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic. Hepatol Intl 2020in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein cell 2020; ;1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Boeckmans J, Rodrigues RM, Demuyser T, Pierard D, Vanhaecke T, Rogiers V. COVID-19 and drug-induced liver injury: a problem of plenty or a petty point? Archives of toxicology 2020; ;94(4):1367–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Cao X. COVID-19: immunopathology and its implications for therapy. Nature reviews Immunology 2020; 20:269–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. The Lancet 2020. ;395(10236):1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Zhang XJ, Cheng X, Yan ZZ, Fang J, Wang X, Wang W, et al. An ALOX12–12-HETE-GPR31 signaling axis is a key mediator of hepatic ischemia-reperfusion injury. Nature medicine 2018;24:73–83. [DOI] [PubMed] [Google Scholar]
- [108].Lau G, Ward J. Synthesis of liver associations recommendations for Hepatology and Liver Transplant Care During the COVID-19 Pandemic. Clinical Liver Disease 2020, ;15(5):204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Tian Y, Rong L, Nian WD, He Y. Review article:gastrointestinal features in COVID-19 and the possibility of fecal transmission. Alimen Pharmacol Ther 2020;51:843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis [published online ahead of print, 2020 May 12]. Lancet Gastroenterol Hepatol. 2020;10.1016/S2468–1253(20)30126–6. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore JAMA. 2020;323(15):1488–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020; 69(6):1002–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, et al. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020; 115(5):766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Cheung E, Ting V, Cheng L. Coronavirus: Hong Kong public housing estate evacuated after cluster of Covid-19 infections found. https://www.scmp.com/news/hong-kong/health-environment/article/3087526/coronavirus-new-cluster-infection-expands-elderly (assessed June 5, 2020)
- [116].Van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020; 382(16):1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wu X, Cai Y, Huang X, Yu X, Zhao L, Wang F, et al. Co-infection with SARS-CoV-2 and Influenza A Virus in Patient with Pneumonia, China Emerg Infect Dis. 2020; 26(6):1324–1326. doi: 10.3201/eid2606.200299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Tian H, Liu Y, Li Y, Wu CH, Chen B, Kraemer MUG, et al. An investigation of transmission control measures during the first 50 days of the COVID-19 epidemic in China. Science 2020;368:638–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Kraemer MUG, Yang CH, Gutierrez B, Wu CH, Klein B, Pigott DM, et al. The effect of human mobility and control measures on the COVID-19 epidemic in China. Science 2020;368:493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Ferretti L, Wymant C, Kendall M, Zhao L, Nurtay A, Abeler-Dorner L, et al. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science 2020;368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al. Drug repurposing: progress, challenges and recommendations. Nature reviews Drug discovery 2019;18:41–58. [DOI] [PubMed] [Google Scholar]
- [122].Colson P, Rolain JM, Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. International journal of antimicrobial agents 2020;55:105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. Journal of critical care 2020. 59:176–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Vastag B. Old drugs for a new bug: influenza, HIV drugs enlisted to fight SARS. Jama 2003; 290:1695–1696. [DOI] [PubMed] [Google Scholar]
- [126].Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults Hospitalized with Severe Covid-19. The New England journal of medicine 2020; 382:1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Choy KT, Wong AY, Kaewpreedee P, Sia SF, Chen D, Hui KPY, et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020;178:104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell research 2020;30:269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. The New England journal of medicine 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19 N Engl J Med. 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Blaising J, Polyak SJ, Pecheur EI. Arbidol as a broad-spectrum antiviral: an update. Antiviral research 2014;107:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Zhu Z, Lu Z, Xu T, Chen C, Yang G, Zha T, et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. The Journal of infection 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, et al. Experimental Tteatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003; 300(5626):1763–7. [DOI] [PubMed] [Google Scholar]
- [135].Freedberg DE, Conigliaro J, Wang TC, Tracey KJ, Callahan MV, Abrams JA, et al. Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 Patients: A propensity score matched retrospective cohort study, Gastroenterology 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. Engl J Med 2020, in press. [Google Scholar]
- [137].Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multi-centre, randomised controlled trial. Lancet Respir Med 2020;8:267–76. [DOI] [PubMed] [Google Scholar]
- [138].He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020;26: 672–5. [DOI] [PubMed] [Google Scholar]
- [139].Hung IF, Lung KC, Tso EY, Liu R, Chung TW, Chu MY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020; 395(10238):1695–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Musumeci F, Greco C, Giacchello I, Fallacara AL, Ibrahim MM, Grossi G, et al. An update on JAK Inhibitors. Current medicinal chemistry 2019;26:1806–1832. [DOI] [PubMed] [Google Scholar]
- [141].Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nature reviews Rheumatology 2017;13:234–243. [DOI] [PubMed] [Google Scholar]
- [142].Gavegnano C, Brehm JH, Dupuy FP, Talla A, Ribeiro SP, Kulpa DA, et al. Novel mechanisms to inhibit HIV reservoir seeding using Jak inhibitors. PLoS pathogens 2017;13:e1006740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Gavegnano C, Detorio M, Montero C, Bosque A, Planelles V, Schinazi RF. Ruxolitinib and tofacitinib are potent and selective inhibitors of HIV-1 replication and virus reactivation in vitro. Antimicrobial agents and chemotherapy 2014;58:1977–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Gavegnano C, Haile WB, Hurwitz S, Tao S, Jiang Y, Schinazi RF, et al. Baricitinib reverses HIV-associated neurocognitive disorders in a SCID mouse model and reservoir seeding in vitro. Journal of neuroinflammation 2019;16:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 2020; 395:e30–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Stebbing J, Krishnan V, de Bono S, Ottaviani S, Casalini G, Richardson PJ, et al. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO Molecular Medicine 2020; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Cantini F, Niccoli L, Matarrese D, Nicastri E, Stobbione P, Goletti D. Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact. J Infect 2020; S0163–4453(20)30228–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Titanji BK, Farley MM, Mehta A, Connor-Schuler R, Moanna A, Cribbs SK, et al. Use of Baricitinib in patients with moderate and severe COVID-19. Clinical Infectious Diseases, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Dyall J, Coleman CM, Hart BJ, Venkataraman T, Holbrook MR, Kindrachuk J, et al. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrobial agents and chemotherapy 2014; 58:4885–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].La Rosée F, Bremer HC, Gehrke I, Kehr A, Hochhaus A, Birndt S, et al. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation. Leukemia 2020; 34(7):1805–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Deng W, Bao L, Liu J, Xiao C, Liu J, Xue J, et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science, 2020; 369(6505):818–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Gudbjartsson DF, Norddahl GL, Melsted P, Gunnarsdottir K, Holm H, Eythorsson E, et al. Humoral Immune Response to SARS-CoV-2 in Iceland. NEJM, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].To KK, Hung IF, Ip JD, Chu AW, Chan WM, Tam AR, et al. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin Infect Dis. 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Guarner J. Three Emerging Coronaviruses in Two Decades. American journal of clinical pathology 2020; 153:420–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S. The spike protein of SARS-CoV--a target for vaccine and therapeutic development. Nature reviews Microbiology 2009; 7:226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nature reviews Drug discovery 2018;17:261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Stadler K, Masignani V, Eickmann M, Becker S, Abrignani S, Klenk HD, et al. SARS--beginning to understand a new virus. Nature reviews Microbiology 2003;1:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al. An mRNA Vaccine against SARS-CoV-2 - Preliminary Report. N Engl J Med. 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Chandrashekar A, Liu J, Martinot AJ, McMahan K, Mercado NB, Peter L et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 2020. May 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Cao W-C, Liu W, Zhang P-H, Zhang F, Richardus JH. Disappearance of antibodies to SARS-associated coronavirus after recovery. N Engl J Med 2007;357:1162–3. [DOI] [PubMed] [Google Scholar]
- [161].Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. , Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Zhu F-C, Guan X-H, Li Y-H, Huang JY, Jiang T, Hou LH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


