Abstract
The habenula is a dorsal diencephalic structure consisting of medial and lateral subnuclei and a principal output tract, the fasciculus retroflexus, which together form a link between the limbic forebrain and ventral midbrain. Here we have used microarray and bioinformatic approaches in the mouse to show that the habenula is a distinctive molecular territory of the CNS, with a unique profile of neurotransmitter, ion channel, and regulatory factor expression. Neurons of the medial habenula and part of the lateral habenula express the transcription factor Brn3a/Pou4f1, and Brn3a-expressing habenular neurons project exclusively to the interpeduncular nucleus in the ventral midbrain. In Brn3a mutant embryos the fasciculus retroflexus is directed appropriately, but habenular neurons fail to innervate their targets. Microarray analysis of Brn3anull embryos shows that this factor regulates an extensive program of habenula-enriched genes, but not generic neural properties. The orphan nuclear receptor Nurr1/Nr4a2 is co-expressed with Brn3a in the developing habenula, is downstream of Brn3a, and mediates expression of a subset of Brn3a regulated transcripts. Together these findings begin to define a gene regulatory pathway for habenula development in mammals.
Keywords: habenula, epithalamus, diencephalon, development, Brn3a, Nurr1
INTRODUCTION
The habenula is a paired midline structure, located on the dorsal surface of the thalamus. It is part of a division of the diencephalon termed the epithalamus, which also includes the pineal organ and in non-mammalian vertebrates, the parapineal. In mammals it consists of two principal parts, the medial habenula (MH) and the lateral habenula (LH). The dorsal MH expresses the neuropeptide SP, while the ventral MH expresses choline acetyltransferase (Contestabile et al., 1987; Lecourtier and Kelly, 2007). LH neurons exhibit greater diversity in cytoarchitecture, neurotransmitter expression, and connectivity, and the LH appears to be comprised of multiple subnuclei (Andres et al., 1999; Geisler et al., 2003).
The habenula mediates a major descending pathway connecting the limbic forebrain to the monoaminergic nuclei of the midbrain tegmentum, via direct connections and also indirectly through the interpeduncular nucleus (IPN, Klemm, 2004; Lecourtier and Kelly, 2007). A hallmark of the habenula is a prominent output tract, the habenulopeduncular tract or fasciculus retroflexus (FR). MH neurons project almost exclusively via this tract to the IPN (Contestabile and Flumerfelt, 1981). The IPN in turn innervates secondary midbrain targets, including the median raphe, ventral tegmental area (VTA) and dorsal tegmental nucleus (Shibata and Suzuki, 1984). Efferents from the LH also project via the FR, but may connect directly to brainstem structures, bypassing the IPN (Herkenham and Nauta, 1979). It has been proposed that these habenular pathways regulate midbrain monoamine systems in parallel with the medial forebrain bundle (Hikosaka et al., 2008).
Little is known about the development of the habenula in mammals. In fish and frogs, the habenula and its projections are bilaterally asymmetrical (Concha and Wilson, 2001; Aizawa et al., 2005), and develop in relationship to the photoreceptive parapineal organ under the influence of nodal signaling (Concha et al., 2003; Gamse et al., 2003; Aizawa et al., 2007; Roussigne et al., 2009). In mammals, which do not have a photoreceptive pineal complex, this asymmetry appears to be lost. However, in both fish and rodents, neuropilin-semaphorin signaling plays a role in directing habenular axons to their midbrain targets (Giger et al., 2000; Kantor et al., 2004; Kuan et al., 2007).
Here we have used microarray data and bioinformatic analysis of high-throughput in situ hybridization (ISH) to define a unique habenular profile of neurotransmitters, receptors, ion channels and regulatory factors. Nearly all MH neurons, and a subset of those in the LH, express the POU-domain transcription factor Brn3a (product of the Pou4f1 gene). Brn3a-expressing habenular neurons project exclusively to the IPN. In Pou4f1−/− embryos the FR has an appropriate initial trajectory, but does not innervate its target. Microarray analysis of Pou4f1−/− embryos shows that Brn3a regulates a large subset of the habenula-enriched genes, but not generic neural properties. One target of Brn3a regulation is the orphan nuclear receptor Nurr1 (product of the Nr4a2 gene), which is co-expressed with Brn3a throughout the habenula, and requires Brn3a for the initiation of its expression. Examination of Nr4a2−/− mice reveals that this factor is required for the expression of some of the genes downstream of Brn3a, defining a pathway for the establishment of a unique molecular profile for habenular neurons.
MATERIALS AND METHODS
Mice and matings
Mice bearing a null allele of Pou4f1 (Xiang et al., 1996), a tauLacZ fusion protein targeted to the Pou4f1 locus (Pou4f1tLacZ, Quina et al., 2005) and a null allele of Nr4a2 (Castillo et al., 1998), and the genotyping of these alleles have been previously described. All alleles were carried on a C57bl/6 genetic background and have been backcrossed to C57bl/6 for at least 5 generations. All targeted alleles of the Pou4f1 and Nr4a2 loci are neonatal lethal when homozygous, and heterozygous mice were mated to generate null embryos and newborn pups. Noon on the day of the detection of a mucous plug was designated E0.5.
Microarray analysis
For studies of global gene expression in the developing habenula, mice bearing Pou4f1− and Pou4f1tLacZ alleles were interbred to generate litters with Pou4f1tLacZ/− knockout embryos and Pou4f1tLacZ/+ controls, which were harvested at E16.5. The embryonic brain was dissected intact, then hemisected in the sagittal plane to expose the habenula near the midline. For transgene-guided microdissection, brain tissue was stained in xgal buffer containing 100 mM potassium phosphate, pH 7.4, 5 mM EGTA, 2 mM MgCl2, 0.5 mg/ml xgal, and 1mM EDTA for 15–30 minutes at 37C, and the stained habenulae were dissected using fine forceps. The tissue was then rinsed in PBS, and stored in RNAlater (Qiagen) at 4C for up to one week, until the completion of genotyping. Knockout and control embryos were then pooled according to genotype and transferred to −80C for subsequent storage. For each microarray sample, RNA was prepared from eleven pairs of habenulae using an RNAqueous-Micro kit (Ambion) according to manufacturer’s protocol, followed by DNase treatment, yielding ~1μg of RNA per sample. For comparison of gene expression between the habenula, cortex and thalamus, tissue was harvested from Pou4f1tLacZ/+ E16.5 embryos and processed as described above. The thalamic sample consisted of the entire thalamus, minus habenula. The cortical sample was a full-thickness explant of the dorsolateral part of the frontoparietal cortex, avoiding the midline and ventral regions. The quality of the extracted RNA was monitored by the ratio of the intensity of 18S and 28S ribosomal RNA bands using an Agilent 2100 Bioanalyzer, and RNA was quantified using a Nanodrop ND-1000 spectrophotometer.
For microarray analysis, aRNA was synthesized from 0.5 ± 0.1μg of total RNA, using the Message Amp II kit (Ambion), following the manufacturer’s instructions for a one-step amplification. Yields of biotin-labeled aRNA ranged from 75–100μg per sample, and 15μg was used for hybridization to Affymetrix Mouse Genome 430 v2.0 array. Hybridization to GeneChip arrays were all performed according to standard protocols provided by the manufacturer (Affymetrix). Comparisons of gene expression for the habenula versus thalamus and cortex, and between habenula samples from Pou4f1tLacZ/+ and Pou4f1tLacZ/− embryos were performed in duplicate, using entirely separate samples (biological replicates).
The primary analysis of microarray data, including determination of the absence/presence of the assayed transcripts, transcript expression levels, and the probability of change in transcript expression between samples (“change-p”) was performed with Microarray Suite 5.0 (MAS5, Affymetrix). Default MAS5 parameters were used for increase (I) and decrease (D) calls. For the 430v2 array set these cutoff values were p<0.002 and p>0.998 for I and D respectively. All array values were initially scaled to a mean value of 500 using global scaling. Microarray probe sets were related to the corresponding mouse transcripts using the NetAffx database (Affymetrix), based on the NCBI Build 36 annotation of the mouse genome.
Detailed methods for the bioinformatic analysis of the adult brain appear in the Supplementary Methods.
Electroporation in utero
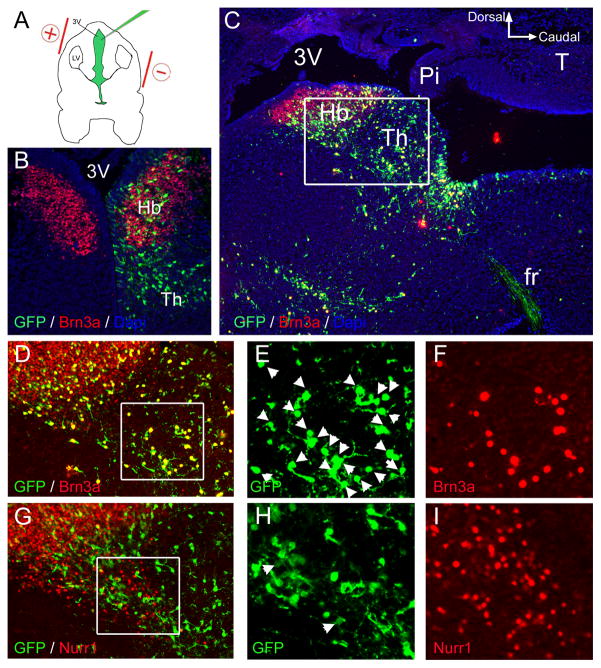
Timed-pregnant ICR mice (Charles River, San Diego, CA) bearing E13.5 embryos were anesthetized by isoflurane inhalation, their abdominal cavity incised, and the uterine horns exposed as described by Saito (Saito, 2006). Approximately 1 μl of solution encoding Brn3a and/or GFP was injected into the third ventricle of embryos using a pulled glass micropipette (Figure 9). Prior to injection, plasmid DNA was diluted to 4 μg/μl in PBS, and Fast Green solution was added to a final concentration of 0.03% to monitor the injection.
Figure 9. Brn3a is not sufficient to induce ectopic expression of Nurr1 in the diencephalon.
(A) Plasmids encoding Brn3a plus cytoplasmic or nuclear targeted GFP (nGFP), or a control plasmid expressing GFP alone were electroporated into habenular precursors by injection into the third ventricle at E13.5. Embryos were harvested at E16.5.
(B) Expression of GFP from a control plasmid was observed within the habenula, and in more ventral and caudal domains within the developing thalamus. The control plasmid did not alter the pattern of endogenous Brn3a expression.
(C) Low power image of the habenula and periventricular thalamus in sagittal section, following electroporation of a Brn3a-GFP expression vector. Immunofluorescence for Brn3a and GFP with DAPI staining reveals co-localization of the expressed proteins in nearly all cells for which the nucleus resides within the plane of section. Inset box indicates area enlarged in (D, G).
(D–I) Expression of Brn3a (D–F) and Nurr1 in adjacent sections (G–I) following electroporation of a Brn3a-GFP plasmid. Nurr1 expression was observed in 4% of GFP+ cells in this region electroporated with Brn3a-GFP (n=400) and 2% of cells electroporated with GFP alone (n=400, not shown), which did not represent a statistically significant difference.
3v, third ventricle; Pin, pineal; fr, fasciculus retroflexus; Hb, habenula; Th, thalamus.
Tweezers-type electrodes (CUY650-P5; Harvard Apparatus) were placed on each side of the fetal head, such that a line between the electrode faces formed a ~20° angle with the horizontal plane, with the anode in the more dorsal position. Square wave electrical pulses (30 V, 50 ms) were then applied five times at 1 s intervals with an electroporator (BTX, T820). Only a few embryos at a time were exposed, and care was taken to quickly place them back into the abdominal cavity to avoid excessive cooling. The wall and skin of the abdominal cavity were then closed, and embryos were allowed to develop normally until harvest at E16.5. Embryos were then processed for immunofluorescence as described below.
Four plasmids were employed for electroporation studies, designed to express Brn3a + cytoplasmic GFP (pTS-Brn3a), Brn3a + nuclear GFP (pTSn-Brn3a), or cytoplasmic/nuclear GFP alone (pTS, pTSn). The parent vector pTS is derived from the vector pMES and has been previously described (Fedtsova et al., 2008). It contains a CMV enhancer, a chick βactin promoter, a multiple cloning site, IRES sequence, eGFP expression cassette, and a βglobin polyadenylation signal. The expression cassette encoding the full mouse Brn3a open reading frame has been described (Gruber et al., 1997) and the coding sequence corresponds to Entrez accession NP_035273. Because electroporation of pTS-Brn3a alone resulted in expression of Brn3a protein which clearly exceeded endogenous levels in habenula neurons, Brn3a expression plasmids were pre-mixed with pTS vector in (1:1 ratio) to allow expression of high levels of the GFP marker and physiologically appropriate levels of Brn3a.
Immunofluorescence, in situ hybridization
Tissue for immunofluorescence and in situ hybridization was fixed by immersion in 4% paraformaldehyde in phosphate-buffered saline at stages up to E16.5, and by cardiac perfusion in newborn and adult mice. Cryostat sections at 15–30μm were used for both techniques. Primary antibodies used included: Rabbit anti-Brn3a (Fedtsova and Turner, 1995), guinea pig anti-Brn3a (Quina et al., 2005), rabbit anti-Nurr1, gift of Thomas Perlmann (Wallen et al., 2001), and from a commercial source (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-Robo3/Rig-1, gift of Fujio Murakami (Tamada et al., 2008), rabbit anti-Etv1/Er81gift of Sylvia Arber (Arber et al., 2000), rabbit anti-Dbx1, gift of Y. Nakagawa (Vue et al., 2007), rabbit and guinea-pig anti-Olig3, gifts of Carmen Birchmeier (Storm et al., 2009), mouse monoclonal Pax3 (Developmental Studies Hybridoma Bank), rat monoclonal anti-Substance P, goat anti-ChAT, rabbit anti-TH and Rabbit anti-Tph2 (Millipore, Billerica, MA), goat anti-βgalactosidase (Biogenesis, Sandown, NH), rabbit anti-βgalactosidase, (5Prime-3Prime, Boulder, CO), and mouse monoclonal anti-Tubulin beta-3 (R&D Systems). Secondary antibodies conjugated to Alexa series fluorophores were obtained from Invitrogen (Carlsbad, CA). In situ hybridization was performed as previously described (Eng et al., 2004). Sources for in situ hybridization probes appear in Supplementary Table 1.
RESULTS
Brn3a expression defines a functionally related population of habenular neurons
To begin to understand the role of Brn3a in habenula development, we first examined Brn3a expression in the adult mouse MH and LH. In order to trace the projections of Brn3a-expressing neurons, we employed a transgenic line with a tauLacZ reporter integrated into the Pou4f1 locus (Quina et al., 2005). Staining for βgalactosidase activity revealed the habenula, fasciculus retroflexus (FR), interpeduncular nucleus (IPN) and fiber tracts originating from other sites of Brn3a expression in these mice (Figure 1A–D). Examination of the habenula by immunofluorescence for Brn3a protein revealed expression in nearly all MH neurons, but only in a subset of LH cells (Figure 1E,F).
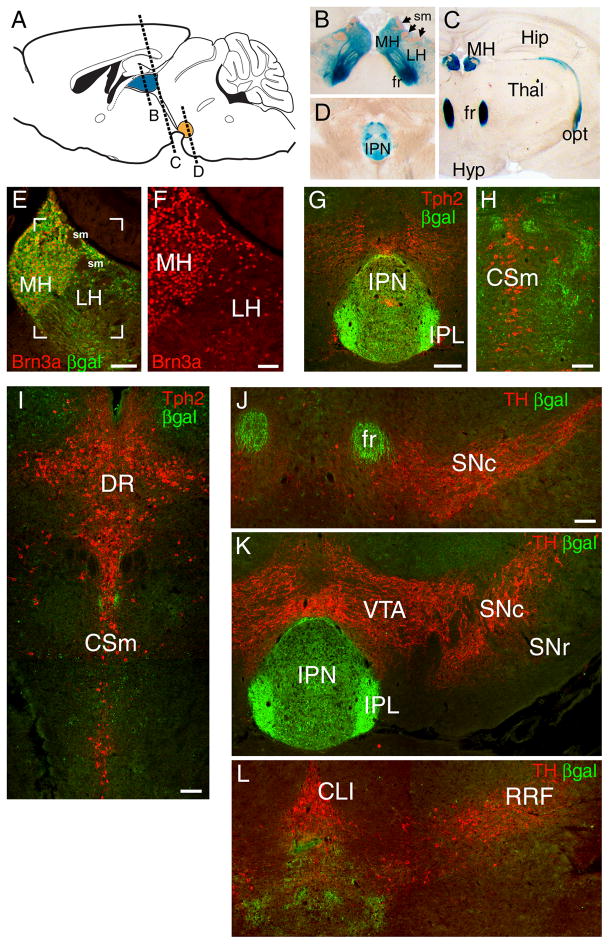
Figure 1. Brn3a is expressed in habenular neurons projecting specifically to the interpeduncular nucleus.
(A–D) Expression of a Pou4f1tLacZ transgene in the habenula and its projections revealed by xgal staining in the adult mouse brain. Afferent habenular fibers of the striae medularis are also indicated.
(E–F) Co-localization of Brn3a protein expression and βgal immunoreactivity in the medial and lateral habenula. Brn3a is expressed in nearly all MH and a subset of LH neurons. Bracketed area in E is enlarged in F.
(G–I) Relationship of habenular projections to serotonergic neurons and fibers revealed by Tph2 immunoreactivity. In (G) βgal labeled habenular fibers terminate in the interpeduncular nucleus. Tph2 staining at this level is predominantly ascending serotonergic fiber tracts. Plane of section is similar to (D). (H,I) Progressively more caudal sections showing that βgal labeled fibers do not associate with cell bodies of serotonergic neurons of the raphe nuclei.
(J–L) Relationship of habenular projections to dopaminergic neurons and fibers, marked by TH immunoreactivity. βgal-labeled fibers do not project to the dopaminergic areas of the basal midbrain.
CLI, central linear nucleus raphe; CSm, Superior central nucleus raphe, medial part; DR, dorsal raphe; fr, fasciculus retroflexus; Hip, hippocampus; Hyp, hypothalamus; IPL, interpeduncular nucleus, lateral subnucleus; IPN, interpeduncular nucleus; LH, lateral habenula; MH, medial habenula; opt, optic tract, RRF, retrorubral field; sm, striae medularis; SNc, substantia nigra, pars compacta; SNr, substantia nigra, pars reticulata; Thal, thalamus; VTA, ventral tegmental area. Scale: E, 100μm; F, 50μm; G–L, 100μm.
Past neuroanatomical studies of the habenula have shown that habenular neurons project via the FR to the IPN, and also directly to ventral monoaminergic nuclei including the dopaminergic SNc and VTA, and the serotonergic raphe nuclei. To resolve the projections of the Brn3a-expressing neurons in the MH and LH, we combined transgenic tract tracing with immunofluorescence for tryptophan hydroxylase 2 (Tph2) and tyrosine hydroxylase (TH), which identify 5-HT and DA neurons respectively. Examination of the entire course of the habenular projections labeled in Pou4f1tLacZ mice revealed that the vast majority of the βgalactosidase- (βgal) labeled fibers terminated in the IPN or immediately caudal to the IPN (Figure 1G,H). Labeling was not observed in the vicinity of the Tph2+ neurons of the median raphe (Figure 1H,I) or the dorsal raphe (Figure 1I), or the TH+ neurons of the SN (Figure 1J), VTA (Figure 1K), central linear nucleus raphe or the retrorubal field (Figure 1L). We conclude that although Brn3a is expressed in a subset of neurons in the LH, which is known to contain neurons that project directly to monoaminergic areas, the Brn3a+ LH neurons do not. Instead, the projections of the Brn3a+ LH neurons resemble those of the MH, exclusively innervating the IPN or contiguous areas.
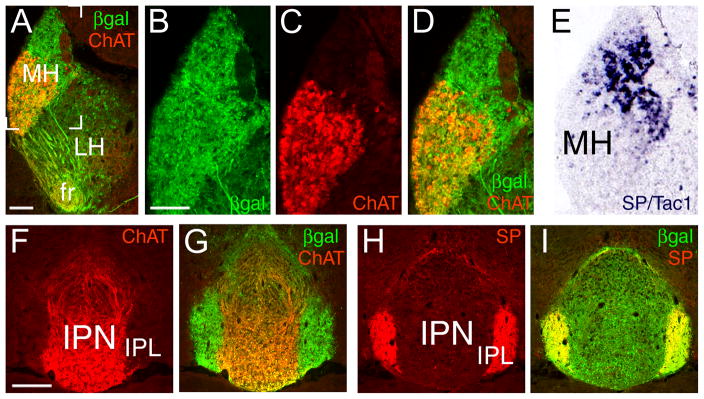
In order to determine the neurotransmitter phenotype of the Brn3a-expressing neurons in the medial habenula, we examined the expression of ChAT and SP in Pou4f1tLacZ mice. ChAT immunoreactivity strongly co-localized with βgal in the ventral two-thirds of the MH (Figure 2A,C,D), and also in the principal part of the IPN (Figure 2F,G). SP/Tac1-expressing neurons were confined to the dorsal third of the MH, which was also positive for βgal (Figure 2D,E), and SP immunoreactivity co-localized with βgal in the lateral subnucleus of the IPN (Figure 2H,I). These results demonstrate that both of these major subclasses of MH neurons, cholinergic and peptidergic, express Brn3a.
Figure 2. Neurotransmitter phenotype of Brn3a-expressing habenular neurons.
(A–D) Immunofluorescence in adult brain sections for ChAT and βgal in expressed from the Pou4f1 locus showed co-localization in the ventral two-thirds of the MH. Bracketed area in (A) appears enlarged in (B–D).
(E) In situ hybridization signal for SP/Tac1 was restricted to the dorsal third of the MH (ABA Tac1_187_2030).
(F,G) ChAT strongly co-localizes with βgal in the principal part of the IPN, but not in the lateral subnucleus.
(H,I) SP immunoreactivity strongly co-localizes with βgal in the lateral subnucleus of the IPN. Fr, fasciculus retroflexus; IPL, interpeduncular nucleus, lateral subnucleus; IPN, interpeduncular nucleus; LH, lateral habenula; MH, medial habenula. Scale: A, 100μm; B–D, 100μm; F–I, 200μm.
The habenula is a unique molecular territory of the CNS
We next used microarray analysis to compare global gene expression in the habenula, thalamus, and cerebral cortex of E16.5 embryos. In order to accurately dissect habenula tissue for RNA extraction, a rapid xgal staining protocol was employed which allowed the habenula to be visualized in the brains of hemisected Pou4f1taulacZ/+ embryos within 15 minutes (Supplementary Figure 1, Methods). Pair-wise comparisons of habenula-thalamus and habenula-cortex gene expression were made using Affymetrix murine 430v2 arrays, and the experiments were performed with two independent samples from each brain region. The patterns of gene expression in replicate samples from each tissue were highly reproducible, while many divergently expressed transcripts were noted in comparisons of the habenula versus thalamus and habenula versus cortex (Supplementary Figure 1). Average expression values from these brain regions were used to determine a set of transcripts most enriched in the habenula (Table 1). Of 45,037 probe sets on the array, Brn3a ranked as second-most enriched in the habenula relative to the other CNS regions, confirming this factor as a habenula-specific marker in the forebrain.
Table 1. Transcripts enriched in E16.5 habenula relative to cerebral cortex and thalamus.
Microarray analysis at E16.5 and bioinformatic analysis of the adult brain were used to identify habenula enriched transcripts. Microarray values are derived from the means of two independent arrays for the habenula, cortex, and thalamus. The ratio of habenula/other is calculated using the mean of the cortex and thalamus values. ABA data were examined by visual inspection and automated signal quantitation (Methods). Key to ABA visual analysis: +++, enriched in the habenula with minimal other CNS expression; ++, enriched in the habenula with a few other CNS sites of expression; +, enriched in the habenula with multiple other CNS sites of expression; nc, expression not correlated with habenula; ne, not expressed, little or no expression detected in any area of CNS; nd, no data in ABA atlas; cp, highly enriched in choroid plexus.
| Microarray ratio | ABA | ||||||
|---|---|---|---|---|---|---|---|
| Gene Symbol | Gene Title | Hab/Ctx | Hab/Thal | Hab/other | visual | Hab/Ctx | Hab/Thal |
| Robo3 | roundabout homolog 3 | 294 | 103 | 152 | + | 1.7 | 0.9 |
| Pou4f1 | POU domain, class 4, transcription factor 1;Brn3a | 180 | 99 | 128 | ++ | 69 | 75 |
| Dcc | Deleted in colorectal carcinoma | 29 | 171 | 49 | ne | -- | -- |
| Gpr151 | G protein-coupled receptor 151 | 51 | 34 | 40 | +++ | 25 | 179 |
| Irx2 | Iroquois related homeobox 2 | 118 | 22 | 38 | ++ | 29 | 68 |
| Kcnma1 | K+ large conductance Ca++-activated channel, subfamily M, alpha member 1 | 63 | 25 | 36 | + | 3.2 | 1.7 |
| Msi2 | Musashi homolog 2 | 32 | 34 | 33 | + | 5.7 | 4.5 |
| Dmt2 | Dorso-medial telencephalon gene 2 | 447 | 17 | 33 | nd | -- | -- |
| Gng8 | Guanine nucleotide binding protein, gamma 8 subunit | 146 | 18 | 32 | nd | -- | -- |
| Scube2 | Signal peptide, CUB domain, EGF-like 2 | 230 | 17 | 32 | ne | -- | -- |
| Ppnr | Per-pentamer repeat gene | 129 | 16 | 29 | + | 1.4 | 5.5 |
| Kcng4 | K+ voltage-gated channel, G4 | 28 | 27 | 27 | ++ | 9.4 | 9.7 |
| Tesc | Tescalcin | 117 | 14 | 25 | + | 8.8 | 2.1 |
| Dbx1 | Developing brain homeobox 1 | 33 | 19 | 24 | ne | -- | -- |
| Tmem16a | Transmembrane protein 16A | 29 | 19 | 23 | +++ | 52 | 123 |
| Irx5 | Iroquois related homeobox 5 | 79 | 12 | 21 | ne | -- | -- |
| Pbx3 | Pre B-cell leukemia transcription factor 3 | 21 | 18 | 19 | ++ | 8.0 | 11 |
| Htr5b | 5-HT (serotonin) receptor 5B | 69 | 11 | 19 | +++ | 33 | 43 |
| Chrnb4 | Cholinergic receptor, nicotinic, beta polypeptide 4 | 27 | 14 | 18 | +++ | 80 | 177 |
| Col2a1 | Procollagen, type II, alpha 1 | 21 | 16 | 18 | nc | 0.7 | 1.5 |
| Irx1 | Iroquois related homeobox 1 | 55 | 10 | 17 | + | 1.7 | 1.5 |
| Robo1 | Roundabout homolog 1 | 15 | 19 | 17 | + | 3.5 | 7.8 |
| Selected | |||||||
| Ttr | Transthyretin | 14 | 17 | 15 | cp | 3.5 | 6.4 |
| 3110047P20Rik | Hematological and neurological expressed 1 | 188 | 7.6 | 15 | +++ | 32 | 15 |
| Efcbp2 | EF hand calcium binding protein 2 | 40 | 8.7 | 14 | ++ | 4 | 23 |
| Etv1 | Ets variant gene 1 | 11 | 19 | 14 | ++ | 9.5 | 2.1 |
| Vav3 | Vav 3 oncogene | 42 | 7.2 | 12 | ++ | 2.9 | 18 |
| Ntng2 | Netrin G2 | 8.0 | 26 | 12 | ++ | 1.9 | 8.5 |
| Kctd8 | Potassium channel tetramerisation domain containing 8 | 23 | 8.3 | 12 | +++ | 28 | 19 |
| Nrp2 | Neuropilin 2 | 20 | 8.6 | 12 | ++ | 15 | 85 |
| Grb10 | Growth factor receptor bound protein 10 | 14 | 10 | 12 | +++ | 11 | 131 |
| Nr4a2 | Nuclear receptor subfamily 4, group A, member 2 | 8.1 | 22 | 12 | + | 1.3 | 0.8 |
To test whether the embryonic pattern of habenula-specific gene expression detected at E16.5 was maintained in the adult, we examined the expression of the enriched transcripts in the extensive atlas of gene expression generated for the mouse brain by the Allen Institute (Allen Brain Atlas, ABA, http://mouse.brain-map.org, Lein et al., 2007). The results of a visual inspection of the ABA for the most habenula-enriched transcripts appears in Table 1, and the habenula ISH results for these transcripts appear in Supplementary Figure 2. Many of the habenula-enriched transcripts identified on the microarray have a similar pattern of expression in the adult brain, suggesting that the ABA may be used to comprehensively examine habenula gene expression. However, the ABA contains expression data for approximately 20,000 transcripts in the mouse brain, beyond the practical limit for determining regional gene expression by visual inspection.
To globally identify habenula-enriched transcripts in the adult brain, we employed a bioinformatic approach. All in situ hybridization (ISH) data in the ABA are spatially registered to a common three-dimensional atlas space derived from the Allen Reference Atlas (ARA, Dong, 2007) using previously described image processing algorithms (Ng et al., 2007). Three-dimensional regions of interest (ROIs) can thus be identified in reference atlas space and projected onto the ISH data for analysis. Three ROIs were defined for this analysis, including the habenula (medial + lateral), the thalamus, and the cerebral cortex, corresponding as closely as possible to the physical dissections performed for the microarray study of the embryonic brain (Methods). From these ROIs, expression signal extraction was performed using morphological filtering and adaptive thresholding techniques, resulting in an expression mask for each ISH image classifying each pixel as either expressing or non-expressing (Ng et al., 2007). This mask was combined with ISH signal intensity data for the expressing pixels to compute an “expression energy” for the ROI (Methods). Habenula/thalamus and habenula/cortex expression energy ratios were then computed as measures of habenula enrichment. Cortex and thalamus expression energies were also averaged to allow a single value for habenula enrichment to be generated (habenula/other CNS).
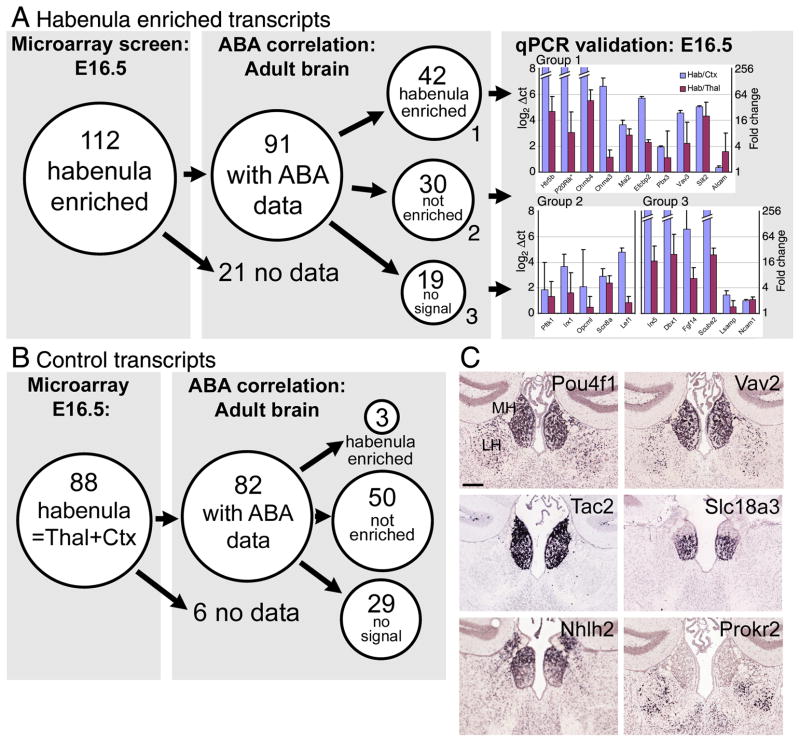
We then compared the set of habenula enriched transcripts identified by microarray to the results of the bioinformatic analysis of the adult brain (Figure 3A). We identified 112 transcripts in the microarray data set which had the most-enriched microarray expression in the habenula compared to the average expression in the thalamus and cortex, and 91 of these had extractable data in the ABA. Of these 91, 42 had enriched expression in the adult habenula, 30 had detectable expression which was not enriched in the habenula, and 19 had habenula expression below the reliable threshold of detection. We also selected 88 control transcripts from the microarray experiment which had equal expression in the habenula and thalamus/cortex (Figure 3B). Of these, only 3 showed increased habenula expression in the analysis of the ABA data. A bionomial test (Supplemental Methods) was employed to assess the probability that the 42 habenula-enriched transcripts and the 3 control transcripts from the microarray analysis that were also enriched in the ABA analysis would be selected by chance from among the 10,108 transcripts with significant habenula expression in the ABA. This test yielded a p-value of 4.44 × 10−16 (highly unlikely) for obtaining greater than 42 enriched probes by random selection, and p-value of 0.98 (highly likely) for obtaining greater than 3 enriched probes by random selection. We conclude that a large subset of habenula-enriched transcripts are stable across developmental time and are identifiable by both array and bioinformatic methods.
Figure 3. Microarray and bioinformatic analysis of habenula gene expression in the embryonic and adult brain.
(A) Microarray analysis of the E16.5 habenula, cortex, and thalamus revealed 112 unique transcripts with >10 fold enriched expression in the habenula compared to the mean expression level in the cortex/thalamus. Of these transcripts, 91 had available data in the Allen Brain Atlas. Quantitative analysis of the ABA data showed that 42 of these transcripts also had enriched expression in the adult brain, while 30 were expressed but not enriched, and 19 were not detectable. Microarray enrichment of transcripts in all three categories were verified by quantitative RT-PCR (mean and S.D. of three assays are shown).
(B) Control transcripts were identified in the microarray analysis which had equal expression in the habenula and cortex/thalamus. Of 88 control transcripts, 82 had available data in the ABA. Three of the control transcripts exhibited habenula-enriched expression in the adult brain, while 50 did not show enriched expression and 29 were not detected.
(C) Characteristic expression patterns of habenula-enriched transcripts. Recurring patterns observed included medial + lateral habenula (Pou4f1, Vav2), medial habenula only (Tac2), and the ventral part of the medial habenula only (Slc18a3). Enriched expression in the dorsal half of the medial habenula or in the lateral habenula alone was much less frequently observed (Nhlh2, Prokr2). See also data for Chat and SP/Tac1, Figure 2. Pou4f1, Brn3a; Vav2, Vav2 oncogene; Tac2, Tachykinin 2; Slc18a3, solute carrier family 18, member 3/vescicular acetylcholine transporter; Nhlh2, nescient helix loop helix 2; Prokr2, Prokineticin receptor 2. In situ hybridization data are derived from the ABA. Scale: 200μm.
Although the microarray and bioinformatic analyses were concordant for a large number of transcripts, we wished to determine why some of the transcripts that were habenula-enriched in the microarray analysis at E16.5 were not enriched in the ABA atlas. To rule out artifacts in the microarray analysis, we examined the expression of 21 habenula-enriched transcripts by RT-PCR (Figure 3A), in addition to those verified by in situ hybridization, described below. RT-PCR confirmed the microarray results for 20/21 comparisons between habenula and cortex regardless of whether enriched expression, equal expression, or no detectable habenula signal was observed in the ABA data. We conclude that the transcripts which are habenula-enriched at E16.5, but show equal expression in the adult, are enriched because of the later developmental program of the thalamus and cortex, and represent a difference in timing rather than true specificity for the habenula. Transcripts that are habenula-enriched at E16.5, but not detectable in the adult, are likely to represent developmental genes which are transiently expressed in the habenula.
The high correlation between the microarray and bioinformatic analysis suggested that the ABA data set could be effectively searched for novel habenula-specific transcripts. Based on parameters derived from the test set of microarray-enriched transcripts, the ABA data set was filtered for transcripts which exhibited expression energy at least 10-fold enriched in habenula/cortex and 4-fold enriched in habenula/thalamus, plus a minimal threshold of habenula expression. This yielded ISH data for 306 unique transcripts, which were then visually inspected in the ABA data set. Of these transcripts, 98 were below the threshold of reliable detection, and 78 exhibited high expression in the choroid plexus or in cells, presumably glia, which lined the ventricle both immediately adjacent to the habenula and thus could not be distinguished from the habenula proper by the automated analysis. The remaining 130 transcripts were verified as habenula-enriched in the atlas (Supplementary Table 2, Supplementary Figure 3).
Specific habenula-enriched transcripts exhibited characteristic expression patterns within the medial and lateral habenula (Figure 3C). The restricted expression of Chat and SP/Tac1 in the ventral and dorsal MH, respectively (Figure 2), were confirmed in the ABA data, although these transcripts did not meet the cutoff for habenula specificity because of significant expression in the neocortex. A large set of transcripts were expressed throughout the medial habenula, with (e.g. Pou4f1, Vav2) or without (Tac2) expression in a subset of lateral habenula neurons. A second large set of transcripts were restricted to the ventral part of the medial habenula, including multiple genes related to cholinergic systems (Slc18a3/VAChT). Expression restricted to the dorsal habenula (Nhlh2) or to neurons in the lateral habenula only (Prokr2), was much less frequently observed. Together these patterns of gene expression begin to define a systematic set of markers for the subnuclear anatomy of the habenula.
Brn3a is required for correct innervation of habenular targets
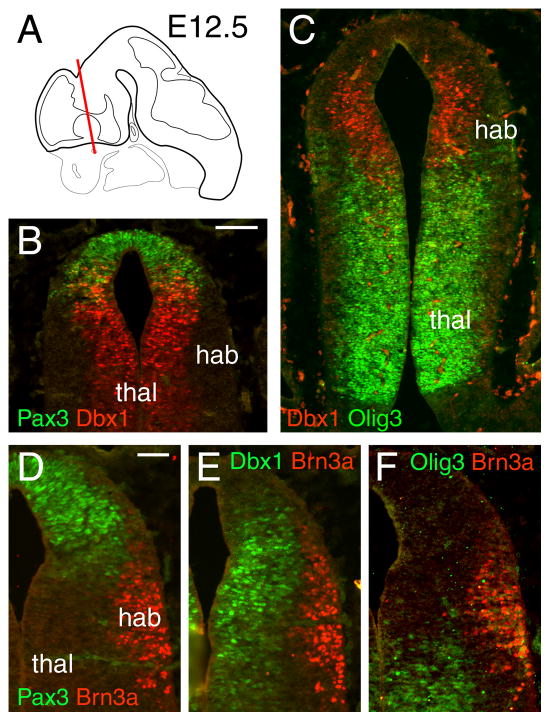
In order to understand the specific role of Brn3a in habenula development, we next examined the effects of loss of Brn3a on the morphology of the habenula and FR and on the innervation of the IPN. Brn3a expression in the dorsal diencephalon was first detected in a few neurons in the mantle layer at 11.5 (data not shown). Using Brn3a as a marker for early habenula neurons in E12.5 embryos, we examined the relationship between the differentiation of the habenula and key markers that pattern the neuroepithelium of the diencephalon (Vue et al., 2007). The transcription factors Pax3, Dbx1, and Olig3 showed minimal overlap in their expression, and portioned the neuroepithelium into three nearly discrete neurogenic domains in the apical, dorsal, and ventral diencephalon, respectively (Figure 4A–C). Differentiation of the habenula, marked by Brn3a+ post-mitotic neurons in the mantle layer, was associated with the Dbx1+ neuroepithelial domain, whereas Pax3+ precursors appeared more dorsal to the habenula neurogenic zone, and Olig3+ precursors were largely ventral to this area (Figure 4D–F).
Figure 4. The habenula develops from Dbx1-expressing precursors in the presumptive dorsal thalamus.
(A) Schematic of the brain at E12.5 showing approximate plane of section for subsequent views.
(B–C) Immunofluorescence for Pax3, Dbx1 and Olig3 reveals nearly discrete domains in the neuroepithelium of the presumptive thalamus.
(D–F) Immunofluorescence for Brn3a with each of the progenitor domain markers reveals an association of the habenula with Dbx1 expressing precursors. Scale: B,C, 100μm, D–F, 50μm.
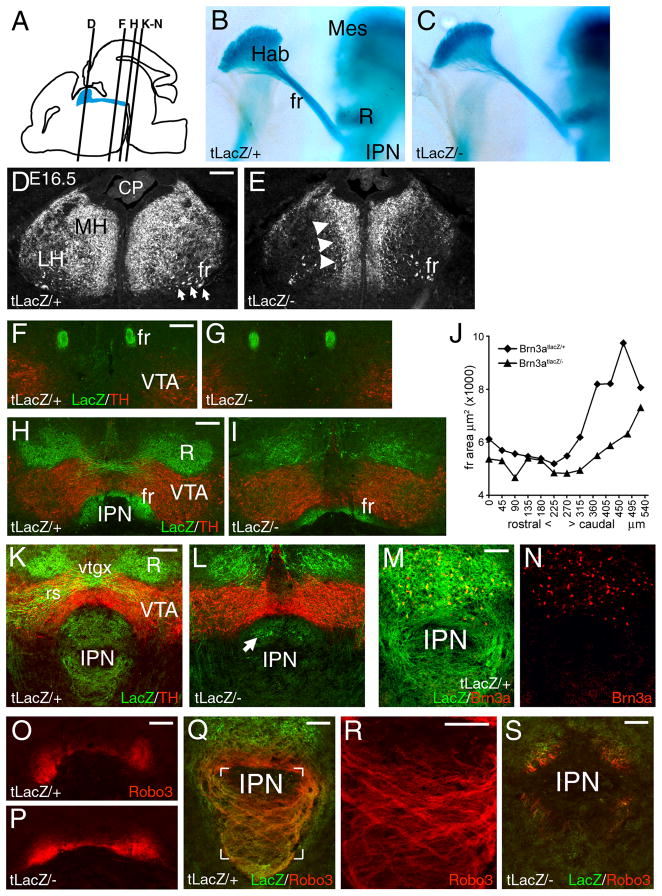
In Pou4f1tLacZ/− mutant embryos, habenula development was examined in the absence of Brn3a protein using the expression of the LacZ transgene as a marker. At E12.5, differentiating habenula neurons in control and Pou4f1tLacZ/− mutant embryos both expressed the early neuronal cytoskeletal marker tubulin beta-3 (Tubb3), and early fibers of the FR projected toward their midbrain targets similarly in both genotypes, but did not yet reach the IPN (Supplementary Figure 4). By E16.5 labeled axons of the FR had reached the IPN, and their general trajectory was normal in Pou4f1tLacZ/− embryos (Figure 5B,C). However, at this stage the morphology of the habenula was clearly abnormal in the mutants, with displacement of the LacZ-expressing neurons toward the midline (Figure 5D,E).
Figure 5. Displacement of habenular neurons and defective target innervation in Brn3a knockout mice.
(A) Sagittal view of the brain at E16.5 showing the location of the habenula, fasciculus retroflexus and interpeduncular nucleus and the plane of sections shown in subsequent views.
(B,C) Midline view of habenula, FR and IPN in hemisected, xgal-stained E16.5 embryos.
(D,E) Structure of the habenula in Pou4f1tLacZ/+ and Pou4f1tLacZ/− E16.5 embryos. Habenular neurons and axons are displaced toward the midline in the knockout.
(F–I) Coronal sections showing the course of the FR in Pou4f1tLacZ/+ and Pou4f1tLacZ/− P0 mice, taken near the middle of its course (F,G) and just prior to termination in the interpeduncular nucleus (H,I).
(J) Cross sectional area of the FR from the caudal end of the habenula (zero coordinate) to the interpedunuclar nucleus in P0 mice. Average values of right and left measurements are shown. Paired T-test for the comparison of all sections from Pou4f1tLacZ/+ and Pou4f1tLacZ/− mice yields p = 1.0 × 10−4.
(K–N) Termination zone of habenular axons in the interpeduncular nucleus at P0. The majority βgalactosidase immunoreactive fibers are missing in the Pou4f1tLacZ/− specimen (K,L), and the remaining expression appears to be associated with a few Brn3a+ neurons which are intrinsic to the interpeduncular nucleus (M,N).
(O–S) Robo3 expression in the habenulopeduncular system. Robo3 is undiminished in the caudal FR (O–P, plane of section is similar to H). Robo3-expressing fibers innervate the IPN in a crossing pattern in a Pou4f1tLacZ/+ embryo (Q, detail R, plane of section is similar to K), but are nearly absent in a Pou4f1tLacZ/− specimen (S).
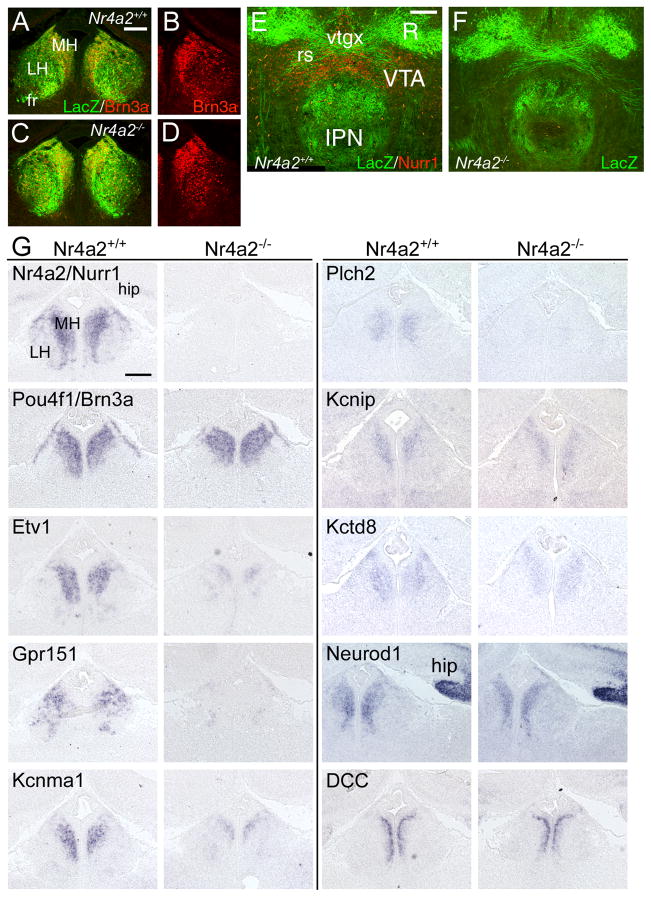
CP, choroid plexus; fr, fasciculus retroflexus; Hab, habenula; IPN, interpeduncular nucleus; LH, lateral habenula; Mes, mesencephalon; MH, medial habenula; R, red nucleus; rs, rubrospinal tract; VTA, ventral tegmental area; vtgx, ventral tegmental decussation. Scale: D–I, 100μm; K,L, 100μM; M,N, 50μM; O–S, 100μM.
Due to neonatal lethality, P0 was the last stage at which the habenulopeduncular system could be studied in Brn3a mutants. At this stage, the FR followed a normal trajectory toward its target (Figure 5F,G), but was significantly diminished as it approached the IPN (Figure 5H-J). Within the IPN, LacZ labeled fibers were absent in the mutant animals, and residual LacZ labeling appeared to be due to a small population of Brn3a-expressing neurons which are intrinsic to the dorsal IPN (Figure 5K–N), outside the entry zone of the FR axons.
Habenular axons strongly expressed the pathfinding signal Robo3/Rig1 (Tamada et al., 2008), and Robo3 expression in the FR was undiminished in Pou4f1tLacZ/− embryos (Figure 5O,P). In control embryos Robo3 immunoreactive fibers innervated the IPN in a intricate crossing pattern (Figure 5Q,R), but Robo3 immunoreactive axons were nearly absent from the IPN of mutant embryos (Figure 5S), confirming the loss of target innervation. Examination of ChAT and SP immunoreactivity in the IPN of Pou4f1tLacZ/− mice revealed that both of these classes of MH neurons fail to effectively innervate their respective targets in the medial and lateral IPN (Supplementary Figure 5).
Brn3a regulates a coordinated program of habenular gene expression
We next adopted a microarray strategy to determine the downstream targets of Brn3a in the habenula. Rapid staining for βgalactosidase activity was again employed to visualize the habenula and facilitate tissue harvesting in unfixed brains of E16.5 Pou4f1tLacZ/− embryos and Pou4f1tLacZ/+ controls (Methods). Although the Pou4f1tLacZ allele is a null allele, heterozygous Pou4f1tLacZ/+ embryos are suitable controls because Brn3a knockout mice have no known heterozygote phenotype, and in prior studies of the sensory ganglia, Pou4f1+/+ and Pou4f1+/− mice have shown little difference in gene expression (Eng et al., 2004; Eng et al., 2007).
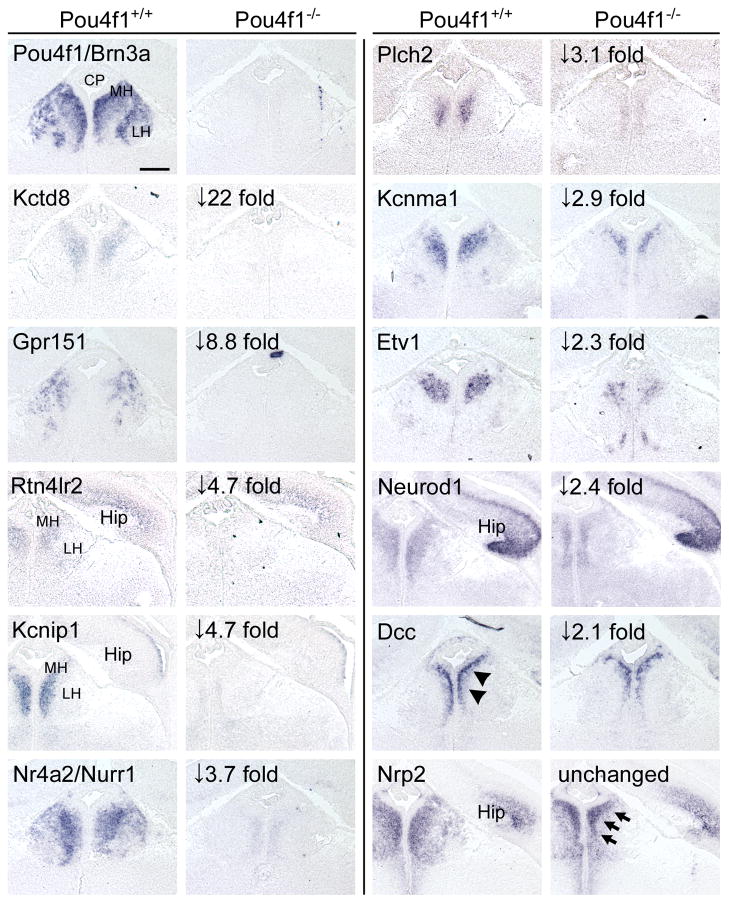
Loss of Brn3a resulted in profound changes in habenular gene expression. Table 2 and Supplementary Table 3 list transcripts which were significantly and reproducibly decreased in the Pou4f1tLacZ/− habenula relative to controls. Prominent among these transcripts are several which encode components of neurotransmitter systems, including Htr5b, Gpr151, Chrnb4, Sstr4, and Chrna3, components or accessory proteins of K+ channels, such as Kctd8, Kcnip1 and Kcnma1, and neurodevelopmental transcription factors, including Nurr1, Irx6, Neurod1 and Etv1. Extensive overlap was immediately apparent between the set of transcripts identified as most habenula-enriched by microarray and bioinformatic analysis, and the Brn3a-dependent transcripts.
Table 2. Transcripts decreased in the habenula of E16.5 Brn3a knockout embryos.
LacZ transgene-guided microdissection was used to isolate the habenula of Brn3atlacZ/+ and Brn3atlacZ/− embryos. Two independent microarray analyses were performed, and results are ranked by the heterozygote/knockout ratio of the mean expression values. Array results were verified by in situ hybridization (Figure 6) and quantitiative RT-PCR (Supplementary Figure 6).
| Expt 1 | Expt 2 | Mean | ||||
|---|---|---|---|---|---|---|
| Gene Symbol | Gene Title | Het1 | KO1 | Het2 | KO2 | HT/KO |
| Pou4f1 | POU domain, class 4, transcription factor 1; Brn3a | 12886 | 118 | 6208 | 182 | 64 |
| Kctd8 | K+ channel tetramerisation domain containing 8 | 99 | 2 | 422 | 22 | 22 |
| Htr5b | 5-hydroxytryptamine (serotonin) receptor 5B | 242 | 6 | 305 | 34 | 14 |
| Sncg | Synuclein, gamma | 257 | 22 | 616 | 58 | 11 |
| Gpr151 | G protein-coupled receptor 151 | 1971 | 209 | 2027 | 248 | 8.8 |
| Irx6 | Iroquois related homeobox 6 | 204 | 13 | 362 | 65 | 7.3 |
| 3110047P20Rik | RIKEN 3110047P20 gene | 13229 | 2801 | 11756 | 2443 | 4.8 |
| Kcnip1 | Kv channel-interacting protein 1 | 581 | 116 | 1641 | 360 | 4.7 |
| Rtn4rl2 | Reticulon 4 receptor-like 2 | 254 | 72 | 825 | 160 | 4.7 |
| Selected: | ||||||
| Chrnb4 | Cholinergic receptor, nicotinic, beta polypeptide 4 | 533 | 163 | 601 | 103 | 4.3 |
| Sstr4 | Somatostatin receptor 4 | 822 | 177 | 434 | 121 | 4.2 |
| 9430028L06Rik | RIKEN 9430028L06 gene | 218 | 57 | 341 | 79 | 4.1 |
| Nr4a2 | Nuclear receptor subfamily 4, group A, member 2; Nurr1 | 7300 | 1902 | 4452 | 1297 | 3.7 |
| Cart | Cocaine and amphetamine regulated transcript | 518 | 154 | 959 | 266 | 3.5 |
| Plch2 | Phospholipase C, eta 2; Phospholipase C-like 4; Plcl4 | 1524 | 442 | 2022 | 703 | 3.1 |
| Cacng5 | Ca++ channel, voltage-dependent, gamma subunit 5 | 396 | 151 | 546 | 155 | 3.1 |
| Syt9 | Synaptotagmin IX | 1921 | 788 | 2744 | 745 | 3.0 |
| Kcnma1 | K+ large conductance calcium- activated channel, subfamily M, alpha member 1 | 2315 | 704 | 849 | 378 | 2.9 |
| Msi2 | Musashi homolog 2 | 3230 | 1067 | 1364 | 637 | 2.7 |
| Cacnb1 | Ca++-dependent, beta 1 subunit | 252 | 107 | 898 | 323 | 2.7 |
| Chrna3 | Cholinergic receptor, nicotinic, alpha polypeptide 3 | 1203 | 523 | 2408 | 835 | 2.7 |
| Cacnb3 | Ca++ channel, voltage-dependent, beta 3 subunit | 5191 | 2260 | 8193 | 3118 | 2.5 |
| Neurod1 | Neurogenic differentiation 1 | 7968 | 3243 | 4988 | 2211 | 2.4 |
| Rgma | RGM domain family, member A | 875 | 462 | 2148 | 850 | 2.3 |
| Etv1 | Ets variant gene 1; ER81 | 9202 | 3665 | 7438 | 3639 | 2.3 |
| Slc6a17 | Solute carrier family 6 (neurotransmitter transporter), member 17 | 275 | 121 | 816 | 364 | 2.2 |
| Epha8 | Eph receptor A8 | 846 | 549 | 1127 | 338 | 2.2 |
| Irx5 | Iroquois related homeobox 5 | 1753 | 846 | 3435 | 1676 | 2.1 |
| Efcbp2 | EF hand Ca++ binding protein 2 | 9409 | 5229 | 13343 | 5974 | 2.0 |
In contrast, relatively few transcripts exhibited increased expression in the Pou4f1tLacZ/− habenula (Supplementary Table 4). The changes were also small in magnitude, and most of the increased transcripts were neither highly expressed nor specific for the habenula. The only generalization which could be drawn from these changes was a modest relative increase in the expression of some components of inhibitory neurotransmitter receptors, including the GABA-A receptor subunits Gabrg1 and Gabra4, and the glycine receptor subunits Glra2 and Glrb.
In order to confirm the array results and to refine our understanding of the expression patterns of the Brn3a-regulated genes, we performed in situ hybridization for several of the downstream transcripts in wild-type and Pou4f1−/− embryos (Figure 6). Changes in the receptor and channel components Gpr151, Kcnip1, Kctd8, and Kcnma1, the transcription factors Nurr1, Neurod1 and Etv1, the axon guidance molecules Rtn4lr2 and DCC, and phospholipase C-eta2 (Plch2) were validated by this method. Only a small change in DCC expression was observed, and the most remarkable aspect of its expression was its restriction to a population of cells immediately adjacent to the ventricle, not observed for other factors. Neuropilin 2 (Nrp2) was confirmed as unchanged, although Nrp2-expressing cells were displaced toward the midline. RT-PCR validation of additional transcripts decreased in the Pou4f1tLacZ/− habenula confirmed the array results in 9/10 cases in which the array change was >2.5 fold (Supplementary Figure 6).
Figure 6. Molecular phenotype of the habenula in Brn3a null mice.
Expression of potential Brn3a target genes identified by microarray were examined in Pou4f1+/+ and Pou4f1−/− embryos at E16.5 using in situ hybridization. In general, the magnitude of the changes in expression detected by in situ hybridization appeared to be greater than the changes observed on the array, and transcripts with >4-fold decreased expression on the microarray were generally undetectable by in situ hybridization in Pou4f1−/− embryos. This may be in part because the use of heterozygous controls in the microarray study somewhat reduced the magnitude of the changes in expression measured by that method. DCC expression was verified but restricted to cells immediately adjacent to the ventricle (arrowheads). Nrp2 expression appeared unchanged, but Nrp2-expressing cells were displaced toward the midline, as observed for other markers (arrows). Expanded gene names appear in Table 2.
Hip, hippocampus; LH, lateral habenula; MH, medial habenula. Scale: 100μM.
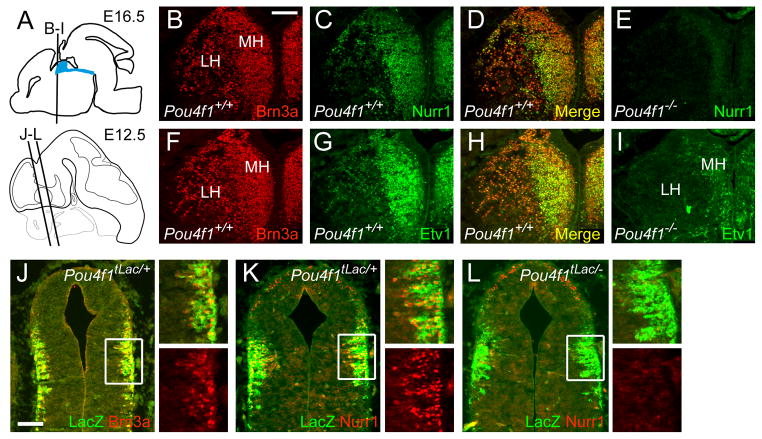
The pattern of in situ hybridization for the transcription factors Brn3a, Nurr1 and Etv1, and the dependence of Nurr1 and Etv1 on Brn3a, suggest that these factors are expressed in the same set of medial and lateral habenular neurons. To confirm this, we examined the co-localization of Brn3a with each of the other factors by immunofluorescence (Figure 7A–I). In control embryos at E16.5, both factors strongly co-localize with Brn3a, although the relative intensity of expression varied across the medial and lateral habenula. In the habenula of Pou4f1−/− embryos, Nurr1 expression was barely detectable (Figure 7E), but a small number of neurons persistently expressed Etv1, and these appeared to be medially displaced (Figure 7I). To determine whether Brn3a null embryos exhibited a primary loss of Nurr1 expression, or a failure to maintain Nurr1, we examined earlier developmental stages. A significant population of Brn3a-immunoreactive habenular neurons are present by E12.5 (Figure 7J). Nurr1 expression followed Brn3a expression closely, and nearly all cells marked by βgalactosidase expression in Pou4f1tLacZ/+ embryos at E12.5 also expressed Nurr1 (Figure 7K). However, in E12.5 Pou4f1tLacZ/− embryos there was no evidence for Nurr1 expression, demonstrating a primary failure to activate this gene (Figure 7L).
Figure 7. Co-expression of transcription factors regulating habenula development.
Expression of Brn3a, Nurr1 and Etv1 were examined by immunofluorescence at E16.5 and E12.5.
(A) Diagram depicting the planes of section in subsequent views.
(B–E) Co-expression of Brn3a and Nurr1 in E16.5 embryos. Nearly all neurons that express Brn3a in the medial and lateral habenula also express Nurr1, although relative expression levels vary (D). Nurr1 is almost undetectable in the Pou4f1−/− habenula (E).
(F–I) Co-expression of Brn3a and Etv1 in E16.5 embryos. In the Pou4f1−/− habenula a small residual population of Etv1+ neurons is noted near the ventricle.
(J–L) Brn3a and Nurr1 expression in early habenular neurons at E12.5. Expression of the tauLacZ transgene faithfully replicates the expression of Brn3a protein in the first habenular neurons to differentiate in Pou4f1tLacZ/+ embryos (J). All or nearly all of these neurons co-express Nurr1 (K), and Nurr1 expression is not initiated in the absence of Brn3a (L).
LH, lateral habenula; MH, medial habenula. Scale: B–I, 100μm; J–L, 100μm.
Nurr1 mediates Brn3a regulation of habenular targets
The early and nearly complete loss of Nurr1 expression in the habenula of Pou4f1−/− embryos suggests that Nurr1 may mediate some of the developmental effects of Brn3a. Nurr1 is required for the normal differentiation of dopaminergic neurons of the ventral tegmentum and substantia nigra (Perlmann and Wallen-Mackenzie, 2004), but the habenula is not dopaminergic, and the role of Nurr1 in the habenula has not been described. Homozygous Nurr1 knockout mice die in the perinatal period, but Nr4a2+/− mice are viable and fertile (Zetterstrom et al., 1997; Castillo et al., 1998). To examine Nurr1 function in habenular development, we obtained Nr4a2+/− mice which were then crossed with the Pou4f1tLacZ reporter line, and the resulting double heterozygotes were bred with Nr4a2+/− mice to generate Nr4a2+/+ and Nr4a2−/− embryos bearing the LacZ reporter allele.
We first examined the cellular phenotype of the habenula in Nr4a2−/− mice at E16.5. As in Brn3a null embryos, the projection of the FR to the ventral midbrain was grossly normal (data not shown; embryos appeared identical to Figure 5B,C). Loss of Nurr1 expression did not replicate the defects in habenular structure and target innervation seen in Brn3a null embryos. Specifically, in Nurr1 knockouts we did not observe the medial displacement of habenular neurons and axons noted in Brn3a mutants at E16.5 (Figure 8A–D), nor did we observe the defects in the innervation of the interpeduncular nucleus seen in Brn3a knockouts at P0 (Figure 8E,F).
Figure 8. Regulation of habenular gene expression by Nurr1.
(A–D) Brn3a and LacZ expression in the habenula of E16.5 Nr4a2+/+ and Nr4a2−/− embryos bearing a Pou4f1tLacZ reporter allele. Neurons and axons do not display the medial displacement observed in Pou4f1−/− embryos, and Brn3a expression is not affected by the loss of Nurr1.
(E–F) Innervation of the interpeduncular nucleus in P0 Nr4a2+/+ and Nr4a2−/− mice carrying a Pou4f1tLacZ reporter allele. Nurr1 expression also identifies dopaminergic neurons in the VTA adjacent to the IPN.
(G) Changes in gene expression in Nr4a2−/− embryos at E16.5. Nurr1 mediates a subset of the gene expression changes observed in the habenula of Brn3a null embryos. Complete gene names appear in Table 2.
Fr, fasciculus retroflexus; Hip, hippocampus; IPN, interpeduncular nucleus; LH, lateral habenula; MH, medial habenula; R, red nucleus; rs, rubrospinal tract; VTA, ventral tegmental area; vtgx, ventral tegmental decussation. Scale: A–D, 50μm; E,F, 100μm; G, 100μM.
To determine whether Nurr1 mediates some of the transcriptional effects of Brn3a, and to establish a gene regulatory pathway for the habenula, we examined the expression of key transcripts downstream of Brn3a in the habenula of Nr4a2−/− embryos at E16.5 (Figure 8G). First, the examination of Brn3a expression in Nurr1 knockouts revealed no change, clearly demonstrating that Nurr1 lies downstream from Brn3a in the program of habenula development. Loss of Nurr1 resulted in decreased expression of Etv1 to an extent resembling that observed in Pou4f1−/− embryos. Similarly Gpr151, Kcnma1 and Plch2 were markedly decreased in Nr4a2−/− embryos. However, Kcnip, Kctd8 and Neurod1 were minimally affected by the loss of Nurr1. Thus Nurr1 regulates a distinct segment of the overall program of gene expression regulated by Brn3a.
Nurr1 and several downstream genes exhibit nearly complete dependence on Brn3a in the habenula. However, Brn3a expression in the superior colliculus, dorsal spinal cord and sensory ganglia is not associated with Nurr1 or most other habenula-enriched genes. To determine whether Brn3a is sufficient to induce Nurr1 in other regions of the CNS, particularly in diencephalic areas adjacent to the habenula, we mis-expressed Brn3a by electroporation in utero. To target the habenula and adjacent thalamus, a dicistronic expression plasmid encoding Brn3a and GFP (pTS-Brn3a, Supplementary Methods) was introduced into the third ventricle (Figure 9A), and a pulsed electric field was applied. A vector encoding GFP alone was used as a control (Figure 9B). Because only dividing neural precursors in the ventricular zone are accessible to electroporation, the procedure was performed at E13.5, prior to cell cycle exit for most habenular neurons, and the embryos were examined at E16.5, when habenular neurogenesis is largely complete. Electroporation of Brn3a-GFP plasmid resulted in nearly 100% co-expression of GFP and Brn3a protein (Figure 9C–F).
Ectopic expression of Brn3a failed to induce Nurr1 expression in the diencephalon, even in the periventricular thalamus adjacent to the habenula (Figure 9G–I). Similarly, Brn3a did not induce Nurr1 in the cerebral cortex, ventral thalamus, hypothalamus or midbrain superior colliculus (Supplementary Figure 7). Thus Brn3a is necessary but not sufficient for induction of the habenula program of gene expression, and is likely to act only in combination with other factors present in this domain.
DISCUSSION
The habenula is a unique molecular territory of the CNS
The results presented here establish the habenula as a highly distinctive molecular compartment of the CNS, yet also underscore the molecular and functional heterogeneity of habenular neurons. Several of the habenula-enriched transcripts show remarkable specificity for the habenula, with little or no expression elsewhere in the mature CNS. Some of these include the orphan 7-transmembrane receptor Gpr151, which may be a galanin receptor (Vassilatis et al., 2003; Ignatov et al., 2004), Tmem16a, recently shown to be a Ca2+-dependent chloride channel (Caputo et al., 2008; Schroeder et al., 2008), the serotonin receptor Htr5b, the acetylcholine receptor subunit Chrnb4, and the potassium channel interacting protein Kctd8, which is known to be a habenula marker in lower vertebrates (Gamse et al., 2005). Numerous other transcripts are expressed in the habenula and a very limited number of other CNS regions.
We did not identify any transcript which was expressed throughout the entire medial and lateral habenula, and also distinguished the habenula from other diencephalic or telencephalic regions, indicating that the habenula as a whole is an anatomical rather than a functional domain. However, Brn3a and several other genes were expressed throughout the medial habenula and also in a subset of lateral habenula neurons. The Brn3a-expressing neurons of the MH and LH are functionally related in that they all appear to project to the IPN via the FR, and do not contribute to the previously characterized direct LH projections to the midbrain monoaminergic nuclei including the SNc, VTA and raphe (Sutherland, 1982; Lecourtier and Kelly, 2007). Thus Brn3a identifies, along with several other markers, an unrecognized set of LH neurons that share gene expression and connectivity characteristics with the MH.
Previous studies have defined subdomains of the MH based on the dorsal and ventral expression of Tac1 (substance P), and Chat (choline acetyltransferase), respectively (Contestabile et al., 1987). Recent work has shown that the MH neurons projecting to the IPN neurons also express glutamatergic markers (Qin and Luo, 2009). It has also been shown that the dorsal MH projects predominantly to the lateral subnucleus of the IPN and the ventral MH to central and intermediate subnuclei of the IPN (Kawaja et al., 1988). The expression patterns of habenula-enriched transcripts in the ABA data set (Supplementary Table 2, Supplementary Figures 2, 3) strongly support the division of the MH into dorsal and ventral subnuclei, although many more ventral-specific transcripts were identified than dorsal. Several of the ventral-specific transcripts are clearly linked to cholinergic pathways (Chat, Slc18a3, Chrnb4, Chrna3), but several have other or unknown functions, including the receptors Gpr151, Npr1, and Tacr1, and molecules of diverse function including Ctnnbip1, Prkcq, Robo3 and Tmem16a.
A series of studies in the rat have defined up to ten subnuclei of the LH based on morphology and immunohistochemistry for a set of molecular markers (Geisler et al., 2003). The expression patterns of a number of transcripts in the LH, examined here and in the ABA data, appear to support a division into lateral and medial subnuclei (e.g. Pou4f1, Gpr151, and Efcpb2 which are expressed in the lateral part of the LH as well as the MH). However, evidence for a finer subnuclear structure is not immediately evident, and will require simultaneous examination of multiple markers and a careful distinction between signals derived from resident neurons and afferent fibers.
In the present study microarray and bioinformatic approaches to habenula-specific gene expression were highly concordant. Future bioinformatic searches for transcripts expressed in specific brain regions will be greatly facilitated by the recently developed Anatomic Gene Expression Atlas (AGEA, http://mouse.brain-map.org/agea, Ng et al., 2009), a relational atlas of the mouse brain computationally derived from the ABA ISH image data. AGEA supports the generation of neuroanatomical maps based on related patterns of gene expression rather than traditional structural boundaries, using either three-dimensional spatial correlation or a hierarchical transcriptome-based parcellation of the brain. A search facility allows the retrieval of lists of genes exhibiting enriched expression in local correlated domains. AGEA analysis of the habenula identifies a majority of the habenula-enriched transcripts described here, and supports the conclusion that the habenula is a distinct domain of the CNS with respect to gene expression.
A hallmark of the habenula in fish, amphibians and reptiles is bilaterally asymmetrical morphology (Concha and Wilson, 2001; Halpern et al., 2003), connectivity (Aizawa et al., 2005), gene expression (Gamse et al., 2005), and timing of neurogenesis (Aizawa et al., 2007), but this asymmetry has not been demonstrated in birds and mammals. Brn3a is symmetrically expressed in the zebrafish habenula (Aizawa et al., 2007), and is not likely to participate in the generation of these asymmetrical features. Members of the KCTD family, related to murine Kctd8 which has been identified here as downstream of Brn3a and Nurr1, are asymmetrically expressed in the zebrafish habenula (Gamse et al., 2005). The zebrafish neuropilin, Nrp1a, is also expressed asymmetrically and interacts with members of the Sema3 family to establish and refine asymmetrical habenulopeduncular projections (Kuan et al., 2007), clearly employing some of the same molecular pathways used for FR axon guidance in mice (Kantor et al., 2004). No lateral asymmetry in expression was observed for any of the habenula-enriched transcripts identified here by microarray or bioinformatic analysis, including Kctd8 and Nrp2, but the methods used here cannot rule out subtle differences in the extent or intensity of gene expression between the left and right habenulae.
A developmental pathway for the mammalian habenula
Microarray results for Pou4f1−/− embryos demonstrate that Brn3a is required for the coordinated expression of a battery of genes that characterize the habenula. The majority of the regulated genes are highly enriched in the habenula, and none are “housekeeping” genes or genes encoding generic neuronal factors. These Brn3a-regulated genes include transcription factors, components of neurotransmitter systems, K+ and Ca++ channels, and mediators of intracellular signal transduction.
Prior work has established neuropilin/semaphorin and netrin signaling mechanisms in the guidance of the FR (Funato et al., 2000; Giger et al., 2000; Kantor et al., 2004). Nrp2 transcripts are highly enriched in E16.5 habenula, but are not changed in Pou4f1−/− embryos. This is consistent with the normal initial trajectory and fasciculation of the FR in Brn3a knockouts, and suggests that these pathways are independent of Brn3a.
Nurr1 is clearly downstream of Brn3a in a regulatory hierarchy, as Nurr1 expression is lost from the onset of its expression in Pou4f1−/− embryos, but Brn3a expression is maintained in Nr4a2−/− mice. Nurr1 mediates the expression of a distinct subset of the genes downstream of Brn3a, including the ets-domain transcription factor Etv1 and the effectors of neural function Gpr151, Plch2, and Kcnma1. In contrast, the Brn3a targets Kcnip, Kctd8, and Neurod1 were not significantly decreased in Nr4a2−/−embryos. These examples are not sufficient to determine whether Nurr1-dependent and independent habenula transcripts belong to distinct functional categories. However, loss of Nurr1 does not produce the severe defect in the innervation of the IPN seen in Pou4f1−/− embryos, suggesting that some critical factors mediating the interaction of habenular axons with their targets are Brn3a-dependent but Nurr1-independent.
Tissue-specific developmental roles for Brn3a
One objective of the current study was to determine whether Brn3a regulates conserved target genes in the sensory nervous system and CNS. In prior studies we have shown that in the trigeminal and dorsal root ganglia, Brn3a represses the expression of transcription factors characteristic of early sensory neurogenesis, such as Neurog1, Neurod1, Neurod4 (Math3), and Tcfap2b (Eng et al., 2004; Eng et al., 2007). Brn3a has also been shown to bind directly to Neurod1 and Neurod4 regulatory sequences in the developing trigeminal ganglion (Lanier et al., 2007). The set of transcripts which are positively regulated by Brn3a (decreased in the knockout) are expressed later and associated with the differentiation of specific sensory subtypes.
In contrast to sensory neurons, in the habenula Brn3a appears to function almost exclusively as a transcriptional activator. Few transcripts exhibit increased expression in the habenula of Pou4f1−/− embryos, and the changes are of small magnitude and may be indirect, effectively ruling out a repressor role analogous to the sensory system. The context-dependent function of Brn3a is illustrated by its effects on Neurod1, one of the few identified target genes in common between the habenula and sensory ganglia, which is repressed by Brn3a in the sensory ganglia and activated in the habenula. The apparent lack of a repressor role for Brn3a in the habenula may be related to the distinct timing of Brn3a expression in the CNS and sensory system. In the CNS, Brn3a is expressed only in post-mitotic neurons, whereas in the peripheral sensory system its expression is initiated prior to cell cycle exit (Fedtsova and Turner, 1995), consistent with a model in which the repression of early neurogenic genes is exclusively a sensory function of Brn3a.
Even when only positively regulated target genes are considered, there is little overlap between the gene expression program regulated by Brn3a in the habenula and sensory system. The majority of Brn3a-regulated targets in the habenula are preferentially expressed there, and are not expressed in developing sensory neurons. Conversely, the majority of genes which require Brn3a for their expression in sensory neurons are not expressed in the habenula, and it is rarely if ever the case that a transcript is expressed in both tissues, and regulated in just one. There is also limited overlap between the regulatory targets of Brn3a in the habenula and the extensive set of downstream targets defined for the closely related factor Brn3b in the retina (Mu et al., 2004; Mu et al., 2008; Qiu et al., 2008).
Much less is known about the downstream targets of Nurr1, which has been characterized mainly with respect to its role in the differentiation of midbrain dopaminergic neurons (Perlmann and Wallen-Mackenzie, 2004). Because the specific genes downstream of Nurr1 identified here, such as Gpr151, Kcnma1, and Etv1, are not expressed in the ventral tegmental area or substantia nigra, the targets of Nurr1 regulation in the midbrain and the habenula are probably distinct.
How these or any transcription factors regulate different sets of downstream targets in different tissues remains largely unknown. It is likely that in each cell type, Brn3a and Nurr1 act combinatorially to exert their effects, and may have different DNA binding partners or co-factors in different tissues. However, we have also recently shown that the binding of Brn3a to high affinity sites in vivo is restricted to regions of transcriptionally active chromatin, characterized by histone H3-K9/14 acetylation and H3-K4 methylation (Lanier et al., 2007). This suggests a model in which Brn3a cannot interact with large segments of the genome, and its tissue-specific are determined in part by uniquely accessible subsets of potential target genes in each cell type.
Supplementary Material
Acknowledgments
We would like to thank Drs. Geraldine Kerjan and Joe Gleeson for help with developing protocols for electroporation in utero. We would also like to thank Drs. Thomas Perlmann, Fujio Murakami, Yasushi Nakagawa and Sylvia Arber, Carmen Birchmeier for their generous gifts of antisera (Methods), and Drs. Qiufu Ma and Keijo Luukko for ISH probes. We are also indebted to Dr. Vera Nikodem for providing Nurr1 knockout mice, Dr. William Wachsman and Lutfunnessa Shireen of the UCSD/VA microarray core facility for assistance with microarray technology, and Gian Carlo Parico for technical assistance. Mouse monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank, maintained under contract 758
NO1-HD23144 from the NICHD. Supported in part by Department of Veterans Affairs MERIT funding, and NIH awards HD33442 and MH065496 (E.E.T). E.E.T. is a NARSAD Investigator.
References
- Aizawa H, Goto M, Sato T, Okamoto H. Temporally regulated asymmetric neurogenesis causes left-right difference in the zebrafish habenular structures. Dev Cell. 2007;12:87–98. doi: 10.1016/j.devcel.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Aizawa H, Bianco IH, Hamaoka T, Miyashita T, Uemura O, Concha ML, Russell C, Wilson SW, Okamoto H. Laterotopic representation of left-right information onto the dorso-ventral axis of a zebrafish midbrain target nucleus. Curr Biol. 2005;15:238–243. doi: 10.1016/j.cub.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres KH, von During M, Veh RW. Subnuclear organization of the rat habenular complexes. J Comp Neurol. 1999;407:130–150. doi: 10.1002/(sici)1096-9861(19990428)407:1<130::aid-cne10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2000;101:485–498. doi: 10.1016/s0092-8674(00)80859-4. [DOI] [PubMed] [Google Scholar]
- Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- Castillo SO, Baffi JS, Palkovits M, Goldstein DS, Kopin IJ, Witta J, Magnuson MA, Nikodem VM. Dopamine biosynthesis is selectively abolished in substantia nigra/ventral tegmental area but not in hypothalamic neurons in mice with targeted disruption of the Nurr1 gene. Mol Cell Neurosci. 1998;11:36–46. doi: 10.1006/mcne.1998.0673. [DOI] [PubMed] [Google Scholar]
- Concha ML, Wilson SW. Asymmetry in the epithalamus of vertebrates. J Anat. 2001;199:63–84. doi: 10.1046/j.1469-7580.2001.19910063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha ML, Russell C, Regan JC, Tawk M, Sidi S, Gilmour DT, Kapsimali M, Sumoy L, Goldstone K, Amaya E, Kimelman D, Nicolson T, Grunder S, Gomperts M, Clarke JD, Wilson SW. Local tissue interactions across the dorsal midline of the forebrain establish CNS laterality. Neuron. 2003;39:423–438. doi: 10.1016/s0896-6273(03)00437-9. [DOI] [PubMed] [Google Scholar]
- Contestabile A, Flumerfelt BA. Afferent connections of the interpeduncular nucleus and the topographic organization of the habenulo-interpeduncular pathway: an HRP study in the rat. J Comp Neurol. 1981;196:253–270. doi: 10.1002/cne.901960206. [DOI] [PubMed] [Google Scholar]
- Contestabile A, Villani L, Fasolo A, Franzoni MF, Gribaudo L, Oktedalen O, Fonnum F. Topography of cholinergic and substance P pathways in the habenulo-interpeduncular system of the rat. An immunocytochemical and microchemical approach. Neuroscience. 1987;21:253–270. doi: 10.1016/0306-4522(87)90337-x. [DOI] [PubMed] [Google Scholar]
- Dong H-W. Allen reference atlas: a digital color brain atlas of the C57Black/6J male mouse. Hoboken, N.J. Chichester: Wiley; John Wiley distributor; 2007. [Google Scholar]
- Eng SR, Lanier J, Fedtsova N, Turner EE. Coordinated regulation of gene expression by Brn3a in developing sensory ganglia. Development. 2004;131:3859–3870. doi: 10.1242/dev.01260. [DOI] [PubMed] [Google Scholar]
- Eng SR, Dykes IM, Lanier J, Fedtsova N, Turner EE. POU-domain factor Brn3a regulates both distinct and common programs of gene expression in the spinal and trigeminal sensory ganglia. Neural Develop. 2007;2:3. doi: 10.1186/1749-8104-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedtsova N, Turner E. Brn-3.0 Expression identifies early post-mitotic CNS neurons and sensory neural precursors. Mechanisms of Development. 1995;53:291–304. doi: 10.1016/0925-4773(95)00435-1. [DOI] [PubMed] [Google Scholar]
- Fedtsova N, Quina LA, Wang S, Turner EE. Regulation of the development of tectal neurons and their projections by transcription factors Brn3a and Pax7. Dev Biol. 2008;316:6–20. doi: 10.1016/j.ydbio.2007.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funato H, Saito-Nakazato Y, Takahashi H. Axonal growth from the habenular nucleus along the neuromere boundary region of the diencephalon is regulated by semaphorin 3F and netrin-1. Mol Cell Neurosci. 2000;16:206–220. doi: 10.1006/mcne.2000.0870. [DOI] [PubMed] [Google Scholar]
- Gamse JT, Thisse C, Thisse B, Halpern ME. The parapineal mediates left-right asymmetry in the zebrafish diencephalon. Development. 2003;130:1059–1068. doi: 10.1242/dev.00270. [DOI] [PubMed] [Google Scholar]
- Gamse JT, Kuan YS, Macurak M, Brosamle C, Thisse B, Thisse C, Halpern ME. Directional asymmetry of the zebrafish epithalamus guides dorsoventral innervation of the midbrain target. Development. 2005;132:4869–4881. doi: 10.1242/dev.02046. [DOI] [PubMed] [Google Scholar]
- Geisler S, Andres KH, Veh RW. Morphologic and cytochemical criteria for the identification and delineation of individual subnuclei within the lateral habenular complex of the rat. J Comp Neurol. 2003;458:78–97. doi: 10.1002/cne.10566. [DOI] [PubMed] [Google Scholar]
- Giger RJ, Cloutier JF, Sahay A, Prinjha RK, Levengood DV, Moore SE, Pickering S, Simmons D, Rastan S, Walsh FS, Kolodkin AL, Ginty DD, Geppert M. Neuropilin-2 is required in vivo for selective axon guidance responses to secreted semaphorins. Neuron. 2000;25:29–41. doi: 10.1016/s0896-6273(00)80869-7. [DOI] [PubMed] [Google Scholar]
- Gruber C, Rhee J, Gleiberman A, Turner E. POU-domain factors of the Brn-3 class recognize functional DNA elements which are distinctive, symmetrical, and highly conserved in evolution. Mol Cell Bio. 1997;17:2391–2400. doi: 10.1128/mcb.17.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern ME, Liang JO, Gamse JT. Leaning to the left: laterality in the zebrafish forebrain. Trends Neurosci. 2003;26:308–313. doi: 10.1016/S0166-2236(03)00129-2. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979;187:19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sesack SR, Lecourtier L, Shepard PD. Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci. 2008;28:11825–11829. doi: 10.1523/JNEUROSCI.3463-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatov A, Hermans-Borgmeyer I, Schaller HC. Cloning and characterization of a novel G-protein-coupled receptor with homology to galanin receptors. Neuropharmacology. 2004;46:1114–1120. doi: 10.1016/j.neuropharm.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ, Kolodkin AL. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron. 2004;44:961–975. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Kawaja MD, Flumerfelt BA, Hrycyshyn AW. Topographical and ultrastructural investigation of the habenulo-interpeduncular pathway in the rat: a wheat germ agglutinin-horseradish peroxidase anterograde study. J Comp Neurol. 1988;275:117–127. doi: 10.1002/cne.902750110. [DOI] [PubMed] [Google Scholar]
- Klemm WR. Habenular and interpeduncularis nuclei: shared components in multiple-function networks. Med Sci Monit. 2004;10:RA261–273. [PubMed] [Google Scholar]
- Kuan YS, Yu HH, Moens CB, Halpern ME. Neuropilin asymmetry mediates a left-right difference in habenular connectivity. Development. 2007;134:857–865. doi: 10.1242/dev.02791. [DOI] [PubMed] [Google Scholar]
- Lanier J, Quina LA, Eng SR, Cox E, Turner EE. Brn3a target gene recognition in embryonic sensory neurons. Dev Biol. 2007;302:703–716. doi: 10.1016/j.ydbio.2006.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci Biobehav Rev. 2007;31:658–672. doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Mu X, Fu X, Beremand PD, Thomas TL, Klein WH. Gene regulation logic in retinal ganglion cell development: Isl1 defines a critical branch distinct from but overlapping with Pou4f2. Proc Natl Acad Sci U S A. 2008;105:6942–6947. doi: 10.1073/pnas.0802627105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X, Beremand PD, Zhao S, Pershad R, Sun H, Scarpa A, Liang S, Thomas TL, Klein WH. Discrete gene sets depend on POU domain transcription factor Brn3b/Brn-3.2/POU4f2 for their expression in the mouse embryonic retina. Development. 2004;131:1197–1210. doi: 10.1242/dev.01010. [DOI] [PubMed] [Google Scholar]
- Ng L, Pathak SD, Kuan C, Lau C, Dong H, Sodt A, Dang C, Avants B, Yushkevich P, Gee JC, Haynor D, Lein E, Jones A, Hawrylycz M. Neuroinformatics for genome-wide 3D gene expression mapping in the mouse brain. IEEE/ACM Trans Comput Biol Bioinform. 2007;4:382–393. doi: 10.1109/tcbb.2007.1035. [DOI] [PubMed] [Google Scholar]
- Ng L, Bernard A, Lau C, Overly CC, Dong HW, Kuan C, Pathak S, Sunkin SM, Dang C, Bohland JW, Bokil H, Mitra PP, Puelles L, Hohmann J, Anderson DJ, Lein ES, Jones AR, Hawrylycz M. An anatomic gene expression atlas of the adult mouse brain. Nat Neurosci. 2009;12:356–362. doi: 10.1038/nn.2281. [DOI] [PubMed] [Google Scholar]
- Perlmann T, Wallen-Mackenzie A. Nurr1, an orphan nuclear receptor with essential functions in developing dopamine cells. Cell Tissue Res. 2004;318:45–52. doi: 10.1007/s00441-004-0974-7. [DOI] [PubMed] [Google Scholar]
- Qin C, Luo M. Neurochemical phenotypes of the afferent and efferent projections of the mouse medial habenula. Neuroscience. 2009;161:827–837. doi: 10.1016/j.neuroscience.2009.03.085. [DOI] [PubMed] [Google Scholar]
- Qiu F, Jiang H, Xiang M. A comprehensive negative regulatory program controlled by Brn3b to ensure ganglion cell specification from multipotential retinal precursors. J Neurosci. 2008;28:3392–3403. doi: 10.1523/JNEUROSCI.0043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quina LA, Pak W, Lanier J, Banwait P, Gratwick K, Liu Y, Velasquez T, O’Leary DD, Goulding M, Turner EE. Brn3a-expressing retinal ganglion cells project specifically to thalamocortical and collicular visual pathways. J Neurosci. 2005;25:11595–11604. doi: 10.1523/JNEUROSCI.2837-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussigne M, Bianco IH, Wilson SW, Blader P. Nodal signalling imposes left-right asymmetry upon neurogenesis in the habenular nuclei. Development. 2009;136:1549–1557. doi: 10.1242/dev.034793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T. In vivo electroporation in the embryonic mouse central nervous system. Nat Protoc. 2006;1:1552–1558. doi: 10.1038/nprot.2006.276. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H, Suzuki T. Efferent projections of the interpeduncular complex in the rat, with special reference to its subnuclei: a retrograde horseradish peroxidase study. Brain Res. 1984;296:345–349. doi: 10.1016/0006-8993(84)90071-4. [DOI] [PubMed] [Google Scholar]
- Storm R, Cholewa-Waclaw J, Reuter K, Brohl D, Sieber M, Treier M, Muller T, Birchmeier C. The bHLH transcription factor Olig3 marks the dorsal neuroepithelium of the hindbrain and is essential for the development of brainstem nuclei. Development. 2009;136:295–305. doi: 10.1242/dev.027193. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- Tamada A, Kumada T, Zhu Y, Matsumoto T, Hatanaka Y, Muguruma K, Chen Z, Tanabe Y, Torigoe M, Yamauchi K, Oyama H, Nishida K, Murakami F. Crucial roles of Robo proteins in midline crossing of cerebellofugal axons and lack of their up-regulation after midline crossing. Neural Develop. 2008;3:29. doi: 10.1186/1749-8104-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilatis DK, Hohmann JG, Zeng H, Li F, Ranchalis JE, Mortrud MT, Brown A, Rodriguez SS, Weller JR, Wright AC, Bergmann JE, Gaitanaris GA. The G protein-coupled receptor repertoires of human and mouse. Proc Natl Acad Sci U S A. 2003;100:4903–4908. doi: 10.1073/pnas.0230374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vue TY, Aaker J, Taniguchi A, Kazemzadeh C, Skidmore JM, Martin DM, Martin JF, Treier M, Nakagawa Y. Characterization of progenitor domains in the developing mouse thalamus. J Comp Neurol. 2007;505:73–91. doi: 10.1002/cne.21467. [DOI] [PubMed] [Google Scholar]
- Wallen AA, Castro DS, Zetterstrom RH, Karlen M, Olson L, Ericson J, Perlmann T. Orphan nuclear receptor Nurr1 is essential for Ret expression in midbrain dopamine neurons and in the brain stem. Mol Cell Neurosci. 2001;18:649–663. doi: 10.1006/mcne.2001.1057. [DOI] [PubMed] [Google Scholar]
- Xiang M, Lin G, Zhou L, Klein WH, Nathans J. Targeted deletion of the mouse POU-domain gene Brn-3a causes a selective loss of neurons in the brainstem and trigeminal ganglion, uncoordinated limb movement, and impaired suckling. Proc Nat Acad Sci. 1996;93:11950–11955. doi: 10.1073/pnas.93.21.11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterstrom RH, Solomin L, Jansson L, Hoffer BJ, Olson L, Perlmann T. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276:248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.