Abstract
shibirets1, a temperature-sensitive mutation of the Drosophila gene encoding a Dynamin orthologue, blocks vesicle endocytosis and thus synaptic transmission, at elevated, or restrictive temperatures. By targeted Gal4 expression, UAS-shibirets1 has been used to dissect neuronal circuits. We investigated the effects of UAS-shibirets1 overexpression in Drosophila photoreceptors at permissive (19°C) and restrictive (31°C) temperatures. At 19°C, overexpression of UAS-shits1 causes decelerated phototransduction and reduced neurotransmitter release. This phenotype is exacerbated with dark-adaptation, age and in white mutants. Photoreceptors overexpressing UAS-shibirets1 contain terminals with widespread vacuolated mitochondria, reduced numbers of vesicles and bundled microtubules. Immuno-EM reveals that the latter are dynamin coated. Further, the microtubule phenotype is not restricted to photoreceptors, as UAS-shibirets1 overexpression in lamina cells also bundles microtubules. We conclude that dynamin has multiple functions that are interrupted by UAS-shibirets1 overexpression in Drosophila photoreceptors, destabilizing their neural communication irreversibly at previously reported permissive temperatures.
Keywords: photoreceptor, endocytosis, shibirets1, ERG, MAP, UAS/Gal4
Introduction
The fly orthologue of Dynamin is encoded by shibire (Chen et al., 1991), a GTPase involved in endocytosis (van der Bliek and Meyerowrtz, 1991; Damke et al., 1994). Under previously tested conditions, the temperature-sensitive allele shibirets1 (shits1) allows, normal endocytosis to occur at temperatures below 22°C, but prevents membrane retrieval at temperatures exceeding 30°C, leading to reduced neurotransmitter release and synaptic blockade (Kosaka and Ikeda, 1983). Overexpression of shits1 (Kitamoto, 2001) using the Gal4/UAS system (Brand and Perrimon, 1993) allows targeted temperature-dependent blockade of synaptic transmission within the Drosophila nervous system (for a review see: Duffy, 2002). Hence, the contribution of individual neurons to identified circuits can be assessed (Luo et al., 2008), such as those involved in color (Gao et al., 2008) or motion perception (Rister et al., 2007).
Temperature-dependent activation of UAS-shits1 was originally studied in Drosophila photoreceptors (Kitamoto, 2001), from which the continuous release (Zheng et al., 2006) of histamine (Hardie, 1987; Sarthy, 1991), to the Large Monopolar Cells (LMC) (Zheng et al., 2006) is blocked by mutant shits1 at restrictive temperatures. In an Electroretinogram (ERG), the eye’s field potential response to a light pulse (Heisenberg, 1971), such blockade is apparent by the lack of “On”- and “Off”-transients (Zheng et al., 2006), reported by Coombe (1986) to derive from the LMCs. The permissive (18°C) and restrictive (31°C) temperatures for shits1 action, originally defined from their effects on the ERG of homozygous mutant flies (Chen and Stark, 1993), have been confirmed for the UAS-shits1 transgene by recording the ERGs of light-adapted flies overexpressing UAS-shits1 in their photoreceptors (Kitamoto, 2001).
Other variables may also affect photoreceptors overexpressing UAS-shits1. For example, light exposure increases endocytosis and membrane turnover in fly photoreceptors (Stark et al., 1988; Bähner et al., 2002; Kosloff et al., 2003; Chorna-Ornan et al., 2005; Frechter et al., 2007; Han et al., 2007). In addition, ageing reduces the time needed for synaptic blockade (Beramendi et al., 2007) and the eye marker gene, white, interacts with shits1 action (Chen and Stark, 1993). A study controlling for these factors in flies overexpressing shibirets1 has hitherto been lacking and should reveal additional functions of dynamin. For example, dynamin may act in vivo as a microtubule associated protein (MAP), an interaction previously demonstrated biochemically (Shpetner and Vallee, 1989).
To address dynamin function in vivo, we examine how interactions between light exposure, temperature, genetic background and age affect the synaptic output of outer photoreceptors (R1–R6) overexpressing UAS-shits1. To evaluate changes in neurotransmission, we compare the ERGs. These results are then related to changes in phototransduction, recorded intracellularly through light-evoked voltage responses, and to changes in ultrastructure using electron microscopy, EM. We show that at 19°C, photoreceptors overexpressing UAS-shits1 have decelerated phototransduction and compromised neurotransmission, both after dark-adaptation and in older flies. At 19°C, photoreceptor terminals contain fewer vesicles, vacuolated mitochondria typical of apoptosis and bundled microtubules decorated with dynamin. These changes reveal the outcome of overexpressing a temperature-sensitive form of dynamin that is functionally impaired even at a permissive temperature of 19°C.
Materials and Methods
Fly Stocks
The reporter line UAS-shits1 was provided by Dr. Toshihiro Kitamoto (University of Iowa); Canton-S (wild-type), w1118 (here referred to simply as white), Rh1-Gal4 and GMR-Gal4 were acquired from the Bloomington Stock Center. Mutant ort5 (US6096) was a gift from Prof. Roger Hardie (University of Cambridge), MJ85b-Gal4 was a gift from Prof. Leslie C. Griffith (Brandeis University), and hdcJK910 flies were from Prof. Erich Buchner (Julius-Maximilians-Universität, Würzburg). Flies were raised at 18°C in a 12-12 dark-light cycle. Female flies were used for all experiments.
UAS-shits1 overexpression in photoreceptors
We used the UAS/Gal4 system (Brand and Perrimon, 1993) to overexpress UAS-shits1 and dominate wild-type Dynamin function by the mutant temperature-sensitive form of the protein. The driver Rh1-Gal4 was chosen to ensure that UAS-shits1 was expressed solely in photoreceptors R1–R6. Rh1-Gal4 is expressed only in later stages of pupal development of R1–R6 (Kumar and Ready, 1995). Given the weak expression of Rh1-Gal4, we generated homozygous flies for both the UAS-shits1 and Rh1-Gal4 insertions, and reared them at the permissive temperature, 18°C. Two different types of homozygous Rh1-shits1 flies were used, having either the wild-type eye color (w+), or the white mutation (w1118). In addition, we used GMR-Gal4, which is strongly expressed in all eight photoreceptors, R1–R8, as well as in the supporting cells in the eye imaginal disc (Freeman, 1996). GMR-Gal4 expression, which starts much earlier than Rh1-Gal4 expression, can induce a rough-eye phenotype in homozygous flies when reared at >23°C (Kramer and Staveley, 2003). To prevent a rough-eye phenotype and preserve the normal optics of the compound eyes, we therefore used only heterozygous +; GMR-Gal4/+; UAS-shits1/+ flies reared at 18°C.
Fly crosses
white Rh1-shits1 flies with insertions of P(ry+, Rh1-Gal4) and P(w+, UAS-shits1) on chromosomes 2 and 3 respectively, were generated by crossing each insertion line to a white double balancer stock (white; If/CyO; MKRS/TM6b) over two generations, to produce white; Rh1-Gal4/CyO; MKRS/TM6b and white; If/CyO; UAS-shits1/TM6b stocks. These stocks were then crossed to each other to produce a balanced Rh1-shits1 stock carrying both insertions (white; Rh1-Gal4/CyO; UAS-shits1/TM6b). To exchange the X-chromosome to a wild-type, a Canton-S double balancer stock was used over two generations. Rh1-Gal4 stock with a Canton-S background was produced as a control line using our Canton-S balancer stock (+; Gla/CyO; +). To gauge the expression levels of UAS-shits1, our balanced stock was further crossed to either Canton-S, UAS-shits1 stock or Canton-S background Rh1-Gal4 stock. Notably, homozygous Rh1-shits1 flies emerged in a proportion less than the Mendelian ratio predicted (10% instead of 25%)
GMR-Gal4/+; UAS-shits1/+ flies with a Canton-S background and insertions of P(w+, GMR-Gal4) and P(w+, UAS-shits1) on chromosomes 2 and 3 respectively, were generated by crossing each insertion line to a Canton-S double balancer stock (+; If/CyO; MKRS/TM6b) over two generations to produce +; GMR-Gal4/CyO; MKRS/TM6b and +; If/CyO; UAS-shits1/TM6b stocks. These stocks were then crossed to each other to produce a balanced GMR-shits1 stock carrying both insertions (+; GMR-Gal4/CyO; UAS-shits1/TM6b). These flies where then crossed in turn to Canton-S to produce +; GMR-Gal4/+; UAS-shits1/+ for testing. A GMR-Gal4 stock with a Canton-S background was produced as a control line using our Canton-S balancer stock (+; Gla/CyO; +). Mutant ort5 flies were first crossed to a Canton-S MKRS/TM6b stock to remove the white mutation and then the +; +; ort5 st1 flies recombined with Canton-S to remove the scarlet marker.
Mj85b-shits1 flies with a Canton-S background and insertions of P(w+, Mj85b-Gal4) and P(w+, UAS-shits1) on chromosomes 1 and 2 respectively, were generated by crossing each insertion line to a Canton-S double balancer stock (FM6/FM7h; If/CyO) over two generations to produce Mj85b-Gal4/FM7h; If/CyO and FM6/FM7h; UAS-shits1/CyO stocks. These stocks were then crossed to each other to produce a balanced Mj85b-shits1 stock carrying both insertions (Mj85b-Gal4/FM7h; UAS-shits1/CyO).
ERGs
Flies were mounted inside a copper cone and their temperature controlled by a Peltier element (Juusola and Hardie, 2001). To test the effects of light history and temperature on neurotransmission, three experiments were undertaken (Fig. S1). The adaptation periods at 19°C were set at 30 min because although long-term dark-adaptation, measured as the percentage of G protein translocated to the rhabdomere membrane (Frechter et al., 2007), takes up to 2 h (Kosloff et al., 2003; Cronin et al., 2004), morphological changes related to dark-adaptation are readily visible after 30 min (Kosloff et al., 2003). In contrast, the restrictive period at 31°C was kept to 10 min, as this has previously been shown sufficient to block neurotransmission in flies overexpressing UAS-shits1 (Kitamoto, 2001). The reference electrode was placed in the ocelli, while a blunt recording electrode was placed on the stimulated eye. Both electrodes were borosilicate glass, filled with fly Ringers. The software and electrophysiology setup were as previously reported (Juusola and Hardie, 2001).
ERG analysis
The influence of fast light-adaptation meant that all flies kept in darkness for 30 min displayed “On”-transient sizes in traces 1 to 4 significantly different from each other and from the rest of the recorded traces (data not shown). It is possible that shibirets1 flies might release neurotransmitter only in the first traces because of the stimulation-dependent depletion of the synaptic vesicle pool (Delgado et al., 2000). Therefore, only traces 5 to 10 were used to obtain a representative figure for each fly or condition. The three main components of an ERG were quantified: the “On”- and “Off”-transients, corresponding to fast response waveforms of lamina cells – claimed to be LMCs (Coombe, 1986), and most likely amacrine cells that share the same histamine receptor (Zheng et al., 2006; Pantazis et al., 2008), and the slower photoreceptor component (Heisenberg, 1971; Coombe and Heisenberg, 1986). Both photoreceptors and LMCs respond to the inputs continuously, because of the tonic graded-potential synapses (feedforward and feedback) that link them (Juusola et al., 1995; Uusitalo et al., 1995a; Zheng et al., 2006). Photoreceptors hyperpolarize and the LMCs depolarize the ERG waveform in a push-pull relationship. For bright stimuli, LMC output adapts rapidly (Juusola et al., 1995) so that only their transients overcome the large photoreceptor component. Nonetheless, the size of the photoreceptor component is further reduced by the residual sustained component in the LMC responses.
For each fly strain, 6 (n) flies were tested. For each genotype or treatment the mean and Standard Error of Mean (SEM) were calculated, and a student t-test with a Bonferroni correction was conducted. The shapes of the photoreceptor response were analyzed by normalizing the ERGs at 1.78 s, the time chosen as the first point of the sustained component in dark-adapted transgenic lines, after the initial hyperpolarization of the ERG. The amplitude of the ERG at this time point is not significantly different from controls. This is important because normalizing ERG at points with marked differences in the absolute amplitude of the photoreceptor response could influence other dynamics, which in turn could be reflected in the shape of the ERG. The mean of all normalized ERGs for each genotype is shown, the SEM was omitted for clarity. The data acquisition and stimulus protocols were executed under Matlab (Mathworks: Natick, MA), using custom-written software. Origin software (Origin Lab: Northampton, MA) was used for the data analysis and plotting.
Light stimulus
To ensure a uniform and reproducible stimulation of the eye, the light source was composed of a matrix of 100 (10×10) white 3 mm LEDs (Nichia LEDs NSPW500BS, 15,500 mcd, 20° half width). The overall stimulus illuminance was 5.92 lumen/cm2. The stimulus was composed of 50 ms of darkness, 700 ms of light, followed by 1,250 ms of darkness. This cycle was flashed 10 times in consecutive square-wave pulses, the stimulus used to test the visual response after each adaptation period in each experiment (e.g. Fig. 1A). The stimulus evoked saturating peak responses without being compromised by a Prolonged Depolarizing After potential, as measured by intracellular recordings in wild-type photoreceptors. Here, the responses followed typical adaptation dynamics, seen commonly in bright-light pulse experiments (Fig. S1). The photoreceptor sensitivity (peak response) first declined and then recovered during subsequent stimulation, indicating that phototransduction maintained responsiveness throughout the experimental protocol.
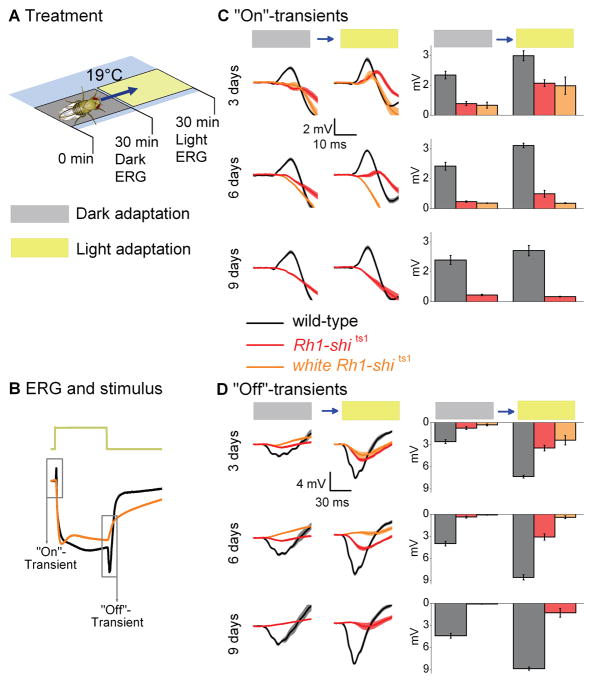
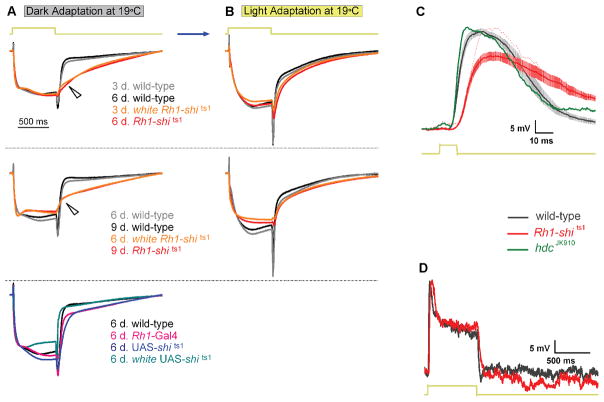
Figure 1.
Rh1-shits1 photoreceptor output depends on light history at 19°C. (A) Dark-adaptation (30 min) followed by light-adaptation (30 min) at 19°C. (B) ERGs were recorded (700 ms stimulus) after each adaptation. Traces are from dark-adapted flies and 6 days post-eclosion. The amplitude of (C) “On”- and (D) “Off”-transients quantified for wild-type (black), Rh1-shi ts1 (red) and white Rh1-shi ts1 (orange). Mean ± SEM shown in traces and histograms with n = 6 for each group. Age refers to days post-eclosion.
Intracellular recordings from photoreceptors in vivo
The setup and preparation were established as previously reported (Juusola and Hardie, 2001). Briefly, a hole of approximately 4×4 ommatidia was made on the cuticle of the left eye (Fig. S5) to allow insertion of a sharp quartz electrode (>95 MΩ), which was filled with 3M KCl. A reference electrode, which was placed inside the head capsule close to the ocelli, was filled with fly Ringer (Juusola and Hardie, 2001). Cells were selected with a resting potential below −60 mV and the point light source was placed to maximize the response. The light source comprised of a single high power LED (Seoul Z-Power LED P4 star, white, 100 Lumens), mounted on a cardan arm system and projected by a lens through a pinhole (subtended angle 0.7°) and driven by an Opto LED (Cairn Research Ltd). Flies were dark-adapted for a minimum of 20 min and then stimulated with either a short (10 ms) or a long light pulse (700 ms) and the response of a single photoreceptor cell recorded. See “ERG analysis” for equipment and software used.
Electron microscopy (EM)
For the retina, flies were adapted to room lighting prior to fixation. For the photoreceptor terminals in the lamina, we replicated the conditions used in Fig. 1. Flies were dark-adapted for 30 min. Following this, half of the flies were dissected and fixed using only red light. The rest of the flies were exposed to intense white light for 30 min and then dissected and fixed in the light. For this experiment, the temperature was held at 17 ± 1°C. The dissection, fixation and embedding protocols for all EM observations have been described previously (Meinertzhagen and O’Neil, 1991; Meinertzhagen, 1996). Serial 60 nm sections were stained with uranyl acetate and lead citrate and examined at 80 kV in a Philips Tecnai 12 electron microscope. Images were captured with a Kodak Megaview II digital camera, by using Analy-SIS software (SIS, Münster, Germany). For the vesicle counts, we sampled 3 flies for each condition tested (dark/light, age, genotype), and counted 15 terminals from each fly. For the microtubule counts, we counted profiles in either 28 terminals from 6 flies that were 3 days post-eclosion or in 26 terminals from 6 flies that were 9 days post-eclosion.
EM immunolabeling
Tissue samples prepared as previously reported (Meinertzhagen, 1996) were fixed for EM in a 3% formaldehyde, 0.25% glutaraldehyde mixture in 0.1M phosphate buffer (PB) (pH7.2) as reported (Yasuhara et al., 2000). Sections 50–60 nm thick were collected on nickel grids covered with 0.25% Pioloform dissolved in chloroform. The postembedding immunogold grid labeling protocol was as previously reported (Goode et al., 2004), but antigen unmasking and silver enhancement were omitted. Primary antibody, (affinity purified Ab 2074), a generous gift from Prof. Mani Ramaswami (Trinity College, Dublin, Ireland), was used at a 1:20 dilution. The Ab 2074 rabbit antiserum was raised against a purified recombinant fusion protein generated by fusing the maltose-binding protein (MBP) to a 66 kDa fragment of Drosophila dynamin lacking the N-terminal 241 amino acids (lacking the GTP-binding domain) (Estes et al., 1996). Specificity has been shown by Estes (1996) through western blot and labeling at the Drosophila neuromuscular junction. Secondary antibody (10 nm gold-conjugated goat anti-rabbit IgG (H+L):10, BB international) was used at a dilution of 1:20. Labeled grids were stained with aqueous uranyl acetate and viewed as above to detect the subcellular localization of gold particles.
Results
To characterize how UAS-shits1 overexpression in R1–R6 photoreceptors influences their synaptic output, we quantified neurotransmission and light-evoked photoreceptor response, while controlling light exposure, temperature, age and eye color. We characterized how these factors affect the photoreceptors’ output to their postsynaptic interneurons in the lamina (Hardie, 1989; Gengs et al., 2002; Zheng et al., 2006; Pantazis et al., 2008). We investigated how the light-evoked response influences the slow component of the ERG, and how this in turn affects interpretation of neurotransmission dynamics. Finally, we linked the physiological changes, induced by UAS-shits1, to the ultrastructure of photoreceptors.
Rh1-shits1 photoreceptor output depends on light history at 19°C
To investigate how light exposure affects neurotransmission at permissive temperatures (19°C), we examined Drosophila which overexpress shits1 in R1–R6. To overexpress shits1 we used Rh1-Gal4, which uses the ninaE promoter (O’Tousa et al., 1985). We refer to their targeted expression of shits1 as Rh1-shits1. We first examined Rh1-shits1 photoreceptors dark-adapted for 30 min, followed by exposure to intense white light for 30 min (Figs. 1A; S1), termed light-adaptation in our protocol. After each adaptation, we recorded ERGs from wild-type, Rh1-shits1 and white Rh1-shits1 flies to volleys of 700-ms-long pulses of intense white light (see Methods for light stimulus) (Fig. 1B). The size of the “On”- and “Off”-transients, which reflect transmission to the lamina, were then compared after dark- and light-adaptation at 3, 6 and 9 days post-eclosion (Figs. 1C–D).
(1) Dark-adaptation reduces synaptic transmission
At a permissive temperature of 19°C, Rh1-shits1 flies were predicted to exhibit ERG responses comparable to wild-type (Kitamoto, 2001). Instead, we found that 30 min of dark-adaptation strongly suppressed neurotransmission of Rh1-shits1 flies (Figs. 1C–D), in which transients were either missing or very small (<10% of the wild-type). Dark-adaptation affected ERG transients even when a different Gal4-line was used, as seen in +; GMR-Gal4/+; UAS-shits1/+ flies (Fig. S2; GMR expressed in R1–R8). After dark-adaptation, the ERGs of shits1 mutants also displayed reduced transient amplitudes (Fig. S2).
(2) Increased age also reduces synaptic transmission
The impairment in transmission after dark-adaptation worsened with age, seen as a progressive decrease in transient size between 3 and 9 days post-eclosion. Dark-adaptation affected white Rh1-shits1 photoreceptors most severely (Figs. 1C–D). Thus, although all tested flies overexpressing UAS-shits1 displayed a similar phenotype, the level of impairment in their synaptic transmission depended on the eye color and age.
(3) Light-adaptation aids synaptic functions
After dark-adaptation, subsequent light helped to restore synaptic function in Rh1-shits1 photoreceptors, seen as the partial return of ERG transients (Figs. 1C–D). Nevertheless, recovery in transmission appeared to be absent at 9 days post-eclosion, revealing age-associated impairment. These findings suggest that at 19°C: (i) dark-adaptation of Rh1-shits1 disrupts the functionality of dynamin-dependent endocytosis that is required to maintain normal synaptic throughput; (ii) the underlying mechanisms for the decline in their transmission are similar in all the tested genotypes; and (iii) light-adaptation partially reverses this phenotype, but only at 3 days post-eclosion, not in older flies.
Temperature shift in light-adapted Rh1-shits1 photoreceptors
Insofar as the transmission defects seen after dark-adaptation in Rh1-shits1 photoreceptors were alleviated by a brief exposure to light, prolonged light-adaptation should further improve synaptic transmission. To test this hypothesis and investigate whether synaptic transmission in photoreceptors that overexpress UAS-shits1 could be blocked by warming, as shown previously (Kitamoto, 2001), flies were exposed to a temperature shift as they were light-adapted (Fig. 2A). We exposed 3- and 6-day post-eclosion wild-type and white Rh1-shits1 (previously kept under bright light for 3 h), to a 30 min period of adaptation to intense white-light at 19°C followed by 10 min of light at 31°C. The ability to support synaptic transmission was examined before (19°C) and after warming (31°C) by turning off the adapting light momentarily before delivering 700 ms pulses of intense white-light (Figs. 2B and S1 B).
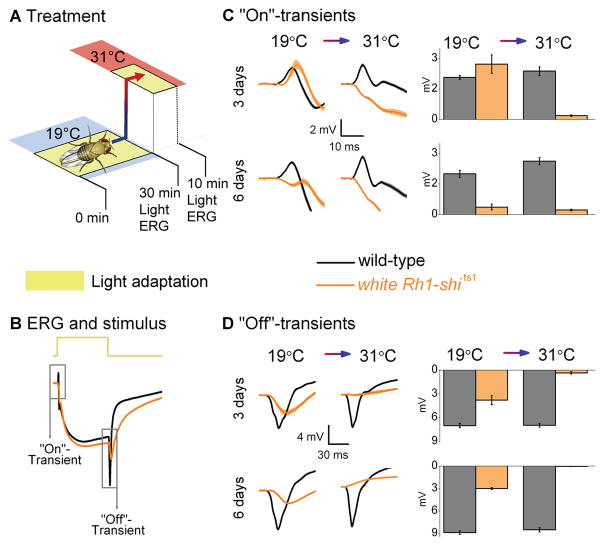
Figure 2.
Temperature shift in light-adapted Rh1-shits1 photoreceptors. (A) Light-adaptation (30 min) at 19°C before light-adaptation (10 min) at 31°C. (B) ERGs were recorded (700 ms stimulus) after each adaptation. Traces are from light-adapted flies at 19°C and 6 days post-eclosion. The amplitude of (C) “On”- and (D) “Off”-transients quantified for wild-type (black) and white Rh1-shi ts1 (orange). Mean ± SEM shown in traces and histograms with n = 6 for each group. Age refers to days post-eclosion.
(1) Prolonged light-adaptation boosts the lamina ERG transients of 3 days post-eclosion photoreceptors
In contrast to the synaptic inactivity of their dark-adapted counterparts (cf. Figs. 1C–D; Dark), after prolonged light-adaptation at 19°C, the “On”-transient of 3 days post-eclosion white Rh1-shits1 photoreceptors appeared slightly delayed and larger in amplitude than those of the wild-type (Figs. 2C–D; 19°C). Nevertheless, even without dark-adaptation, white Rh1-shits1 neurotransmission deteriorated with age. At 6 days post-eclosion, the lamina transients were severely reduced and delayed, resembling the recovering “On”- and “Off”-transients of 3-day post-eclosion dark-adapted flies (cf. Figs. 1C–D; Dark).
(2) Restrictive temperatures extinguish synaptic transmission in light-adapted photoreceptors
As shown previously, warming flies to 31°C appeared to block synaptic transmission from white Rh1-shits1 photoreceptors (Figs. 2C–D; 31°C). These findings confirm that UAS-shits1 silences ERG transients at restrictive temperatures (Kitamoto, 2001).
Neurotransmission from transgenic controls
The neurotransmission reduction seen in transgenic photoreceptors may stem from the independent actions of either the Gal4 or UAS insertions. Thus, as controls, we next tested flies homozygous for a single insertion. We conditioned the wild-type and transgenic flies using increased age, darkness and warming, as each of these factors on their own was sufficient to alter severely the synaptic output of Rh1-shits1 photoreceptors (Fig. 3A). Transgenic 6-day post-eclosion control flies were dark-adapted for 30 min at 19°C, ERG tested and rapidly warmed to 31°C, dark-adapted for another 10 min, and retested.
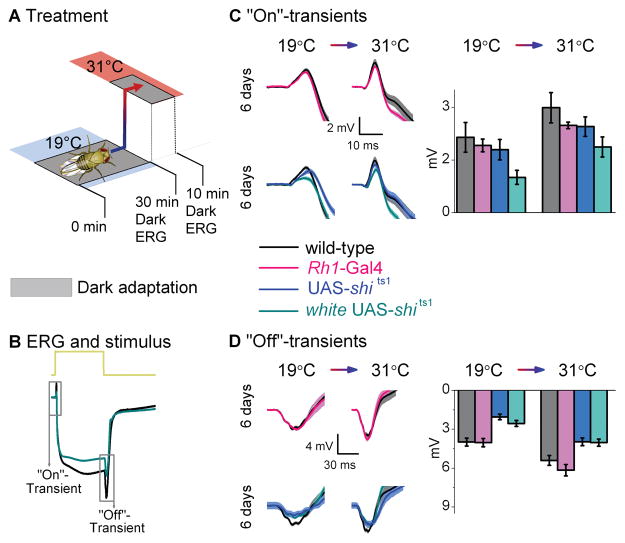
Figure 3.
Neurotransmission from transgenic controls. (A) Dark-adaptation (30 min) at 19°C before dark-adaptation (10 min) at 31°C. (B) ERGs were recorded (700 ms stimulus) after each adaptation. Traces are from dark-adapted flies at 19°C and 6 days post-eclosion. The amplitude of (C) “On”- and (D) “Off”-transients quantified for wild-type (black) and homozygous transgenic controls; Rh1-Gal4 (pink), UAS-shi ts1 (dark blue) and white UAS-shi ts1 (blue-green). Mean ± SEM shown in traces and histograms with n = 6 for each group. Age refers to days post-eclosion.
Despite these conditions, “On”-transients were similar, if not identical, to wild-type transients (Fig. 3C). Therefore, none of the insertions acting on their own impaired synaptic transmission. The only deviation was from white UAS-shits1 6 days post-eclosion, which after dark-adaptation at 19°C had “On”-transients reduced by ~50% (0.93 ± 0.21 vs. 2.04 ± 0.20 of wild-type; mean ± SEM; p = 0.003, Bonferroni-test; n = 6). At 31°C, white UAS-shits1 photoreceptor responses approached the performance of wild-type. These findings highlight that white has a deleterious effect on photoreceptor synaptic output (cf. Fig. 1C), an effect likely attributable to the reduced histamine in white mutants (Borycz et al., 2008). The “On”-transient delay in UAS-shits1 flies (Fig. 3C), suggests a small leakage effect from the UAS-shits1 insertion.
Assessment of Gal4 and UAS insertions
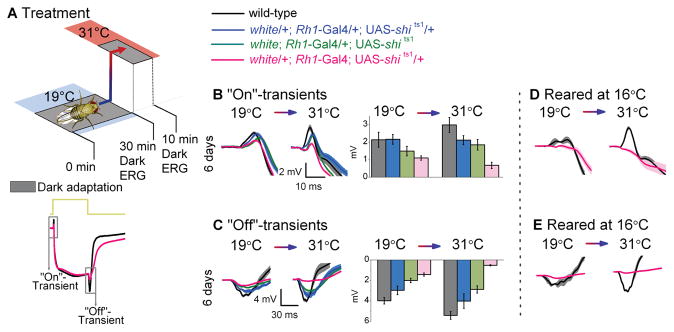
To reduce the effects of the partial activation of UAS-shits1 at low temperatures while blocking neurotransmission at high temperatures, we varied the number of Rh1-Gal4 and UAS-shits1 insertions (Fig. 4). Altering the number of insertions or the temperature at which the flies were raised, did not yield a workable compromise to block transmission at >31°C while keeping photoreceptors unaffected at 19°C.
Figure 4.
Assessment of Gal4 and UAS insertions. (A) Dark-adaptation (30 min) at 19°C before dark-adaptation (10 min) at 31°C. ERGs were recorded (700 ms stimulus) after each adaptation. Traces are from dark-adapted flies at 19°C and 6 days post-eclosion. Genotypes range in number of Rh1-Gal4 and UAS-shi ts1 insertions. The amplitude of (B) “On”-and (C) “Off”-transients quantified for wild-type (black), white/+; Rh1-Gal4/+; UAS-shi ts1/+ (blue), white; Rh1-Gal4/+; UAS-shi ts1 (green), and white/+; Rh1-Gal4; UAS-shi ts1/+ (pink). (D) “On”- and (E) “Off”-transients from white/+; Rh1-Gal4; UAS-shi ts1/+ and wild-type flies raised at 16°C up to 3 days post-eclosion and thereafter shifted to 18°C. Mean ± SEM shown in traces and histograms with n = 6 for each group. Age refers to days post-eclosion.
Rh1-shits1 photoreceptors show decelerated voltage responses to light
The ERG’s slow component echos mostly mass-activity of photoreceptors (Heisenberg, 1971), attenuated by resistance barriers in the tissue (Pantazis et al., 2008). Thus, observing the slow component allowed us to assess the general health of phototransduction (Wang and Montell, 2007; Hardie and Postma, 2008; Pantazis et al., 2008) under each stimulus condition, tested in the prior light-adaptation experiment (cf. Fig. 1A).
(1) Dark-adaptation extends the time course of the Rh1-shits1 ERG slow component
To clarify if UAS-shits1 overexpression had affected the time course of the photoreceptor response, the ERGs recorded at 19°C (cf. Fig. 1) were normalized. After dark-adaptation, we found a slower return to baseline, in both Rh1-shits1 and white Rh1-shits1 flies (Fig. 5A) but after light-adaptation the return to baseline appeared similar to wild-type (Fig. 5B). The slow return to baseline has been previously reported for homozygous shits1 mutants, which had been shifted to high temperatures (Kelly and Suzuki, 1974). The amplitude of the slow component in an ERG appeared to be smaller in Rh1-shits1 flies after dark-adaptation at 19°C (Fig. S 3) but the exact photoreceptor contribution to the ERG is unknown. Consequently, to examine how well changes in the slow ERG component could be attributed to changes in phototransduction, we used intracellular recordings.
Figure 5.
Rh1-shits1 photoreceptors show decelerated voltage responses to light. (A) Mean normalized ERGs (n = 6) to 700 ms stimulus, taken from Figs. 1 and 3 after dark-adaptation and (B) after light-adaptation. Wild-type is plotted for young (grey) and older (black) flies. white Rh1-shi ts1 (orange) and Rh1-shi ts1 (red) at 3 and 6 days post-eclosion, respectively, have been plotted together. Similarly, 6 and 9 days post-eclosion are shown together. Wild-type (black) and homozygous transgenic controls; Rh1-Gal4 (pink), UAS-shi ts1 (dark blue) and white UAS-shi ts1 (blue-green) have been plotted together (bottom). Arrows indicate differences in response waveforms between overexpressed UAS-shi ts1 and wild-type. Age refers to days post-eclosion. (C) Intracellular recordings of 3 days post-eclosion wild-type (black) and Rh1-shi ts1 (red) photoreceptors. Mean ± SEM shown in traces (n = 9). When Rh1-shi ts1 displayed the same light response amplitude (red dotted line) as wild-type, the dynamics were as slow as the rest of the cells. The histamine mutant hdcJK910 (green) does not show such delay in its response. (D) Representative intracellular recordings of wild-type (black) and Rh1-shi ts1 (red) for a 700 ms light stimulus.
(2) Rh1-shits1 exhibit slow photoreceptor voltage responses shown by intracellular recordings
We carried out single-cell intracellular recordings from dark-adapted 3-day post-eclosion Rh1-shits1 and wild-type flies at 19°C by opening a small “window” in the cornea (Fig. S 4). Voltage responses to short light pulses (10 ms) from Rh1-shits1 photoreceptors were slower and smaller than wild-type (Fig. 5C). These differences could have arisen from a slowed phototransduction sequence, from reduced feed-forward and feed-back synaptic communication between photoreceptors and visual interneurons (Zheng et al., 2006; Zheng et al., 2009), or from both.
To test the impact of UAS-shits1 on phototransduction we compared the intracellular responses of Rh1-shits1 photoreceptors with those of blind hdcJK910 (hdc) mutants. Mutant hdc flies cannot produce histamine (Burg et al., 1993). These flies generate an ERG response purely through phototransduction, with responses that changed voltage at a rate similar to that of wild-type photoreceptors (Fig. 5C). The delayed and much slower waveforms of Rh1-shits1 photoreceptors could not arise from synaptic silence, but instead must have resulted from slowed phototransduction. Thus, UAS-shits1 overexpression not only compromises the synaptic output from photoreceptors (cf. Fig. 1), but it also decelerates the phototransduction in their cell bodies (Fig. 5C).
Slow photoreceptors evoke slow LMC responses (van Hateren, 1992; Juusola et al., 1995), so decelerated phototransduction must be partly responsible for the sluggish synaptic output of Rh1-shits1 photoreceptors. To clarify this effect, we recorded intracellular responses of Rh1-shits1 and wild-type photoreceptors to a 700 ms light pulse and compared these waveforms to our ERG results. The delay in the response termination, observed intracellularly (Fig. 5D), was small when compared with the decaying ERGs (Fig. 5A). Hence, the latter could not reflect a slowing of the individual photoreceptor responses.
This sluggish transmission, observed as the decrease of “On”- transients, could arise from: weak voltage and calcium changes, which fail to drive exocytosis normally (Juusola et al., 1996), enlarged vesicles that lead to slower emptying (Juusola et al., 1995), reduced vesicle availability or proximity to the release site, or direct alterations to vesicle transport processes. We addressed some of these alternatives by ultrastructural examination.
Overexpression of UAS-shits1 changes photoreceptor morphology at 19°C
To examine the ultrastructure corresponding to the conditions under which ERG recordings were made (Fig. 1A), we first exposed 3- and 9-day post-eclosion wild-type and Rh1-shits1 to either 30 min of dark-adaptation, or to 30 min of dark-adaptation followed by 30 min of light-adaptation, at 18 ± 1°C.
(1) Photoreceptor cell body and rhabdomere shape are altered
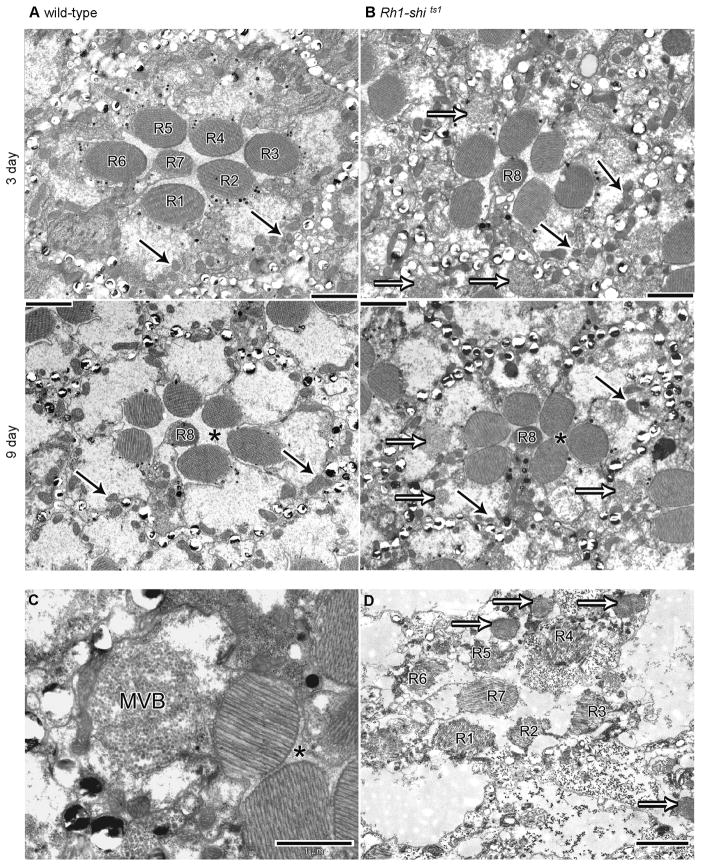
One striking phenotype observed in 3 days post-eclosion Rh1-shits1 was the presence of multivesicular bodies (MVBs) in the photoreceptor cell bodies (Figs. 6A–B). By day 9, the photoreceptors were enlarged to the extent that their rhabdomeres touched. Additionally, MVBs were found in almost every photoreceptor (Fig. 6C). Further, Rh1-shits1 flies raised at 25°C as a control treatment showed apoptotic R1–R6, as previously reported (Acharya et al., 2003), and contained MVBs and distorted rhabdomeres. In contrast, R7/R8 had well formed and positioned rhabdomeres (Fig. 6D), consistent with their being spared from the Rh1-driven overexpression of shits1 that occurred exclusively in R1–R6. Given physiological changes in synaptic transmission in Rh1-shits1, we searched for morphological changes in their photoreceptor terminals.
Figure 6.
Overexpression of UAS-shits1 changes retinal morphology at 19°C. (A) EM cross sections of 3 and 9 days post-eclosion wild-type and (B) Rh1-shi ts1 retinas. Scale = 2 μm. (C) Multi-vesicular body (MVB) present in Rh1-shi ts1 photoreceptor. Scale = 1 μm. (D) Rh1-shi ts1 retina, raised at 25°C, with photoreceptor R7 in a healthy state, demonstrating that Rh1-Gal4 overexpresses UAS-shi ts1 only in photoreceptors R1–R6. Scale = 2 μm. Mitochondria (solid arrows), Multi-vesicular bodies (open arrows), inter-photoreceptor space (asterisk).
(2) Lamina axons of R1–R6 contain coated microtubules and vacuolated mitochondria
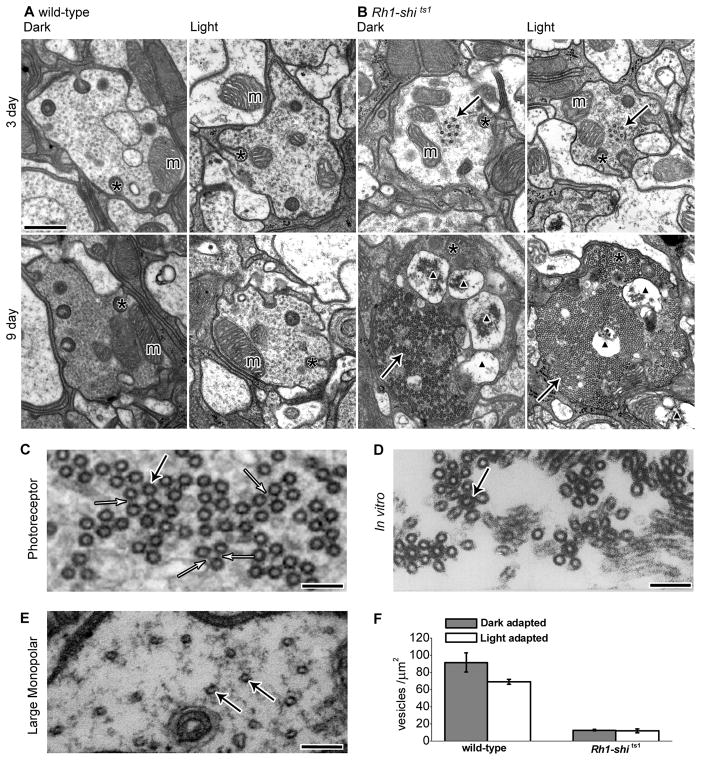
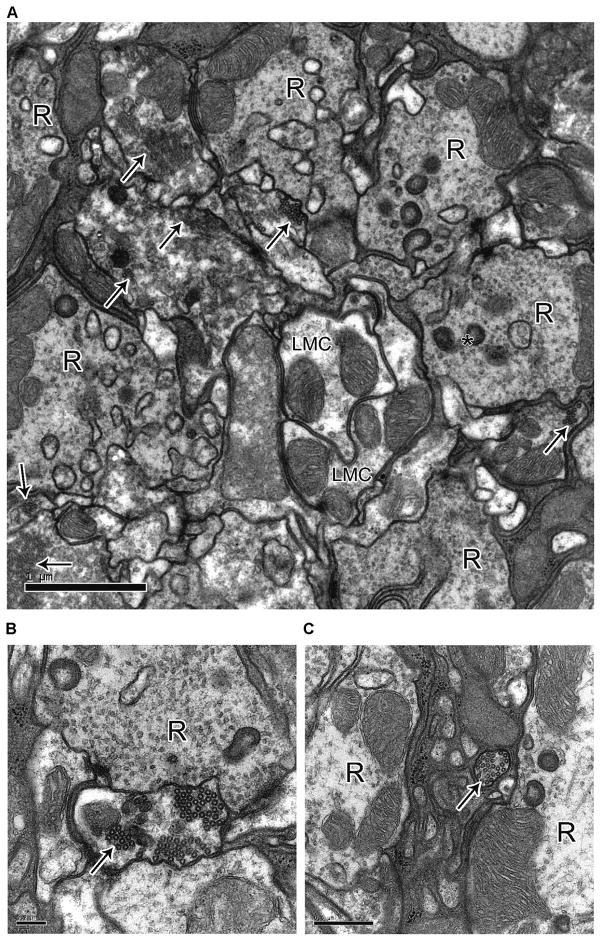
Wild-type photoreceptor terminals had well-defined membranes and mitochondria (Fig. 7A). Conversely, Rh1-shits1 terminals contained novel electron-dense profiles (Fig. 7B). These profiles matched those found previously after overexpressing UAS-shits1 with GMR-Gal4 (Sun, 1998). In agreement with Sun (1998), we confirmed that these profiles were bundles of coated microtubules, based on the following criteria. When cut in longitudinal plane, each coated microtubule formed an elongate profile (Fig. S 5). The diameter of the coated microtubules was 38.92 nm ± 0.17 (mean ± SEM) and the profiles appeared dark, with a surrounding electron-dense coat (Fig. 7C). R1–R6 terminals from flies at 9 days post-eclosion showed bundles of cross-linked microtubules akin to those reported from in vitro experiments (Shpetner and Vallee, 1989) (Fig. 7D). In contrast, un-coated microtubules seen in LMCs (Fig. 7E), had an average diameter of 24.19 nm ± 0.40 (mean ± SEM; n = 27), as seen elsewhere (Friedman, 1971). The bundling of microtubules became extensive with age. The number of microtubules per μm2 in the terminals of Rh1-shi ts1 in 3 days post-eclosion was 15.44 ± 4.07 and in 9 days post-eclosion was 155.04 ± 41.33 (n=28 or 26 terminals respectively). Coated microtubules were not observed in wild-type terminals. In many cases the coated microtubules occupied the entire profile of 9-days post-eclosion photoreceptor terminals (cf. Fig. 7B; Fig. S5). In addition, mitochondrial profiles in 9-days post-eclosion terminals were enlarged, vacuolated, and appeared typical of apoptotic neurons (cf. Fig. 7B).
Figure 7.
Overexpression of UAS-shits1 changes photoreceptor terminal morphology at 19°C. (A) EM cross sections of 3 and 9 days post-eclosion wild-type and (B) Rh1-shi ts1 terminals, reared at 18°C. Flies were exposed to 30 min of darkness (Dark) or to 30 min of darkness followed by 30 min of light (Light) at 18°C. Scale = 500 nm. Mitochondria (m), coated microtubules (solid arrows), capitate projections (asterisk), apoptotic mitochondria profiles (black triangle). (C) High magnification image of coated microtubules with cross-bridges (open arrow). (D) in vitro coated microtubules, reprinted from Cell, 59(3), Shpetner & Vallee, Identification of dynamin, a novel mechanochemical enzyme that mediates interactions between microtubules, 421–432, Copyright 1989, with permission from Elsevier and the authors. (E) Image of LMC wild-type microtubules. C–E Scale = 100 nm, microtubules (solid arrow). (F) Number of vesicles per μm2 for 3 days post-eclosion wild-type (dark- adapted; 91.40 ± 11.02, light-adapted; 68.86 ± 3.07) and Rh1-shi ts1 terminals (dark- adapted; 12.70 ± 0.78, light-adapted; 11.70 ± 2.26). Mean ± SEM given.
In contrast to mitochondria in the terminals of Rh1-shits1 photoreceptors at 9 days post-eclosion, the cell bodies had healthy mitochondria (Fig. 6D). We take this observation to indicate that widespread microtubule bundling in the axon interferes with the normal function of a neuron, preventing the proper transport of organelles to the synaptic terminal and consequently perhaps also affecting synaptic transmission. It appears that to keep axonal transport functional, Rh1-shits1 photoreceptors progressively accrete additional microtubules, because these clearly outnumber those seen in control terminals (Fig. 7A–B).
(3) Rh1-shits1 photoreceptor terminals contain fewer vesicles than wild-type
In Rh1-shits1 terminals 3 days post-eclosion, there were significantly fewer vesicle profiles than in wild-type, but their numbers did not differ between dark- and light-adaptation (Fig. 7F). The dense packing of microtubule bundles in most Rh1-shits1 terminals at 9 days post-eclosion left little space for synaptic vesicles, which consequently were not counted.
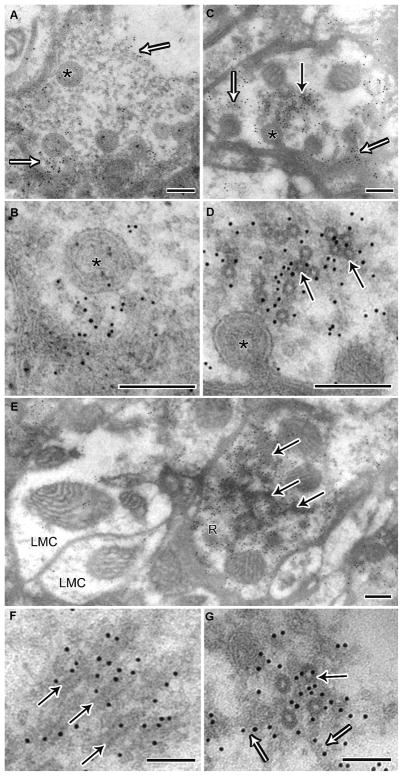
Immunogold labeling confirms that the microtubule coat is dynamin
Given that dynamin behaves as a microtubule associated protein (MAP) in vitro (Shpetner and Vallee, 1989), we investigated whether the electron-dense microtubule coating was overexpressed dynamin (Figs. 8A–G). We directed an antibody against dynamin, using a post-embedding immunogold method. Gold particles were distributed near the membranes and capitate projections of wild-type and Rh1-shits1 terminals (Figs. 8A–D). In addition, gold particles tagged the cytoplasmic space close to coated microtubules in many Rh1-shits1 terminals (Figs. 8C–G), in a pattern not observed in wild-type. Adjacent LMC profiles, which acted as an internal control, lacked such cytoplasmic labeling, confirming the specificity of gold labeling to sites with dynamin overexpression (Fig. 8E). At high magnification, gold particles were observed adjacent to coated microtubules and near the filaments that extended from microtubules (Fig. 8G).
Figure 8.
Immunogold labeling confirms that the microtubule coat is dynamin. (A) Wild-type terminal showing 10 nm gold immuno-labeling in proximity to the membrane, indicated by open arrows and capitate projections (asterisk). (B) High magnification of A. (C) Photoreceptor terminal from Rh1-shi ts1, which in addition to labeling near capitate projections (open arrows), has labeling in the cytoplasm near coated microtubules (solid arrow). (D) High magnification of C. (E) Terminal from Rh1-shi ts1 showing labeling of oblique coated microtubules in the cytoplasm. Note that adjacent LMC profiles do not have cytoplasmic labeling, thus confirming the specificity of gold labeling to overexpressed shits1 dynamin. A–E Scale = 200 nm. (F) High magnification of labeled oblique and (G) cross section coated microtubules (solid arrow) showing that labeling extends to filaments adjacent (open arrow). F–G Scale = 100 nm.
Microtubule coat is present in other neurons when UAS-shits1 is overexpressed
To test if the interaction between overexpressed UAS-shits1 and microtubules is exclusive to photoreceptor cells, we targeted UAS-shits1 to a selection of lamina cells with the driver MJ85b-Gal4 (Joiner and Griffith, 1997). TEM sectioning revealed extensive microtubule bundling in L3 and other unidentified neurons (tentatively amacrine or T1 profiles; Fig. 9AB), which extend thin spines between photoreceptor cells (Fig. 9C).
Figure 9.
Microtubule coat is present in other neurons when UAS-shits1 is overexpressed. (A) MJ85b-shi ts1 lamina cartridge showing coated microtubules (arrows) in lamina cells and not in photoreceptor terminals (R). Scale = 1 μm. (B) Close up of coated microtubules in a lamina cell. Scale = 0.2 μm. (C) Lamina cell spine packed with coated microtubules. Scale = 0.5 μm.
Discussion
We have shown that, at 19°C, transgenic photoreceptors that overexpress UAS-shits1 display anomalous synaptic transmission when dark-adapted or aged. Even at previously reported permissive temperatures (Kitamoto, 2001), overexpressing UAS-shits1 in photoreceptors causes a significant deceleration in phototransduction, a significant reduction of neurotransmitter release and severe alterations in cell shape when compared with wild-type. At 19°C, the induced morphological changes in Rh1-shits1 photoreceptors included: widespread vacuolation of mitochondrial profiles, significantly reduced numbers of vesicles, and photoreceptors filled with dynamin-coated microtubules. The physiological and morphological changes shown here seem to extend to changes in behavior, insofar as Keller (2002) reported a significant drop in visual performance in flies overexpressing UAS-shits1 at 19°C in photoreceptors R1–R8 (GMR-Gal4).
When we replicated the conditions used by Kitamoto (2001) and recorded ERGs from light-adapted flies at 3 days post-eclosion, we also obtained transientless ERGs at 31°C. However, at 19°C dark-adaptation and age made significant changes to phototransduction and neurotransmission not found by Kitamoto (2001). We also showed that the onset of these phenotypic changes is accelerated in flies containing the white null mutation (w1118). Thus caution is needed when using white mutant background flies (Borycz et al., 2008). In an attempt to yield a complete blockade of endocytosis at the restrictive temperature while retaining wild-type responses at lower temperatures, we decreased the number of transgenic insertions. We failed to achieve such a phenotype, possibly because dynamin has an intrinsic thermo-liable nature (Grant et al., 1998) rather than a discrete switch mechanism.
Mutant dynamin overexpression decelerates phototransduction at 19°C
Intracellular recordings from photoreceptors showed that phototransduction was slowed by UAS-shits1 overexpression after dark-adaptation. This finding suggests that UAS-shits1 overexpression might compete with or hinder endocytotic or translocation-dependent reactions in phototransduction, such as Rh1, trpl, Arr1 or Arr2 cycles for the normal initiation and termination of light-evoked responses (Satoh and Ready, 2005; Frechter and Minke, 2006; Hardie and Postma, 2008). Decelerated phototransduction would in turn slow down the rate of transmitter release (van Hateren, 1992; Juusola et al., 1995). After dark-adaptation, the return to base line of the Rh1-shits1 ERG was slowed at 19°C, as also documented in shits1 mutants shifted to 28°C (Kelly and Suzuki, 1974). The reasons for such a delay are elusive. Possibly the altered neurotransmission resulting in small and delayed “Off”-transients integrates with the slow component of the waveform, changing the shape of the ERG. Alternatively, overexpressing UAS-shits1 could in some way alter the metabolic homeostasis in the retina. Increased extracellular K+ concentration depolarizes slow responding pigment cells in the bee retina (Coles and Orkand, 1983), for example, so that increased K+ concentrations may contribute in the ERG to a delayed return to baseline.
Mutant dynamin overexpression changes photoreceptor shape
UAS-shits1 overexpression at 19°C induced enlarged photoreceptor cell bodies, noticeable by their reduced inter-rhabdomeric space, which resemble changes previously reported in HtTA cells induced to express the active dynamin mutant form ele1 for 24 h (Damke et al., 1994). Furthermore, large numbers of multivesicular bodies were observed, a phenotype reported for a mutant with defective endocytotic machinery and neurotransmitter recycling (Dermaut et al., 2005). For many published behavioral assays, flies overexpressing UAS-shits1 are commonly reared at a reported permissive temperature of 25°C. However, when UAS-shits1 is overexpressed in photoreceptors, and flies are reared at 23°C (Acharya et al., 2003) or at 25°C (as in this study), the effects of dynamin overexpression are cell lethal, and result in apoptosis.
Dynamin coats and cross-links microtubules
The action of shibirets1 in photoreceptors has been widely interpreted in terms of its effects on endocytosis (Fabian-Fine et al., 2003). While shibire’s role in endocytosis (Kosaka and Ikeda, 1983) has overshadowed dynamin’s other actions in Drosophila, dynamin was in fact initially discovered as a MAP (Vallee, 1992). Thus, the interaction between dynamin and microtubules has long been known to occur in vitro (Shpetner and Vallee, 1989), but to our knowledge dynamin has not previously been shown to coat and cross-link microtubules in living cells. The microtubules we find decorated with dynamin are a hallmark of UAS-shits1 overexpression in the terminals of Rh1-shits1 photoreceptors. We also confirmed that shibire can induce microtubule bundling in other lamina neurons.
Cross-linked microtubules, which aggregate into bundles, are a distinctive feature of many neurons; for example in the “tubular body”, a dendritic specialization necessary for transduction in the wild-type campaniform mechanoreceptors of Drosophila (Toh, 1985). Initial axonal segments of multipolar neurons (Palay et al., 1968; Westrum and Gray, 1976) are likewise recognized by the presence of cross-linked microtubules. The protein causing this structural phenotype in mammalian neurons has not been reported.
Our results demonstrate that shibire can indeed act as a MAP, and induce microtubule bundling in vivo. While the bundling of microtubules seen in the axon and terminals of Rh1-shits1 photoreceptors incorporates a dynamin coat, and thus appears to result from shibirets1 overexpression, the exact wild-type location of the dynamin-microtubule interaction is unknown. It is possible that only cells that require most cytoskeletal support, or place particular demands on axoplasmic transport, translocate MAPs in sufficient concentration to bundle their microtubules in specific locations. Even when dynamin is expressed at high concentrations, the presence of GTP may prevent dynamin self assembly around microtubules, as shown in membrane-bead preparations (Pucadyil and Schmid, 2008). It is feasible that the reduced GTPase activity induced by a single nucleotide change in the GTP-binding domain of shits1 (Grant et al., 1998), mimics low GTP availability. If this were the case, the self assembly of dynamin and cross-linking of microtubules observed in Rh1-shits1 terminals is in accordance with the suggestion that without GTP, self assembled dynamin is in a “kinetically trapped state” (Pucadyil and Schmid, 2008). Furthermore, the shibire gene has seven splice variants (Chen et al., 1991) the intracellular location of which may not be uniform. Splice variation could be important, because another MAP protein, tau (MAPt), causes microtubule bundling in vitro, but only when specific splice variants are used and only at high concentrations (Scott et al., 1992).
Dynamin-microtubule interactions may be involved in functions other than microtubule bundling or endocytosis, such as sorting of vesicular cargo. In mammals, dynamin 2 associates with the Golgi apparatus (Maier et al., 1996). More recently, dynamin 2 has been shown to be necessary for maintaining dynamic instability of microtubules and to form mature Golgi complexes (Tanabe and Takei, 2009). Given that Drosophila has a single dynamin isoform, one of the shibire splice variants could take on a similar role to that of dynamin 2. It is conceivable that the microtubule interaction here described for shibirets1 may be crucial for dynamin-like proteins (DLPs). DLPs, which possess a GTPase domain and can self assemble but lack the pleckstrin homology domain and the proline rich domain, are involved in a multitude of processes related to membrane shape (Hinshaw, 2000; Praefcke and McMahon, 2004). For example, DLP1 is necessary for mitochondrial fission (Yu et al., 2005) and has also been shown to localize to vesicles that coalign with microtubules and the endoplasmic reticulum (Yoon et al., 1998).
Even though the endocytotic function of the shibirets1 homozygous mutant is altered at 19°C, as shown by the reduced number of its R1–R6 vesicles (MacIntosh, 1996), it must be emphasized that bundled microtubules have not been found in the photoreceptor terminals of the shibirets1 mutant (MacIntosh, 1996). Additionally, Zheng et al., (2006) carried out intracellular recordings of shibirets1 mutant photoreceptors and reported wild-type like responses at 19°C and reversible silencing of neurotransmission at 29°C. We therefore suggest that it is the high levels of dynamin overexpression, in common with its mutant structure, which together overcome the normal function of wild-type dynamin, and caution against uncritical use of the UAS-shits1 transgene to interrupt synaptic function reversibly. In graded neurons with high levels of neurotransmitter release (Juusola et al., 1995; Uusitalo et al., 1995b; Juusola et al., 1996), high levels of shibirets1 overexpression may be required to block neurotransmission but may potentially cause apoptosis. In comparison, low overexpression of shibirets1 in presynaptic spiking neurons may prevent action potential generation in postsynaptic cells without completely blocking the synapse. In either case, however, optimal synapse control may be difficult to achieve. Gal4 drivers are in fact notorious for the wide range of intensity in their expression between different lines; the remaining elements of the neural network, that lack shibirets1 overexpression, may moreover reroute and reshape neural communication to compensate for any altered function in the Ga4-shits1 expressing neuron (Vähäsöyrinki et al., 2006; Zheng et al., 2006; Nikolaev et al., 2009). Furthermore, it would be paramount to confirm, with a physiological diagnostic, that shibirets1 overexpression prevents adequate information flow. Finally, ceramidase overexpression prevents photoreceptor degeneration induced by shits1 (Acharya et al., 2003), providing one avenue to spare the UAS-shits1 generated defects in endocytosis at low temperatures.
We conclude that the use of UAS-shits1 in photoreceptors and lamina neurons of Drosophila at 19°C causes non-reversible phenotypes and confirms an additional action of dynamin in microtubule binding. Our results indicate that careful review on the toxicity and secondary effects caused by overexpressing mutant dynamin is needed.
Supplementary Material
Acknowledgments
We thank M Ramaswami for the anti-dynamin primary antibody, A Whitworth and W Marcotti for discussions; JA Borycz for help with immunolabeling; P Li and Z Lu and C Hill for assistance with EM; and RC Hardie, LC Griffith, E Buchner, and T Kitamoto for fly stocks. This work was funded by The University of Sheffield, Biotechnology and Biological Sciences Research Council (BBF0120711 and BBD0019001 to MJ), Gatsby Charitable Foundation (GAT2839 to MJ), the Royal Society (UF991042 to MJ) and the National Eye Institute of the NIH (EY-03592 to IAM).
Footnotes
Author contributions. PTGB, TJW and MJ conceived and designed the experiments. PTGB and TJW undertook the fly crossing. PTGB performed the immuno-EM, ERGs, intracellular experiments and analysis. PTGB and RK performed EM. MJ wrote the software for stimulation, acquisition, and analysis. MJ designed the experimental set-ups, and MJ and TW constructed them. PTGB, TJW, MJ and IAM co-wrote the paper.
References
- Acharya U, Patel S, Koundakjian E, Nagashima K, Han X, Acharya JK. Modulating sphingolipid biosynthetic pathway rescues photoreceptor degeneration. Science. 2003;299:1740–1743. doi: 10.1126/science.1080549. [DOI] [PubMed] [Google Scholar]
- Bähner M, Frechter S, Da Silva N, Minke B, Paulsen R, Huber A. Light-regulated subcellular translocation of Drosophila TRPL channels induces long-term adaptation and modifies the light-induced current. Neuron. 2002;34:83–93. doi: 10.1016/s0896-6273(02)00630-x. [DOI] [PubMed] [Google Scholar]
- Beramendi A, Peron S, Casanova G, Reggiani C, Cantera R. Neuromuscular junction in abdominal muscles of Drosophila melanogaster during adulthood and aging. Journal of Comparative Neurology. 2007;501:498–508. doi: 10.1002/cne.21253. [DOI] [PubMed] [Google Scholar]
- Borycz J, Borycz JA, Kubów A, Lloyd V, Meinertzhagen IA. Drosophila ABC transporter mutants white, brown and scarlet have altered contents and distribution of biogenic amines in the brain. Journal of Experimental Biology. 2008;211:3454–3466. doi: 10.1242/jeb.021162. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Burg MG, Sarthy PV, Koliantz G, Pak WL. Genetic and molecular identification of a Drosophila histidine-decarboxylase gene required in photoreceptor transmitter synthesis. Embo Journal. 1993;12:911–919. doi: 10.1002/j.1460-2075.1993.tb05732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DM, Stark WS. Effects of temperature on visual receptors in temperature-sensitive paralytic shibire (shits) mutants in Drosophila. Journal of Insect Physiology. 1993;39:385–392. [Google Scholar]
- Chen MS, Obar RA, Schroeder CC, Austin TW, Poodry CA, Wadsworth SC, Vallee RB. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature. 1991;351:583–586. doi: 10.1038/351583a0. [DOI] [PubMed] [Google Scholar]
- Chorna-Ornan I, Tzarfaty V, Ankri-Eliahoo G, Joel-Almagor T, Meyer NE, Huber A, Payre F, Minke B. Light-regulated interaction of Dmoesin with TRP and TRPL channels is required for maintenance of photoreceptors. J Cell Biol. 2005;171:143–152. doi: 10.1083/jcb.200503014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles JA, Orkand RK. Modification of potassium movement through the retina of the drone (Apis mellifera ♂) by glial uptake. Journal of Physiology. 1983;340:157–174. doi: 10.1113/jphysiol.1983.sp014756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombe PE. The large monopolar cells L1 and L2 are responsible for ERG transients in Drosophila. Journal of Comparative Physiology A Sensory Neural and Behavioral Physiology. 1986;159:655–665. [Google Scholar]
- Coombe PE, Heisenberg M. The structural brain mutant Vacuolar medulla of Drosophila melanogaster with specific behavioral defects and cell degeneration in the adult. Journal of Neurogenetics. 1986;3:135–158. doi: 10.3109/01677068609106845. [DOI] [PubMed] [Google Scholar]
- Cronin MA, Diao F, Tsunoda S. Light-dependent subcellular translocation of Gqα in Drosophila photoreceptors is facilitated by the photoreceptor-specific myosin III NINAC. J Cell Sci. 2004;117:4797–4806. doi: 10.1242/jcs.01371. [DOI] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado R, Maureira C, Oliva C, Kidokoro Y, Labarca P. Size of vesicle pools, rates of mobilization, and recycling at neuromuscular synapses of a Drosophila mutant, shibire. Neuron. 2000;28:941–953. doi: 10.1016/s0896-6273(00)00165-3. [DOI] [PubMed] [Google Scholar]
- Dermaut B, Norga KK, Kania A, Verstreken P, Pan H, Zhou Y, Callaerts P, Bellen HJ. Aberrant lysosomal carbohydrate storage accompanies endocytic defects and neurodegeneration in Drosophila benchwarmer. J Cell Biol. 2005;170:127–139. doi: 10.1083/jcb.200412001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JB. GAL4 System in Drosophila: a fly geneticist’s Swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- Estes PS, Roos J, vanderBliek A, Kelly RB, Krishnan KS, Ramaswami M. Traffic of dynamin within individual Drosophila synaptic boutons relative to compartment-specific markers. Journal of Neuroscience. 1996;16:5443–5456. doi: 10.1523/JNEUROSCI.16-17-05443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian-Fine R, Verstreken P, Hiesinger PR, Horne JA, Kostyleva R, Zhou Y, Bellen HJ, Meinertzhagen IA. Endophilin promotes a late step in endocytosis at glial invaginations in Drosophila photoreceptor terminals. J Neurosci. 2003;23:10732–10744. doi: 10.1523/JNEUROSCI.23-33-10732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frechter S, Minke B. Light-regulated translocation of signaling proteins in Drosophila photoreceptors. J Physiol Paris. 2006;99:133–139. doi: 10.1016/j.jphysparis.2005.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frechter S, Elia N, Tzarfaty V, Selinger Z, Minke B. Translocation of Gqα mediates long-term adaptation in Drosophila photoreceptors. J Neurosci. 2007;27:5571–5583. doi: 10.1523/JNEUROSCI.0310-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell. 1996;87:651–660. doi: 10.1016/s0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- Friedman MH. Arm-bearing microtubules associated with an unusual desmosome-like junction. Journal of Cell Biology. 1971;49:916. doi: 10.1083/jcb.49.3.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Takemura S-y, Ting C-Y, Huang S, Lu Z, Luan H, Rister J, Thum AS, Yang M, Hong S-T, Wang JW, Odenwald WF, White BH, Meinertzhagen IA, Lee C-H. The neural substrate of spectral preference in Drosophila. Neuron. 2008;60:328–342. doi: 10.1016/j.neuron.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengs CX, Leung HT, Skingsley DR, Iovchev MI, Yin Z, Semenov EP, Burg MG, Hardie RC, Pak WL. The target of Drosophila photoreceptor synaptic transmission is a histamine-gated chloride channel encoded by ort (hclA) J Biol Chem. 2002;277:42113–42120. doi: 10.1074/jbc.M207133200. [DOI] [PubMed] [Google Scholar]
- Goode NP, Shires M, Crellin DM, Khan TN, Mooney AF. Post-embedding double-labeling of antigen-retrieved ultrathin sections using a Silver enhancement-controlled sequential immunogold (SECSI) technique. J Histochem Cytochem. 2004;52:141–144. doi: 10.1177/002215540405200114. [DOI] [PubMed] [Google Scholar]
- Grant D, Unadkat S, Katzen A, Krishnan KS, Ramaswami M. Probable mechanisms underlying interallelic complementation and temperature-sensitivity of mutations at the shibire locus of Drosophila melanogaster. Genetics. 1998;149:1019–1030. doi: 10.1093/genetics/149.2.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Reddig K, Li HS. Prolonged Gq activity triggers fly rhodopsin endocytosis and degradation, and reduces photoreceptor sensitivity. Embo J. 2007;26:4966–4973. doi: 10.1038/sj.emboj.7601929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC. A histamine-activated chloride channel involved in neurotransmission at a photoreceptor synapse. Nature. 1989;339:704–706. doi: 10.1038/339704a0. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Postma M. Phototransduction In Microvillar Photoreceptors Of Drosophila And Other Invertebrates. In: Albright TD, Masland R, editors. The Senses: A Comprehensive Reference. Vol. 1. San Diego: Academic Press; 2008. pp. 77–130. Vision I. [Google Scholar]
- Heisenberg M. Separation of receptor and lamina potentials in the electroretinogram of normal and mutant Drosophila. J Exp Biol. 1971;55:85–100. doi: 10.1242/jeb.55.1.85. [DOI] [PubMed] [Google Scholar]
- Hinshaw JE. Dynamin and its role in membrane fission. Annual Review of Cell and Developmental Biology. 2000;16:483–519. doi: 10.1146/annurev.cellbio.16.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner MLA, Griffith LC. CaM kinase II and visual input modulate memory formation in the neuronal circuit controlling courtship conditioning. Journal of Neuroscience. 1997;17:9384–9391. doi: 10.1523/JNEUROSCI.17-23-09384.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juusola M, Hardie RC. Light adaptation in Drosophila photoreceptors: I. Response dynamics and signaling efficiency at 25°C. Journal of General Physiology. 2001;117:3–25. doi: 10.1085/jgp.117.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juusola M, Uusitalo RO, Weckström M. Transfer of graded potentials at the photoreceptor-interneuron synapse. Journal of General Physiology. 1995;105:117–148. doi: 10.1085/jgp.105.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juusola M, French AS, Uusitalo RO, Weckström M. Information processing by graded-potential transmission through tonically active synapses. Trends Neurosci. 1996;19:292–297. doi: 10.1016/S0166-2236(96)10028-X. [DOI] [PubMed] [Google Scholar]
- Keller A. Dr rer nat thesis Genetik und Neurobiologie. Würzburg: der Bayerischen Julius-Maximilians-Universität Würzburg; 2002. Genetic intervention in sensory systems of a fly. [Google Scholar]
- Kelly LE, Suzuki DT. The effects of increased temperature on electroretinograms of temperature-sensitive paralysis mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1974;71:4906–4909. doi: 10.1073/pnas.71.12.4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Ikeda K. Reversible blockage of membrane retrieval and endocytosis in the garland cell of the temperature-sensitive mutant of Drosophila melanogaster, shibirets1. J Cell Biol. 1983;97:499–507. doi: 10.1083/jcb.97.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosloff M, Elia N, Joel-Almagor T, Timberg R, Zars TD, Hyde DR, Minke B, Selinger Z. Regulation of light-dependent Gqα translocation and morphological changes in fly photoreceptors. Embo J. 2003;22:459–468. doi: 10.1093/emboj/cdg054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JM, Staveley BE. GAL4 causes developmental defects and apoptosis when expressed in the developing eye of Drosophila melanogaster. Genet Mol Res. 2003;2:43–47. [PubMed] [Google Scholar]
- Kumar JP, Ready DF. Rhodopsin plays an essential structural role in Drosophila photoreceptor development. Development. 1995;121:4359–4370. doi: 10.1242/dev.121.12.4359. [DOI] [PubMed] [Google Scholar]
- Luo L, Callaway E, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntosh S. Drosophila melanogaster mutant shibirets1. Dalhousie University; Halifax, Nova Scotia, Canada: 1996. Membrane recycling at photoreceptor synapses of the temperature-sensitive. [Google Scholar]
- Maier O, Knoblich M, Westermann P. Dynamin II binds to the trans-Golgi network. Biochemical and Biophysical Research Communications. 1996;223:229–233. doi: 10.1006/bbrc.1996.0876. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA. Ultrastructure and quantification of synapses in the insect nervous system. Journal of Neuroscience Methods. 1996;69:59–73. doi: 10.1016/S0165-0270(96)00021-0. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA, O’Neil SD. Synaptic organization of columnar elements in the lamina of the wild type in Drosophila melanogaster. Journal of Comparative Neurology. 1991;305:232–263. doi: 10.1002/cne.903050206. [DOI] [PubMed] [Google Scholar]
- Nikolaev A, Zheng L, Wardill TJ, O’Kane CJ, de Polavieja GG, Juusola M. Network adaptation improves temporal representation of naturalistic stimuli in Drosophila eye: II mechanisms. PLoS ONE. 2009;4:e4306. doi: 10.1371/journal.pone.0004306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, Sotelo C, Peters A, Orkand PM. Axon hillock and initial segment. Journal of Cell Biology. 1968;38:193. doi: 10.1083/jcb.38.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazis A, Segaran A, Liu CH, Nikolaev A, Rister J, Thum AS, Roeder T, Semenov E, Juusola M, Hardie RC. Distinct roles for two histamine receptors (hclA and hclB) at the Drosophila photoreceptor synapse. J Neurosci. 2008;28:7250–7259. doi: 10.1523/JNEUROSCI.1654-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praefcke GJK, McMahon HT. The dynamin superfamily: Universal membrane tubulation and fission molecules? Nature Reviews Molecular Cell Biology. 2004;5:133–147. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- Pucadyil TJ, Schmid SL. Real-time visualization of dynamin-catalyzed membrane fission and vesicle release. Cell. 2008;135:1263–1275. doi: 10.1016/j.cell.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rister J, Pauls D, Schnell B, Ting CY, Lee CH, Sinakevitch I, Morante J, Strausfeld NJ, Ito K, Heisenberg M. Dissection of the peripheral motion channel in the visual system of Drosophila melanogaster. Neuron. 2007;56:155–170. doi: 10.1016/j.neuron.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Satoh AK, Ready DF. Arrestin1 mediates light-dependent rhodopsin endocytosis and cell survival. Curr Biol. 2005;15:1722–1733. doi: 10.1016/j.cub.2005.08.064. [DOI] [PubMed] [Google Scholar]
- Scott CW, Klika AB, Lo MMS, Norris TE, Caputo CB. Tau protein induces bundling of microtubules In vitro: Comparison of different Tau isoforms and a Tau protein fragment. Journal of Neuroscience Research. 1992;33:19–29. doi: 10.1002/jnr.490330104. [DOI] [PubMed] [Google Scholar]
- Shpetner HS, Vallee RB. Identification of dynamin, a novel mechanochemical enzyme that mediates interactions between microtubules. Cell. 1989;59:421–432. doi: 10.1016/0092-8674(89)90027-5. [DOI] [PubMed] [Google Scholar]
- Stark WS, Sapp R, Schilly D. Rhabdomere turnover and rhodopsin cycle: maintenance of retinula cells in Drosophila melanogaster. J Neurocytol. 1988;17:499–509. doi: 10.1007/BF01189805. [DOI] [PubMed] [Google Scholar]
- Sun X. Long-term action of shibirets1 in photoreceptor terminals. Dalhousie University; Halifax, Nova Scotia, Canada: 1998. [Google Scholar]
- Tanabe K, Takei K. Dynamic instability of microtubules requires dynamin 2 and is impaired in a Charcot-Marie-Tooth mutant. Journal of Cell Biology. 2009;185:939–948. doi: 10.1083/jcb.200803153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh Y. Structure of campaniform sensilla on the haltere of Drosophila prepared by cryofixation. Journal of Ultrastructure Research. 1985;93:92–100. [Google Scholar]
- Uusitalo RO, Juusola M, Weckstrom M. Graded responses and spiking properties of identified first-order visual interneurons of the fly compound eye. J Neurophysiol. 1995a;73:1782–1792. doi: 10.1152/jn.1995.73.5.1782. [DOI] [PubMed] [Google Scholar]
- Uusitalo RO, Juusola M, Kouvalainen E, Weckstrom M. Tonic transmitter release in a graded potential synapse. J Neurophysiol. 1995b;74:470–473. doi: 10.1152/jn.1995.74.1.470. [DOI] [PubMed] [Google Scholar]
- Vähäsöyrinki M, Niven JE, Hardie RC, Weckström M, Juusola M. Robustness of neural coding in Drosophila photoreceptors in the absence of slow delayed rectifier K+ channels. Journal of Neuroscience. 2006;26:2652–2660. doi: 10.1523/JNEUROSCI.3316-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee RB. Dynamin: motor protein or regulatory GTPase. Journal of Muscle Research and Cell Motility. 1992;13:493–496. doi: 10.1007/BF01737991. [DOI] [PubMed] [Google Scholar]
- van der Bliek AM, Meyerowrtz EM. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature. 1991;351:411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]
- van Hateren JH. Theoretical predictions of spatiotemporal receptive fields of fly LMCs, and experimental validation. Journal of Comparative Physiology a-Sensory Neural and Behavioral Physiology. 1992;171:157–170. [Google Scholar]
- Wang T, Montell C. Phototransduction and retinal degeneration in Drosophila. Pflugers Arch. 2007;454:821–847. doi: 10.1007/s00424-007-0251-1. [DOI] [PubMed] [Google Scholar]
- Westrum LE, Gray EG. Microtubules and membrane specializations. Brain Research. 1976;105:547–550. doi: 10.1016/0006-8993(76)90601-6. [DOI] [PubMed] [Google Scholar]
- Yasuhara JC, Baumann O, Takeyasu K. Localization of Na/K-ATPase in developing and adult Drosophila melanogaster photoreceptor. Cell and Tissue Research. 2000;300:239–249. doi: 10.1007/s004410000195. [DOI] [PubMed] [Google Scholar]
- Yoon Y, Pitts KR, Dahan S, McNiven MA. A novel dynamin-like protein associates with cytoplasmic vesicles and tubules of the endoplasmic reticulum in mammalian cells. Journal of Cell Biology. 1998;140:779–793. doi: 10.1083/jcb.140.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TZ, Fox RJ, Burwell LS, Yoon Y. Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hFis1. Journal of Cell Science. 2005;118:4141–4151. doi: 10.1242/jcs.02537. [DOI] [PubMed] [Google Scholar]
- Zheng L, Polavieja GG, Wolfram V, Asyali MH, Hardie RC, Juusola M. Feedback network controls photoreceptor output at the layer of first visual synapses in Drosophila. Journal of General Physiology. 2006;127:495–510. doi: 10.1085/jgp.200509470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Nikolaev A, Wardill TJ, O’Kane CJ, de Polavieja GG, Juusola M. Network adaptation improves temporal representation of naturalistic stimuli in Drosophila eye: I dynamics. PLoS ONE. 2009;4:e4307. doi: 10.1371/journal.pone.0004307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.