Abstract
Background
Cannabis is among the most frequently used substance in United States (U.S.). Studies evaluating the association between cannabis use and inflammation in humans have been few and have not explored potential sex-dependent effects.
Objective
To examine the relationship between self-reported cannabis use and high-sensitivity C-reactive protein (hsCRP), Interleukin 6 (IL-6) and fibrinogen.
Methods
We used Wave 1 of the Population Assessment of Tobacco and Health (PATH) – a nationally representative sample of adults in the U.S. Weighted linear regression models were used to determine associations of self-reported cannabis use with natural log-transformed hs-CRP, IL-6 and fibrinogen adjusting for sociodemographic and psychosocial factors.
Results
Self-reported cannabis use, particularly cannabis use within the past 30 days, was associated with lower levels of each biomarker of systemic inflammation, although findings were imprecise. Specifically, in multivariable models, the associations between respondents who self-reported cannabis use in the past 30 days compared to never use was imprecise for hs-CRP (β= −0.15, 95% confidence interval (CI): −0.32, 0.00), IL-6 (β= - 0.02, 95% CI: −0.10, 0.05) and fibrinogen (β= - 0.01, 95% CI: −0.04, 0.02). We did not find that these associations differed significantly by sex.
Discussions
Data from this nationally representative study suggest potential anti-inflammatory effects of recent cannabis use. Additional studies that biologically measure the THC and CBD concentrations of the cannabis used and employ prospective and or experimental study designs investigate cannabis and inflammation associations are needed.
Keywords: Cannabis, inflammation, adults
1. Introduction
Chronic, as opposed to acute inflammation, has been proposed to be involved in the pathophysiology of several chronic physical health conditions, including coronary artery disease, diabetes, cancer, Alzheimer’s, osteoarthritis, and autoimmune diseases (Bennett et al., 2018; Chung et al., 2011; Furman et al., 2019; Newcombe et al., 2018). In 2017, these four chronic conditions accounted for nearly 1.5 million deaths in the United States (U.S.) (CDC, 2017). Additionally, a growing body of new evidence suggests that chronic low-grade inflammation is involved in mental health conditions and recurrence such as depression (Liu et al., 2019; Miller & Cole, 2012; Opel et al., 2019; Osimo et al., 2019; Slavich & Irwin, 2014; Tannous et al., 2020), as well as chronic pain (Bennett et al., 2018; Karshikoff et al., 2016). As such, patients and clinicians, are increasingly exploring novel therapies for chronic health conditions that target or mitigate chronic inflammation (Daily et al., 2016; Edwards, 2005; Goldfine & Shoelson, 2017; Ricker & Haas, 2017).
The active constituents of cannabis, particularly tetrahydrocannabinol (THC) and cannabidiol (CBD), have been shown to have immunomodulatory effects (Persidsky et al., 2015; Rom & Persidsky, 2013), specifically anti-inflammatory properties (Klein, 2005; Nagarkatti et al., 2009a). In non-human primates, THC administration attenuated tissue inflammation (Chandra et al., 2014; Kumar et al., 2019; Molina et al., 2011). Consequently, there has been an increase in cannabis use for medical purposes (Han et al., 2018), particularly among conditions with an inflammatory component including HIV, cancer and chronic pain (Boehnke et al., 2016; DʼSouza et al., 2012). Currently, 33 states have laws allowing medical cannabis use for a wide range of conditions, with 11 states allowing recreational cannabis use (Legal Medical Cannabis States and DC - Medical Cannabis - ProCon.Org, n.d.).
Yet, there have not been many studies evaluating the association between cannabis use and inflammation in humans and findings from the few studies published have been mixed. Cannabis use was significantly associated with lower levels of C reactive protein (CRP), but only among those whose CRP levels were below the median (Alshaarawy & Anthony, 2015). Lifetime but not recent cannabis use was associated with lower levels of fibrinogen (Alshaarawy et al., 2019). Further, studies have not found any significant association between cannabis use and interleukin 6 (IL-6) and CRP, high sensitivity CRP (hs-CRP)(Alshaarawy et al., 2019; Ferguson et al., 2019; Fond et al., 2017). Furthermore, cumulative cannabis use (for 20 years) and cannabis dependence was not associated with levels of CRP (Meier et al., 2016). In contrast, any cannabis was significantly associated with elevated levels of CRP (Costello et al., 2013) and lifetime cannabis use was significantly associated with lower levels of IL-6 (Keen et al., 2014).
We aim to evaluate three biomarkers of systemic inflammation including IL-6, fibrinogen and hs-CRP – a more sensitive CRP test that can detect small changes in CRP levels (Ridker, 2004; Vodolazkaia et al., 2011), which may be more apt to estimating cannabis inflammation effect. Furthermore, to understand the cannabis-inflammation association better, studies need to address for confounding variables particularly sociodemographic, behavioral and pharmacological confounders. Finally, studies on a potential sex-dependent effect of cannabis use on inflammation is scarce. In other studies, women reported higher ratings of subjective effects of cannabis use compared to men (Cooper & Haney, 2014), while men exhibited greater cannabis-induced analgesia compared to women (Cooper & Haney, 2016). Women are more likely to report loss of appetite, while men are more likely to report increased appetite associated with cannabis use (Cuttler et al., 2016). The mechanisms underlying potential sex-dependent effects of cannabis use may be attributed to sex-dependent differences in cannabis metabolism and interactions between the endocannabinoid system and sex hormones (Calakos et al., 2017; Cooper & Craft, 2018; Craft et al., 2013; Rubino & Parolaro, 2015). As such, the evidence suggests that women are more sensitive than males to the behavioral and physiological effects of cannabis. This emerging evidence on potential sex-dependent effects of cannabis call for investigating cannabis use by sex interactions on health, including chronic inflammation.
Therefore, the objective of this analysis was the examine the relationship between self- reported cannabis use and biomarkers of systemic inflammation, specifically high-sensitivity C- reactive protein (hsCRP), Interleukin 6 (IL-6) and fibrinogen. In light of findings from the extant literature (Alshaarawy & Anthony, 2015), we hypothesized that self-reported cannabis use will be associated with significantly lower levels of each biomarker of systemic inflammation compared to nonuse. Additionally, we investigated self-reported cannabis use by sex interactions to determine whether sex-dependent differences emerge on the impact of self-reported of cannabis use on biomarkers of systemic inflammation.
2. Methods
2.1. Study sample
Data came from Wave 1 (2013–2014) of the Population Assessment of Tobacco and Health (PATH) study Biomarker Restricted-Use files (NAHDAP, 2018a). The PATH study is a nationally representative, longitudinal cohort study of tobacco use and health outcomes in the United States (U.S.). The PATH study is a collaboration between the National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH), the Center for Tobacco Products (CTP), and the Food and Drug Administration (FDA) to collect data on tobacco use patterns and related health outcomes from non-institutionalized residents of the U.S. aged 12 years and older.
Extensive details of the PATH study design and methods has been published previously (Hyland et al., 2017). Of the 32,320 respondents who completed the Wave 1 adult interview, 21,801 (67.4 percent) provided a urine specimen. Among these, a stratified probability sample of 11,522 adults were selected from a diverse mix of six tobacco product use groups including: (i) current exclusive established users of cigarettes (ii), current established users of one or more tobacco products other than cigarettes (who may also be current established users of cigarettes or experimental users of other products, including cigarettes), (iii) current experimental users of any tobacco products, (iv) former established users of any tobacco product (last use within the past 12 months), (v) never users of any tobacco products and (vi) current established users of cigarettes who are experimental users of at least one other tobacco products. Of the 11,522 adults, 7,159 also provided a blood specimen. Blood was collected from consenting adults at a separate study visit by a phlebotomist, who visited the respondent’s home. Blood specimens were shipped to the Division of Laboratory Sciences, National Center for Environmental Health (NCEH), Centers for Disease Control and Prevention (CDC) for analysis of biomarkers of inflammation. Thus, the analysis sample comprised of 7,159 adults of PATH Wave 1.
2.2. Measures
2.2.1. Outcome: Biomarkers of systemic inflammation
High Sensitivity C Reactive Protein (hsCRP) was measured in serum or plasma using the Cardiac C-reactive Protein (Latex) Sensitive immunoturbidimetric assay on the automated Roche/Hitachi cobas c 311 module. The lower limit of detection, as determined by the assay manufacturer, is 0.5 mg/L. Additional details of the laboratory procedures can be found here (NAHDAP, 2018b).
Interleukin 6 (IL-6) IL-6 assays were performed following GenWay Biotech’s Standard Operating Procedure ANA015 (High Sensitivity Human IL-6 in Serum ELISA). IL-6 was measured in serum using the Quantikine Human IL-6 ELISA KIT (R&D Systems Cat# HS600B and HS600C) and Immunoassay Control Group 10 (R&D Systems Cat#QC41). Optical density was read using the Emax precision microplate reader (Molecular Devices) set to 490 nm. The assay range is 0.255 to 9.755 pg/mL.
Fibrinogen was measured using the Clauss fibrinogen assay, a quantitative, clot-based, functional assay. The assay measures the ability of fibrinogen to form fibrin clots after being exposed to a high concentration of purified thrombin. The assay was run on the ACL Top 300, which adds a predetermined number of units of bovine thrombin to citrated human plasma and measures the clotting kinetics turbidometrically. The assay range is 150 to 1000 mg/dL.
2.2.2. Predictor
Self-reported cannabis use.
We used three questions from wave 1 of the adult survey to identify the participant’s self-reported recency of cannabis use. Respondents were asked the following questions “Have you ever used marijuana, hash, THC, grass, pot or weed and “Have you ever smoked part or all of a cigar, cigarillo or filtered cigar with cannabis in it”. All respondents who had ever used cannabis (based on affirmative responses above) were additionally asked “How long has it been since you last used marijuana, hash, THC, grass, pot or weed?” We then used responses to all three questions to create a four-level categorical variable for self-reported cannabis use: (1) never used, (2) more than a year ago, (3) more than 30 days, but within the past year and (4) within the past 30 days.
2.2.3. Covariates
Sociodemographic covariates include age (in years), sex (male/female), race/ethnicity (Non-Hispanic white, Non-Hispanic Black, Hispanic, and others), educational attainment (Less than high school/GED, high school graduate, some college (no degree)/associate degree and college degree or more. In light of a recent study (Ferguson et al., 2019), we included anti- inflammatory medications use as a covariate and included aspirin, Tylenol (acetaminophen), Cox-2 inhibitors, ibuprofen, Motrin, Advil, Naprosyn and Aleve. Weight was measured in pounds and height in inches; using weight and height measured, body mass index (BMI) was calculated in kgm2. Alcohol, tobacco smoking and illicit drug use were measured of all respondents via self-report. Recency of alcohol use was categorized using similar categories as the self-reported cannabis use variable, while recent tobacco use was categorized as never/none, more than three days, but within the past year and within the past year. We operationalized any illicit drug (including cocaine/crack, methamphetamine/speed, heroin, inhalants, solvents, or hallucinogens) use in the past year as a binary variable.
2.3. Data analysis
We used weighted frequencies, percentages (for categorical variables), medians, and interquartile range (for continuous variables) to describe the characteristics of the Wave 1 PATH sample by their self-reported cannabis use. The primary predictor in this analysis was the four- level variable of self-reported cannabis use and the primary outcomes were the four biomarkers of inflammation (hsCRP, IL-6, and Fibrinogen). All the outcome variables were highly skewed and were subsequently natural log-transformed to stabilize their distributions. We used linear regression models to examine the association of self-reported cannabis use and each of the biomarkers of inflammation. We conducted the crude and adjusted models. We conducted the adjusted models in stages, model 1 adjusted for age and sex, model 2 additionally adjusted for race/ethnicity status, educational attainment, anti-inflammatory medication use, recency of alcohol use, recency of tobacco smoking and any illicit drug use in past 12 months, model 3 additionally adjusted for BMI. The set of covariates included in the final models were based on a priori knowledge of their relationship to biomarkers of systemic inflammation (Alshaarawy et al., 2019; Alshaarawy & Anthony, 2015; Ferguson et al., 2019; Meier et al., 2016). To explore potential sex differences in association between self-reported cannabis use and the biomarkers of systemic inflammation, we repeated all analysis above and included a self-reported cannabis use by sex interaction term in the fully adjusted model (i.e. model 3). The analysis was conducted in SAS version 9.4 (SAS Institute Inc., Cary, North Caroline, USA) and accounted for the PATH study’s multi-stage stratified area probability sampling design and nonresponse adjustments by using Wave 1 blood biomarker weights (Westat, 2017), balanced repeated replication (McCarthy, 1966) and Fay’s adjustment set to 0.3 to increase estimate stability (Judkins,1990). The replication weights is also recommended for subpopulation analysis (NAHDAP, 2018c).
2.4. Sensitivity analysis
Because a hs-CRP level greater than 10 mg/L is indicative of serious infection, trauma or chronic disease (Pearson et al., 2003; Shanahan et al., 2014), we further explored the impact of high hs-CRP levels on our findings by limiting the analysis for hs-CRP among those with hs- CRP in the normal range (i.e. hs-CRP ≤ 10 mg/L).
3. Results
3.1. Population characteristics
Among the total population (N=7,159), we excluded respondents who reported that a doctor told them that they had a heart disease or cancer (N=1,796), for a final analysis sample of N=5,363. Among these, majority were male (51%), non-Hispanic white (63%), and over half had attended some college, attained an Associate, college degree or more (53%; Table 1). Sixty-three percent had used alcohol and over a third (35%) had smoked tobacco within the past year, while 3% had used any illicit drugs within the past year. Median age of the sample was 38 years IQR (27, 53) and median BMI was 26 (23, 30; Table 1). Among the total population, 10.9% self- reported cannabis use within the past 30 days, 5.8% within the past year and 21.8% used more than a year ago (Table 1).
Table 1.
Characteristics of respondents in Wave 1 of the PATH
| Recency of marijuana use | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (N/n, Weighted %) | Total sample (N=5,363, 100%) | Never (n=2,132, 61.5) | More than a year ago (n=1,669, 21.8) | Within the past year (n=462, 5.8) | Within the past 30 days (n=1,086, 10.9) | P-value | |||||
| N | Weighted % | n | Weighted % | n | Weighted % | n | Weighted % | n | Weighted % | ||
| Median age (IQR) | 38 (27, 53) | 43 (29, 56) | 37 (28, 50) | 26 (21, 38) | 28 (21, 39) | <0.001 | |||||
| Sex | <0.001 | ||||||||||
| Male | 2,976 | 50.7 | 1,057 | 44.3 | 952 | 58.9 | 279 | 56.3 | 680 | 67.3 | |
| Female | 2,385 | 49.3 | 1,073 | 55.7 | 717 | 41.1 | 183 | 43.7 | 406 | 32.7 | |
| Race/ethnicity | <0.001 | ||||||||||
| Non-Hispanic White | 3,193 | 63.0 | 1,235 | 60.8 | 1,123 | 71.8 | 273 | 67.0 | 552 | 55.6 | |
| Non-Hispanic Black | 757 | 13.1 | 273 | 11.7 | 178 | 11.5 | 74 | 15.5 | 232 | 22.7 | |
| Hispanic | 941 | 17.3 | 441 | 20.4 | 232 | 10.9 | 76 | 13.0 | 188 | 14.3 | |
| Others | 408 | 6.6 | 155 | 7.1 | 119 | 5.6 | 34 | 4.5 | 100 | 7.4 | |
| Educational attainment | 0.070 | ||||||||||
| Less than high school /GED | 1,339 | 20.0 | 535 | 19.4 | 376 | 18.8 | 102 | 19.1 | 324 | 26.2 | |
| High school graduate | 1,306 | 27.0 | 590 | 28.9 | 362 | 24.3 | 100 | 24.7 | 253 | 22.8 | |
| Some college /AA/ College degree or more | 2,703 | 53.0 | 1,001 | 51.7 | 929 | 56.9 | 260 | 56.2 | 505 | 51.0 | |
| Anti-inflammatory medication use¶ | 0.127 | ||||||||||
| No | 1,318 | 23.1 | 564 | 23.6 | 348 | 20.5 | 97 | 20.0 | 307 | 26.8 | |
| Yes | 4,028 | 76.9 | 1,557 | 76.4 | 1,320 | 79.5 | 365 | 80.0 | 776 | 73.2 | |
| Alcohol use in the past 12 months | <0.001 | ||||||||||
| Never/none | 1,531 | 37.4 | 941 | 49.8 | 397 | 23.7 | 48 | 7.2 | 143 | 11.5 | |
| Yes | 3,815 | 62.6 | 1,184 | 50.2 | 1,270 | 76.3 | 414 | 92.8 | 940 | 88.5 | |
| Smoking | <0.001 | ||||||||||
| Never/none | 2,147 | 63.8 | 1,212 | 82.1 | 492 | 37.1 | 163 | 40.8 | 277 | 27.4 | |
| More than 3 days, but within the past yr. | 307 | 3.2 | 83 | 1.7 | 116 | 6.0 | 33 | 5.2 | 74 | 5.3 | |
| Within the past 3 days | 2,908 | 33.0 | 837 | 16.2 | 1,061 | 56.9 | 266 | 54.0 | 734 | 67.3 | |
| Illicit drug use† | <0.001 | ||||||||||
| No | 5,002 | 96.8 | 2109 | 99.8 | 1,614 | 97.6 | 410 | 92.0 | 861 | 81.0 | |
| Yes | 315 | 3.2 | 9 | 0.2 | 40 | 2.4 | 51 | 8.0 | 215 | 19.0 | |
| Median BMI (IQR) | 26 (23, 30) | 27 (23, 30) | 26 (23, 30) | 25 (22, 29) | 25 (22, 29) | <0.001 | |||||
Note: PATH = Population Assessment of Tobacco use and Health
Includes aspirin, Tylenol (acetaminophen), Cox-2 inhibitors, ibuprofen, Motrin, Advil, Naprosyn and Aleve
Includes cocaine/crack, methamphetamine/speed, heroin, inhalants, solvents or hallucinogens in the 12 months; BMI=Body mass index, AA=Associated Degree
3.2. Self-reported cannabis use and biomarkers of inflammation
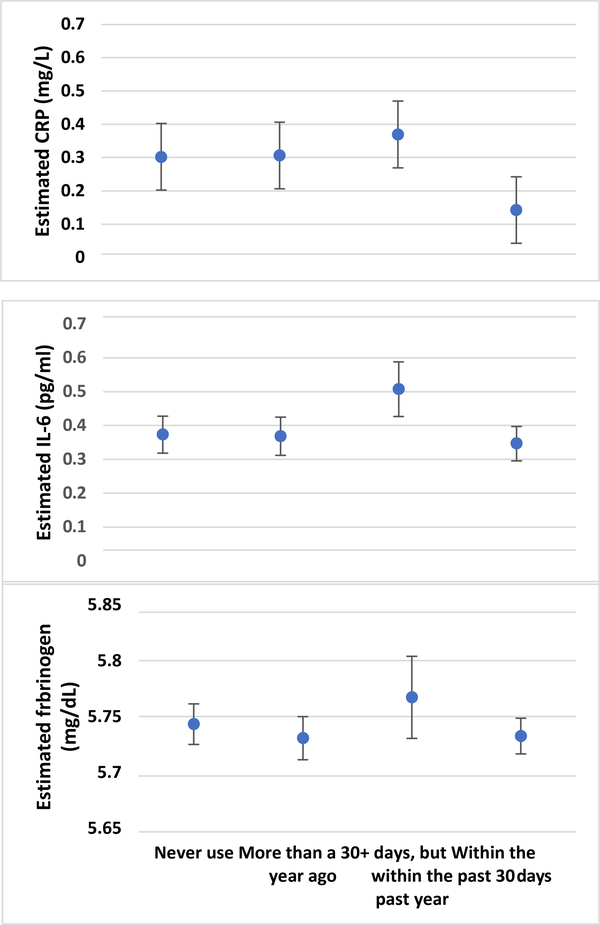
Distribution of the untransformed biomarkers of systemic inflammation in the total population and by cannabis use groups and relationships among the biomarkers of inflammation are included in the Supplementary Material Tables S1 & S2. Figure 1 displays mean estimated hsCRP, IL-6 and fibrinogen levels (on logarithm scale) among categories of cannabis use. These results show a general pattern of lower mean biomarkers of systemic inflammation among respondents self-reporting cannabis use in the past 30 days compared to other categories of cannabis use. We compared each category of cannabis use with the group that self-reported never use.
Figure 1.
Estimated levels of biomarkers of systemic inflammation by self-reported cannabis use. Estimates represent logarithm transformed scale. Error bars represent 95% confidence intervals.
hsCRP.
In bivariate analysis, cannabis use within the past 30 days was associated with lower levels (i.e. negative association) of hs-CRP compared to nonuse, with narrow confidence intervals around the beta estimates that excluded null and positive values (Table 2). Adjusting for covariates in model 2 attenuated the beta estimate of this association, with confidence intervals also excluding null and positive values. In model 3 that additionally adjusted for BMI, the beta estimate was further attenuated, indicating lower levels of hsCRP in respondents who self- reported past 30 days cannabis use compared to nonuse, with confidence intervals around the point estimates excluding null value. All other categories of self-reported cannabis use indicated higher levels of hsCRP compared to nonuse (i.e. positive beta estimates), but the confidence intervals around the points estimates spanned both the null and negative values.
Table 2.
Association between self-reported recency of marijuana use and biomarkers of inflammation in Wave 1 of PATH
| β (95% Confidence interval) | ||||
|---|---|---|---|---|
| Crude | Model 1 | Model 2 | Model 3 | |
| Recency of marijuana use | High sensitivity C reactive protein (mg/L) | |||
| Never | Reference | Reference | Reference | Reference |
| More than a year ago | 0.01 (−0.15, 0.17) | 0.09 (−0.08, 0.26) | 0.01 (−0.17, 0.19) | 0.00 (−0.16, 0.17) |
| Within the past year | −0.06 (−0.31, 0.19) | 0.08 (−0.14, 0.31) | 0.00 (−0.21, 0.22) | 0.06 (−0.16, 0.30) |
| Within the past 30 days | −0.26 (−0.43, −0.10) | −0.09 (−0.26, 0.06) | −0.20 (−0.38, −0.02) | −0.15 (−0.32, 0.00) |
| Recency of marijuana use X Sex | ||||
| P-value for interaction | 0.1263 | |||
| Interleukin-6 (pg/ml) | ||||
| Never | Reference | Reference | Reference | Reference |
| More than a year ago | −0.00 (−0.08, 0.08) | 0.06 (−0.01, 0.14) | −0.00 (−0.07, 0.07) | −0.00 (−0.07, 0.06) |
| Within the past year | −0.04 (−0.19, 0.10) | 0.14 (0.00, 0.27) | 0.09 (−0.04, 0.24) | 0.13 (−0.00, 0.27) |
| Within the past 30 days | −0.13 (−0.21, −0.05) | 0.05 (−0.02, 0.13) | −0.04 (−0.12, 0.04) | −0.02 (−0.10, 0.05) |
| Recency of marijuana use X Sex | ||||
| P-value for interaction | 0.8134 | |||
| Fibrinogen (mg/dL) | ||||
| Never | Reference | Reference | Reference | Reference |
| More than a year ago | −0.02 (−0.05, 0.00) | 0.00 (−0.02, 0.02) | −0.01 (−0.03, 0.01) | −0.01 (−0.03, 0.01) |
| Within the past year | −0.03 (−0.13, 0.05) | 0.02 (−0.06, 0.10) | 0.01 (−0.06, 0.09) | 0.02 (−0.05, 0.10) |
| Within the past 30 days | −0.06 (−0.10, −0.03) | −0.00 (−0.03, 0.03) | −0.01 (−0.05, 0.01) | −0.01 (−0.04, 0.02) |
| Recency of marijuana use X Sex | ||||
| P-value for interaction | 0.5411 | |||
Note:
Mode 1 was adjusted for age and sex
Model 2 was adjusted for age, sex, race/ethnicity status, educational attainment, anti-inflammatory medication use, alcohol use, recency of smoking and any illicit drug use in past 12 months
Model 3 was additionally adjusted for BMI
IL-6.
In bivariate analysis, self-reported cannabis use within the past 30 days was associated with lower levels of IL-6 compared to nonuse, with confidence intervals around the beta point estimates excluding null and positive values. However, adjusting for covariates in models 2 and 3 progressively attenuated the beta estimate and increased the width of the confidence intervals to include null and positive values. All other categories of self-reported cannabis use produced wide confidence intervals that include null values.
Fibrinogen.
In bivariate analysis, self-reported cannabis use within the past 30 days was associated with lower levels of fibrinogen compared to nonuse with confidence intervals around the beta point estimates excluding null and positive value. Like IL-6, adjusting covariates in models 2 and 3 attenuated the point estimates and widened the confidence intervals to include the null value. Full model estimates with covariate results are included in the Supplementary Material Table S3).
3.3. Interactions between self-reported cannabis use and sex and biomarkers of inflammation
In the fully adjusted analysis (model 3), the interaction terms between self-reported cannabis use and sex were not statistically significant (all p’s = >0.05), indicating that the associations between self-reported cannabis use and biomarkers of systemic inflammation did not significantly differ by sex (Table 2). All other categories of self-reported cannabis use and hs-CRP produced wide confidence intervals that included null and positive values.
3.4. Sensitivity analysis
The model that excluded respondents with hs-CRP level greater than 10 mg/L showed a negative association between self-reported cannabis use in the past 30 days and hs-CRP, although the confidence intervals around the beta estimate included null and positive values (β = −0.12, 95% CI: −0.28, 0.04) (included in the Supplementary Material Tables S4). All other categories of self-reported cannabis use and biomarkers of systemic inflammation included both the null and positive values.
4. Discussion
In this analysis of nationally representative data from Wave 1 of the PATH study, there was a pattern of lower levels of biomarkers of systemic inflammation, particularly hs-CRP among respondents self-reporting recent (past 30 days’ use) cannabis use compared to never use, although the wide confidence intervals around the point estimates indicated findings were not statistically significant. Furthermore, statistical tests to determine whether the association between self-reported cannabis use and biomarkers of systemic inflammation differed by sex were also not statistically significant. The findings from our study are based on a multivariable analysis that adjusted for important confounding variables, including respondent’s BMI and use of anti-inflammatory medications.
The potential therapeutic effects of cannabis have been proposed to be mediated via its anti-inflammatory properties (Lowin et al., 2019; Nagarkatti et al., 2009b; Perisetti et al., 2020). Findings from preclinical and animal studies converge to suggest that the active constituents in the cannabis plant – particularly THC and CBD induce anti-inflammatory properties (Weiss et al., 2006). Our study found that more recent cannabis use is associated with lower levels of biomarkers of systemic inflammation is consistent with anti-inflammatory hypothesis and with findings from prior research. Data from the National Health and Nutrition Examination Survey (NHANES) showed lower serum CRP levels in active cannabis users compared to never users, but only when CRP levels were below the median (Alshaarawy & Anthony, 2015). Also, self- reported cannabis use was not statistically associated with lower levels of CRP in a recent analysis using data from the Adolescent to Adult Health study, after adjusting for sociodemographic characteristics, tobacco exposure, BMI and anti-inflammatory medication use (Ferguson et al., 2019). In a recent longitudinal analysis of participants in the CARDIA cohort study, self-reported recent cannabis use was not statistically associated with lower levels of CRP, IL-6 and fibrinogen among the CARDIA sample (Alshaarawy et al., 2019).
In sum, most epidemiologic studies investigating the association between self-reported cannabis use and biomarkers of inflammation have found negative (i.e. inverse) associations that were nonsignificant with the exception of a few that reported statistically significant negative associations (Karoly et al., 2018; Keen et al., 2014, 2015). The reason for the disparate findings may be related to differences in the study sample that focused on primarily African Americans (Keen et al., 2014, 2015) and in the set of confounders included in the multivariable models (Karoly et al., 2018). Our study adjusted for important confounders such as anti-inflammatory medication use and BMI, which these prior studies did not address in their analysis (Karoly et al., 2018). Indeed, our study found statistically significant associations between recent cannabis use and lower levels of hs-CRP, IL-6 and fibrinogen in limited models (i.e. models 1 and 2), which was no longer statistically significant in the fully adjusted model 3, underscoring the importance of fully adjusting for important confounders when investigating the relationships between cannabis use and inflammation.
Our study extends findings from the extant literature by utilizing a large nationally representative data to analyze the association between self-reported cannabis use and three biomarkers of systemic inflammation. Although our results suggest lower levels of biomarkers of systemic inflammation in respondents self-reporting cannabis use in the past 30 days (compared with never use), the estimates produced had wide confidence intervals. The wide confidence intervals observed in our study might be a source of measurement error related to the imprecise measurement of cannabis. Our study relied on self-report of cannabis use, which is prone to inaccuracies. Specifically, respondents may not accurately recall the last time they used cannabis, making it possible that some respondents who reported that they used cannabis within the past year, but more than 30 days ago, might have used in the past 30 days. Also, the cannabis use measurement in this analysis did not collect data on the amount and or concentrations of the different active compounds in marijuana. This is particularly relevant as emerging evidence suggest that the two primary active constituents in cannabis – tetrahydrocannabinol (THC) and cannabidiol (CBD) – may have opposing immunomodulatory effects (Bidwell et al., 2018).
Therefore, to move the field forward, better measurement of cannabis use, that includes the amount, THC and CBD content and mode of consumption (i.e. smoking, vaping, consumption) would help us ascertain the dose-response relationship between cannabis, inflammatory markers, and symptomatology and whether findings differ by THC/CBD concentrations and mode of consumption.
Our study also analyzed hs-CRP data which is a more stable and sensitive molecule (Ridker, 2004; Vodolazkaia et al., 2011) than the standard CRP test. The hs-CRP assay can detect trace amounts of CRP than the standard CRP test. Although statistically nonsignificant, our analysis showed that the differences between recent cannabis use and other categories of cannabis use were more prominent for hs-CRP than other sensitive biomarkers of systemic inflammation. This suggests that more sensitive biomarkers of systemic inflammation should be employed in future research. Our analysis was based on only three biomarkers of systemic inflammation; with the blood specimen to measure these biomarkers collected on a separate visit from the visit cannabis data was collected. Future studies should include a broad panel of biomarkers, particularly anti-inflammatory molecules in order to increase our understanding of the immunomodulatory effects of cannabis use.
We note that mean levels of all biomarkers of systemic inflammation were higher among respondent self-reporting cannabis use within the past year, but not in the past 30-days (Figure 1). It is possible that this group includes respondents who recently ceased cannabis use due to illness that can drive inflammation response. The cross-sectional design of our analysis precluded the assessment of directional relationships. Specifically, a cross-sectional finding indicates a correlational association, precluding a directional or causal relationship between cannabis use and biomarkers of systemic inflammation. Therefore, future research using longitudinal and experimental designs that follow subjects across multiple time points are needed to broaden our understanding of the cannabis and inflammation relationship, specifically whether reductions in inflammatory markers mediate the association between cannabis use and reduced self-reported chronic disease symptomatology or differences in incident or recurring inflammatory-related disease (e.g., depression relapse).
5. Conclusion
In this nationally representative study of adults in the U.S. from the PATH study, we found lower levels of biomarkers of systemic inflammation among respondents with self- reported cannabis use in the past 30 days, compared to those reporting that they never used, although the findings were not statistically significant. Future studies that biologically measure the THC and CBD concentrations of the cannabis used and that employ prospective and or experimental study designs are needed to move the field forward and provide robust data on the impact of cannabis use on biomarkers of systemic inflammation.
Supplementary Material
Highlights.
Studies assessing cannabis use and peripheral inflammation in humans have been few
We investigated associations of cannabis use and peripheral inflammation in adults
Cannabis use was not statistically associated with hs-CRP, IL-6 and fibrinogen
Studies with biological measures of cannabis use is warranted
Acknowledgments
Funding Source: Chukwuemeka N. Okafor is supported by the National Institute on Drug Abuse (K01-DA047912).
Footnotes
Declarations of Interest: none
References
- Alshaarawy O, & Anthony JC (2015). Cannabis smoking and serum C-reactive protein: A quantile regressions approach based on NHANES 2005–2010. Drug and Alcohol Dependence, 147, 203–207. 10.1016/j.drugalcdep.2014.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshaarawy O, Sidney S, Auer R, Green D, Soliman EZ, Goff DC, & Anthony JC (2019). Cannabis Use and Markers of Systemic Inflammation: The Coronary Artery Risk Development in Young Adults Study. The American Journal of Medicine. 10.1016/j.amjmed.2019.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JM, Reeves G, Billman GE, & Sturmberg JP (2018). Inflammation–Nature’s Way to Efficiently Respond to All Types of Challenges: Implications for Understanding and Managing “the Epidemic” of Chronic Diseases. Frontiers in Medicine, 5 10.3389/fmed.2018.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell LC, Mueller R, YorkWilliams SL, Hagerty S, Bryan AD, & Hutchison KE (2018). A Novel Observational Method for Assessing Acute Responses to Cannabis: Preliminary Validation Using Legal Market Strains. Cannabis and Cannabinoid Research, 3(1), 35–44. 10.1089/can.2017.0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehnke KF, Litinas E, & Clauw DJ (2016). Medical Cannabis Use Is Associated With Decreased Opiate Medication Use in a Retrospective Cross-Sectional Survey of Patients With Chronic Pain. The Journal of Pain, 17(6), 739–744. 10.1016/j.jpain.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Calakos KC, Bhatt S, Foster DW, & Cosgrove KP (2017). Mechanisms Underlying Sex Differences in Cannabis Use. Current Addiction Reports, 4(4), 439–453. 10.1007/s40429-017-0174-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2017). Leading Causes of Death. https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm
- Chandra LC, Kumar V, Torben W, Stouwe CV, Winsauer P, Amedee A, Molina PE, & Mohan M (2014). Chronic Administration of Δ9-Tetrahydrocannabinol Induces Intestinal Anti-Inflammatory MicroRNA Expression during Acute Simian Immunodeficiency Virus Infection of Rhesus Macaques. Journal of Virology, 89(2), 1168–1181. 10.1128/JVI.01754-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HY, Lee EK, Choi YJ, Kim JM, Kim DH, Zou Y, Kim CH, Lee J, Kim HS, Kim ND, Jung JH, & Yu BP (2011). Molecular inflammation as an underlying mechanism of the aging process and age-related diseases. Journal of Dental Research, 90(7), 830–840. 10.1177/0022034510387794 [DOI] [PubMed] [Google Scholar]
- Cooper ZD, & Craft RM (2018). Sex-Dependent Effects of Cannabis and Cannabinoids: A Translational Perspective. Neuropsychopharmacology, 43(1), 34–51. 10.1038/npp.2017.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, & Haney M (2014). Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug and Alcohol Dependence. 10.1016/j.drugalcdep.2013.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, & Haney M (2016). Sex-dependent effects of cannabis-induced analgesia. Drug and Alcohol Dependence, 167, 112–120. 10.1016/j.drugalcdep.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Copeland WE, Shanahan L, Worthman CM, & Angold A (2013). C- reactive protein and substance use disorders in adolescence and early adulthood: A prospective analysis. Drug and Alcohol Dependence, 133(2), 712–717. 10.1016/j.drugalcdep.2013.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Marusich JA, & Wiley JL (2013). Sex differences in cannabinoid pharmacology: A reflection of differences in the endocannabinoid system? Life Sciences, 92(8–9), 476–481. 10.1016/j.lfs.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttler C, Mischley LK, & Sexton M (2016). Sex Differences in Cannabis Use and Effects: A Cross-Sectional Survey of Cannabis Users. Cannabis and Cannabinoid Research, 1(1), 166–175. 10.1089/can.2016.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daily JW, Yang M, & Park S (2016). Efficacy of Turmeric Extracts and Curcumin for Alleviating the Symptoms of Joint Arthritis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Journal of Medicinal Food, 19(8), 717–729. 10.1089/jmf.2016.3705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DʼSouza G, Matson PA, Grady CD, Nahvi S, Merenstein D, Weber KM, Greenblatt R, Burian P, & Wilson TE (2012). Medicinal and Recreational Marijuana Use Among HIV-Infected Women in the Womenʼs Interagency HIV Study (WIHS) Cohort, 1994–2010. JAIDS Journal of Acquired Immune Deficiency Syndromes, 61(5), 618–626. 10.1097/QAI.0b013e318273ab3a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards T (2005). Inflammation, pain, and chronic disease: An integrative approach to treatment and prevention. Alternative Therapies in Health and Medicine, 11(6), 20–27; quiz 28, 75. [PubMed] [Google Scholar]
- Ferguson EG, Mannes ZL, & Ennis N (2019). Is marijuana use associated with lower inflammation? Results from waves III and IV of the national longitudinal study of adolescent to adult health. Drug and Alcohol Dependence, 198, 162–167. 10.1016/j.drugalcdep.2019.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fond G, Berna F, Andrianarisoa M, Godin O, Leboyer M, Brunel L, Aouizerate B, Capdevielle D, Chereau I, D’Amato T, Denizot H, Dubertret C, Dubreucq J, Faget C, Gabayet F, Llorca PM, Mallet J, Misdrahi D, Passerieux C, … Schurhoff F (2017). Chronic low-grade peripheral inflammation is associated with severe nicotine dependence in schizophrenia: Results from the national multicentric FACE-SZ cohort. European Archives of Psychiatry and Clinical Neuroscience, 267(5), 465–472. 10.1007/s00406-017-0771-4 [DOI] [PubMed] [Google Scholar]
- Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, & Slavich GM (2019). Chronic inflammation in the etiology of disease across the life span. Nature Medicine, 25(12), 1822–1832. 10.1038/s41591-019-0675-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine AB, & Shoelson SE (2017). Therapeutic approaches targeting inflammation for diabetes and associated cardiovascular risk. The Journal of Clinical Investigation, 127(1), 83–93. 10.1172/JCI88884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Compton WM, Blanco C, & Jones CM (2018). Trends in and correlates of medical marijuana use among adults in the United States. Drug and Alcohol Dependence, 186, 120–129. 10.1016/j.drugalcdep.2018.01.022 [DOI] [PubMed] [Google Scholar]
- Hyland A, Ambrose BK, Conway KP, Borek N, Lambert E, Carusi C, Taylor K, Crosse S, Fong GT, Cummings KM, Abrams D, Pierce JP, Sargent J, Messer K, Bansal-Travers M, Niaura R, Vallone D, Hammond D, Hilmi N, … Compton WM (2017). Design and methods of the Population Assessment of Tobacco and Health (PATH) Study. Tobacco Control, 26(4), 371–378. 10.1136/tobaccocontrol-2016-052934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly HC, Bidwell LC, Mueller RL, & Hutchison KE (2018). Investigating the Relationships Between Alcohol Consumption, Cannabis Use, and Circulating Cytokines: A Preliminary Analysis. Alcoholism, Clinical and Experimental Research, 42(3), 531–539. 10.1111/acer.13592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karshikoff B, Jensen KB, Kosek E, Kalpouzos G, Soop A, Ingvar M, Olgart Höglund C, Lekander M, & Axelsson J (2016). Why sickness hurts: A central mechanism for pain induced by peripheral inflammation. Brain, Behavior, and Immunity, 57, 38–46. 10.1016/j.bbi.2016.04.001 [DOI] [PubMed] [Google Scholar]
- Keen L, Pereira D, & Latimer W (2014). Self-reported lifetime marijuana use and interleukin-6 levels in middle-aged African Americans. Drug and Alcohol Dependence, 140(Supplement C), 156–160. 10.1016/j.drugalcdep.2014.04.011 [DOI] [PubMed] [Google Scholar]
- Keen L, Turner AD, Callender C, & Campbell A (2015). Differential Effects of Self- Reported Lifetime Marijuana Use on Interleukin-1 Alpha and Tumor Necrosis Factor in African American Adults. Journal of Behavioral Medicine, 38(3), 527–534. 10.1007/s10865-015-9625-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TW (2005). Cannabinoid-based drugs as anti-inflammatory therapeutics. Nature Reviews. Immunology, 5(5), 400–411. 10.1038/nri1602 [DOI] [PubMed] [Google Scholar]
- Kumar V, Torben W, Mansfield J, Alvarez X, Vande Stouwe C, Li J, Byrareddy SN, Didier PJ, Pahar B, Molina PE, & Mohan M (2019). Cannabinoid Attenuation of Intestinal Inflammation in Chronic SIV-Infected Rhesus Macaques Involves T Cell Modulation and Differential Expression of Micro-RNAs and Pro-inflammatory Genes. Frontiers in Immunology, 10, 914 10.3389/fimmu.2019.00914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legal Medical Marijuana States and DC - Medical Marijuana—ProCon.org. (n.d.). Medical Marijuana; Retrieved February 20, 2020, from https://medicalmarijuana.procon.org/legal-medical-marijuana-states-and-dc/ [Google Scholar]
- Liu C-H, Zhang G-Z, Li B, Li M, Woelfer M, Walter M, & Wang L (2019). Role of inflammation in depression relapse. Journal of Neuroinflammation, 16(1), 90 10.1186/s12974-019-1475-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowin T, Schneider M, & Pongratz G (2019). Joints for joints: Cannabinoids in the treatment of rheumatoid arthritis. Current Opinion in Rheumatology, 31(3), 271–278. 10.1097/BOR.0000000000000590 [DOI] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Cerdá M, Hancox RJ, Harrington H, Houts R, Poulton R, Ramrakha S, Thomson WM, & Moffitt TE (2016). Associations Between Cannabis Use and Physical Health Problems in Early Midlife: A Longitudinal Comparison of Persistent Cannabis vs Tobacco Users. JAMA Psychiatry, 73(7), 731–740. 10.1001/jamapsychiatry.2016.0637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, & Cole SW (2012). Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biological Psychiatry, 72(1), 34–40. 10.1016/j.biopsych.2012.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina PE, Winsauer P, Zhang P, Walker E, Birke L, Amedee A, Stouwe CV, Troxclair D, McGoey R, Varner K, Byerley L, & LaMotte L (2011). Cannabinoid administration attenuates the progression of simian immunodeficiency virus. AIDS Research and Human Retroviruses, 27(6), 585–592. 10.1089/aid.2010.0218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarkatti P, Pandey R, Rieder SA, Hegde VL, & Nagarkatti M (2009a). Cannabinoids as novel anti-inflammatory drugs. Future Medicinal Chemistry, 1(7), 1333–1349. 10.4155/fmc.09.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarkatti P, Pandey R, Rieder SA, Hegde VL, & Nagarkatti M (2009b). Cannabinoids as novel anti-inflammatory drugs. Future Medicinal Chemistry, 1(7), 1333–1349. 10.4155/fmc.09.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAHDAP. (2018a). Population Assessment of Tobacco and Health (PATH) Study Series. https://www.icpsr.umich.edu/icpsrweb/NAHDAP/series/606
- NAHDAP. (2018b). Population Assessment of Tobacco and Health (PATH) Study [United States] Biomarker Restricted-Use Files (ICPSR 36840). https://www.icpsr.umich.edu/icpsrweb/NAHDAP/studies/36840
- NAHDAP. (2018c). Population Assessment of Tobacco and Health (PATH) User Guide. https://www.icpsr.umich.edu/icpsrweb/NAHDAP/series/606
- Newcombe EA, Camats-Perna J, Silva ML, Valmas N, Huat TJ, & Medeiros R (2018). Inflammation: The link between comorbidities, genetics, and Alzheimer’s disease. Journal of Neuroinflammation, 15(1), 276 10.1186/s12974-018-1313-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opel N, Cearns M, Clark S, Toben C, Grotegerd D, Heindel W, Kugel H, Teuber A, Minnerup H, Berger K, Dannlowski U, & Baune BT (2019). Large-scale evidence for an association between low-grade peripheral inflammation and brain structural alterations in major depression in the BiDirect study. Journal of Psychiatry & Neuroscience: JPN, 44(6), 423–431. 10.1503/jpn.180208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osimo EF, Baxter LJ, Lewis G, Jones PB, & Khandaker GM (2019). Prevalence of low-grade inflammation in depression: A systematic review and meta-analysis of CRP levels. Psychological Medicine, 49(12), 1958–1970. 10.1017/S0033291719001454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Taubert K, Tracy RP, & Vinicor F (2003). Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: A Statement for Healthcare Professionals From the Centers for Disease Control and Prevention and the American Heart Association. Circulation, 107(3), 499–511. 10.1161/01.CIR.0000052939.59093.45 [DOI] [PubMed] [Google Scholar]
- Perisetti A, Rimu AH, Khan SA, Bansal P, & Goyal H (2020). Role of cannabis in inflammatory bowel diseases. Annals of Gastroenterology, 33(2), 134–144. 10.20524/aog.2020.0452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Fan S, Dykstra H, Reichenbach NL, Rom S, & Ramirez SH (2015). Activation of Cannabinoid Type Two Receptors (CB2) Diminish Inflammatory Responses in Macrophages and Brain Endothelium. Journal of Neuroimmune Pharmacology : The Official Journal of the Society on NeuroImmune Pharmacology, 10(2), 302–308. 10.1007/s11481-015-9591-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricker MA, & Haas WC (2017). Anti-Inflammatory Diet in Clinical Practice: A Review. Nutrition in Clinical Practice, 32(3), 318–325. 10.1177/0884533617700353 [DOI] [PubMed] [Google Scholar]
- Rom S, & Persidsky Y (2013). Cannabinoid Receptor 2: Potential Role in Immunomodulation and Neuroinflammation. Journal of Neuroimmune Pharmacology, 8(3), 608–620. 10.1007/s11481-013-9445-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, & Parolaro D (2015). Sex-Dependent Vulnerability to Cannabis Abuse in Adolescence. Frontiers in Psychiatry, 6 10.3389/fpsyt.2015.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan L, Freeman J, & Bauldry S (2014). Is very high C-reactive protein in young adults associated with indicators of chronic disease risk? Psychoneuroendocrinology, 40, 76–85. 10.1016/j.psyneuen.2013.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, & Irwin MR (2014). From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological Bulletin, 140(3), 774–815. 10.1037/a0035302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannous J, Godlewska BR, Tirumalaraju V, Soares JC, Cowen PJ, & Selvaraj S (2020). Stress, inflammation and hippocampal subfields in depression: A 7 Tesla MRI Study. Translational Psychiatry, 10(1), 1–7. 10.1038/s41398-020-0759-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L, Zeira M, Reich S, Har-Noy M, Mechoulam R, Slavin S, & Gallily R (2006). Cannabidiol lowers incidence of diabetes in non-obese diabetic mice. Autoimmunity, 39(2), 143–151. 10.1080/08916930500356674 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.