Abstract
We disclose a novel class of 6-amino-tetrahydroquinazoline derivatives that inhibit human topoisomerase II (topoII), a validated target of anticancer drugs. In contrast to topoII-targeted drugs currently in clinical use, these compounds do not act as topoII poisons that enhance enzyme-mediated DNA cleavage, a mechanism that is linked to the development of secondary leukemias. Instead, these tetrahydroquinazolines block topoII function with no evidence of DNA intercalation. We identified a potent lead compound [Compound 14 (ARN-21934) IC50 = 2 µM for inhibition of DNA relaxation, as compared to an IC50 = 120 μM for the anticancer drug etoposide] with excellent metabolic stability and solubility. This new compound also shows great selectivity for topoIIα, a broad antiproliferative activity toward cultured human cancer cells, a favorable in vivo pharmacokinetic profile, and the ability to penetrate the blood-brain barrier. Thus, ARN-21934 is a highly promising lead for the development of novel and potentially safer topoII-targeted anticancer drugs.
Keywords: Topoisomerase II, enzyme inhibition, drug design, structure-activity relationship
Graphical Abstract
INTRODUCTION
Human topoisomerase II (topoII) is a crucial enzyme that controls the topology of DNA in cells, and regulates vital cellular processes such as DNA replication, transcription, recombination, and chromosome segregation.1–5 In addition to its important cellular functions, topoII is a validated target for the treatment of cancer,1, 6–7 and several topoII-targeted drugs are in clinical use. 1–2 Notably, all of these drugs act by trapping the covalent enzyme/DNA cleavage complex that is formed during DNA topology modifications.6–8 As a result, topoII generates double-stranded breaks in the genome. Although effective against cancer, this mechanism of action, referred to as topoII poisoning, is linked to severe side effects including the development of secondary leukemias in patients.6–10 These unwanted effects represent timely and urgent issues that have motivated the recent renaissance of research to seek new and safer topoII-targeted drugs.4, 11–13
To address this issue, we have previously reported synthesis and modeling of new small molecules that act as topoII poisons.14–20 Here, we outline the discovery of novel 6-amino-tetrahydroquinazoline derivatives that are potent topoII inhibitors and that, notably, do not act as topoII poisons. Remarkably, these molecules preferentially inhibit the α isoform of human topoII, over the β one.21–22 Furthermore, they have broad antiproliferative activity against cultured human cancer cells and display a favorable in vivo pharmacokinetic profile. These findings highlight the potential of these tetrahydroquinazoline derivatives for the development of novel anticancer agents.
RESULTS AND DISCUSSION
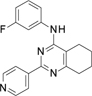
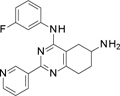
A virtual screening of an internal library of small compounds docked against the cleavage complex returned promising hits against human topoIIα. From these, ~70 compounds were measured for their ability to inhibit human topoIIα in a DNA relaxation assay. We identified the small molecule with formula N4-(3-fluorophenyl)-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-di-amine (compound 1, Table 2), as a novel inhibitor with an IC50 of 160 μM.
Table 2.
Topoisomerase IIα inhibition. Values shown correspond to the IC50 ± SD.
| Compound 1 | Compound 2 | Compound 3 | Compound 4 | Compound 5 | Compound 6 | Compound 7 |
|---|---|---|---|---|---|---|
 |
 |
 |
 |
 |
 |
 |
| 160 ± 20 µM | not active | 160 ± 20 µM | 160 ± 20 µM | 200 ± 15 µM | not active | not active |
| Compound 8 | Compound 9 | Compound 10 | Compound 11 | Compound 12 | Compound 13 | Compound 14 |
 |
 |
 |
 |
 |
 |
 |
| not active | not active | 220 ± 30 µM | 170 ± 15 µM | not active | not active | 2 ± 0.5 µM |
Notably, the core of the starting hit 1 is based on the privileged scaffold of functionalized quinazolines (Figure 2), which display a large spectrum of biological activities, including antibacterial, antifungal, anticonvulsant, anti-inflammatory, anti-HIV, anticancer, and analgesic effects.23
Figure 2.
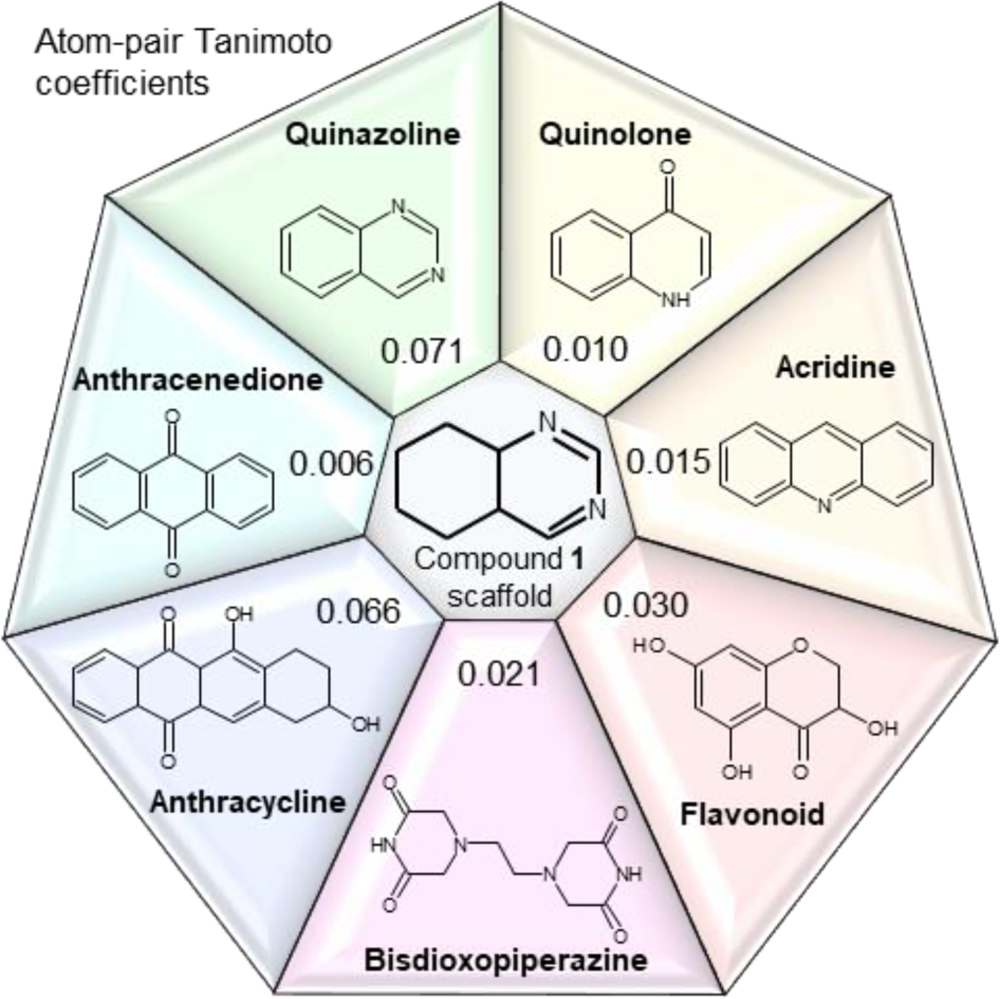
Chemical similarity between chemotypes commonly employed to target topoisomerase IIα. The values in each box correspond to the atom-pair Tanimoto coefficient calculated between the partially-saturated quinazoline scaffold of compound 1 and seven other substructures, namely quinazoline, quinolone, acridine, flavonoid, bisdioxopiperazine, anthracycline, and anthracenedione.
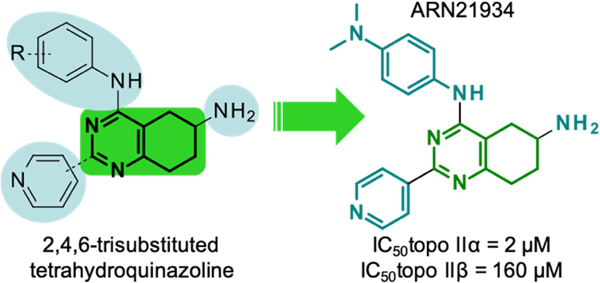
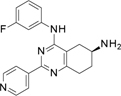
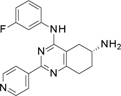
Compound 1 includes a partially saturated quinazoline heterocycle bearing a pyridine, an aniline, and an amino group in positions 2, 4 and 6, respectively (Figure 1). Thus, we started a focused structure-activity relationship (SAR) study and explored an initial set of 13 close analogs that were synthesized and tested against topoIIα-catalyzed DNA relaxation (summarized in Schemes 1.b, 1.c and 1.d and Table 1). This systematic exploration of 1 led to 6 additional new active compounds. In particular, we identified compound 14 (named ARN-21934, Figure 1), which has an IC50 of 2 µM (Figure 1). Thus, 14 is ~80-fold more potent than the starting hit 1 and is more potent than the anticancer drug etoposide (~120 µM), which was used as a reference compound in our topoIIα inhibition assay. 11, 24
Figure 1.
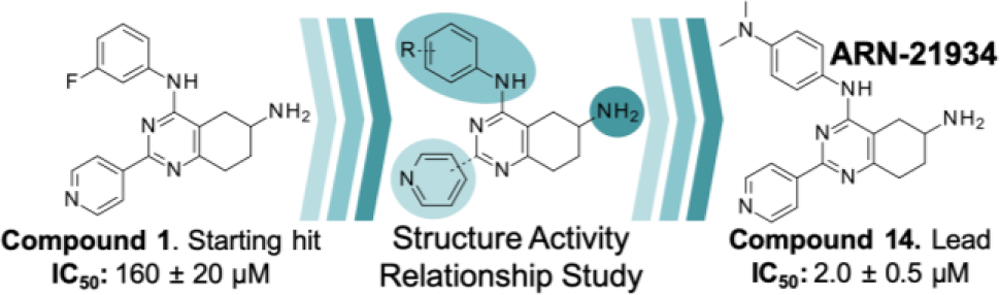
The virtual screening of the D3 library of compounds and the experimental test of the most promising hits resulted in the identification of a new type of topoII inhibitor consisting of bicyclic pyrimidines derivatives, which was used to build a structure-activity relationship, from compound 1 (IC50 of 160 µM) to the lead compound 14 (IC50 of 2 µM, ARN-21934).
Scheme 1.
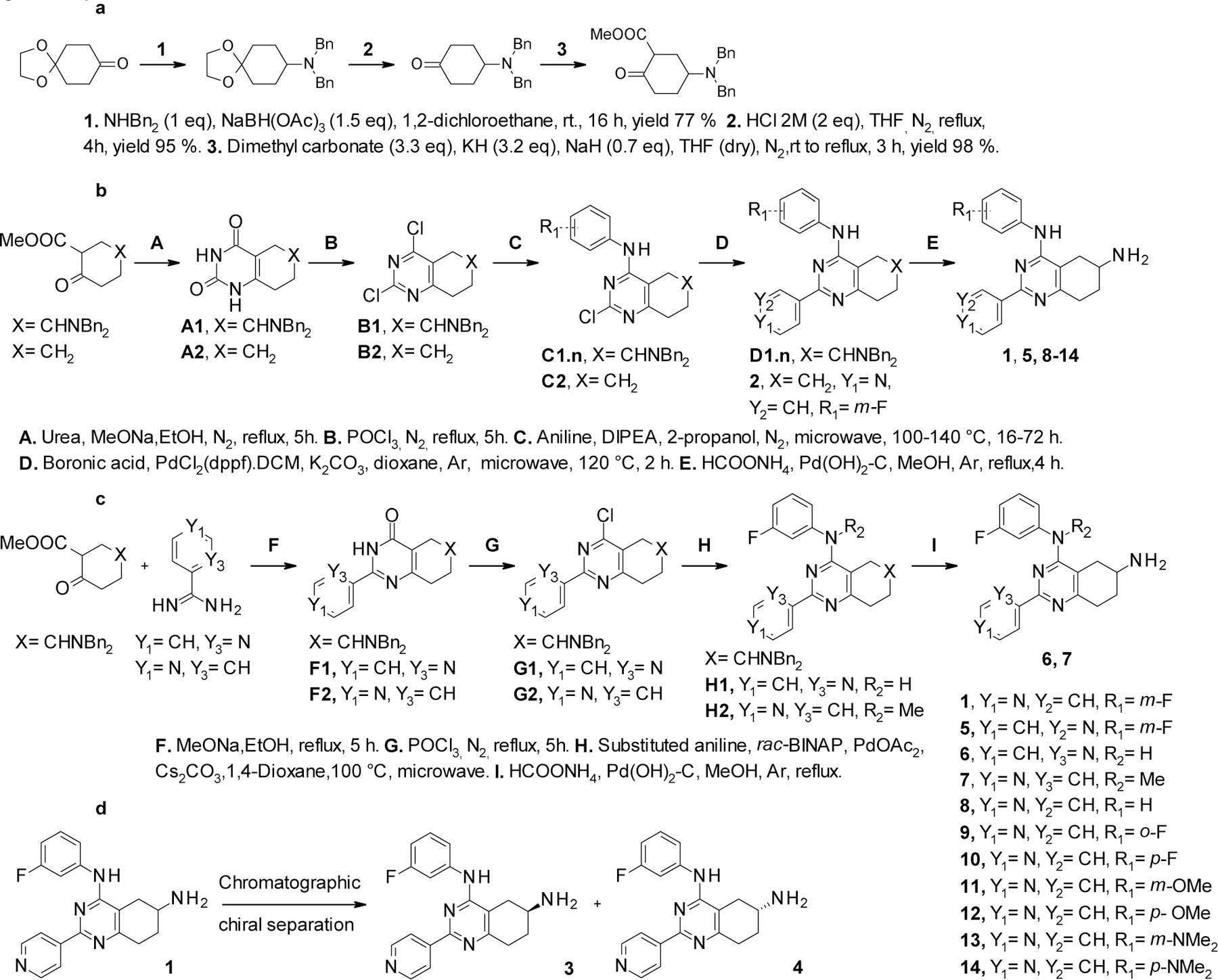
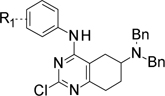
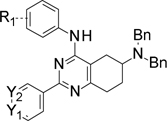
Table 1.
Structures of synthesized intermediates C1.n and D1n.
 |
 |
||||
|---|---|---|---|---|---|
| Intermediate | R1 | Intermediate | R1 | Y1 | Y2 |
| C1.1 | m-F | D1.1 | m-F | N | CH |
| C1.2 | H | D1.2 | m-F | CH | N |
| C1.3 | o-F | D1.3 | H | N | CH |
| C1.4 | p-F | D1.4 | o-F | N | CH |
| C1.5 | m-MeO | D1.5 | p-F | N | CH |
| C1.6 | p-MeO | D1.6 | m-MeO | N | CH |
| C1.7 | m-NMe2 | D1.7 | p-MeO | N | CH |
| C1.8 | p- NMe2 | D1.8 | m-NMe2 | N | CH |
| D1.9 | p- NMe2 | N | CH | ||
In view of its potency, we further characterized 14 at the cellular level, analyzed its solubility and metabolic stability, and evaluated its pharmacokinetic properties in mice. We show that 14 represents a new, isoform-specific and highly promising topoIIα lead inhibitor that may be an excellent starting point for a full-fledge future anticancer drug discovery program. First, we investigated the chiral center at 6 position in 1. We removed the 6-amino group in 1 using the procedure in scheme 1.b, obtaining 2. This, however, led to an inactive compound, suggesting that the amino group is essential for topoIIα inhibition. We then determined that the pure enantiomers 3 and 4, obtained with a semi preparative chiral HPLC purification (Scheme 1.d, see the Experimental Section for detailed chromatographic conditions), were equipotent to the starting racemic mixture in 1. This finding suggests that position 6 stereo configuration does not significantly affect topoIIα inhibition, although the poor inhibitory activity of compounds 1, 3 and 4 advises that further investigation on this point is needed.
Compounds 5, 8-14 were then obtained using a five-step synthetic route (Scheme 1.b) that was characterized by the initial formation of the bicyclic scaffold, followed by the selective introduction of different anilines at position 4, via aromatic nucleophilic substitutions. Different pyridines were then introduced using microwave assisted Suzuki coupling conditions in position 2. Compounds 6 and 7 were synthesized with an alternative procedure (Scheme 1.c). The bicyclic heterocycle was formed functionalized in 2 position and, in a second step, corresponding anilines were introduced via microwave assisted Buchwald coupling conditions.
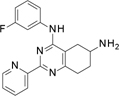
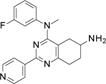
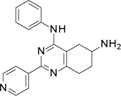
TopoIIα inhibition results (Table 2) indicate that the replacement of the pyridine substituent at 2 position in 1 with the corresponding 3-pyridyl group, as in 5, was partially tolerated, with an IC50 of 200 µM. Alternatively, compound with a 2-pyridyl substituent, which generated 6, was inactive. We therefore decided to maintain the initial pyridine ring at position 2 of the active scaffold. At this point, we investigated the effect of substituents at the 4-aniline position, generating an additional eight compounds (7 to 14). The introduction of a methyl group in the 2-aniline nitrogen, 7, and removal of the fluorine substituent, 8, led to an inactive compound. While a fluorine substituent in the ortho position generated an inactive compound, 9, a para-fluorine substitution was tolerated leading to an IC50 of 220 µM, in 10. However, alternative H-bond acceptor groups did not retain this activity pattern if moved from the initial meta-position as in hit 1. For example, a methoxy substituent in the meta-position, 11, produced an IC50 of 170 µM, but led to an inactive compound for the dimethylamino derivative (compound 13). In contrast, when replacing the fluorine atom in para position, the methoxy substituent in 12 led to the complete loss of activity, while the dimethylamino one in 14 led to a significant boost of the inhibitory activity (IC50 of 2 µM). These results suggest a steric effect of aniline substituents on topoIIα inhibition.
We further characterized 14 for the mechanism by which it inhibited topoII (see SI, figure S2–S5). First, we evaluated its ability to act as a topoII poison, like etoposide, using a DNA cleavage assay (See SI). Surprisingly, the compound displayed no ability to increase levels of enzyme-mediated DNA cleavage, although docking suggested a favorable fit of this scaffold into the cleavage site. We then evaluated the ability of 14 to intercalate into DNA in the absence of enzyme using an unwinding assay. In contrast to quinazolines that intercalate into DNA,[25] 14 did not show this behavior (Figure 3). This finding confirms that this tetrahydroquinazoline derivative can be classified as a topoII inhibitor. Thus, 14 acts differently than all of the currently approved topoII-targeted drugs, which act as poisons.[6–8, 26]
Figure 3.
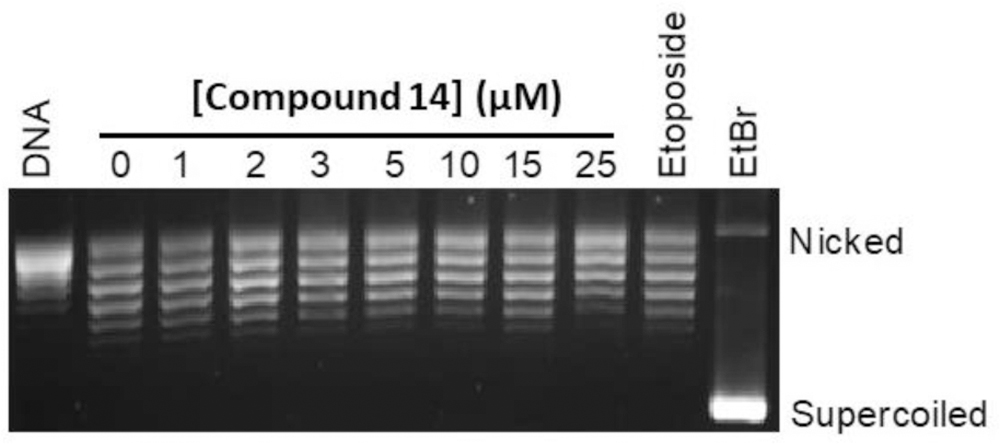
Compound 14 does not intercalate into DNA in the concentration range in which it inhibits topoIIα. A topoisomerase I-DNA unwinding assay was used to monitor intercalation. An ethidium bromide-strained agarose gel is shown. A relaxed DNA control (DNA) is included. The positions of nicked and supercoiled DNA are indicated. Relaxed DNA bands display an electrophoretic mobility between the nicked (more relaxed) and supercoiled (less relaxed) bands. Intercalation is indicated by the shift in the position of the plasmid from relaxed to negatively supercoiled. The effects of 10 µM ethidium bromide (EtBr, a strong intercalator) and 100 µM etoposide (a non-intercalator) on the DNA unwinding assay are shown as controls. The gel is representative of at least three independent experiments.
Most topoisomerase II-targeted agents display a similar affinity for topoIIα and topoIIβ.11, 24 Remarkably, 14 was much more potent against the α isoform (Figure 4). Whereas the IC50 for the inhibition of DNA relaxation by topoIIα was ~2 µM, the value for inhibition of DNA relaxation by topoIIβ was ~100 µM. Thus, 14 is ~50-fold more specific for topoIIα over topoIIβ. Although additional studies will need to be carried out to determine the mechanistic basis for the actions of 14 against topo IIα, the compound is likely to interact at the DNA cleavage/ligation active site of the enzyme, as it displays little ability to inhibit topoIIα-catalyzed ATP hydrolysis in the presence of DNA, and none in the absence of the nucleic acid substrate (see SI, Figure S1–S4).
Figure 4.

Inhibition of DNA relaxation catalyzed by human type II topoisomerases by 14. The inhibition of DNA relaxation by human topoIIα (hTIIα, red) and topoIIβ (hTIIβ, blue) are shown. The inset shows an expanded scale for results with topoIIα. Error bars represent the standard deviations of three independent experiments.
The solubility and metabolic stability of compounds 1, 5, 10, 11 and 14 were also evaluated. While the initial hit 1 showed a moderate kinetic solubility of 126 µM (measured in PBS, 2.5 % di DMSO), all the following derivatives showed an excellent kinetic solubility (>250 µM, see SI table S4). In terms of stability, all of the compounds displayed an excellent profile both in mouse plasma (t1/2>120 min) and in mouse liver microsomes (t1/2>60 min). Taken together, these data confirm a favorable drug-like profile of these tetrahydroquinazoline derivatives.
Lastly, the lead compound 14 was tested at 2 µM for off-target activity (both as agonist and antagonist) on a set of 47 proteins for safety profiling. These include GPRCs, non-kinase and kinase enzymes, transporters, ion channels (including the hERG channel) and nuclear receptors. The compound showed no noteworthy activity toward these unwanted targets (See SI), only a mild inhibitory effect against COX1 was observed with IC50 4–4.5 µM, thus much less potent than the reference COX1 inhibitor indomethacin (IC50 0.051 µM, see SI). The mechanistic implications of mild COX-1 inhibition remain to be investigated.
Encouraged by these results, we also evaluated the antiproliferative activity of the compounds in a small panel of human cancer cell lines (Table 3). We found an anti-proliferative activity with IC50 values in the low µM range against melanoma (A375 and G-361), breast (MCF7), endometrial (HeLa), lung (A549), and androgen-independent prostate (DU145) cancer cell lines. In some cases, the anti-proliferative activity was comparable to that of etoposide, used as the reference compound, while in others, such as lung and endometrial cancer cells, it was less sensitive (IC50 values ~20 µM). It is notable that the most potent compound in vitro, 14, did not induce the phosphorylation of H2AX (see SI, figure S1), which further indicates that the measured cytotoxicity is most likely due to cellular inhibition of topoII, rather than poisoning. Reassuringly, also the enantiomers 3 and 4 were equally active compared to the racemic mixture 1, reinforcing the evidence of no/little influence on cytotoxic mechanisms and activity, of the chiral center in position 6 of the tetrahydroquinazoline scaffold.
Table 3.
Cell proliferation results in various cancer cell lines (A549: lung adenocarcinoma, DU145: androgen-independent prostate cancer, MCF7: breast adenocarcinoma, HeLa: cervical carcinoma, A375: Human melanoma and G-361: Human melanoma. Values shown correspond to the IC50 (µM) ± SD of at least two independent experiments.
| Cell line | 1 | 3 | 4 | 10 | 11 | 14 | Etoposide |
|---|---|---|---|---|---|---|---|
| DU145 | 15.1 ± 5.5 | 17.9 ± 7.4 | 11.1 ± 6.5 | 10.5 ± 6.0 | 8.8 ± 1.5 | 11.5 ± 1.3 | 0.5 ± 0.2 |
| A549 | 19.4 ± 1.8 | 23.3 ± 2.3 | 17.8 ± 0.6 | 22.9 ± 6.5 | 18.0 ± 0.1 | 17.1 ± 8.7 | 2.4 ± 2.0 |
| HeLa | 17.0 ± 1.9 | 17.1 ± 3.2 | 21.8 ± 4.6 | 20.8 ± 4.5 | 17.2 ± 2.6 | 38.2 ± 9.1 | 1.2 ± 0.4 |
| MCF7 | 13.9 ± 2.3 | 14.2 ± 2.5 | 16.2 ± 2.6 | 16.0 ± 0.5 | 15.8 ± 1.4 | 15.8 ± 1.3 | 8.9 ± 1.6 |
| A375 | 9.9 ± 0.1 | 10.7 ± 0.1 | 10.8 ± 0.1 | 12.3 ± 1.8 | 15.1 ± 4.8 | 12.6 ± 0.7 | 0.5 ± 0.1 |
| G361 | 1.4 ± 0.4 | 1.6 ± 0.5 | 1.8 ± 0.6 | 3.0 ± 0.7 | 6.6 ± 3.1 | 8.1 ± 2.9 | 3.8 ± 3.0 |
Finally, we evaluated the pharmacokinetic (PK) profile of 14, in mice (Table 3 and Figure 5). We found that 14 quickly enters the bloodstream after a single intraperitoneal (ip 10 mg kg−1) administration, reaching a maximal plasma concentration of 0.68 μg/ml after 15 min. The half-life was 149 min in circulation, still being present in plasma 360 min after injection. The compound also showed good clearance values (0.116 l/min/kg). Finally, 14 was able to reach the brain, with a maximum concentration of compound at 60 min, and was still present in the brain 300 min after injection. This favorable pharmacokinetic profile confirmed 14 as a highly promising lead compound for future efficacy studies in animal models for cancer, including brain tumors.
Figure 5.
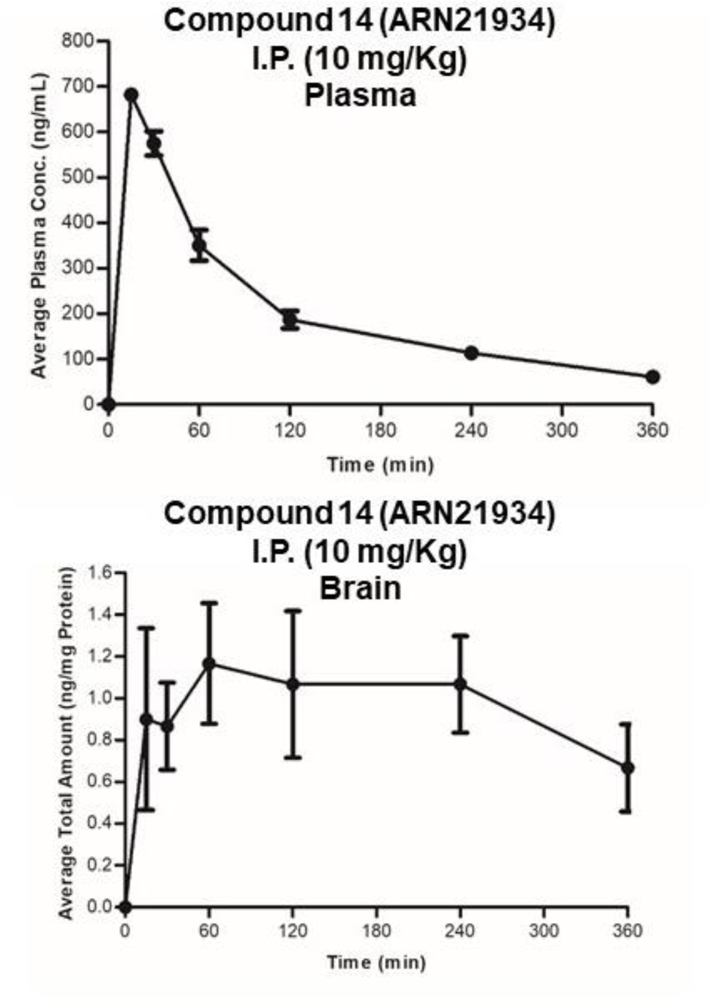
Pharmacokinetic profile of compound 14 (ARN21934) in mouse plasma (top) and brain (down). Strain: C57B6/J. Route of administration: IP Dose: 10 mg/Kg.
CONCLUSIONS
In summary, we have identified a class of potent topoII inhibitors that are based on a tetrahydroquinazoline core. We show that these compounds act as potent topoII inhibitors, with no accumulation of the covalent enzyme/DNA cleavage complex. Therefore, these compounds act as topoII inhibitors as opposed to poisons. Focused SAR studies showed that 2-pyridine, 4-substituted aniline and 6-amino substitutions are mandatory for activity. Indeed, the introduction of a dimethylamino group significantly boosts topoII inhibition and yields 14, which is highly selective for topoIIα over topoIIβ. Notably, these tetrahydroquinazoline derivatives inhibit cell proliferation in various cancer cell lines, especially G-361 melanoma cells. These few novel prototype compounds also show high solubility and a favorable metabolic stability profile. In addition, compound 14 (ARN-21934) displays favorable pharmacokinetics in vivo, including brain penetration. Thus, our results support ARN-21934 for further lead optimization studies to develop novel topoII-targeted therapeutic agents that may lack the development of secondary leukemias associated to topoII poisoning. Further expansion of this chemical class will be reported in due course.
EXPERIMENTAL SECTION
Chemistry.
All the commercially available reagents and solvents were used as purchased from vendors without further purification. Dry solvents were purchased from Sigma-Aldrich. Automated column chromatography purifications were done using a Teledyne ISCO apparatus (CombiFlash® Rf) with pre-packed silica gel columns of different sizes (from 4 g up to 120 g) and mixtures of increasing polarity of cyclohexane and ethyl acetate (EtOAc), cyclohexane and tert-butylmethyleter (TBME) or dicloromethane (DCM) and ethanol (EtOH). NMR experiments were run on a Bruker Avance III 400 system (400.13 MHz for 1H, and 100.62 MHz for 13C), equipped with a BBI probe and Z-gradients. Spectra were acquired at 300 K, using deuterated dimethyl sulfoxide (DMSO–d6), deuterated methanol (CD3OD) or deuterated chloroform (CDCl3) as solvents. For 1H-NMR, data are reported as follows: chemical shift, multiplicity (s= singlet, d= doublet, dd= doublet of doublets, dt= doublet of triplets, td= triplet of doublets, t= triplet, q= quartet, m= multiplet), coupling constants (Hz) and integration. UPLC/MS analyses were run on a Waters ACQUITY UPLC/MS system consisting of a SQD (single quadrupole detector) mass spectrometer equipped with an electrospray ionization interface and a photodiode array detector. The PDA range was 210–400 nm. Analyses were performed on an ACQUITY UPLC BEH C18 column (50×2.1mmID, particle size 1.7 µm) with a VanGuard BEH C18 pre-column (5×2.1 mmID, particle size 1.7 µm). Mobile phase was 10 mM NH4OAc in H2O at pH 5 adjusted with CH3COOH (A) and 10 mM NH4OAc in CH3CN–H2O (95:5) at pH 5.0 (B). Two types of gradients were applied depending on the analysis, gradient 1 (5 % to 95 % mobile phase B in 2.5 min) or gradient 2 (50 % to 100 % mobile phase B in 2.5 min). Electrospray ionization in positive and negative mode was applied. Unless otherwise indicated, all LC/MS instruments, columns and software were from Waters Inc. (Milford, MA, USA). A LC/MS Quality Check (QC) was performed for all compounds before testing. A 10 mM stock solution of the compound was prepared in DMSO-d6, diluted 20-fold with ACN-H2O (1:1) and analyzed on a Waters ACQUITY UPLC/MS system consisting of a SQD (Single Quadrupole Detector) Mass Spectrometer equipped with an Electrospray Ionization interface and a Photodiode Array Detector. Electrospray ionization in positive and negative mode was applied in the mass scan range 100–500 Da. The PDA range was 210–400 nm. The analyses were run on an ACQUITY UPLC BEH C18 column (100×2.1 mmID, particle size 1.7 µm) with a VanGuard BEH C18 pre-column (5×2.1 mmID, particle size 1.7 µm). The mobile phase was 10 mM NH4OAc in H2O at pH 5 adjusted with AcOH (A) and 10 mM NH4OAc in ACN-H2O (95:5) at pH 5 (B) with 0.5 mL/min as flow rate. A linear gradient was applied: 0–0.2min: 10%B, 0.2–6.2min: 10–90%B, 6.2–6.3min: 90–100%, 6.3–7.0min: 100%B. All tested compounds showed ≥ 95% purity by NMR and UPLC/MS analysis
A. General procedure reaction A Scheme 1.a. Pyrimidone fused ring formation
A suspension of corresponding cyclic 2-oxo ethylester (1 mmol) in EtOH (4.5 ml), urea (5 mmol) and sodium methoxide (4.5 mmol) was stirred at reflux temperature for 16 hours. Afterwards the reaction crude was concentrated to dryness at low pressure, resulting solid triturated in water (0.5 ml), ice cooled, pH adjusted to 8–9 with concentrated HCl and filtrated. Resulting solid was then rinsed with methanol (0.5 ml) and diethyl ether (0.5 ml) yielding title compound.
B. General procedure reaction B Scheme 1.a. Pyrimidone fused ring chlorination
A suspension of corresponding pyrimidone fused ring obtained from general method A (1 mmol) in POCl3 (1.5 ml) was stirred at 120 °C under N2 atmosphere for 5 hours (total reaction crude solution observed). POCl3 was then evaporated at low pressure, resulting residue solved in dichloromethane (3 ml), poured onto ice cold NaHCO3 saturated solution (18 ml), aqueous pH adjusted to 7–8 with NaHCO3 (no gas evolution observed after addition), organic layer separated, dried over Na2SO4 and concentrated to dryness at low pressure. Final normal phase chromatography purification yielded title compound.
C. General procedure reaction C Scheme 1.a. Aniline introduction
A suspension of dichlorinated pyrimidinic fused ring obtained from general method B (1 mmol), corresponding aniline (1.1 mmol) and diisopropylethylamine (5 mmol) in 2-propanol (2 ml) was stirred in a CEM® microwave apparatus at 100–160 °C (depending on corresponding aniline) until reaction completion or no crude evolution was observed. Then reaction crude was concentrated to dryness at low pressure, solved in dichloromethane (20 ml), extracted with NaHCO3 saturated solution (20 ml), dried over Na2SO4 and concentrated to dryness at low pressure. Final normal phase purification yielded title compound.
D. General procedure reaction D Scheme 1.a. Suzuki coupling reaction
A suspension of compound obtained from general method C (1 mmol), corresponding boronic acid (1.2 mmol), PdCl2(dppf) dichloromethane complex (0.1 mmol) and K2CO3 2 M solution (2 mmol) in 1,4-dioxane (10 ml) was stirred in a CEM ® microwave apparatus at 120 °C for 2 hours. Resulting crude was portioned between dichloromethane (25 ml), NaHCO3 saturated solution (25 ml), the organic layer dried over Na2SO4 and concentrated to dryness at low pressure. Final normal phase purification yielded title compound.
E. General procedure reaction E Scheme 1.a. Benzyl group removal
Under N2 atmosphere, a suspension of compound to be deprotected (1 mmol), ammonium formate (4 mmol), Pd(OH)2/C (20 % of starting material weight) was stirred at reflux temperature until reaction completion. Catalyst was filtered off trough a celite coarse patch and resulting filtrate concentrated to dryness at low pressure. Final normal phase purification yielded title compound.
F. General procedure reaction F Scheme 1.b. 2-pyrido-4-pyrimidone fused ring formation
A suspension of methyl 5-(dibenzylamino)-2-oxo-cyclohexanecarboxylate (1 mmol) in ethanol (4.5 ml), corresponding pyridinecarboxamidine hydrochloride (5 mmol) and sodium methoxide (5.5 mmol) was stirred at reflux temperature for 16 hours. Afterwards, the reaction crude was concentrated to dryness at low pressure, triturated in water (4.5 ml) and filtered. Resulting solid was then rinsed with MeOH (0.5 ml) yielding title compound.
G. General procedure reaction G Scheme 1.b. 2-pyrido-4-pyrimidone fused ring chlorination
A suspension of corresponding pyrimidone fused ring obtained from general procedure reaction A (1mmol) in POCl3 (1.5 ml) was stirred at 120 °C under N2 atmosphere until total solution was observed (around 4 hours). POCl3 was then evaporated at low pressure, resulting residue solved in dichloromethane (15 ml), poured onto ice cold NaHCO3 saturated solution (15 ml), organic layer separated, dried over Na2SO4 and concentrated to dryness at low pressure. Purification by normal phase chromatography finally yielded title compound.
H. General procedure reaction H Scheme 1.b. Buchwald coupling reaction
A mixture of Pd(OAc)2 (0.10 mmol) and rac-BINAP (0.10 mmol) in 1,4-dioxane (5 ml) was stirred under Ar flushing for 10 minutes. Then were stepwise added corresponding 2-chloro-4-pyrido-pyrimidine fused ring obtained from general procedure reaction G (1 mmol), corresponding substituted aniline (1.2 mmol) and Cs2CO3 (1.4 mmol). The reaction mixture was stirred in a CEM® microwave apparatus at 100 °C until reaction completion, filtrated through a celite coarse patch, rinsed with DCM and concentrated to dryness at low pressure. Final normal phase purification yielded title compound.
I. General procedure reaction I Scheme 1.b. Benzyl group removal
Under N2 atmosphere, a suspension of compound to be deprotected obtained from general procedure reaction H (1 mmol), ammonium formate (4 mmol), Pd(OH)2/C (20% of starting material weight) was stirred at reflux temperature until reaction completion. Catalyst was filtered off through a celite coarse patch and resulting filtrate concentrated to dryness at low pressure. Final normal phase purification yielded title compound.
J. Synthesis of compound 1. N4-(3-fluorophenyl)-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine
Step 1. Synthesis of N,N-dibenzyl-1,4-dioxaspiro[4.5]decan −8-amine (Reaction 1 Scheme 1.a)
A solution of 1,4-cyclohexanedionemonoethyleneacetal (5000 mg, 31.05 mmol) in dry 1,2-dichloroethane (129 ml), dibenzylamine (6.3 ml, 31.05 mmol) and acetic acid (1.8 ml, 31.05 mmol) was stirred for 15 minutes at room temperature. Then sodium triacetoxyborohydride (10391.9 mg, 46.58 mmol) was portionwise added and the reaction mixture stirred at room temperature for 16 hours, afterwards was diluted with DCM (100 ml), extracted with NaHCO3 10 % solution (100 ml), the aqueous layer extracted twice with DCM (2×100 ml), combined organic layers were dried over Na2SO4 and concentrated to dryness at low pressure. Purification by typical silica gel flash chromatography (cyclohexane/TBME from 100/0 to 90/10) afforded the pure title compound (8070 mg, yield 77 %) as a white solid. Rt = 2.15 min (gradient 1); MS (ESI) m/z: 338.3 [M-H]+, [M-H]+ calculated: 338.5. 1H NMR (400 MHz, CDCl3) δ 7.39 – 7.33 (m, 4H), 7.28 (dd, J = 8.5, 7.0 Hz, 4H), 7.24 – 7.16 (m, 2H), 3.96 – 3.87 (m, 4H), 3.64 (s, 4H), 2.57 (tt, J = 11.5, 3.6 Hz, 1H), 1.90 – 1.81 (m, 2H), 1.81 – 1.72 (m, 2H), 1.67 (td, J = 12.5, 3.5 Hz, 2H), 1.45 (td, J = 13.0, 4.3 Hz, 2H).
Step 2. Synthesis of 4-(dibenzylamino)cyclohexanone (Reaction 2 Scheme 1.a)
HCl 2M solution (62 ml, 124.70 mmol) was added to a of N,N-dibenzyl-1,4-dioxaspiro[4.5]decan-8-amine (8070 mg, 23.19 mmol) solution in tetrahydrofuran (62 ml). The reaction mixture was stirred under N2 atmosphere at reflux temperature for 4 hours, then cooled in an ice/water bath, basified with NaOH 5M solution, extracted with EtOAc (3×50 ml), combined organic layers dried over Na2SO4 and concentrated to dryness at low pressure. Purification by typical silica gel flash chromatography (cyclohexane/TBME from 100/0 to 90/10) afforded the pure title compound (6464 mg, yield 95 %). Rt = 1.83 min (gradient 1); MS (ESI) m/z: 294.2 [M-H]+, [M-H]+ calculated: 294.2. 1H NMR (400 MHz, CDCl3) δ 7.40 – 7.35 (m, 4H), 7.34 – 7.28 (m, 4H), 7.26 – 7.20 (m, 2H), 3.66 (s, 4H), 3.02 (tt, J = 11.5, 3.4 Hz, 1H), 2.43 (p, J = 2.4 Hz, 1H), 2.39 (p, J = 2.4 Hz, 1H), 2.31 – 2.22 (m, 2H), 2.20 – 2.14 (m, 2H), 1.88 – 1.78 (m, 2H).
Step 3. Synthesis of methyl 5-(dibenzylamino)-2-oxo-cyclohexanecarboxylate (Reaction 3 Scheme 1.a)
Under N2 atmosphere, a solution of 4-(dibenzylamino)cyclohexanone (6200 mg, 20.50 mmol) in tetrahydrofuran (2.5 ml) was dropwise added to a suspension of KH (5261.3 mg, 65.59 mmol) and NaH (414.2 mg, 16.40 mmol) in dry tetrahydrofuran (256 ml) at room temperature. The reaction mixture was stirred for 30 minutes and dimethyl carbonate was added (5.9 ml, 69.08 mmol), then stirred at reflux temperature under for 3 hours, cooled to room temperature, added to cold NaHCO3 saturated solution (100 ml), the organic layer separated, the aqueous one extracted with ethyl acetate (100 ml), the combined organic layers dried over Na2SO4 and concentrated to dryness at low pressure. Purification by typical silica gel flash chromatography (cyclohexane/TBME from 100/0 to 90/10) afforded the pure title compound (7058 mg, yield 98 %). Retention time 2.54 min (gradient 1); MS (ESI) m/z: 352.2 [M-H]+, [M-H]+ calculated: 352.2. 1H NMR (400 MHz, CDCl3) δ 12.06 (s, 1H), 7.42 – 7.34 (m, 4H), 7.29 (dd, J = 8.3, 6.7 Hz, 4H), 7.24 – 7.18 (m, 2H), 3.76 (s, 3H), 3.72 (d, J = 14.0 Hz, 2H), 3.62 (d, J = 14.0 Hz, 2H), 2.88 – 2.73 (m, 1H), 2.54 – 2.43 (m, 1H), 2.43 – 2.18 (m, 3H), 2.05 – 1.94 (m, 1H), 1.74 – 1.57 (m, 1H).
Step 4. Synthesis of 6-(dibenzylamino)-5,6,7,8-tetrahydro-1H-quinazoline-2,4-dione (compound A1 Scheme 1.b)
This compound was obtained using 5-(dibenzylamino)-2-oxo-cyclohexanecarboxylate (7040.0 mg, 20.03 mmol) following the general procedure A previously described affording a brown solid (5865 mg, yield 81 %). Rt = 0.94 min (gradient 1); MS (ESI) m/z: 362.1 [M-H]+, [M-H]+ calculated: 362.4. 1H NMR (400 MHz, DMSO-d6) δ 10.85 (br s, 1H), 10.56 (br s, 1H), 7.36 (d, J = 7.2 Hz, 4H), 7.30 (t, J = 7.2 Hz, 4H), 7.20 (t, J = 7.2 Hz, 2H), 3.67 (d, J = 14.2 Hz, 2H), 3.59 (d, J = 14.2 Hz, 2H), 2.76 – 2.61 (m, 1H), 2.48 – 2.37 (m, 2H), 2.37 – 2.22 (m, 1H), 2.25 – 2.11 (m, 1H), 2.06 – 1.93 (m, 1H), 1.64 (qd, J = 12.1, 5.6 Hz, 1H).
Step 5. Synthesis of N,N-dibenzyl-2,4-dichloro-5,6,7,8-tetrahydroquinazolin-6-amine (compound B1 Scheme 1.b)
This compound was obtained using compound A1 (5862 mg, 16.22 mmol) following the general procedure B previously described and affording a white solid (5685 mg, yield 88 %). Rt = 2.60 min (gradient 2); MS (ESI) m/z: 398.2/400.2/402.2 [M-H]+, [M-H]+ calculated: 398.2/400.2/402.2. 1H NMR (400 MHz, CDCl3) δ 7.41 (d, J = 7.4 Hz, 4H), 7.32 (t, J = 7.4 Hz, 4H), 7.23 (d, J = 7.4 Hz, 2H), 3.82 (d, J = 13.7 Hz, 2H), 3.69 (d, J = 13.7 Hz, 2H), 3.19 – 3.00 (m, 1H), 3.02 – 2.86 (m, 1H), 2.87 – 2.65 (m, 2H), 2.42 – 2.12 (m, 1H), 2.01 – 1.56 (m, 2H).
Step 6. Synthesis of N6, N6-dibenzyl-2-chloro-N4-(3-fluorophenyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound C1.1 Scheme 1.b)
This compound was obtained using compound B1 (400 mg, 1.0 mmol) and 3-Fluoroaniline (0.12 ml, 1.21 mmol) following the general procedure C previously described at 100 °C for 72 h. Final normal phase purification (cyclohexane/TBME from 100/0 to 80/20) afforded pure title compound (152 mg, yield 32 %). Rt = 2.61 min (gradient 2); MS (ESI) m/z: 471.1/473.1 [M-H]+, [M-H]+ calculated: 471.2/473.2. 1H NMR (400 MHz, CDCl3) δ 7.56 (dt, J = 11.0, 2.3 Hz, 1H), 7.45 – 7.38 (m, 4H), 7.37 – 7.28 (m, 5H), 7.30 – 7.19 (m, 3H), 6.82 (tdd, J = 8.2, 2.5, 1.2 Hz, 1H), 6.38 (s, 1H), 3.86 (d, J = 14.1 Hz, 2H), 3.67 (d, J = 14.0 Hz, 2H), 3.17 – 3.05 (m, 1H), 2.95 (ddd, J = 18.2, 5.0, 2.4 Hz, 1H), 2.71 (ddd, J = 18.1, 12.1, 5.6 Hz, 1H), 2.55 – 2.46 (m, 2H), 2.32 – 2.21 (m, 1H), 1.78 (qd, J = 12.3, 5.1 Hz, 1H).
Step 4. N6, N6-dibenzyl-N4-(3-fluorophenyl)-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound D1.1 Scheme 1.b)
This compound was obtained using compound C1.1 (150 mg, 0.32 mmol) and Pyridine-4-boronic acid (52 mg, 0.38 mmol) following the general procedure D previously described. Final normal phase purification (cyclohexane/TBME from 80/20 to 50/20) afforded pure title compound (157 mg, yield 96%). Rt = 2.74 min (gradient 2); MS (ESI) m/z: 516.4 [M-H]+, [M-H]+ calculated: 516.2. 1H NMR (400 MHz, DMSO-d6) δ 8.72 (s, 1H), 8.70 – 8.67 (m, 2H), 8.10 – 8.05 (m, 2H), 7.73 (dt, J = 12.0, 2.3 Hz, 1H), 7.63 (ddd, J = 8.2, 2.0, 0.9 Hz, 1H), 7.48 – 7.39 (m, 5H), 7.32 (dd, J = 8.3, 6.9 Hz, 4H), 7.25 – 7.17 (m, 2H), 6.92 (tdd, J = 8.4, 2.5, 0.9 Hz, 1H), 3.80 (d, J = 14.2 Hz, 2H), 3.70 (d, J = 14.2 Hz, 2H), 3.05 – 2.84 (m, 3H), 2.84 – 2.63 (m, 2H), 2.16 (d, J = 12.3 Hz, 1H), 1.85 (tt, J = 12.0, 6.2 Hz, 1H).
Step 5. N4-(3-fluorophenyl)-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound 1 Scheme 1.b)
This compound was obtained using compound D1.1 (154.3 mg, 0.29 mmol) following the general procedure E previously described for 4 hours. Final normal phase purification (DCM/DCM:NH3 1M MeOH 4:1 from 90/10 to 60/40) afforded pure title compound as a solid (62.9 mg, yield 64 %). Rt = 1.53 min (gradient 1); MS (ESI) m/z: 336.2 [M-H]+, [M-H]+ calculated: 336.2. HRMS m/z: 336.1623, calculated for C19H19FN5+: 336.1624. QC analysis: Rt= 2.58 min, m/z: 336.10 [M-H]+, UV (215 nm): 98 %. 1H NMR (400 MHz, DMSO-d6) δ 8.73 (s, 1H), 8.72 – 8.67 (m, 2H), 8.16 – 8.05 (m, 2H), 7.75 (dt, J = 12.1, 2.3 Hz, 1H), 7.64 (dd, J = 8.0, 1.9 Hz, 1H), 7.41 (td, J = 8.2, 6.9 Hz, 1H), 6.90 (td, J = 8.4, 2.6 Hz, 1H), 3.27 (s, 3H), 2.89 (dddd, J = 19.8, 15.1, 8.6, 5.4 Hz, 3H), 2.46 – 2.35 (m, 1H), 2.09 – 1.89 (m, 1H), 1.70 (dtd, J = 12.6, 9.5, 5.9 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 163.08 (C), 162.05 (CF, d, JCF = 240.7 Hz), 158.61 (C), 157.26 (C), 150.24 (CH), 145.23 (C), 141.69 (C, d, JCF = 11.1 Hz), 129.86 (CH, d, JCF = 9.6 Hz), 121.29 (CH), 117.23 (CH, d, JCF = 1.5 Hz), 112.76 (C), 109.11 (CH, d, JCF = 21.0 Hz), 108.11 (CH, d, JCF = 25.9 Hz), 46.01 (CH), 31.84 (CH2), 30.60 (CH2), 30.35 (CH2).
K. Synthesis of compound 2. N-(2-fluorophenyl)-2-(4-pyridyl)-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-4-amine
Step 1. Synthesis of 5,6,7,8-tetrahydro-1H-quinazoline-2,4-dione (compound A2, Scheme 1.b)
This compound was obtained using ethyl 2-oxocyclohexanecarboxylate (10000.0 mg, 55.81 mmol) following the general procedure A previously described and affording a brown solid (8440 mg, yield 91 %). Rt = 1.01 min (gradient 1); MS (ESI) m/z: 167.1 [M-H]+, [M-H]+ calculated: 167.1. 1H NMR (400 MHz, DMSO-d6) δ 10.71 (s, 2H), 2.29 (t, J = 6.1 Hz, 2H), 2.13 (t, J = 6.0 Hz, 2H), 1.61 (dddd, J = 17.5, 9.3, 7.6, 4.6 Hz, 4H).
Step 2. Synthesis of 2,4-dichloro-5,6,7,8-tetrahydroquinazoline (compound B2, Scheme 1.a)
This compound was obtained using compound A2 (3000 mg, 18.05 mmol) following the general procedure B previously described and affording pure white solid (2055 mg, yield 56 %). Rt = 2.21 min (gradient 1); MS (ESI) m/z: 203.1/205.1/207.1 [M-H]+, [M-H]+ calculated: 203.1/205.1/207.1. 1H NMR (400 MHz, CDCl3) δ 2.88 (ddt, J = 5.4, 4.1, 2.5 Hz, 2H), 2.73 (ddt, J = 6.6, 4.6, 2.3 Hz, 2H), 1.88 (h, J = 3.8, 3.3 Hz, 4H).
Step 3. Synthesis of 2-Chloro-N-(3-fluorophenyl)-5,6,7,8-tetrahydroquinazolin-4-amine (compound C2, Scheme 1.b)
This compound was obtained using compound B2 (300 mg, 1.48 mmol) and 3-Fluoroaniline (0.16 ml, 1.21 mmol) following the general procedure C previously described at 120 °C for 72 h. Final normal phase purification (cyclohexane/EtOAc from 100/0 to 80/20) afforded pure title compound (115 mg, yield 28 %). Rt = 2.32 min (gradient 1); MS (ESI) m/z: 278.1/280.1 [M-H]+, [M-H]+ calculated: 278.1/280.1. 1H NMR (400 MHz, DMSO-d6) δ 8.76 (s, 1H), 7.61 (dt, J = 11.9, 2.3 Hz, 1H), 7.48 (ddd, J = 8.2, 2.0, 0.9 Hz, 1H), 7.37 (m, 1H), 6.91 (tdd, J = 8.5, 2.6, 0.9 Hz, 1H), 2.69 – 2.60 (m, 2H), 2.58 – 2.52 (m, 2H), 1.86 – 1.69 (m, 4H).
Step 4. Synthesis of N-(2-fluorophenyl)-2-(4-pyridyl)-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-4-amine (compound 2 Scheme 1.b)
This compound was obtained using compound C2 (110 mg, 0.40 mmol) and Pyridine-4-boronic acid (64.9 mg, 0.48 mmol) following the general procedure D previously described. Final normal phase purification (cyclohexane/EtOAc from 80/20 to 60/40) afforded pure title compound (65 mg, yield 51 %). Rt = 2.41 min (gradient 1); MS (ESI) m/z: 321.1 [M-H]+, [M-H]+ calculated: 321.1. HRMS m/z: 321.1509, calculated for C19H18FN4+: 321.1515. QC analysis: Rt= 4.82 min, m/z: 321.79 [M-H]+, UV (215 nm): >99.5 %. 1H NMR (400 MHz, DMSO-d6) δ 8.72 – 8.69 (m, 2H), 8.62 (s, 1H), 8.15 – 8.08 (m, 2H), 7.77 (dt, J = 12.1, 2.3 Hz, 1H), 7.64 (dd, J = 8.1, 1.8 Hz, 1H), 7.41 (td, J = 8.2, 6.9 Hz, 1H), 6.90 (td, J = 8.4, 2.6 Hz, 1H), 2.79 (t, J = 5.9 Hz, 2H), 2.64 (t, J = 5.8 Hz, 2H), 1.94 – 1.77 (m, 4H). 13C NMR (151 MHz, DMSO-d6) δ 163.53 (C), 162.03 (CF, d, JCF = 240.9 Hz), 158.42 (C), 157.12 (C), 150.26 (CH), 145.21 (C), 141.64 (C, d, JCF = 11.0 Hz), 129.88 (CH, d, JCF = 9.2 Hz), 121.25 (CH), 117.25 (CH, d, JCF = 2.1 Hz), 114.08 (C), 109.14 (CH, d, JCF = 21.0 Hz), 108.12 (CH, d, JCF = 26.1 Hz), 31.98 (CH2), 22.59 (CH2), 21.62 (CH2).
L. Chiral Chromatographic separation of Compound 3 Scheme 1.d ((6R)-N4-(3-fluorophenyl)-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine) and Compound 4 Scheme 1.d ((6S)-N4-(3-fluorophenyl)-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine)
Chiral semi-preparative separation of both enantiomers by HPLC was run on a Waters Alliance HPLC instrument consisting of an e2695 Separation Module and a 2998 Photodiode Array Detector. The PDA range was 210–400 nm. The separation was performed isocratic on a ChiralCel OD-H column (250×10mm ID, particle size: 5µm) using 0.1% TEA Heptane-EtOH (95:5) as mobile phase at 5 ml/min. Post-analysis of each isolated enantiomer was performed on a ChiralCel OD-H column (250×4.6mm ID, particle size: 5µm) using 0.1% TEA Heptane-EtOH (95:5) as mobile phase at 1 ml/min. Chiral structure of enantiomers 3 and 4 was arbitrary assigned.
(6R)-N4-(3-fluorophenyl)-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (3, Scheme 1.d):
Enantiomeric purity of 95.2% ee at 290 nm of the first eluting enantiomer at Rt = 63.3 min in ChiralCel OD-H column. Rt = 1.53 min (gradient 1); MS (ESI) m/z: 336.2 [M-H]+, [M-H]+ calculated: 336.2. HRMS m/z: 336.1623, calculated for C19H19FN5+: 336.1624. QC analysis: Rt= 2.57 min, m/z: 336.11 [M-H]+, UV (215 nm): >99.5 %. 1H NMR (400 MHz, DMSO-d6) δ 8.73 (s, 1H), 8.72 – 8.67 (m, 2H), 8.16 – 8.05 (m, 2H), 7.75 (dt, J = 12.1, 2.3 Hz, 1H), 7.64 (dd, J = 8.0, 1.9 Hz, 1H), 7.41 (td, J = 8.2, 6.9 Hz, 1H), 6.90 (td, J = 8.4, 2.6 Hz, 1H), 3.27 (s, 3H), 2.89 (dddd, J = 19.8, 15.1, 8.6, 5.4 Hz, 3H), 2.46 – 2.35 (m, 1H), 2.09 – 1.89 (m, 1H), 1.70 (dtd, J = 12.6, 9.5, 5.9 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 163.08 (C), 162.05 (CF, d, JCF = 240.7 Hz), 158.61 (C), 157.26 (C), 150.24 (CH), 145.23 (C), 141.69 (C, d, JCF = 11.1 Hz), 129.86 (CH, d, JCF = 9.6 Hz), 121.29 (CH), 117.23 (CH, d, JCF = 1.5 Hz), 112.76 (C), 109.11 (CH, d, JCF = 21.0 Hz), 108.11 (CH, d, JCF = 25.9 Hz), 46.01 (CH), 31.84 (CH2), 30.60 (CH2), 30.35 (CH2).
(6S)-N4-(3-fluorophenyl)-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (4, Scheme 1.d):
Enantiomeric purity >99.5% ee at 290 nm of the second eluting enantiomer at Rt = 67. 6 min in ChiralCel OD-H column. Rt = 1.53 min (gradient 1); MS (ESI) m/z: 336.2 [M-H]+, [M-H]+ calculated: 336.2. HRMS m/z: 336.1623, calculated for C19H19FN5+: 336.1624. QC analysis: Rt= 2.56 min, m/z: 336.14 [M-H]+, UV (215 nm): >99.5 %. 1H NMR (400 MHz, DMSO-d6) δ 8.73 (s, 1H), 8.72 – 8.67 (m, 2H), 8.16 – 8.05 (m, 2H), 7.75 (dt, J = 12.1, 2.3 Hz, 1H), 7.64 (dd, J = 8.0, 1.9 Hz, 1H), 7.41 (td, J = 8.2, 6.9 Hz, 1H), 6.90 (td, J = 8.4, 2.6 Hz, 1H), 3.27 (s, 3H), 2.89 (dddd, J = 19.8, 15.1, 8.6, 5.4 Hz, 3H), 2.46 – 2.35 (m, 1H), 2.09 – 1.89 (m, 1H), 1.70 (dtd, J = 12.6, 9.5, 5.9 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 163.08 (C), 162.05 (CF, d, JCF = 240.7 Hz), 158.61 (C), 157.26 (C), 150.24 (CH), 145.23 (C), 141.69 (C, d, JCF = 11.1 Hz), 129.86 (CH, d, JCF = 9.6 Hz), 121.29 (CH), 117.23 (CH, d, JCF = 1.5 Hz), 112.76 (C), 109.11 (CH, d, JCF = 21.0 Hz), 108.11 (CH, d, JCF = 25.9 Hz), 46.01 (CH), 31.84 (CH2), 30.60 (CH2), 30.35 (CH2).
M. Synthesis of compound 5. N4-(3-fluorophenyl)-2-(3-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine
Step 1. Synthesis of N6,N6-dibenzyl-N4-(3-fluorophenyl)-2-(3-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound D1.2 Scheme 1.b)
This compound was obtained using compound C1.1 (55 mg, 0.12 mmol) and Pyridine-3-boronic acid (19.2 mg, 0.14 mmol) following the general procedure D previously described. Final normal phase purification (cyclohexane/TBME from 80/20 to 50/50) afforded pure title compound (58.0 mg, yield 96 %). Rt = 3.0 min (gradient 2); MS (ESI) m/z: 516.4 [M-H]+, [M-H]+ calculated: 516.2. 1H NMR (400 MHz, CDCl3) δ 9.54 (dd, J = 2.2, 0.9 Hz, 1H), 8.65 (dd, J = 4.8, 1.7 Hz, 1H), 8.58 (dt, J = 7.9, 2.0 Hz, 1H), 7.68 (dt, J = 11.2, 2.3 Hz, 1H), 7.46 (d, J = 7.8 Hz, 5H), 7.41 – 7.29 (m, 7H), 7.26 (s, 2H), 6.81 (tdd, J = 8.3, 2.6, 1.2 Hz, 1H), 6.49 (s, 1H), 3.91 (d, J = 13.7 Hz, 2H), 3.71 (d, J = 14.0 Hz, 2H), 3.29 – 3.10 (m, 1H), 3.11 – 2.98 (m, 1H), 2.80 (ddd, J = 17.9, 12.2, 5.4 Hz, 1H), 2.65 (d, J = 8.3 Hz, 2H), 2.46 – 2.22 (m, 1H), 1.84 (qd, J = 12.2, 4.8 Hz, 1H)
Step 2. Synthesis of N4-(3-fluorophenyl)-2-(3-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound 5 Scheme 1.b)
This compound was obtained using compound D1.2 (58.0 mg, 0.11 mmol) following the general procedure E previously described for 4 hours. Final normal phase purification (DCM / DCM:NH3 1N MeOH 4:1 from 95/5 to 75/25) afforded pure title compound (12.2 mg, yield 33 %). Rt = 1.71 min (gradient 1); MS (ESI) m/z: 336.1 [M-H]+, [M-H]+ calculated: 336.2. HRMS m/z: 336.1620, calculated for C19H19 FN5+: 336.1624. QC analysis: Rt= 2.67 min, m/z: 336.10 [M-H]+, UV (215 nm): 95 %. 1H NMR (400 MHz, CD3OD) δ 9.45 – 9.32 (m, 1H), 8.64 (dt, J = 8.0, 1.9 Hz, 1H), 8.58 (dd, J = 4.9, 1.7 Hz, 1H), 7.70 (dt, J = 11.7, 2.3 Hz, 1H), 7.56 – 7.44 (m, 2H), 7.35 (td, J = 8.2, 6.6 Hz, 1H), 6.83 (tdd, J = 8.4, 2.6, 0.9 Hz, 1H), 3.29 – 3.22 (m, 1H), 3.04 – 2.83 (m, 3H), 2.40 (dd, J = 16.3, 9.1 Hz, 1H), 2.21 – 2.08 (m, 1H), 1.77 (dtd, J = 12.8, 10.3, 6.2 Hz, 1H). 13C NMR (101 MHz, CD3OD) δ 164.21 (CF, d, JCF = 241.9 Hz), 163.95 (C), 159.68 (C), 150.93 (CH), 149.77 (CH), 137.16 (CH), 135.75 (C), 130.75 (CH, d, JCF = 9.3 Hz), 125.04 (CH), 118.43 (CH, d, JCF = 2.6 Hz), 112.15 (C), 110.71 (CH, d, JCF = 21.4 Hz), 109.86 (CH, d, JCF = 26.0 Hz), 47.71 (CH), 31.91 (CH2), 31.62 (CH2), 31.25 (CH2).
N. Synthesis of compound 6. N4-(3-fluorophenyl)-2-(2-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine
Step 1. Synthesis of 6-(dibenzylamino)-2-(2-pyridyl)-5,6,7,8-tetrahydro-3H-quinazolin-4-one (compound F1 Scheme 1.c)
This compound was obtained using 5-(dibenzylamino)-2-oxo-cyclohexanecarboxylate (335.0 mg, 0.95 mmol) and pyridine-2-carboxamidine hydrochloride (774 mg, 4.77 mmol) following the general procedure F previously described and affording a pure white solid (300 mg, yield 74 %). Rt = 1.89 min (gradient 2); MS (ESI) m/z: 423.5 [M-H]+, [M-H]+ calculated: 423.2. 1H NMR (400 MHz, DMSO-d6) δ 11.81 (s, 1H), 8.70 (dt, J = 4.6, 1.3 Hz, 1H), 8.23 (d, J = 7.9 Hz, 1H), 7.99 (td, J = 7.8, 1.7 Hz, 1H), 7.60 (ddd, J = 7.7, 4.7, 1.2 Hz, 1H), 7.43 – 7.36 (m, 4H), 7.31 (dd, J = 8.3, 6.8 Hz, 4H), 7.25 – 7.15 (m, 2H), 3.73 (d, J = 14.3 Hz, 2H), 3.65 (d, J = 14.2 Hz, 2H), 2.91 – 2.76 (m, 1H), 2.73 (s, 1H), 2.70 – 2.54 (m, 2H), 2.12 – 2.05 (m, 1H), 1.77 (qd, J = 12.0, 5.2 Hz, 1H).
Step 2. Synthesis of N,N-dibenzyl-4-chloro-2-(2-pyridyl)-5,6,7,8-tetrahydroquinazolin-6-amine (compound G1 Scheme 1.c)
This compound was obtained using compound F1 (300.0 mg, 0.71 mmol) following the general procedure G previously described. Normal phase purification (CHCl3/ CHCl3:MeOH 4:1 from 100/0 to 50/50) afforded pure title compound (275 mg, yield 88 %). Rt = 2.29 min (gradient 2); MS (ESI) m/z: 441/443 [M-H]+, [M-H]+ calculated: 441/443. 1H NMR (400 MHz, CDCl3) δ 8.82 (ddd, J = 4.7, 1.8, 0.9 Hz, 1H), 8.44 (dt, J = 8.0, 1.1 Hz, 1H), 7.82 (td, J = 7.8, 1.8 Hz, 1H), 7.46 – 7.39 (m, 4H), 7.37 (ddd, J = 7.5, 4.8, 1.2 Hz, 1H), 7.35 – 7.28 (m, 4H), 7.25 – 7.20 (m, 2H), 3.83 (d, J = 14.0 Hz, 2H), 3.70 (d, J = 14.1 Hz, 2H), 3.28 (ddd, J = 18.3, 5.0, 2.5 Hz, 1H), 3.16 – 3.00 (m, 2H), 2.98 – 2.79 (m, 2H), 2.29 (ddd, J = 12.5, 5.4, 2.6 Hz, 1H), 1.85 (qd, J = 12.3, 5.0 Hz, 1H).
Step 3. Synthesis of N6,N6-dibenzyl-N4-(3-fluorophenyl)-2-(2-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound H1 Scheme 1.c)
This compound was obtained using compound G1 (135 mg, 0.31 mmol) and 3-Fluoroaniline (0.036 ml, 0.37 mmol) following the general procedure H previously described for 1 hour. Final normal phase purification (cyclohexane/EtOAc from 40/60 to 60/40) afforded pure title compound (131.1 mg, yield 83 %). Rt = 2.26 min (gradient 1); MS (ESI) m/z: 516.5 [M-H]+, [M-H]+ calculated: 516.2. 1H NMR (400 MHz, DMSO-d6) δ 8.69 (ddd, J = 4.7, 1.8, 0.9 Hz, 1H), 8.63 (s, 1H), 8.20 (dt, J = 8.0, 1.1 Hz, 1H), 8.06 (dt, J = 12.5, 2.3 Hz, 1H), 7.90 (td, J = 7.7, 1.8 Hz, 1H), 7.70 (ddd, J = 8.3, 2.0, 0.9 Hz, 1H), 7.48 – 7.41 (m, 5H), 7.38 (td, J = 8.3, 7.0 Hz, 1H), 7.32 (t, J = 7.6 Hz, 4H), 7.26 – 7.18 (m, 2H), 6.86 (tdd, J = 8.4, 2.6, 0.9 Hz, 1H), 3.80 (d, J = 14.2 Hz, 2H), 3.70 (d, J = 14.2 Hz, 2H), 3.06 – 2.86 (m, 3H), 2.85 – 2.69 (m, 1H), 2.25 – 2.10 (m, 1H), 1.85 (qd, J = 12.2, 5.0 Hz, 1H).
Step 4. Synthesis of N4-(3-fluorophenyl)-2-(2-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound 6 Scheme 1.c)
This compound was obtained using compound H1 (131 mg, 0.25 mmol) following the general procedure I previously described for 4 hours. Final normal phase purification (DCM / DCM:NH3 1N MeOH 4:1 from 90/10 to 60/40) afforded pure title compound (51.1 mg, yield 60 %). Rt = 1.57 min (gradient 1); MS (ESI) m/z: 336.1 [M-H]+, [M-H]+ calculated: 336.2. HRMS m/z: 336.1623, calculated for C19H19FN5+: 336.1624. QC analysis: Rt= 2.70 min, m/z: 336.50 [M-H]+, UV (215 nm): 97 %. 1H NMR (400 MHz, DMSO-d6) δ 8.71 (ddd, J = 4.7, 1.8, 0.9 Hz, 1H), 8.58 (s, 1H), 8.23 (dt, J = 8.0, 1.1 Hz, 1H), 8.10 (dt, J = 12.6, 2.3 Hz, 1H), 7.92 (td, J = 7.7, 1.8 Hz, 1H), 7.78 – 7.68 (m, 1H), 7.46 (ddd, J = 7.5, 4.7, 1.2 Hz, 1H), 7.35 (td, J = 8.2, 7.0 Hz, 1H), 6.90 – 6.79 (m, 1H), 3.18 (tdd, J = 8.4, 5.0, 3.0 Hz, 1H), 2.96 – 2.71 (m, 3H), 2.35 (dd, J = 17.0, 8.3 Hz, 1H), 2.00 – 1.89 (m, 1H), 1.64 (dtd, J = 12.7, 9.6, 5.8 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 163.48 (C), 162.56 (d, JCF = 240.1 Hz), 159.52 (C), 159.02 (C), 155.95 (C), 149.91 (CH), 142.61 (C, d, JCF = 11.5 Hz), 137.19 (CH), 130.14 (CH, d, JCF = 9.5 Hz), 124.82 (CH), 123.27 (CH), 117.00 (CH), 112.75 (C), 108.95 (CH, d, JCF = 21.2 Hz), 108.06 (CH, d, JCF = 26.8 Hz), 46.58 (CH), 32.65 (CH2), 31.62 (CH2), 30.89 (CH2).
O. Synthesis of compound 7. N4-(3-fluorophenyl)-N4-methyl-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine
Step 1. Synthesis of 6-(dibenzylamino)-2-(4-pyridyl)-5,6,7,8-tetrahydro-3H-quinazolin-4-one (compound F2 Scheme 1.c)
This compound was obtained using 5-(dibenzylamino)-2-oxo-cyclohexanecarboxylate (2000.0 mg, 5.69 mmol) and pyridine-4-carboxamidine hydrochloride (4624.0 mg, 28.46 mmol) following the general procedure F previously described and affording a pure white solid (1620 mg, yield 67 %). Rt = 1.34 min (gradient 2); MS (ESI) m/z: 423.5 [M-H]+, [M-H]+ calculated: 423.2. 1H NMR (400 MHz, DMSO-d6) δ 12.78 (s, 1H), 8.79 – 8.69 (m, 2H), 8.03 – 7.93 (m, 2H), 7.40 (d, J = 7.1 Hz, 4H), 7.32 (t, J = 7.5 Hz, 4H), 7.21 (t, J = 7.3 Hz, 2H), 3.74 (d, J = 14.2 Hz, 2H), 3.66 (d, J = 14.2 Hz, 2H), 2.90 – 2.76 (m, 1H), 2.78 – 2.70 (m, 1H), 2.71 – 2.55 (m, 2H), 2.17 – 2.05 (m, 1H), 1.77 (qd, J = 12.0, 5.4 Hz, 1H).
Step 2. Synthesis of N,N-dibenzyl-4-chloro-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazolin-6-amine (compound G2 Scheme 1.c)
This compound was obtained using compound F2 (1620.0 mg, 3.83 mmol) following the general procedure G previously described. Normal phase purification (CHCl3/CHCl3:MeOH 4:1 from 95/5 to 65/35) afforded pure title compound (1488 mg, yield 88 %). Rt = 2.67 min (gradient 2); MS (ESI) m/z: 441/443 [M-H]+, [M-H]+ calculated: 441/443. 1H NMR (400 MHz, DMSO-d6) δ 8.99 – 8.89 (m, 2H), 8.55 – 8.39 (m, 2H), 7.73 (s, 2H), 7.62 (d, J = 6.0 Hz, 2H), 7.51 – 7.26 (m, 6H), 4.72 (d, J = 13.7 Hz, 2H), 4.60 (d, J = 12.2 Hz, 2H), 3.84 – 3.68 (m, 1H), 3.62 – 3.50 (m, 1H), 3.47 – 3.32 (m, 1H), 3.25 – 3.17 (m, 1H), 3.09 – 2.91 (m, 1H), 2.81 – 2.64 (m, 1H), 2.29 – 2.11 (m, 1H).
Step 3. Synthesis of N6,N6-dibenzyl-N4-(3-fluorophenyl)-N4-methyl-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound H2 Scheme 1.c)
This compound was obtained using compound G1 (150.0 mg, 0.34 mmol) and 3-Fluoro-N-methylaniline (52.7 mg, 0.41mmol) following the general procedure H previously described for 6.5 hours. Final normal phase purification (cyclohexane/EtOAc from 95/5 to 70/30) afforded pure title compound (80.7 mg, yield 46 %). Rt = 1.72 min (gradient 1); MS (ESI) m/z: 530.5 [M-H]+, [M-H]+ calculated: 530.3. 1H NMR (400 MHz, DMSO-d6) δ 8.76 – 8.67 (m, 2H), 8.22 – 8.18 (m, 2H), 7.45 – 7.36 (m, 2H), 7.35 – 7.26 (m, 1H), 7.29 – 7.19 (m, 3H), 7.21 – 7.16 (m, 2H), 7.16 – 7.10 (m, 4H), 7.03 (td, J = 8.4, 2.5 Hz, 1H), 6.97 (dd, J = 8.0, 2.0 Hz, 1H), 3.52 (s, 3H), 3.45 (d, J = 14.2 Hz, 2H), 3.40 (d, J = 14.2 Hz, 2H), 3.07 – 2.90 (m, 1H), 2.80 – 2.60 (m, 1H), 2.13 – 1.90 (m, 4H), 1.67 (qd, J = 12.0, 5.6 Hz, 1H).
Step 4. Synthesis of N4-(3-fluorophenyl)-N4-methyl-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound 7 Scheme 1.c)
This compound was obtained using compound H2 (80.0 mg, 0.25 mmol) following the general procedure I previously described for 2 hours. Final normal phase purification (DCM/DCM:NH3 1N MeOH 4:1 from 95/5 to 75/25) afforded pure title compound (11.9 mg, yield 23 %). Rt = 1.57 min (gradient 1); MS (ESI) m/z: 350.1 [M-H]+, [M-H]+ calculated: 350.4. HRMS m/z: 350.1778, calculated for C20H21FN5+: 350.1781. QC analysis: Rt= 2.77 min, m/z: 350.20 [M-H]+, UV (215 nm): >99.5 %. 1H NMR (400 MHz, DMSO-d6) 8.79 – 8.66 (m, 2H), 8.27 – 8.17 (m, 2H), 7.36 (td, J = 8.2, 6.8 Hz, 1H), 7.02 (dt, J = 11.0, 2.3 Hz, 1H), 6.97 (td, J = 8.4, 2.5 Hz, 1H), 6.88 – 6.83 (m, 1H), 3.54 (s, 3H), 3.07 – 2.91 (m, 1H), 2.91 – 2.75 (m, 2H), 2.20 – 2.07 (m, 1H), 1.93 – 1.76 (m, 1H), 1.64 (dd, J = 16.8, 8.3 Hz, 1H), 1.51 (dtd, J = 12.7, 9.3, 6.2 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 165.74 (C), 162.94 (C), 162.69 (CF, d, JCF = 243.3 Hz), 157.78 (C), 150.26 (CH), 144.90 (C), 130.69 (CH, d, JCF = 9.6 Hz), 121.42 (CH), 118.87 (CH, d, JCF = 2.3 Hz), 117.94 (C), 110.72 (CH, d, JCF = 20.9 Hz), 110.01 (CH, d, JCF = 23.5 Hz), 45.79 (CH), 40.78 (CH3), 35.40 (CH2), 30.58 (CH2), 30.44 (CH2).
P. Synthesis of compound 8. N4-phenyl-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine
Step 1. Synthesis of N6,N6-dibenzyl-2-chloro-N4-phenyl-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound C1.2 Scheme 1.b)
This compound was obtained using compound B1 (200 mg, 0.500 mmol) and aniline (0.05 ml, 0.55 mmol) following the general procedure C previously described at 100 °C for 16 h. Final normal phase purification (cyclohexane/TBME from 95/5 to 75/25) afforded pure title compound (141.7 mg, yield 62 %). Rt = 2.50 min (gradient 2); MS (ESI) m/z: 455.2/457.2 [M-H]+, [M-H]+ calculated: 455.2/457.2. 1H NMR (400 MHz, CDCl3) δ 7.62 – 7.53 (m, 2H), 7.43 (d, J = 7.5 Hz, 4H), 7.39 – 7.28 (m, 6H), 7.29 – 7.19 (m, 2H), 7.12 (t, J = 7.6 Hz, 1H), 6.42 (s, 1H), 3.87 (d, J = 14.1 Hz, 2H), 3.67 (d, J = 14.0 Hz, 2H), 3.18 – 3.02 (m, 1H), 2.97 – 2.86 (m, 1H), 2.68 (ddd, J = 18.1, 12.1, 5.5 Hz, 1H), 2.54 (d, J = 8.1 Hz, 2H), 2.33 – 2.16 (m, 1H), 1.77 (qd, J = 12.3, 5.0 Hz, 1H).
Step 2. Synthesis of N6,N6-dibenzyl-N4-phenyl-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound D1.3 Scheme 1.b)
This compound was obtained using compound C1.2 (75 mg, 0.16 mmol) and Pyridine-4-boronic acid (27.0 mg, 0.20 mmol) following the general procedure D previously described. Final normal phase purification (cyclohexane/TBME from 75/25 to 45/55) afforded pure title compound (57 mg, yield 69 %). Rt = 2.72 min (gradient 2); MS (ESI) m/z: 514.4 [M-H]+, [M-H]+ calculated: 514.3. 1H NMR (400 MHz, CDCl3) δ 8.75 – 8.64 (m, 2H), 8.22 – 8.12 (m, 2H), 7.75 – 7.65 (m, 2H), 7.43 (ddd, J = 16.0, 7.8, 1.6 Hz, 6H), 7.33 (t, J = 7.5 Hz, 4H), 7.29 – 7.20 (m, 3H), 7.15 (td, J = 7.3, 1.1 Hz, 1H), 6.44 (s, 1H), 3.90 (d, J = 14.0 Hz, 2H), 3.71 (d, J = 14.0 Hz, 2H), 3.28 – 3.10 (m, 1H), 3.11 – 2.96 (m, 1H), 2.80 (ddd, J = 17.9, 12.3, 5.4 Hz, 1H), 2.64 (d, J = 8.1 Hz, 2H), 2.43 – 2.24 (m, 1H), 1.83 (qd, J = 14.6, 13.4, 6.1 Hz, 1H).
Step 3. Synthesis of N4-phenyl-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound 8 Scheme 1.b)
This compound was obtained using compound D1.3 (56.6 mg, 0.11 mmol) following the general procedure E previously described for 4 hours. Final normal phase purification (DCM / DCM:NH3 1N MeOH 4:1 from 95/5 to 75/25) afforded pure title compound (20.2 mg, yield 57 %). Rt = 1.43 min (gradient 1); MS (ESI) m/z: 318.3 [M-H]+, [M-H]+ calculated: 318.2. HRMS m/z: 318.1716, calculated for C19H20N5+: 318.1719. QC analysis: Rt= 2.36 min, m/z: 318.19 [M-H]+, UV (215 nm): >99.5 %. 1H NMR (400 MHz, DMSO-d6) δ 8.74 – 8.61 (m, 2H), 8.54 (s, 1H), 8.22 – 8.03 (m, 2H), 7.85 – 7.70 (m, 2H), 7.39 (dd, J = 8.5, 7.3 Hz, 2H), 7.14 – 7.03 (m, 1H), 3.24 – 3.10 (m, 1H), 2.98 – 2.72 (m, 3H), 2.34 (dd, J = 16.9, 8.2 Hz, 1H), 2.06 – 1.88 (m, 1H), 1.64 (dtd, J = 12.6, 9.6, 5.8 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 162.60 (C), 158.85 (C), 157.23 (C), 150.16 (CH), 145.40 (C), 139.73 (C), 128.36 (CH), 122.96 (CH), 121.91 (CH), 121.34 (CH), 112.34 (C), 46.09 (CH), 32.06 (CH2), 30.90 (CH2), 30.35 (CH2).
Q. Synthesis of compound 9. N4-(2-fluorophenyl)-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine.
Step 1. Synthesis of N6,N6-dibenzyl-2-chloro-N4-(2-fluorophenyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound C1.3 Scheme 1.b)
This compound was obtained using compound B1 (500 mg, 1.26 mmol) and 2-Fluoroaniline (0.15 ml, 1.51 mmol) following the general procedure C previously described at 160 °C for 72 h. Final normal phase purification (cyclohexane/TBME from 100/0 to 80/20) afforded title compound pure enough to be used in the next step (235 mg). Rt = 2.38 min (gradient 2); MS (ESI) m/z: 473.1/475.1 [M-H]+, [M-H]+ calculated: 473.2/475.2. 1H NMR (400 MHz, CDCl3) δ 8.39 (t, J = 8.5 Hz, 1H), 7.41 (d, J = 7.4 Hz, 4H), 7.32 (t, J = 7.5 Hz, 4H), 7.23 (t, J = 7.6 Hz, 2H), 7.18 (d, J = 8.6 Hz, 1H), 7.13 (dd, J = 9.9, 2.5 Hz, 1H), 7.10 – 7.04 (m, 1H), 6.63 (d, J = 3.8 Hz, 1H), 3.85 (d, J = 14.0 Hz, 2H), 3.68 (d, J = 14.0 Hz, 2H), 3.12 (q, J = 9.0 Hz, 1H), 3.03 – 2.89 (m, 1H), 2.72 (ddd, J = 18.2, 12.1, 5.7 Hz, 1H), 2.31 – 2.20 (m, 1H), 2.11 – 2.00 (m, 1H), 1.80 (qd, J = 12.2, 5.0 Hz, 1H), 1.31 – 1.21 (m, 1H).
Step 2. Synthesis of N6,N6-dibenzyl-N4-(2-fluorophenyl)-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound D1.4 Scheme 1.b)
This compound was obtained using crude C1.3 (230 mg, 0.49 mmol) and Pyridine-4-boronic acid (79.7 mg, 0.58 mmol) following the general procedure D previously described. Final normal phase purification (cyclohexane/TBME from 80:20 to 60:40) afforded pure title compound (175 mg, overall yield 70 % for steps 1 and 2). Rt = 2.63 min (gradient 2); MS (ESI) m/z: 516.4 [M-H]+, [M-H]+ calculated: 516.2. 1H NMR (400 MHz, CDCl3) δ 8.70 (dt, J = 4.8, 0.8 Hz, 2H), 8.49 (t, J = 8.2 Hz, 1H), 8.22 – 8.14 (m, 2H), 7.45 (d, J = 7.5 Hz, 4H), 7.33 (t, J = 7.5 Hz, 4H), 7.25 – 7.20 (m, 3H), 7.20 – 7.14 (m, 1H), 7.09 (q, J = 7.5, 7.0 Hz, 1H), 6.66 (s, 1H), 3.91 (d, J = 14.0 Hz, 2H), 3.73 (d, J = 14.0 Hz, 2H), 3.20 (s, 1H), 3.07 (dd, J = 17.1, 4.6 Hz, 1H), 2.82 (ddd, J = 17.9, 12.2, 5.4 Hz, 1H), 2.69 (d, J = 10.2 Hz, 2H), 2.33 (d, J = 12.5 Hz, 1H), 1.87 (qd, J = 12.3, 4.9 Hz, 1H).
Step 3. Synthesis of N4-(2-fluorophenyl)-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound 9 Scheme 1.b)
This compound was obtained using compound D1.4 (170 mg, 0.33 mmol) following the general procedure E previously described for 4 hours. Final normal phase purification (DCM / DCM:NH3 1N MeOH 4:1 from 95/5 to 75/25) afforded pure title compound (55.2 mg, yield 22 %). Rt = 1.41 min (gradient 1); MS (ESI) m/z: 336.2 [M-H]+, [M-H]+ calculated: 336.2. HRMS m/z: 336.1617, calculated for C19H19FN5+: 336.1624. QC analysis: Rt= 2.29 min, m/z: 336.20 [M-H]+, UV (215 nm): 99 %. 1H NMR (400 MHz, DMSO-d6) δ 8.64 – 8.58 (m, 2H), 8.51 (s, 1H), 7.97 – 7.91 (m, 2H), 7.58 (td, J = 7.6, 2.4 Hz, 1H), 7.36 – 7.21 (m, 4H), 3.18 (d, J = 11.0 Hz, 1H), 3.03 – 2.67 (m, 3H), 2.32 (dd, J = 16.9, 8.3 Hz, 1H), 2.06 – 1.87 (m, 1H), 1.64 (dtd, J = 12.7, 9.6, 5.7 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 163.09 (C), 157.69 (C), 150.51 (CH), 145.71 (C), 144.87 (C), 127.82 (CH, d, JCF = 2.0 Hz), 127.41 (CH, d, JCF = 3.0 Hz), 124.65 (CH, d, JCF = 3.5 Hz), 121.64 (CH), 116.12 (CH, d, JCF = 20.0 Hz), 112.52 (C), 46.48 (CH), 32.67 (CH2), 31.66 (CH2), 30.66 (CH2).
R. Synthesis of compound 10. N4-(4-fluorophenyl)-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine.
Step 1. Synthesis of N6,N6-dibenzyl-2-chloro-N4-(4-fluorophenyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound C1.4 Scheme 1.b)
This compound was obtained using compound B1 (300 mg, 0.75 mmol) and 4-Fluoroaniline (0.07 ml, 0.75 mmol) following the general procedure C previously described at 100 °C for 16 h. Final normal phase purification (cyclohexane/TBME from 100/0 to 90/10) afforded pure title compound (303 mg, yield 73 %). Rt = 2.70 min (gradient 2); MS (ESI) m/z: 473.1 [M-H]+, [M-H]+ calculated: 473.2. 1H NMR (400 MHz, CDCl3) δ 7.70–7.58 (m, 3H), 7.45 – 7.38 (m, 4H), 7.37 – 7.28 (m, 6H), 7.12–7.02 (m, 2H), 3.67 (d, J = 14.0 Hz, 2H), 3.17 – 3.05 (m, 1H), 2.95 (ddd, J = 18.2, 5.0, 2.4 Hz, 1H), 2.71 (ddd, J = 18.1, 12.1, 5.6 Hz, 1H), 2.55 – 2.46 (m, 2H), 2.32 – 2.21 (m, 1H), 1.78 (qd, J = 12.3, 5.1 Hz, 1H).
Step 2. Synthesis of N6,N6-dibenzyl-N4-(4-fluorophenyl)-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound D1.5 Scheme 1.b).
This compound was obtained using compound C1.4 (75 mg, 0.16 mmol) and Pyridine-4-boronic acid (26.0 mg, 0.19 mmol) following the general procedure D previously described. Final normal phase purification (cyclohexane/TBME from 80/20 to 50/50) afforded pure title compound (75.7 mg, yield 92 %). Rt = 2.97 min (gradient 2); MS (ESI) m/z: 516.4 [M-H]+, [M-H]+ calculated: 516.2. 1H NMR (400 MHz, CDCl3) δ 8.74 – 8.62 (m, 2H), 8.21 – 8.05 (m, 2H), 7.69 – 7.55 (m, 2H), 7.50 – 7.39 (m, 4H), 7.38 – 7.29 (m, 4H), 7.26 (s, 1H), 7.16 – 7.05 (m, 2H), 6.41 (s, 1H), 3.91 (d, J = 14.0 Hz, 2H), 3.71 (d, J = 14.0 Hz, 2H), 3.29 – 3.10 (m, 1H), 3.11 – 2.98 (m, 1H), 2.79 (ddd, J = 17.9, 12.3, 5.5 Hz, 1H), 2.65 (d, J = 8.1 Hz, 2H), 2.43 – 2.22 (m, 1H), 1.95 – 1.81 (m, 1H).
Step 3. Synthesis of N4-(4-fluorophenyl)-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (Compound 10, Scheme 1.b).
This compound was obtained using compound D1.6 (75.7 mg, 0.14 mmol) following the general procedure E previously described for 4 hours. Final normal phase purification (DCM / DCM:NH3 1N MeOH 4:1 from 95/5 to 75/25) afforded pure title compound (45.3 mg, yield 94 %). Rt = 1.65 min (gradient 1); MS (ESI) m/z: 336.1 [M-H]+, [M-H]+ calculated: 336.2. HRMS m/z: 336.1619, calculated for C19H19FN5+: 336.1624. QC analysis: Rt= 2.52 min, m/z: 336.10 [M-H]+, UV (215 nm): 96 %. 1H NMR (400 MHz, DMSO-d6) δ 8.67 (d, J = 5.2 Hz, 2H), 8.59 (s, 1H), 8.08 (d, J = 5.2 Hz, 2H), 7.76 (ddd, J = 9.3, 5.0, 2.3 Hz, 2H), 7.22 (t, J = 8.9 Hz, 2H), 2.93 – 2.71 (m, 3H), 2.31 (dd, J = 16.9, 8.1 Hz, 1H), 1.94 (ddt, J = 11.1, 7.8, 4.4 Hz, 1H), 1.63 (dtd, J = 12.4, 9.6, 5.7 Hz, 1H), 1.23 (t, J = 7.2 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 162.58 (C), 158.84 (C), 151.25 (CF, d, JCF = 1193.8 Hz), 150.16 (CH), 135.98 (C, d, JCF = 2.1 Hz), 123.91 (CH, d, JCF = 7.8 Hz), 121.33 (CH), 114.89 (CH, d, JCF = 22.2 Hz), 112.24 (C), 46.10 (CH), 32.20 (CH2), 31.09 (CH2), 30.37 (CH2).
S. Synthesis of compound 11. N4-(3-methoxyphenyl)-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine.
Step 1. Synthesis of N6,N6-dibenzyl-2-chloro-N4-(3-methoxyphenyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound C1.5 Scheme 1.b)
This compound was obtained using compound B1 (250 mg, 0.63 mmol) and 3-methoxyaniline (0.08 ml, 0.69 mmol) following the general procedure C previously described at 100 °C for 16 h. Final normal phase purification (cyclohexane/TBME from 100/0 to 90/10) afforded pure title compound (140 mg, yield 46 %). Rt = 2.43 min (gradient 2); MS (ESI) m/z: 485.2 [M-H]+, [M-H]+ calculated: 469.2. 1H NMR (400 MHz, CDCl3) δ 7.48 – 7.38 (m, 5H), 7.33 (t, J = 7.4 Hz, 5H), 7.24 (dd, J = 7.8, 2.5 Hz, 3H), 6.95 (d, J = 7.5 Hz, 1H), 6.35 (s, 1H), 3.86 (d, J = 14.1 Hz, 2H), 3.67 (d, J = 14.0 Hz, 2H), 3.11 (q, J = 9.0 Hz, 1H), 2.92 (ddd, J = 18.3, 5.1, 2.2 Hz, 1H), 2.69 (ddd, J = 18.1, 12.2, 5.5 Hz, 1H), 2.52 (d, J = 8.5 Hz, 2H), 2.38 (s, 3H), 2.30 – 2.19 (m, 1H), 1.85 – 1.70 (m, 1H).
Step 2. Synthesis of N6,N6-dibenzyl-N4-(3-methoxyphenyl)-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound D1.6 Scheme 1.b)
This compound was obtained using compound C1.5 (135 mg, 0.28 mmol) and Pyridine-4-boronic acid (45.6 mg, 0.33 mmol) following the general procedure D previously described. Final normal phase purification (cyclohexane/EtOAc from 75/25 to 60/40) afforded pure title compound (132 mg, yield 90 %). Rt = 2.62 min (gradient 2); MS (ESI) m/z: 528.4 [M-H]+, [M-H]+ calculated: 528.3. 1H NMR (400 MHz, DMSO-d6) δ 8.71 – 8.63 (m, 2H), 8.55 (s, 1H), 8.15 – 8.05 (m, 2H), 7.48 – 7.40 (m, 5H), 7.38 (ddd, J = 8.1, 2.0, 1.0 Hz, 1H), 7.31 (q, J = 7.7 Hz, 5H), 7.25 – 7.18 (m, 2H), 6.69 (ddd, J = 8.1, 2.5, 1.0 Hz, 1H), 3.82 – 3.78 (m, 5H), 3.70 (d, J = 14.2 Hz, 2H), 3.03 – 2.84 (m, 3H), 2.84 – 2.63 (m, 2H), 2.16 (d, J = 12.2 Hz, 1H), 1.84 (qd, J = 12.1, 5.1 Hz, 1H).
Step 3. Synthesis of N4-(3-methoxyphenyl)-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound 11 Scheme 1.b)
This compound was obtained using compound D1.4 (130.0 mg, 0.25 mmol) following the general procedure E previously described for 4 hours. Final normal phase purification (neutral alumina, DCM/DCM:MeOH 4:1 from 90/10 to 60/40) afforded pure title compound (51.4 mg, yield 60 %). Rt = 1.45 min (gradient 1); MS (ESI) m/z: 348.2 [M-H]+, [M-H]+ calculated: 348.2. HRMS m/z: 348.1818, calculated for C20H22N5O+: 348.1824. QC analysis: Rt= 2.44 min, m/z: 348.16 [M-H]+, UV (215 nm): >99.5 %. 1H NMR (400 MHz, DMSO-d6) δ 8.80 – 8.63 (m, 2H), 8.48 (s, 1H), 8.30 – 8.02 (m, 2H), 7.50 (t, J = 2.2 Hz, 1H), 7.43 (ddd, J = 8.2, 2.0, 0.9 Hz, 1H), 7.27 (t, J = 8.1 Hz, 1H), 6.65 (ddd, J = 8.2, 2.5, 0.9 Hz, 1H), 3.78 (s, 3H), 3.17 (qd, J = 5.5, 2.2 Hz, 1H), 2.95 – 2.70 (m, 3H), 2.40 – 2.25 (m, 1H), 2.01 – 1.88 (m, 1H), 1.63 (dtd, J = 12.3, 9.4, 5.5 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 162.70 (C), 159.33 (C), 158.78 (C), 157.17 (C), 150.14 (CH), 145.38 (C), 141.03 (C), 129.02 (CH), 121.27 (CH), 113.82 (CH), 112.63 (C), 108.70 (CH), 106.95 (CH), 55.01 (CH3), 46.07 (CH), 32.28 (CH2), 31.13 (CH2), 30.37 (CH2).
T. Synthesis of compound 12. N4-(4-methoxyphenyl)-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine
Step 1. Synthesis of N6,N6-dibenzyl-2-chloro-N4-(4-methoxyphenyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound C1.6 Scheme 1.b)
This compound was obtained using compound B1 (250 mg, 0.63 mmol) and 4-methoxyaniline (0.087 ml, 0.69 mmol) following the general procedure C previously described at 120 °C for 4 h. Final normal phase purification (cyclohexane/TBME from 80/20 to 60/40) afforded pure title compound (216 mg, yield 71 %). Rt = 2.30 min (gradient 2); MS (ESI) m/z: 485.3/487.3 [M-H]+, [M-H]+ calculated: 485.0/487.0. 1H NMR (400 MHz, DMSO-d6) δ 8.53 (s, 1H), 7.66 – 7.54 (m, 2H), 7.42 – 7.38 (m, 4H), 7.35 (t, J = 7.5 Hz, 4H), 7.25 – 7.14 (m, 2H), 7.06 – 6.97 (m, 2H), 3.82 (d, J = 14.2 Hz, 2H), 3.81 (s, 3H), 3.72 (d, J = 14.2 Hz, 2H), 3.05 – 2.83 (m, 3H), 2.73 (dt, J = 17.2, 11.2 Hz, 2H), 2.17 (d, J = 12.2 Hz, 1H), 1.85 (qd, J = 12.2, 5.1 Hz, 1H).
Step 2. Synthesis of N6,N6-dibenzyl-N4-(4-methoxyphenyl)-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound D1.7 Scheme 1.b)
This compound was obtained using crude C1.6 (210 mg, 0.43 mmol) and Pyridine-4-boronic acid (71.0 mg, 0.52 mmol) following the general procedure D previously described. Final normal phase purification (cyclohexane/TBME from 70/30 to 50/50) afforded pure title compound (170 mg, overall yield 74 % from step 1). Rt = 2.50 min (gradient 2); MS (ESI) m/z: 528.4 [M-H]+, [M-H]+ calculated: 528.7. 1H NMR (400 MHz, DMSO-d6) δ 8.69 – 8.60 (m, 2H), 8.51 (s, 1H), 8.10 – 8.00 (m, 2H), 7.68 – 7.57 (m, 2H), 7.47 – 7.40 (m, 4H), 7.32 (t, J = 7.5 Hz, 4H), 7.26 – 7.18 (m, 2H), 7.03 – 6.94 (m, 2H), 3.80 (d, J = 14.2 Hz, 2H), 3.79 (s, 3H), 3.70 (d, J = 14.2 Hz, 2H), 3.02 – 2.81 (m, 3H), 2.71 (dt, J = 17.2, 11.2 Hz, 2H), 2.15 (d, J = 12.2 Hz, 1H), 1.83 (qd, J = 12.2, 5.1 Hz, 1H).
Step 3. Synthesis of N4-(4-methoxyphenyl)-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound 12 Scheme 1.b)
This compound was obtained using compound D1.6 (165 mg, 0.31 mmol) following the general procedure E previously described for 4 hours. Final normal phase purification (DCM / DCM:NH3 1N MeOH 4:1 from 95/5 to 75/25) afforded pure title compound (78.8 mg, yield 76 %). Rt = 1.49 min (gradient 1); MS (ESI) m/z: 348.2 [M-H]+, [M-H]+ calculated: 348.4. HRMS m/z: 348.1815, calculated for C20H22N5O+: 348.1824. QC analysis: Rt= 2.43 min, m/z: 348.09 [M-H]+, UV (215 nm): >99.5. 1H NMR (400 MHz, DMSO-d6) δ 8.71 – 8.61 (m, 2H), 8.43 (s, 1H), 8.14 – 8.02 (m, 2H), 7.69 – 7.59 (m, 2H), 7.00 – 6.92 (m, 2H), 3.77 (s, 3H), 3.22 – 3.10 (m, 1H), 2.93 – 2.68 (m, 3H), 2.29 (dd, J = 16.9, 8.3 Hz, 1H), 2.02 – 1.87 (m, 1H), 1.62 (dtd, J = 12.7, 9.6, 5.7 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 162.01 (C), 158.95 (C), 157.18 (C), 155.25 (C), 150.07 (CH), 145.47 (C), 132.59 (C), 123.74 (CH), 121.29 (CH), 113.50 (CH), 111.78 (C), 55.20 (CH3), 46.12 (CH), 32.10 (CH2), 31.05 (CH2), 30.30 (CH2).
U. Synthesis of compound 13. N4-[3-(dimethylamino)phenyl]-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine
Step 1. Synthesis of N6,N6-dibenzyl-2-chloro-N4-[3-(dimethylamino)phenyl]-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound C1.7 Scheme 1.b)
This compound was obtained using compound B1 (250 mg, 0.63 mmol) and N,N-Dimethyl-1,3-phenylenediamine dihydrochloride (145.8 mg, 0.69 mmol) following the general procedure C previously described at 100 °C for 6 h. Final normal phase purification (cyclohexane/EtOAc from 100/0 to 80/20) afforded pure title (175 mg, yield 56 %). Rt = 2.51 min (gradient 2); MS (ESI) m/z: 498.3/500.3 [M-H]+, [M-H]+ calculated: 498.2/500.2. 1H NMR (400 MHz, DMSO-d6) δ 8.61 (s, 1H), 7.48 – 7.38 (m, 4H), 7.32 (dd, J = 8.3, 6.8 Hz, 4H), 7.26 – 7.19 (m, 2H), 7.16 (t, J = 8.1 Hz, 1H), 7.02 (t, J = 2.2 Hz, 1H), 6.92 (ddd, J = 8.0, 2.0, 0.8 Hz, 1H), 6.52 (ddd, J = 8.4, 2.6, 0.9 Hz, 1H), 3.78 (d, J = 14.2 Hz, 2H), 3.67 (d, J = 14.2 Hz, 2H), 2.91 (s, 6H), 2.99 – 2.84 (m, 0H), 2.75 (dd, J = 7.9, 3.6 Hz, 1H), 2.73 – 2.56 (m, 2H), 2.10 (d, J = 12.4 Hz, 1H), 1.79 (tt, J = 12.1, 6.0 Hz, 1H).
Step 2. Synthesis of N6,N6-dibenzyl-N4-[3-(dimethylamino)phenyl]-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound D1.8 Scheme 1.b)
This compound was obtained using crude C1.7 (170 mg, 0.34 mmol) and Pyridine-4-boronic acid (55.9 mg, 0.41 mmol) following the general procedure D previously described. Final normal phase purification (cyclohexane/EtOAc from 90/10 to 60/40) afforded pure title compound (150 mg, overall yield 81 % from step 1). 1H NMR (400 MHz, DMSO-d6) δ 8.71 – 8.62 (m, 2H), 8.41 (s, 1H), 8.14 – 8.06 (m, 2H), 7.48 – 7.41 (m, 4H), 7.35 – 7.29 (m, 4H), 7.25 – 7.17 (m, 4H), 7.08 (ddd, J = 7.9, 1.9, 0.9 Hz, 1H), 6.50 (ddd, J = 8.3, 2.5, 0.9 Hz, 1H), 3.80 (d, J = 14.2 Hz, 2H), 3.70 (d, J = 14.2 Hz, 2H), 2.94 (s, 6H), 3.00 – 2.83 (m, 2H), 2.82 – 2.69 (m, 1H), 2.15 (d, J = 12.4 Hz, 1H), 1.83 (qd, J = 12.0, 5.1 Hz, 1H).
Step 3. Synthesis of N4-[3-(dimethylamino)phenyl]-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound 13 Scheme 1.b)
This compound was obtained using compound D1.8 (150 mg, 1.39 mmol) following the general procedure E previously described for 4 hours. Final normal phase purification (DCM / DCM:NH3 1N MeOH 4:1 from 95/5 to 75/25) afforded pure title compound (83.0 mg, yield 83 %). Rt = 1.57 min (gradient 1); MS (ESI) m/z: 361.2 [M-H]+, [M-H]+ calculated: 361.2. HRMS m/z: 361.2134, calculated for C21H25N6+: 361.2141. QC analysis: Rt= 2.65 min, m/z: 361.11 [M-H]+, UV (215 nm): 99 %. 1H NMR (600 MHz, DMSO-d6) δ 8.70 – 8.65 (m, 2H), 8.30 (s, 1H), 8.19 – 8.09 (m, 2H), 7.28 – 7.21 (m, 1H), 7.19 – 7.12 (m, 2H), 6.51 – 6.44 (m, 1H), 3.17 (dddd, J = 11.1, 8.3, 5.1, 2.9 Hz, 1H), 2.92 (s, 6H), 2.88 (p, J = 2.3 Hz, 1H), 2.86 (d, J = 5.0 Hz, 1H), 2.78 (ddd, J = 17.4, 9.7, 5.9 Hz, 1H), 2.31 (dd, J = 16.8, 8.2 Hz, 1H), 1.94 (dp, J = 12.8, 4.4, 3.6 Hz, 1H), 1.71 (s, 2H), 1.67 – 1.57 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 162.39 (C), 158.90 (C), 157.14 (C), 150.70 (C), 150.13 (CH), 145.48 (C), 140.44 (C), 128.64 (CH), 121.30 (CH), 112.46 (C), 110.02 (CH), 107.55 (CH), 105.90 (CH), 46.14 (CH), 40.29 (CH3), 32.33 (CH2), 31.23 (CH2), 30.38 (CH2).
V. Synthesis of compound 14. N4-[4-(dimethylamino)phenyl]-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine.
Step 1. Synthesis of N6,N6-dibenzyl-2-chloro-N4-[4-(dimethylamino)phenyl]-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound C1.8 Scheme 1.b)
This compound was obtained using compound B1 (250 mg, 0.63 mmol) and N4,N4-dimethylbenzene-1,4-diamine (0.085 ml, 0.69 mmol) following the general procedure C previously described at 100 °C for 16 h. Final normal phase purification (cyclohexane/EtOAc from 100:0 to 70:30) afforded pure title (240 mg, yield 77 %). Rt = 2.44 min (gradient 2); MS (ESI) m/z: 498.1/500.1 [M-H]+, [M-H]+ calculated: 498.2/500.2. 1H NMR (400 MHz, CDCl3) δ 7.42 (d, J = 7.5 Hz, 4H), 7.34 (dt, J = 14.7, 8.1 Hz, 6H), 7.24 (t, J = 7.4 Hz, 2H), 6.72 (s, 2H), 6.28 (s, 1H), 3.84 (d, J = 14.1 Hz, 2H), 3.66 (d, J = 14.0 Hz, 2H), 3.16 – 3.01 (m, 1H), 3.01 – 2.81 (m, 1H), 2.73 – 2.58 (m, 1H), 2.48 (s, 2H), 2.30 – 2.16 (m, 1H), 1.74 (qd, J = 12.3, 5.1 Hz, 1H), 1.43 (s, 6H).
Step 2. Synthesis of N6,N6-dibenzyl-N4-[4-(dimethylamino)phenyl]-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound D1.9 Scheme 1.b)
This compound was obtained using compound C1.8 (240 mg, 0.48 mmol) and Pyridine-4-boronic acid (85.7 mg, 0.58 mmol) following the general procedure D previously described. Final normal phase purification (cyclohexane/EtOAc from 75/25 to 50/50) afforded pure title compound (197 mg, yield 76 %). Rt = 2.62 min (gradient 2); MS (ESI) m/z: 541.2 [M-H]+, [M-H]+ calculated: 541.7. 1H NMR (400 MHz, DMSO-d6) δ 8.68 – 8.60 (m, 2H), 8.40 (s, 1H), 8.09 – 8.01 (m, 2H), 7.57 – 7.49 (m, 2H), 7.47 – 7.39 (m, 4H), 7.32 (t, J = 7.5 Hz, 4H), 7.25 – 7.17 (m, 2H), 6.84 – 6.74 (m, 2H), 3.79 (d, J = 14.2 Hz, 2H), 3.69 (d, J = 14.2 Hz, 2H), 3.02 – 2.92 (m, 1H), 2.91 (s, 6H), 2.89 – 2.78 (m, 2H), 2.76 – 2.60 (m, 2H), 2.14 (d, J = 12.1 Hz, 1H), 1.81 (qd, J = 12.3, 5.1 Hz, 1H).
Step 3. Synthesis of N4-[4-(dimethylamino)phenyl]-2-(4-pyridyl)-5,6,7,8-tetrahydroquinazoline-4,6-diamine (compound 14 Scheme 1.b)
This compound was obtained using compound D1.9 (197.2 mg, 0.48 mmol) following the general procedure E previously described for 5 hours. Final normal phase purification (DCM/DCM:NH3 1M MeOH 4:1 from 95/5 to 40/60) afforded pure title compound (79.4 mg, yield 46 %). Rt = 1.32 min (gradient 1); MS (ESI) m/z: 361.2 [M-H]+, [M-H]+ calculated: 361.2. HRMS m/z: 361.2137, calculated for C21H25N6+: 361.2141. QC analysis: Rt= 2.52 min, m/z: 361.17 [M-H]+, UV (215 nm): 96 %. 1H NMR (400 MHz, DMSO-d6) δ 8.73 – 8.61 (m, 2H), 8.52 (s, 1H), 8.12 – 8.00 (m, 2H), 7.57 – 7.44 (m, 2H), 6.84 – 6.75 (m, 2H), 3.50 – 3.60 (m, 1H), 2.97 (dd, J = 17.0, 5.4 Hz, 1H), 2.91 (s, 6H), 2.86 (d, J = 6.6 Hz, 2H), 2.60 (dd, J = 16.6, 8.8 Hz, 1H), 2.17 (d, J = 19.0 Hz, 1H), 1.90 (p, J = 8.2 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 160.97 (C), 158.93 (C), 157.76 (C), 150.11 (CH), 147.27 (C), 145.34 (C), 128.75 (C), 123.86 (CH), 121.31 (CH), 112.32 (CH), 109.52 (C), 45.74 (CH), 40.50 (CH3), 29.34 (CH2), 27.82 (CH2), 26.26 (CH2).
Topoisomerase II Activity Assay.
The activity of topoIIα was measured using a decatenation assay (Inspiralis) following the manufacturer’s instructions. Compounds were dissolved in DMSO and used at a concentration ranging from 200 to 1 μM. Final DMSO concentration in the assay was ≤1%. Reaction mixtures were incubated for 30 min at 37 °C and terminated with STEB buffer (40% (w/v) sucrose, 100 mM Tris-HCl, pH 8, 1 mM EDTA, 0.5 mg/ mL bromophenol blue). Reaction products were resolved by electrophoresis in 1% agarose gels containing SYBR Safe DNA stain (Invitrogen), scanned, and quantified using the ChemiDoc system (BioRad). IC50 values were obtained with GraphPad Prism software (version 5.03) using the band intensities of the dose−response gels. Values are reported as the mean ± SD of two independent experiments.
Biology.
Cell Viability Assay.
Human cancer cell lines A549 (lung adenocarcinoma, ATCC CCL-185), DU-145 (androgen- independent prostate cancer, ATCC HTB-81), and HeLa (cervical carcinoma, ATCC CCL-2) were obtained from ATCC. Cells were routinely grown in minimal essential medium containing Eagle’s salts and L-glutamine supplemented with 10% heat-inactivated FBS in a humidified atmosphere of 5% CO2 at 37 °C. To assess the antiproliferative activity of the compounds, cells were seeded at a density of 2500 cells/well (HeLa) or 5000 cells/well (A549, DU-145) in 96-well plates, and cell viability was measured using the MTT assay. Values are reported as the mean ± SD of two independent experiments.
TopoII inhibition mechanism characterization of compound 14 - Materials.
Recombinant wild-type human topoisomerase IIα (topoIIα) and topoisomerase IIβ (topoIIβ) were expressed in Saccharomyces cerevisiae JEL-1Δtop1 and purified as described previously. 25–27 The enzymes were stored at −80 °C as 1.5 mg/mL stocks in 50 mM Tris-HCl, pH 7.9, 0.1 mM NaEDTA, 750 mM KCl, and 40% glycerol. Negatively supercoiled pBR322 DNA was prepared from Escherichia coli using a Plasmid Mega Kit (Qiagen) as described by the manufacturer and exonuclease treated to remove any chromosomal DNA contaminants from the plasmid. 28 Analytical grade etoposide and ethidium bromide were purchased from Sigma-Aldrich. [γ32P]ATP (3000 Ci/mmol stock) was purchased from Perkin Elmer. Compound 14 was stored at −20 °C as a 20 mM solution in dimethyl sulfoxide.
DNA Relaxation.
DNA relaxation reactions were carried out using the procedure of Fortune and Osheroff. 29 Reaction mixtures contained 5 nM negatively supercoiled pBR322 DNA, 3 nM human topoIIα or 4 nM topoIIβ, 1 mM ATP and 0–200 µM compound 14 in a total of 20 µL of 10 mM Tris-HCl, pH 7.9, 5 mM MgCl2, 175 mM KCl, 0.1 mM NaEDTA, and 2.5% (v/v) glycerol. Mixtures were incubated at 37 °C for 4 min. Reactions were stopped by the addition of 3 µL of 0.77% SDS-77 mM NaEDTA, pH 8.0. Samples were mixed with 2 µL of agarose loading dye [60% sucrose (w/v), 10 mM Tris-HCl, pH 7.9, 0.5% bromophenol blue, 0.5% xylene cyanol], heated for 2 min at 45 °C, and subjected to electrophoresis in a 1% agarose gel in 100 mM Tris-borate, pH 8.3, and 2 mM EDTA. Gels were stained for 30 min using 1.0 µg/mL ethidium bromide and rinsed in deionized water. DNA bands were visualized by medium wave UV light and quantified using an Alpha Innotech digital imaging system. DNA Relaxation was monitored by the loss of supercoiled plasmid molecules.
DNA Cleavage.
DNA cleavage reactions were performed using the procedure of Fortune and Osheroff. 29Reaction mixtures contained 10 nM negatively supercoiled pBR322 DNA and 150 nM human topoIIα in a final volume of 20 µL of cleavage buffer [10 mM Tris-HCl, pH 7.9, 5 mM MgCl2, 100 mM KCl, 0.1 mM NaEDTA, and 2.5% (v/v) glycerol] containing 0–200 µM compound 14. Reactions were incubated for 6 min at 37 °C and enzyme-DNA cleavage complexes were trapped by the addition of 2 μL of 4% SDS followed by 2 μL of 250 mM NaEDTA, pH 8.0. Proteinase K (2 μL of a 0.8 mg/mL solution) was added, and samples were incubated for 30 min at 45 °C to digest the enzyme. Samples were mixed with 2 µL of agarose loading dye, heated for 2 min at 45 °C, and subjected to electrophoresis in a 1% agarose gel in 40 mM Tris-acetate, pH 8.3, and 2 mM EDTA containing 0.5 μg/mL ethidium bromide. DNA bands were visualized and quantified as described above. DNA cleavage was monitored by the conversion of supercoiled plasmid to linear molecules.
DNA Intercalation.
DNA intercalation was carried out using the protocol of Fortune et al. 30 Calf thymus DNA topoisomerase I (Invitrogen, 0.5 U) and 5 mM of relaxed pBR322 were incubated in 10 mM Tris-HCl, pH 7.5, 10 mM KCl, 2 mM MgCl2, 0.02 mM EDTA, 0.1 mM dithiothreitol, 6 µg/mL bovine serum albumin. Assays were carried out in the presence of 0–25 µM compound 14. Mixtures were incubated for 15 min at 37 °C. Reactions containing 10 µM ethidium bromide (a well-characterized intercalator), or 100 µM etoposide (a non-intercalative topoisomerase II poison) were used as positive or negative controls, respectively. Samples were extracted using 20 µL of phenol:chloroform:isoamyl alcohol (25:24:1), and the aqueous layer was mixed with 2 µL of agarose loading dye and heated for 5 min at 45 °C. Reactions were stopped by the addition of 3 µL of 0.77% SDS-77 mM NaEDTA, pH 8.0. Samples were mixed with 2 µL of agarose loading dye, heated for 2 min at 45 °C, and subjected to electrophoresis in a 1% agarose gel in 100 mM Tris-borate, pH 8.3, and 2 mM EDTA. Gels were stained for 30 min using 1.0 µg/mL ethidium bromide and rinsed in deionized water. DNA bands were visualized and quantified as described above. DNA intercalation was monitored by the conversion of relaxed to supercoiled plasmid molecules.
ATP hydrolysis.
ATPase assays were performed as described by Osheroff et al. 31 Reaction mixtures contained 30 nM human topoII𝛂, 1 mM [γ32P]ATP, and 0–25 µM compound 14 in a total of 20 µL of cleavage buffer. Some assays were carried out in the presence of 5 nM negatively supercoiled pB322 DNA. Reactions were initiated by the addition of topoIIα and mixtures were incubated at 37 °C for 8 min. Samples (2.0 µL) were removed and spotted on to polyethyleneimine-impregnated thin layer cellulose chromatography plates. Plates were developed by chromatography in freshly prepared 400 mM NH4HCO3, and released 32P was visualized and quantified using a Bio-Rad Pharos FX Plus Molecular Imager.
Supplementary Material
Table 4.
Pharmacokinetic parameters of 14. Maximum plasma concentration (Cmax); Time to reach maximum concentration (tmax), Area under the curve (AUC); Half-life (t½); Volume of distribution (VD); and Clearance (CL).
| Cmax | 0.68 µg/ml | t½ | 149 min |
| tmax | 15 min | VD | 24.9 l/kg |
| AUC | 73.1 μg-min/ml | CL | 0.116 l/min/kg |
ACKNOWLEDGMENT
M.D.V. and V. G. thank Paolo Bisignano for technical assistance.
Funding Sources
This work was supported in part by the Italian Association for Cancer Research (AIRC) through the “IG 23679” and the National Institutes of Health (Grant R01 GM126363 to NO).
ABBREVIATIONS
- topoII
topoisomerase II enzyme
- topoIIα
topoisomerase II enzyme α isoform
- hTIIα
human topoisomerase II enzyme α isoform
- topoIIβ
topoisomerase II enzyme β isoform
- hTIIβ
human topoisomerase II enzyme β isoform
- SI
supporting information
- SD
Standard Deviation
- Cmax
maximum plasma concentration
- tmax
Time to reach maximum concentration
- VD
Volume of distribution
- CL
Clearance
- ACN
acetonitrile
- EtOAc
ethyl acetate
- EtOH
ethanol
- MeOH
methanol
- SQD
single quadrupole detector
- PDA
Photodiode array
- QC
quality check
- UPLC
Ultra Performance Liquid Chromatography
- PdCl2(dppf)
[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium (II)
- Pd(OH)2/C
Pd(OH)2 on activated carbon
- rac-BINAP
(±)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthalene
- Rt
retention time
- TEA
trimethylamine
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website including General procedures and spectra, additional molecular biology data, docking and virtual screening procedure, pharmacokinetics procedures, compounds chemical characterization (PDF) and Molecular Formula Strings.
The authors declare the following competing financial interest(s): One patent application protecting the class of compounds disclosed in this manuscript has been filed by the following authors: Marco De Vivo, Jose Antonio Ortega, Jose M. Arencibia, Claudia Sissi and Anna Minarini.
REFERENCES
- 1.Deweese JE; Osheroff MA; Osheroff N, DNA topology and topoisomerases: teaching a “Knotty” Subject. Biochem. Mol. Biol. Educ 2008, 37 (1), 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nitiss JL, DNA topoisomerase II and its growing repertoire of biological functions. Nat. Rev. Cancer 2009, 9 (5), 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vos SM; Tretter EM; Schmidt BH; Berger JM, All tangled up: how cells direct, manage and exploit topoisomerase function. Nat. Rev. Mol. Cell Biol 2011, 12 (12), 827–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pommier Y; Sun Y; Huang SN; Nitiss JL, Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat. Rev. Mol. Cell Biol 2016, 17 (11), 703–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashley RE; Osheroff N, Regulation of DNA Topology by Topoisomerases: Mathemtics at the Molecular Level. In Knots, Low-Dimensional Topology and Applications, Adams CC; Gordon CM; Jones VFR; Kauffman LH; Lambropoulou S; Millett KC; Przytycki JH; Ricca R; Sazdanovic R, Eds. Springer: Switzerland, 2019; pp 411–433. [Google Scholar]
- 6.Deweese JE; Osheroff N, The DNA cleavage reaction of topoisomerase II: wolf in sheep’s clothing. Nucleic Acids Res 2009, 37 (3), 738–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pommier Y, Drugging topoisomerases: lessons and challenges. ACS Chem. Biol 2013, 8 (1), 82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nitiss JL, Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9 (5), 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joannides M; Mays AN; Mistry AR; Hasan SK; Reiter A; Wiemels JL; Felix CA; Coco FL; Osheroff N; Solomon E; Grimwade D, Molecular pathogenesis of secondary acute promyelocytic leukemia. Mediterr. J. Hematol. Infect. Dis 2011, 3 (1), e2011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pendleton M; Lindsey RH Jr.; Felix CA; Grimwade D; Osheroff N, Topoisomerase II and leukemia. Ann. N Y Acad. Sci 2014, 1310, 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailly C, Contemporary challenges in the design of topoisomerase II inhibitors for cancer chemotherapy. Chem Rev 2012, 112 (7), 3611–3640. [DOI] [PubMed] [Google Scholar]
- 12.Palermo G; Minniti E; Greco ML; Riccardi L; Simoni E; Convertino M; Marchetti C; Rosini M; Sissi C; Minarini A; De Vivo M, An optimized polyamine moiety boosts the potency of human type II topoisomerase poisons as quantified by comparative analysis centered on the clinical candidate F14512. Chem Commun (Camb) 2015, 51 (76), 14310–14313. [DOI] [PubMed] [Google Scholar]
- 13.Hu W; Huang XS; Wu JF; Yang L; Zheng YT; Shen YM; Li ZY; Li X, Discovery of novel topoisomerase II Inhibitors by medicinal chemistry approaches. J Med Chem 2018, 61 (20), 8947–8980. [DOI] [PubMed] [Google Scholar]
- 14.Arencibia JM; Brindani N; Franco-Ulloa S; Nigro M; Akkarapattiakal Kuriappan J; Ottonello G; Bertozzi SM; Summa M; Girotto S; Bertorelli R; Armirotti A; De Vivo M, Design, synthesis, dynamic docking, biochemical characterization, and in vivo pharmacokinetics studies of novel topoisomerase II poisons with promising antiproliferative activity. J Med Chem 2020, 63 (7), 3508–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riccardi L; Genna V; De Vivo M, Metal–ligand interactions in drug design. Nature Reviews Chemistry 2018, 2 (7), 100–112. [Google Scholar]
- 16.Oviatt AA; Kuriappan JA; Minniti E; Vann KR; Onuorah P; Minarini A; De Vivo M; Osheroff N, Polyamine-containing etoposide derivatives as poisons of human type II topoisomerases: differential effects on topoisomerase IIalpha and IIbeta. Bioorg. Med. Chem. Lett 2018, 28 (17), 2961–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortega JA; Riccardi L; Minniti E; Borgogno M; Arencibia JM; Greco ML; Minarini A; Sissi C; De Vivo M, Pharmacophore hybridization to discover novel topoisomerase II poisons with promising antiproliferative activity. J Med Chem 2018, 61 (3), 1375–1379. [DOI] [PubMed] [Google Scholar]
- 18.Minniti E; Byl JAW; Riccardi L; Sissi C; Rosini M; De Vivo M; Minarini A; Osheroff N, Novel xanthone-polyamine conjugates as catalytic inhibitors of human topoisomerase II. Bioorg. Med. Chem. Lett 2017, 27 (20), 4687–4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palermo G; Cavalli A; Klein ML; Alfonso-Prieto M; Dal Peraro M; De Vivo M, Catalytic metal ions and enzymatic processing of DNA and RNA. Acc Chem Res 2015, 48 (2), 220–228. [DOI] [PubMed] [Google Scholar]
- 20.Palermo G; Stenta M; Cavalli A; Dal Peraro M; De Vivo M, Molecular simulations highlight the role of metals in catalysis and inhibition of type II topoisomerase. J Chem Theory Comput 2013, 9 (2), 857–862. [DOI] [PubMed] [Google Scholar]
- 21.Bau JT; Kang Z; Austin CA; Kurz EU, Salicylate, a catalytic inhibitor of topoisomerase II, inhibits DNA cleavage and is selective for the alpha isoform. Mol Pharmacol 2014, 85 (2), 198–207. [DOI] [PubMed] [Google Scholar]
- 22.Kuriappan JA; Osheroff N; De Vivo M, Smoothed potential MD simulations for dissociation kinetics of etoposide to unravel isoform specificity in targeting human topoisomerase II. J Chem Inf Model 2019, 59 (9), 4007–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alagarsamy V; Chitra K; Saravanan G; Solomon VR; Sulthana MT; Narendhar B, An overview of quinazolines: Pharmacological significance and recent developments. Eur J Med Chem 2018, 151, 628–685. [DOI] [PubMed] [Google Scholar]
- 24.Wu CC; Li TK; Farh L; Lin LY; Lin TS; Yu YJ; Yen TJ; Chiang CW; Chan NL, Structural basis of type II topoisomerase inhibition by the anticancer drug etoposide. Science 2011, 333 (6041), 459–462. [DOI] [PubMed] [Google Scholar]
- 25.Worland ST; Wang JC, Inducible overexpression, purification, and active site mapping of DNA topoisomerase II from the yeast Saccharomyces cerevisiae. J. Biol. Chem 1989, 264 (8), 4412–4416. [PubMed] [Google Scholar]
- 26.Wasserman RA; Austin CA; Fisher LM; Wang JC, Use of yeast in the study of anticancer drugs targeting DNA topoisomerases: expression of a functional recombinant human DNA topoisomerase II alpha in yeast. Cancer Res 1993, 53 (15), 3591–3596. [PubMed] [Google Scholar]
- 27.Kingma PS; Greider CA; Osheroff N, Spontaneous DNA lesions poison human topoisomerase IIalpha and stimulate cleavage proximal to leukemic 11q23 chromosomal breakpoints. Biochemistry 1997, 36 (20), 5934–5939. [DOI] [PubMed] [Google Scholar]
- 28.Minniti E; Byl JAW; Riccardi L; Sissi C; Rosini M; De Vivo M; Minarini A; Osheroff N, Novel xanthone-polyamine conjugates as catalytic inhibitors of human topoisomerase IIalpha. Bioorg Med Chem Lett 2017, 27 (20), 4687–4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fortune JM; Osheroff N, Merbarone inhibits the catalytic activity of human topoisomerase II. J. Biol. Chem 1998, 273 (28), 17643–17650. [DOI] [PubMed] [Google Scholar]
- 30.Fortune JM; Velea L; Graves DE; Utsugi T; Yamada Y; Osheroff N, DNA topoisomerases as targets for the anticancer drug TAS-103: DNA interactions and topoisomerase catalytic inhibition. Biochemistry 1999, 38 (47), 15580–15586. [DOI] [PubMed] [Google Scholar]
- 31.Osheroff N; Shelton ER; Brutlag DL, DNA topoisomerase II from Drosophila melanogaster. Relaxation of supercoiled DNA. J. Biol. Chem 1983, 258 (15), 9536–9543. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


