Abstract
Background—
Sex is a well-recognized risk factor for sudden cardiac death (SCD). We hypothesized that sex modifies the association of electrophysiological (EP) substrate with SCD.
Methods—
Participants from the Atherosclerosis Risk in Communities study with analyzable ECGs (n=14,725; age, 54.2±5.8 yrs; 55% female, 74% white) were included. EP substrate was characterized by heart rate, QRS, QTc, Cornell voltage, spatial ventricular gradient (SVG), and sum absolute QRST integral (SAI QRST) ECG metrics. Two competing outcomes were adjudicated SCD and nonSCD. Interaction of ECG metrics with sex was studied in Cox proportional hazards and Fine-Gray competing risk models. Model 1 was adjusted for prevalent cardiovascular disease (CVD) and risk factors. Time-updated model 2 was additionally adjusted for incident non-fatal CVD. Relative hazard ratio (RHR) and relative sub-hazard ratio (RSHR) with a 95% confidence interval for SCD and nonSCD risk for women relative to men was calculated. Model 1 was adjusted for prevalent CVD and risk factors. Time-updated model 2 was additionally adjusted for incident non-fatal CVD.
Results—
Over a median follow-up of 24.4 years, there were 530 SCDs (incidence 1.72 (1.58-1.88)/1000 person-years). Women as compared to men experienced a greater risk of SCD associated with Cornell voltage (RHR 1.18(1.06-1.32); P=0.003), SAI QRST (RHR 1.16(1.04-1.30); P=0.007), and SVG magnitude (RHR 1.24(1.05-1.45); P=0.009), independently from incident CVD.
Conclusion—
In women, the global EP substrate is associated with up to 24% greater risk of SCD than in men, suggesting differences in underlying mechanisms and the need for sex-specific SCD risk stratification.
Keywords: sudden cardiac death, women, sex, ECG, global electrical heterogeneity
Graphical Abstract

Sex is a well-recognized risk factor for sudden cardiac death (SCD), which is a leading cause of death in the United States.1 SCD more commonly occurs in men as compared to women. Women have a lower prevalence of obstructive coronary heart disease (CHD), and systolic dysfunction preceding SCD.2 Women are also less likely than men to receive implantable cardioverter defibrillators (ICD) for primary and secondary prevention of SCD.3 Moreover, regardless of the underlying etiology of heart disease,4 women with ICDs are less likely to receive appropriate ICD therapies5. Therefore, a better understanding of the relationship between sex and SCD is warranted and is especially important for women.
Electrophysiological (EP) substrate of SCD can be characterized by a widely available routine surface 12-lead electrocardiogram (ECG).6 Sex differences in EP substrate have been recognized: women are known to have faster heart rate, narrower QRS, and longer QT interval than men.7 In addition to these traditional ECG metrics, recently, we expanded the armamentarium for global ECG measures of EP substrate with global electrical heterogeneity (GEH).1 GEH is quantified by spatial ventricular gradient (SVG) magnitude and direction (elevation and azimuth), its scalar value sum absolute QRST integral (SAI QRST), and spatial QRS-T angle. The addition of GEH to demographic and risk factors improves reclassification of SCD.1 However, sex is not routinely considered a potential effect modifier of the association between EP substrate and SCD. As women develop CHD approximately ten years later than men, women are commonly viewed as “younger men.”
We sought to determine whether sex can modify the association of ECG measures with SCD. We hypothesized that (1) there are sex differences in ECG measures, and (2) sex modifies the association of ECG measures of EP substrate with SCD.
Methods
Informed consent was obtained from all participants in this study. The study was approved by the OHSU IRB. The ARIC Study data are available through the BioLINCC.
Study population
The Atherosclerosis Risk in Communities (ARIC) study is a prospective cohort that recruited 15,792 men and women, age 45-64 years, selected as a probability sample from four United States communities. Participants were recruited in 1987-1989. Standardized examinations were conducted as previously described.8 Included in the analysis were ARIC cohort participants with recorded resting 12-lead ECG and measured GEH; n=15,777. Excluded were participants self-identifying as non-white or non-black race (n=48), or as black at the Washington County, and Minneapolis field centers (n=55), those with missing covariates (n=903), and non-sinus median beat (n=46). The final sample of participants with normal sinus median beat included 14,725 participants. Definitions of clinical characteristics are provided in the Supplement.
Exposures of sex and electrocardiographic global electrical heterogeneity
Resting 12-lead ECGs of the first five study visits were analyzed. Visit 1 was conducted in 1987-1989, visit 2 in 1990-1992, visit 3 in 1993-1995, visit 4 in 1996-1998, and visit 5 in 2011-2013. Traditional ECG amplitudes and intervals were measured by the 12 SL algorithm (GE Marquette Electronics, Milwaukee, WI). Sex-specific Cornell product was calculated to define ECG-left ventricular hypertrophy (LVH).
GEH was measured as previously described9, by spatial QRS-T angle, SVG magnitude, azimuth, and elevation, and SAI QRST. The MATLAB (MathWorks, Natick, MA, USA) software code for GEH measurement is provided at https://physionet.org/physiotools/geh. Both area and peak SVG vectors9 and QRS-T angles were included in the analysis. Previously reported1 area-based GEH metrics were used in this study. To measure peak-vector-based GEH metrics, we constructed a time-coherent median beat and defined isoelectric heart vector origin point.10 The MATLAB (MathWorks, Natick, MA, USA) software code for the heart vector origin definition is provided at https://github.com/Tereshchenkolab/Origin. In this study, we included only participants with a normal sinus median beat.10
Incident non-fatal cardiovascular events
Incident atrial fibrillation was defined as either detected on follow-up 12-lead ECG or hospital discharge records (ICD-9 code 427.3).11 Incident stroke was physician-adjudicated, as previously described.12 Definite or probable incident strokes are included in this study. Expert-adjudicated incident CHD was defined as a definite or probable myocardial infarction, angina, or a coronary revascularization procedure.13, 14 Incident HF was defined based on the HF codes in a death certificate or an International Classification of Diseases (ICD-9) discharge code, in any position, as previously described.15
Primary outcome: sudden cardiac death
Follow-up of ARIC participants14 and adjudication of SCD was previously described.16 Physician-adjudicated SCD was defined as a sudden pulseless condition in a previously stable individual without evidence of a non-cardiac cause of cardiac arrest if the cardiac arrest occurred out of the hospital or in the emergency room. Definite, probable, or possible SCD was included in this study as a primary outcome.
Competing mortality outcome: non-sudden cardiac death
Non-sudden cardiac death (nonSCD) was defined as an SCD exclusion, composite of fatal CHD, HF death, death in a participant with baseline HF, or incident hospitalized HF. Cases of fatal CHD were adjudicated by the ARIC Morbidity and Mortality Classification Committee.13, 14
Statistical analyses
A detailed description of the statistical methods is provided in the Supplement. To determine differences in GEH between men and women, we constructed two linear regression models with sex as a predictor and normally distributed GEH variables (one-by-one) as an outcome. Model 1 was adjusted for age, race, and study center. To determine whether sex differences in GEH can be explained by sex differences in clinical and traditional ECG characteristics, Model 2 was additionally adjusted for prevalent cardiovascular disease (CVD) and known cardiovascular risk factors. Circular variables were analyzed using circular statistics.
In survival analysis, we constructed three models, performed a statistical test for interaction with sex in each model, and constructed sex-stratified Cox models for men and women. Relative hazard ratio (RHR) with a 95% confidence interval (CI) of SCD risk for women relative to men was reported, assuming hazard ratio for men is a reference (equal to 1). Model 1 was extensively adjusted for demographic and clinical characteristics. Associations of continuous ECG variables with SCD were also evaluated using adjusted Cox regression models incorporating cubic splines with 4 knots. The positions of the 4 knots in the cubic spline models are reported in Supplemental Table 1. To determine whether global ECG measures associated with SCD independently from the substrate of structural heart disease over time, time-updated model 2 included time-updated ECG predictors (one-by-one), all baseline covariates included in model 1, and time-updated incident non-fatal CVD (atrial fibrillation, HF, CHD, and stroke). To determine whether GEH is associated with SCD independently from traditional ECG measures, time-updated model 3 also included time-updated traditional ECG measurements.
To study competing risks of SCD and nonSCD, we constructed Fine and Gray’s competing risk models for SCD and nonSCD outcomes, using the same covariates as for Cox models. Relative sub-hazard ratio (RSHR) with 95% CI of SCD risk for women relative to men was reported, assuming the sub-hazard ratio for men is a reference.
Statistical analyses were performed using STATA MP 15.1 (StataCorp LP, College Station, TX); code is provided at https://github.com/Tereshchenkolab/statistics. Statistical significance at the 0.05 level should be interpreted cautiously.
Results
Study population
Women comprised more than half of the study population (Table 1). Greater than half of the women were postmenopausal. At baseline, women had a lower prevalence of CVD as compared to men. At baseline, women had a lower prevalence of CVD as compared to men. Men had less favorable lipid profiles, were more likely current smokers and alcohol users, and were less physically active.
Table 1.
Comparison of baseline clinical and ECG characteristics in men and women
| Characteristics | Men(n=6,601) | Women(n=8,124) | P-value |
|---|---|---|---|
| Age(SD), y | 54.6(5.8) | 53.8(5.7) | <0.0001 |
| White, n(%) | 5,229(78.1) | 5,886(71.4) | <0.0001 |
| Postmenopause, n(% of women) | 4,834(59.5) | ||
| Heart Failure, n(%) | 204(3.1) | 475(5.9) | <0.0001 |
| Coronary heart disease, n(%) | 528(8.0) | 169(2.1) | <0.0001 |
| Stroke, n(%) | 142(2.2) | 107(1.3) | <0.0001 |
| Body mass index(SD), kg/m2 | 27.5(4.2) | 27.8(6.1) | 0.0002 |
| Diabetes, n(%) | 784(12.0) | 948(11.7) | 0.697 |
| Hypertension, n(%) | 2,227(33.7) | 2,811(34.6) | 0.272 |
| Antihypertensive drugs, n(%) | 1,782(27.0) | 2,664(32.8) | <0.0001 |
| Current tobacco smoker, n(%) | 1,809(27.4) | 2,020(24.9) | <0.0001 |
| Current alcohol drinker, n(%) | 4,282(64.9) | 4,010(49.4) | <0.0001 |
| Leasure physical activity score(SD) | 2.34(0.56) | 2.38(0.59) | 0.0001 |
| Education less than high school, n(%) | 1,543(23.4) | 1,863(22.9) | 0.526 |
| Total cholesterol(SD), mmol/L | 5.46(1.03) | 5.64(1.12) | <0.0001 |
| HDL cholesterol(SD), mg/dL | 44.3(13.8) | 57.6(17.3) | <0.0001 |
| Triglycerides(SD), mmol/L | 1.60(1.13) | 1.39(0.92) | <0.0001 |
| Blood urea nitrogen(SD), mg/dL | 16.1(4.3) | 14.5(4.3) | <0.0001 |
| Chronic kidney disease stage≥2, n(%) | 2,310(35.0) | 2,247(27.7) | <0.0001 |
| Use of antiarrhythmic drugs, n(%) | 1,006(15.2) | 1,043(12.8) | <0.0001 |
| Heart rate(SD), bpm | 64.6(10.2) | 67.5(10.0) | <0.0001 |
| QRS duration(SD), ms | 96.9(12.5) | 88.4(10.7) | <0.0001 |
| QTc(SD), ms | 411.6(17.0) | 420.0(20.0) | <0.0001 |
| Cornell voltage(SD), mV | 1.40(0.6) | 1.10(0.5) | <0.0001 |
| Sex-specific ECG-LVH, n(%) | 423(6.4) | 419(5.2) | 0.001 |
| QRST(SD), degrees | 69.9(28.7) | 54.4(26.0) | <0.0001 |
| SVG magnitude(SD), mV | 1.75(0.5) | 1.68(0.5) | <0.0001 |
| SVG elevation(SD), degrees | 70.3(18.6) | 65.4(15.9) | <0.0001 |
| SVG azimuth(SD), degrees | 21.6(27.6) | 26.2(22.6) | <0.0001 |
| SAI QRST(SD), mV*ms | 161(55) | 129(41) | <0.0001 |
Differences in electrocardiographic parameters between men and women
Women had a faster heart rate, longer QTc, and a narrower QRS (Table 1). The QRS-T angle and SAI QRST were significantly larger in men as compared to women. (Figure 1, Supplemental Table 2 and Supplemental Figure 1). Sex differences in SVG magnitude were explained by covariates. SVG vector pointed more upward and forward in men. There were statistically significant two-way interactions of all GEH variables with sex and heart rate and hypertension (Supplemental Figure 2).
Figure 1. GEH comparison in men and women.

Estimated adjusted marginal (least-squares) means and 95% CI of GEH variables for men and women.
EP substrate of sudden cardiac death in men and women in Cox regression analysis
Over a median follow-up of 24.4 years, there were 530 SCDs (incidence 1.72; 95% CI 1.58-1.88 per 1000 person-years), 2,178 non-sudden cardiac deaths (nonSCDs; incidence 7.09; 95% CI 6.80-7.39 per 1000 person-years), and 2,535 noncardiac deaths (incidence 8.25; 95%CI 7.93-8.58 per 1000 person-years). Incidence of SCD was higher in men (2.56; 95%CI 2.30-2.84 per 1000 person-years) than in women (1.10; 95%CI 0.95-1.26 per 1000 person-years). Incidence of nonSCD was also higher in men (8.51; 95%CI 8.03-9.03 per 1000 person-years) than in women (6.01; 95%CI 5.66-6.38 per 1000 person-years).
We observed a statistically significant interaction of sex with SAI QRST, SVG magnitude, and QRS duration (Figure 2 and Supplemental Table 3A). In women, one standard deviation (SD) of SVG magnitude (0.5 mV), SAI QRST (51 mV*ms), and QRS duration (12 ms) carried a higher risk of SCD than in men, by 21-27% (model 1). Importantly, we observed opposite trends in hazard ratio change across the distribution of SVG magnitude and SAI QRST in men and women (Figure 3 and Supplemental Figure 3). Adjustment for incident non-fatal CVD in model 2 strengthened the interaction of sex with SVG magnitude and revealed significant interaction with Cornell voltage, but attenuated the interaction with SAI QRST and wiped out the interaction with the QRS duration.
Figure 2. The relative risk of sudden cardiac death for women as compared to men.

Forest plot shows relative hazard ratio (RHR) with 95%CI for women as compared to men, with a hazard ratio for men equals 1.0, in model 1 (green diamond) and model 2 (gold diamond). Black lines correspond to 95% CI bounds.
Figure 3.
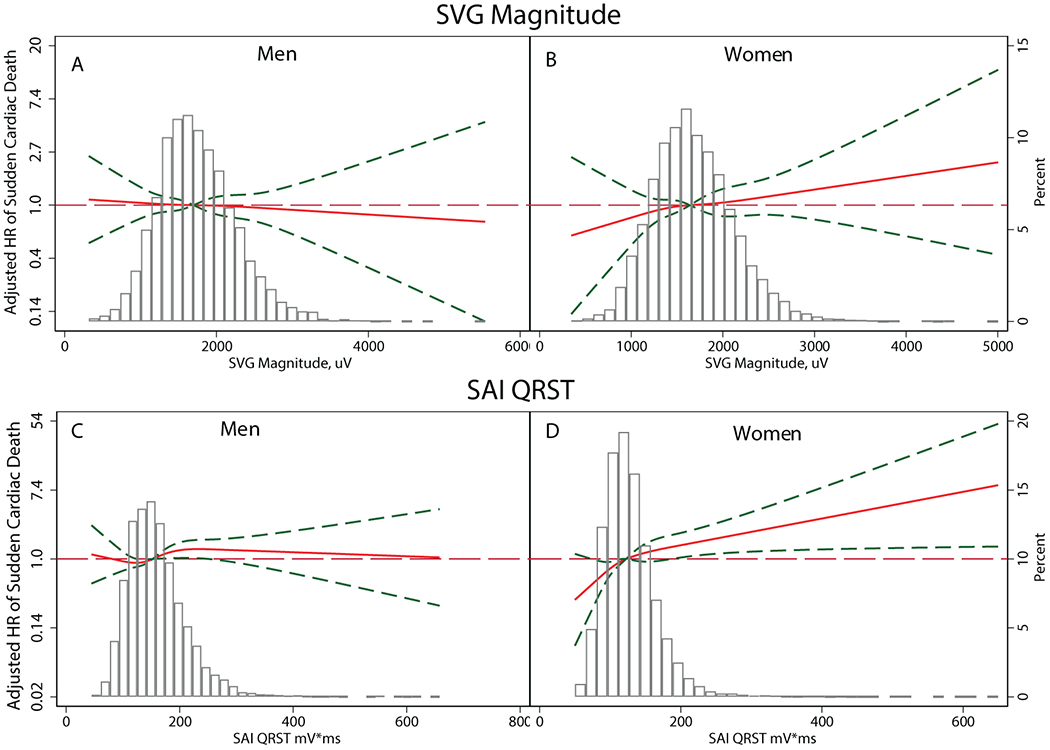
Adjusted (model 2) risk of SCD associated with SVG magnitude (A-B) and SAI QRST (C-D) in men and women. Restricted cubic spline with 95% CI shows a change in the hazard ratio (Y-axis) in response to GEH variable change (X-axis). The 50th percentile of the GEH variable is selected as a reference.
Sex-stratified Cox models confirmed a significant association of traditional and novel global ECG metrics with SCD (Figure 4 and Supplemental Table 3B). After full adjustment for non-fatal incident CVD, there was a 24% increase SCD risk in women versus 10% in men with one SD (0.6 mV) increase in Cornell voltage. Similarly, there was a 19% increase in SCD risk in women versus 9% in men with one SD (51 mV*ms) of SAI QRST. Interaction of SVG magnitude and SAI QRST with sex remained significant in model 3 (Supplemental Table 3).
Figure 4. Sex-stratified risk of sudden cardiac death.

Sex-stratified adjusted (models 1 and 2) Cox proportional hazard ratio and 95% CI of SCD for ECG metrics in men (blue diamond) and women (red diamond). Black lines correspond to 95% CI bounds.
Relative competing risk of SCD and nonSCD in women as compared to men
In competing risk model 1, a statistically significant interaction of sex with competing risk of SCD was observed for QTc, QRS duration, and SAI QRST (Figure 5 and Supplemental Table 3A). Adjustment in model 2 eliminated the interaction with QTc and QRS duration, suggesting that sex differences in SCD risk conveyed by QTc and QRS duration were explained by sex differences in structural heart disease substrate. However, model 2 revealed significant interaction of sex with SVG magnitude and Cornell voltage, in addition to interaction with SAI QRST. After full adjustment for incident CVD, SVG magnitude, SAI QRST, and Cornell voltage were associated with a 16-23% increase in odds of SCD occurrence in women as compared to men (Figure 5 and Supplemental Table 4A).
Figure 5. Relative competing risk of sudden cardiac death.

Forest plot shows relative sub-hazard ratio (RSHR) with 95%CI for women as compared to men, with SHR for men equal 1.0, in model 1 (green diamond), and model 2 (gold diamond). Black lines correspond to 95% CI bounds.
A few interactions were observed for competing risk of nonSCD in model 1, but not in model 2. This suggests that sex differences in the risk of nonSCD were explained by non-fatal incident CVD.
In sex-stratified analyses, model 1 demonstrated that QTc, QRS, and SAI QRST were associated with an increased rate of SCD occurrence by 18-26% in women, but not in men (Figure 6 and Supplemental Table 4B). In men, but not in women, QTc prolongation and smaller peak SVG magnitude were associated with an increased incidence of nonSCD. When adjusted for time-updated CVD substrate in model 2, in women, larger SAI QRST, QRS duration, SVG magnitude, and Cornell voltage were associated with greater probabilities of SCD.
Figure 6. Sex-stratified competing risks of sudden and non-sudden cardiac death.

Sex-stratified adjusted (models 1 and 2) competing risk sub-hazard ratio (SHR) and 95% CI of SCD and nonSCD for ECG metrics in men (blue diamond) and women (red diamond). Black lines correspond to 95% CI bounds.
Across all comparisons and models, peak-based and area-based GEH metrics displayed consistent results, reassuring robustness of analyses.
Discussion
Our study of a large, community-based prospective cohort of over 14,000 participants with greater than 24 years median follow-up showed that sex is a significant modifier with respect to the association of EP substrate with SCD. In women, global EP substrate (QRS duration, Cornell voltage, SAI QRST, SVG magnitude, heart rate, and QTc) was associated with up to 24% greater risk of SCD than in men. Our findings have important clinical implications: our findings support the inclusion of these ECG metrics in risk scores of SCD and suggest the need for sex-specific risk scores. Our results indicate that significant improvement in SCD risk prediction for women can be made. Further studies of mechanisms behind global ECG metrics in men and women, as well as validation of our findings in a separate cohort, are needed for the development of sex-specific prevention of SCD. Theoretically, there are two major groups of mechanisms behind the observed effect modification: differences in the cardiac EP substrate between men and women and differences in structural heart disease substrate.
Our study showed that after rigorous adjustment for baseline demographic and clinical risk factors of SCD, several traditional ECG metrics (QRS duration, heart rate, and QTc), Cornell voltage, and voltage-based GEH metrics (SAI QRST and SVG magnitude) were associated with greater SCD risk in women than in men.
The most remarkable difference in the risk of SCD between men and women was conveyed by amplitude-based ECG metrics: Cornell voltage, SAI QRST, and SVG magnitude. Importantly, the interaction of sex with amplitude-based ECG metrics was independent not only from baseline CVD and its risk factors but also from incident CVD, and it was consistently observed in both Cox regression analysis and competing risk models. One SD (0.6 mV) increase in Cornell voltage was associated with more than 20% higher risk of SCD in women as compared to men. Our finding is consistent with a recent autopsy SCD study in the Finnish population, which observed ECG-LVH more commonly in female than male SCD victims.17
We observed that one SD increase in the magnitude of SVG was associated with approximately 20% higher risk of SCD in women as compared to men. A recent Finnish study demonstrated results consistent with our findings of sex differences in SAI QRST and its association with fatal CVD18, although it did not specifically include SCD. The magnitude of SVG and SAI QRST are global measures of the dispersion of total recovery time in the heart, encompassing dispersion of activation and refractoriness.19–21 We speculate that SVG magnitude and SAI QRST reflect differences in cardiac electrophysiology between men and women, which are responsible for the stronger association of SAI QRST and SVG magnitude with SCD in women than in men. Consistently with this hypothesis, a recent study of healthy volunteers showed that quinidine caused a significantly larger drop in SVG magnitude and SAI QRST in men than in women.22 As quinidine affects multiple ion channels, further studies are needed to discern responsible mechanisms.
We demonstrated that QRS duration is associated with more than 20% higher SCD risk in women than in men. Sex differences in SCD risk conveyed by QRS duration were largely explained by sex differences in time-updated structural heart disease substrate. The collider effect23 can possibly explain the strengthening of the association of QRS with SCD in men after adjustment for incident non-fatal CVD. More likely in men (than in women), unobserved true EP substrate (e.g., distal nonspecific ventricular conduction abnormality manifested by measured ECG variables QRS and QTc) and an unobserved CHD substrate (manifested by SCD outcome) cause a collider (e.g., incident HF). Existing literature on the association between QRS duration and SCD is inconsistent, likely owing at least in part to the study populations having very few women (1-16%).24
Our study showed that in women, QTc is associated with greater odds of SCD, whereas in men, QTc is associated with a greater incidence of nonSCD. The association of QT interval with SCD is controversial.25 Consistent with our findings, both the Rotterdam and OregonSUDS studies showed an association of QT prolongation with SCD only in the absence of cardiac dysfunction and diabetes, respectively.26 Women have a longer QT interval due to reduced expression of potassium channels.27 Estrogens inhibit the rapid delayed rectifier current, ultimately leading to increased calcium release mediated by the ryanodine receptor, which can predispose to triggered activity.28 Two-thirds of the drug-induced TdP cases occur in women.29 Thus, in women, QTc carries an additional risk of SCD due to sex-specific EP mechanisms, independent of CVD substrate.
In this study, resting heart rate was associated with greater odds of SCD in women but not in men. Women have faster resting heart rate7 mostly because of smaller LV mass and volume, resulting in lesser exercise capacity in women than in men.30 Exercise capacity is associated with cardiac arrhythmias.31 Our results suggest that lesser exercise capacity in women, manifesting by faster resting heart rate, translates into the stronger association of heart rate with SCD in women, which is independent of the CVD development.
In this study, non-fatal incident CVD explained the stronger association of QTc and QRS duration with SCD in women, as compared to men. On the other hand, sex did not modify the association of studied ECG features with nonSCD. This finding is in accord with known differences in structural heart disease and coronary microvasculature between men and women. Women have greater arteriolar wall thickness than men.32 In spite of less frequent obstructive CHD, women with angina or MI have greater cardiac mortality than men.33 Thus, in women, QTc and QRS duration reflect an underlying structural heart disease with greater than in men risk of proarrhythmia, whereas, in men, QTc and QRS duration reflect an underlying structural heart disease leading to pump failure and eventually, more likely to nonSCD.
Consistent with previous studies in healthy young individuals34 and young athletes9, we observed a wider QRS-T angle, larger SAI QRST, and SVG vector pointing more upward and forward in middle-aged men than in middle-aged women. Therefore, sex-specific thresholds1 of QRS-T angle, SVG direction, and SAI QRST should be recommended.
Strengths and limitations
This is a large community-based prospective cohort study with long-term follow-up, well-adjudicated SCD, and approximately equal representation of men and women, providing unique opportunity to study sex exposure as an effect modifier. The well-characterized population of the ARIC study allowed us to perform comprehensive adjustment for confounders accounting for important non-cardiac differences between men and women. However, limitations of the study have to be taken into account. The study population was predominantly white; only 26% of the study participants were black. Nevertheless, our recent study showed that race does not modify an association of ECG metrics with SCD.35 Due to the lack of information on baseline LVEF for most of the study participants, we did not adjust our analyses for baseline LVEF. Nevertheless, we adjusted our analyses for incident HF and conducted competing risk analyses, sufficiently accounting for competing risk of a pump failure death.
Supplementary Material
Acknowledgments:
The authors thank the staff and participants of the ARIC study for their important contributions. We would like to acknowledge the SCD mortality classification committee members: Nona Sotoodehnia (lead), Selcuk Adabag, Sunil Agarwal, Lin Chen, Rajat Deo, Leonard Ilkhanoff, Liviu Klein, Saman Nazarian, Ashleigh Owen, Kris Patton, and Larisa Tereshchenko.
Funding:
The ARIC study has been funded in whole or in part with Federal funds from the NHLBI, NIH, DHHS, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, HHSN268201700005I). This work was supported by HL118277 (LGT), and OHSU President Bridge funding (LGT).
Footnotes
Conflict of interest: None.
References
- 1.Waks JW, Sitlani CM, Soliman EZ, et al. Global Electric Heterogeneity Risk Score for Prediction of Sudden Cardiac Death in the General Population: The Atherosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Circulation 2016;133:2222–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chugh SS, Uy-Evanado A, Teodorescu C, et al. Women have a lower prevalence of structural heart disease as a precursor to sudden cardiac arrest: The Ore-SUDS (Oregon Sudden Unexpected Death Study). J Am Coll Cardiol 2009;54:2006–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtis LH, Al-Khatib SM, Shea AM, Hammill BG, Hernandez AF, Schulman KA. Sex differences in the use of implantable cardioverter-defibrillators for primary and secondary prevention of sudden cardiac death. JAMA 2007;298:1517–1524. [DOI] [PubMed] [Google Scholar]
- 4.Tompkins CM, Kutyifa V, Arshad A, et al. Sex Differences in Device Therapies for Ventricular Arrhythmias or Death in the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy (MADIT-CRT) Trial. J Cardiovasc Electrophysiol 2015;26:862–871. [DOI] [PubMed] [Google Scholar]
- 5.MacFadden DR, Crystal E, Krahn AD, et al. Sex differences in implantable cardioverter-defibrillator outcomes: findings from a prospective defibrillator database. Ann Intern Med 2012;156:195–203. [DOI] [PubMed] [Google Scholar]
- 6.Tereshchenko LG. Electrocardiogram as a screening tool in the general population: A strategic review. Journal of Electrocardiology 2013;46:553–556. [DOI] [PubMed] [Google Scholar]
- 7.Gillis AM. Atrial Fibrillation and Ventricular Arrhythmias: Sex Differences in Electrophysiology, Epidemiology, Clinical Presentation, and Clinical Outcomes. Circulation 2017;135:593–608. [DOI] [PubMed] [Google Scholar]
- 8.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 9.Thomas JA, A. P-AE, Junell A, et al. Vectorcardiogram in athletes: The Sun Valley Ski Study. Ann Noninvasive Electrocardiol 2019;24:e12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez-Alday EA, Li-Pershing Y, Bender A, et al. Importance of the heart vector origin point definition for an ECG analysis: The Atherosclerosis Risk in Communities (ARIC) study. Comput Biol Med 2019;104:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso A, Agarwal SK, Soliman EZ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2009;158:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke 1999;30:736–743. [DOI] [PubMed] [Google Scholar]
- 13.Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation 2003;108:2543–2549. [DOI] [PubMed] [Google Scholar]
- 14.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. Journal of clinical epidemiology 1996;49:223–233. [DOI] [PubMed] [Google Scholar]
- 15.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol 2008;101:1016–1022. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Alday EA, Bender A, German D, et al. Dynamic predictive accuracy of electrocardiographic biomarkers of sudden cardiac death within a survival framework: the Atherosclerosis Risk in Communities (ARIC) study. BMC cardiovascular disorders 2019;19:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haukilahti MAE, Holmstrom L, Vahatalo J, et al. Sudden Cardiac Death in Women. Circulation 2019;139:1012–1021. [DOI] [PubMed] [Google Scholar]
- 18.Lipponen JA, Kurl S, Laukkanen JA. Global electrical heterogeneity as a predictor of cardiovascular mortality in men and women. Europace 2018;20:1841–1848. [DOI] [PubMed] [Google Scholar]
- 19.Waks JW, Tereshchenko LG. Global electrical heterogeneity: A review of the spatial ventricular gradient. J Electrocardiol 2016;49:824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tereshchenko LG, McNitt S, Han L, Berger RD, Zareba W. ECG marker of adverse electrical remodeling post-myocardial infarction predicts outcomes in MADIT II study. PLoS One 2012;7:e51812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tereshchenko LG, Cheng A, Fetics BJ, et al. A new electrocardiogram marker to identify patients at low risk for ventricular tachyarrhythmias: sum magnitude of the absolute QRST integral. J Electrocardiol 2011;44:208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stabenau HF, Shen C, Tereshchenko LG, Waks JW. Changes in global electrical heterogeneity associated with dofetilide, quinidine, ranolazine, and verapamil. Heart Rhythm 2020;17:460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenland S Quantifying biases in causal models: classical confounding vs collider-stratification bias. Epidemiology 2003;14:300–306. [PubMed] [Google Scholar]
- 24.Tereshchenko LG, Cheng A, Fetics BJ, et al. Ventricular arrhythmia is predicted by sum absolute QRST integralbut not by QRS width. J Electrocardiol 2010;43:548–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surawicz B, Knoebel SB. Long QT: good, bad or indifferent? J Am Coll Cardiol 1984;4:398–413. [DOI] [PubMed] [Google Scholar]
- 26.Chugh SS, Reinier K, Singh T, et al. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation 2009;119:663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mellor G, Laksman ZWM, Tadros R, et al. Genetic Testing in the Evaluation of Unexplained Cardiac Arrest: From the CASPER (Cardiac Arrest Survivors With Preserved Ejection Fraction Registry). Circ Cardiovasc Genet 2017;10. [DOI] [PubMed] [Google Scholar]
- 28.Odening KE, Koren G. How do sex hormones modify arrhythmogenesis in long QT syndrome? Sex hormone effects on arrhythmogenic substrate and triggered activity. Heart Rhythm 2014;11:2107–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drici MD, Clement N. Is gender a risk factor for adverse drug reactions? The example of drug-induced long QT syndrome. Drug Saf 2001;24:575–585. [DOI] [PubMed] [Google Scholar]
- 30.Burke JH, Goldberger JJ, Ehlert FA, Kruse JT, Parker MA, Kadish AH. Gender differences in heart rate before and after autonomic blockade: Evidence against an intrinsic gender effect. The American Journal of Medicine 1996;100:537–543. [DOI] [PubMed] [Google Scholar]
- 31.Andersen K, Rasmussen F, Held C, Neovius M, Tynelius P, Sundstrom J. Exercise capacity and muscle strength and risk of vascular disease and arrhythmia in 1.1 million young Swedish men: cohort study. BMJ 2015;351:h4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell DJ, Somaratne JB, Jenkins AJ, et al. Differences in myocardial structure and coronary microvasculature between men and women with coronary artery disease. Hypertension 2011;57:186–192. [DOI] [PubMed] [Google Scholar]
- 33.Hemingway H, McCallum A, Shipley M, Manderbacka K, Martikainen P, Keskimaki I. Incidence and prognostic implications of stable angina pectoris among women and men. JAMA 2006;295:1404–1411. [DOI] [PubMed] [Google Scholar]
- 34.Sur S, Han L, Tereshchenko LG. Comparison of sum absolute QRST integral, and temporal variability in depolarization and repolarization, measured by dynamic vectorcardiography approach, in healthy men and women. PLoS One 2013;8:e57175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jensen K, Howell SJ, Phan F, et al. Bringing Critical Race Praxis Into the Study of Electrophysiological Substrate of Sudden Cardiac Death: The ARIC Study. J Am Heart Assoc 2020;9:e015012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


