Abstract
On March 16, 2020, Ukraine’s Ministry of Health issued nonspecific interim guidance to continue enrolling patients in opioid agonist therapies (OAT) and transition existing patients to take-home dosing to reduce community COVID-19 transmission. Though the number of OAT patients increased modestly, the proportion receiving take-home dosing increased from 57.5% to 82.2%, which translates on average to 963,952 fewer clinic interactions annually (range: 728,652–1,016,895) and potentially 80,329 (range: 60,721–84,741) fewer hours of in-person clinical encounters. During the transition, narcologists (addiction specialists) expressed concerns about overdoses, the guidance contradicting existing legislation, and patient dropout, either from incarceration or inadequate public transportation. Though clinicians did observe some overdoses, short-term overall mortality remained similar to the previous year. As the country relaxes the interim guidance, we do not know to what extent governmental guidance or clinical practice will change to adopt the new guidance permanently or revert to preguidance regulations. Some future considerations that have come from COVID-19 are should dosing schedules continue to be flexible, should clinicians adopt telehealth, and should there be more overdose education and naloxone distribution? OAT delivery has improved and become more efficient, but clinicians should plan long-term should COVID-19 return in the near future. If the new efficiencies are maintained, it will free the workforce to further scale up OAT.
Keywords: Opioid agonist therapies, Methadone, Buprenorphine, Policy, COVID-19, Ukraine
The first reported COVID-19 case in Ukraine was on March 3, 2020, with the first person dying 15 days later. By March 16, when there were 5 reported cases (Statistica, 2020), the Ministry of Health issued nonspecific interim guidance to chief narcologists (the administrative addiction treatment expert in each region) to 1) encourage continued access for starting new patients on OAT to reach national targets that the Ministry of Health (MoH) recommended and 2) provide physicians more flexibility with take-home dosing (range: 3–10 days) to safely transition as many patients as possible to reduce patient contact with clinical staff, exposure to others in the community when using public transportation, and clustering in the clinic during the limited hours of medication administration. We describe the consequences of this interim guidance for managing patients with opioid use disorder (OUD) who are treated with opioid agonist therapies (OAT) in the Ukrainian context. We focus on OAT delivery during two 60-day time periods in 2020: a) pre-COVID guidance from January 1 to March 1; and b) post-COVID guidance from April 1 to June 1.
Ukraine introduced OAT in 2004 (Bruce, Dvoryak, Sylla, & Altice, 2007) yet scale-up has been slow, constrained by patient, clinic and policy factors (Bojko et al., 2016; Carroll, 2019; LaMonaca et al., 2019; L. Madden et al., 2017; Zelenev et al., 2018). Ukraine introduced treatment of OUD using OAT primarily for HIV prevention, and Order 200 highly regulates OAT (LaMonaca et al., 2019). The Network for Improvement of Addiction Treatment (NIATx) model for behavioral health (McCarty et al., 2007), an evidence-based implementation strategy, started in November 2014 to scale-up OAT. Four trained NIATx coaches (AM, IP, TF, MF) meet weekly with the chief narcologist in all administrative regions, except Crimea, to facilitate rapid cycle change projects; we used notes from calls to contextualize data. We also analyzed data from the national OAT registry (SYREX) to monitor patient census, entry, attrition (including death), type of OAT, and whether a patient receives take-home dosing (Farnum et al., 2020; Tan, Altice, Madden, & Zelenev, 2020).
Order 200 changed in late 2016, guided by NIATx change projects, to allow “take-home” dosing every 3, 7, or 10 days if clincians documented sobriety for 6 consecutive months; this change reflects the narcologists’ recommendations to reduce demand on patients (L. Madden et al., 2017). During 2019, OAT increased by 9% (11,385 to 12,411), resulting in 4.4% coverage for the estimated 284,022 people who inject drugs (PWID) with OUD; 40–45% of OAT patients have HIV (Mazhnaya et al., 2018). OAT scale-up in 2019 occurred despite 2,067 patients discontinuing treatment, with 538 deaths (annualized mortality=4.3%) among them (cause unknown).
Before interim COVID-19 guidance, 7,381 (57.5%) of the 12,837 OAT patients at 214 sites received take-home dosing. Thereafter, OAT clinics focused mostly on transitioning patients to take-home dosing. By June 1, 10,766 (82.2%) of 13,097 OAT patients were receiving take-home medications (3-, 7- or 10-day supply; mostly 10 days), a 45.9% net increase in take-home dosing. Using a mid-point of 7-day take-homes and 5 minutes per clinical encounter, this translates into a reduction in 963,952 direct annual clinical contacts (range: 728,652–1,016,895 if take-homes were every 3 and 10 days, respectively) and 80,329 (range: 60,721–84,741) fewer hours (Figure 1). OAT scale-up increased by 1,272 patients from January through May with fewer (N=360) during the post-COVID guidance period. Though complete mortality data are not available yet (death certificates required), annualized mortality during this period did not change appreciably (5.0%−4.2%).
Figure 1:
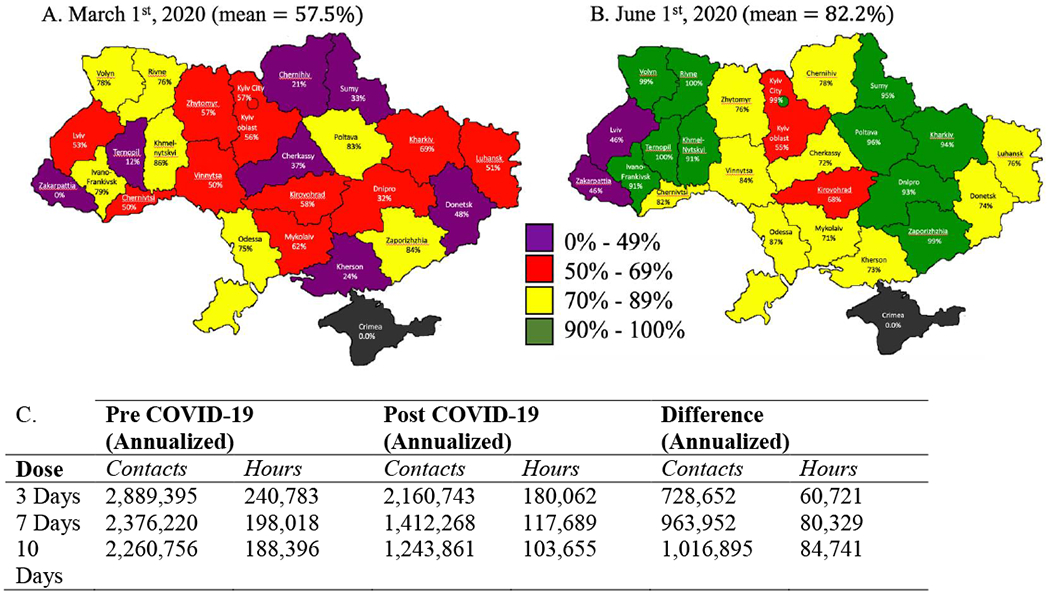
Proportion of patients receiving opioid agonist therapies as take-home dosing and clinical contact requirements in each administrative region in Ukraine both before (a) and during (b) the COVID-19 response, and (c) estimation of reduction in contacts and hours of in-person treatment.
Both scale-up and take-home dosing varied geographically (Figure 1). Six regions (Kyiv city, Odesa, Ivano-Frankivsk, Ternopil, Rivne, Luhansk) reduced the number of OAT patients during COVID-19 restrictions. Most regions increased their number of patients, with two regions doing so by more than 30% (Cherkasy, Dnipro). These two regions had increased their number of patients in 2019 by more than 70% and 20%, respectively. Take-home dosing increased from 53.4% to 82.2%, with 13 regions that increased the proportion of OAT patients on take-home dosing to >90%: only two regions had <50% on take-homes (Lviv, Zakarpattya).
We have learned several important lessons, with some questions still unanswered. The country quickly responded to COVID-19 guidance by modestly expanding treatment coverage, but also safely transitioned a substantial number of patients to take-home dosing. Chief narcologists’ expressed major concerns during weekly NIATx collaborative calls, including concerns about overdoses, the guidance’s contradiction with Order 200 (take-home dosing despite not meeting 6-month sobriety requirements), and patient drop-out, either from decreased or no public transportation. Before COVID-19, most clinicians felt Order 200 sobriety requirements were too stringent, and the order provided no clear guidance about “which patients” should transition to take-home dosing. Clinicians were concerned that shelter-in-place restrictions would increase stress among patients and may facilitate overdose and polysubstance use. They also recognized that the police, who often extract bribes from patients coming for OAT, may arrest patients who were coming less often for OAT or had less money; research has documented such practices previously (Izenberg et al., 2013; Kutsa et al., 2016; Metiluk, 2020).
Though clinicians increased telephone contact with patients to provide support, they felt it was insufficient because COVID-19 came just as a new national healthcare service started, which had substantially increased reporting requirements. With their heightened concerns, chief narcologists reported 15 fatal overdoses among OAT patients after the COVID guidance. Although the circumstances surrounding these overdoses have not been reported, they underline the need to strengthen overdose education and naloxone distribution (OEND) to patients and families. Naloxone is inexpensive in Ukraine, but harm reduction using naloxone has traditionally been the perview of nongovernmental organizations (NGOs) and not medical providers.
Our findings here align with OAT scale-up strategies elsewhere (Bachireddy, Weisberg, & Altice, 2015; L. M. Madden et al., 2018; Strang, Hall, Hickman, & Bird, 2010), where reduced demand and supervision of patients can create more efficient program delivery, promote scale-up, and not contribute to mortality. As a consequence of COVID-19, both clinicians and patients have markedly adapted their professional and personal lives. A major lesson that we have learned is that allowing more take-home medications is extraordinarily efficient. The times saved in clinical encounters and for supervised dosing could be used to scale-up OAT by allowing clinicians to enroll new patients who may need more time for stabilization and focus more on telehealth for counseling. Though a closer review is required, initial mortality data suggest that transitioning to take-home dosing appears safe despite observations of some fatal overdoses. As the country begins to relax shelter-in-place guidelines, to what extent governmental guidance or clinical practice will change in response to relaxation of pandemic restrictions is unclear. A crucial strategy for clinicians will be to identify patients at highest risk for overdose and either reduce the amount of take-homes or shift clinical time to telehealth to assist with patient coping and provide ongoing counseling support. Potentially more challenging will be how narcologists interpret Order 200 regarding take-home dosing. Officially, the order states that no patient may receive take-home dosing until that patient has demonstrated sobriety for 6 months. It does not, however, state that patients who have successfully transitioned to take-home dosing (e.g., during this pandemic and at the request of the government) must be returned to daily supervision once clinicians have transitioned them. In the absence of governmental guidance, narcologists often yield to a very stringent legal framework. Their most conservative interpretation is that all patients previously on daily supervision must return, irrespective of how well they have done clinically, because police may demand to review urine drug testing results to confirm Order 200 compliance. A more liberal interpretation, however, is that if patients have been successfully transitioned to take-home dosing, there is no need to return them to daily supervision unless clinically indicated. Last, we do not know the the extent to which narcologists will learn about and use telehealth and opioid overdose education and naloxone distribution to manage patients with take-home medications. Telehealth principles are nascent in Ukraine, but could optimize patient care and keep patients and communities safe. Learning from COVID-19 in Ukraine could reform healthcare, especially for OAT patients, and may help advance OAT scale-up efforts by improving efficiencies in treatment delivery while keeping both patients and the broader community safe.
Highlights.
82.2% of OAT patients on take-home medication
963,952 annual clinical contacts reduced
Acknowledgments
Author Statement
We are grateful for the thoughtful reviewer comments and input by the editor. We have carefully listed the comments from the reviewers and responded to each. The responses are reflected in the revised submission.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachireddy C, Weisberg DF, & Altice FL (2015). Balancing access and safety in prescribing opioid agonist therapy to prevent HIV transmission. Addiction, 770(12), 1869–1871. doi: 10.1111/add.13055 [DOI] [PubMed] [Google Scholar]
- Bojko MJ, Mazhnaya A, Marcus R, Makarenko I, Islam Z, Filippovych S, … Altice FL (2016). The Future of Opioid Agonist Therapies in Ukraine: A Qualitative Assessment of Multilevel Barriers and Ways Forward to Promote Retention in Treatment. Journal of Substance Abuse Treatment, 66, 37–47. doi: 10.1016/j.jsat.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce RD, Dvoryak S, Sylla L, & Altice FL (2007). HIV treatment access and scale-up for delivery of opiate substitution therapy with buprenorphine for IDUs in Ukraine--programme description and policy implications. Int J Drug Policy, 75(4), 326–328. doi:S0955-3959(06)00254-4 [pii] 10.1016/j.drugpo.2006.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JJ (2019). Narkomania: Drugs, HIV, and Citizenship in Ukraine. Ithaca, NY: Cornell University Press. [Google Scholar]
- Famum SO, Makarenko I, Madden L, Mazhnaya A, Marcus R, Prokhorova T, …Altice FL (2020). The Real-World Impact of Dosing of Methadone and Buprenorphine on Retention on Opioid Agonist Therapies in Ukraine. Addiction, doi: 10.1111/add.15115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izenberg JM, Bachireddy C, Soule M, Kiriazova T, Dvoryak S, & Altice FL (2013). High rates of police detention among recently released HIV-infected prisoners in Ukraine: Implications for health outcomes. Drug and Alcohol Dependence, 755(1), 154–160. doi: 10.1016/j.drugalcdep.2013.05.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsa O, Marcus R, Bojko MJ, Zelenev A, Mazhnaya A, Dvoriak S, … Altice FL (2016). Factors associated with physical and sexual violence by police among people who inject drugs in Ukraine: implications for retention on opioid agonist therapy. Journal of International AIDS Society, 19(4 Suppl 3), 20897. doi: 10.7448/IAS.19.4.20897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMonaca K, Dumchev K, Dvoriak S, Azbel L, Morozova O, & Altice FL (2019). HIV, drug injection, and harm reduction trends in Eastern Europe and Central Asia: Implications for international and domestic policy. Current Psychiatry Reports, 21(1), 47. doi: 10.1007/s11920-019-1038-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden L, Bojko MJ, Farnum S, Mazhnaya A, Fomenko T, Marcus R, … Altice FL (2017). Using nominal group technique among clinical providers to identify barriers and prioritize solutions to scaling up opioid agonist therapies in Ukraine. International Journal of Drug Policy, 49, 48–53. doi: 10.1016/j.drugpo.2017.07.025 [DOI] [PubMed] [Google Scholar]
- Madden LM, Farnum SO, Eggert KF, Quanbeck AR, Freeman RM, Ball SA, … Barry DT (2018). An investigation of an open-access model for scaling up methadone maintenance treatment. Addiction, 775(8), 1450–1458. doi: 10.1111/add.14198 [DOI] [PubMed] [Google Scholar]
- Mazhnaya A, Marcus R, Bojko MJ, Zelenev A, Makarenko I, Pykalo I, … Altice FL (2018). Opioid Agonist Treatment and Improved Outcomes at Each Stage of the HIV Treatment Cascade in People Who Inject Drugs in Ukraine. Journal of Acquired Immune Deficiency Syndrome, 79(3), 288–295. doi: 10.1097/QAI.0000000000001827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty D, Gustafson DH, Wisdom JP, Ford J, Choi D, Molfenter T, … Cotter F (2007). The Network for the Improvement of Addiction Treatment (NIATx): Enhancing access and retention. Drug and Alcohol Dependence, 88(2–3), 138–145. doi:S0376-8716(06)00408-X [pii] 10.1016/j.drugalcdep.2006.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistica. (2020). Cumulative coronavirus (COVID-19) confirmed cases, recoveries, and deaths in Ukraine as of June 12, 2020, by date of report. Retrieved from https://www.statista.com/statistics/1102288/coronavirus-deaths-development-europe/
- Strang J, Hall W, Hickman M, & Bird SM (2010). Impact of supervision of methadone consumption on deaths related to methadone overdose (1993–2008): Analyses using OD4 index in England and Scotland. BMJ, 341, c4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Altice FL, Madden LM, & Zelenev A (2020). Effect of expanding opioid agonist therapies on the HIV epidemic and mortality in Ukraine: a modelling study. Lancet HIV, 7(2), el21–el28. doi: 10.1016/S2352-3018(19)30373-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenev A, Shea P, Mazhnaya A, Rozanova J, Madden L, Marcus R, & Altice FL (2018). Assessment of barrier severity and willingness to enter opioid agonist treatment among people who inject drugs in Ukraine. Drug and Alcohol Dependence, 190, 82–88. doi: 10.1016/j.drugalcdep.2018.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]


