Abstract
Objectives:
To report the initial compliance with new infection control regulations and geographic disparities in nursing homes (NHs) in the United States.
Design:
Retrospective cohort study from November 27, 2017 to November 27, 2019.
Setting and Participants:
In total, 14,894 NHs in the continental United States comprising 26,201 inspections and 176,841 deficiencies.
Methods:
We measured the cumulative incidence of receiving F880: Infection Prevention and Control deficiencies, geographic variability of F880 citations across the United States, and the scope and severity of the infection control deficiencies.
Results:
A total of 6164 NHs (41%) in the continental United States received 1 deficiency for F880, and 2300 NHs (15%) were cited more than once during the 2-year period. Geographic variation was evident for F880 deficiencies, ranging from 20% of NHs in North Carolina to 79% of NHs in West Virginia. Between 0% (Vermont) and 33% (Michigan) of states’ NHs were cited multiple times over 2 years. Facilities receiving 2 or more F880 deficiencies were more reliant on Medicaid, for-profit, and served more acute residents. Infection Prevention and Control deficiencies were of similar severity but of greater scope in NHs that were cited multiple times.
Conclusions and Implications:
As the coronavirus disease 2019 pandemic challenges hospitals with an increased surge of patients from the community, NHs will be asked to accept convalescing patients who were previously infected with the virus. NHs will need to rely on infection control practices to mitigate the effects of the virus in their facilities. Particular attention to NHs that have fared poorly with repeat infection control practices deficiencies might be a good first step to improving care overall and preventing downstream morbidity and mortality among the highest-risk patients.
Keywords: Infection prevention, deficiencies, geographic disparities
Nursing homes (NHs) across the country face a mounting challenge from coronavirus disease 2019 (COVID-19), with early data showing vastly increased fatality rates among those with advanced age and comorbid illness.1,2 The deaths of 19 residents from a single NH near Seattle, Washington, including the first death reported in the United States, had raised the chilling prospect that the 1.3 million NH residents will be among the most severely affected population.3 By June 2020, more than 43,000 NH residents and staff died from COVID-19.4 Absent effective treatment against COVID-19 and devoid of existing herd immunity among patients and staff, compliance with recommended infection control practices will be more important than ever to keep residents safe from the COVID-19 pandemic.
Historically, infection control practices within NHs have been important against more conventional illnesses such as influenza and norovirus.5–7 Recognizing the challenge of infection control within NHs, the Centers for Medicare and Medicaid Services (CMS) implemented enhanced standards through phases from November 2016 through November 2019,8 though some surveyor guidelines from phase III are still delayed in 2020. Standards phased in so far require NHs to have programs to prevent, identify, investigate, and control infections and communicable diseases. Unfortunately, professionals responsible for infection control in NHs often have little to no specific training in infection prevention and control, and have additional responsibilities in the NH.9 The CMS guidance focuses on standard precautions such as hand hygiene, the use of gloves and masks, and procedures to disinfect equipment. NHs have not been required to have specialized equipment such as respirators or airborne infection isolation rooms.10
Recent research suggests that infection control has improved since these standards went into effect in 2016,11 which is promising given the high rates of infection control deficiencies reported in the mid-2000s.12 However, there is also evidence that infection control lapses continue to occur in NHs.13 It is conceivable that NHs that receive multiple infection control citations over time will be at greatest risk for adverse events because of communicable diseases. Given the heightened risk of mortality among older adults who contract COVID-19,14 highlighting these facilities for urgent quality improvement efforts may be in the best interest of public health professionals who seek to curb downstream mortality from the virus. We examined NH compliance with new CMS infection control regulations and assessed variability by state. Specific attention was paid to identifying facilities that have received multiple citations for infection control.
Methods
This study used data from the Certification and Survey Provider Enhanced Reports (CASPER) to examine 26,201 inspections of 14,894 freestanding NHs in the continental United States from November 27, 2017 to November 27, 2019. Hospital-based NHs (n = 640) and facilities with fewer than 10 beds (n = 23) were excluded as they do not represent typical NHs and may have drastically different resources. The final sample represents 96% of all NHs in the continental United States. We analyzed all F880: Infection Prevention and Control deficiencies (Appendix 1 lists the federal regulations). Facilities with consecutive citations for F880 over the 2-year period were examined.
Deficiencies related to infection control were evaluated for the breadth of the problem (scope) and the relative harm caused to residents (severity). In accordance with classifications developed by CMS (Supplementary Table 1), scope of deficiencies was categorized as isolated, pattern, or widespread, and severity was categorized as potential for minimal harm, potential for more than minimal harm, actual harm, or immediate jeopardy. Bivariate results (t-tests and χ2 tests) with associated P values are reported in Table 1 to compare NHs with no F880 citation to NHs with more than one F880 citation.
Table 1.
Characteristics of NH that Received 0, 1, or More than 1 Deficiency for F880: Infection Prevention and Control from 2017 to 2019
| Zero F880 Tags | One F880 Tag | ≥Two F880 Tags | ||
|---|---|---|---|---|
| Number of NHs | 43% (6430) | 41% (6164) | 15% (2300) | |
| Total number of beds | 682,870 | 687,913 | 262,291 | |
| Total number of residents | 533,904 | 540,557 | 201,440 | |
| M (SD) | M (SD) | M (SD) | P (0 vs ≥2) | |
| Organizational structure (yes/no) | ||||
| For-profit facility | 69.04% | 73.46% | 78.00% | <.001 |
| Not for-profit facility | 25.09% | 20.77% | 16.96% | <.001 |
| Government facility | 5.88% | 5.78% | 5.04% | .14 |
| Chain-facility | 59.08% | 60.03% | 57.35% | .15 |
| Part of a CCRC | 12.88% | 10.14% | 8.22% | <.001 |
| Size (number of beds) | 106.20 (61.37) | 111.60 (59.19) | 114.04 (53.75) | <.001 |
| Occupancy rate (% out of 100) | 78.06% (18.67) | 78.62% (16.33) | 77.31% (15.75) | .08 |
| ADRD special care unit | 13.72% | 14.88% | 13.57% | .86 |
| Non-ADRD special care unit | 3.14% | 3.80% | 4.52% | .002 |
| Any special care unit | 16.86% | 18.67% | 18.09% | .18 |
| Payer-mix (% residents) | ||||
| Medicare | 13.55% (14.20) | 12.98% (12.56) | 12.58% (11.52) | .003 |
| Medicaid | 57.30% (25.29) | 60.65% (22.97) | 62.41% (22.11) | <.001 |
| Private pay and other | 29.15% (21.43) | 26.37% (18.86) | 25.01% (18.14) | <.001 |
| Resident characteristics (% residents) | ||||
| Acuindex (patient acuity) | 10.36 (1.44) | 10.43 (1.39) | 10.52 (1.41) | <.001 |
| Behavioral healthcare needs | 21.38% (18.99) | 21.49% (18.67) | 21.44% (18.59) | .89 |
| ADRD | 45.13% (17.81) | 44.57% (16.84) | 43.47% (17.03) | <.001 |
| Depression | 37.18% (23.81) | 36.82% (23.38) | 35.58% (23.42) | .005 |
| Intellectual disability | 2.20% (5.57) | 2.30% (5.40) | 2.35% (4.64) | .23 |
| Serious mental illness | 32.67% (18.79) | 33.85% (18.25) | 33.94% (18.70) | .005 |
| Physical restraint use | 0.56% (3.68) | 0.65% (3.99) | 0.75% (4.56) | .04 |
| Antipsychotic use | 17.98% (13.52) | 19.33% (13.97) | 19.80% (13.96) | <.001 |
| Infection risk factors (% residents) | ||||
| Pressure ulcer on admission | 3.35% (4.15) | 3.40% (3.81) | 3.64% (3.93) | .004 |
| Pressure ulcer developed | 5.97% (5.43) | 6.07% (4.99) | 6.33% (4.95) | .005 |
| Ostomy care | 2.16% (3.22) | 2.31% (3.45) | 2.40% (3.50) | .003 |
| Tracheostomy care | 0.92% (5.29) | 1.11% (5.64) | 1.47% (6.52) | <.001 |
| Suctioning care | 1.04% (5.53) | 1.26% (5.86) | 1.63% (6.64) | <.001 |
| Tube feeding care | 3.79% (6.91) | 4.16% (7.10) | 4.80% (7.83) | <.001 |
| Received influenza vaccine | 65.47% (23.07) | 63.53% (22.01) | 62.31% (21.20) | <.001 |
| Received pneumococcal Vaccine | 63.28% (26.12) | 60.97% (25.59) | 58.44% (25.80) | <.001 |
| Received antibiotics | 8.47% (6.76) | 8.72% (6.65) | 8.50% (5.99) | .82 |
ADRD, Alzheimer’s disease and related dementias; CCRC, continuing care retirement community; M, mean; SD, standard deviation.
These results use all freestanding nursing homes in the continental United States with at least 10 beds (N = 14,894). Characteristics of nursing homes were estimated from the closest inspection to the midpoint of the 2-year time period (ie, November 27, 2018). All F880 deficiencies were summed between November 27, 2017 and November 27, 2019.
Results
In total, 176,841 deficiencies were given to 14,894 NHs between November 27, 2017 and November 27, 2019. Of those deficiencies, 10,806 (6.11%) were for F880: Infection Prevention and Control (Table 1). A total of 8464 NHs (57%) in the continental United States received at least 1 deficiency for F880; 2300 NHs (15%) were cited more than once during the 2-year period. Infection Prevention and Control deficiencies were of similar severity but of greater scope in NHs that were cited multiple times (Supplementary Table 2).
In general, facilities receiving more than 1 Infection Prevention and Control deficiency in comparison to facilities receiving none were larger, for-profit, less likely to be a continuing care retirement community, more reliant on Medicaid, and had greater utilization of antipsychotics (all P < .001; Table 1). Residents dwelling in NHs that received multiple F880 deficiencies were more acutely ill and were more likely to require services that may increase the risk of contracting infectious diseases (eg, tracheostomy care, suctioning, and tube feeding; all P < .001). Though statistically significant, the effect size differences between resident characteristics are small (all Cohen d 0.21).
Geographic variation was evident for Infection Prevention and Control deficiencies, ranging from 20% of NHs in North Carolina to 79% of NHs in West Virginia (Figure 1); 31 states cited 50% or more of their NHs. One-third of NHs in Michigan and Missouri were cited for multiple F880 deficiencies over 2 years, though receiving more than 1 deficiency varied by state (0% in Vermont) (Figure 2).
Fig. 1.
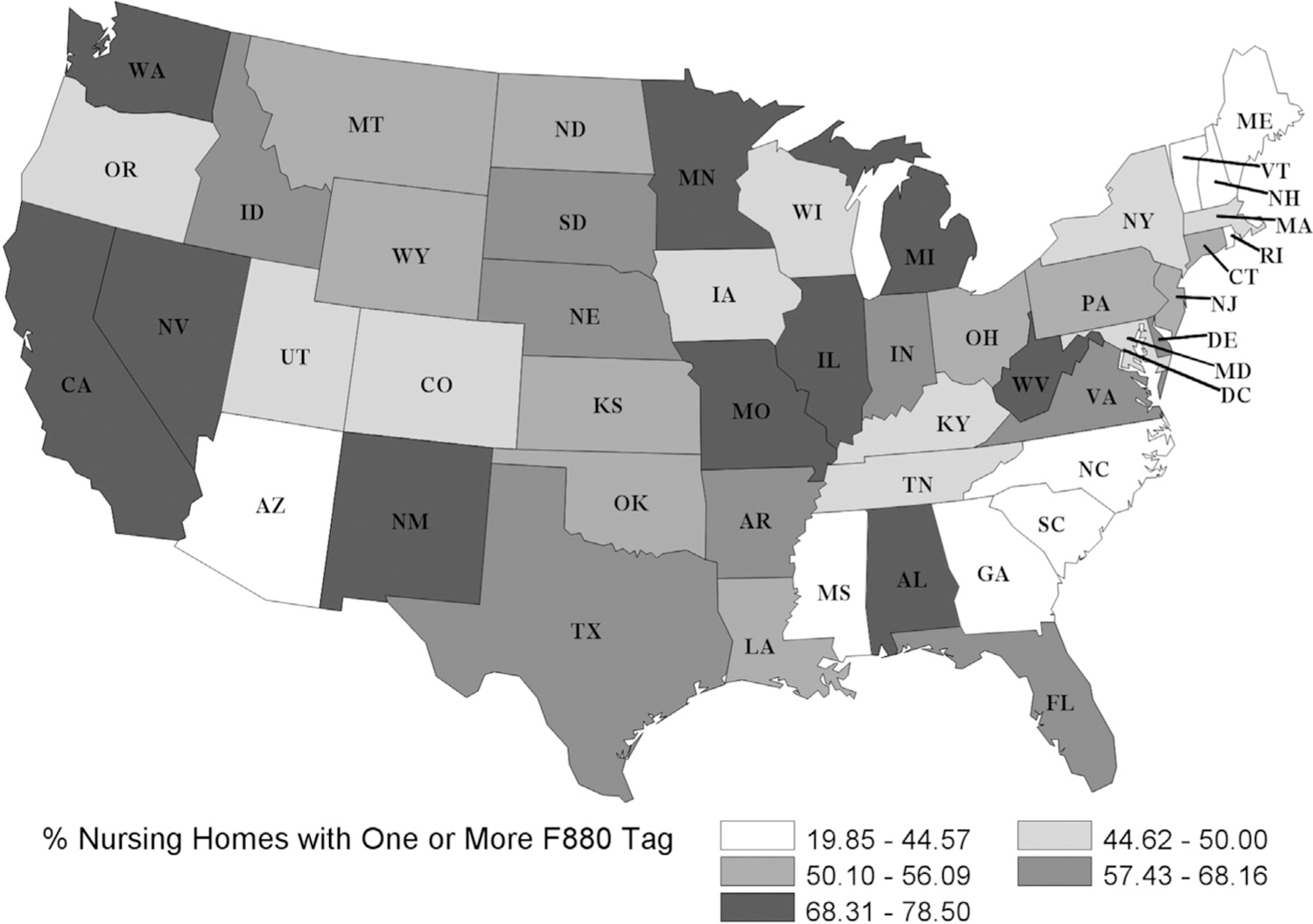
Percentage of NHs that received at least 1 deficiency for F880: Infection Prevention and Control from 2017 to 2019. Of US NHs, 57% (n = 8464) received at least 1 F880 deficiency over 2 years. Cumulative incidence was lowest in North Carolina at 19.85% and highest in West Virginia at 78.50%.
Fig. 2.
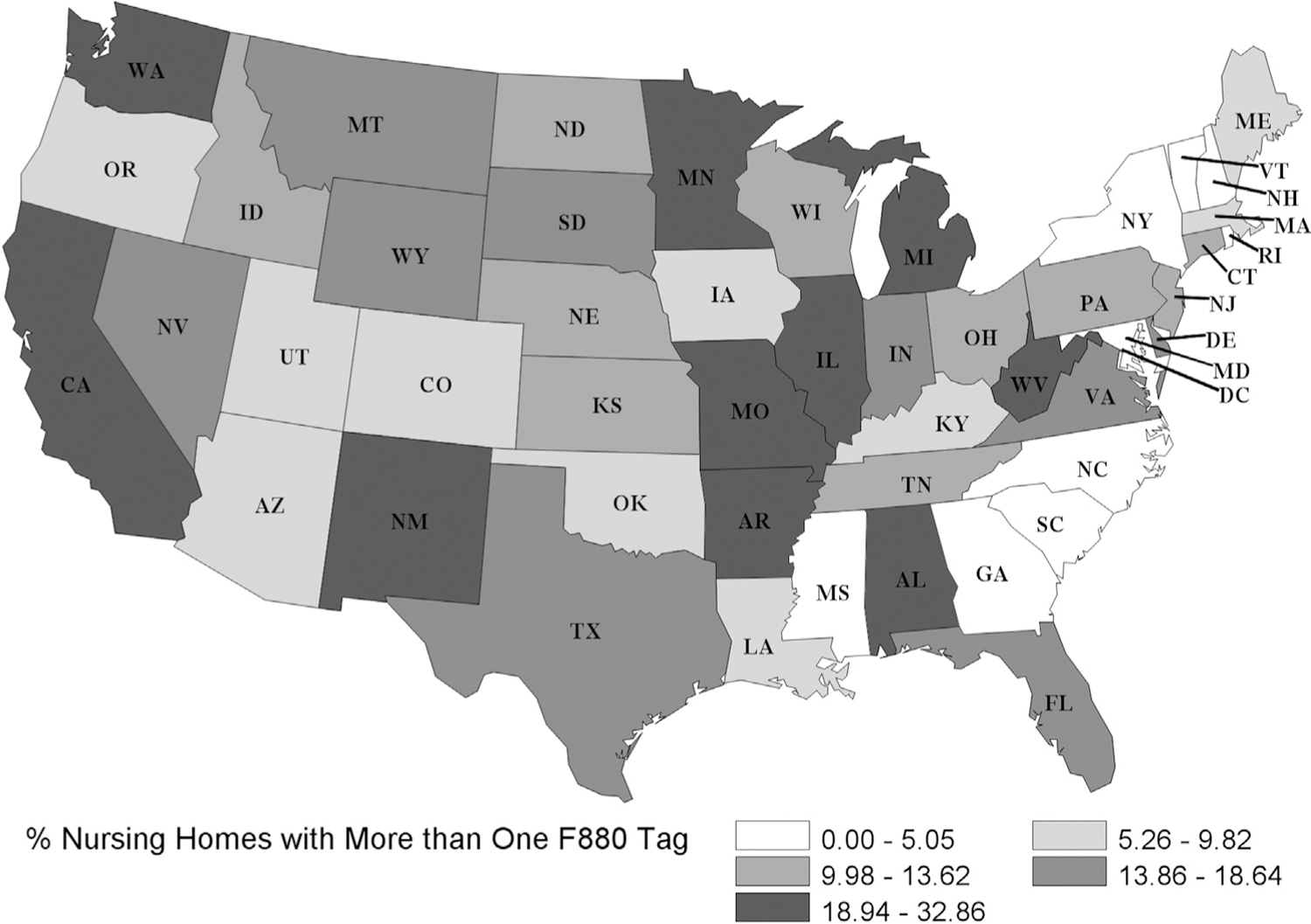
Percentage of NH that received more than 1 deficiency for F880: Infection Prevention and Control from 2017 to 2019. Of US NHs, 15% (n = 2300) received more than 1 F880 deficiency over 2 years. Cumulative incidence was lowest in Vermont at 0% and highest in Michigan at 32.86%.
Discussion
Over a 2-year period, greater than one-half of all NHs in the United States received at least 1 regulatory citation for infection control practices, with 31 states citing 50% or more of their facilities. More than 15% of all NHs received multiple Infection Prevention and Control citations over 2 years, whereas 43% received none. The effect size differences were small when comparing resident characteristics of NHs with none vs more than 1 Infection Prevention and Control deficiency. This may suggest that receiving an F880 deficiency is more related to process measures (eg, staffing levels, staff compliance with regulations) or geographic variability (ie, differences in vigilance and stringency may be found across states) rather than resident predispositions.
Considering geographic variability, prior research has found geographic differences in deficiency citations overall. CMS contracts with state agencies to conduct annual surveys to assess NHs’ federal regulatory compliance. Several studies have found differences among states in the training and availability of regulatory staff to conduct surveys15–17 and in numbers of deficiencies cited.18–20 Walshe and Harrington21 found a significant relationship between state NH regulatory funding and deficiencies, with more well-funded states citing NHs for more deficiencies and a higher percentage of severe deficiencies. Others have highlighted regulatory differences among states that affect NH quality outcomes.22
Whether the numbers of infection control deficiencies we found are a result of differences in survey practices, varying attention to infection control or differences in quality is unknown. However, the data do suggest that infection control practices are lagging in some regions and lapses may be worse among NHs with more vulnerable residents. Prior work by the United States Government Accountability Office23 found similar results when analyzing deficiencies prior to the new F880 regulations. From 2013 to 2017, 82% of NHs received at least 1 Infection Prevention and Control deficiency, with 48% receiving multiple deficiencies in consecutive years and 19% receiving multiple deficiencies in nonconsecutive years.23 Future work should examine if these patterns persist with the new tags and regulations.
To allow NHs the ability to focus on resident care and avoid distractions with routine inspections, CMS initially suspended inspections for at least 3 weeks in March 2020. On March 23, 2020 in congruence with guidelines set by the Centers for Disease Control and Prevention (CDC), CMS announced a focused survey process that would highlight complaint inspections, targeted infection control inspections, and self-assessments to drastically limit the increasing number of NH residents with COVID-19.24 By May 2020, CMS developed COVID-19 deficiencies (F884: COVID-19 Reporting to CDC, F885: Reporting to Residents, their Representatives, and Families) to enhance compliance with national guidelines on mandatory screening and reporting.25 As the COVID-19 pandemic challenges hospitals with an increased surge of patients from the community, NHs will be asked to accept convalescing patients who were previously infected with the virus. NHs will need to rely on infection control practices to mitigate the effects of COVID-19 among other threats such as the increase in multidrug resistant organisms and influenza outbreaks in their facilities.26 Paying particular attention to NHs with repeated infection control deficiencies would be a good first step to improving care overall and preventing downstream morbidity and mortality among the highest risk patients.
The relationship between past infection control citations and current COVID-19 cases and deaths is complex given the time-sensitive nature of a developing pandemic. Some have suggested that NHs with at least 1 COVID-19 case are larger, situated within an urban area, and have a greater proportion of residents identifying as racial or ethnic minorities, though 5-star rating and prior infection control deficiencies do not yet appear to be associated in the national data.27 However, the relationship between 5-star quality and COVID-19 cases or deaths may be different between states. In California, a 5-star rating was associated with having fewer COVID-19 cases and deaths.28 As the pandemic progresses quickly across the country, researchers must continuously update the literature on emerging associations. In all likelihood, it may be several months until we better understand the predisposing factors that led to heightened COVID-19 cases and deaths in some—but not all—NHs.
Equally as important are 2 additional infection control measures requiring NHs to hire infectious disease preventionists and to provide appropriate infection control training (F882 Infection Preventionist Qualifications and Role; F945 Infection Control Training).29 These new regulations were to have been implemented in November 2019 as part of phase III of the enhanced standards. However, CMS delayed the guidelines and training that would enable NH inspectors to assess for compliance of the new requirements. Whether this impacted the preparedness of NHs to mitigate particularly virulent infectious diseases like COVID-19 will never be known. The guidance is now scheduled to be released in the second quarter of the calendar year of 2020.29 Given that the staffing of infection preventionists has not changed from 2014 to 2018,30 these requirements may be especially impactful.
Conclusions and Implications
As the world tackles an unprecedented challenge to its infection control infrastructure, greater attention must be paid to NH infection control practices and to the resources required to assist those with deficiencies, particularly those with repeated deficiencies, who serve the most vulnerable residents.
Supplementary Material
Acknowledgments
This work was supported by the National Institute on Aging of the National Institutes of Health under award number R01AG060581–01.
The National Institute on Aging (R01AG060581–01) did not have a role in the design, methods, analysis, or preparation of this manuscript.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Dosa D, Jump RL, LaPlante K, et al. Long-term care facilities and the coronavirus epidemic: Practical guidelines for a population at highest risk. J Am Med Dir Assoc 2020;21:569–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 3.Golden H Washington nursing home at center of US coronavirus outbreak reports 13 deaths. The Guardian. 2020. Available at: https://www.theguardian.com/world/2020/mar/07/coronavirus-washington-kirkland-life-care-center-deaths.
- 4.Paulin E How to track COVID-19 nursing home cases and deaths in your state: What state are reproting and how to find it. AARP. 2020. Available at: https://www.aarp.org/caregiving/health/info-2020/coronavirus-nursing-home-cases-deaths.html.
- 5.Gravenstein S, Davidson HE, Taljaard M, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: A cluster-randomised trial. Lancet Respir Med 2017;5:738–746. [DOI] [PubMed] [Google Scholar]
- 6.Petrignani M, van Beek J, Borsboom G, et al. Norovirus introduction routes into nursing homes and risk factors for spread: A systematic review and meta-analysis of observational studies. J Hosp Infect 2015;89:163–178. [DOI] [PubMed] [Google Scholar]
- 7.Pop-Vicas A, Rahman M, Gozalo PL, et al. Estimating the effect of influenza vaccination on nursing home residents’ morbidity and mortality. J Am Geraitr Soc 2015;63:1798–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Medicare and Medicaid Services. State Operations Manual-Appendix PP Guidance to surveyors for long-term care facilities (Rev. 173, 11-22-17); 2017.
- 9.Herzig CT, Stone PW, Castle N, et al. Infection prevention and control programs in US nursing homes: Results of a national survey. J Am Med Dir Assoc 2016;17: 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Corona-virus Disease 2019 (COVID-19) in Healthcare Settings. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fhcp%2Finfection-control.html.
- 11.Agarwal M, Dick AW, Sorbero M, et al. Changes in US nursing home infection prevention and control programs from 2014 to 2018. J Am Med Dir Assoc 2020; 21:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castle NG, Wagner LM, Ferguson-Rome JC, et al. Nursing home deficiency citations for infection control. Am J Infect Control 2011;39:263–269. [DOI] [PubMed] [Google Scholar]
- 13.Davis C Most-cited F-tags nationwide: F880 and F689 are the top concerns. 2018. Available at: https://www.aadns-ltc.org/Resources/DNS-Navigator/details/post/most-cited-f-tags-nationwide-f880-and-f689-are-the-top-concerns/2018-09-11.
- 14.CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)dUnited States, February 12eMarch 16, 2020. Morbid Mortal Week Rep (MMWR) 2020;69:343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United States Government Accountability Office. Prevalence of serious problems, while declining, reinforces importance of enhanced oversight. 2003. Available at: https://www.gao.gov/products/GAO-03-561.
- 16.United States Government Accountability Office. Addressing the factors underlying understatement of serious care problems requires sustained CMS and state commitment. 2009. Available at: https://www.gao.gov/products/GAO-10-70.
- 17.United States Office of Inspector General. A few states fell short in timely investigation of the most serious nursing home complaints: 2011–2015. 2017. Available at: https://oig.hhs.gov/oei/reports/oei-01-16-00330.pdf.
- 18.Stevenson DG. Nursing home consumer complaints and quality of care: A national view. Med Care Res Rev 2006;63:347–368. [DOI] [PubMed] [Google Scholar]
- 19.Hansen KE, Hyer K, Holup AA, et al. Analyses of complaints, investigations of allegation, and deficiency citations in United States nursing homes. Med Care Res Rev 2019;76:736–757. [DOI] [PubMed] [Google Scholar]
- 20.United States Government Accountability Office. CMS should continue to improve data and oversight. 2015. Available at: https://www.gao.gov/products/GAO-16-33.
- 21.Walshe K, Harrington C. Regulation of nursing facilities in the United States: An analysis of resources and performance of state survey agencies. The Gerontologist 2002;42:475–486. [DOI] [PubMed] [Google Scholar]
- 22.Mukamel DB, Weimer DL, Harrington C, et al. The effect of regulatory stringency and nursing home quality. Health Serv Res 2012;47:1791–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.United States Government Accountability Office. Infection control deficiencies were widespread and persistent in nursing homes prior to COVID-19 pandemic. 2020. Available at: https://www.gao.gov/assets/710/707069.pdf.
- 24.Centers for Medicare and Medicaid Services. Kirkland, Washington update and survey prioritization fact sheet. 2020. Available at: https://www.cms.gov/newsroom/fact-sheets/kirkland-washington-update-and-survey-prioritization-fact-sheet.
- 25.Centers for Medicare & Medicaid Services. Interim final rule updating requirements for notification of confirmed and suspected COVID-19 cases among residents and staff in nursing homes. 2020. Available at: https://www.cms.gov/files/document/qso-20-29-nh.pdf.
- 26.Sloane PD, Zimmerman S, Nace DA. Progress and challenges in the management of nursing home infections. J Am Med Dir Assoc 2020;21:1–4. [DOI] [PubMed] [Google Scholar]
- 27.Abrams HR, Loomer L, Gandhi A, et al. Characteristics of US nursing homes with COVID-19 cases. J Am Geriatr Soc; 2020. [DOI] [PMC free article] [PubMed]
- 28.He M, Li Y, Fang F. Is there a link between nursing home reported quality and COVID-19 cases? Evidence from California skilled nursing facilities. J Am Med Dir Assoc; 2020. [DOI] [PMC free article] [PubMed]
- 29.Centers for Medicare and Medicaid Services. Updates and initiatives to ensure safety and quality in nursing homes. 2019. Available at: https://www.cms.gov/files/document/qso-20-03-nh.pdf.
- 30.Stone PW, Agarwal M, Pogorzelska-Maziarz M. Infection preventionist staffing in nursing homes. Am J Infect Control 2020;48:330–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


