Abstract
Background
Increased arterial stiffness and vascular endothelial dysfunction are important nontraditional cardiovascular risk factors evident in patients with CKD. Vascular oxidative stress and inflammation may contribute to vascular dysfunction in CKD, but direct evidence is lacking.
Methods
We assessed carotid-femoral pulse-wave velocity (arterial stiffness) and brachial artery flow-mediated dilation (vascular endothelial function) in participants with moderate-to-severe CKD (eGFR 15–59 ml/min per 1.73 m2) and in healthy controls. Change in brachial artery flow-mediated dilation after an acute infusion of ascorbic acid to inhibit vascular oxidative stress (versus saline) was also measured. Protein expression of vascular endothelial cells collected from a peripheral vein and ELISAs to assess circulating markers were also performed.
Results
A total of 64 participants with CKD (mean±SD, 65±8 years) and 17 healthy controls (60±5 years) were included. Carotid-femoral pulse-wave velocity was greater in participants with CKD compared with healthy controls (1071±336 versus 732±128 cm/s; P<0.001). Brachial artery flow-mediated dilation was lower in participants with CKD compared with healthy controls (3.5%±2.8% versus 5.5%±3.2%; P=0.02). Circulating inflammation markers (C-reactive protein and IL-6) were elevated in the CKD group (P≤0.02). Endothelial cell protein expression of NADPH (intensity versus human umbilical vein endothelial cell control, 1.48±0.28 versus 1.25±0.31; P=0.05) was greater in participants with CKD. However, ascorbic acid significantly improved brachial artery flow-mediated dilation in control participants (saline, 5.5±3.2; ascorbic acid, 6.8±3.6); as compared with participants with CKD (saline, 3.5±2.8; ascorbic acid, 3.6±3.2) (group×condition interaction P=0.04), suggesting vascular oxidative stress could not be overcome with ascorbic acid in participants with CKD.
Conclusions
Vascular oxidative stress is present in CKD, which cannot be overcome with acute infusion of ascorbic acid.
Introduction
Patients with CKD are more likely to die of cardiovascular disease (CVD) than to progress to ESKD (1), and the risk of cardiovascular mortality or a cardiovascular event is significantly increased compared with the general population (2). However, although patients with CKD exhibit a high presence of traditional CVD risk factors, they only partially explain the increased incidence of CVD in this population (3,4).
Patients with CKD exhibit vascular endothelial dysfunction (impaired endothelium-dependent dilation, commonly assessed as brachial artery flow-mediated dilation [FMDBA]) (5–7) and increased arterial stiffness (commonly assessed as carotid-femoral pulse-wave velocity [PWV]) (8–10), as well as chronic oxidative stress and inflammation (11,12). Oxidative stress and inflammation are important nontraditional risk factors for CVD (4) and may contribute to the development of vascular dysfunction; however, the mechanisms contributing to vascular dysfunction in CKD are incompletely understood.
Circulating markers of oxidative stress are associated with endothelial dysfunction in patients with CKD (13), and evidence suggests that oxidative stress may contribute to cutaneous microvascular dysfunction in patients with stage 3–4 CKD (14). However, the role of vascular oxidative stress in large conduit arteries is currently unclear (5,15,16). It is plausible but currently unknown if local vascular endothelial oxidative stress and inflammation are increased in CKD.
We sought to compare vascular function and measures of vascular oxidative stress and inflammation in a group of participants with moderate-to-severe CKD and a group of age-matched healthy controls. We used novel methods to assess FMDBA during normal versus inhibited oxidative stress (via an acute supraphysiologic infusion of ascorbic acid) and by measuring expression of proteins involved in oxidative stress and inflammation in endothelial cells collected from participants. We hypothesized that participants with CKD would exhibit increased vascular oxidative stress and inflammation in conjunction with vascular dysfunction.
Materials and Methods
Study Design and Participants
This was a cross-sectional study assessing mechanisms of vascular dysfunction in adults with moderate-to-severe CKD as compared with age-matched healthy controls. Patients with CKD had participated in one of two randomized, placebo-controlled trials: administration of an IL-1 inhibitor (rilonacept; n=10; trial 1) (17) or lanthanum carbonate (NCT02209636; n=54; trial 2). Included data were collected at baseline. Trial 1 enrolled between September 2012 and September 2014 and trial 2 enrolled between September 2014 and December 2018. Healthy controls were prospectively recruited through advertisements at the University and in the community, with enrollment between December 2015 and November 2018. The study was conducted at the University of Colorado Anschutz Medical Campus Division of Renal Diseases and Hypertension Clinical Vascular Physiology Laboratory. Analysts were blinded to status (CKD or healthy control) when assessing outcome measures (vascular function and circulating/cellular markers).
All participants with CKD in either clinical trial who had successful baseline mechanistic vascular measurements (i.e., change in FMDBA with acute infusion of ascorbic acid and/or endothelial cell protein expression [see details below]) were included in this analysis, to focus the analysis on these novel parameters. Inclusion criteria for trial 1 were as follows: 18–80 years of age, eGFR (by the Modified Diet Renal Disease [MDRD] equation) of 15–59 ml/min per 1.73 m2, and evidence of chronic inflammation (high-sensitivity C-reactive protein (CRP) >2.0 mg/L on at least two consecutive weekly determinations). All women from this trial included in the present analysis were postmenopausal for better matching to trial 2 and healthy controls. Inclusion criteria for trial 2 were as follows: 40–79 years of age (post-menopausal for women), MDRD eGFR of 15–45 ml/min per 1.73 m2, and baseline serum phosphorous of 2.8–5.5 mg/dl (stable in the past month and not taking phosphate binders). All participants with CKD were on optimal, stable, antihypertensive, diabetic, and lipid-lowering regimens as appropriate for at least 1 month before inclusion. To eliminate the influence of smoking, all participants included in this analysis were nonsmokers. Individuals who participated in both trials (n=2) were only included in the analysis using data from their most recent trial participation (CKD trial 2), because this time point was the most likely to have sufficient remaining samples (e.g., endothelial cells, blood).
Healthy control participants were 50–72 years of age (recruited to best match the age of participants with CKD after partial completion of CKD enrollment; women were postmenopausal). Inclusion criteria were as follows: healthy (i.e., free from kidney disease, CVD, diabetes, and other chronic disease [assessed via self-report, physical exam including a resting 12-lead electrocardiogram, and screening laboratory tests]), free from hypertension based on guidelines at the time (BP <140/90 mm Hg and no antihypertensive agents), an eGFR ≥60 ml/min per 1.73 m2 by the CKD Epidemiology Collaboration equation (18), and nonsmoking.
Procedures
Vascular Measurements.
The number of participants in each group with each outcome measurement are shown in Supplemental Table 1. All measurements were made under supine, overnight-fasted (water only) conditions, following standard recommendations including 24-hour abstention from physical activity, and in a climate-controlled room (19). Participants refrained from nonprescription medications for 48 hours before testing, but prescription medications were not withheld to maintain BP control. FMDBA was determined using duplex ultrasonography (Xario 200; Toshiba, Tustin, CA) with electrocardiogram-gated end-diastolic ultrasound images analyzed by a single blinded analyst using a commercially available software package (Vascular Analysis Tools 5.8.1; Medical Imaging Applications, Coralville, IA), as described in detail previously (17,20,21). Doppler flow of the brachial artery was also measured and peak shear rate was calculated as a potential covariate (17,20,21). Endothelium-independent dilation (brachial artery dilation to 0.4 mg of sublingual nitroglycerin) was assessed as a standard index of smooth muscle cell sensitivity to exogenous nitric oxide (NO) (17,20,21). A total of 14 control and 48 participants with CKD were administered nitroglycerin (missing data due to low heart rate and/or low systolic BP [n=2 control; n=12 CKD], contraindication [n=2 CKD], arrhythmia precluding analysis [n=1 CKD], failed intravenous [i.v.] insertion [n=1 control]).
The assessment of carotid-femoral PWV has been described in detail previously (17,20,21). Briefly, carotid-femoral PWV and carotid-radial PWV (an index of peripheral stiffness) were noninvasively measured by positioning a trans-cutaneous custom tonometer (Noninvasive Hemodynamics Workstation [NIHem]; Cardiovascular Engineering Inc., Norwood, MA) at the carotid, brachial, radial, and femoral arteries. Distances between sites were measured using a custom raised ruler (NIHem for suprasternal notch and femoral artery) or tape measure (all other distances). The distance from the suprasternal notch to the carotid was subtracted from the distance between the two recording sites, and carotid-femoral PWV was calculated as the distance divided by time between the foot of waveforms recorded at each site, as described previously (22). A total of 61 participants with CKD and 16 control participants had successful carotid-femoral PWV (n=62 and n=17, respectively, for carotid-radial PWV) that met quality assurance.
Ultrasound imaging of the carotid artery was obtained in conjunction with the tonometry to provide blinded assessment of carotid artery compliance and carotid artery β-stiffness index (secondary indices of arterial stiffness), as described previously (n=60 participants with CKD and n=17 control participants) (17,20). Carotid systolic BP and carotid intimal medial thickness were also assessed (n=62 CKD and n=17 control participants) (17,20).
An acute supraphysiologic dose of ascorbic acid or isovolumic saline was infused to determine the influence of oxidative stress on FMDBA. FMDBA was measured during the “drip infusion” when peak plasma concentrations occur, as described previously (17,20,23) The plasma concentrations with this dose have been shown to inhibit superoxide production in vitro (24). A priming bolus of 0.075 g of ascorbic acid/kg of fat-free mass was dissolved in 150 ml of saline and infused i.v. at 5 ml/min for 20 minutes (maximal dosage was set at 5.0 g). This was immediately followed by a drip infusion of 0.5 ml/min and FMDBA was again measured. All 17 controls and 60 participants with CKD received infusions. Before and after the ascorbic acid infusion, plasma ascorbic acid levels were measured (quantitative high-performance liquid chromatography by ARUP Laboratories) to demonstrate effective elevation of circulating levels in a small subgroup of participants with CKD (n=4) and controls (n=5).
Cellular Markers of Oxidative Stress and Inflammation.
We have described the details and rigor of the technique to measure endothelial cell protein expression previously (17,21,25–27). Vascular endothelial cells from the intima of an antecubital vein were obtained immediately before vascular measurements (n=8–11 control participants and n=24–38 participants with CKD per protein analyzed; not available for all participants and all proteins due to i.v. failure or low cell yield; additionally, only limited endothelial cells were available from CKD trial 1, because most slides were previously analyzed using a different microscope). Cells were recovered and fixed. Slides were prepared and then frozen for subsequent staining. VE-cadherin primary antibody (1:500; Abcam, Cambridge, MA) was used to identify endothelial cells. Primary antibodies used for the assessment of markers included NAD(P)H oxidase (1:1000, P47phox; Millipore, Billerica, MA), IL-6 (1:50; Santa Cruz, Dallas, TX); NFκB (1:300; Santa Cruz), and phosphorylated endothelial NO synthase (PeNOS; 1:100; Cell Signaling, Danvers, MA). Expression of these proteins was determined by a blinded analyst using immunofluorescence (Nikon Eclipse Ti, Melville, NY), as described previously (17,20,21,25). These markers were selected as indicators of oxidative stress, inflammation, and vascular endothelial NO production.
Circulating Markers of Oxidative Stress and Inflammation.
ELISA (MSD, Rockville, MD) was used to measure serum CRP and IL-6 concentrations as markers of inflammation. Oxidized LDL was also measured by ELISA (Mercodia, Uppsala, Sweden) as an index of oxidative stress. Stored samples were not available from n=1 from CKD trial 1 and n=5 from CKD trial 2, thus n=57–58 participants with CKD and n=17 (all) controls were included in the assessment of these circulating markers.
Statistical Analyses
The Shapiro–Wilk test was used to test for normality. Independent sample t tests, chi-squared tests, or Fisher exact tests were used to evaluate differences between groups in baseline variables. An independent-samples t test was used to determine differences between groups in vascular parameters and circulating markers. A 2×2 ANOVA was used to assess group differences in change in FMDBA after ascorbic acid infusion. Analysis of covariance was used to evaluate the influences of mean arterial pressure on carotid-femoral PWV (28) and shear rate and baseline diameter on FMDBA. Log-transformation was performed on non-normally distributed variables before analysis. All data are reported as means±SD or medians (interquartile range) unless otherwise noted, with figures presented as means±SEM. Analysis was completed only on individuals with complete data for the outcome of interest (missing data for any variables are described above). Analyses were performed using SPSS 25 and statistical significance was set at P<0.05. Adjustment was not made for multiple comparisons because the study was mechanistic and hypothesis generating.
A sample size of 17 control subjects was calculated based on approximately 90% power at an α level of 0.05 (two sided) to detect a group difference of 1.9 for the outcome of change in FMDBA after ascorbic acid infusion. This calculation was based on previously published data assessing change in FMDBA after ascorbic acid infusion in healthy older adults compared with young healthy controls (mean±SD change in percent FMDBA: young healthy controls, 0.2±2.0; older adults, 2.1±0.9) (23); we assumed a similar effect size in CKD. Although only 17 participants with CKD were required to provide approximately 90% power, we included all participants from the two clinical trials in our CKD group. Based on previous publications in CKD, these sample sizes (n=17 controls and n=62 individuals with CKD) also provided 99% power to detect a group difference of 2.3±0.5 in percent FMDBA (7) and 99% power to detect a group difference of 390±275 cm/s in carotid-femoral PWV (8).
Study Approval
All procedures were approved by the Colorado Multiple Institutional Review Board and adhere to the Declaration of Helsinki. The nature, benefits, and risks of the study were explained to volunteers and volunteers provided written informed consent before study participation.
Results
Demographic and Clinical Characteristics
A total of 64 individuals with CKD from two previous clinical trials were included in this analysis. A total of 22 control participants were assessed for eligibility for this study. Five were excluded from enrollment due to not meeting inclusion/exclusion criteria, leading to a total cohort of 17. Individuals in the CKD group were slightly older; more likely to be male (trial 2 was a mostly veteran population); more likely to be a former smoker; and had higher BP, higher cholesterol, higher body mass index, and lower eGFR than healthy controls (Table 1). The majority of participants with CKD had a history of hypertension and BP was controlled. Because of the inclusion criteria for enrollment, no control participants were hypertensive. Participants with CKD were more likely to use antihypertensive agents and statins. Use of other medications did not differ between groups, nor did race/ethnicity. Etiology of CKD was attributed to diabetes (45%), hypertension (27%), nephrolithiasis (3%), autosomal dominant polycystic kidney disease (3%), drugs or toxins (3%), AKI (3%), and/or other or unknown (34%).
Table 1.
Demographics and clinical characteristics of participants with CKD and controls
| Variable | CKD (n=64) | Control (n=17) |
|---|---|---|
| Age (yr)a | 65±8 | 60±5 |
| Sex (% male)a | 86 | 53 |
| Race/ethnicity (% non-Hispanic white) | 70 | 76 |
| Smoking (%) | ||
| Never smoker | 48 | 94 |
| Former smoker | 52 | 6 |
| BMI (kg/m2)a | 31.3±4.1 | 26.3±4.3 |
| Systolic BP (mm Hg)a | 130±14 | 117±15 |
| Diastolic BP (mm Hg) | 74±10 | 70±10 |
| eGFR (ml/min per 1.73 m2)a | 33±9 | 84±13 |
| LDL cholesterol (mg/dl)a | 80±30 | 113±27 |
| HDL cholesterol (mg/dl)a | 37±11 | 60±20 |
| Total cholesterol (mg/dl)a | 154±45 | 185±31 |
| Hypertension (%)a | 91 | 0 |
| Diabetes (%)a | 56 | 0 |
| ACEi/ARB (%)a | 75 | 0 |
| Diuretic (%)a | 56 | 0 |
| β Blocker (%)a | 55 | 0 |
| Calcium channel blockers (%)a | 41 | 0 |
| Statin (%)a | 69 | 12 |
| Antidepressant or antianxiety medication (%) | 27 | 18 |
| Thyroid medication (%) | 20 | 6 |
Data are mean±SD or n (%). BP measured in the seated position; eGFR measured by the Modification of Diet in Renal Disease study equation for the CKD group and by the CKD Epidemiology Collaboration equation for the control group. BMI, body mass index; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
P<0.05 by chi-squared or Fisher exact tests for categoric data and independent sample t test for continuous variables.
Vascular Parameters
Participants with CKD had a 36% lower FMDBA, indicating impaired endothelium-dependent dilation, and 46% higher carotid-femoral PWV, indicating greater aortic stiffness, compared with healthy controls (Table 2). The time to peak FMDBA was also longer in participants with CKD compared with healthy controls (P=0.03). Peak hyperemic (P=0.01) but not resting (P=0.39) shear rate differed between participants with CKD and healthy controls; the fold-increase in shear rate during reactive hyperemia was thus greater in healthy controls (6.9±1.3) than in the CKD group (5.0±1.5; P<0.001). The difference in FMDBA was no longer significant between groups after adjustment for peak shear rate (P=0.47). Endothelium-independent dilation to sublingual nitroglycerin was reduced in participants with CKD compared with healthy controls (P=0.01). Participants with CKD also had greater carotid systolic BP, carotid intimal medial thickness, and carotid β-stiffness index compared with controls, with no difference in carotid-radial PWV (an index of peripheral stiffness) or supine brachial artery mean arterial pressure. Consistent with the lack of difference in mean arterial pressure between groups, carotid-femoral PWV remained significantly different between groups after statistically adjusting for mean arterial pressure (P<0.001).
Table 2.
Vascular parameters in participants with CKD and controls
| Variable | CKD (n=64) | Control (n=17) | P Value |
|---|---|---|---|
| FMDBA (%) | 3.5±2.8 | 5.5±3.2 | 0.02 |
| FMDBA (mm) | 0.14±0.10 | 0.20±0.11 | 0.04 |
| Baseline FMD diameter (mm) | 4.1±0.7 | 3.7±0.6 | 0.02 |
| Resting shear rate (s−1) | 132±47 | 121±38 | 0.39 |
| Hyperemic shear rate (s−1) | 644±225 | 831±271 | 0.01 |
| Time to peak FMDBA (s) | 57±22 | 44±16 | 0.03 |
| Brachial artery dilation to nitroglycerin (%) | 18.2±9.7 | 26.0±7.3 | 0.01 |
| Baseline nitroglycerin diameter (mm) | 4.1±0.7 | 3.8±0.6 | 0.13 |
| Carotid-femoral PWV (cm/s) | 1071±336 | 732±128 | <0.001 |
| Carotid-radial PWV (cm/s) | 1003±246 | 955±165 | 0.46 |
| Carotid artery compliance (mm/mm Hg×10−1) | 0.76±0.32 | 0.78±0.18 | 0.65 |
| Carotid β-stiffness index (A.U.) | 11.8±4.6 | 8.7±2.0 | <0.001 |
| Carotid IMT (mm) | 0.67±0.19 | 0.58±0.09 | 0.04 |
| Carotid systolic BP (mm Hg) | 134±19 | 11±617 | 0.001 |
| Brachial mean arterial BP (mm Hg) | 90±14 | 87±10 | 0.41 |
Data are mean±SD. n=14 controls and n=49 participants with CKD were administered nitroglycerin. n=56 participants with CKD and all (n=17) control participants had measurements of carotid artery compliance and β-stiffness index. n=64 participants with CKD and all (n=17) control participants had measurements of carotid IMT. N=62/N=63 participants with CKD and n=16/n=17 control participants had successful measurements of carotid-femoral PWV and carotid-radial PWV, respectively. All vascular parameters were assessed in the supine position. FMDBA, brachial artery flow-mediated dilation; PWV, pulse-wave velocity; IMT, intimal medial thickness.
Acute Inhibition of Vascular Oxidative Stress
Following an acute infusion of ascorbic acid previously shown inhibit superoxide production in vitro, plasma ascorbic acid levels were significantly elevated in both the control (preinfusion, 73±7 μmol/L; postinfusion, 1236±81 μmol/L; 17-fold increase; P<0.001) and CKD group (preinfusion, 36±7 μmol/L; postinfusion, 1664±429 μmol/L; 48-fold increase; P<0.01). However, the infusion (compared with isovolumetric saline) differentially improved FMDBA in healthy controls as compared with participants with CKD (absolute change in percent FMDBA: healthy controls, 1.3±0.6; participants with CKD, 0.12±0.2 [mean±SEM]; group×condition interaction P=0.04) (Figure 1).
Figure 1. |. Acute infusion of ascorbic acid differentially improved brachial artery flow-mediated dilation in healthy controls as compared to participants with CKD.
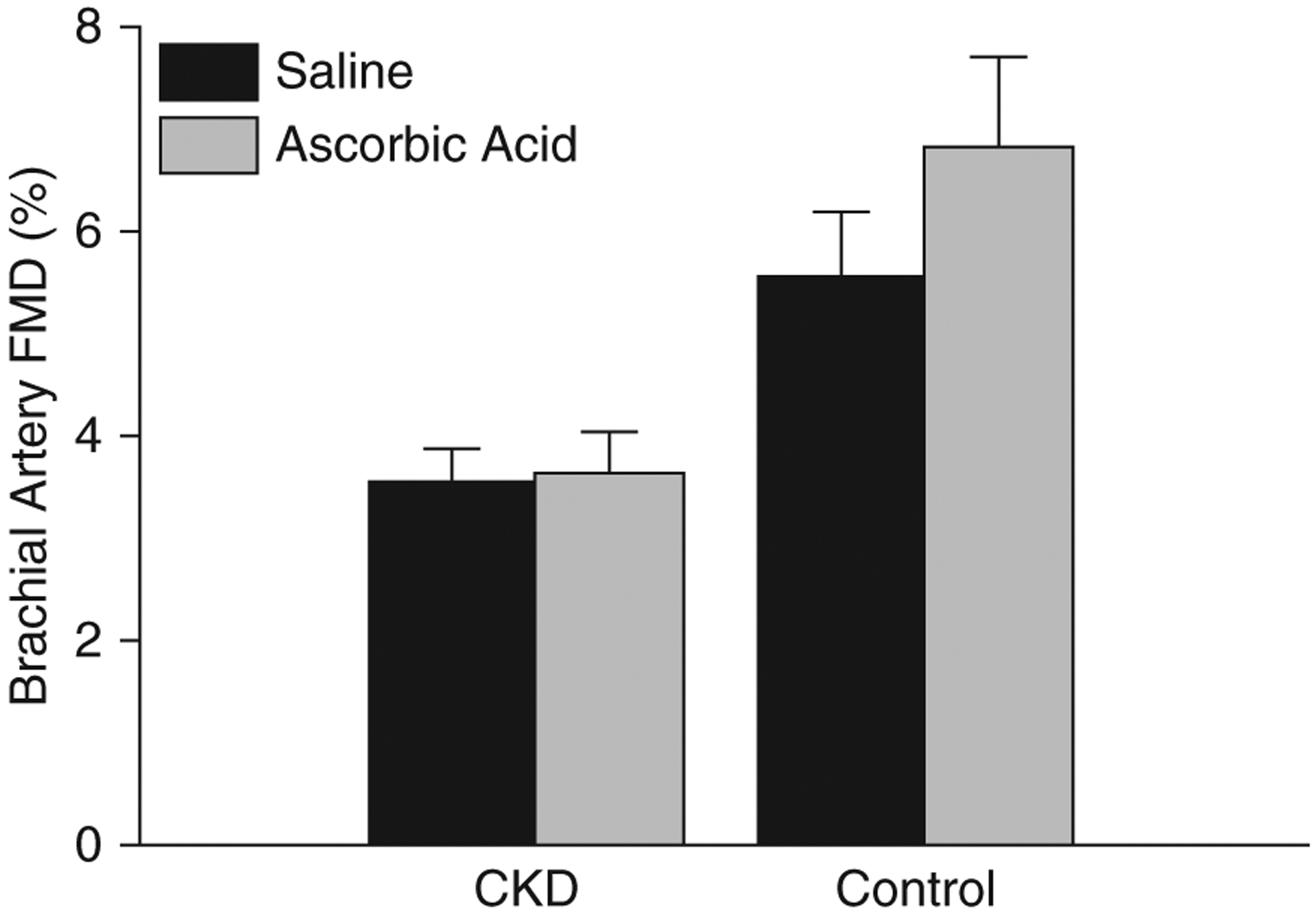
Brachial artery flow-mediated dilation (FMD) after an acute infusion of saline (black bars) and ascorbic acid (gray bars) in participants with CKD and healthy controls. Infusions were performed in all control participants (n=17) and n=60 participants with CKD. Ascorbic acid significantly improved brachial artery FMD in control participants (saline, 5.5%±0.8%; ascorbic acid, 6.8%±0.9%) as compared with participants with CKD (saline, 3.5%±0.4%; ascorbic acid, 3.6%±0.4%) (group×condition interaction P=0.04). Values are mean±SEM.
Cellular and Circulating Markers of Oxidative Stress and Inflammation
Cellular Markers.
Figure 2 displays vascular endothelial cell protein expression of NADPH oxidase, IL-6, NFκB, and PeNOS. Expression of the oxidant enzyme NADPH oxidase was greater in the CKD compared with control group (intensity versus human umbilical vein endothelial cell control, 1.48±0.05 versus 1.25±0.11 [mean±SEM]; P=0.05). The proinflammatory transcription factor NFκB (0.78±0.02 versus 0.67±0.08; P=0.19), proinflammatory cytokine IL-6 (0.94±0.02 versus 0.98±0.05; P=0.43), and PeNOS (1.34±0.04 versus 1.23±0.10; P=0.34) did not differ in the CKD group compared with controls.
Figure 2. |. Greater vascular endothelial cell oxidative stress in participants with CKD as compared to controls.
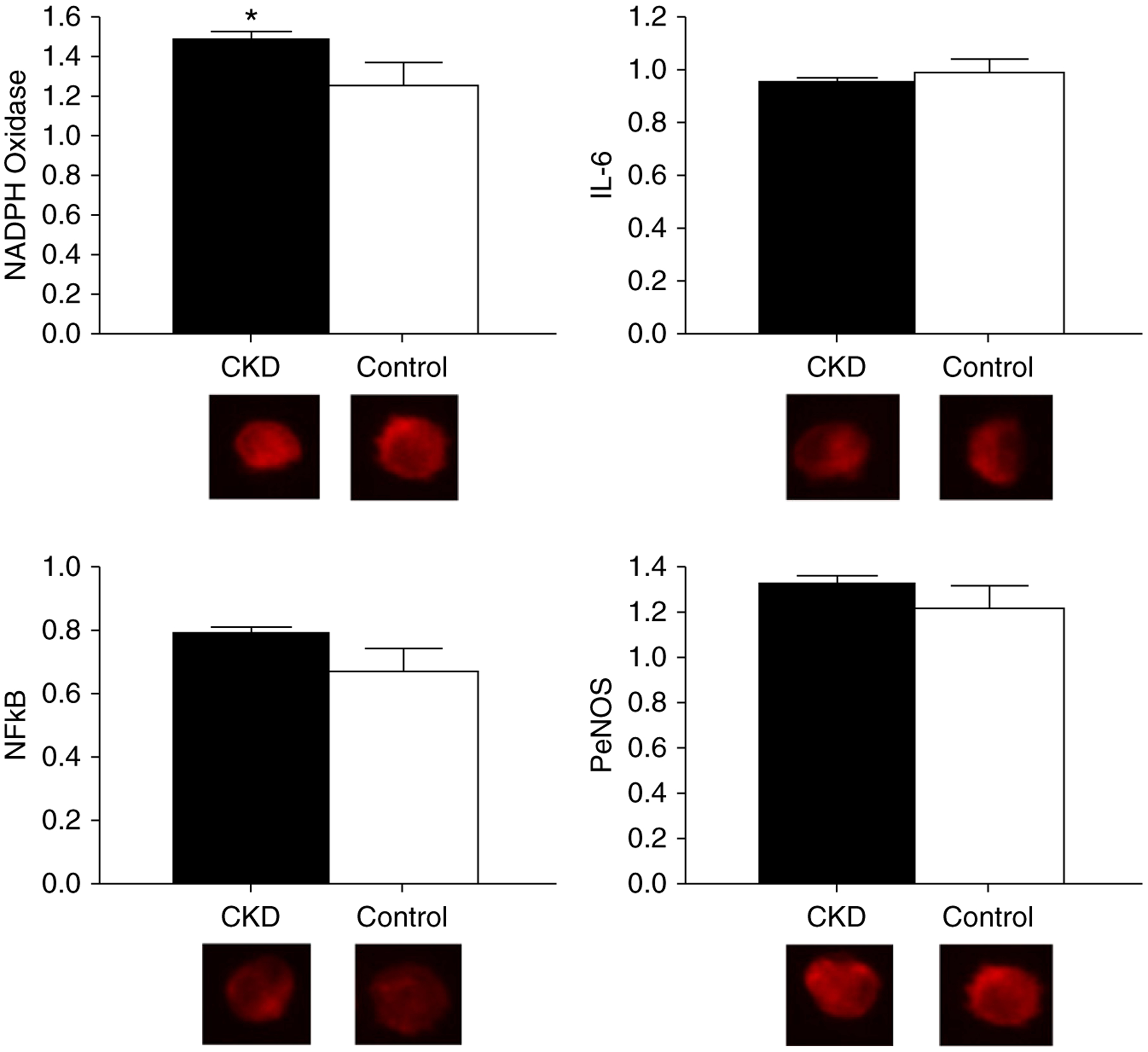
Protein expression of NAD(P)H oxidase (CKD, 1.48±0.05; control, 1.25±0.11; P=0.05), IL-6 (CKD, 0.94±0.02; control, 0.98±0.05; P=0.43), NFκB (CKD, 0.78±0.02; control, 0.67±0.08; P=0.19), and phosphorylated endothelial cell nitric oxide synthase (PeNOS; CKD, 1.34±0.04; control, 1.23±0.10; P=0.34) in vascular endothelial cells collected from a peripheral vein of participants with CKD (black bars) compared with healthy controls (white bars). Expression is relative to human umbilical vein endothelial cell control, with representative images shown below (quantitative immunofluorescence). Values are mean±SEM. *P≤0.05.
Circulating Markers.
Circulating proinflammatory markers CRP and IL-6 were elevated in the CKD compared with the control group, with no difference in the marker of oxidative damage, oxidized LDL (Table 3).
Table 3.
Circulating markers of oxidative stress, inflammation, and nitric oxide production in participants with CKD and controls
| Variable | CKD (n=57–58) | Control (n=17) | P Value |
|---|---|---|---|
| CRP (mg/L) | 2.60 (1.08–6.15) | 0.70 (0.50–3.53) | 0.01 |
| IL-6 (pg/ml) | 1.39 (0.94–2.23) | 0.71 (0.43–0.96) | <0.001 |
| oxLDL (mU/L) | 62,302 (49,508–79,326) | 71,213 (57,265–84,198) | 0.18 |
Data are median (interquartile range). P values are independent t test comparisons between groups using log-transformed variables. CRP, C-reactive protein; oxLDL, oxidized LDL.
Discussion
In this translational study comparing adults with moderate-to-severe CKD and middle-aged and older healthy controls, we confirmed the presence of vascular dysfunction (impaired FMDBA and increased carotid-femoral PWV). Additionally, although the study is hypothesis generating in nature, we have provided the first direct evidence in humans with CKD suggesting vascular oxidative stress. Endothelial cell protein expression of the oxidant enzyme NADPH oxidase was increased with CKD, providing the first cellular evidence that vascular oxidative stress may be increased in adults with moderate-to-severe CKD.
Additionally, we administered an acute supraphysiologic infusion of ascorbic acid that produces plasma concentrations known to inhibit superoxide production in vitro (24). This infusion failed to improve FMDBA in the participants with CKD, despite improvements in the control group. We believe these unexpected findings indicate that the level of oxidative stress in the CKD group (as reflected by endothelial cell protein expression and circulating markers) was too great to be overcome by the ascorbic acid infusion, despite a substantial rise in plasma ascorbic acid levels. The improvement in the control group comprised of healthy middle-aged and older adults is consistent with previous literature demonstrating an improvement in age-associated impairment in FMDBA in healthy middle-aged and older adults (23,29). Acute infusion of ascorbic acid has also been shown to improve conduit artery or microvascular endothelium-dependent dilation in individuals with diabetes (30), hypertension (31), and those who smoke (32). Additionally, we recently demonstrated that our ascorbic acid infusion protocol improved FMDBA in adults with early-stage autosomal dominant polycystic kidney disease and preserved kidney function (20).
An acute ascorbic acid infusion previously failed to improve radial artery FMD; however, in this study kidney disease was severe (eGFR<20 ml/min per 1.73 m2) and a different artery was assessed (15). Additionally, oral ascorbic acid has failed to improve FMDBA in adults with CKD (5), but oral administration does not raise plasma ascorbic acid levels (which were not assessed in this study) nearly as much as an acute supraphysiologic infusion (23). A recent small study including both patients with CKD and peritoneal dialysis showed no change in FMDBA after an ascorbic acid infusion (16). However, microvascular endothelium-dependent dilation in the cutaneous microvasculature is improved to the level of healthy controls in adults with moderate-to-severe CKD after local ascorbic acid administration, indicating potential differences across vascular beds (14). Overall, these data support that CKD may have extensive oxidative stress that is not overcome by ascorbic acid, and this should be taken into account when testing future antioxidant therapies in patients with kidney disease.
We observed a 35% lower FMDBA in the CKD group, reiterating the presence of impaired endothelium-dependent dilation in CKD (5–7). Of interest, the CKD group also demonstrated a longer duration to peak dilation than the control group after cuff release. Time to peak dilation has also been shown to be delayed in older sedentary versus young healthy adults (33), individuals with the metabolic syndrome (34) and type 2 diabetes mellitus (35) as compared with healthy controls, as well as adults with moderate versus low cardiovascular risk (36). This has not been reported previously in CKD and may be an additional reflection of vascular dysfunction. Suggested mechanisms that may contribute to impaired time to peak dilation include reduced arterial wall compliance, changes in enzyme rate production, and free radicals interacting with endothelium-derived vasodilators (33).
Shear rate is produced by the hyperemic blood flow response to the cuff deflation and is the mechanical stimulus that promotes vasodilation (37). Notably, peak shear rate has been shown to differ according to Framingham risk factors (38), as well as the presence of metabolic syndrome (39), diabetes (40), and advanced age (40). Peak shear rate has typically not been quantified in previous CKD studies, although hyperemic blood flow or peak velocity have been reported to be similar to controls (5,6,41). In the Framingham Heart Study, inclusion of shear rate attenuated the association between cardiovascular risk factors and FMDBA, suggesting that impaired FMDBA in the presence of cardiovascular risk factors may represent an attenuated hyperemic stimulus rather than brachial endothelium dysfunction (38). However, it has also been proposed that shear rate should be presented rather than corrected for when comparing FMDBA between groups (42). We observed a difference in shear rate between the CKD and control group, and the difference in FMDBA was no longer significant after adjustment for shear rate, suggesting at the minimum an importance of the hyperemic stimulus when evaluating FMDBA in participants with CKD.
In addition to reduced FMDBA, we observed impaired brachial artery dilation to the NO donor nitroglycerin, suggesting there is also smooth muscle cell impairment (i.e., impaired endothelium-independent dilation) in nondialysisdependent CKD. Previous literature has demonstrated mixed results regarding the presence of impaired brachial artery dilation to nitroglycerin (5–7).
Carotid-femoral PWV was 46% greater in participants with moderate-to-severe CKD compared with healthy controls. The results are consistent with previous literature demonstrating greater large elastic artery stiffness in nondialysis CKD (8–10). The group difference remained highly significant after adjustment for mean arterial pressure, suggesting structural changes contributing to increased arterial stiffness. Additionally, individuals with CKD in this study had elevated carotid systolic BP compared with controls, consistent with higher brachial systolic BP (although still controlled according to guidelines at the time). Evidence on local arterial compliance, such as the carotid artery, has been much less reported, but our result of increased β-stiffness index is also consistent with limited available evidence (9).
Circulating markers of increased oxidative stress or reduced antioxidant defenses (7,15), as well as increased inflammation (43,44), were previously shown to be altered in moderate-to-severe CKD. We have provided the first direct evidence that oxidative stress is increased at the level of the vascular endothelium in humans with CKD. This was observed despite a lack of difference in oxidized LDL, a circulating marker of oxidative damage. Notably, consistent with previous evidence, circulating markers of inflammation were elevated in the CKD group.
Increased oxidative stress and inflammation are both likely promoters of a decline in NO bioavailability. Reduced NO is a contributing mechanism common to both large elastic artery stiffness and endothelial dysfunction. However, no difference in endothelial cell PeNOS protein expression was observed in participants with CKD compared with healthy controls.
The major strength of this study is that we used novel methodology to evaluate physiologic mechanisms contributing to vascular dysfunction in CKD—the most comprehensive assessment to date. We have extended existing literature indicating circulating markers of oxidative stress in CKD by collecting vascular endothelial cells to provide direct evidence of vascular oxidative stress. We also assessed FMDBA after acute inhibition of oxidative stress. Given the comprehensive nature of these assessments, these measurements were performed in a relatively large number of participants with CKD.
This study also has several notable limitations. Given that the participants with CKD also had other comorbidities, it is difficult to separate the contributions of these factors from other contributing mechanisms. Differences between the two groups besides the presence of CKD may have contributed to the observed results, beyond the primary disease process alone. For example, ages were not precisely matched and there were more males in the CKD group because trial 2 was a Veterans Affairs–funded trial. Importantly, our findings are still clinically meaningful, despite any residual group differences. The results are cross-sectional and cannot provide insight into changes in vascular function and associated mechanisms over time. Additionally, we recognize that the sample size was smaller than the overall cohort for endothelial cell protein expression, due limitations in the technique (e.g., i.v. failure, inadequate cell recovery) and lack of remaining slides from CKD trial 1, which may have introduced selection bias or increased the likelihood of a type-1 error in the comparison of NAD(P)H oxidase protein expression between groups.
In conclusion, we have provided initial evidence that oxidative stress may be a physiologic mechanism contributing to vascular dysfunction in moderate-to-severe CKD. Our results also reiterate that vascular dysfunction is present in CKD, before the initiation of dialysis. Future research should follow changes in vascular function and associated mechanisms longitudinally. Additionally, physiologic mechanisms contributing to vascular oxidative stress and inflammation should continue to be delineated, including how targeting these processes influence vascular function. Interventions to reduce oxidative stress in individuals with moderate-to-severe CKD could potentially reduce the risk of cardiovascular events and mortality in patients with CKD.
Supplementary Material
Funding
This study was supported by National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases grant K01DK103678 (to K. Nowak), Center for Integrated Health-care, US Department of Veterans Affairs Career Development Award Program grant 5IK2CX001030-03 (to A. Jovanovich), and American Heart Association grant 12POST11920023 (to K. Nowak). Additional support was provided by the NIH National Center for Advancing Translational Sciences Clinical and Translational Science Award grant UL1 TR001082.
Disclosures
A. Jovanovich reports non-financial support from Shire, outside the submitted work. All remaining authors have nothing to disclose.
Footnotes
Supplemental Material
This article contains supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/K3602019000096/-/DCSupplemental.
Supplemental Table 1. Number of participants included in the assessment of each outcome measure.
References
- 1.Dalrymple LS, Katz R, Kestenbaum B, Shlipak MG, Sarnak MJ, Stehman-Breen C, Seliger S, Siscovick D, Newman AB, Fried L: Chronic kidney disease and the risk of end-stage renal disease versus death. J Gen Intern Med 26: 379–385, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW; American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and Epidemiology and prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Sarnak MJ, Coronado BE, Greene T, Wang SR, Kusek JW, Beck GJ, Levey AS: Cardiovascular disease risk factors in chronic renal insufficiency. Clin Nephrol 57: 327–335, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Kendrick J, Chonchol MB: Nontraditional risk factors for cardiovascular disease in patients with chronic kidney disease. Nat Clin Pract Nephrol 4: 672–681, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Ghiadoni L, Cupisti A, Huang Y, Mattei P, Cardinal H, Favilla S, Rindi P, Barsotti G, Taddei S, Salvetti A: Endothelial dysfunction and oxidative stress in chronic renal failure. J Nephrol 17: 512–519, 2004 [PubMed] [Google Scholar]
- 6.Thambyrajah J, Landray MJ, McGlynn FJ, Jones HJ, Wheeler DC, Townend JN: Abnormalities of endothelial function in patients with predialysis renal failure. Heart 83: 205–209, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yilmaz MI, Saglam M, Caglar K, Cakir E, Sonmez A, Ozgurtas T, Aydin A, Eyileten T, Ozcan O, Acikel C, Tasar M, Genctoy G, Erbil K, Vural A, Zoccali C: The determinants of endothelial dysfunction in CKD: Oxidative stress and asymmetric dimethylarginine. Am J Kidney Dis 47: 42–50, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Temmar M, Liabeuf S, Renard C, Czernichow S, Esper NE, Shahapuni I, Presne C, Makdassi R, Andrejak M, Tribouilloy C, Galan P, Safar ME, Choukroun G, Massy Z: Pulse wave velocity and vascular calcification at different stages of chronic kidney disease. J Hypertens 28: 163–169, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Briet M, Bozec E, Laurent S, Fassot C, London GM, Jacquot C, Froissart M, Houillier P, Boutouyrie P: Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Int 69: 350–357, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Wang MC, Tsai WC, Chen JY, Huang JJ: Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis 45: 494–501, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmelfarb J: Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int 65: 1009–1016, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Cachofeiro V, Goicochea M, de Vinuesa SG, Oubiña P, Lahera V, Luño J: Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl [111]: S4–S9, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Costa-Hong V, Bortolotto LA, Jorgetti V, Consolim-Colombo F, Krieger EM, Lima JJ: Oxidative stress and endothelial dysfunction in chronic kidney disease. Arq Bras Cardiol 92: 413–418, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Dupont JJ, Farquhar WB, Townsend RR, Edwards DG: Ascorbic acid or L-arginine improves cutaneous microvascular function in chronic kidney disease. J Appl Physiol (1985) 111: 1561–1567, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cross JM, Donald AE, Nuttall SL, Deanfield JE, Woolfson RG, Macallister RJ: Vitamin C improves resistance but not conduit artery endothelial function in patients with chronic renal failure. Kidney Int 63: 1433–1442, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Gillis K, Stevens KK, Bell E, Patel RK, Jardine AG, Morris STW, Schneider MP, Delles C, Mark PB: Ascorbic acid lowers central blood pressure and asymmetric dimethylarginine in chronic kidney disease. Clin Kidney J 11: 532–539, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nowak KL, Chonchol M, Ikizler TA, Farmer-Bailey H, Salas N, Chaudhry R, Wang W, Smits G, Tengesdal I, Dinarello CA, Hung AM: IL-1 inhibition and vascular function in CKD. J Am Soc Nephrol 28: 971–980, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 155: 408, 2011] Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris RA, Nishiyama SK, Wray DW, Richardson RS: Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nowak KL, Wang W, Farmer-Bailey H, Gitomer B, Malaczewski M, Klawitter J, Jovanovich A, Chonchol M: Vascular dysfunction, oxidative stress, and inflammation in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 13: 1493–1501, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jablonski KL, Decker E, Perrenoud L, Kendrick J, Chonchol M, Seals DR, Jalal D: Assessment of vascular function in patients with chronic kidney disease. J Vis Exp [88]: 51478, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D: Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: The Framingham Heart study. Hypertension 43: 1239–1245, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Eskurza I, Monahan KD, Robinson JA, Seals DR: Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson TS, Xu A, Vita JA, Keaney JF Jr.: Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res 83: 916–922, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR: Direct evidence of endothelial oxidative stress with aging in humans: Relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100: 1659–1666, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T; American Heart Association Council on Hypertension: Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement From the American Heart association. Hypertension 66: 698–722, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, Seals DR: Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol 61: 335–343, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T; American Heart Association Council on Hypertension: Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the American Heart association. Hypertension 66: 698–722, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreau KL, Stauffer BL, Kohrt WM, Seals DR: Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab 98: 4507–4515, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Timimi FK, Ting HH, Haley EA, Roddy MA, Ganz P, Creager MA: Vitamin C improves endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. J Am Coll Cardiol 31: 552–557, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A: Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation 97: 2222–2229, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Motoyama T, Kawano H, Kugiyama K, Hirashima O, Ohgushi M, Yoshimura M, Ogawa H, Yasue H: Endothelium-dependent vasodilation in the brachial artery is impaired in smokers: Effect of vitamin C. Am J Physiol 273: H1644–H1650, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Black MA, Cable NT, Thijssen DH, Green DJ: Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension 51: 203–210, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Fernandes IA, Sales ARK, Rocha NG, Silva BM, Vianna LC, da Nóbrega ACL: Preserved flow-mediated dilation but delayed time-to-peak diameter in individuals with metabolic syndrome. Clin Physiol Funct Imaging 34: 270–276, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Irace C, Tschakovsky ME, Carallo C, Cortese C, Gnasso A: Endothelial dysfunction or dysfunctions? Identification of three different FMD responses in males with type 2 diabetes. Atherosclerosis 200: 439–445, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Padilla J, Johnson BD, Newcomer SC, Wilhite DP, Mickleborough TD, Fly AD, Mather KJ, Wallace JP: Adjusting flow-mediated dilation for shear stress stimulus allows demonstration of endothelial dysfunction in a population with moderate cardiovascular risk. J Vasc Res 46: 592–600, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Pyke KE, Dwyer EM, Tschakovsky ME: Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J Appl Physiol (1985) 97: 499–508, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF Jr., Keyes MJ, Levy D, Vasan RS, Benjamin EJ: Local shear stress and brachial artery flow-mediated dilation: The Framingham Heart study [published correction appears in Hypertension 45: e9, 2005]. Hypertension 44: 134–139, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Title LM, Lonn E, Charbonneau F, Fung M, Mather KJ, Verma S, Anderson TJ: Relationship between brachial artery flow-mediated dilatation, hyperemic shear stress, and the metabolic syndrome. Vasc Med 13: 263–270, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Silber HA, Lima JA, Bluemke DA, Astor BC, Gupta SN, Foo TK, Ouyang P: Arterial reactivity in lower extremities is progressively reduced as cardiovascular risk factors increase: Comparison with upper extremities using magnetic resonance imaging. J Am Coll Cardiol 49: 939–945, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Foster MC, Keyes MJ, Larson MG, Vita JA, Mitchell GF, Meigs JB, Vasan RS, Benjamin EJ, Fox CS: Relations of measures of endothelial function and kidney disease: The Framingham Heart study. Am J Kidney Dis 52: 859–867, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thijssen DH, Bullens LM, van Bemmel MM, Dawson EA, Hopkins N, Tinken TM, Black MA, Hopman MT, Cable NT, Green DJ: Does arterial shear explain the magnitude of flow-mediated dilation?: A comparison between young and older humans. Am J Physiol Heart Circ Physiol 296: H57–H64, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yilmaz MI, Sonmez A, Qureshi AR, Saglam M, Stenvinkel P, Yaman H, Eyileten T, Caglar K, Oguz Y, Taslipinar A, Vural A, Gok M, Unal HU, Yenicesu M, Carrero JJ: Endogenous testosterone, endothelial dysfunction, and cardiovascular events in men with nondialysis chronic kidney disease. Clin J Am Soc Nephrol 6: 1617–1625, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Peyster E, Chen J, Feldman HI, Go AS, Gupta J, Mitra N, Pan Q, Porter A, Rahman M, Raj D, Reilly M, Wing MR, Yang W, Townsend RR; CRIC Study Investigators: Inflammation and arterial stiffness in chronic kidney disease: Findings From the CRIC study. Am J Hypertens 30: 400–408, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


