Abstract
Objective:
The antiretroviral-based dapivirine vaginal ring reduced HIV risk among women in Phase III clinical trials. However, limited data exists on the impact of dapivirine on the vaginal microenvironment in adolescents.
Design:
A comprehensive metaproteomics approach was used to assess host proteome and microbiome changes in cervicovaginal mucus with dapivirine ring use in adolescents enrolled in the MTN-023/IPM 030 (MTN-023) trial.
Methods:
Participants were randomized 3:1 to use dapivirine or placebo vaginal rings monthly for 6 months. Cervicovaginal samples from a subset of 35 participants (8 placebo, 27 dapivirine) were analyzed.
Results:
Mass spectrometry analysis identified 405 human and 2467 bacterial proteins belonging to 15 unique genera. The host proteome belonged to many functional pathways primarily related to inflammation. When stratified by study treatment arm, 18 (4.4%) and 28 (6.9%) human proteins were differentially abundant (p adj.<0.05) between baseline and follow-up in the placebo and dapivirine arms respectively. The vaginal microbiome was predominantly composed of Lactobacillus, Gardnerella, and Prevotella. While bacterial taxa did not differ by arm or change significantly, L. crispatus increased (p<0.001) and L. iners decreased (p<0.001) during the six month follow-up. There were no significant differences in bacterial functions by arm or time in the trial. Protected vaginal sex significantly associated with decreased neutrophil inflammatory biomarkers and may be associated with changes in bacterial taxa and metabolism.
Conclusions:
Condom use may associate with differences to inflammation and bacterial function, but dapivirine ring use does not, thereby supporting the mucosal safety profile of this vaginal ring for adolescents.
Keywords: dapivirine, vaginal ring, adolescents, microbiome, HIV
Introduction
One million women are infected with human immunodeficiency virus (HIV) annually[1]. Young women (15–24) are disproportionally infected with HIV, with rates up to 8-fold higher[1] and infection acquired 5–7 years earlier[2] than aged-matched males. Young women represent a major target population for HIV prevention strategies, yet clinical trials rarely include adolescents.
Female-controlled HIV prevention technologies, which could include antiretrovirals formulated as oral tablets, or vaginal gels and rings, are critical for limiting the HIV epidemic. Adherence to daily or coitally dependent strategies has been low[3–5], indicating longer-acting approaches are warranted. Vaginal rings provide sustained local release of drug, do not require daily adherence and can be removed any time. The safety and acceptability of rings for contraception and estrogen replacement therapies support the use of a vaginal ring for HIV prevention, and the technology provides the option for co-formulating multiple active ingredients for dual-purpose prevention of HIV and pregnancy[6]. Two Phase III clinical trials investigating a vaginal ring containing the non-nucleoside HIV reverse transcriptase inhibitor dapivirine reported HIV risk was reduced by approximately 30% across both trials[7, 8]. A post-hoc analysis of one study showed a 56% reduction in HIV risk among women over 21 years of age, likely due to more consistent ring use[7, 8]. Furthermore, open label extension studies have reported HIV incidence decreased by an estimated 39% (HOPE/MTN-025)[9] and 63% (DREAM-IPM 032)[10] with dapivirine.
While the dapivirine ring has been shown to be well-tolerated in adult women, studies in adolescents are lacking. As the vaginal microenvironment differs between adolescent and adult women, it is important to investigate safety and efficacy in adolescents. Biological changes associated with puberty and sexual debut can influence both the immune responses and the microbiome in the vaginal tract[11–18]. Some studies have reported high levels of Lactobacillus in adolescent girls, with compositional and structural similarity to adults, while others have reported decreased Lactobacillus, higher vaginal pH, and increased levels of bacterial vaginosis associated bacteria in adolescents, particularly after sexual debut, all of which could impact the safety or efficacy of the dapivirine ring[15, 18–26].
This is an exploratory analysis of MTN-023, a Phase 2a trial assessing product safety and acceptability in adolescents[27]. A proteomics approach was used to evaluate the influence of the dapivirine ring on the mucosal proteome and microbiome in a substudy of 35 participants.
Methods
Study population:
MTN-023 was a Phase 2a study of dapivirine vaginal ring in adolescents. The study enrolled 96 healthy, HIV-uninfected adolescents aged 15–17. Participants were randomized 3:1 to a 25mg dapivirine or placebo ring to be inserted once every 4 weeks for 24 weeks. Participants were sexually experienced, non-pregnant and using an effective method of hormonal contraception, including intrauterine devices, implantable hormonal contraceptives, or oral contraceptives. Screening for sexually transmitted infections (STIs) was performed at screening visits and young women with STIs were excluded from enrolment. Participants agreed to use condoms and to refrain from inserting anything into the vagina for 72 hours prior to each sampling visit. Participants provided written informed consent and obtained written parental/guardian permission for screening and enrolment. Thirty-five participants (36%) who reported continuous ring use were selected for proteomic analysis (n=8 placebo, n=27 dapivirine). This substudy was approved by institutional review boards at the University of Pittsburgh, the University of Alabama, and the University of Manitoba.
Sample collection:
Cervicovaginal lavage (CVL) was performed at clinic visits by bathing the cervical os with 10mL of sterile phosphate buffered saline. CVL specimens were kept cool and processed within 8 hours. CVL was spun at 800xg for 10 minutes then supernatant was aliquoted and stored at ≤70°C until use. Substudy samples were available from a baseline time point, prior to ring use, and 2 follow-up visits. All follow-up visits in the placebo arm were at 3 and 6 months post-enrolment. In the dapivirine arm, 23/27 women (85%) had samples available at 3 and 6 months of follow up. A total of 3/27 (11%) participants had samples at 1 and 3 months post-enrolment, and 1/27 (4%) participants had samples at months 3 and 4 post-enrolment used for follow up visits 1 and 2.
Sample preparation for mass spectrometry:
Sample preparation was performed as previously described[28–30]. Briefly, 50μg of protein per sample was denatured for 10 minutes at room temperature with urea exchange buffer (8M urea, (GE HealthCare, Mississauga, ON, Canada), 50mM HEPES pH 8.0 (Sigma, St. Louis, MO)), reduced with 25mM dithiothreitol (Sigma), alkylated with 50mM iodoacetamide (Sigma), and digested with trypsin (Promega, Madision, WI). Peptides were eluted and dried via vacuum centrifugation. Reversed-phase liquid chromatography (high pH RP, Agilent 1200 series microflow pump, Water XBridge column) was used for desalting and detergent removal of peptides using a step-function gradient as described[31]. Peptides were quantified using the FluoroProfile® quantification kit (Sigma) following the Lava Pep quantification protocol. Samples were randomized and aliquoted to a final peptide concentration of 0.5μg/μL in 15μL LC buffer (2% acetronitrile, 0.1% formic acid).
Mass spectrometry analysis:
Samples were analyzed by label-free tandem mass spectrometry as described[31]. Equal amounts of peptides were injected into a nanoflow LC system (1200 Easy nLC, Thermo Fisher) connected inline to a Q Exactive Plus mass spectrometer (Thermo Fisher). Data-dependent acquisition was used, and the 15 most abundant precursor ions in the survey scan were selected for high collision dissociation fragmentation.
Human proteome analysis:
Protein levels were normalized to total ion current using Progenesis QI (v21.38.1432, Nonlinear Dynamics, Durham, USA). Mascot (Matrix Science, v2.6.0) was used to search peptide sequences against the UniProt SwissProt (2015) human database. A decoy database was included to determine the rate of false discovery. Protein identifications were confirmed using Scaffold (v4.4.1, Proteome Software) with confidence thresholds set at 95% protein identification confidence, requiring at least 2 unique peptides and 80% peptide identification confidence. Proteins underwent a log transformation (base 2) prior to analysis. Only proteins that had an average coefficient of variance (CV) <25% (405 proteins), determined through measurements of a standard reference sample run at 10 sample intervals (total 10 times) were used in downstream analysis. Proteins associated with either dapivirine or placebo ring use (adj. p<0.05) underwent pathway enrichment analysis using Ingenuity Pathway Analysis (v.01–07, IPA software).
Microbial proteome analysis:
Protein database searches were initially conducted against all bacterial proteins in the UniProt-TrEMBL database (Aug-2015), from both reviewed and unreviewed sources, using Mascot (v2.4.0, Matrix Science). Searches for bacterial peptides were then performed a second time using a manually curated database limited to the major genera (≥0.2% of the total protein) identified in the initial search including (from most to least abundant): Lactobacillus, Gardnerella, Ruegeria, Chlamydia, Prevotella, Nitrosospira, Mobiluncus, Escherichia, Azospirillum, Bifidobacterium, Desulfovibrio, Ruminococcus, Megasphaera, Atopobium, Clostridium, Pseudomonas, Hylemonella, Acidovorax, Sneathia, Bradyrhizobium, and Congregibacter as well as reviewed sequence data from Homo sapiens (UniProtKB/SwissProt, Sept 2015) to exclude potential homologies. Scaffold was used to validate protein identifications using the following criteria: ≤0.1% FDR for peptide identification, ≤1% FDR for protein identification, and at least two unique peptides identified per protein. Microbial abundance was calculated by taking the sum of normalized total spectral counts from Scaffold for all proteins associated with each genus. Lactobacillus was analyzed at the species level. Non-homologous bacterial proteins were mapped against the KEGG ontology database using GhostKOALA (v.2.0, Kyoto University Bioinformatics Center).
Statistical Analysis:
Follow-up visits were separated based on follow-up visit 1 and follow-up visit 2. Longitudinal differences in the human proteome and in bacterial taxa levels were assessed using one-way repeated measures ANOVA with Tukey’s correction for multiple comparisons on paired data (n=3 comparisons between sample visits). Four participants were removed from the host proteome analysis (1 placebo, 3 dapivirine) as they either did not have samples available at baseline and all follow-up visits or at least one sample was identified as an outlier (above or below 1.5 IQR). Based on conservative estimates of variance (CV = 100%, adj. alpha = 0.0167) and CVL proteome coverage, the study was adequately powered (80%) to detect 1.8- and 0.73-fold changes in protein expression from baseline in the placebo and dapivirine arms over time, respectively. Proteins significantly associated with dapivirine or placebo ring use were analyzed using hierarchical clustering analysis of median centered protein abundances, using a Euclidean distance with Fisher’s exact test or Chi square as appropriate (NMF package in R v.3.5.1[32, 33]).
Metaproteome taxonomic diversity was assessed using Shannon’s H diversity index. Using a conservative estimate of 150% CV across all taxa, and a two-tailed alpha=0.05, adjusted for n=17 taxa comparisons, the study was adequately powered (80%) to detect changes of 2.9- and 1.1-fold between time points within women using placebo and dapivirine rings, respectively.
A total of 18 bacterial functions were identified that could be assessed at 80% power (CV=144%, power=0.80, FD=1, 20% sample coverage). Bacterial functional composition profiles were calculated using three zero replacement methods to ensure robustness of findings: undetected pathways were assumed to be true zeros, a pseudocount = 0.0001 was applied, or a half minimum pathway-level replacement was applied; statistical results from the true-zero estimates are reported. Pathway differences were assessed using either proportional data (two-tailed, Wilcoxon signed-rank tests) over time or between women with above/below median levels of each pathway (two-tailed Fisher’s exact test) between treatment arms.
For the condom use sub-analysis Mann-Whitney U test and Kruskal-Wallis test with Dunn’s correction for multiple comparisons were used to determine differences between groups while Spearman’s r value was used for correlations.
Results
Participant characteristics
At enrolment 4 (50%) and 18 (66.7%) participants in the placebo and dapivirine arms of this substudy, respectively, reported having a primary sexual partner (p=0.69). At follow-up visits, 50% of participants in the placebo arm and 53% in the dapivirine arm reported having a primary sexual partner (p>0.99). Vaginal sex with a condom in the previous 30 days was reported by 37.5–55% of participants at study visits, whereas 25–31% of participants reported unprotected vaginal sex in the previous 30 days. There was no difference in reports of receptive oral sex. All participants in the placebo arm reported ring use at follow-up visits. One participant in the dapivirine arm reported no ring use in the past 30 days at one follow-up visit. (Table 1) Ninety-five percent of returned vaginal rings had levels of less than 23.5mg of dapivirine, which is indicative of adherence over the past month, and has been associated with protection from HIV[27].
Table 1:
Participant characteristics
| Placebo (n=8) | Dapivirine (n=27) | |||||
|---|---|---|---|---|---|---|
| Baseline (n=8) | Follow-up visits¶ (n=16) | P value | Baseline (n=27) | Follow-up visits¶ (n=54) | P value | |
| Primary partner (n; % yes) | 4 (50%) | 8 (50%) | >0.9999 | 18 (66.7%) | 28 (52.8%) | 0.2403 |
| Vaginal sex last 30 days (all) (n; % yes) | 3 (37.5%) | 8 (50%) | 0.6792 | 15 (55.6%) | 25 (47.2%) | 0.4852 |
| Vaginal sex with condom last 30 days (n; % yes) | 3 (37.5%) | 7 (43.8%) | >0.9999 | 14 (51.8%) | 24 (45.3%) | 0.6381 |
| Vaginal sex without condom last 30 days (n; % yes) | 2 (25%) | 5 (31.3%) | >0.9999 | 8 (29.6%) | 14 (26.4%) | 0.7933 |
| Oral sex last 30 days (receptive) (n; % yes) | 3 (37.5%) | 3 (18.8%) | 0.3618 | 10 (37.0%) | 21 (38.9%) | >0.999 |
| Anal sex last 30 days (n; % yes) | 0 (0%) | 0 (0%) | N/A | 1 (3.7%) | 1 (1.9%) | N/A |
| Ring use last 30 days (n; % yes) | N/A | 16 (100%) | N/A | 53 (98.1%) | >0.9999§ | |
Follow-up visits were pooled for each treatment arm
Fisher’s exact test comparing ring use between placebo and dapivirine arms. All other p values represent Fisher’s exact tests comparing baseline versus all follow-up visits in each arm.
Cervicovaginal proteome alterations between baseline and follow-up visits in dapivirine and placebo arms
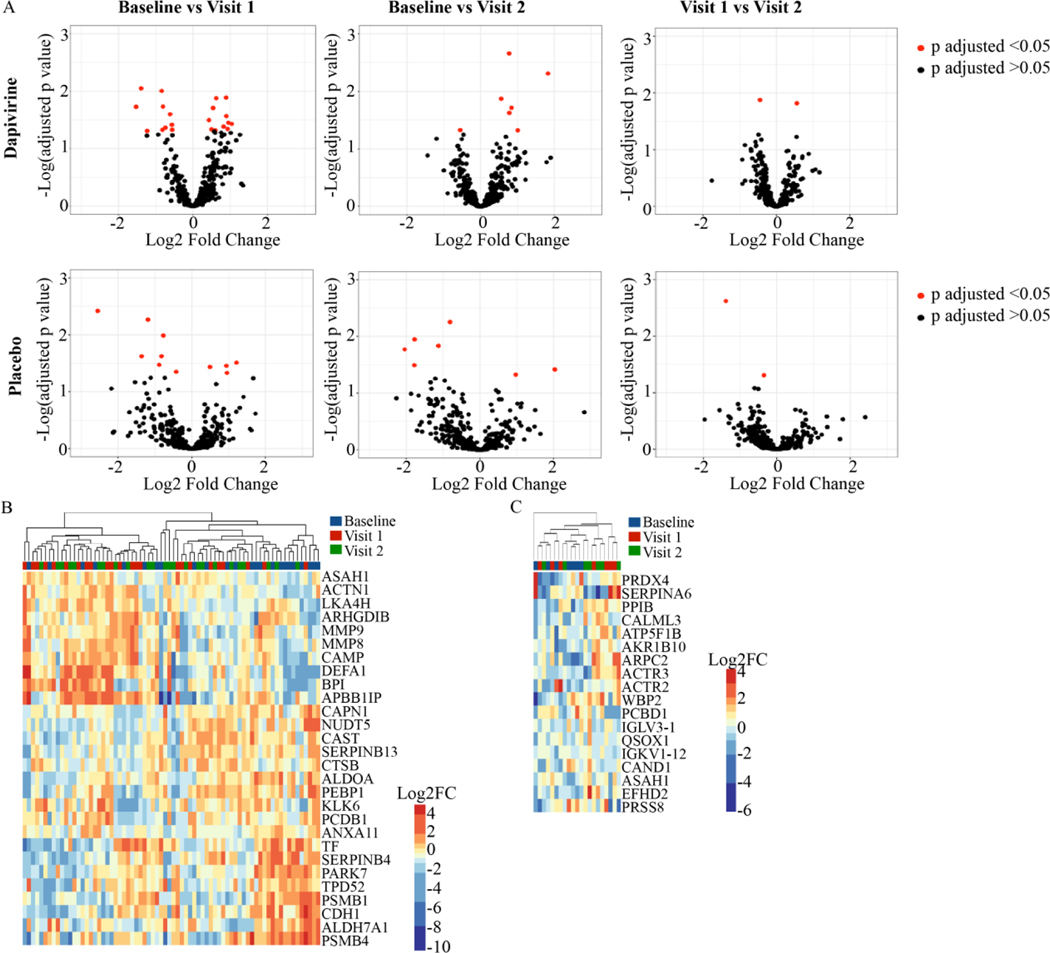
Mass spectrometry analysis of CVL samples identified 405 human proteins. In the dapivirine arm 28 proteins (6.9%) were differentially abundant (p adj.<0.05) between baseline and follow-up visits, with 21 proteins (5.2%) differentially abundant between baseline and follow-up visit 1, 7 proteins (1.7%) between baseline and follow-up visit 2, and 2 proteins (0.5%) between follow-up visits 1 and 2. (Figure 1a, Supplementary Table 1). ANXA11 and PSMB1 were differentially abundant compared to baseline at both follow-up visits. Differentially abundant proteins between baseline and follow-up visit 1 were related to necrosis, degranulation of cells, and quantity of granulocytes (Table 2). Leukocyte extravasation signalling and ILK signalling were canonical pathways associated with proteins that were differentially abundant between baseline and visit 1 (Table 3). Hierarchical cluster analysis of all differentially abundant proteins trended to cluster by visit (Chi square p=0.068) (Figure 1b). In the placebo arm 18 proteins (4.4%) were differentially abundant (p adj.<0.05) between baseline and follow-up visits, with 11 (2.7%) proteins differentially abundant between baseline and follow-up visit 1, 7 proteins (1.7%) between baseline and follow-up visit 2, and 2 proteins (0.5%) between follow-up visits 1 and 2 (Figure 1a, Supplementary Table 2). CALML3 was significantly different compared to baseline at both follow-up visits. Cell movement and degranulation of neutrophils were biofunctions significantly associated with proteins differentially abundant between baseline and visit 1 in the placebo arm (Table 2). Canonical pathways associated with differentially abundant proteins between baseline and visit 1 were related to inflammation including fMLP signalling in neutrophils and integrin signalling. Hierarchical clustering of differentially abundant proteins indicated that the samples tended to cluster by visit (Figure 1c).
Figure 1: Host proteome changes in cervicovaginal mucus over time in adolescent girls using dapivirine and placebo vaginal rings.
Volcano plots displaying human cervicovaginal proteins differentially abundant between visits in both the dapivirine (A) and placebo (B) arms using one-way repeated measures ANOVA with Tukey’s correction for multiple comparisons. (C) Hierarchical clustering of differentially abundant (p adjusted<0.05) proteins in dapivirine arm and placebo (D) arm. Overabundant proteins are represented in red and those that are underabundant are represented in blue. Baseline, follow-up visit 1, and follow-up visit 2 are shown.
Table 2:
Biofunctions associated with dapivirine and placebo vaginal ring use
| Arm | Comparison | Pathway | Proteins | p value (z score) |
|---|---|---|---|---|
| Dapivirine | Baseline vs Visit 1 | Necrosis | ASAH1, BPI, CAMP, CAPN1, CAST, CDH1, MMP8, MMP9, PEBP1, PSMB1, SERPINB4, TF, TPD52 | 7.34E-06 (−2.035) |
| Dapivirine | Baseline vs Visit 1 | Degranulation of cells | ACTN1, ASAH1, BPI, CAMP, CAPN1, CDH1, LTA4H, MMP8, MMP9, PEBP1, PSMB1, TF | 4.64E-13 (−1.025) |
| Dapivirine | Baseline vs Visit 1 | Angiogenesis | BPI, CAMP, CAPN1, CDH1, MMP8, MMP9, TF | 1.83E-04 (−0.798) |
| Dapivirine | Baseline vs Visit 1 | Cell movement of leukocytes | APBB1IP, CAMP, CAST, CDH1, MMP8, MMP9 | 5.20E-04 (−0.865) |
| Dapivirine | Baseline vs Visit 1 | Quantity of granulocytes | ARHGDIB, CAMP, MMP8, MMP9, TF | 3.64E-05 (1.89) |
| Dapivirine | Baseline vs Visit 1 | Inflammation of body cavity | CAMP, CDH1, MMP8, MMP9, PSMB1, SERPINB13, TF | 2.04E-04 (2.188) |
| Placebo | Baseline vs Visit 1 | Cell movement | ACTR2, ACTR3, ARPC2, ATP5F1B, CALML3, IGLV3–1 | 4.56E-03 (−2.224) |
| Placebo | Baseline vs Visit 1 | Degranulation of neutrophils | ACTR2, CAND1, PRDX4, QSOX1 | 6.74E-05 (NA) |
| Placebo | Baseline vs Visit 1 | Organization of cytoplasm | ACTR2, ACTR3, ARPC2, ATP5F1B, CALML3 | 4.10E-03 (−1.612) |
Table 3:
Canonical pathways associated with dapivirine or placebo vaginal ring use
| Arm | Comparison | Pathway | Proteins | P value |
|---|---|---|---|---|
| Dapivirine | Baseline vs Visit 1 | ILK signaling | ACTN1, CDH1, MMP9 | 5.89E-04 |
| Dapivirine | Baseline vs Visit 1 | Leukocyte extravasation signaling | ACTN1, MMP8, MMP9 | 6.61E-04 |
| Placebo | Baseline vs Visit 1 | Remodeling of epithelial adherens junctions | ACTR2, ACTR3, ARPC2 | 4.17E-06 |
| Placebo | Baseline vs Visit 1 | FcÎ3 receptor-mediated phagocytosis in macrophages and monocytes | ACTR2, ACTR3, ARPC2 | 1.10E-05 |
| Placebo | Baseline vs Visit 1 | fMLP signalling in neutrophils | ACTR2, ACTR3, ARPC2 | 2.09E-05 |
| Placebo | Baseline vs Visit 1 | CD28 signaling in T Helper cells | ACTR2, ACTR3, ARPC2 | 2.29E-05 |
| Placebo | Baseline vs Visit 1 | Integrin signalling | ACTR2, ACTR3, ARPC2 | 1.26E-04 |
To determine if background longitudinal proteome variability contributed to proteins associated with treatment arm proteome, differences in all substudy participants was assessed. When both treatment arms were combined there were 29 (7.2%) proteins differentially abundant between baseline and follow-up visits (p adj.<0.05), with 23 (5.7%) differentially abundant between baseline and visit 1, 9 (2.2%) between baseline and visit 2, and 2 (0.5%) between visit 1 and visit 2 (Supplementary Figure 1a, Supplementary Table 3). Four proteins were differentially abundant compared to baseline at both follow-up visits. There was no clustering of these proteins by treatment arm (p=0.615 Fisher’s Exact Test) but they trended to cluster by time in trial (p=0.066, Chi square) (Supplementary Figure 1b). Cell movement, apoptosis, and degranulation of cells were biofunctions associated with baseline to visit 1 while degranulation of cells was also associated with baseline to visit 2. Samples clustered by reported condom use in the past 30 days (Fisher’s Exact p=0.0012, Supplementary Figure 1b), but not by reported unprotected vaginal sex (Fisher’s Exact p=0.4715) or reported vaginal sex in the past 30 days (Fisher’s Exact p=0.2083) (data not shown). Condom use did associate with reported receptive oral sex (Fisher’s Exact p<0.0001), but samples did not cluster by oral sex (Fisher’s Exact p=0.5045).
Vaginal microbiome does not change with dapivirine ring use
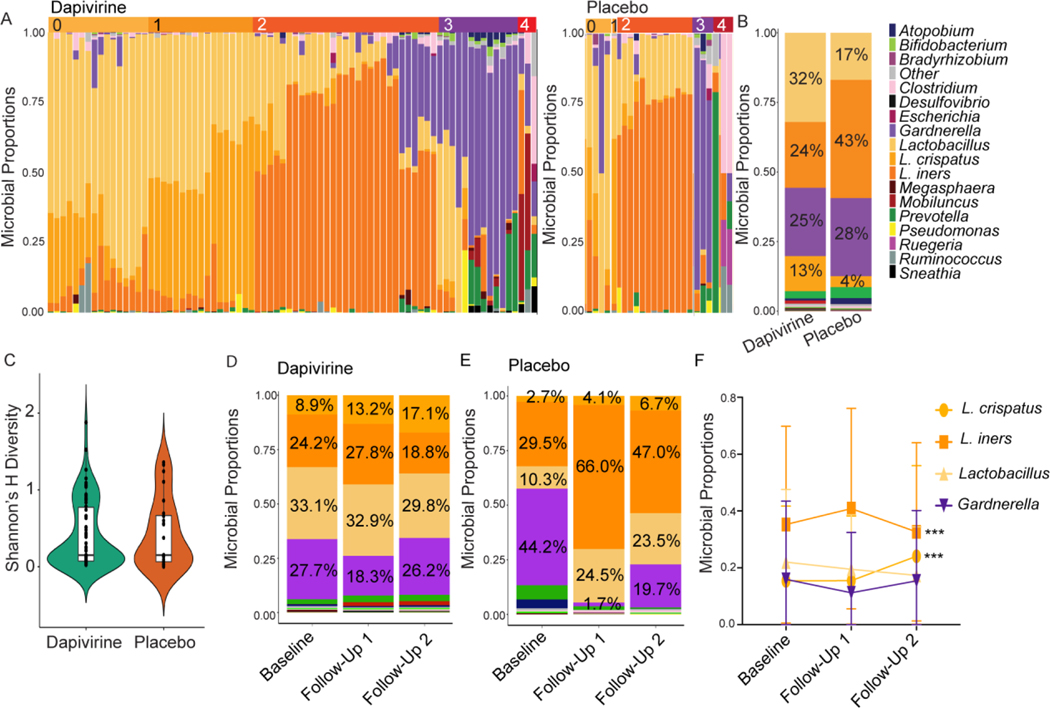
Mass spectrometry identified 2467 bacterial proteins from 15 genera. Lactobacillus was the most commonly identified bacterium, followed by Gardnerella, Prevotella, Atopobium, and Mobiluncus (Figure 2a, b, Supplementary Figure 2). Lactobacillus dominance (>50% Lactobacillus proteins[29]) was not associated with treatment arm (p=0.3745) or time in the trial (p=0.7965). Overall, 7.8% of participants (8) belonged to microbiome group 0 (other Lactobacillus), 28.4% (29) to microbiome group 1 (L. crispatus), 41.2% (42) to microbiome group 2 (L. iners), 16.7% (17) to microbiome group 3 (Gardnerella), and 6% (6) were polymicrobial (Supplementary Figure 2). These microbiome groups were represented in both treatment arms (Figure 2a). There was no difference in proportion of bacterial genera (Figure 2b) or bacterial diversity (Shannon’s H) by treatment arm (Figure 2c). There were no significant changes in the proportion of any bacterial genera between baseline and all follow-up visits, or within each treatment arm (Figure 2d, e). However, the proportion of L. crispatus significantly increased over time (paired data, Baseline vs Follow-up 1, p=0.0010; Follow-up 1 vs 2, p<0.001) and this was accompanied by a significant decrease in L. iners (paired data, Follow-up 1 vs 2 p<0.001) (Figure 2f).
Figure 2: Vaginal microbial proteome in adolescents using dapivirine or placebo vaginal rings.
A) Taxa proportion plots of each individual in dapivirine and placebo treatment arms. Lactobacillus is displayed to the species level for the two most abundant species detected, L. crispatus and L. iners. Microbiome groups are indicated on top of the graphs. B) Summary of distribution of bacterial taxa by treatment arm. The average percentages for the top 4 bacterial taxa are shown. C) Shannon’s Diversity Index by treatment arm. D/E) Bacterial proportion plots over time in the trial in the dapivirine arm and placebo arms, respectively. Visits are grouped by baseline, first follow-up visit or second follow-up visit. All participants are included. F) Changes in the proportion of the major taxa groups over time in all participants with paired samples available regardless of treatment arm (baseline and 2 independent follow-up visits, n=30). Data points represent mean ± SD. *** p<0.001.
Bacterial functional pathway differences with ring use
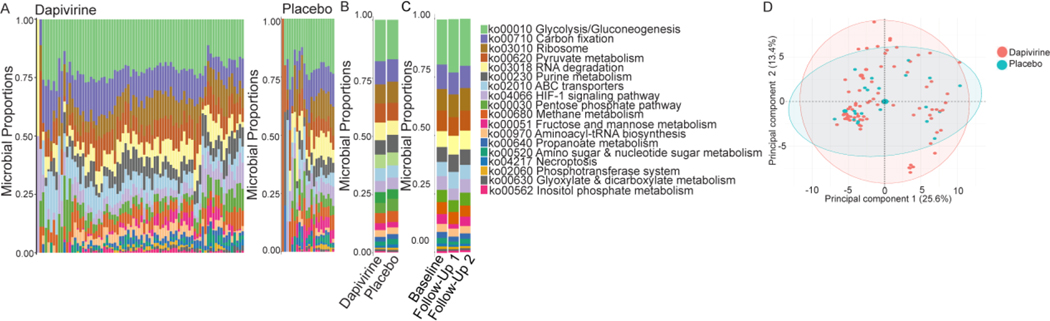
Bacterial metabolic pathways were examined to determine if ring use was associated with any functional differences. Sixty-four percent (64.7%) of the bacterial proteins matched to one or more functions using KEGG gene ontology. These bacterial functions were primarily related to carbohydrate metabolism (43.9%), energy metabolism (17.2%), and translation (10.2%) at the higher-level categorization of functions (b-level). At the lower level of categorization (ko-level) 18 high-coverage bacterial functions were identified (Figure 3a). The most abundant ko-level functions were Glycolysis/Gluconeogenesis (ko00010) (20.9%), carbon fixation (ko00710) (12.0%), and pyruvate metabolism (ko00620) (9.5%). No bacterial functions were differentially abundant between treatment arm or between baseline and all follow-up visits in each treatment arm (Figure 3b-d). In a paired analysis aminoacyl-tRNA biosynthesis (ko00970) was significantly decreased at follow-up visit 1 compared to baseline in the dapivirine arm, although this did not pass correction for multiple comparisons (−0.56% decrease, p=0.077).
Figure 3: Functional microbiome differences associated with dapivirine and placebo vaginal ring use in adolescent girls.
A) Curated ko-level bacterial functions present in the dapivirine and placebo arms using KEGG gene ontology. B) Summary of ko-level bacterial functional proportions between dapivirine and placebo arms. C) Summary of ko-level bacterial functional proportions over time in the trial. Samples are grouped by baseline, first follow-up visit, and second follow-up visit. Both treatment arms have been combined. D) Principal component analysis of ko-level bacterial functions.
Sexual behaviours associated with the cervicovaginal proteome and functional microbiome
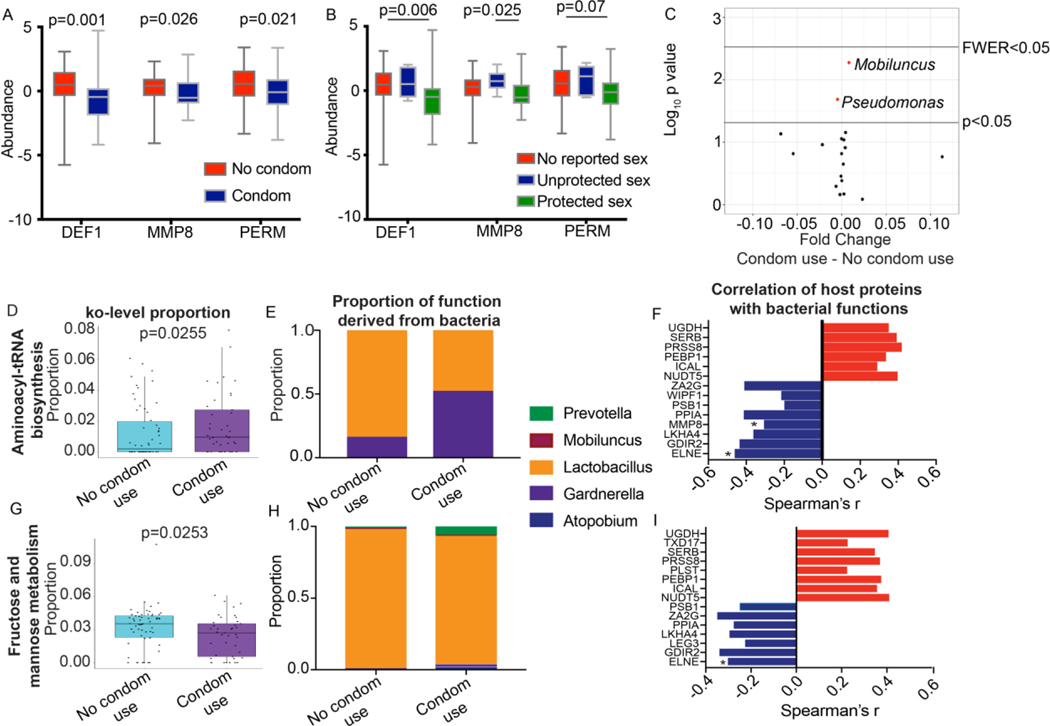
Although participants agreed to use condoms during the trial, approximately a third of substudy participants (31.3% placebo, 26.4% dapivirine) reported unprotected vaginal sex in the previous 30 days during at least one follow-up visit. Differentially abundant proteins between baseline and follow-up visits clustered by reported condom use (Fisher’s Exact p=0.0012), with proteins associated with neutrophils (DEF1 p=0.001, MMP8 p=0.026, and PERM p=0.021) increased in participants reporting no condom use (Figure 4a, Supplementary Figure 1b). When stratified by condom use and reported vaginal sex, these neutrophil factors remained lowest in adolescents reporting both vaginal sex and condom use (“protected vaginal sex”), and highest in those reporting vaginal sex without condom use (“unprotected vaginal sex”) (Figure 4b). Condom use in the past 30 days was associated with a significant increase in Mobiluncus (p=0.0053, fold change=8.09E-03) and a significant decrease in Pseudomonas (p=0.0206, fold change=−4.52E-03), although this did not pass multiple comparisons correction (Figure 4c). There was no significant difference in Mobiluncus based on oral sex in the previous 30 days. The fold change of these bacteria between adolescent girls reporting condom use and those with no reported condom use were small and these bacterial species were present at low levels (<0.5% of microbiome). The identified neutrophil factors were negatively correlated with Mobiluncus counts (DEF1 Spearman’s r=−0.348, p=0.00039; MMP8 r=−0.209, p=0.037; PERM r=−0.300, p=0.0024) but not with Pseudomonas. In adolescents reporting no condom usage the bacterial function ko00051 fructose and mannose metabolism was significantly increased (abundance 3.6% vs. 2.7%, p=0.0255) and ko00970 aminoacyl-tRNA biosynthesis was significantly decreased (1.4% vs 1.9%, p=0.0255) (Figure 4d, g), although these did not pass multiple comparisons correction. Proteins involved in aminoacyl-tRNA biosynthesis were primarily derived from Lactobacillus in adolescents who did not report condom use (83.4% of proteins from Lactobacillus (L. crispatus, L. iners), 16.1% from Gardnerella) and equally from Lactobacillus and Gardnerella in those reporting condom use (47.8% from Lactobacillus (L. iners, L. crispatus), 52.1% from Gardnerella) (Figure 4e). Fructose and mannose metabolism proteins were primarily derived from Lactobacillus (predominantly L. iners) in both groups (89.9% of condom users, 97.6% non-condom users), although reported condom users did have low levels of Prevotella (relative abundance=6%) and Atopobium (relative abundance=2%) that contributed to this pathway (Figure 4h). Proteins from these two functional pathways did not cluster by reported condom use (Supplementary Figure 3). When total bacterial count for each of these pathways was correlated to host proteins that were differentially abundant between baseline and follow-up visits, several proteins were significantly correlated (Figure 4f,i, Supplementary Table 4). In particular, several proteins related to immune system functioning (LKHA4, MMP8, PSB1) were negatively correlated with each bacterial function whereas proteins related to nucleotide sugar metabolism (NUDT5), protein kinase binding (PEBP1), and glycosaminoglycan biosynthesis (UGDH) were positively correlated with these bacterial functions.
Figure 4: Host and functional microbiome differences based on reported condom use in the past 30 days.
A) Relative abundance of neutrophil associated proteins DEF1, MMP8, PERM based on reported condom use in the past 30 days. Statistics were calculated using Mann-Whitney test. B) Relative abundance of neutrophil associated proteins DEF1, MMP8 and PERM based on reported condom use and reported acts of vaginal sex. No reported sex indicates no vaginal sex was reported in the past 30 days and no condom use was reported in the past 30 days. Unprotected sex indicates the participant reported vaginal sex with inconsistent condom use (reported both condom use and unprotected vaginal sex in the past 30 days). Protected sex indicates the participant reported vaginal sex with no acts of unprotected vaginal sex in the past 30 days. Statistics were calculated using Kruskal-Wallis test with Dunn’s correction for multiple comparisons. C) Volcano plot displaying differences in bacterial taxa between women reporting condom use and those reporting no condom use using Mann-Whitney U test. p<0.05 and family wise error rate (FWER, Bonferroni corrected p value) are indicated. Boxplot of ko00970 Aminoacyl-tRNA biosynthesis (D) and ko00051 Fructose and mannose metabolism (G) in the microbiome based on reported condom usage in the past 30 days, as well as contributing microbial taxa to these functions (E, H) (F, I) Relationship between host proteins associating with altered functional pathways. Spearman’s r values are shown for significant (p<0.05) correlations. Neutrophil proteins that independently associated with the host proteome are indicated with *.
Discussion
While the dapivirine ring was seen in clinical trials to be well-tolerated and reduce HIV risk in adult women, it is important to evaluate this product in adolescents. MTN-023 was conducted in American adolescents and demonstrated the ring was acceptable and well tolerated over 6 months of use[34]. This substudy examined cervicovaginal inflammation and the microbiome in a subset of participants and showed dapivirine ring use did not associate with any large changes to host inflammatory pathways or the vaginal microbiome over time. Reported condom usage was associated with changes in inflammation and functional metabolic pathways in vaginal bacteria. Overall, this trial supports the safety of a dapivirine ring for use in adolescents.
It is important to evaluate potential inflammatory changes associated with topical agents as previous vaginal products have demonstrated an increase in vaginal inflammation, resulting in increased susceptibility to HIV[35–38]. Tenofovir gel, a microbicide that provided significant reduction in HIV acquisition in the CAPRISA 004 trial[39], has been reported to have some immunoregulatory properties[40–42]. While cluster analysis showed enrichment for pathways relating to neutrophil degranulation and cell movement that differed over time, this was observed in both treatment arms, and only between baseline and the first follow-up visit suggesting that ring use and not treatment arm was responsible for these observed changes. Quantity of granulocytes and leukocyte extravasation signalling were uniquely associated with dapivirine use between baseline and follow-up visit 1, which suggests that any differences in inflammation with dapivirine use could be transient. However, due to the low number of participants in the placebo arm, we may not have had the power to observe similar effects in the placebo arm. Taken together, these results should be validated in a larger study.
While there have been concerns about differences in the microbiome between adolescents and adults, several studies examining the microbiome in girls prior to the onset of puberty and into adolescence and sexual debut have found similarities to the microbiome of adults, with similar rates of bacterial vaginosis between adolescents and adults[12, 15, 19–22, 24, 43, 44]. As women with bacterial vaginosis are at increased risk for acquisition of several STIs including HIV[45–53], it is important to determine if topical agents change the vaginal microbiota. Previous vaginal products including nonoxynol-9 and cellulose sulfate significantly altered the vaginal microbiome, increasing bacteria associated with bacterial vaginosis and shifting the microbiome towards a community lacking Lactobacillus[18, 54, 55]. Furthermore, studies have found that vaginal bacteria modify tenofovir gel efficacy[29]. Phase 1 studies of dapivirine vaginal rings found that the prevalence of pigmented anaerobic gram-negative rods increased significantly during ring use, E. coli levels decreased, and there were no significant changes in Nugent score, or Lactobacillus[56, 57]. In our study, Lactobacillus was the most common bacterium identified. While there were no significant changes in bacterial genera over time, there were significant increases in L. crispatus when both treatment arms were combined, which was accompanied by a significant decrease in L. iners within this study. As L. iners has been associated with cervical inflammation[58–61] and increased susceptibility to STIs including HIV and C. trachomatis[62–65], these results may represent a shift to a healthier microbiome with either dapivirine or placebo ring use. Indeed, studies using vaginal rings for contraception have found that the mean Nugent score decreased significantly with ring use and Lactobacillus prevalence increased[66]. While those results are likely due to the estrogen contained in the contraceptive ring, promoting Lactobacillus colonization, our observation may indicate that enrolment in a clinical trial could have positive influences on the microbiome due to enhanced awareness regarding safe sex practices.
Alterations to functional pathways were observed in the microbiome with reported condom use. These functions were related to protein translation and fructose and mannose metabolism, predominantly from L. iners. Fructose and mannose metabolism are important pathways for energy production by Lactobacillus. These functional pathways were negatively correlated with host proteins associated with neutrophils, suggesting a relationship between condom use, changes in bacteria metabolic activity, and inflammation. When these neutrophil proteins were stratified by vaginal sex (no sex, unprotected sex, protected sex) we found the lowest levels of the neutrophil factors in adolescents reporting protected vaginal sex. Follow-up studies investigating soluble metabolites may help better understand these relationships between sexual behaviours, neutrophils, and bacteria metabolism.
There were several limitations of this study. As the sample size in this study allowed us to detect protein level changes >1.8 fold with 80% power, smaller effects may have gone unnoticed. The data on condom use and vaginal sex were self-reported which may lead to over-reporting of condom use. Microbiome results were reported using mass spectrometry. While we have previously demonstrated good concordance between 16S rRNA sequencing and mass spectrometry to identify bacterial taxa, 16S rRNA sequencing tends to detect greater bacterial diversity, which may be a result of the increased dynamic range of the proteome compared to the genome as well as limitations in available databases for proteomics[29, 30]. While we have previously shown mass spectrometry has high sensitivity to identify bacterial species that were present at 0.1% of the population[30] we cannot discount that low abundance bacterial species were not identified in this study. However, mass spectrometry provides species-level identification of bacteria as well as information on functional and metabolic activity, which is not readily available from 16S-based approaches.
The availability of safe and acceptable HIV prevention options are of critical importance for adolescent girls to protect themselves from HIV infection. This study supports the safety profile of dapivirine rings in adolescents.
Supplementary Material
Supplementary Figure 1: Differentially abundant proteins between baseline and follow-up visits in both treatment arms combined. A) Volcano plots displaying host proteins differentially abundant between each visit using one-way repeated measures ANOVA with Tukey’s correction for multiple comparisons. B) Hierarchical clustering of differentially abundant proteins (p adjusted <0.05). Overabundant proteins are represented in red and those that are underabundant are represented in blue. Arm, visit dates, and condom use in the past 30 days are shown.
Supplementary Figure 2: Vaginal microbial proteome in adolescents using dapivirine or placebo vaginal rings. Taxa proportion plot of each individual in the trial. Lactobacillus is displayed to the species level for the two most abundant species detected, L. crispatus and L. iners. Microbiome groups are indicated on top of the graphs. Both treatment arms and all visits are combined.
Supplementary Figure 3: Functional microbiome proteins do not group by condom use. Heatmaps showing count for bacterial proteins identified in the fructose and mannose metabolism and aminoacyl-tRNA biosynthesis pathways. Condom use data in the past 30 days is overlaid.
Acknowledgements:
The authors thank Max Abou and Kenzie Birse for technical support. The MTN is funded by the National Institute of Allergy and Infectious Diseases (UM1AI068633, UM1AI068615, UM1AI106707), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health. Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) is funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD 040533).
Conflicts of Interest and Source of Funding: The MTN-023/IPM 030 trial was designed and implemented by the Microbicide Trials Network (MTN) in conjunction with the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN). The MTN is funded by the National Institute of Allergy and Infectious Diseases (UM1AI068633, UM1AI068615, UM1AI106707), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health. Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) is funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD 040533). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The vaginal rings used in this study were developed and supplied by the International Partnership for Microbicides (IPM).
References
- 1.2016 UFSN. In; 2016.
- 2.Glynn JR, Carael M, Auvert B, Kahindo M, Chege J, Musonda R, et al. Why do young women have a much higher prevalence of HIV than young men? A study in Kisumu, Kenya and Ndola, Zambia. AIDS 2001; 15 Suppl 4:S51–60. [DOI] [PubMed] [Google Scholar]
- 3.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. The New England journal of medicine 2015; 372(6):509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. The New England journal of medicine 2012; 367(5):411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delany-Moretlwe S, Lombard C, Baron D, Bekker LG, Nkala B, Ahmed K, et al. Tenofovir 1% vaginal gel for prevention of HIV-1 infection in women in South Africa (FACTS-001): a phase 3, randomised, double-blind, placebo-controlled trial. The Lancet infectious diseases 2018; 18(11):1241–1250. [DOI] [PubMed] [Google Scholar]
- 6.Thurman AR, Clark MR, Hurlburt JA, Doncel GF. Intravaginal rings as delivery systems for microbicides and multipurpose prevention technologies. Int J Womens Health 2013; 5:695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. The New England journal of medicine 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nel A, van Niekerk N, Kapiga S, Bekker LG, Gama C, Gill K, et al. Safety and Efficacy of a Dapivirine Vaginal Ring for HIV Prevention in Women. The New England journal of medicine 2016; 375(22):2133–2143. [DOI] [PubMed] [Google Scholar]
- 9.Baeten J, Palanee-Phillips T, Mgodi N, Ramjee G, Gati B, Mhlanga F, Hunidzarira P, Manoor L, Siva S, Govender V, Makanani B, Naidoo L, Singh N, Nair G, Chinula L, Mayo A, Szydlo D, Soto-Torres L, Nel A, Rosenberg Z, Hillier S, Brown E, MTN-025/HOPE Study Team. High adherence and sustained impact on HIV-1 incidence: Final results of an open-label extension trial of the dapivirine vaginal ring. In: IAS. Mexico City, Mexico; 2019. [Google Scholar]
- 10.Nel A, Malherbe M, Mans W, Van Baelen B, van Niekerk N, Louw C, Gwetu T, Mabude Z, Kotze P, Moraba R, Tempelman H, Gill K, Kusemererwa S, Solai L, Bekker L, Rosenberg Z. Safety, Adherence, and HIV-1 Seroconversion in DREAM - An Open-label Dapivirine Vaginal Ring Trial. In: 9th SA AIDS Conference. Durban, South Africa; 2019. [Google Scholar]
- 11.Roberts L, Liebenberg L, Barnabas S, Passmore JA. Vaginal microbicides to prevent human immunodeficiency virus infection in women: perspectives on the female genital tract, sexual maturity and mucosal inflammation. Best practice & research Clinical obstetrics & gynaecology 2012; 26(4):441–449. [DOI] [PubMed] [Google Scholar]
- 12.Porter KA, Turpin J, Begg L, Brown G, Chakhtoura N, Church E, et al. Understanding the Intersection of Young Age, Mucosal Injury, and HIV Susceptibility. AIDS research and human retroviruses 2016; 32(10–11):1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghanem KG, Shah N, Klein RS, Mayer KH, Sobel JD, Warren DL, et al. Influence of sex hormones, HIV status, and concomitant sexually transmitted infection on cervicovaginal inflammation. The Journal of infectious diseases 2005; 191(3):358–366. [DOI] [PubMed] [Google Scholar]
- 14.Hwang LY, Scott ME, Ma Y, Moscicki AB. Higher levels of cervicovaginal inflammatory and regulatory cytokines and chemokines in healthy young women with immature cervical epithelium. Journal of reproductive immunology 2011; 88(1):66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez-Olmos MI, Barousse MM, Rajan L, Van Der Pol BJ, Fortenberry D, Orr D, et al. Vaginal lactobacilli in adolescents: presence and relationship to local and systemic immunity, and to bacterial vaginosis. Sexually transmitted diseases 2004; 31(7):393–400. [DOI] [PubMed] [Google Scholar]
- 16.Barousse MM, Van Der Pol BJ, Fortenberry D, Orr D, Fidel PL Jr., Vaginal yeast colonisation, prevalence of vaginitis, and associated local immunity in adolescents. Sexually transmitted infections 2004; 80(1):48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shrier LA, Bowman FP, Lin M, Crowley-Nowick PA. Mucosal immunity of the adolescent female genital tract. The Journal of adolescent health : official publication of the Society for Adolescent Medicine 2003; 32(3):183–186. [DOI] [PubMed] [Google Scholar]
- 18.Pellett Madan R, Dezzutti CS, Rabe L, Hillier SL, Marrazzo J, McGowan I, et al. Soluble Immune Mediators and Vaginal Bacteria Impact Innate Genital Mucosal Antimicrobial Activity in Young Women. Am J Reprod Immunol 2015; 74(4):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell CM, Fredricks DN, Winer RL, Koutsky L. Effect of sexual debut on vaginal microbiota in a cohort of young women. Obstetrics and gynecology 2012; 120(6):1306–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto T, Zhou X, Williams CJ, Hochwalt A, Forney LJ. Bacterial populations in the vaginas of healthy adolescent women. Journal of pediatric and adolescent gynecology 2009; 22(1):11–18. [DOI] [PubMed] [Google Scholar]
- 21.Madan RP, Carpenter C, Fiedler T, Kalyoussef S, McAndrew TC, Viswanathan S, et al. Altered biomarkers of mucosal immunity and reduced vaginal Lactobacillus concentrations in sexually active female adolescents. PloS one 2012; 7(7):e40415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hickey RJ, Zhou X, Settles ML, Erb J, Malone K, Hansmann MA, et al. Vaginal microbiota of adolescent girls prior to the onset of menarche resemble those of reproductive-age women. mBio 2015; 6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jespers V, Hardy L, Buyze J, Loos J, Buve A, Crucitti T. Association of Sexual Debut in Adolescents With Microbiota and Inflammatory Markers. Obstetrics and gynecology 2016; 128(1):22–31. [DOI] [PubMed] [Google Scholar]
- 24.Vodstrcil LA, Twin J, Garland SM, Fairley CK, Hocking JS, Law MG, et al. The influence of sexual activity on the vaginal microbiota and Gardnerella vaginalis clade diversity in young women. PloS one 2017; 12(2):e0171856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fethers KA, Fairley CK, Morton A, Hocking JS, Hopkins C, Kennedy LJ, et al. Early sexual experiences and risk factors for bacterial vaginosis. The Journal of infectious diseases 2009; 200(11):1662–1670. [DOI] [PubMed] [Google Scholar]
- 26.Fethers K, Twin J, Fairley CK, Fowkes FJ, Garland SM, Fehler G, et al. Bacterial vaginosis (BV) candidate bacteria: associations with BV and behavioural practices in sexually-experienced and inexperienced women. PloS one 2012; 7(2):e30633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bunge KE, Levy L, Szydlo DW, Zhang J, Gaur AH, Reirden D, et al. Brief Report: Phase IIa Safety Study of a Vaginal Ring Containing Dapivirine in Adolescent Young Women. J Acquir Immune Defic Syndr 2020; 83(2):135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birse K, Arnold KB, Novak RM, McCorrister S, Shaw S, Westmacott GR, et al. Molecular Signatures of Immune Activation and Epithelial Barrier Remodeling Are Enhanced during the Luteal Phase of the Menstrual Cycle: Implications for HIV Susceptibility. Journal of virology 2015; 89(17):8793–8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klatt NR, Cheu R, Birse K, Zevin AS, Perner M, Noel-Romas L, et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 2017; 356(6341):938–945. [DOI] [PubMed] [Google Scholar]
- 30.Zevin AS, Xie IY, Birse K, Arnold K, Romas L, Westmacott G, et al. Microbiome Composition and Function Drives Wound-Healing Impairment in the Female Genital Tract. PLoS pathogens 2016; 12(9):e1005889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burgener A, Tjernlund A, Kaldensjo T, Abou M, McCorrister S, Westmacott GR, et al. A systems biology examination of the human female genital tract shows compartmentalization of immune factor expression. Journal of virology 2013; 87(9):5141–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaujoux R, Seoighe C. A flexible R package for nonnegative matrix factorization. BMC bioinformatics 2010; 11:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Team R. RStudio: Integrated Development for R. RStudio Inc 2015. [Google Scholar]
- 34.Bunge LL Katherine E, Szydlo Daniel W, Zhang Jingyang, Gaur Aditya H, Reirden Daniel, Mayer Kenneth H, Futterman Donna, Hoesley Craig, Hillier Sharon L, Marzinke Mark A, Hendrix Craig, Gorbach Pamina M, Wilson Craig M, Lydia Soto-Torres, Kapagiannis Bill, Annalene Nel, Kathleen E Squires for the MTN-023/IPM 030 Study Team. Phase IIa safety study of a vaginal ring containing dapivirine in adolescent young women. JAIDS 2019; Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fields S, Song B, Rasoul B, Fong J, Works MG, Shew K, et al. New candidate biomarkers in the female genital tract to evaluate microbicide toxicity. PloS one 2014; 9(10):e110980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Damme L, Chandeying V, Ramjee G, Rees H, Sirivongrangson P, Laga M, et al. Safety of multiple daily applications of COL-1492, a nonoxynol-9 vaginal gel, among female sex workers. COL-1492 Phase II Study Group. AIDS 2000; 14(1):85–88. [DOI] [PubMed] [Google Scholar]
- 37.Van Damme L, Govinden R, Mirembe FM, Guedou F, Solomon S, Becker ML, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. The New England journal of medicine 2008; 359(5):463–472. [DOI] [PubMed] [Google Scholar]
- 38.Van Damme L, Ramjee G, Alary M, Vuylsteke B, Chandeying V, Rees H, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 2002; 360(9338):971–977. [DOI] [PubMed] [Google Scholar]
- 39.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010; 329(5996):1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hladik F, Burgener A, Ballweber L, Gottardo R, Vojtech L, Fourati S, et al. Mucosal effects of tenofovir 1% gel. eLife 2015; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGowan I, Hoesley C, Cranston RD, Andrew P, Janocko L, Dai JY, et al. A phase 1 randomized, double blind, placebo controlled rectal safety and acceptability study of tenofovir 1% gel (MTN-007). PloS one 2013; 8(4):e60147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castillo-Mancilla JR, Meditz A, Wilson C, Zheng JH, Palmer BE, Lee EJ, et al. Reduced immune activation during tenofovir-emtricitabine therapy in HIV-negative individuals. J Acquir Immune Defic Syndr 2015; 68(5):495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ravel J, Brotman RM, Gajer P, Ma B, Nandy M, Fadrosh DW, et al. Daily temporal dynamics of vaginal microbiota before, during and after episodes of bacterial vaginosis. Microbiome 2013; 1(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brotman RM, Ravel J, Cone RA, Zenilman JM. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sexually transmitted infections 2010; 86(4):297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. American journal of obstetrics and gynecology 2013; 209(6):505–523. [DOI] [PubMed] [Google Scholar]
- 46.Thurman AR, Kimble T, Herold B, Mesquita PM, Fichorova RN, Dawood HY, et al. Bacterial Vaginosis and Subclinical Markers of Genital Tract Inflammation and Mucosal Immunity. AIDS research and human retroviruses 2015; 31(11):1139–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myer L, Denny L, Telerant R, Souza M, Wright TC, Jr., Kuhn L. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. The Journal of infectious diseases 2005; 192(8):1372–1380. [DOI] [PubMed] [Google Scholar]
- 48.Brotman RM. Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. The Journal of clinical investigation 2011; 121(12):4610–4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen CR, Lingappa JR, Baeten JM, Ngayo MO, Spiegel CA, Hong T, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African couples. PLoS medicine 2012; 9(6):e1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. The Journal of infectious diseases 1999; 180(6):1863–1868. [DOI] [PubMed] [Google Scholar]
- 51.Taha TE, Hoover DR, Dallabetta GA, Kumwenda NI, Mtimavalye LA, Yang LP, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS 1998; 12(13):1699–1706. [DOI] [PubMed] [Google Scholar]
- 52.van de Wijgert JH, Morrison CS, Brown J, Kwok C, Van Der Pol B, Chipato T, et al. Disentangling contributions of reproductive tract infections to HIV acquisition in African Women . Sexually transmitted diseases 2009; 36(6):357–364. [DOI] [PubMed] [Google Scholar]
- 53.van de Wijgert JH, Morrison CS, Cornelisse PG, Munjoma M, Moncada J, Awio P, et al. Bacterial vaginosis and vaginal yeast, but not vaginal cleansing, increase HIV-1 acquisition in African women. J Acquir Immune Defic Syndr 2008; 48(2):203–210. [DOI] [PubMed] [Google Scholar]
- 54.McGowan I, Gomez K, Bruder K, Febo I, Chen BA, Richardson BA, et al. Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 gel (VivaGel) in sexually active young women (MTN-004). AIDS 2011; 25(8):1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ravel J, Gajer P, Fu L, Mauck CK, Koenig SS, Sakamoto J, et al. Twice-daily application of HIV microbicides alter the vaginal microbiota. mBio 2012; 3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen BA, Panther L, Marzinke MA, Hendrix CW, Hoesley CJ, van der Straten A, et al. Phase 1 Safety, Pharmacokinetics, and Pharmacodynamics of Dapivirine and Maraviroc Vaginal Rings: A Double-Blind Randomized Trial. J Acquir Immune Defic Syndr 2015; 70(3):242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nel A, Haazen W, Nuttall J, Romano J, Rosenberg Z, van Niekerk N. A safety and pharmacokinetic trial assessing delivery of dapivirine from a vaginal ring in healthy women. AIDS 2014; 28(10):1479–1487. [DOI] [PubMed] [Google Scholar]
- 58.Petrova MI, Reid G, Vaneechoutte M, Lebeer S. Lactobacillus iners: Friend or Foe? Trends in microbiology 2017; 25(3):182–191. [DOI] [PubMed] [Google Scholar]
- 59.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proceedings of the National Academy of Sciences of the United States of America 2011; 108 Suppl 1:4680–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jespers V, Kyongo J, Joseph S, Hardy L, Cools P, Crucitti T, et al. A longitudinal analysis of the vaginal microbiota and vaginal immune mediators in women from sub-Saharan Africa. Sci Rep 2017; 7(1):11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dabee S, Barnabas SL, Lennard KS, Jaumdally SZ, Gamieldien H, Balle C, et al. Defining characteristics of genital health in South African adolescent girls and young women at high risk for HIV infection. PloS one 2019; 14(4):e0213975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Edwards VL, Smith SB, McComb EJ, Tamarelle J, Ma B, Humphrys MS, et al. The Cervicovaginal Microbiota-Host Interaction Modulates Chlamydia trachomatis Infection. mBio 2019; 10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amabebe E, Anumba DOC. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front Med (Lausanne) 2018; 5:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Balle C, Lennard K, Dabee S, Barnabas SL, Jaumdally SZ, Gasper MA, et al. Endocervical and vaginal microbiota in South African adolescents with asymptomatic Chlamydia trachomatis infection. Sci Rep 2018; 8(1):11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tamarelle J, de Barbeyrac B, Le Hen I, Thiebaut A, Bebear C, Ravel J, et al. Vaginal microbiota composition and association with prevalent Chlamydia trachomatis infection: a cross-sectional study of young women attending a STI clinic in France . Sexually transmitted infections 2018; 94(8):616–618. [DOI] [PubMed] [Google Scholar]
- 66.Crucitti T, Hardy L, van de Wijgert J, Agaba S, Buyze J, Kestelyn E, et al. Contraceptive rings promote vaginal lactobacilli in a high bacterial vaginosis prevalence population: A randomised, open-label longitudinal study in Rwandan women. PloS one 2018; 13(7):e0201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Differentially abundant proteins between baseline and follow-up visits in both treatment arms combined. A) Volcano plots displaying host proteins differentially abundant between each visit using one-way repeated measures ANOVA with Tukey’s correction for multiple comparisons. B) Hierarchical clustering of differentially abundant proteins (p adjusted <0.05). Overabundant proteins are represented in red and those that are underabundant are represented in blue. Arm, visit dates, and condom use in the past 30 days are shown.
Supplementary Figure 2: Vaginal microbial proteome in adolescents using dapivirine or placebo vaginal rings. Taxa proportion plot of each individual in the trial. Lactobacillus is displayed to the species level for the two most abundant species detected, L. crispatus and L. iners. Microbiome groups are indicated on top of the graphs. Both treatment arms and all visits are combined.
Supplementary Figure 3: Functional microbiome proteins do not group by condom use. Heatmaps showing count for bacterial proteins identified in the fructose and mannose metabolism and aminoacyl-tRNA biosynthesis pathways. Condom use data in the past 30 days is overlaid.