Abstract
Isolated cleft lip with or without cleft palate and cleft palate are among the most common human birth defects. Several candidate gene studies of MSX1 have shown significant association between markers in MSX1 and risk of oral clefts, and resequencing studies have identified multiple mutations in MSX1 in a small minority of cases, which may account for 1–2% of all isolated oral clefts. We explored the two megabase region around MSX1, using a marker map of 393 single nucleotide polymorphisms in 297 cleft lip with or without cleft palate case-parent trios and 84 cleft palate trios from Maryland, Taiwan, Singapore, and Korea. Both individual markers and haplotypes of 2-5 SNPs showed several regions yielding statistical evidence of linkage and disequilibrium. Two genes (STK32B and EVC) yielded consistent evidence from cleft lip with or without cleft plate trios in all four populations. These two genes plus EVC2 also yielded suggestive evidence of linkage and disequilibrium among CP trios. This analysis suggests several genes, not just MSX1, in this region may influence risk of oral clefts.
Keywords: Oral Clefts, Cleft lip with or without cleft palate, Cleft Palate, MSX1, Chromosome 4p16
Oral clefts are among the most common birth defects, and appear to be controlled by several genes. Conventionally, isolated cleft palate (CP) is considered distinct from isolated cleft lip with or without cleft palate (CL/P) and several genes may influence both, e.g. for CL/P, at least 6 regions of linkage have been identified in genome wide studies1. Birth prevalence rates differ significantly among populations and vary by gender2. Prevalence among liveborn infants ranges from 1/500–1/1000 births, and is highest in Native Americans and lowest in populations of African descent3. Classification into CL/P and CP cannot assure etiologic homogeneity, however, and non-syndromic oral clefts appear to be influenced by multiple genes as well as environmental risk factors4, which may differ between CL/P and CP5. Both linkage and candidate gene studies have found several genes influence risk6, furthermore private mutations7 (i.e. those seen only in one family) and microdeletions8 have been identified. The exact number of causal genes, however, and their mechanism of action remains unclear7.
MXS1 [Entrez GeneID: 4487]9 is a small muscle segment homeobox gene on chromosome 4p16, consisting of two exons and one intron spanning less than 4,300 bp. An antisense transcript of the second exon has been reported10. Build 129 of dbSNP11 reports 78 known SNPs in or near this gene, six in the coding sequence.
A linkage study of a single, large Dutch family segregating for both tooth agenesis and oral clefts identified a specific causal mutation in MSX112. Multiple candidate gene studies in largely European-derived populations have shown consistent association between MSX1 and CL/P, although studies in Asians were less consistent13. Park et al14, Tongkobpetch et al15, and Otero et al16 have recently reported associations in Korean, Thai and Colombian populations, respectively, adding two Asian and one admixed South American population to those giving evidence of association. We previously analyzed several SNPs, along with a common, intronic CA repeat polymorphism, and found significant evidence of association17. Jezewski et al. reported complete sequencing of MSX1 in over 900 individuals from different ethnic backgrounds18, and identified several novel SNPs that may be directly causal as well as three intronic SNPs showing association in either Asian or Caucasian populations. They estimated about 2% of oral clefts could be attributed to MSX118. Because markers in this region have shown association with oral clefts, yet obvious causal mutations in MSX1 are extremely rare, we examined a 2 Mb region around MSX1 using a map of 393 SNPs in an international case-parent trio study. This region includes eleven known genes and six putative genes (Supplemental Figure 1). The function of most of these genes is unknown, but mutations in two genes (EVC and EVC2) cause Ellis-Van Creveld Syndrome [MIM 22550] or Weyers acrofacial dysostosis [MIM 193530] and CRMP1 is a suppressor of tumor cell invasion controlling neural development and axonal growth19.
Material and Methods
Sample description
We collected data on case-parent trios recruited through treatment centers in Maryland (Johns Hopkins and the University of Maryland), Taiwan (Chang Gung Memorial Hospital), Singapore (KK Women’s & Children’s Hospital) and Korea (Yonsei University in Seoul). Research protocols were reviewed and approved by institutional review boards (IRB) at each institution. Table 1 lists gender and the type of oral cleft for all trios used here. The racial/ethnic background of the Maryland probands were 92.1% European American, 6.6% African American & 1.3% other. The cases from Korea and Taiwan were exclusively Asian, as were 94.2% of cases from Singapore. All Singaporean parents were unaffected, but five parents (4 CL/P & 1 CP) among the 103 Maryland trios and two parents of the 172 Taiwanese trios were affected (two affected brothers from a single multiplex CP family, one of whom fathered two affected children (one with no DNA available) and one of whom had a single CP child; these cases are first cousins). All probands were given a clinical genetics evaluation to check for congenital anomalies or major developmental delays, and all were classified as having an isolated, non-syndromic oral cleft.
Table 1.
Proband gender and type of oral cleft, by recruitment site.
| CL/P | CP | ||||||
|---|---|---|---|---|---|---|---|
| # Trios (# incompletea) | Male | Female | Total | Male | Female | Total | |
| Maryland | 102 | 44 | 32 | 76 | 13 | 13 | 26 |
| Koreab | 42 (2) | 22 | 18 | 40 | 2 | 0 | 2 |
| Singapore | 66 (13) | 24 | 11 | 35 | 12 | 19 | 31 |
| Taiwan | 171 | 95 | 51 | 146 | 6 | 19 | 25 |
| Total: | 381 (15) | 185 | 112 | 297 | 33 | 51 | 84 |
only one parent contributed DNA
data on Korean CP families were not analyzed separately (due to small numbers), but they were included in any analysis of combined populations.
SNP selection, DNA & genotyping
SNPs were selected for fine mapping in a 2 Mb region surrounding MSX1 on chromosome 4p16 from position 4,000,000 to 6,000,000 of NCBI Build 35. When these SNPs were selected, the HapMap project was still in its genotyping phase, so dbSNP contained little allele frequency information for Caucasians and less for Asians. Thus, we could not identify tagging SNPs, but SNPs were selected with a goal of having one SNP per 5kb of physical distance. Variants with Illumina (San Diego, CA) scores >0.6, dbSNP validation by submitters on multiple platforms, and high heterozygosity levels (particularly in multiple populations), were ranked higher in the selection process. In total, 429 unique SNPs were selected (see Supplemental Table 1 for positions and Minor Allele Frequency (MAF) in each population), but not all met Hardy-Weinberg expectations in each population.
Genomic DNA samples were prepared as described previously20. DNA concentration was determined using the PicoGreen® dsDNA Quantitation Kit (Molecular Probes, Inc., Eugene OR) and all DNA samples were stored at −20°C. A 4µg aliquot of each genomic DNA sample was dispensed onto a bar-coded 96-well microtiter plate at a concentration of 100ng/µl and genotyped for SNP markers using the Illumina Golden-Gate™ chemistry with Sentrix® Array Matrices from the manufacturer21 at the SNP Center, Johns Hopkins School of Medicine. Two cross-plate duplicates and four CEPH controls were included on each plate to evaluate genotyping consistency within and between plates. Genotypes were generated on a BeadLab 1000 system22. Of the 429 SNPs attempted, 393 were successfully genotyped.
Statistical methods
Single SNP and 2-5 SNP haplotypes were analyzed using a family based association test (FBAT) program separately for CL/P and CP trios23, 24. Trios from all populations were combined for analysis of individual SNPs, but each population was analyzed separately when haplotypes were considered. Haplotype analysis assumes parents are drawn from a single population, but single SNP analysis can be implemented on combined samples because the case-parent trio design compares alleles transmitted to the case against those present in the mating type but not transmitted.
We screened all markers for parent-of-origin effects using Clayton’s extension of the TDT test in Stata 8.225, and the Transmission Asymmetry Test (TAT) suggested by Weinberg26, which is similar to the TDT but excludes matings between two heterozygotes (where transmission can be ambiguous). When evidence of parent specific over- or under-transmission of alleles at several SNPs was observed, haplotypes were examined for transmission distortion using the FAMHAP program which calculates maximum likelihood estimates (MLEs) of haplotype frequencies via the expectation-maximization algorithm and can handle missing data27,28 Haplotype-based tests were used where the transmitted/nontransmitted status was permuted in each replicate, and the typical chi-square statistic for marker combinations was replaced with the maximum chi-square over all individual haplotypes (maximum TDT statistic). This approach gives an empiric p-value, corrected for the multiple haplotypes in each analysis, separately for maternal and paternal transmission29.
Results
The 393 SNPs were grouped in one of the known/putative genes in this region or in one of twelve intergenic regions (labeled ‘No Locus #’) beginning at position 4 Mb and ending at 6 Mb (Supplemental Table 2). Among these 393 SNPs, 62 had a minor allele frequency (MAF) between 0.5% and 5% in at least one population, while 54 SNPs were effectively monomorphic (MAF≤0.005) in one or more populations, and 19 were monomorphic in all populations. One SNP was not in Hardy-Weinberg Equilibrium in one population. In total, 352 SNPs were analyzed in the Maryland (MD) trios, 311 in the Taiwanese (TW) trios, 324 in Singaporean (SP) trios, 314 in the Korean (KR) trios. Among these SNPs, 308 are included in the HapMap II data and they picked up 48% of the linkage disequilibrium (LD) across this region in CEU samples and 49% in CHB/JPT samples. Patterns of LD across the entire region were calculated for each population using Haploview30 (Supplemental Figure 2), and are comparable with only minor differences between populations.
Genetic distance between populations (CL/P and CP trios combined) was measured using eight SNPs in and around MSX1 polymorphic in all populations [rs12651676, rs3821949, rs3116581, rs4075, rs12532, rs13104352, rs4473606, and rs4689955]. As expected, the vast majority of the variation in haplotype frequencies occurred within populations (97.7%), while the among-population variation was only 2.3%. Pairwise FST values between these four populations revealed anticipated patterns of genetic distance: genetic distances (FST) among Asian populations were an order of magnitude smaller than those between Maryland and any of three Asian populations (data not shown).
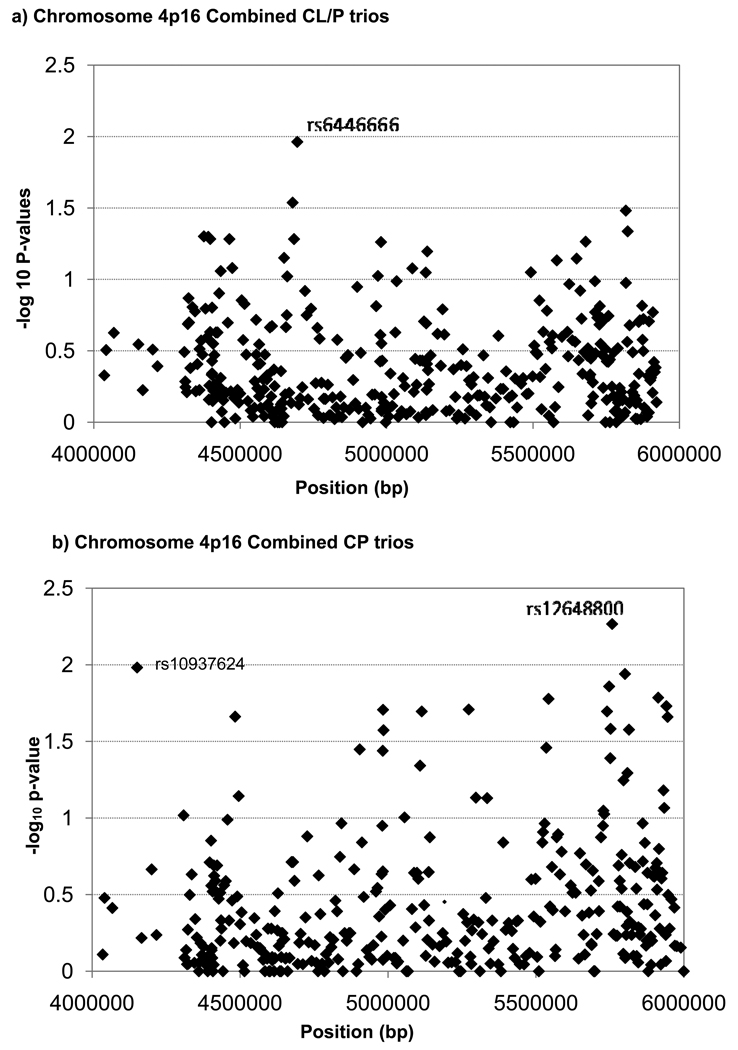
Individual SNP markers were used to test the composite null hypothesis of no linkage or no LD, and their empiric p-values are summarized in Figure 1, for the combined sample of 297 CL/P case-parent trios and for the 84 CP trios. Among CL/P trios, only one SNP (rs6446666) approached the 1% significance level. On the other hand, rs10937624 (p=0.01, in LOC389145) and rs12648800 (p=0.0054, in EVC2) at opposites ends of this 2Mb region reached the 1% threshold among CP trios. Pooled analysis of all populations is valid for individual markers because the case-parent trio design is robust to population heterogeneity. This approach, however, is less suitable for haplotypes because estimation of haplotype frequencies typically assumes samples were drawn from a single population. Therefore, we stratified by population for haplotype analyses.
Figure 1.
Empirical p-values from a family based test for linkage in the presence of disequilbrium for 393 individual SNPs from a 2 Mb region on Chromosome 4p16.
a. 297 CL/P case-parent trios from four populations (Maryland, Taiwan, Singapore, Korea)
b. 84 CP case-parent trios from four populations (Maryland, Taiwan, Singapore and Korea)
Analysis of CL/P trios
Graphical Assessment of Sliding Windows (GrASP)31 was used to summarize statistical evidence of linkage and LD for individual SNPs and sliding windows of 2-5 SNPs (see Supplemental Figure 3). Markers in MSX1 reached the 5% significance level in CL/P trios from 3 of 4 of populations (MD, TW & KR). Among MD CL/P trios, four SNPs (rs6826372, rs10937678, rs2279252 and rs1031919) in EVC included in several haplotypes of different sizes gave p-value≤0.001. These were found at the 3’ end of a region of 16 SNPs where several haplotypes had p-values<0.05 (Supplemental Figure 3/Supplemental Table 3). SNP rs1031919 had a p-value=0.0495 in single SNP analysis in SP trios, and the first three SNPs were part of a 5-SNP haplotype in the TW samples yielding p=0.0098, indicating some consistency of signal for these SNPs. The MD samples also had five other regions showing 0.001<p<0.01 (Supplemental Table 3).
SP trios had one 5-SNP haplotype near the 3’ end of STK32B (rs6856163, rs12511127, rs7434429, rs7672067 and rs7440723) which gave p=0.00087. The first four of these SNPs were involved in a 4 SNP haplotype in the MD samples giving p=0.029, but none was significant in any other population, nor was there much signal around this haplotype in SP samples (Supplemental Figure 3).
While there were no haplotypes with p<0.001 in the TW samples, two fell in between 0.01–0.001. A 5-SNP haplotype in the intergenic region between ZNF509 and D4S234E (consisting of rs7692312, rs7698300, rs4689340, rs1212085 and rs4318596) gave p=0.00263. This same haplotype was also significant in MD trios (p=0.00498), and overlapped the last two SNPs of another significant 5-SNP haplotype (p=0.00801, Supplemental Table 3). None of these SNPs, however, were significant in either SP or KR trios. Among the KR CL/P trios, one 5-SNP haplotype (rs4450871, rs4522817, rs13117014, rs12171324, rs4689973) reached p<0.001. This haplotype is just upstream from C17. The last three of these SNPs were included in a 5-SNP haplotype yielding p=0.01775 among the TW trios (Supplemental Figure 3), but these SNPs were not significant in MD or SP trios.
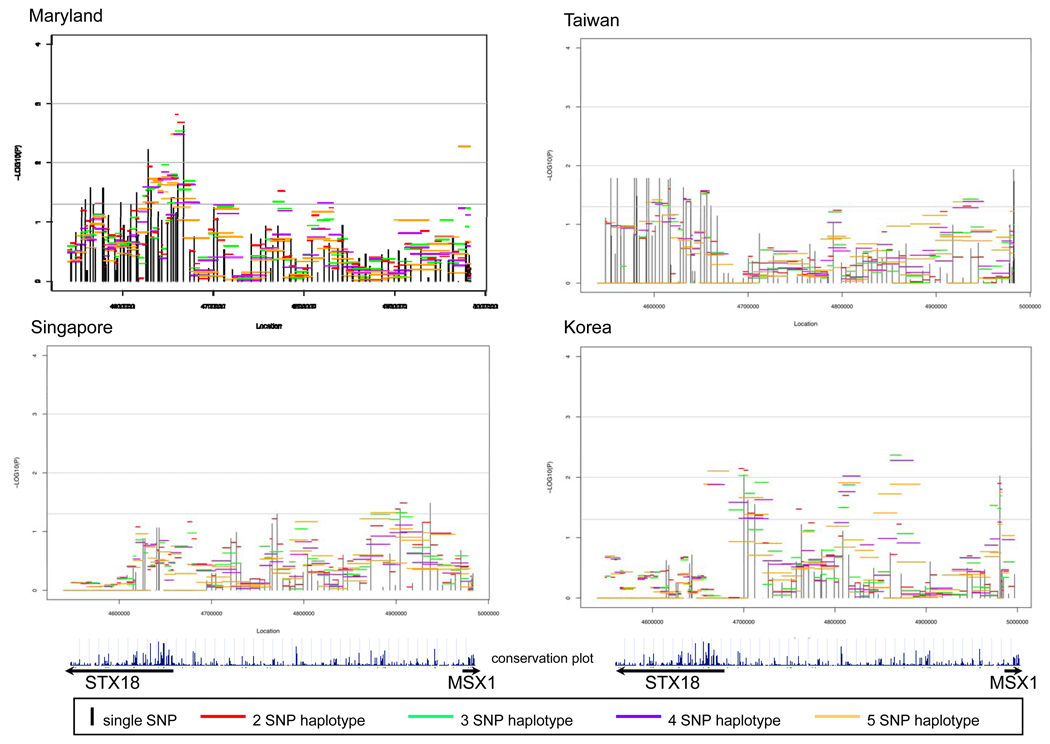
Figure 2 summarizes evidence from analysis of individual SNPs and haplotypes for STX18, MSX1 and the intergenic region between them. Here −log10(p-value) is plotted against physical location for 99 SNPs separately for each population. In these plots, vertical lines represent the strength of evidence from individual SNPs and horizontal lines represent strength of evidence from haplotypes of 2-5 SNPs. Of these 99 SNPs, four had MAF <0.05 in all four populations and therefore contributed little information (Supplemental Table 1). Among 95 SNPs polymorphic in one or more populations, the number of fully polymorphic SNPs (MAF>0.05) varied by population: MD had 94 SNPs, TW 74, SP 80, and KR 76. Among CL/P trios (Figure 2), MD and TW showed a consistent signal in STX18. In MD trios, haplotypes near the 5’ end of this gene showed the strongest signal, while among TW trios, individual SNPs yielded fairly consistent signals but haplotypes did not provide additional evidence due to strong LD. All four populations showed some signal in the intergenic region, but its location was not consistent across populations. Three populations (MD, TW & KR) gave statistical evidence just upstream of MSX1, but none within the gene itself.
Figure 2.
Significance of individual SNPs and sliding window haplotypes in STX18 and MSX1 for CL/P trios from 4 populations. Vertical lines represent −log10(p-value) for individual SNPs and horizontal lines represent −log10(p-value) for haplotypes of 2-5 SNPs over the entire region. The conservation plot indicates percent conservation of the sequence across vertebrates, as calculated by the UCSC genome browser web-site29. Arrows below indicate the direction of transcription of each gene.
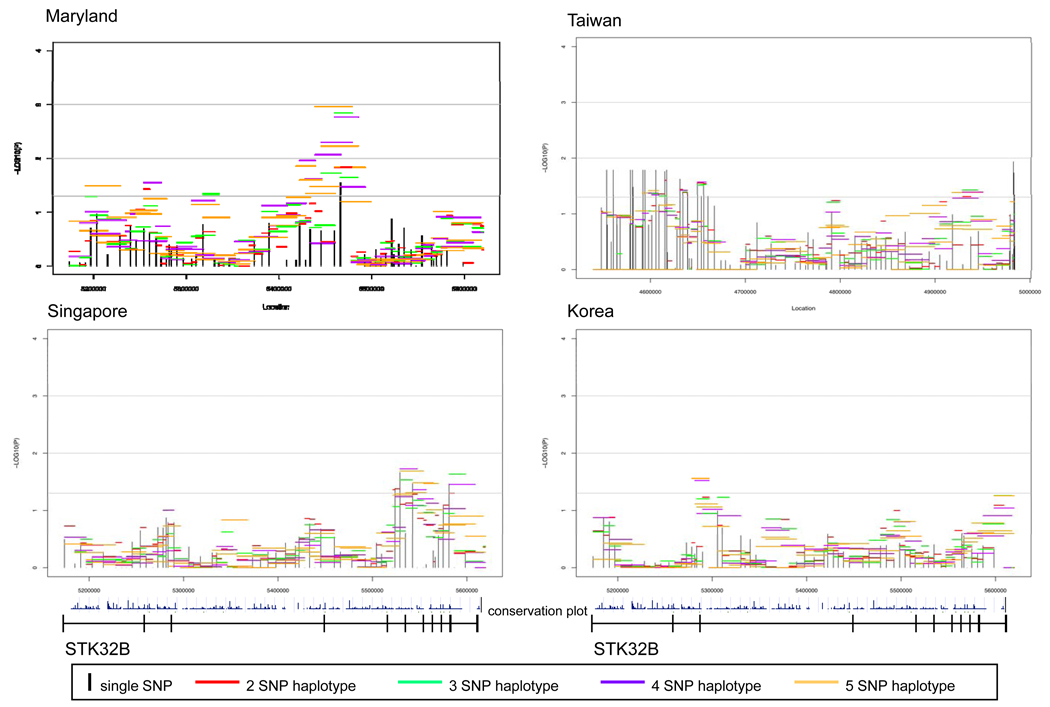
Sixty-eight SNPs were successfully genotyped in STK32B, but four were effectively monomorphic (MAF<0.05, Supplemental Table 1). Again in this gene, the number of SNPs with a MAF>0.05 varied by population: MD had 59; TW 62; SP 63; and KR 62. As seen in Figure 3, CL/P trios had at least one region in STK32B yielding evidence of linkage and association at the 5% significance level from all populations although the location of signal varied. The MD CL/P trios had the strongest signal, located in and around exon 4.
Figure 3.
Significance of individual SNPs and sliding window haplotypes in STK32B in CL/P trios from 4 populations. Vertical lines represent −log10(p-value) for individual SNPs and horizontal lines represent −log10(p-value) for haplotypes of 2-5 SNPs over the gene. The gene is transcribed from left to right in these figures. The conservation plot indicates percent conservation of the sequence across vertebrates, as calculated by the UCSC genome browser web-site29. Exons are indicated by vertical lines on the line below the plots.
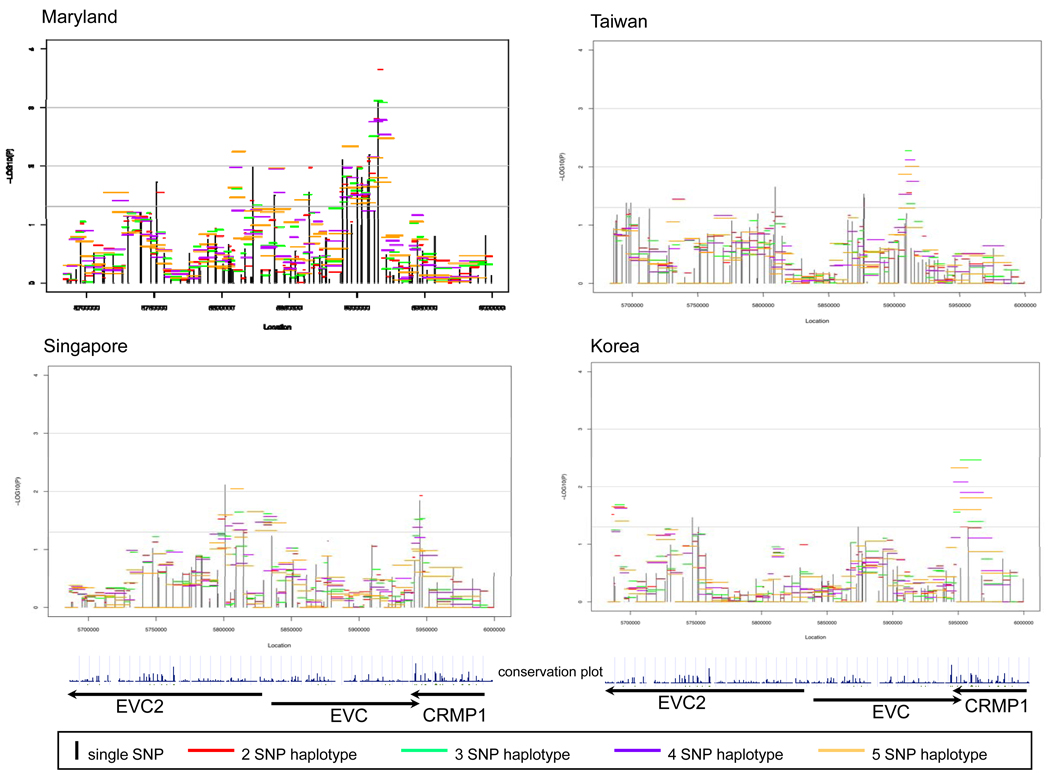
The EVC2-EVC-CRMP1 gene cluster included 100 polymorphic SNPs (of 106 tested, Supplemental Table 1) and showed evidence of linkage and association, though once again the location of statistical signals varied by population (Figure 4). The number of polymorphic SNPs varied by population: MD had 92, TW 85, SP 85, and KR 81. MD CL/P trios gave the strongest signal near the 3’ end of EVC. Interestingly, there was also a strong signal at the junction between the 3’ end of EVC and the 3’ end of CRMP1 among both SP and KR CL/P trios. Among SP CL/P trios, the strongest signal was in EVC2. Within this gene cluster, screening individual SNPs revealed an intriguing pattern of stronger transmission distortion when alleles were inherited from fathers as opposed to mothers. Supplemental Table 4 shows allelic TAT analysis separately for maternal and paternal transmission at 41 informative SNPs in all CL/P trios. Fifteen SNPs in EVC2 showed statistically significant results for the TAT when paternally transmitted, but only two among the maternally inherited alleles. Further analysis of haplotypes in EVC2 using the FAMHAP program showed consistent evidence of over-transmission from fathers but not from mothers, with greater evidence provided among Asian trios. For example, considering 2 SNP haplotypes of rs6446384 and rs4689273 (which were not in the same LD block) showed significant over-transmission of paternally derived haplotypes (maximum TDT=6.821, empiric p-value=0.0145 among all Asian trios and maximum TDT=1.78, empiric p-value=0.389 among MD trios). Because this distortion of transmission patterns was seen among paternally derived alleles and haplotypes, it raises the possibility of imprinting at this gene.
Figure 4.
Significance of individual SNPs and sliding window haplotypes in EVC2, EVC and CRMP1 in CL/P trios from 4 populations. Vertical lines represent −log10(p-value) for individual SNPs and horizontal lines represent −log10(p-value) for haplotypes of 2-5 SNPs over the entire region. Arrows indicate direction of transcription. The conservation plot indicates percent conservation of the sequence across vertebrates, as calculated by the UCSC genome browser web-site29.
Analysis of CP trios
Although the number of CP trios was smaller (Table 1), similar analyses yielded some evidence of linkage and LD. MD CP trios gave evidence in MSX1 and at the 5’ end of STK32B, as well as near the 3’ end of EVC (data not shown). The SP trios showed some signal in STK32B, near both ends of EVC and in the intergenic region between EVC and EVC2 (data not shown). TW trios on the other hand, showed strong signal in the region from LYAR through ZNF509 and the intergenic region between STX18 and MSX1. There was also some signal near MSX1 and EVC2 (data not shown).
Discussion
Based on multiple reports of association between markers in MSX1 and oral clefts and few reports of causal mutations identified in this gene, we examined the region around MSX1 for evidence of linkage and disequilibrium using case-parent trios identified through a case with an isolated, non-syndromic oral clefts recruited in an international study. Here we report results from a fine mapping panel of 393 SNPs extending from physical position 4 Mb to 6 Mb on chromosome 4p16, covering 1 Mb on each side of MSX1.
We found evidence of linkage in the presence of disequilibrium in several genes and intergenic regions, raising the possibility multiple genes in this region may influence risk of oral clefts. Three gene regions are of particular interest because of their consistent evidence in case-parent trios from four populations for both CL/P and CP: STK32B, the EVC/EVC2/CRMP1 region, and the STX18/MSX1 region.
EVC [Entrez gene ID 2121]9 and EVC2 [Entrez gene ID 132884]9 are novel and intriguing candidate genes for oral clefts. Mutations in these either of these genes can lead to Ellis-Van Creveld syndrome or Weyers acrofacial dysostosis. Ellis-Van Creveld syndrome is an autosomal recessive Mendelian syndrome not usually associated with oral clefts, but there have been reports of cases with “partial hare-lip” 32, fully erupted teeth at birth32, tooth agenesis33 or dental abnormalities34. Weyers acrofacial dysostosis is associated with anomalies of the lower jaw, dentition and oral vestibule35. EVC and EVC2 are arranged head to head on the chromosome, separated by less than 3kb, and appear to have a common promoter sequence. EVC encodes a protein containing a leucine–zipper and a transmembrane domain. EVC2 also has a transmembrane domain, but is not homologous to EVC. These genes are less than one megabase from marker D4S431, the locus where Hecht et. al. found evidence suggestive of linkage36. Both are expressed in the skeleton, but their function is unknown. As these genes may influence development of the mouth and jaw, and it seems reasonable to look for possible causal mutations, given the consistency of signal and their proximity to MSX1. Several SNPs in EVC2 (and their haplotypes) showed intriguing evidence of paternal transmission distortion, raising the possibility of imprinting in this region.
STX18 [Entrez gene ID 15942]9 is a SNAP receptor protein that functions in the endoplasmic reticulum, intermediate compartment and cis-Golgi vesicle trafficking37. It has been implicated in cell apoptosis through its binding of BNIP138. Down regulation of STX18 expression is also associated with growth of human breast cancer cells39. Cell growth regulation could be important in the etiology of oral clefts, as apoptosis must occur in the embryonic nasal fin for the developing lip to form properly40. Programmed cell death also occurs at several other stages in palate development41. Improper timing and/or failure of apoptosis at any of these developmental stages could create an oral cleft. A recent analysis of higher rates of cancer among families ascertained through an isolated oral cleft has been documented42, and the tumor suppressor gene TP63 is also a candidate gene for clefting43, which makes the association between STX18 and both cancer and clefting intriguing.
STK32B [Entrez gene ID 55351]9 is a member of the serine/threonine kinase family. This family is involved in phosphorylation and ATP binding. Its specific function in the cell is currently unknown, making it difficult to speculate on its potential role in clefting. Interestingly, MSX1 is the only one of these genes well conserved among vertebrates44 (see figures 2–4). Our statistical evidence of linkage and disequilibrium did not correlate with regions of conservation.
The issue of multiple comparisons is never simple when analyzing multiple SNPs and haplotypes in a small physical region. Since SNPs in strong LD generate highly correlated p-values, adjusting significance levels using a Bonferroni correction would be overly conservative. In our analysis, we used empirical p-values whenever possible, and relied on replication across the four populations to build valid inferences. We acknowledge confirmation from additional studies is still needed, and any study design based on LD between markers and unobserved causal variant(s) will have limited power to detect multiple rare variants that could also be important. Nonetheless, this analysis contributes to the evidence that genes in the 4p16 region influence risk to oral clefts, and suggests MSX1 may not be the only causal gene. Since none of the novel genes yielding intriguing evidence of linkage and disequilibrium (STK32B, EVC, EVC2 and STX18) have been tested for association with oral clefts before, it is critical to confirm this evidence of association in future studies.
Supplementary Material
Supplemental Figure 1: Map of genes in chromosomal region 4p16 from 4,000,000 bp to 6,000,000 bp, according to NCBI genome build 35. Arrows indicate direction of transcription.
Supplemental Figure 2: Linkage disequilibrium in all 4 populations on chromosome 4p16 among parents of CL/P and CP children. Genes are indicated along the top of the figure, separated by vertical hash marks.
Supplemental Figure 3: GrASP20 “heat-map” display of p-values over 2 Mb region of chromosome 4p16 in 297 CL/P trios from Maryland, Taiwan, Singapore, and Korea. White cells indicate SNPs or haplotypes where there was insufficient information to calculate empiric p-values; green cells represent non-significant p-values; pale green cells represent marginally significant p-values (0.10>p>0.05), yellow represents 0.05>p>0.01, orange represents 0.01>p>0.001 and red represents 0.001>p. Corresponding p-values for individual SNPs are listed to the right of the heat-map, and the smallest p-value from sliding windows of haplotypes of size 2-5 SNPs are listed next. Individual SNP names and the corresponding gene/locus name are listed in the rightmost column. Known genes are listed at the far right of the diagram, along with ‘rs’ numbers, in map order. To the right of each population is the most significant p-value for that SNP in any haplotype. The second column to the right of each diagram indicates the p-value from single point analysis.
Acknowledgements
We gratefully acknowledge all participants and staff at each institution who contributed data for this project. We would like to thank the Johns Hopkins SNP Center for carrying out the genotyping. This work was supported by NIH R01-DE-01448 (Beaty, PI) and NIH R01-DE-013939 (Scott, PI).
References
- 1.Marazita ML, Lidral AC, Murray JC. Genome Scan, Fine-Mapping, and Candidate Gene Analysis of Non-Syndromic Cleft Lip with or without Cleft Palate Reveals Phenotype-Specific Differences in linkage and association results. Hum Hered. 2009;68:151–170. doi: 10.1159/000224636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mossey PA, Little J. Epidemiology of oral clefts: an international perspective. In: Wysnynski DF, editor. Cleft Lip and Palate. 2002. pp. 127–158. [Google Scholar]
- 3.Saal HM. In: Classification and description of nonsyndromic clefts. Wysnynski DF, editor. New York: Cleft Lip and Palate; 2002. pp. 47–52. [Google Scholar]
- 4.Jugessur A, Murray JC. Orofacial clefting: recent insights into a complex trait. Curr Opin Genet Dev. 2005;15(3):270–278. doi: 10.1016/j.gde.2005.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes C. In: Environmental risk factors and oral clefts. Wysnynski DF, editor. New York: Cleft Lip and Palate; 2002. pp. 159–168. [Google Scholar]
- 6.Marazita ML, Neiswanger K. Association studies. New York: Cleft Lip and Palate; 2002. pp. 240–253. [Google Scholar]
- 7.Vieira AR. Unraveling Human Cleft Lip and Palate Research. J Dent Res. 2008;87(2):119–125. doi: 10.1177/154405910808700202. [DOI] [PubMed] [Google Scholar]
- 8.Shi M, Mostowska A, Jugessur A, et al. Identification of microdeletions in candidate genes for cleft lip and/or palate. Birth Defects Res A Clin Mol Teratol. 2009;85(1):42–51. doi: 10.1002/bdra.20571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Entrez Gene. http://www.ncbi.nlm.nih.gov/entrez/
- 10.Blin-Wakkach C, Lezot F, Ghoul-Mazgar S, et al. Endogenous Msx1 antisense transcript: in vivo and in vitro evidences, structure, and potential involvement in skeleton development in mammals. Proc Natl Acad Sci U S A. 2001;98(13):7336-4. doi: 10.1073/pnas.131497098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sherry ST, Ward MH, Kholodov M, et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001;29(1):308–311. doi: 10.1093/nar/29.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Boogaard MJ, Dorland M, Beemer FA, et al. MSX1 mutation is associated with orofacial clefting and tooth agenesis in humans. Nat Genet. 2000;24(4):342–343. doi: 10.1038/74155. Erratum in: Nat Genet; 25(1): 125. [DOI] [PubMed] [Google Scholar]
- 13.Marazita ML, Field LL, Cooper ME, et al. Nonsyndromic cleft lip with or without cleft palate in China: assessment of candidate regions. Cleft Palate Craniofac J. 2002;39(2):149–156. doi: 10.1597/1545-1569_2002_039_0149_nclwow_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 14.Park J, Park BY, Kim HS, et al. MSX1 polymorphism associated with risk of oral cleft in Korea: evidence from case-parent trio and case control studies. Yonsei Med J. 2007;48(1):101–108. doi: 10.3349/ymj.2007.48.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tongkobpetch S, Siriwan P, Shotelersuk V, et al. MSX1 mutations contribute to nonsyndromic cleft lip in a Thai population. J Hum Genet. 2006;51(8):671–676. doi: 10.1007/s10038-006-0006-4. [DOI] [PubMed] [Google Scholar]
- 16.Otero L, Gutiérrez S, Cháves M, Vargas C, Bérmudez L. Association of MSX1 with nonsyndromic cleft lip and palate in a Colombian population. Cleft Palate Craniofac J. 2007;44(6):653–656. doi: 10.1597/06-097.1. [DOI] [PubMed] [Google Scholar]
- 17.Fallin MD, Hetmanski JB, Park J, et al. Family-based analysis of MSX1haplotypes for association with oral clefts. Genet Epidemiol. 2003;25(2):168–175. doi: 10.1002/gepi.10255. [DOI] [PubMed] [Google Scholar]
- 18.Jezewski PA, Vieira AR, Nishimura C, et al. Complete sequencing shows a role for MSX1 in non-syndromic cleft lip and palate. J Med Genet. 2003;40(6):399–407. doi: 10.1136/jmg.40.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shih JY, Yang SC, Hong TM, Yuan A, Chen JJ, Yu CJ, Chang YL, Lee YC, Peck K, Wu CW, Yang PC. Collapsin response mediator protein-1 and the invasion and metastasis of cancer cells. J Natl Cancer Inst. 2001;93(18):1392–1400. doi: 10.1093/jnci/93.18.1392. [DOI] [PubMed] [Google Scholar]
- 20.Bellus GA, Hefferon TW, Luna RIO, et al. Achondroplasia is defined by recurrent G380R mutations of FGFR3. Am J Hum Genet. 1995;56:368–373. [PMC free article] [PubMed] [Google Scholar]
- 21.Oliphant A, Barker DL, Stuenlpnagel JR. BeadArray™ Technology: Enabling an accurate, cost-efficient approach to high-throughput genotyping. Biotechniques. 2002;32:S56–S61. [PubMed] [Google Scholar]
- 22.Fan JB, Oliphant A, Shen R. Highly parallel SNP genotyping. Cold Spring Harb Symp Quant Biol. 2003;68:69–78. doi: 10.1101/sqb.2003.68.69. [DOI] [PubMed] [Google Scholar]
- 23.Laird NM, Horvath S, Xu X. Implementing a unified approach to family-based tests of association. Genet Epidem. 2000;19(Suppl 1):S36–S42. doi: 10.1002/1098-2272(2000)19:1+<::AID-GEPI6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 24.Rabinowitz D, Laird N. A unified approach to adjusting association tests for population admixture with arbitrary pedigree structure and arbitary missing marker information. Hum Hered. 2000;50:211–223. doi: 10.1159/000022918. [DOI] [PubMed] [Google Scholar]
- 25.Stata Corporation. Intercooled Stata for Mac, version 8.2. College Station TX: 2005. [Google Scholar]
- 26.Weinberg CR. Methods for detection of parent-of-origin effects in genetic studies of case-parents triads. Am J Hum Genet. 1999;65:229–235. doi: 10.1086/302466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knapp M, Becker T. Family-based association analysis with tightly linked markers. Hum Hered. 2003;56:2–9. doi: 10.1159/000073727. [DOI] [PubMed] [Google Scholar]
- 28.Becker T, Knapp M. Maximum-likelihood estimation of haplotype frequencies in nuclear families. Genet Epidemiol. 2004;27:21–32. doi: 10.1002/gepi.10323. [DOI] [PubMed] [Google Scholar]
- 29.Becker T, Baur MP, Knapp M. Detection of parent-of-origin effects in nuclear families using haplotype analysis. Hum Hered. 2006;62:64–76. doi: 10.1159/000095942. [DOI] [PubMed] [Google Scholar]
- 30.Barrett JC, Fry B, Maller J, et al. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 31.Mathias RA, Gao P, Goldstein JL, et al. A graphical assessment of p-values from sliding window haplotype tests of association to identify asthma susceptibility loci on chromosome 11q. BMC Genet. 2006;7(1):38. doi: 10.1186/1471-2156-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKusick VA, Egeland JA, Eldridge R, Krusen DE. Dwarfism in the Amish. The Ellis-van Creveld syndrome. Bull. Johns Hopkins Hosp. 1964;115:306–336. [PubMed] [Google Scholar]
- 33.Digilio MC, Marino B, Giannotti A, Dallapiccola B. Single atrium, atrioventricular canal/postaxial hexodactyly indicating Ellis-van Creveld syndrome. Hum. Genet. 1995;96:251–253. doi: 10.1007/BF00207395. [DOI] [PubMed] [Google Scholar]
- 34.Spranger S, Tariverdian G. Symptomatic heterozygosity in the Ellis-van-Creveld syndrome? Clin. Genet. 1995;47:217–220. doi: 10.1111/j.1399-0004.1995.tb03963.x. [DOI] [PubMed] [Google Scholar]
- 35. [accessed October 31];Online Mendelian Inheritance in Man, Weyers Acrofacial Dysostosis [193530] 2006 http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=193530.
- 36.Hecht JT, Mulliken JB, Blanton SH. Evidence for a Cleft Palate only locus on Chromosome 4 near MSX1. Am J Med Genet. 2002;110:406–407. doi: 10.1002/ajmg.10497. [DOI] [PubMed] [Google Scholar]
- 37.Hatsuzawa K, Hirose H, Tani K. Syntaxin 18, a SNAP receptor that functions in the endoplasmic reticulum, intermediate compartment, and cis-Golgi vesicle trafficking. J Biol Chem. 2000;275(18):13713–13720. doi: 10.1074/jbc.275.18.13713. [DOI] [PubMed] [Google Scholar]
- 38.Nkajima K, Hirose H, Taniguchi M, et al. Involvement of BNIP1 in apoptosis and endoplasmic reticulum membrane fusion. EMBO J. 2004;23(16):3216–3226. doi: 10.1038/sj.emboj.7600333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bassett T, Harpur B, Poon HY, et al. Effective stimulation of growth in MCF-7 human breast cancer cells by inhibition of syntaxin18 by external guide sequence and ribonuclease P. Cancer Lett. 2008;272(1):167–175. doi: 10.1016/j.canlet.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Sperber G. In: Formation of the primary palate. Wysnynski DF, editor. New York: Cleft Lip and Palate; 2002. pp. 6–7. [Google Scholar]
- 41.Sperber G. In: Palatogenesis: closure of the secondary palate. Wysnynski DF, editor. New York: Cleft Lip and Palate; 2002. pp. 14–23. [Google Scholar]
- 42.Menezes R, Marazita ML, Goldstein McHenry T, Cooper ME, Bardi K, Brandon C, Letra A, Martin RA, Vieira AR. AXIS inhibition protein 2, orofacial clefts and a family history of cancer. J Am Dent Assoc. 2009;140(1):80–84. doi: 10.14219/jada.archive.2009.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. [accessed June 21];Online Mendelian Inheritance in Man, Orofacial Cleft 1 [119530] 2009 http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=119530.
- 44.Karolchik D, Baertsch R, Diekhans M, et al. The UCSC Genome Browser Database. Nucl. Acids Res. 2003;31(1):51–54. doi: 10.1093/nar/gkg129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Map of genes in chromosomal region 4p16 from 4,000,000 bp to 6,000,000 bp, according to NCBI genome build 35. Arrows indicate direction of transcription.
Supplemental Figure 2: Linkage disequilibrium in all 4 populations on chromosome 4p16 among parents of CL/P and CP children. Genes are indicated along the top of the figure, separated by vertical hash marks.
Supplemental Figure 3: GrASP20 “heat-map” display of p-values over 2 Mb region of chromosome 4p16 in 297 CL/P trios from Maryland, Taiwan, Singapore, and Korea. White cells indicate SNPs or haplotypes where there was insufficient information to calculate empiric p-values; green cells represent non-significant p-values; pale green cells represent marginally significant p-values (0.10>p>0.05), yellow represents 0.05>p>0.01, orange represents 0.01>p>0.001 and red represents 0.001>p. Corresponding p-values for individual SNPs are listed to the right of the heat-map, and the smallest p-value from sliding windows of haplotypes of size 2-5 SNPs are listed next. Individual SNP names and the corresponding gene/locus name are listed in the rightmost column. Known genes are listed at the far right of the diagram, along with ‘rs’ numbers, in map order. To the right of each population is the most significant p-value for that SNP in any haplotype. The second column to the right of each diagram indicates the p-value from single point analysis.