Abstract
Cellular senescence is a complex stress response that induces an essentially permanent cell cycle arrest and a complex secretory phenotype termed the senescence-associated secretory phenotype (SASP), which drives numerous aging pathologies. Characterization of the SASP can provide insights into aging and disease mechanisms, aging biomarker candidates and targets for counteracting the deleterious effects of senescent cells. Here, we describe a mass spectrometry (MS)-compatible protocol to i) generate senescent cells using different stimuli, ii) collect conditioned media containing proteins secreted by senescent cells (SASP), and iii) prepare the SASP for quantitative proteomic analysis using data-independent acquisition (DIA) MS.
Basic Protocol 1: Generating ionizing radiation-induced senescent cells and control cells
Alternate Protocol 1A: Generating doxorubicin-induced senescent and control cells
Alternate Protocol 1B: Generating oncogenic Ras-induced senescent cells and control cells
Alternate Protocol 1C: Generating mitochondrial dysfunction-induced senescent and control cells
Alternate Protocol 1D: Generating atazanavir-induced senescent and control cells
Support Protocol 1: A multiple-assay approach to confirm the phenotype of senescent cells
Basic Protocol 2: Generating conditioned media from senescent cells cultured in low serum and quiescent control cells
Alternate Protocol 2: Generating conditioned media from senescent cells cultured in complete media and quiescent control cells
Basic Protocol 3: Quantitative proteomic analysis of the SASP
Keywords: senescence, secretome, mass spectrometry, data-independent acquisition, aging
INTRODUCTION
Cellular senescence is a complex stress response that leads to an exit from the cell cycle that is essentially permanent, although it can in some cases be overcome by genetic or pharmacological manipulation in culture (Beausejour et al., 2003; Lee and Schmitt, 2019). It is not known whether senescence reversal occurs in vivo. Most dividing cell types can become senescent, and subsequently influence both physiological and pathological processes (Campisi and d’Adda di Fagagna, 2007; Gorgoulis et al., 2019). Mouse models and human cell and tissue experiments show that senescent cells can contribute to frailty and many age-related conditions, including type II diabetes, pulmonary fibrosis and cancer, making cellular senescence a key driver of aging (Campisi, 2013; Munoz-Espin and Serrano, 2014). Many biological effects (both beneficial and detrimental) that senescent cells exert on organisms are triggered by their secretome, termed Senescence-Associated Secretory Phenotype (SASP) (Coppe et al., 2008).
The SASP is a complex mixture of biologically active molecules, including lipids, metabolites and proteins (Basisty et al., 2020a; Coppe et al., 2008; Schafer et al., 2020). Among the SASP factors are inflammatory cytokines, growth factors, hormones, proteases, hemostasis factors (Wiley et al., 2019) and many other still unknown components. Interestingly, senescent cells release not only soluble metabolites, lipids and proteins, but also extracellular vesicles, including exosomes (Basisty et al., 2020a; Borghesan et al., 2019; Jeon et al., 2019). Overall, the SASP is extremely heterogeneous and varies depending on cell type and the senescence-inducing stimulus (Basisty et al., 2020a; Ozcan et al., 2016).
To add even more complexity, the SASP is very dynamic and changes significantly with time after the initial senescence induction (Basisty et al., 2020a; James et al., 2018). With newer technological developments we are now able to use proteomic unbiased discovery workflows to gain deeper insights into highly complex SASP profiles (Basisty et al., 2020b), and are only beginning to appreciate their true heterogeneity. A better characterization of the SASP is of importance, as it will allow us to better understand the biological effects of cellular senescence, as well as offer new potential biomarkers and therapeutic targets that could be key tools in the fight against age-related diseases.
Given the vast number and heterogeneity of protein components in the SASP, quantitative and comprehensive mass spectrometry-based proteomic approaches, such as data-independent acquisition (DIA) mass spectrometry (MS), are excellent techniques for unbiased profiling of the SASP. This protocol will guide researchers in the generation and validation of cultured senescent cells, collection and processing of conditioned medium containing the SASP, and discovery and quantification of SASP proteins using a comprehensive DIA-MS-based acquisition workflow (Collins et al., 2017; Gillet et al., 2012; Schilling et al., 2017). These SASP isolation protocols are also compatible with other downstream quantitative mass spectrometric methods, such as data-dependent acquisition (DDA) MS.
Protocols List
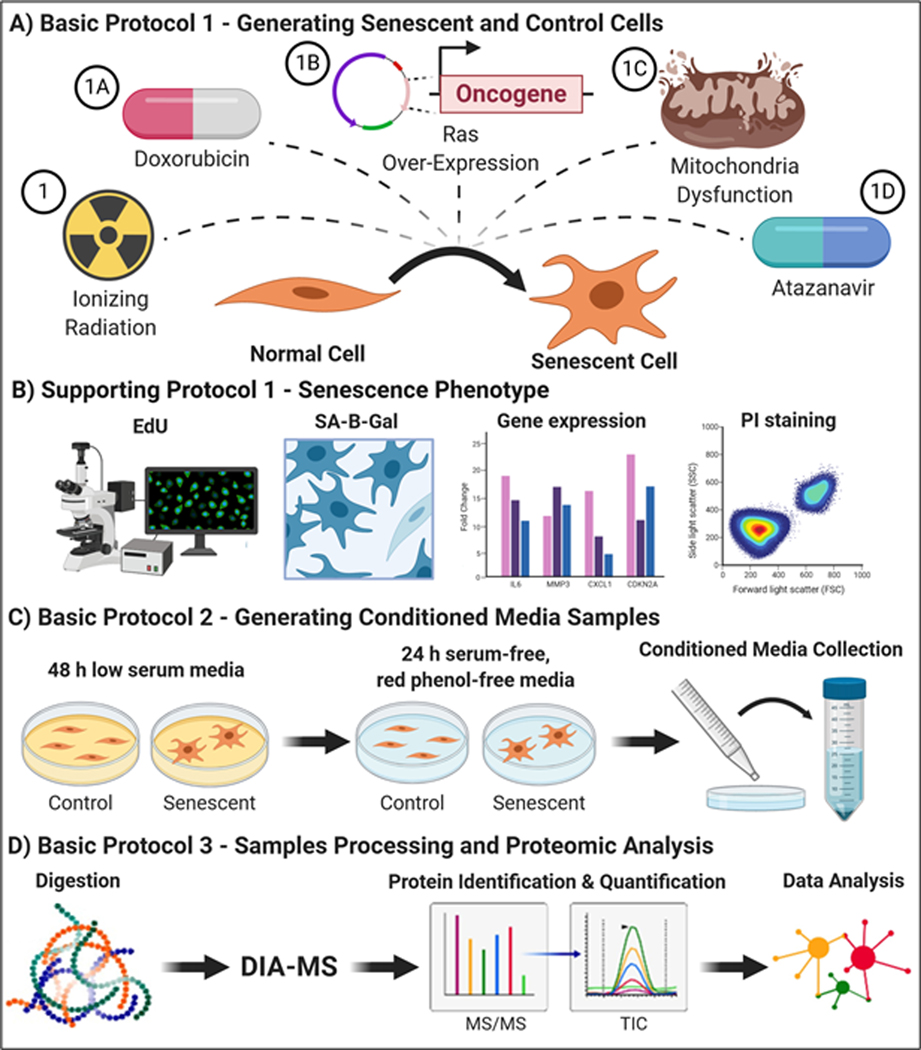
See Figure 1 for a schematic of the protocols. Basic Protocol 1 and its Alternate Protocols 1A-D describe in detail the preparation of senescent cells and appropriate control cells using some of the most common senescence inducers: ionizing radiation (Basic Protocol 1), the chemotherapeutic drug doxorubicin (Alternate Protocol 1A), oncogenic RAS overexpression (Alternate Protocol 1B), mitochondrial disruption (Alternate Protocol 1C) and the HIV protease inhibitor atazanavir (Alternate Protocol 1D).
Figure 1.
Overview of the senescence induction protocols, collection of conditioned media and proteomics analysis.
Support Protocol 1 describes assays to check for the presence of senescence-associated markers and confirm successful senescence induction.
Basic Protocol 2 and Alternate Protocol 2 describe the procedure for generating and collecting conditioned media (CM) from senescent and quiescent control cells.
Basic Protocol 3 describes the proteomic workflow that we recommend to identify and quantify the SASP proteins secreted by senescent cells in culture, including sample processing, mass spectrometric acquisition by DIA-MS, and analysis of raw mass spectrometric data files for protein identification and quantification.
STRATEGIC PLANNING
Each researcher will choose a specific senescence inducer and version of Protocol 1 to use based on the best models for the biological system and context under investigation. For example, if the objective is to identify SASP factors involved in the side-effects of chemotherapy, then the doxorubicin-induced senescence protocol below (Alternate Protocol 1A) may be the most appropriate approach. Another important factor in deciding which senescence inducer to use is the availability of materials/instrumentation to perform senescence induction. For example, senescence induction using oncogenic RAS overexpression requires having cells harboring an inducible vector such as pLVX-RASV12, while induction of senescence by ionizing radiation requires access to an x-ray generator. The same rationale applies to the decision about which cell type should be investigated. The selection of cell type will be based on what is most relevant to the tissue or disease of interest.
It should be noted that the Protocols outlined here are optimized for IMR-90 human fetal lung fibroblasts cultured at physiological O2 concentration (3% O2, 10% CO2 at 37° C), and the inducer doses and culture conditions should be individually tailored and optimized for other cell types. It should also be pointed out that the approaches described in Basic Protocol 1 and Alternate Protocols 1A-D are not the only means of senescence induction.
To generate SASP-containing and quiescent control conditioned media (CM), Basic Protocol 2 should be followed. If after senescence induction the cell type investigated does not remain viable in low-serum, then Alternate Protocol 2 should be used. Also, when using any combination of cell type and senescence induction for the first time, successful induction of senescence should be verified. Support Protocol 1 describes several assays that are commonly used to confirm senescence induction. Please note that other assays, such as assessing lack of proliferation through Ki67 staining or detecting increased lysosomal activity by staining for lipofuscin granules, could be used to confirm the presence of senescent cells (Gonzalez-Gualda et al., 2020). However, the assays listed in Support Protocol 1 are widely used, robust and easy to perform.
BASIC PROTOCOL 1 GENERATING IONIZING RADIATION-INDUCED SENESCENT AND CONTROL CELLS
The method described here allows for the generation of senescent cells by X-rays (ionizing radiation; IR) and appropriate control cells. From these two cell populations, the researcher will later generate the SASP-containing and control CM (see Basic Protocol 2 / Alternate Protocol 2). Cells are seeded at the desired density, cultured overnight, and irradiated the following day. After IR, media are replaced with fresh complete media. Then, media are switched to fresh complete media every two days. Three days before the irradiated cells reach a full senescent state, control cells are seeded and cultured overnight. These control cells are derived from the same stock culture used to seed the irradiated cells and have been cultured in parallel up to this point. The next day, control cells are mock irradiated. This protocol has been optimized for IMR-90 primary human fetal lung fibroblasts and can serve as a guide for the use of other adherent human cells.
Materials
Desired cell type
Such as human primary fibroblasts strain IMR-90 (ATCC, cat. no. CCL¬186), WI-38 (ATCC, cat. no. CCL-75), or BJ (ATCC, cat. no. CRL-2522)
Complete medium appropriate for the cell type used
When using IMR-90 fibroblasts, the complete medium composition is the following: DMEM (Gibco, cat. no. 12430–054) supplemented with 100 U/mL penicillin-streptomycin (Gibco, cat. no. 15070063) and 10% fetal bovine serum (Gibco, cat. no. 2614079)
Serum-free, phenol red-free media
When culturing IMR-90 fibroblasts, use phenol red-free DMEM (Gibco, cat. no. 21063–029) supplemented with 100 U/mL penicillin-streptomycin (Gibco, cat. no. 15070063) to avoid interferences for subsequent protein concentration measurements, such as BCA assays.
Dulbecco’s phosphate-buffered saline (PBS) (Gibco, cat. no. 21600–010)
Trypsin-EDTA (Corning, cat. no. 25–051-CI)
Incubator set at the optimal conditions of temperature and partial pressures of air gases to mimic the physiological requirement of the cell type used
IMR-90 cells are cultured at 37° C, 3% O2, 10% CO2 in HERA CELL 240i CO2 incubators (ThermoFisher, cat. no. 51026331)
T175 Tissue culture flasks (Genesee Scientific, cat. no. 25–211)
X-ray generator (X-Ray associates LLC, Polaris 320 kV X-ray Generator set)
Generation of senescent cells
-
1Seed 3 T175 flasks with 2 × 106 fibroblasts each (or other cell type of interest), using complete DMEM medium (see “Materials”); culture overnight. These cells will be used to generate SASP-containing CM. Seed (at least) 3 replicates per condition to ensure that changes observed in the MS data can be statistically analyzed.The number of cells needed to obtain enough SASP protein amount for MS analysis may vary depending on the cell type and MS instrumentation available. Ideally, the total amount of secreted proteins for each replicate should be above 50 μg. If less protein is available, the protocol should be optimized for smaller protein amounts and tested. To estimate the level of protein secretion, conduct a pilot experiment by seeding the desired number of cells, allowing them to recover overnight, then wash them twice with PBS, add serum-free, phenol red-free media, collect CM 24 h later, and perform a Bicinchoninic Acid (BCA) assay. This quantification will allow an estimation of the number of cells needed to obtain enough SASP proteins for MS analysis. Culture media used during this step must be free of serum and, as much as possible, of protein components/contaminants. This way, the protein concentration measured will reflect only the proteins secreted by the cells. Also, the CM needs to be phenol red-free because this compound interferes with the quantification of proteins using BCA.
-
2Treat fibroblasts the next day with 15 Gy IR to induce senescence and then replace the media with fresh complete media.Fibroblasts will develop a full senescent phenotype approximately 10 days later. By then, almost all fibroblasts should develop the enlarged, flattened morphology associated with senescence. The replacement of media should not wash off cells as senescent fibroblasts actually tend to stick to plates more firmly than their control counterpart. The radiation dose and time needed may vary depending on the cell type.
-
3
Replace media with fresh complete media every 2 days until day 6 (including on day 6) after IR treatment. On day 8, proceed with the “Generation of CM samples” step in Basic Protocol 2 (or Alternate Protocol 2) to prepare the cells for subsequent CM collection.
Generation of control cells
-
4
Seed flasks with control fibroblasts 7 days after IR treatment of experimental cells (that is, 3 days before the IR-treated fibroblasts reach a full senescent phenotype) and allow them to recover overnight. These cells will be used to generate control CM.
-
5
On the next day, mock irradiate the control cells by placing them into the X-ray chamber without irradiating them. Leave control cells in the X-ray chamber for as long as it took to give the irradiated cells their full dose of radiation.
-
6
After mock irradiation, proceed to Basic Protocol 2 (or Alternate Protocol 2).
ALTERNATE PROTOCOL 1A GENERATING DOXORUBICIN-INDUCED SENESCENT AND CONTROL CELLS
This method allows for the generation of senescent cells upon treatment with the chemotherapeutic drug doxorubicin and appropriate control cells. From these two cell populations, the researcher will later generate the SASP-containing and control CM (see Basic Protocol 2 / Alternate Protocol 2). Cells are seeded at the desired density, cultured overnight, and treated with doxorubicin for 24 h. Thereafter, cells are washed with PBS, fresh media is added and cells are cultured until the development of a full senescent phenotype. Four days before the doxorubicin-treated cells reach full senescence, the control cells are seeded and cultured overnight. These control cells are derived from the same stock culture used to seed the doxorubicin-treated cells and have been cultured in parallel up to this point. The next day, control cells are treated with vehicle (DMSO) for 24 h. This protocol has been optimized for IMR-90 primary human fetal lung fibroblasts and can serve as a guide for the use of other adherent human cells.
Materials
(see also Basic Protocol 1)
Doxorubicin stock solution (2.5 mM in DMSO) (see recipe details in the “Reagent and Solutions” section)
Dimethyl sulfoxide (DMSO) (MilliporeSigma, cat. no. 67–68-5)
Generation of senescent cells
-
1Seed 3 T175 flasks with 2 × 106 fibroblasts each (or other cell type) using the appropriate complete medium (see “Materials”), and culture overnight. These cells will be used to generate SASP-containing CM. Seed (at least) 3 replicates to ensure changes observed in the MS data can be statistically analyzed.The number of cells needed to obtain enough SASP proteins for MS analysis may vary depending on the cell type and MS instrumentation available. To verify that protein concentrations are sufficient for downstream MS analyses, see step 1 comment in Basic Protocol 1.
-
2
On the next day, dilute the doxorubicin stock solution (2.5 mM doxorubicin in DMSO) in complete media for a final concentration of 250 nM doxorubicin (dilution 1:10,000).
-
3Remove culture media from the flasks and then switch to doxorubicin-containing media. Incubate for 24 h to induce senescence.Fibroblasts will develop a full senescent phenotype 10 days later. By then, almost all fibroblasts should develop the enlarged, flattened morphology associated with senescence. The dose of doxorubicin and time needed may vary depending on the cell type.
-
4
After doxorubicin treatment, wash cells twice with 25 mL PBS and add fresh complete media. Then replace media every 2 days until day 6 (including on day 6) after the beginning of doxorubicin treatment. On day 8, proceed with the “Generation of CM samples” step in Basic Protocol 2 (or Alternate Protocol 2) to prepare the cells for subsequent CM collection.
Generation of control cells
-
5
Seed new flasks with fibroblasts 6 days after doxorubicin treatment (that is, 4 days before the doxorubicin-treated cells reach senescence) and allow the cells to recover overnight. These cells will be used to generate quiescent control CM.
-
6
On the next day, treat the control cells with vehicle (DMSO) for 24 h.
-
7
After treatment with vehicle DMSO, proceed to Basic Protocol 2 (or Alternate Protocol 2) to prepare the cells for subsequent CM collection.
ALTERNATE PROTOCOL 1B GENERATING ONCOGENIC RAS-INDUCED SENESCENT CELLS AND CONTROL CELLS
The method described here allows for the generation of senescent cells via oncogenic RAS overexpression and appropriate control cells. From these cell populations, the researcher will later generate SASP-containing and control CM (see Basic Protocol 2 / Alternate Protocol 2). Cells transduced with a pLVX-RASV12 vector or GFP control vector are seeded at the desired density, allowed to recover overnight, then cultured with doxycycline to induce RAS or GFP overexpression. Media are changed every 2 days with fresh complete media containing doxycycline. Three days before the RAS-overexpressing cells reach a full senescent phenotype, GFP-overexpressing control cells are re-seeded and cultured overnight. Until then, it is important to passage the control cells when appropriate (after reaching 80–90% confluency) since these cells keep proliferating. This protocol has been optimized for IMR-90 primary human fetal lung fibroblasts and can serve as a guide for the use of other adherent human cells.
Materials
(see also Basic Protocol 1)
Desired cell type transduced with the inducible vector pLVX (Lenti-X™ Tet On®Advanced Inducible Expression System; Takara Bio, cat.no. 632162) expressing either a constitutively active RASV12 oncogene or GFP (Basisty et al., 2020a).
For example, IMR-90 primary human fetal lung fibroblasts (ATCC, cat. no. CCL¬186) transduced with pLVX-RASV12 or pLVX-GFP (Basisty et al., 2020a)
Tetracycline-free FBS (Tet-free FBS) (Takara Bio, cat. no. 631105)
Doxycycline hyclate (MilliporeSigma, cat. no. D9891–5G)
Generation of senescent and control cells
- Seed 3 T175 flasks with 1.5 × 106 fibroblasts transduced with pLVX-RASV12 (RAS cells), and 3 T175 flasks with 1.5 × 106 fibroblasts transduced with pLVX-GFP (GFP cells). Use the appropriate complete medium (see “Materials”) and allow them to recover overnight. Use media containing Tet-free FBS (see “Materials”) to ensure that RASV12 or GFP is not expressed until the addition of doxycycline. These cell populations will be used to generate SASP-containing or control CM, respectively. Seed (at least) 3 replicates for each condition to ensure changes observed in the MS data can be statistically analyzed.The number of cells needed to obtain enough SASP proteins for MS analysis may vary depending on the cell type and MS instrumentation available. To verify that protein secretion is sufficient for downstream MS analyses, see step 1 comment in Basic Protocol 1.
- The next day, treat the two fibroblast populations with 1 μg/mL doxycycline, which will trigger senescence in RAS cells while serving as a control treatment for GFP cells.Fibroblasts will develop a full senescent phenotype approximately 7 days later. By then, almost all fibroblasts should develop the enlarged, flattened morphology associated with senescence. The time needed may vary depending on the cell type used.
- Replace media for both cell populations with fresh culture media containing doxycycline every 2 days until day 4 (including on day 4) after the beginning of treatment. On day 5, proceed with the “Generation of CM samples” step in Alternate Protocol 2 (or in Basic Protocol 2) to prepare the cells for subsequent CM collection. Note that RAS cells will undergo a hyper-proliferative phase before undergoing growth arrest. Importantly, IMR-90 fibroblasts induced to senescence by oncogenic RAS overexpression are not viable in low-serum. Therefore, after completing Alternate Protocol 1B, we suggest using Alternate Protocol 2 (rather than Basic Protocol 2) when using this method of senescence induction in fibroblasts.Note that when instructed to feed cells with fresh media in Alternate Protocol 2 (or Basic Protocol 2), such media must be supplemented with doxycycline. Only serum-free media given to both cell populations 24 h before CM collection (“Collection of CM” section) should be devoid of doxycycline.
ALTERNATE PROTOCOL 1C GENERATING MITOCHONDRIAL DYSFUNCTION-INDUCED SENESCENT AND CONTROL CELLS
The method described here allows for generating senescent cells by mitochondrial dysfunction (mitochondrial dysfunction-associated senescence or MiDAS) and appropriate control cells. From these cell populations, the researcher will later generate the SASP-containing and control CM (see Basic Protocol 2 / Alternate Protocol 2). Cells are seeded at the desired density, cultured overnight, and treated with either the mitochondrial complex III inhibitor antimycin A or vehicle DMSO. Media are replaced every 2 days with fresh complete media containing either antimycin A or DMSO. Three days before the antimycin A-treated cells reach a full senescent phenotype, DMSO treated cells are re-seeded and cultured overnight. Until then, it is important to passage the DMSO-treated cells when appropriate (after reaching 80–90% confluency) since these cells keep proliferating. This protocol has been optimized for IMR-90 primary human fetal lung fibroblasts and can serve as a guide for other types of adherent human cells.
Materials
(see also Basic Protocol 1)
Antimycin A from Streptomyces species. (Sigma Aldrich, cat. no. A8674–25MG)
Dimethyl sulfoxide (DMSO) (MilliporeSigma, cat. no. 67–68-5)
Generation of senescent and control cells
- Seed 6 T175 flasks (3 for antimycin A treatment, 3 for DMSO treatment) with 2 × 106 fibroblasts each using appropriate complete medium (see “Materials”), and culture overnight. These cells will be used to generate SASP-containing or control CM. Seed (at least) 3 replicates for each condition to ensure that changes observed in the MS data can be statistically analyzed.The number of cells needed to obtain enough SASP proteins for MS analysis may vary depending on the cell type and MS instrumentation available. To verify that protein secretion is sufficient for downstream MS analyses, see step 1 comment in Basic Protocol 1.
- The next day, treat fibroblasts with 250 nM antimycin A or vehicle DMSO. Cells treated with antimycin A will become senescent and generate the SASP-containing CM. Cells treated with DMSO will be used as quiescent controls to generate the control CM.Fibroblasts will develop a full senescent phenotype approximately 10 days later. By then, almost all fibroblasts should develop the enlarged, flattened morphology associated with senescence. The concentration of antimycin A and time needed may vary depending on the cell type used.
Replace media for both cell populations with fresh culture media containing antimycin A or DMSO every 2 days until day 6 (including on day 6) after the beginning of treatment. On day 8, proceed to the “Generation of CM samples” step in Basic Protocol 2 (or in Alternate Protocol 2) to prepare the cells for subsequent CM collection. Note that DMSO-treated cells will keep proliferating, so they must be passaged when appropriate (after reaching 80%–90% confluency).
To generate control cells, re-seed proliferating DMSO-treated control cells 7 days after the beginning of vehicle treatment (that is, 3 days before antimycin A-treated cells reach a full senescent phenotype). By doing so, it will be possible to obtain similar numbers of senescent and control cells when collecting their CM.
- Allow the control DMSO-treated cells to recover overnight and then, on the next day, proceed to Basic Protocol 2 (or Alternate Protocol 2) to prepare the cells for subsequent CM collection.Note that when instructed to feed cells with fresh media in Basic Protocol 2 (or Alternate Protocol 2), media must be supplemented with 250 nM antimycin A or vehicle DMSO. Only serum-free media given to both cell populations 24 h before CM collection (“Collection of CM” section) should be devoid of antimycin A or DMSO.
ALTERNATE PROTOCOL 1D GENERATING ATAZANAVIR/RITONAVIR-INDUCED SENESCENT AND CONTROL CELLS
The method described here allows for the generation of senescent cells upon treatment with the anti-HIV drugs atazanavir and ritonavir (ATV/r), as well as appropriate control cells. From these cell populations, the researcher can generate SASP-containing and control CM (see Basic Protocol 2 / Alternate Protocol 2). Cells are seeded at the desired density, cultured overnight, and treated with either ATV/r or vehicle (DMSO). Media are changed every 2 days with fresh complete media containing either ATV/r or DMSO. Three days before the ATV/r-treated cells reach a full senescent phenotype, DMSO treated cells are re-seeded and cultured overnight. Until then, it is important to passage the DMSO-treated cells when appropriate (after reaching 80–90% confluency) since these cells keep proliferating. This protocol has been optimized for IMR-90 primary human fetal lung fibroblasts and can serve as a guide for the use of other adherent human cells.
Materials
(see also Basic Protocol 1)
Atazanavir/ritonavir solution (ATV/r) (see recipe details in the “Reagent and Solutions” section)
Dimethyl sulfoxide (DMSO) (MilliporeSigma, cat. no. 67–68-5)
Generation of senescent and control cells
- Seed 6 T175 flasks (3 for ATV/r treatment, 3 for DMSO treatment) with 2 × 106 fibroblasts each using the appropriate complete medium (see “Materials”), and culture them overnight. These cells will be used to generate SASP-containing or control CM. Seed (at least) 3 replicates for each condition to ensure that changes observed in the MS data can be statistically analyzed.The number of cells needed to obtain enough SASP proteins for MS analysis may vary depending on the cell type and MS instrumentation available. To verify that protein secretion is sufficient for downstream MS analyses, see step 1 comment in Basic Protocol 1
- On the next day, treat fibroblasts with 25 μM ATV/r or vehicle DMSO. Cells treated with ATV/r will become senescent and generate SASP-containing CM. Cells treated with DMSO will be used as quiescent controls to generate the control CM.Fibroblasts will develop a full senescent phenotype approximately 14 days later. By then, almost all fibroblasts should develop the enlarged, flattened morphology associated with senescence. The concentration of ATV/r and time needed may vary depending on the cell type.
Replace media for both cell populations with fresh complete media containing either ATV/r or DMSO every 2 days until day 10 (including on day 10), and on day 12 proceed with the “Generation of CM samples” step in Basic Protocol 2 (or in Alternate Protocol 2) to prepare the cells for subsequent CM collection. Note that DMSO-treated cells will keep proliferating, so they will have to be passaged when appropriate (after reaching 80%−90% confluency).
Re-seed the proliferating DMSO-treated control cells 11 days after the beginning of vehicle treatment (that is, 3 days before ATV/r-treated cells reach full senescence). By doing so, it will be possible to obtain similar numbers of senescent and control cells when collecting CM.
- Allow the control DMSO-treated cells to recover overnight and then proceed to Basic Protocol 2 (or Alternate Protocol 2) to prepare the cells for subsequent CM collection.Note that when instructed to feed cells with fresh media in Basic Protocol 2 (or Alternate Protocol 2), such media must be supplemented with ATV/r or DMSO for the culture of senescent or control cells, respectively. Only serum-free media given to both cell populations 24 h before CM collection (“Collection of CM” section) will be devoid of ATV/r and DMSO.
SUPPORT PROTOCOL 1 A MULTIPLE-ASSAYS APPROACH TO CONFIRM THE PHENOTYPE OF SENESCENT CELLS
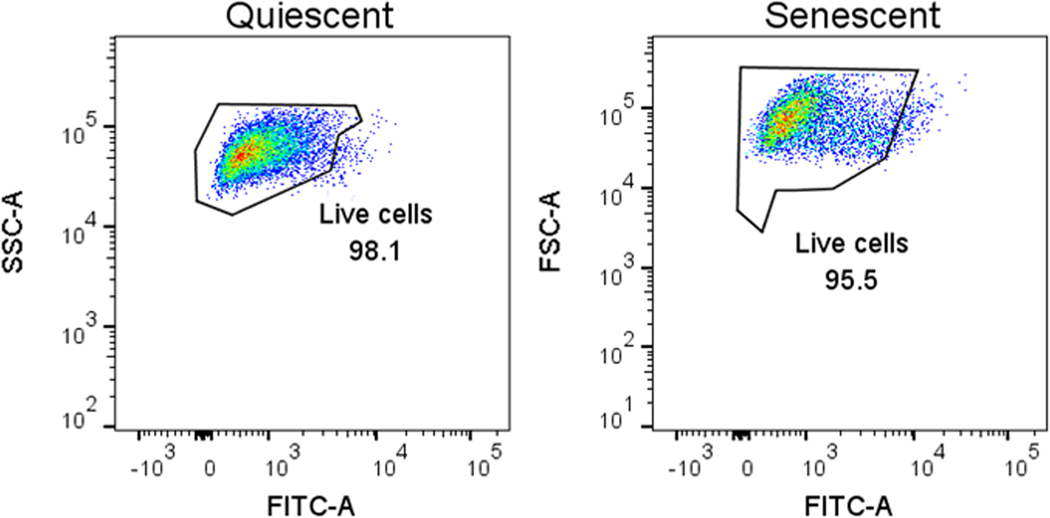
This protocol describes multiple assays to verify that the senescence induction performed in Basic Protocol 1 (or Alternate Protocol 1 versions) has been successful. Supporting Protocol 1 is used to prove that the secreted proteins to be analyzed by MS are produced by senescent cells and therefore part of a SASP. This supporting protocol should be performed when the researcher uses any combination of a senescence inducer (Basic Protocol 1 or Alternate Protocol 1 versions) and a desired cell type. These assays are not compatible with the generation of CM described in Basic Protocol 2 (or Alternate Protocol 2) because they require fewer cells compared to the number needed to analyze secreted proteins by MS. Therefore, cells should be seeded in the appropriate tissue culture dishes (specified in each of the corresponding assay protocols below) to perform the assays described, and then the chosen senescence induction method (Basic Protocol 1 or Alternate Protocol 1 versions) is performed. Once senescent and control cells are generated, the steps described below must be performed for each assay. The assays we recommend are used to verify reduced cell proliferation (see “EdU incorporation”), upregulation of senescence-associated β-galactosidase (SA-β-Gal) activity (see “Senescence-associated β-galactosidase”), and a gene transcription signature associated with senescence (see “Senescence-associated gene expression analysis by qPCR”). We also recommend performing a viability assay (see “Propidium iodide inclusion”) to verify that both senescent and control cells are healthy before generating CM for MS analysis.
Materials
(see also Basic Protocol 1 and its Alternate Protocol 1 versions)
Reverse Transcriptase (Thermo Fisher Scientific, cat. no. 43–112-35)
Serum-free media
For IMR-90 fibroblasts, use DMEM (Gibco, cat. no. 12430–054) supplemented with 100 U/mL penicillin-streptomycin (Gibco, cat. no. 15070063)
Click-iT EdU Kit Alexa Fluor 488 HCS Assay (Thermo Fisher Scientific, cat. no. C10351)
Senescence Detection Kit (BioVision, cat. no. K320–250)
X-Gal (Life Technologies, cat. no. 15520–018)
ISOLATE II RNA Micro Kit (Bioline, cat. no. BIO-52075)
Senescence-associated human genes-specific primers (Eurofins)
Upregulated genes:
For CDKN1A (p21)
Forward: TCACTGTCTTGTACCCTTGTGC
Reverse: GGCGTTTGGAGTGGTAGAAA
For CDKN2A (p16)
Forward: GAGCAGCATGGAGCCTTC
Reverse: CGTAACTATTCGGTGCGTTG
For CDKN2B (p15)
Forward: CTCCCGAAACGGTTGACTC
Reverse: GCGGGGACTAGTGGAGAAG
For CXCL1
Forward: GCTGAACAGTGACAAATCCAAC
Reverse: CTTCAGGAACAGCCACCAGT
For CXCL10
Forward: GAAAGCAGTTAGCAAGGAAAGGT
Reverse: GACATATACTCCATGTAGGGAAGTGA
For IL1B
Forward: CTGTCCTGCGTGTTGAAAGA
Reverse: TTGGGTAATTTTTGGGATCTACA
For IL6
Forward: GCCCAGCTATGAACTCCTTCT
Reverse: GAAGGCAGCAGGCAACAC
For MMP3
Forward: CAAAACATATTTCTTTGTAGAGGACAA
Reverse: TTCAGCTATTTGCTTGGGAAA
For SERPINE1
Forward: CCAGCTGACAACAGGAGGAG
Reverse: CCCATGAGCTCCTTGTACAGAT
Downregulated genes:
For LAMNB1
Forward: TTGGATGCTCTTGGGGTTC
Reverse: AAGCAGCTGGAGTGGTTGTT
Propidium iodide staining solution (eBioscience, cat. no. 00–6990-50)
8-well chamber slides Lab-Tek II (Sigma-Aldrich, cat. no. C7057–1CS)
Inverted fluorescence microscope (Olympus, IX70 Fluorescence Microscope)
6-well cell culture plates (Genesee Scientific, 25–105)
T75 Tissue culture flasks (Genesee Scientific, cat. no. 25–211)
LightCycler® 480 Instrument II (Roche, cat. no. 05015278001)
Flow cytometer (BD™ LSR II with BD FACS Diva v8.0.2 acquisition software)
EdU incorporation
-
1
Follow the steps listed in Basic protocol 1 (or in one of Alternate Protocol 1 versions) to generate senescent and control cells of the chosen cell type. For this immunostaining assay, seed 10,000 fibroblasts per well in 8-well chamber slides for each replicate.
-
2
Wash senescent and control cells twice with PBS, then switch them to media containing 0.2% FBS (instead of 10% FBS) and culture for 48 h to induce quiescence by serum starvation in control cells while maintaining viability for a few days for both cell populations.
-
3The following day (24 h after switching to low serum-containing media), seed cells in new wells using complete media and allow recovery overnight. These cells will be used as a positive control for EdU staining (proliferating control cells).Use complete media (containing 10% FBS when using fibroblasts) to keep cells in a proliferative state.
-
4The next day, remove half the volume of media in each well and replace it with fresh media containing EdU (Click-iT EdU kit) as instructed by the manufacturer’s protocol. Use serum-free media for the senescent and quiescent cells. Use complete media (10% FBS) for the proliferating control cells.For example, if each well of the 8-well chamber slide contains 250 μL of media, remove 125 μL and add 125 μL of the appropriate media (serum-free or complete media) containing EdU (see manufacturer’s protocol for the EdU concentration).
-
5
Incubate for 24 h.
-
624 h later, follow the instructions (manufacturer’s protocol) to fix, permeabilize, detect EdU incorporation and stain cellular DNA with Hoechst 33342.EdU staining is also compatible with DAPI DNA staining.
-
7
Remove all liquid from the wells, remove the well chambers, add mounting media, and then apply coverslips onto the slides (avoid air bubbles).
-
8
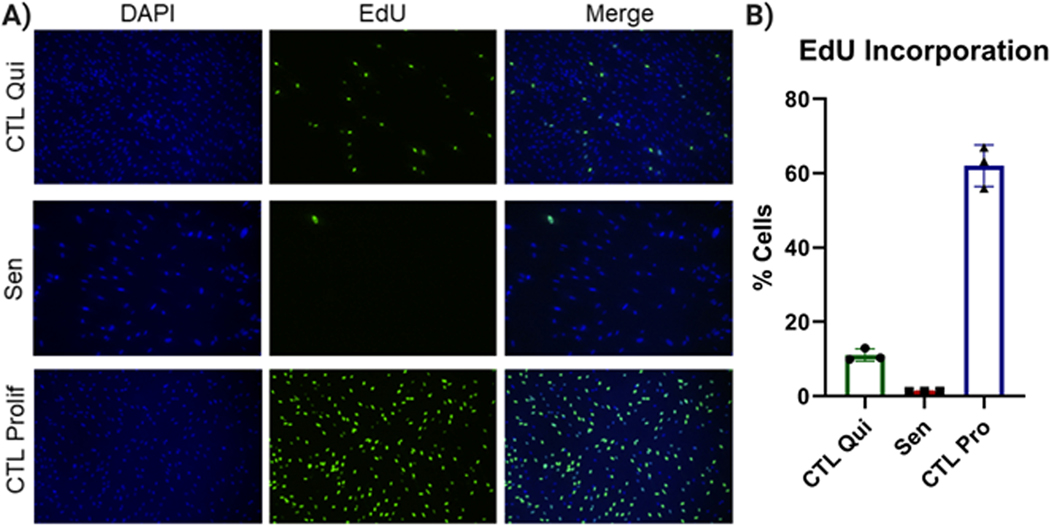
Compare DNA staining with EdU staining to confirm that senescent and quiescent control populations have low numbers of EdU positive cells (whereas proliferating control cells should have high numbers of EdU positive cells) (see Figure 2).
Figure 2.
EdU incorporation. A) Representative images of quiescent control (left), IR-induced senescent (center), and proliferating control IMR-90 cells (right). DAPI staining is shown at the top, EdU staining at the bottom. B) Quantification of EdU-positive cells for quiescent control, IR-induced senescent, and proliferating control cells. Data shown are means of 3 replicates ± SD.
Senescence-associated β-galactosidase
-
9
Follow the steps listed in Basic protocol 1 (or one of the Alternate Protocol 1 versions) to generate senescent and control cells of the chosen cell type. For each replicate, seed 80,000 fibroblasts in a well of a 6-well plate.
-
10
Wash senescent and control cells twice with PBS, then switch to media containing 0.2% FBS (instead of 10% FBS) and culture for 48 h. This will induce quiescence by serum starvation in control cells while maintaining viability for a few days in both cell populations.
-
11
After 48 h, wash the cells twice with PBS, then switch to serum-free media and culture for 24 h.
-
12Fix and incubate the cells with the X-Gal staining solution at 37° C overnight (as instructed in the manufacturer’s protocol from the Senescence Detection Kit).When preparing the staining solution as instructed by the manufacturer’s protocol, we recommend using X-Gal from Life Technologies (see “Materials”) rather than X-Gal provided with the Senescence Detection Kit to optimize staining. Use the suggested solvent and concentration of X-Gal described in the Senescence Detection Kit manufacturer’s manual.
-
13On the next day, remove the staining solution and leave the cells in PBS.The staining reaction might require incubations longer than the overnight suggested in the manufacturer’s protocol. Cells should be monitored under a microscope before removing the staining solution. If senescent cells appear to be poorly stained, they can remain in the staining solution at 37° C for longer times. Observe the cells under a microscope about every 2 h until the senescent cells appear clearly stained, while quiescent control cells should remain unstained. However, avoid extended incubation times as the quiescent cells might turn positive. We recommend acquiring images right after removing the staining solution and adding PBS, even though cells can be stored at 4° C for 1–2 days before imaging.
-
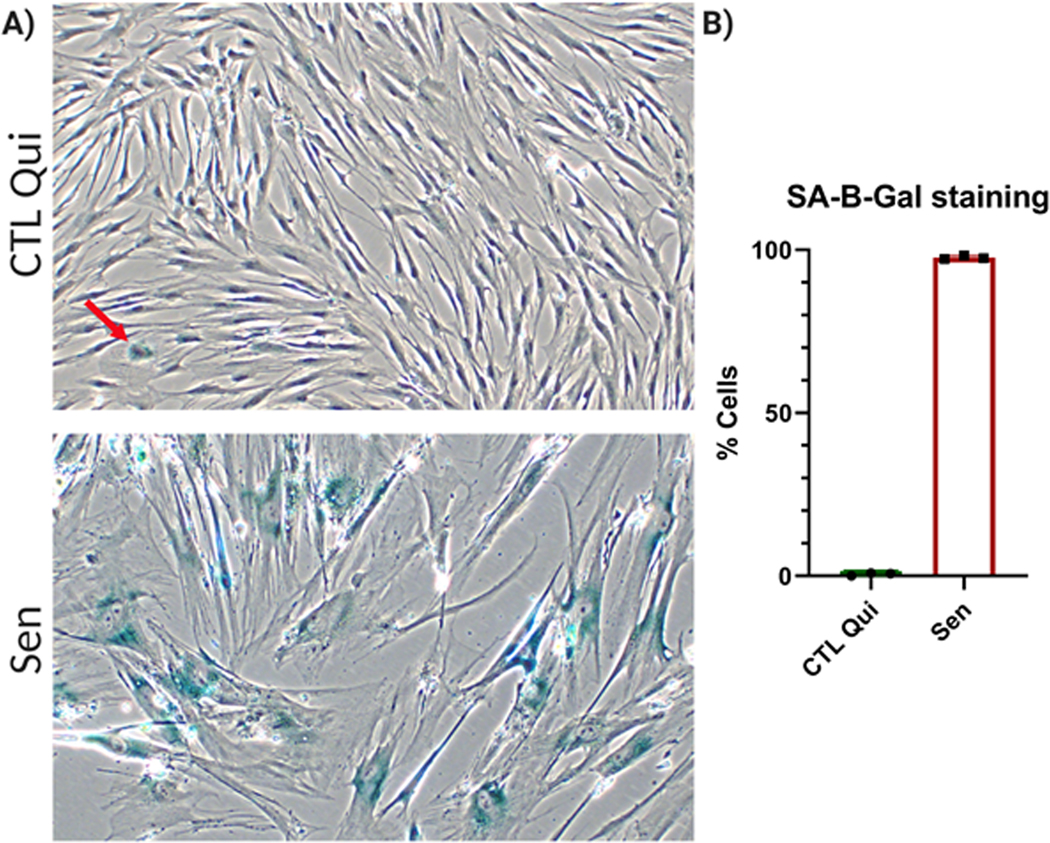
14
Acquire bright-field images using an inverted microscope and confirm that senescent cells have high SA-β-Gal staining compared to quiescent control cells (see Figure 3).
Figure 3.
SA-β-Gal staining. A) Representative images of quiescent control (left) and IR senescent IMR90 cells (right). B) Quantification of SA-β-Gal positive cells for quiescent control and senescent cells. Data shown are means of 3 replicates ± SD.
Senescence-associated gene expression analysis by qPCR
-
15
Follow the steps listed in Basic protocol 1 (or in one of the Alternate Protocol 1 versions) to generate senescent and control cells of the chosen cell type. For each replicate, seed 200,000 fibroblasts in a well of a 6-well plate.
-
16
Wash both senescent and control cells twice with PBS, then switch them to media containing 0.2% FBS (instead of 10% FBS) and culture for 48 h to induce quiescence by serum starvation in control cells while maintaining viability for a few days in both cell populations.
-
17
After 48 h, wash the cells twice with PBS, then switch them to serum-free media and culture for 24 h.
-
18
24 h later, remove media from the wells and extract total RNA using the ISOLATE II RNA Micro Kit. We recommend adding Lysis Buffer RLY directly to the wells after removing the media, making sure to detach all cells with the help of a cell scraper.
-
19
After RNA extraction, convert the RNA samples into cDNA using the Applied Biosystems MultiScribe Reverse Transcriptase kit.
-
20Use the cDNA to analyze by qPCR mRNA levels of the senescence-associated genes.See “Materials” for a list of genes that are significantly upregulated (all genes except LAMNB1) or downregulated (LAMNB1) upon senescence induction (Hernandez-Segura et al., 2017). The primer sequences provided have been tested with the Universal Probe Library System. If using another probe system, the primer sequences listed are not guaranteed to work, and primer sequence optimization might be needed.
-
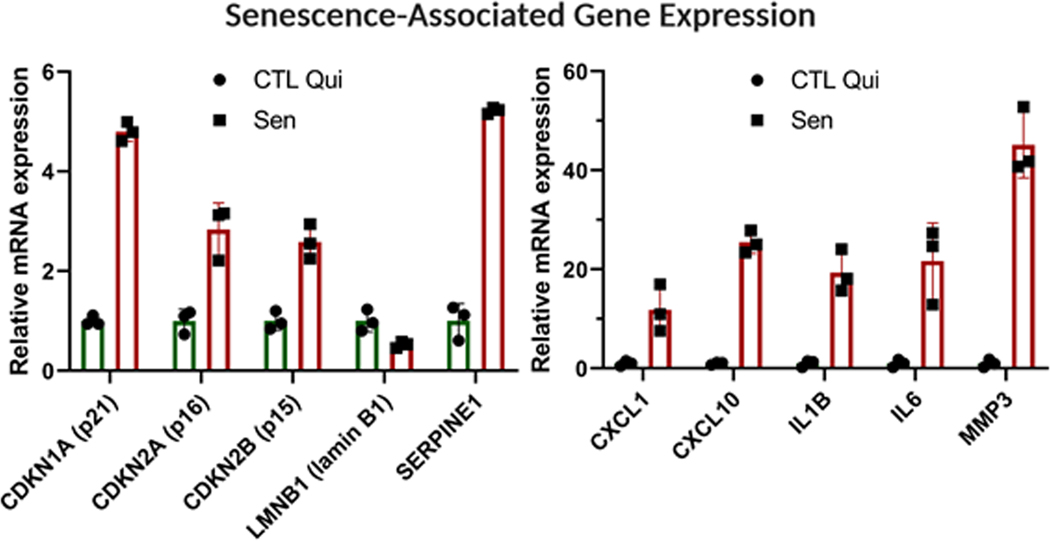
21Analyze qPCR data to compare the relative expression of senescence-associated genes between senescent and quiescent control cells. Confirm that most genes have the same upregulation or downregulation trends shown in Figure 4.Different cell types may express different complements of SASP factors. The senescence-associated genes listed here are optimized for fibroblasts.
Figure 4.
Gene expression analysis using qPCR. Relative mRNA levels of the indicated genes in senescent cells normalized to quiescent IMR-90 cells. Data shown are means of 3 replicates ± SD.
Propidium iodide inclusion
-
22
Follow the steps listed in Basic protocol 1 (or Alternate Protocol 1 versions) to generate senescent and control cells of the chosen cell type. Seed 1 × 106 fibroblasts in a T75 flask for each replicate.
-
23
Wash senescent and control cells twice with PBS, then switch to media containing 0.2% FBS (instead of 10% FBS) and culture for 48 h. This will induce quiescence by serum starvation in control cells while maintaining viability for a few days in both cell populations
-
24
After 48 h, wash the cells twice with PBS, then switch to serum-free media and culture them for 24 h.
-
25
Wash the cells with PBS and detach them from the substratum by incubation with Trypsin/EDTA at 37°C for 5 min.
-
26Resuspend the cells in complete media to inhibit the trypsin, then wash the cell suspensions twice with PBS.To perform a PBS wash, pellet the cells by centrifugation at 250 × g for 5 min, remove the supernatant, resuspend the pellet in PBS, centrifuge the cells again at 250 × g for 5 min, and remove the PBS.
-
27
Resuspend the cell pellets in PBS to a final concentration of 106–107 cells/μl.
-
28
Aliquot 100 μl of each cell suspension in a flow cytometry tube and add 5 μl PI staining solution.
-
29
Mix the solution by pipetting and incubate for 1 min at room temperature.
-
30Analyze the samples for PI staining by flow cytometry. Confirm that the number of PI positive cells are low in both senescent and control cells (see Figure 5).PI dye is impermeable to cells, so will not stain cells unless their membrane integrity is compromised (that is, the cells are dead or dying).
Figure 5.
Propidium iodide (PI) staining for cell death. Percentage of PI-positive cells in quiescent control and IR-induced senescent IMR-90 cell populations. Data shown are means of 3 replicates ± SD.
BASIC PROTOCOL 2 GENERATING CONDITIONED MEDIA FROM SENESCENT CELLS CULTURED IN LOW SERUM AND QUIESCENT CONTROL CELLS
With this method, SASP-containing and control CM are generated from senescent and control cells, prepared following Basic Protocol 1 (or an Alternate Protocol 1 version). Both senescent and control cells are cultured in low-serum media (0.2% FBS) for 48 h, which allows control cells to become quiescent. Then, cells are cultured in phenol red-free, serum-free media for 24 h. CM from senescent and control quiescent cells are collected, and the cells are counted, which will allow normalization for proteomic analysis. Protein levels normalized to cell count accounts for changes due to differences in the number of cells and represents a more meaningful measure of ‘proteins secreted per cell’. This protocol has been optimized for IMR-90 primary human fetal lung fibroblasts and can serve as a guide for the use of other adherent human cells.
Materials
(also see Basic Protocol 1)
Senescent and control cells generated using Basic Protocol 1 or an Alternate Protocol 1 versions
Low-serum media
When using IMR-90 fibroblasts, the low-serum medium composition is the following: DMEM (Gibco, cat. no. 12430–054) supplemented with 100 U/mL penicillin-streptomycin (Gibco, cat. no. 15070063) and 0.2% fetal bovine serum (Gibco, cat. no. 2614079)
Phenol red-free, serum-free media
When using IMR90 fibroblasts, use phenol-red free DMEM (Gibco, cat. no. 21063–029) supplemented only with 100 U/mL penicillin-streptomycin (Gibco, cat. no. 15070063)
Beckman Coulter Z1 Particle Counter (Beckman, cat. no. 6605698)
Swing Rotor Centrifuge 5804 (Eppendorf, cat. no. 022622501)
Generation of CM samples
-
1Aspirate culture media from senescent and control cells, then wash cells twice by adding PBS and subsequently aspirate it. After the washes, switch to low-serum media (see “Materials”) for both senescent and control cells and culture for 48 h.Using low-serum media induces quiescence in control cells by serum starvation, while maintaining viability for a few days in both cell populations.
-
2After 48 h, aspirate culture media, then wash senescent and quiescent control cells twice by adding PBS, and subsequently aspirate it. After the washes, switch to serum-free and phenol red-free media, and incubate for 24 h.The collected CM need to be phenol red-free because this compound interferes with quantification of protein using BCA. Also, culture media used during this step must be free of serum and, as much as possible, of protein components/contaminants. Abundant exogenous protein contamination can limit the identification and quantification of secreted proteins. High concentrations of proteins present in serum and other cell culture supplements interfere with and suppress the ionization of secreted proteins during MS analysis. If for some reasons the culture media contain protein components, these proteins must be excluded from later proteomic analysis.
Collection of CM
-
3After the 24 h incubation, collect CM from each replicate and perform cell counts.Right after collection, keep the CM on ice to prevent protein degradation during cell counting. We recommend keeping CM on ice no longer than 1 hours before proceeding to step 3. Cell counts are important to normalize the levels of secreted proteins during proteomic analysis (see Basic Protocol 3). Senescent fibroblasts tend to attach more firmly to plates, so a slightly longer incubation (1–2 min) with trypsin-EDTA might be required for the senescent cells to detach.
-
4
Centrifuge the collected CM at 10,000 × g for 15 min to pellet cell debris and transfer the supernatant into a new tube.
-
5
Proceed to Basic Protocol 3 (“Conditioned media processing and proteomic analysis”) or store the samples at −80°C for later processing.
ALTERNATE PROTOCOL 2 GENERATING CM FROM SENESCENT CELLS CULTURED IN COMPLETE MEDIA AND QUIESCENT CONTROL CELLS
If after senescence induction the cell type investigated does not remain viable in low-serum, then Alternate Protocol 2 should be used to generate CM for MS analysis. Some indicators of poor viability are loss of cell number and cell detachment. Loss of viability can be quantitatively confirmed by increasing cell death, as measured by cell viability assays (for example, see “Propidium iodide inclusion” in Support Protocol 1). In this protocol, CM containing the SASP are collected from senescent cells that are cultured in complete media up to 24 h before CM collection. Control cells are still cultured in low serum to induce quiescence. However, when comparing these two conditions it is difficult to determine whether differences are due to a senescent vs non-senescence state or due to culturing in complete media vs low-serum media. This protocol is optimized for primary lung fibroblasts, which remain viable in low serum under control conditions. If the cell type under investigation is not viable under control conditions, we recommend optimization of culture conditions appropriate to the cell type under investigation. A possible alternative approach may be to compare CM collected from senescent cells cultured in complete media versus CM collected from non-senescent cells cultured in complete media. However, under these conditions, one cannot distinguish if changes in protein secretion are the result of comparing proliferating cells versus non-proliferating cells, differences in cell density between control and senescent conditions, or differences between senescent and non-senescent cells. Also see section “Cell cultures in low-serum media before CM collection” under “Critical Parameters and Troubleshooting”.
Materials
See Basic Protocol 2 Materials section.
Generation of CM samples
-
1Aspirate culture media from senescent and quiescent control cells, then wash cells twice by adding PBS, and subsequently aspirate it. After the washes, switch control cells to low serum-media (see “Materials”) and add complete media to senescent cells. Culture both cell populations for 48 h.Using low-serum media induces quiescence in control cells by serum starvation, while maintaining viability for a few days.
-
2After 48 h, remove culture media by aspiration, then wash senescent and quiescent control cells twice by adding PBS, and subsequently aspirate it. After the washes, add serum-free and phenol red-free media, and incubate for 24 h.The CM needs to be phenol red-free because this compound interferes with the quantification of protein using BCA. Also, culture media used during this step must be free of serum and, as much as possible, of protein components/contaminants. Abundant exogenous protein contamination can limit the identification and quantification of secreted proteins. High concentrations of proteins present in the serum and other cell culture supplements interfere with and suppress the ionization of secreted proteins during MS analysis. If for some reasons the culture media contain protein components, these proteins must be excluded from later MS data analysis.
Collection of CM
-
3
For the collection of CM, follow steps 3 through 5 from Basic Protocol 2.
BASIC PROTOCOL 3 QUANTITATIVE PROTEOMIC ANALYSIS OF THE SASP
This protocol describes a comprehensive unbiased mass spectrometry-based approach to identify and quantify the secreted proteins of cultured cells. CM prepared in Basic protocol 2 (or Alternate Protocol 2) are concentrated, digested and desalted. The processed samples are analyzed using a liquid chromatography-mass spectrometry (LC-MS) method, specifically a data-independent acquisition (DIA) workflow. The protocol illustrates in detail how to use a LC-MS system composed of a nano-LC 2D HPLC coupled to a TripleTOF 6600 high-resolution mass spectrometer. The DIA quantitative proteomics analysis software Spectronaut (Biognosys) is used to perform relative quantification of protein levels and create reports of protein abundance, fold changes, and statistics.
Materials
SASP CM and control CM (CTL CM) generated in Basic Protocol 2 (or Alternate Protocol 2)
Ammonium bicarbonate (NH4HCO3) (SigmaAldrich, cat. no. A 6141)
Dithiothreitol (DTT) (Sigma, cat. no. D9779)
Iodoacetamide (IAA) (Sigma, cat. no. I1149)
Sequencing grade trypsin (Promega, cat. no. V5113)
Acetic acid (Sigma-Aldrich, cat. no. 695092–100ML)
Formic acid (Sigma, cat. no. 94318)
Acetonitrile (Burdick & Jackson, cat. no. AH015)
Indexed retention time iRT peptide standards (Biognosys, cat. no. Ki-3002)
Sequencing grade trypsin (Promega, cat. no. V5113)
Amicon® Ultra centrifugal filters with 3 kDa molecular weight cutoff (MilliporeSigma, cat. no. UFC9003)
Bicinchoninic Acid (BCA) kit (Thermo Fisher Scientific, cat. no. PI23225)
Litmus strips (VWR International, cat. no. EM1095350007)
Heating Shaking Drybath (Thomas Scientific, cat. no. 1199A66)
Benchtop microcentrifuge (ThermoFisher, cat. no. 75002401)
Oasis HLB Solid-Phase Extraction (SPE) cartridges, 10 mg Sorbent per Cartridge, 30 μm (Waters, cat. no. 186006339)
SpeedVac concentrator (Thermo Scientific, Savant™ SPD131DDA)
Ultrasonic Bath Sonicator (ThomasScientific)
Autosampler vials for HPLC (Agilent Technologies, cat. no. 5190–3155)
Nano-LC 2D HPLC system (Eksigent Ultra Plus, Eksigent)
cHiPLC system (Eksigent)
200 μm × 0.4 mm ChromXP C18-CL chip, 3 μm, 120 Å (SCIEX)
75 μm × 15 cm ChromXP C18-CL chip, 3 μm, 120 Å (SCIEX)
High-resolution mass spectrometer TripleTOF 6600 System (SCIEX) – or other high-resolution mass spectrometer
Spectronaut™ software (Biognosys, Schlieren)
Concentrating the CM
-
1Transfer CM to Amicon ultrafilter tubes, without exceeding the maximum volume capacity (see manufacturer instructions).It is likely that the volumes of CM samples are higher than the ultrafilter tubes’ capacity. If so, use multiple centrifugations until the volume of every CM sample has been reduced to <0.5 mL (see next step).
-
2
Following manufacturer instructions, buffer exchange the protein samples into 50 mM NH4HCO3 buffer. After initially concentrating the protein samples in the columns, we recommend performing one buffer exchange with a maximum volume of 50 mM NH4HCO3, followed by a buffer exchange with a maximum volume of 8 M urea in 50 mM NH4HCO3. After the addition of 8 M urea in 50 mM NH4HCO3, centrifuge the samples until reaching a final volume <0.5 mL in NH4HCO3 buffer.
-
3
Buffer exchanged samples can be stored long term at −80°C.
In-solution proteolytic digestion
-
4Quantify secreted protein concentrations by BCA assay.Use at least a 1:3 dilution of sample in milliQ water for the BCA assay. The BCA assay is compatible with urea concentrations up to 3M.
-
5Aliquot 50 μg of each sample into new 1.5 mL tubes.If sample yields are lower than 50 μg, we recommend aliquoting at least 20 μg of secreted proteins due to sample losses during subsequent steps. Still, sample amounts as low as 10 μg can be used.
-
6
Bring all samples to equal volumes with 8M urea in 50 mM NH4HCO3.
-
7
Vortex to mix.
-
8
Add DTT (1 M in 50 mM NH4HCO3 buffer stock solution) to a final concentration of 20 mM (in 50 mM NH4HCO3 buffer) to each sample to reduce disulfide bridges.
-
9
Incubate samples for 30 min at 37° C with shaking/agitation.
-
10
Allow samples to cool to room temperature.
-
11Add IAA (200 mM in 50 mM NH4HCO3 buffer stock solution) to a final concentration of 40 mM IAA (in 50 mM NH4HCO3 buffer) to each sample to alkylate reduced thiols.It is important to use an IAA concentration at least double that of DTT to ensure that all the reduced thiols are alkylated.
-
12
Incubate samples in the dark at room temperature for 30 min.
-
13
Dilute all samples 1:6 in 50 mM NH4HCO3 to reduce the concentration of urea for trypsin digestion.
-
14
Verify that the pH of samples is between 7.0–8.5 by pipetting small volumes (<1 μL) onto litmus strips. Adjust the pH as needed.
-
15Resuspend lyophilized trypsin in 50 mM acetic acid to a final concentration of 0.1 mg/mL.For example, 20 μg of lyophilized trypsin are resuspended in 200 μL of solution at pH 8, for a final concentration of 0.1 μg/μl trypsin.
-
16
Digest protein samples by adding trypsin at a protease to substrate protein ratio of 1:50 (wt:wt). Incubate samples overnight at 37° C on a shaking dry bath.
-
17Quench the protein digestion by adding formic acid to a final concentration of 1% by volume from a 10% formic acid (in water) stock.Stock concentrations of formic acid less than or equal to 10% can be pipetted safely with plastic pipette tips. Higher concentrations may dissolve plasticware and should be handled with glass to avoid polymer contamination of samples.
-
18
Spin sample at 5,000 × g for 15 min at room temperature to pellet insoluble material. The supernatants, which contain peptides, are desalted in the next steps.
Desalting samples
-
19
Samples are desalted using commercially available Solid-Phase Extraction (SPE) cartridges. The next steps describe a desalting process using Oasis HLB SPE cartridges.
-
20
Wet each HLB SPE cartridge twice with 800 μL 50% acetonitrile (ACN) in 0.2% formic acid in water.
-
21
Equilibrate each cartridge 3 times with 800 μL 0.2% formic acid in water.
-
22
Load peptide samples onto HLB SPE cartridges.
-
23
Wash each cartridge 3 times with 800 μL 0.2% formic acid in water.
-
24
Elute peptides once with 800 μL 50% ACN in 0.2% formic acid in water and once with 400 μL 50% ACN in 0.2% formic acid in water in the same tube.
-
25Dry samples completely in a SpeedVac.If desired, one can pause sample processing; dried peptide pellets can be safely stored long-term at −80° C.
-
26Resuspend dry pellets in 0.2% formic acid to a final concentration of 1 μg/μL.Calculate the final peptide concentration based on the initial protein sample mass aliquoted for digestion. For example, if the initial aliquot contained 50 μg of protein, the dried pellet will be resuspended in 50 uL of 0.2% formic acid.
-
27
Sonicate samples in a water bath sonicator for 5 min.
-
28
Vortex samples at 4° C for 10 min.
-
29
Centrifuge samples at 15,000 × g for 15 min.
-
30
Transfer supernatants to autosampler vials.
-
31Add retention time standard peptides to each autosampler vial.For example, use iRT peptide standards at a 1:20 dilution.
-
32
Centrifuge samples in autosampler vials at 5,000 × g in a clinical centrifuge for 1 min to remove bubbles.
Mass spectrometry acquisition
The following steps describe in detail the use of a nano-LC 2D HPLC coupled to a TripleTOF 6600. The protocol can be adjusted depending on the available MS instruments or preference.
-
33
Transfer the autosampler vials into the autosampler tray with cooling set at 4° C.
-
34
Create loading, injection and analytical gradient methods with the following settings:
Load a total of 1 μg sample into the autosampler loop.
After injection, transfer the peptide mixtures onto a C18 pre-column chip (200 μm × 0.4 mm ChromXP C18-CL chip, 3 μm, 120 Å) (or column) and desalt by washing with aqueous mobile phase A at 2 μL/min for 10 min. Then, transfer the peptides to an analytical chip (75 μm × 15 cm ChromXP C18-CL chip, 3 μm, 120 Å) (or column) and elute at a flow rate of 300 nL/min with a gradient method using mobile phases A (aqueous) and B (organic). Apply a linear gradient from 5% mobile phase B to 35% mobile phase B over 120 min.
Subsequently, ramp the mobile phase B to 80% over 5 min, then hold at 80% B for 8 min before returning to 5% B for a 25 min re-equilibration.
-
35
Build a DIA MS instrument method and define the following instrument scan experiments:
Experiment 1: perform MS1 precursor ion scan from m/z 400–1,250 (accumulation time of 250 ms).
Experiment 2–65: perform MS/MS product ion scans for 64 variable window segments with a MS2 scan range from m/z 100–1,500 (accumulation time of 45 ms per each of the 64-product ion scans per cycle). Set the collision energy spread to CES = 10, then select the “high sensitivity product ion scan mode”.
Use the 64 variable window DIA acquisition strategy as described by Schilling et al. (Schilling et al., 2017) for a total cycle time of ~3.2 s. In this acquisition, a series of variable window width (5 −90 m/z) is stepped over the full mass range (m/z 400 −1,250 over 64 SWATH segments, each with a 45 ms accumulation time, yielding a cycle time of 3.2 s, which includes one MS1 scan with a 250 ms accumulation time). NOTE: The variable window width is adjusted according to the complexity of the typical MS1 ion current observed within a certain m/z range using a variable window calculator algorithm (more narrow windows are chosen in “busy” m/z ranges, wide windows in m/z ranges with few eluting precursor ions). On other MS instrument platforms, other DIA window strategies (isolation schemes) may be implemented.
-
36Create a sample queue/batch for all biological replicate samples.To obtain consistent and reliable data, regular LC-MS system suitability assessments need to be performed before and during the entire SWATH study. Initially, use pre-digested Beta Galactosidase standards and/or more complex human HeLa cell digests and perform QC acquisitions, or mass calibration acquisitions typically used in your laboratory or proteomics core. Also, randomize study samples to avoid systematic errors; block-randomization of biologically different samples is often applied in proteomics studies.
Assign file names for each sample.
Set injection volumes to corresponding 1μg of sample (for example, 1 μL of a 1 μg/μL sample).
Assign the acquisition method generated above.
-
37
Submit the samples for MS acquisition.
Mass spectrometry data analysis
Here we describe a workflow using the commercially available software Spectronaut (alternative software packages can also be used). The procedure for entering settings and processing data will vary depending on the analysis software.
-
38
To analyze and quantify protein levels, open the DIA analysis software Spectronaut.
-
39
To start the Quantification Analysis, select the “Pipeline” tab, click “Set up a DIA Analysis from File”, and open the MS DIA raw files of interest for relative quantification.
-
40Select “Assign Spectral Library” and select the ‘Pan Human Library’, click “load” | “next”.The Pan Human Spectral Library (Rosenberger et al., 2014) should be used only for human samples (however other Spectral Library approaches can also be applied).
-
41
Select the “BGS Factory Settings” analysis schema. Verify that the peptide FDR settings are set to Q-value < 0.01 with sparse identifications and click “next”.
-
42
Select the appropriate human database FASTA file (the default UniProt FASTA file assigned to the Pan Human Library) and click “next”.
-
43
Define the condition set-up (e.g., ‘senescent’ and ‘non-senescent’).
-
44In the condition set-up form, to each sample assign a correction factor that is equal to the inverse of its cell count (1 divided by the cell count) and click “next”.It is critical to assign correction factors based on cell counts to account for differences in protein secretion levels due to differences in the number of cells in each cell culture flask.
-
45
Select “goa_human” as the gene annotation (ontology) file and click “next”.
-
46
Review the analysis overview (summary of the experiment set-up) and select “output directory” to assign an output directory. Click “finish”.
-
47
Finally, click “Run Pipeline” to perform the label-free quantitative analysis.
-
48
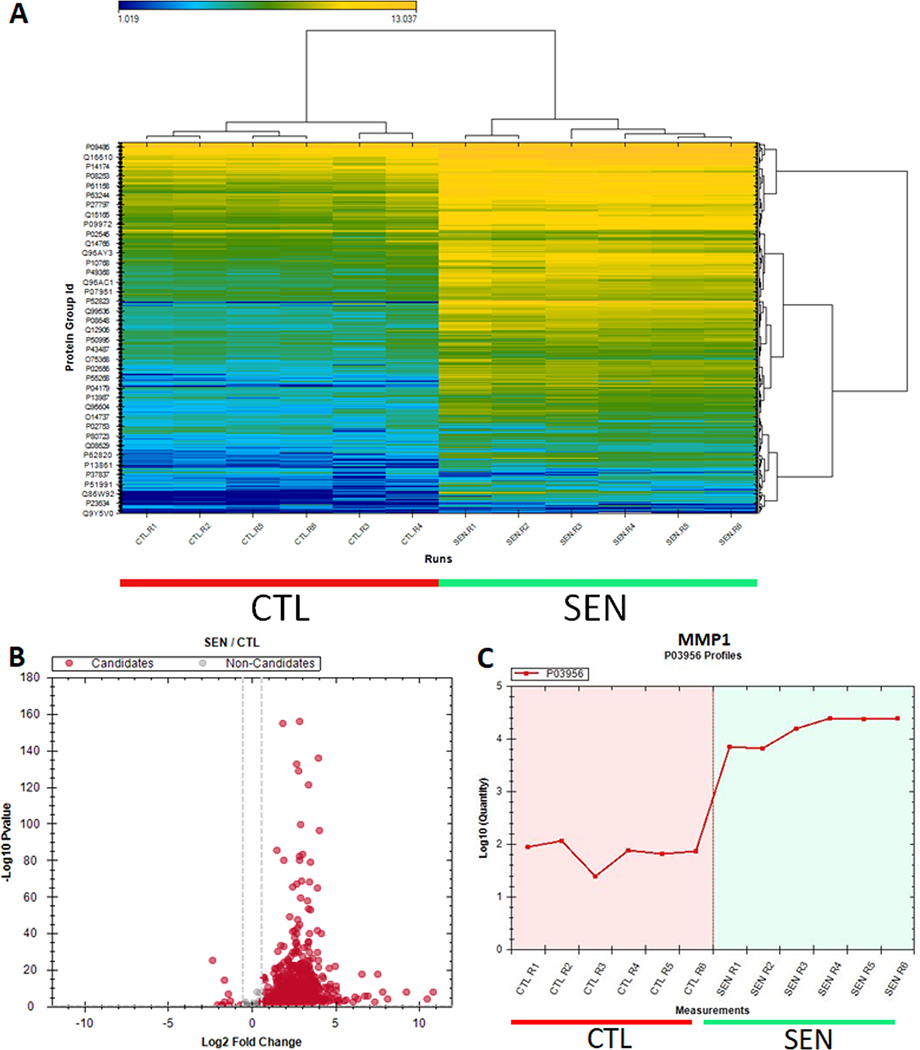
Review the results in the “output directory”. The Spectronaut DIA Quantitative Analysis Software automatically performs FDR analysis, generates heat maps and volcano plots (Figure 6). It generates lists of identified and quantified peptides and proteins, and provides Q-values along with relative fold changes comparing different conditions in a “candidates.tsv” file.
Figure 6.
SASP proteomic analysis figures generated by the Spectronaut software. A) Heatmap depicting the abundance of all proteins identified in the conditioned medium of oncogenic Ras-induced senescent (n=6) and control (n=6) IMR-90 cells. B) Volcano plot of the log2 fold changes in abundance of proteins secreted by senescent versus control cells. Red dots represent fold changes greater than 1.5-fold, and p-values less than 0.05. C) Line plot showing the abundance of a classical SASP protein, MMP1, in the secretomes of senescent and non-senescent cells.
Expected results:
The elevation of several SASP factors is expected in a successful proteomic analysis, including GDF15, MMP1, STC1, CXCL1 (these are known ‘core’ SASP factors for fibroblasts). The number of proteins identified in an experiment with 5 replicates per each condition (5 senescent and 5 controls) is typically approximately 1,000 proteins identified in the secretome. The number of significantly changed proteins (SASP proteins) will typically vary depending on the senescence inducer or cell type used. For example, we have previously reported 548 proteins significantly increased in the SASP of irradiated senescent fibroblasts, but 332 proteins in the SASP of ATV-treated senescent fibroblasts, and 180 proteins in the SASP of irradiated epithelial cells (Basisty et al., 2020a). Significant SASP factors change heterogeneously with senescence induction (and cell type). In senescent fibroblasts, it is typically expected that the majority of significant protein changes are increases in protein secretion. Note: In parallel, it is important to independently verify senescence in the cells using the Support Protocol Assays described above.
REAGENTS AND SOLUTIONS
Doxorubicin stock solution
Prepare a stock solution of doxorubicin by dissolving doxorubicin hydrochloride (Tocris, cat. no. 2252) in DMSO (MilliporeSigma, cat. no. 67–68-5) to obtain a final concentration of 2.5 mM. This solution can be aliquoted and stored at −20° C short-term, and at −80° C for long-term storage.
Atazanavir/ritonavir stock solution (ATV/r)
Prepare a stock solution of ATV/r by mixing atazanavir (sulfate) (MedChem Express, cat. no. HY-17367A) with ritonavir (MedChem Express, cat. no. HY-90001) (wt ratio atazanavir:ritonavir of 4:1) and dissolving in DMSO (MilliporeSigma, cat. no. 67–68-5) to obtain a concentration of 8 mg/mL and 2 mg/mL respectively. For example, mix 32 mg of atazanavir with 8 mg of ritonavir and dissolve in 4 mL DMSO. In a sterile environment, filter the solution using a sterile 0.2 μm filter compatible with DMSO (Fisherbrand Syringe Filters Sterile, Fisher Scientific, 09–719C). After filtration, keep the solution sterile. The stock solution can be stored at −20° C short-term, and at −80° C for long-term storage. The working concentration of ATV/r described in Alternate Protocol 1D (25 μM) is calculated considering only the atazanavir sulfate concentration in the stock solution (that is 8 mg/mL, approximately 10 mM). Therefore, to prepare media with a final concentration of 25 μM ATV/r, dilute the stock solution by a factor of 400 in media.
COMMENTARY
Background Information
Although senescent cells participate in several physiological functions, they are known to contribute to frailty as well as many age-related diseases and therefore are considered a major driver of aging and age-related diseases (Campisi and d’Adda di Fagagna, 2007; Gorgoulis et al., 2019) The secretome of senescent cells, termed the senescence-associated secretory phenotype (SASP), is known to cause many of these effects (both beneficial and detrimental) (Campisi, 2013; Munoz-Espin and Serrano, 2014). For example, the SASP is linked to optimal wound healing (Demaria et al., 2014), but also to pathological states such as osteoarthritis and tumor growth and metastasis (Coppe et al., 2010; Jeon et al., 2018) The SASP is extremely heterogeneous and dynamic, varying with the cell type and senescence-inducing stimulus, as well as how much time has passed since senescence was induced (Hernandez-Segura et al., 2017). Characterization of the SASP on a molecular level may provide novel mechanistic insights into how cellular senescence drives aging, potentially leading to the discovery of aging biomarkers and targets for preventing or counteracting the deleterious effects of senescent cells (Basisty et al., 2020a).
Previously, antibody arrays were used to study the SASP protein composition (Coppe et al., 2008). However, antibody arrays can detect only a pre-selected number of proteins. This method is therefore both biased and limited. Another approach to study the SASP is unbiased gene expression analysis of senescent cells (Hernandez-Segura et al., 2017). This approach enables researchers to analyze a much larger number of genes compared to a small number of proteins using antibody arrays. Nevertheless, gene expression analysis cannot directly provide information about proteins secreted by cells. Recently, more comprehensive and unbiased mass spectrometric studies have been used to characterize and quantify SASP protein profiles directly (Basisty et al., 2020a; Schafer et al., 2020; Wiley et al., 2019). A study by Basisty et al. (Basisty et al., 2020a) found the SASP to be much larger than previously thought and extremely heterogeneous, depending on the cell type and senescence inducer. Interestingly, many of the SASP proteins overlapped with the plasma protein signature of aging identified in the Baltimore Longitudinal Study of Aging (BLSA). This overlap demonstrates the extent to which this approach not only expands our understanding of the SASP but also permits the identification of promising candidate protein biomarkers of organismal senescent cell burden in a biofluid that is easily accessible. The step-by-step procedure described here is meant as a guide to i) generate senescent cells, ii) collect SASP-containing conditioned media, and iii) perform a quantitative, unbiased analysis of the SASP using MS with a DIA workflow.
Critical Parameters and Troubleshooting
The methods for senescence induction described in this protocol collection have been developed and optimized for human IMR90 fibroblasts. Therefore, some adjustments might be required when using other cell strains. Nevertheless, these protocols have been successfully used in our laboratory to induce senescence in multiple human primary cell strains with little to no modification. Hence, we are confident that they can be applied as they are, or with little optimization, to many human primary cell strains.
For compatibility with downstream mass spectrometry analysis, there are several important general considerations. First, the conditioned medium in which the final collection of secreted proteins takes place should be free of highly abundant protein additives and free of serum because abundant exogenously added proteins will interfere with the detection of secreted proteins. Secondly, all buffers and reagents used during the sample processing for mass spectrometry should be mass spectrometry grade. Thirdly, during all steps of sample processing, the sample should be completely free of detergents and chemicals that are incompatible with mass spectrometry analysis.
Senescence Induction
Observation: cells do not develop a senescent phenotype.
Possible causes and solutions:
The dose of senescence inducer is too low. The cell strain or line used might be resistant to the senescence inducer. Increase the dose and perform a dose titration checking for senescence induction using only a couple of easily performed assays, such as SA-β-Gal activity and cell counts as a proxy for growth arrest. Once a promising dose is identified, perform Support Protocol 1 to more thoroughly confirm that the cells developed a senescent phenotype.
Observation: significant cell death upon treatment with the senescence inducer.
Possible causes and solutions:
The senescence inducer chosen is toxic to the cell type used. Reduce the dose (perform a dose titration) and check for senescence induction using only a couple of easily performed assays, as suggested above. Alternatively, use another senescence inducer. Once a promising inducer and dose are identified, perform Support Protocol 1 to more thoroughly confirm that the cells developed a senescent phenotype.
The senescence-inducing compound was not sterile. Ensure compounds given to cells are sterile by filtering the solutions with 0.2 μm filters compatible with the compound solvent.
Cell cultures in low-serum media before CM collection
Observation: significant death of senescent cells (viability ≤ 80%, as measured by PI inclusion assay) when cultured in low-serum media for a prolonged period.
Possible causes and solutions:
The cell strain or line is highly sensitive to low-serum media upon senescence induction. Increase the percentage of serum in the media, performing a titration. Check that the concentration at which senescent cells remain viable still induces quiescence in the control cells. Otherwise, do not culture the senescent cells in low-serum media (except for the last 24 h before CM collection) as described in Alternate Protocol 2.
Observation: significant death in control cells when cultured in low-serum media for a prolonged period.
Possible causes and solutions:
The cell strain or line is highly sensitive to low-serum media. Increase the percentage of serum in the media, performing a titration. Check that the concentration at which control cells remain viable still induces quiescence. Otherwise, try to induce quiescence by other means, such as contact inhibition. If quiescence cannot be achieved in the control cells, then culture both control and senescent cells in complete media (except for the last 24 h before CM collection) and compare the SASP-containing CM to CM generated from these proliferating control cells. However, under these conditions, one cannot distinguish if changes in protein secretion are the result of comparing proliferating cells versus non-proliferating cells, major differences in cell density between control and senescent conditions, or differences between senescent and non-senescent cells.
Sample processing and MS analysis
Observation: High background signal or poor standard curve in BCA results.
Possible causes and solutions:
Residual phenol red or incompletely buffer-exchanged samples. Ensure phenol red-free media is used for the collection of CM containing secreted proteins.
Buffer exchange was not complete. Ensure the buffer exchange is followed according to the manufacturer protocol, making sure to follow multiple rounds of exchanging buffer with 50 mM NH4HCO3.
Observation: Albumin is the only protein detected.
Possible causes and solutions:
The final CM is contaminated with residual serum. Take care to wash cells 2x or more with PBS before addition of serum-free medium for collection of CM.
Observation: Unexpected direction of protein changes between senescent and control cells, or unexpectedly high biological variation among samples.
Possible causes and solutions:
Mass spectrometry analysis is not corrected for cell count. Verify that correction factors are properly assigned in the MS software before sample acquisition. To ensure protein levels reflect changes in secretion levels, rather differences in cell numbers, it is critical to correct for cell counts.
Understanding Results
It is important to confirm that the desired cell line develops a senescent phenotype following senescence induction. To this end, multiple senescence-associated markers should be tested.
EdU incorporation
Senescent cells are characterized by an essentially irreversible growth arrest. Since the senescent cells do not divide, they should not incorporate nucleotides in DNA. The Click-iT EdU kit detects nucleotide incorporation by supplementing cells with a nucleotide analog (EdU) that can be later tagged by a selective chemical reaction. The tag contains a fluorophore and thus incorporation into DNA can be detected with a fluorescence microscope. Senescent cells, as well as quiescent control cells, should have low EdU incorporation (Figure 2). Conversely, non-senescent, proliferating cells should have high EdU staining.
SA-β-Gal activity
Senescent cells increase lysosomes and lysosomal β-galactosidase activity (β-Gal). Therefore, the β-Gal substrate X-Gal is broken down at a higher rate in senescent cells and at a suboptimal pH compared to control cells. The breakdown of X-Gal causes the formation of blue indole crystals, visible by light microscopy. Senescent cells should have high SA-β-Gal staining, whereas the opposite should be true for control cells (Figure 3). Note that if cells are close to confluency, staining can increase significantly, leading to false SA-β-Gal positivity in control cells.
Senescence-associated gene expression
The transcriptome of senescent cells changes significantly compared to that of non-senescent cells. While the transcriptome is very heterogeneous – dependent on cell strain and senescence inducer – there is a transcription signature that associates with the senescent phenotype. This signature includes upregulated (e.g. p16INK4a) and down-regulated (e.g. LaminB1) genes. These changes in transcription can be detected by qPCR. Figure 4 shows a panel of selected genes and their expected relative mRNA levels in senescent cells compared to quiescent control cells. Depending on the cell type and senescence inducer, the expression of some of these genes might remain unchanged or trend in the opposite direction. However, most of the genes in Figure 4 should show similar results, regardless of the cell type and inducer.
Propidium iodide inclusion
Cells used to generate SASP and control CM should be viable and healthy when producing their secretome, otherwise proteins from dying cells might significantly contaminate the CM and falsely being assigned to the SASP. To verify that the cells are viable and healthy when producing CM, staining for dead or unhealthy cells, for example using propidium iodide, can be used with flow cytometry. Both senescent and control cells should have a low percentage of positive cells.
Time Considerations:
Characterizing the SASP of a desired cell type using a chosen senescence inducer will require about 2 months of dedicated effort. The procedure can be divided into sequential and independent stages.
Validation of senescence induction: generating senescent and control cells and checking for senescence markers takes about 2 weeks. It might be necessary to invest more time if the senescence induction protocol requires optimization.
Production of CMs: the generation of senescent and control cells and collection of CMs should take 10–14 days.
Processing of CMs for MS analysis: proteins in CMs samples can be concentrated, digested, and desalted in 2 to 3 days.
LC-MS analysis: running one sample takes approximately 1.5 h. When planning experiments, keep in mind that the LC-MS in your laboratory/MS core might not be able to process your samples immediately. Data analysis and identification of SASP proteins can be done in as little as a few hours to a few days, depending on the complexity of the MS data.
Acknowledgments
This work was supported by the National Institutes of Health under award numbers: U01 AG060906–02 and U01 AG060906–02S1 (PI: Schilling), under award numbers P01 AG017242 and R01 AG051729 (PI: Campisi), K99 AG065484 (PI: Basisty) and under award number 1S10 OD016281 (Buck Institute).
Footnotes
Conflict of Interest
J.C. is a founder and share-holder of Unity Biotechnology which develops senolytic drugs. All other authors declare that there is no conflict of interest.
Internet Resources
The data regarding SASP profiles obtained by Basisty et al. is freely available on the SASP Atlas website: http://www.saspatlas.com/
Literature Cited
- Basisty N, Kale A, Jeon OH, Kuehnemann C, Payne T, Rao C, Holtz A, Shah S, Sharma V, Ferrucci L, et al. (2020a). A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol 18, e3000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basisty N, Kale A, Patel S, Campisi J, and Schilling B (2020b). The power of proteomics to monitor senescence-associated secretory phenotypes and beyond: toward clinical applications. Expert Rev Proteomics 17, 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, and Campisi J (2003). Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J 22, 4212–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghesan M, Fafian-Labora J, Eleftheriadou O, Carpintero-Fernandez P, Paez-Ribes M, Vizcay-Barrena G, Swisa A, Kolodkin-Gal D, Ximenez-Embun P, Lowe R, et al. (2019). Small Extracellular Vesicles Are Key Regulators of Non-cell Autonomous Intercellular Communication in Senescence via the Interferon Protein IFITM3. Cell Rep 27, 3956–3971 e3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J (2013). Aging, cellular senescence, and cancer. Annu Rev Physiol 75, 685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, and d’Adda di Fagagna F (2007). Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8, 729–740. [DOI] [PubMed] [Google Scholar]
- Collins BC, Hunter CL, Liu Y, Schilling B, Rosenberger G, Bader SL, Chan DW, Gibson BW, Gingras AC, Held JM, et al. (2017). Multi-laboratory assessment of reproducibility, qualitative and quantitative performance of SWATH-mass spectrometry. Nat Commun 8, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Desprez PY, Krtolica A, and Campisi J (2010). The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5, 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, and Campisi J (2008). Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6, 2853–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dolle ME, et al. (2014). An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 31, 722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillet LC, Navarro P, Tate S, Rost H, Selevsek N, Reiter L, Bonner R, and Aebersold R (2012). Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: a new concept for consistent and accurate proteome analysis. Mol Cell Proteomics 11, O111 016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gualda E, Baker AG, Fruk L, and Munoz-Espin D (2020). A guide to assessing cellular senescence in vitro and in vivo. FEBS J. [DOI] [PubMed] [Google Scholar]
- Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, et al. (2019). Cellular Senescence: Defining a Path Forward. Cell 179, 813–827. [DOI] [PubMed] [Google Scholar]
- Hernandez-Segura A, de Jong TV, Melov S, Guryev V, Campisi J, and Demaria M (2017). Unmasking Transcriptional Heterogeneity in Senescent Cells. Curr Biol 27, 2652–2660 e2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James ENL, Bennett MH, and Parkinson EK (2018). The induction of the fibroblast extracellular senescence metabolome is a dynamic process. Scientific reports 8, 12148–12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon OH, David N, Campisi J, and Elisseeff JH (2018). Senescent cells and osteoarthritis: a painful connection. J Clin Invest 128, 1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon OH, Wilson DR, Clement CC, Rathod S, Cherry C, Powell B, Lee Z, Khalil AM, Green JJ, Campisi J, et al. (2019). Senescence cell-associated extracellular vesicles serve as osteoarthritis disease and therapeutic markers. JCI Insight 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, and Schmitt CA (2019). The dynamic nature of senescence in cancer. Nat Cell Biol 21, 94–101. [DOI] [PubMed] [Google Scholar]
- Munoz-Espin D, and Serrano M (2014). Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 15, 482–496. [DOI] [PubMed] [Google Scholar]
- Ozcan S, Alessio N, Acar MB, Mert E, Omerli F, Peluso G, and Galderisi U (2016). Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging (Albany NY) 8, 1316–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger G, Koh CC, Guo T, Rost HL, Kouvonen P, Collins BC, Heusel M, Liu Y, Caron E, Vichalkovski A, et al. (2014). A repository of assays to quantify 10,000 human proteins by SWATH-MS. Sci Data 1, 140031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MJ, Zhang X, Kumar A, Atkinson EJ, Zhu Y, Jachim S, Mazula DL, Brown AK, Berning M, Aversa Z, et al. (2020). The senescence-associated secretome as an indicator of age and medical risk. JCI Insight 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling B, Gibson BW, and Hunter CL (2017). Generation of High-Quality SWATH((R)) Acquisition Data for Label-free Quantitative Proteomics Studies Using TripleTOF((R)) Mass Spectrometers. Methods Mol Biol 1550, 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CD, Liu S, Limbad C, Zawadzka AM, Beck J, Demaria M, Artwood R, Alimirah F, Lopez-Dominguez JA, Kuehnemann C, et al. (2019). SILAC Analysis Reveals Increased Secretion of Hemostasis-Related Factors by Senescent Cells. Cell Rep 28, 3329–3337 e3325. [DOI] [PMC free article] [PubMed] [Google Scholar]