Abstract
Background:
Older adults with Alzheimer’s disease (AD) may be unable to perform treadmill testing due to balance issues. We investigated whether older adults with AD could successfully complete a peak cycle ergometer test.
Methods:
Peak oxygen consumption (peak ) assessed via a cycle ergometer test in 44 participants with AD (age 78.4 ± 6.8). Physical function was assessed via the incremental shuttle walk, 6-minute walk, and the Short Physical Performance Battery (SPPB).
Results:
All participants completed the cycle ergometer test successfully. Peak was correlated with SPPB (r = .35, P = .023), shuttle walk (r = .35, P = .024), 6-minute walk (r = .31, P = .05), and inversely with age (r =−.4, P = .009). There was no correlation between peak and cognition.
Conclusion:
Older adults with AD are able to safely complete a peak cycle ergometer exercise testing protocol. We provide an individualized cycle ergometer test for determining aerobic capacity in older adults with AD who may be unable to perform treadmill testing due to balance or gait issues.
Keywords: dementia, Alzheimer’s disease, aging, cycle ergometer, physical function, cardiorespiratory fitness
Introduction
Aerobic exercise and aerobic capacity have been associated with improved or maintained brain structure and cognitive function in healthy individuals and in people with mild cognitive impairment.1-5 Recent studies found associations between aerobic capacity and cognitive and cerebral structural changes in persons with Alzheimer’s disease (AD) as well, suggesting that aerobic exercise training may play a therapeutic role in AD through biologically sound pathways, although this remains to be fully elucidated.6,7 However, the current understanding of aerobic capacity in persons with AD is limited to a few studies that have investigated peak oxygen consumption ( peak) obtained via graded treadmill testing in patients with relatively mild AD.1,2,4,8,9 Less is known about peak using other methods such as cycle ergometer testing in older adults with mild to moderate AD. More importantly, older adults with AD have significant gait instability and balance problems which are partially attributable to both the process of aging and the pervasiveness of cognitive impairment present in persons with AD, raising safety concerns during treadmill walking.10-16 In addition, gait instability could artificially reduce peak due to handlebar gripping, despite the walking familiarity of treadmill testing. This is particularly important because older adults represent the majority of the AD population. Alternative forms of exercise testing in this population are therefore required to ensure safety and accurate assessment of aerobic capacity. Hence, the purpose of this study was to determine whether older adults with mild–moderate AD can successfully complete an individualized peak cycle ergometer exercise test with assessment of peak and to assess the relationship of peak with physical and cognitive function. We hypothesized that at least 80% of persons with AD would be able to complete the peak cycle ergometer test and that aerobic capacity would be associated with measures of physical and cognitive function.
Methods
Of the first 57 participants who completed all screening tests in the FIT-AD trial, 44 who met the eligibility criteria were included for this study. The FIT-AD trial is an ongoing exercise intervention study that aims to enroll a total of 90 participants and its protocol and inclusion/exclusion criteria have previously been described in detail.17 Briefly, English-speaking older (>66 years) community-dwelling adults with diagnosed mild to moderate AD (defined as 0.5-2 on the Clinical Dementia Rating [CDR] scale and a score of 15-26 on the Mini-Mental State Examination [MMSE]) were enrolled in the study. Participants were recruited via a variety of methods including mailings, advertisements, and clinical referrals. Potential participants who have a resting heart rate of <50 or >100 beats/min, cardiac ischemia, significant arrhythmias, neurological/psychiatric disorders other than AD, chemical dependency, inability to cycle, or any contraindications to exercise per American College of Sports Medicine (ACSM) guidelines were excluded.18 This study was approved by the local university’s institutional review board, and informed consent or surrogate consent/participant assent was obtained during an in-person screening visit. All testing procedures took place at the University’s Clinical Translational Science Institute and the School of Nursing Laboratory of Clinical Physiology.
Outcome Variables and Their Measures
All assessors were trained and certified by the investigators. The training included 3 months of readings, lectures, demonstration testing, practice testing, and supervised test runs with the initial participants. All assessors participated in ongoing training to maintain proper procedures. Fidelity checks were conducted on a regular basis and assessors were briefed and debriefed to ensure compliance with the study protocol. All peak exercise testing was conducted by a team that consisted of a trained exercise technician, study staff, and a physician coinvestigator. All physical function tests were conducted by trained research staff.
Aerobic capacity and fitness levels were measured by a peak cycle ergometer test, the incremental shuttle walk test (ISWT),19 and the 6-minute walk test.20 Peak cycle ergometer test: Participants began pedaling at a speed comfortable to them (40-60 rpm). The intensity of cycling was then increased every 3 minutes by increasing the watt to achieve an increase in energy expenditure of 1 metabolic equivalent (MET; 1 MET = 3.5 mL oxygen/kg body weight/minute: estimated resting oxygen consumption). The Borg rating of perceived exertion (RPE) was used to assess the perceived level of exercise exertion based on participant’s report during the last minute of each stage and at peak exercise. The participants continued to exercise until meeting one of the predefined stopping criteria or reaching volitional fatigue/asked to stop or had symptoms that indicated test termination as outlined by the ACSM.18 The predefined stopping criteria were: (a) inability to maintain the required 40 to 60 rpm with increasing exercise intensity above ventilatory threshold; (b) achieving an RPE (Borg RPE) of ≥17/20; or (c) no increase in oxygen consumption or heart rate with increasing exercise intensity. The test was performed in the postabsorptive state with no tobacco or caffeinated beverages in the previous 8 hours. Heart rate and blood pressure were assessed at rest, during the last minute of each stage, at peak exercise, immediately after cessation of exercise, and every 3 minutes until return to baseline levels. Heart rhythm was continuously monitored via electrocardiogram. Expired gases were measured continuously by mouthpiece and nose clip using breath-by-breath analysis averaged by 5 to 7 seconds. The metabolic cart was calibrated prior to each testing session using known precision calibration gases according to manufacturer’s instruction. Peak oxygen consumption was defined as the median oxygen consumption during the last 30 seconds before cessation of exercise. Outcome variables of interest obtained from the cycle ergometer tests were peak (considered aerobic capacity), peak MET (multiples of resting energy expenditure and considered to be a measure of peak exercise capacity), peak heart rate, and peak RPE.
Shuttle walk test, an externally paced incremental test that stresses participants to peak performance, was conducted as following: Participants walked a circuit around a pair of markers placed 9 m apart while being paced by a recording of beeps. The participant had to arrive from one marker to another marker at the time of the beep (1 shuttle). Failure to arrive at a marker by the time of the beep 2 consecutive times marked the end of the test. Verbal cues “stop” and “walk” were given to coincide with the beeps. The distance walked in meters was considered the outcome variable.19
The 6-minute walk test was conducted according to standard guidelines. The distance covered in 6 minutes a walking speed intended to cover as much distance as possible was the used as the outcome variable. The distance walked in meters was considered the outcome variable. The 6-minute walk test has a reported 0.58 to 0.96 validity and 0.65 to 0.92 reliability.20 We have previously tested the symptom-limited peak cycle ergometer test without measuring peak, the shuttle walk test, and the 6-minute walk test in persons with AD.21,22
Physical function was measured by the Short Physical Performance Battery (SPPB), which has 3 subscales: balance, gait speed, and sit to stand (each scored 0-4) with a sum score of 0 to 12 (higher score = better performance). The test–retest and interrater reliabilities are both >.90 in older adults.23 The subscores and total scores on the SPPB were considered the outcome variables of interests in this study.
The AD stage was assessed using the CDR during the in-person interview. The AD stage was used as a measure of dementia severity.
Cognition was measured using the MMSE. The MMSE assesses orientation, memory, recall, constructional praxia, and language with a total score of 30. Higher score indicates better global cognition. The MMSE has a reliability of .98.24
Demographics of interest included age, gender, race, education level, comorbidities, and anthropometrics since these covariates have been linked to exercise capacity.
Statistical Analysis
Continuous variables were summarized by means and standard deviations (SDs) and categorical variables were summarized by frequency and percentage and were used to describe the sample. Pearson bivariate correlation was used to assess simple association between variables for the entire sample. Correlational analyses for CDR were conducted by point biserial correlation due to low distribution (see “Results” section). One participant, an avid cyclist, achieved a substantially higher peak. We subsequently conducted the correlation analyses involving peak and cognitive measures with and without this participant to ensure that results were not affected by this individual. A post hoc analysis was performed to investigate whether associations were different between participants who met criteria for disability versus participants with adequate peak to perform meaningful work. We separated participants into 2 groups based on achieving a peak of >15 mL/ kg/min or not achieving this level. The peak limit of 15 mL/kg/min was chosen because it is considered to be the criteria for objective evidence of functional disability affecting activities of daily living (ADLs) and where no meaningful work can be performed.25 Independent t test was used to compare continuous demographic, cognitive, and fitness variables between high and low fitness levels. A χ2 test was used to compare categorical variables. We conducted the same correlation analyses as completed for the entire sample using Pearson bivariate correlation and point biserial for CDR correlational analyses.
Results
Demographics are presented in Table 1. Of the 59 participants screened in person, 10 participants were excluded and referred for evaluation due to abnormal stress test response and 3 were further excluded due to not meeting the AD criteria on magnetic resonance imaging (also known as having a non-AD dementia) and 2 were excluded due to an inability to bend the knees. The final sample consisted of 44 participants (mean age 78.4 [SD 6.8], 55% male, 98% white), with a CDR range of 0.5 to 2 and a mean MMSE score of 21.6 (SD 3.3). The CDR 0.5 (n = 19), CDR 1 (n = 24), and CDR 2 (n = 1) are considered mild–moderate AD, and the majority of the sample was treated pharmaceutically for AD (70.5%).
Table 1.
Demographics and Health Characteristics.
| Variable | n | Mean (SD) |
|---|---|---|
| Age (years) | 44 | 78.4 (6.8) |
| Weight (kg) | 44 | 77.1 (18.1) |
| BMI (kg/m2) | 44 | 28.1 (5.4) |
| CDR | 44 | 0.81 (0.31) |
| MMSE | 44 | 21.6 (3.3) |
| Education (years) | 43 | 15.7 (3.3) |
| Variable | n | % |
| Hypertension | 24 | 55 |
| Dyslipidemia | 21 | 48 |
| Diabetes | 8 | 18 |
| CVD | 9 | 21 |
| β-blocker | 6 | 14 |
| ACEI | 11 | 25 |
| ARB | 6 | 14 |
| Diuretic | 4 | 9 |
| Statin drugs | 16 | 36 |
| ASA | 16 | 36 |
Abbreviations: ACEI, currently on angiotensin-converting enzyme inhibitor; ARB, currently on angiotensin receptor blocking agent; ASA, currently on aspirin; β-blocker, currently on β-blocking agent; BMI, body mass index; CDR, Clinical Dementia Rating scale (0-3, higher score is worse dementia); CVD, cardiovascular disease, reported yes on medical history; diabetes, reported yes on medical history; dyslipidemia, reported yes on medical history; hypertension, reported yes on medical history; MMSE, Mini-Mental State Examination (0-30, lower score is worse cognitive function); SD, standard deviation; statin, currently on statin drug.
Success of Cycle Ergometer Test Completion
There were no unanticipated adverse events related to exercise testing procedures. We were unable to obtain peak in 1 participant due to instrumentation failure to assess oxygen consumption and 1 participant refused to wear the mouthpiece. All participants were able to complete peak cycle ergometer testing successfully, with all participants (100%) stopping due to meeting one of the predefined stopping criteria for successful completion of the test. This supports our hypothesis of at least 80% success rate (Figure 1). Among those with reliable respiratory exchange ratio (RER) readings (n = 40, 3 participants were excluded due to faulty CO2 capture and 1 participant refused the mouthpiece), 11 participants achieved an RER >1.1, the criterion for achieving an adequate exercise effort per standard guidelines for determination of peak exercise capacity.18,26 However, reports in the literature suggest that an RER ≥1.0 should be used for determination of adequate exercise effort in older adults due to the age-associated shift to an oxidative metabolic profile.27 All 40 participants were able to achieve an RER >1.0 (mean peak RER = 1.07 [SD 0.06]). Nine participants met ACSM’s criteria for maximal exercise testing (ie, having met 2 of the following: RER >1.15, RPE ≥17, plateau of <150 mL/min/kg, heart rate of 10 bpm of predicted heart rate max).18 The mean reported RPE was 15.3 (2.1). The most frequently reported peak RPE were 15 (n = 13) and 17/20 (n = 12). One participant was unwilling to provide an RPE estimate and 11 participants gave questionable RPE estimates compared to objectively determined work rate based on RER defined as an RPE of ≤14 with an RER >1.0.
Figure 1.
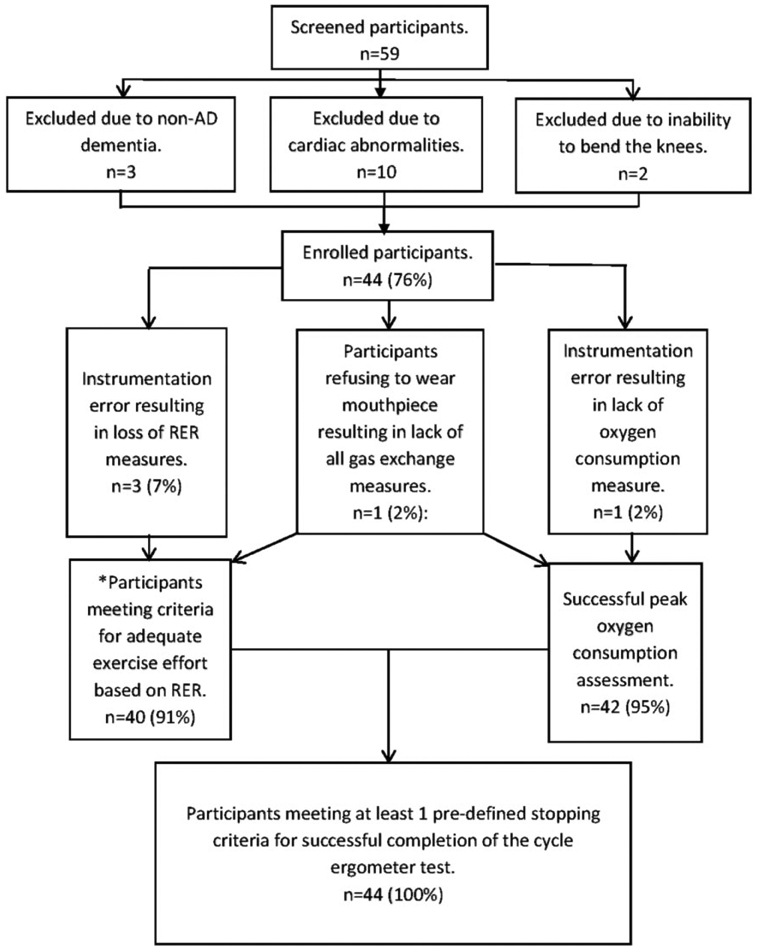
Flow diagram of success rate of the cycle ergometer test. *All participants with reliable respiratory gas exchange measures were able to reach adequate work effort for determining peak in older adults. RER indicates respiratory exchange ratio.
Descriptives of Exercise Capacity and Physical Function Measures
The mean aerobic capacity (peak ) was 16.5 (3.1) mL/ kg/min, mean peak MET was 6.3 (1.2), and mean SPPB score = 10 (2.3), shuttle walk = 257 (124) m, 6-minute walk = 381 (105) m (Table 2). One participant refused to participate in the ISWT and 6-minute walk distance (6 MWD) and was subsequently excluded from analyses involving ISWT and 6 MWD.
Table 2.
Physical Fitness and Physical Functioning Measuresa
| Variable | n | Mean (SD) |
|---|---|---|
| Peak (mL/kg/min) | 42 | 16.5 (3.1) |
| Peak MET | 44 | 6.3 (1.2) |
| Peak RPE | 44 | 15.3 (2.1) |
| Peak RER | 40 | 1.07 (0.06) |
| Peak heart rate (beats per minute) | 44 | 115 (17) |
| Double product (mm Hg·bpm) | 44 | 20 498 (4693) |
| SPPB score | 44 | 10.0 (2.3) |
| SPPB balance | 44 | 3.6 (0.69) |
| SPPB gait speed | 44 | 3.6 (0.65) |
| SPPB chair rise | 44 | 2.8 (1.3) |
| ISWT (m) | 43 | 257 (124) |
| 6-MWD (m) | 43 | 381 (105) |
Abbreviations: peak , peak oxygen consumption; ISWT, incremental shuttle walk test; 6 MWD, 6-min walk distance; Peak MET, peak metabolic equivalent (1 MET = 3.5 mL/kg/min in oxygen consumption and is considered a measure of multiples of resting energy expenditure); RER, respiratory exchange ratio (ratio of carbon dioxide produced/exhaled and oxygen used); RPE, rating of perceived exertion (6-20, higher is more exertion); SD, standard deviation; SPPB, Short Physical Performance Battery (0-12, higher score = better physical function); SPPB balance score, 0-4, higher is better balance; SPPB chair rise score, 0-4, higher is better; SPPB gait speed score, 0-4, higher is better gait speed.
See text for the commonly reported age-appropriate normative values.
Associations of Aerobic Capacity With Physical Functioning and Cognitive Functioning
Peak was strongly correlated with peak MET levels (r = .67, P ≤ .001); moderately correlated with SPPB score (r = .36, P = .023), shuttle walk (r = .35, P = .024), 6-minute walk (r = .33, P = .038), and peak heart rate (r = .36, P = .021); and inversely correlated with age (r = −.40, P = .01), but not with peak RPE, weight, cardiovascular disease, or other comorbidities for the entire sample. Subscales of the SPPB showed moderate correlations with the 6-minute walk test and the shuttle walk. peak was correlated with chair rise score but did not correlate significantly with balance or 4-m walking speed scores (Table 3).
Table 3.
Correlational Analyses.
| Peak | MET | ISWT | 6 MWD | SPPB | SPPB-CR | SPPB-Bal | 4-m walk | CDR | MSSE | Age | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak | XX | .67b | .36a | .33a | .35a | .32a | .28 | .30 | .22 | −.25 | −.40a |
| Peak MET | .48b | .51a | .35a | .33a | .25 | .29 | −.05 | −.04 | −.37a | ||
| ISWT | .89b | .65b | .62b | .42b | .59b | −.11 | .01 | −.52b | |||
| 6 MWD | .58b | .57b | .30a | .55b | −.08 | .03 | −.46b | ||||
| SPPB | .92b | .77b | .84b | .07 | −.22 | −.35a | |||||
| SPPB-CR | .51b | .65b | .12 | −.19 | −.27 | ||||||
| SPPB-Bal | .59b | .02 | −.17 | −.32a | |||||||
| 4-m walk | −.03 | −.19 | −.36a | ||||||||
| CDR | −.60b | −.05 | |||||||||
| MMSE | .16 |
Abbreviations: CDR, Clinical Dementia Rating; ISWT, incremental shuttle walk test; peak MET, peak metabolic equivalent; peak , peak oxygen consumption; MMSE, Mini-Mental State Examination; 6 MWD, 6-minute walk distance; 4-m walk, Short Physical Performance Battery–4-m walking speed; SPPB, Short Physical Performance Battery total score; SPPB-CR, Short Physical Performance Battery–chair rise.
P < .05.
P < .01.
Associations between aerobic capacity and physical function measures are presented in Table 3. The SPPB score was inversely correlated with age and strongly correlated with peak heart rate during the cycle ergometer exercise test, shuttle walk distance, and 6 MWD. The shuttle walk distance was inversely correlated with age, moderately correlated with peak MET achieved during the cycle ergometer exercise test, and strongly correlated with 6 MWD. The 6 MWD correlated moderately with peak MET achieved during the cycle ergometer exercise test and inversely correlated with age. Age remained the only variable that consistently correlated with physical functioning variables and aerobic capacity, with the exception for the SPPB subscale score on chair rise.
Correlational analyses between aerobic capacity and AD severity and cognition indicated no significant correlations for the entire sample (Table 3). The CDR score was inversely correlated with peak RPE (r = −.32, P = .035). Analysis controlling for age did not change outcomes between AD severity, cognitive function measures, and physical function and fitness variables.
Participants with a peak of <15 had significantly more hypertension burden, higher body mass index, and lower physical function on the ISWT (Table 4). There were no statistically significant differences between groups in age, medication use, gender, MMSE, or CDR scores. There was a strong inverse correlation between peak MET level and CDR score in the low fitness group only (r = −.55, P = .028). This association remained after controlling for age and gender. Age remained significantly correlated across all physical fitness variables in the high fitness group only, except for SPPB score (data not shown).
Table 4.
Demographics of Participants With a Peak <15 mL/kg/min or >15 mL/kg/min.
| Peak <15 (n = 16), Mean (SD) |
Peak >15 (n = 28), Mean (SD) |
Significance Independent t Test P Value |
|
|---|---|---|---|
| Age (years) | 80.3 (6.7) | 77.4 (6.7) | .18 |
| BMI (kg/m2) | 31 (6) | 27 (4.5) | .03 |
| Physical functioning | |||
| 6-minute walk distance (m) | 348 (85) | 401 (112) | .08 |
| ISWT (m) | 206 (95) | 288 (131) | .02 |
| SPPB score | 9.4 (2.4) | 10.4 (2.2) | .19 |
| Cognitive functioning | |||
| CDR (score) | 0.81 (.25) | 0.80 (.34) | .92 |
| MMSE (score) | 21.9 (3.0) | 21.4 (3.5) | .61 |
| n (%) | n (%) | Significance (χ2) P Value |
|
| Gender (male) | 9 (56) | 15 (54) | .87 |
| Hypertension (yes) | 12 (75%) | 12 (43%) | .04 |
| Presence of CVD (yes) | 4 (33%) | 5 (18%) | .58 |
Abbreviations: BMI, body mass index; CDR, Clinical Dementia Rating scale score (0-3, higher score = more dementia); CVD, cardiovascular disease reported yes on medical history; hypertension, reported yes on medical history; ISWT, incremental shuttle walk test; MMSE, Mini-Mental State Examination score (0-30, lower score = lower cognitive function); SPPB, Short Physical Performance Battery total score (0-12, higher score better physical function).
Discussion
The results demonstrate that older adults with mild to moderate AD can successfully and safely perform a peak cycle ergometer test for determination of peak aerobic capacity, shuttle walk test, 6-minute walk test, and the SPPB. All participants (100%) met at least one of the predefined stopping criteria for successful completion of the test. This finding meets our consensus a priori criteria that at least 80% of enrolled participants would be able to successfully complete the cycle ergometer test. All participants with reliable respiratory gas exchange measures were able to reach an RER of >1.0, suggesting that adequate work effort was reached for determining peak in older adults. These findings support the use of cycle ergometer exercise testing in older adults with AD. Previous studies have shown that treadmill testing in persons with early AD is safe and reliable but have not focused on older adults with AD.8,9 Treadmill testing has also been more commonly used when assessing aerobic capacity in persons with AD. However, even though treadmill testing has the advantage of walking familiarity, older adults with AD have gait, balance, and cognitive problems that make an electronically powered treadmill predispose them to falls and injuries.10,11 Moreover, persons with AD are known to have gait instability and balance issues beginning even before mild AD symptoms are manifested. In fact, progressive AD pathology in the brain structure appears to cause gait and motor problems and AD-related brain functional connectivity further contributes to disorganization in the locomotion area of the brain.10-16 Cognitive impairment further causes persons with AD to become disoriented in the middle of a task, for example, a person with AD may suddenly stop walking to do something else. This could potentially affect outcomes and relationships between aerobic capacity and cognition, especially if persons are allowed to hold the handlebar for balance purposes and thereby artificially lowering oxygen consumption.18 The balance problems that may occur with treadmill testing are effectively eliminated by using a cycle ergometer exercise test. We specifically chose a recumbent cycle ergometer to prevent any balance issues that could occur with an upright cycle ergometer. By utilizing a cycle ergometer protocol, we were able to strictly control workload, while individualizing the exercise test to match each individual’s abilities in order to reach a peak exercise level. The possible disadvantage of cycle ergometer testing is related unfamiliarity of cycling leading to participants stopping due to peripheral fatigue rather than central fatigue and thereby lowering achieved peak aerobic capacity, which has been observed in other populations.18
We also experienced an unanticipated problem with participants’ range of motion preventing them from completing a full revolution, related to participants being poststatus total knee replacement and an inability to keep feet firmly on the pedal and in the pedal straps. We addressed this problem by allowing slower rpms (40 rpm) than initially anticipated and adjusting the cycle ergometer to allow for less than optimal knee flexion and by adjusting the pedal straps to allow unusually large footwear and ankle angles to fit securely in the pedal straps.18 Despite these adjustments, we were unable to test 2 participants due to an inability to perform sufficient knee flexion to allow for cycle ergometer testing or training; these participants were subsequently excluded from the study and not counted as part of the study sample. Our findings that people with AD can safely and successfully perform the SPPB, 6-minute walk test, and the ISWT are supportive of previous reports. However, to our knowledge, this is the first study that have compared peak obtained via cycle ergometer testing to the SPPB, 6-min walk test, and the ISWT in this population that included older participants with more advanced AD and worse cognition than previously assessed.
Aerobic capacity and physical function in our study sample were all below age- and sex-matched normative levels, suggesting that older adults with AD have reduced physical fitness levels contributing to early loss of independence.28-30 Normative values at the study sample mean age of 78 are commonly considered to be 514 m on the 6-minute walk test, with the 10th percentile reported at approximately 334 to 361 m.28,29 The 25th percentile is commonly reported as <430 for women and <470 for men.29 Importantly, any score lower than the 25th percentile is considered as low functioning status.18 Our mean 6-minute walk test was substantially lower than the 25th percentile, suggesting low physical functioning status with only 17% of men and 40% of women scoring over the 25th percentile. The normative values for the ISWT in healthy adults over age 70 has been reported to be 633 m.30 The mean of our sample was 59% below the ISWT normative value, with all participants scoring well below the normative value of 633 m, further underscoring the reduction in the physical fitness levels in older adults with AD. Moreover, 36% of the study sample scored <10 on the SPPB and would be considered at moderate risk of functional disability.31,32 However, the range of SPPB scores was large with participants ranging from a score of 4 to 12 (total score possible = 12), suggesting that physical functioning varies substantially in this population and according to our data is mostly related to age. The SPPB scores were similar to the Finnish Alzheimer disease exercise trial (FINALEX) study.33
Aerobic capacity that is adequate to allow individuals to perform ADLs is essential to maintain independence. A large subgroup (37%) of older AD patients were not able to achieve a cardiorespiratory fitness level above the minimum of 15 mL/kg/min required for maintenance of independent ADLs, suggesting that more effort should be placed on maintaining physical fitness levels via exercise training.25 Moreover, only 15% of participants were able to achieve a peak >20 mL/kg/ min, suggesting that the older people with AD have overall poor aerobic capacity predisposing them to premature loss of independence.4 Peak in older adults and octogenarians is commonly reported to range between 23 and 30 mL/kg/min, although there are no definitive normative values in older adults firmly established as of yet.18,34,35 Our sample had substantially lower mean peak compared to previous reports in patients with AD using treadmill testing. Burns et al1 reported a mean peak of 34.7 mL/kg/min and Vidoni et al2 reported a mean peak of 20.5 mL/kg/min. Anderson et al9 also reported mean peak levels at 20 to 20.3 mL/kg/ min, while Billinger et al8 reported levels ranging from 18 to 21 mL/kg/min. The differences between these reports and the findings in the present study may be related to the use of treadmill testing versus cycle ergometer testing in the present study. Moreover, our study sample was older and included a sample with over 50% CDR-1, which were more cognitively impaired on the MMSE compared to these studies.
The significant correlations between aerobic capacity and physical function suggest that the SPPB, 6-minute walk test, and the shuttle walk may be useful in identifying individuals who have low aerobic capacity in a clinical setting. Determination of peak requires specific equipment and expertise commonly not available in the usual clinic setting, and although the 6-minute walk test and the shuttle walk test are feasible to complete in a usual clinical setting, it still requires space and provider time, making it less likely for regular clinical use. It is possible that the SPPB or even the SPPB subscale of chair rise alone, as suggested by the correlation with peak in our study, can be used for identification of individuals with poor aerobic capacity in a short clinic visit. Moreover, the finding that age is the only continuously correlated variable emphasizes the need for clinicians to evaluate physical function in older people with AD and initiate behavioral therapies such as exercise when needed in order to maintain physical function and ADLs.
Contrary to our hypothesis, there were no significant correlations between aerobic capacity and cognition, possibly due to the small sample size and limited range of AD severity. Our findings are in support of previous studies that have shown no correlations between cardiorespiratory fitness and CDR.1,2 However, other studies have indicated a moderate correlation between cognitive function measures, brain structure, and aerobic capacity in healthy persons and persons with AD.1,3,5,36,37 One possible explanation of our finding may be related to the use of a cycle ergometer test instead of treadmill testing. Treadmill testing is known to result in higher peak values, but is also dependent on participants being able to maintain balance while walking on an incline.18 Thus, the use of a cycle ergometer test may be more reflective of aerobic capacity independent on balance issues. However, the use of the measures of CDR, MMSE, instead of brain imaging may also have contributed to the present findings. The MMSE and the CDR may not be sensitive enough to pick up on neurocognitive changes consistent with AD (such as delayed recall measures or the Trails B). The FIT-AD study plans on using a battery of neuropsychological measures that are more sensitive to domain-specific cognitive changes following intervention. It is plausible that a larger study with more sensitive neuropsychological and imaging measures would yield different results.
The observation of a significant correlation between peak MET level and CDR in the low fitness group suggests that the association between AD severity and aerobic capacity may not become apparent until aerobic capacity has deteriorated below levels required for meaningful work to be performed. This is in support of previous studies that suggest that aerobic capacity is related to cognitive function and that an increase in physical fitness levels may moderate age-related brain changes and possible AD-related brain changes, the so-called cardiorespiratory fitness hypothesis.36,38,39 It is also possible that people with AD subconsciously reduce usual physical activity levels, which results in lower aerobic capacity and physical fitness levels. This in turn increases the risk of frailty and loss of independence. The results from this study do not support this hypothesis for the entire sample, but rather only in the low fitness sample. It is also plausible that patients with AD are unwilling to reach uncomfortable levels of effort and thereby artificially reduce peak aerobic capacity levels. A large group of participants in this study did not reach a peak RPE >15 (n = 11), and we observed a moderate correlation with RPE and AD severity as measured by CDR score. This suggests that AD severity may affect peak testing outcomes due to apathy or an inability or unwillingness to perform exercise at an uncomfortable effort level or an inability to cognitively understand peak effort.40 This finding is different from Burns et al who did not find a correlation between RPE and dementia.1 This difference was likely due to our study sample having a larger range of AD severity and included participants with more severe AD. Researchers should take these considerations into account when designing exercise testing and training programs40 and possibly use criteria other than RPE for determination of exercise intensity (eg, heart rate or metronome pacing).
Although much research is needed, there is evidence suggesting that the AD disease process follows a biological pathway (the biological hypothesis) that contributes to a reduction in aerobic capacity. Specifically, there appears to be a reduction in both peripheral and central mitochondrial capacity in AD, which may result in low aerobic capacity.41 Moreover, animal and human studies have shown that an increase in fitness levels leads to enhanced survival of brain neurons following neuronal insults and that exercise training increases brain vascularization and growth factors and thereby stimulates hippocampal and cortical neurogenesis as reviewed by Paillard et al6 and Bherer et al42 Future studies should focus on these areas of research.
Strength and Limitations
We enrolled a sample consisting of older adults with AD that has been largely neglected in the majority of published AD studies and we introduced a battery of testing procedures not performed in previous studies in order to fully investigate aerobic capacity and physical function in this population. We present the first study comparing peak obtained via cycle ergometer testing to the 6-minute walk test, the ISWT, and the SPPB and we provide a safe alternative to treadmill-assessed aerobic capacity in older adults with AD. The results are limited due to a small sample size, which does not allow for generalization nor temporal associations. The use of the MMSE and CDR may not have been sensitive enough to detect subtle changes in neurocognition associated with AD. The sample was a convenience sample of participants taking part in a randomized controlled exercise intervention trials with mild to moderate AD, and we were not able to compare the results to a treadmill test. Lastly, the study design does not allow for causal determination of these correlations.
Conclusions
This is the first study to our knowledge that have investigated peak achieved during a peak cycle ergometer test in older adults with AD and its association with physical and cognitive function. Older adults with AD can safely and successfully complete a peak cycle ergometer exercise testing and physical function assessment. These data suggest that older adults with AD have a below population norm aerobic capacity and physical functioning and that aerobic capacity is associated with physical function, but not cognitive function. Although the aerobic capacity and physical function measures are correlated, we suggest that all measures be used to adequately quantify physical functioning in this population. Future studies are clearly needed to elucidate and define the role of aerobic capacity and physical function in AD progression and to clarify the use of exercise training as an adjunctive therapeutic modality in the prevention and management of AD. The FIT-AD trial is poised to provide the starting point to many of these unanswered questions.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health National Institute on Aging award number R01AG043392 (Yu). Trials registration (NCT01954550). The CTSI were supported by the National Institutes of Health National Center for Advancing Translational Sciences (UL1TR000114).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Burns JM, Cronk BB, Anderson HS, et al. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71(3):210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vidoni ED, Gayed MR, Honea RA, Savage CR, Hobbs D, Burns JM. Alzheimer disease alters the relationship of cardiorespiratory fitness with brain activity during the Stroop task. Phys Ther. 2013; 93(7):993–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wendell CR, Gunstad J, Waldstein SR, Wright JG, Ferrucci L, Zonderman AB. Cardiorespiratory fitness and accelerated cognitive decline with aging. J Gerontol A Biol Sci Med Sci. 2014; 69(4):455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vidoni ED, Billinger SA, Lee C, Hamilton J, Burns JM. The physical performance test predicts aerobic capacity sufficient for independence in early-stage Alzheimer disease. J Geriatr Phys Ther. 2012;35(2):72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hess NCL, Dieberg G, McFarlane JR, Smart NA. The effect of exercise intervention on cognitive performance in persons at risk of, or with, dementia: a systematic review and meta-analysis. Healthy Aging Res. 2014;3(3): 1–10. doi: 10.12715/har.2014.3.3. [DOI] [Google Scholar]
- 6.Paillard T, Rolland Y, de Souto Barreto P. Protective effects of physical exercise in Alzheimer’s disease and Parkinson’s disease: a narrative review. J Clin Neurol. 2015;11(3): 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim MJ, Han CW, Min KY, et al. Physical exercise with multicomponent cognitive intervention for older adults with Alzheimer’s disease: a 6-month randomized controlled trial. Dement Geriatr Cogn Dis Extra. 2016;6(2):222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Billinger SA, Vidoni ED, Honea RA, Burns JM. Cardiorespiratory response to exercise testing in individuals with Alzheimer’s disease. Arch Phys Med Rehabil. 2011;92(12): 2000–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson HS, Kluding PM, Gajewski BJ, Donnelly JE, Burns JM. Reliability of peak treadmill exercise tests in mild Alzheimer disease. Int J Neurosci. 2011;121(8):450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zidan M, Arcoverde C, Araujo NB, Rios A, Laks J. Motor and functional changes in different stages of Alzheimer’s disease. Rev Psiquiatr Clin. 2012;29(5):161. [Google Scholar]
- 11.Sheridan PL, Hausdorf JM. The role of higher-level cognitive function in gait: executive dysfunction contributes to fall risk in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24(2): 125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coelho FG, Stella F, de Andrade LP, et al. Gait and risk of falls associated with frontal cognitive functions at different stages of Alzheimer’s disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2012;19(5):644–656. [DOI] [PubMed] [Google Scholar]
- 13.Sheridan PL, Solomont J, Kowall N, Hausdorff JM. Influence of executive function on locomotor function: divided attention increases gait variability in Alzheimer’s disease. J Am Geriatr Soc. 2003;51(11):1633–1637. [DOI] [PubMed] [Google Scholar]
- 14.Manckoundia P, Mourey F, Pfitzenmeyer P, Papaxanthis C. Comparison of motor strategies in sit-to-stand and back-to-sit motions between healthy and Alzheimer’s disease elderly subjects. Neuroscience. 2006;137(2):385–392. [DOI] [PubMed] [Google Scholar]
- 15.Verghese J, Robbins M, Holtzer R, et al. Gait dysfunction in mild cognitive impairment syndromes. J Am Geriatr Soc. 2008;56(7): 1244–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010;67(8):980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu F, Bronas UG, Konety S, et al. Effects of aerobic exercise on cognition and hippocampal volume in Alzheimer’s disease: study protocol of a randomized controlled trial (the FIT-AD trial). Trials. 2014;15:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American College of Sports Medicine. Guidelines for Exercise Testing and Prescription. 9th ed. Baltimore, MD: Lippincott Williams and Wilkins; 2014. [Google Scholar]
- 19.Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax. 1992;47(12):1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001; 119(1):256–270. [DOI] [PubMed] [Google Scholar]
- 21.Yu F, Thomas W, Nelson NW, Bronas UG, Dysken M, Wyman JF. Impact of 6-month aerobic exercise on Alzheimer’s symptoms. J Appl Gerontol. 2015;34(4):484–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu F, Savik K, Wyman JF, Bronas UG. Maintaining physical fitness and physical function in Alzheimer’s disease: a pilot study. Am J Alzheimers Dis Other Demen. 2011;26(5):406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guralnik JM, Simonsick EM, Ferrucci L, et al. A Short Physical Performance Battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontology. 1994; 49(2):M85. [DOI] [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 25.Institute of Medicine, ed. Cardiovascular Disability: Updating the Social Security Listings. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 26.Balady GJ, Arena R, Sietsema K, et al. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2): 191–225. [DOI] [PubMed] [Google Scholar]
- 27.Edvardsen E, Hem E, Anderssen SA. End criteria for reaching maximal oxygen uptake must be strict and adjusted to sex and age: a cross-sectional study. PLoS One. 2014;9(1):e85276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casanova C, Celli BR, Barria P, et al. The 6-min walk distance in healthy subjects: reference standards from seven countries. Eur Respir J. 2011;37(1):150–156. [DOI] [PubMed] [Google Scholar]
- 29.Rikli RE, Jones J. Functional fitness normative scores for community-residing older adults, ages 60-94. J Aging Phys Act. 1999;7:162–181. [Google Scholar]
- 30.Harrison SL, Greening NJ, Houchen-Wolloff L, et al. Age-specific normal values for the incremental shuttle walk test in a healthy British population. J Cardiopulm Rehabil Prev. 2013; 33(5):309–313. [DOI] [PubMed] [Google Scholar]
- 31.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the Short Physical Performance Battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–M231. [DOI] [PubMed] [Google Scholar]
- 32.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9): 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pitkälä KH, Pöysti MM, Laakkonen ML, et al. Effects of the Finnish Alzheimer disease exercise trial (FINALEX): a randomized controlled trial. JAMA Intern Med. 2013;173(10):894–901. [DOI] [PubMed] [Google Scholar]
- 34.Fleg JL, Morrell CH, Bos AG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112(5):674–682. [DOI] [PubMed] [Google Scholar]
- 35.Sidney KH, Shephard RJ. Maximum and submaximum exercise tests in men and women in the seventh, eighth, and ninth decades of life. J Appl Physiol Respir Environ Exerc Physiol. 1977;43(2): 280–287. [DOI] [PubMed] [Google Scholar]
- 36.Erickson KI, Kramer AF. Aerobic exercise effects on cognitive and neural plasticity in older adults. Br J Sports Med. 2009;43(1): 22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erickson KI, Prakash RS, Voss MW, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippo-campus. 2009;19(10):1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Etnier JL, Nowell PM, Landers DM, Sibley BA. A metaregression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Rev. 2006;52(1):119–130. [DOI] [PubMed] [Google Scholar]
- 39.Smiley-Oyen AL, Lowry KA, Francois SJ, Kohut ML, Ekkekakis P. Exercise, fitness, and neurocognitive function in older adults: the “selective improvement” and “cardiovascular fitness” hypotheses. Ann Behav Med. 2008;36(3):280–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cummings J, Friedman JH, Garibaldi G, et al. Apathy in neuro-degenerative diseases: recommendations on the design of clinical trials. J Geriatr Psychiatry Neurol. 2015;28(3):159–173. [DOI] [PubMed] [Google Scholar]
- 41.Swerdlow RH, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis: an update. Exp Neurol. 2009;218(2):308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bherer L, Erickson KI, Liu-Ambrose T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J Aging Res. 2013;2013:657508. [DOI] [PMC free article] [PubMed] [Google Scholar]


