Abstract
Background:
Knowledge gaps remain in the epidemiology and clinical implications of myocardial injury in COVID-19. Our goal was to determine the prevalence and outcomes of myocardial injury in severe COVID-19 compared to acute respiratory distress syndrome (ARDS) unrelated to COVID-19.
Methods:
We included intubated COVID-19 patients from 5 hospitals between March 15 and June 11, 2020 with troponin levels assessed. We compared them to patients from a cohort study of myocardial injury in ARDS. We performed survival analysis with primary outcome of in-hospital death associated with myocardial injury. We performed linear regression to identify clinical factors associated with myocardial injury in COVID-19.
Results:
Of 243 patients intubated with COVID-19, 51% had troponin levels > upper limit of normal (ULN). Chronic kidney disease, lactate, ferritin and fibrinogen were associated with myocardial injury. Mortality was 22.7% among COVID-19 patients with troponin < ULN and 61.5% for those with troponin levels > 10xULN (P< 0.001). The association of myocardial injury with mortality was not statistically significant after adjusting for age, sex and multi-system organ dysfunction. Compared to non-COVID ARDS patients, patients with COVID-19 were older with higher creatinine and less favorable vital signs. After adjustment, COVID-19 was associated with lower odds of myocardial injury compared to non-COVID ARDS (OR 0.55 95% CI 0.36–0.84, P=0.005).
Conclusion:
Myocardial injury in severe COVID-19 is a function of baseline comorbidities, advanced age and multisystem organ dysfunction similar to traditional ARDS. The adverse prognosis of myocardial injury in COVID-19 relates largely to multisystem organ involvement and critical illness.
Keywords: COVID-19, troponin, myocardial injury, ARDS
Introduction
Myocardial injury manifested by elevations in cardiac troponin is common in COVID-19 patients1–3. Myocardial injury has also been proposed as a prognostic factor 4, 5. The pathogenesis of myocardial injury in COVID-19 is not established but is likely multifactorial due to patient, disease, and treatment specific factors 6. Important knowledge gaps remain in understanding the epidemiology and clinical implications of myocardial injury in COVID-19.
First, although crude mortality rates are higher in COVID-19 patients with myocardial injury compared to those without, variable covariate adjustment has been performed in studies to date 2, 4, 7–12. The dictum that a single marker of myocardial injury is independently prognostic in severe COVID-19 should be investigated with comparative studies. Second, the determinants of myocardial injury in COVID-19 are not well established. A conceptual model of COVID-19 myocardial injury includes systemic inflammation, hypoxemia and vasopressor requirement, and thrombophilia which remain to be clarified 6. Finally, it is not clear whether the incidence of myocardial injury in hospitalized COVID-19 patients is truly higher than that observed in traditional acute respiratory distress syndrome (ARDS). ARDS is one of the most common causes of hypoxemic respiratory failure 13 and manifests as acute hypoxemia within a week of a known pulmonary insult with bilateral pulmonary infiltrates not referable to alternate causes such as atelectasis or left heart failure 14. Up to 38% of ARDS patients have been shown to have troponin levels above the 99th percentile cut-point 15. Understanding whether the prevalence and pattern of myocardial injury in COVID-19 differ from ARDS unrelated to COVID-19 is important in defining the COVID-19 clinical phenotype, particularly given the ongoing debate as to whether COVID-19 related respiratory failure represents typical ARDS or not 16.
To contribute to these knowledge gaps, we performed 1) a retrospective cohort study of clinical factors and outcomes associated with myocardial injury in hospitalized patients critically ill with COVID-19 and 2) a comparative study of myocardial injury within a COVID-19 cohort to myocardial injury within a cohort of patients with ARDS unrelated to COVID-19. We hypothesized that myocardial injury would be present in a significant number of COVID-19 patients, and that after adjusting for degree of critical illness, the association of myocardial injury with mortality in COVID-19 would be mitigated. We also hypothesized that the prevalence and prognostic value of troponin in COVID-19 would be similar to that of general ARDS after covariate adjustment.
Methods
Study population
Data for patients with COVID-19 who required intubation were obtained from the Johns Hopkins Health System COVID-19 Precision Medicine Analytic Platform Registry (JH-CROWN). This registry aggregates electronic health data for all patients with COVID-19 across the 5 hospitals in the Johns Hopkins Healthcare System. We included all patients with confirmed COVID-19 who were intubated within our health system between March 15 and June 11, 2020 and who had troponin levels assessed within 24 hours of intubation. We focused our analysis only on intubated patients because of a priori scientific interest, because of high patient risk, and to facilitate a comparison of myocardial injury and outcomes with a prior cohort of intubated ARDS patients.
For a comparison with non-COVID ARDS patients, we used a subset of patients from a prior cohort study to assess myocardial injury in ARDS 15, 17, the MI-ARDS study. This cohort consists of patients with ARDS who were enrolled in clinical trials via the ARDS Network. The initial MI ARDS study consisted of patients with ARDS due to diverse causes including sepsis, trauma, transfusions and pneumonia. To enable a comparison of diseases with similar pathophysiology, we chose to use only ARDS patients who had ARDS due to pneumonia. We previously measured troponin using plasma taken within 24 hours of intubation in all patients 15, 17. Clinical and demographic data and outcomes were collected as part of trial protocols 18, 19. For this comparison, we used 506 patients with ARDS due to primary pneumonia. We chose to use primary pneumonia-ARDS as the comparison group because of the inherent heterogeneity of the ARDS syndrome 16, 20 and because COVID-19 patients manifest primary hypoxemic respiratory failure as an indication for intubation 21. The inclusion criteria and ARDS definition for patients in the MI ARDS study included a ratio of partial pressure of oxygen (PaO2) to fraction of inspired oxygen (FiO2) less than 300, bilateral pulmonary infiltrates, and no clinical evidence of elevated left atrial pressure.
The study was approved by the Johns Hopkins University Institutional Review Board (IRB00251735, committee IRB-3) as “exempt from review” due to the anonymized nature of the COVID JH-CROWN registry. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Troponin classification
All included patients in both cohorts had troponin levels assessed within the 24 hours prior to or after intubation to ensure uniform temporality of the exposure variable. The 5 hospitals contributing data to the JH-CROWN registry use either clinical troponin-T and troponin-I assays. For patients with multiple troponin measurements within the 24 hours surrounding intubation, we used the highest value. To enable pooling of the cohort for analysis, we classified troponin as a categorical variable. Troponin was classified as a) < upper limit of normal (ULN) for each assay, b) between 1 and 5 times ULN c) between 5 and 10 times ULN and d) greater than 10 times ULN. The ULN for each assay is displayed in the Table I in the Supplement. The distribution of patients within each clinical category was similar among the patients with troponin T and troponin I and similar when troponin was considered in quartiles. This categorization is analogous to the predetermined classification scheme in the MI-ARDS study. For troponin measurements in the ARDS population, a highly sensitive troponin-I assay was used (Abbott ARCHITECT) which allows for detection of circulating troponin to a limit of detection of 2 ng/dL; the assays used in the COVID-19 patients were clinical assays that were not highly sensitive (Table I in the Supplement). To enable comparison to the clinical troponin assays used in the COVID-19 patients, we categorized troponin-I as a) < 26 ng/L (corresponding to 99th percentile of a healthy reference population), b) between 26 and 130 ng/L, c) 130 to 260 ng/L and d) greater than 260 ng/L. We also considered troponin as a binary variable in both cohorts as “positive versus negative” with negative being <ULN for the COVID-19 patients and <26 ng/L for the ARDS cohort. Finally, troponin-I and troponin-T were considered as log-transformed continuous variables in separate linear regression models in the COVID-19 group to determine clinical characteristics associated with myocardial injury. For patients with troponin < ULN, we set values to halfway between 0 and ULN.
Study outcomes and covariates
The primary outcome for both groups was in-hospital mortality. Follow-up was complete for all patients. Covariates of interest included demographic and clinical information, laboratory values, inflammatory biomarkers, and ventilator parameters. The registry includes Elixhauser comorbidities 22 generating using ICD-10 codes to identify past medical history and chronic medical problems. Lab data were set to the worst value within the 24 hours prior to or after intubation. Ventilator data were set to the worst value within 24 hours after intubation. A secondary outcome of ventilator-free days was calculated as the number of days free of mechanical ventilation within the first 28 days after intubation, by convention 15.
Statistical analysis
Missing data
Because data in the JH-CROWN registry were drawn from the electronic medical record, data were not complete for all covariates, as shown in the Table II in the Supplement. Data were complete for the exposure of troponin assessment and for the outcome of in-hospital death, for demographics and for comorbidities. To address missing data, we performed multiple imputation to obtain unbiased estimates of the association between myocardial injury and outcome. Multiple imputation was performed using chained equations and 50 imputations 23. The variables which were complete were used as auxiliary variables. We used the “mi estimate” command in Stata which combines the multiply imputed data sets using Rubin’s formula 24. The results with and without multiple imputation were similar, therefore we report the results using multiple imputed datasets. Interleukin-6 and fibrinogen had high levels of missingness and so were not imputed. Analyses incorporating those biomarkers was by complete-case analysis only. For descriptive analysis, comparisons were made using linear regression for continuous variables and logistic regression for categorical variables across the independent variable of interest (category of troponin, death and COVID status).
Survival analysis
We categorized the exposure variable of troponin into clinical categories as described above: 1. < ULN, 2. < 5x ULN, 3. between 5 and 10 x ULN and 4. > 10x ULN. We also classified troponin as a dichotomous variable “positive or negative.” In the COVID-19 population, we performed Cox proportional hazard models and Kaplan-Meier survival analysis to determine the association of myocardial injury with in-hospital mortality. We performed univariable analyses followed by progressive adjustment for 1. Age and sex and then 2. Age, sex, and multi-organ dysfunction (represented by creatinine, bilirubin, PaO2/FIO2 ratio, vasopressor use and lactate levels). We chose these covariates based on factors used to calculate the Sequential Organ Failure (SOFA) score 25. SOFA score itself was not used because not all components were directly captured in the CROWN database. The proportional hazard assumption was assessed by inspection of Schoenfeld residuals and time-dependence of covariates. The assumption of proportionality was met. We also compared the association of myocardial injury with death in COVID-19 and non-COVID ARDS patients. We assessed the interaction of COVID-19 status and myocardial injury with death with Cox proportional hazard models.
Determinants of myocardial injury
To identify determinants of troponin-T and troponin-I in COVID-19, we performed linear regression with log-transformed troponin levels as the dependent variable and clinical and demographic factors as the independent variable in univariable models. Factors with p<0.1 in univariable models were then assessed in adjusted models. The adjusted models included the factor of interest and covariates known to be associated with myocardial injury: age, sex, and creatinine level.
ARDS comparison
Finally, to determine the degree of association of COVID-19 with myocardial injury as compared to traditional ARDS, we performed logistic regression with positive troponin as the dependent variable (corresponding to 26 ng/L for non-COVID ARDS and troponin > ULN for this COVID-19 cohort) and COVID-19 status compared to ARDS as the independent variable in univariable and multivariable models adjusting for age, sex, and multi-organ dysfunction. Analyses were performed using STATA 15. P value < 0.05 was considered statistically significant.
Results
Factors associated with myocardial injury in COVID-19 patients
Of 328 patients in our health system intubated with COVID-19, 243 had troponin assessed within 24 hours of intubation and were included in the study. There were no major differences in age, sex, ethnicity, comorbidities or death rates among patients who did versus did not have troponin checked (supplemental Table III). Of the study population, 54% had troponin I assessed and 46% troponin T. Patients assessed with troponin T were older with greater risk for death (supplemental Table IV). For included patients, mean age was 62.8 years, and substantial minority had comorbidities of congestive heart failure, chronic lung disease, chronic kidney disease or diabetes with complication (Table 1). Over 87% of patients received vasopressors, and mean body-mass index was 30.9 kg/m2. Of intubated COVID-19 patients, 51% had troponin levels > ULN including 16.1% with levels > 10x ULN. With higher troponin levels, patients had older age, higher proportion of chronic hypertension, immunodeficiency, and chronic kidney disease (Table 1). With higher troponin levels, patients had higher creatinine levels, higher lactate levels and greater degree of anemia and thrombocytopenia as well as lower fibrinogen levels and higher ferritin levels (Table 1). Factors associated with troponin-I and troponin-T levels are displayed in Table V in the Supplement. After adjusting for age, sex, and creatinine, independent associations with troponin level included chronic kidney disease, higher lactate levels and white blood cell count, higher ferritin and lower fibrinogen (Table V in the Supplement).
Table 1:
Characteristics of intubated COVID-19 patients by category of troponin level.
| Total | Troponin < ULN | Positive troponin < 5x ULN | Positive troponin 5–10 x ULN | Positive troponin> 10x ULN | P | |
|---|---|---|---|---|---|---|
| Number | 243 | 119 (49.0) | 55 (22.6) | 30 (12.4) | 39 (16.1) | |
| Age (y) | 62.8 (14.9) | 57.8 (14.8) | 66.5 (14.2) | 69.2 (13.4) | 67.8 (12.0) | <0.0001 |
| Female sex (%) | 39.1 | 44.5 | 36.4 | 23.3 | 38.4 | 0.2 |
| Hispanic ethnicity (%) | 22.2 | 27.7 | 16.3 | 16.7 | 22.2 | 0.26 |
| African-American race (%) | 35.4 | 37.0 | 32.7 | 26.7 | 41.0 | 0.61 |
| Chronic lung disease (%) | 22.2 | 21.0 | 27.2 | 16.7 | 23.0 | 0.69 |
| Congestive heart failure (%) | 28.8 | 22.6 | 30.9 | 43.3 | 33.3 | 0.13 |
| Hypertension (%) | 60.9 | 52.1 | 67.3 | 73.3 | 69.2 | 0.051 |
| Diabetes with complication (%) | 19.2 | 16.0 | 14.5 | 33.3 | 25.6 | 0.1 |
| Chronic kidney disease (%) | 20.2 | 4.2 | 25.5 | 43.3 | 43.6 | <0.0001 |
| Temperature (C) | 38.2 (1.1) | 38.3 (1.0) | 38.3 (1.1) | 38.2 (1.0) | 38.0 (1.3) | 0.5 |
| Heart rate (beats per minute) | 113 (21) | 111 (19) | 115 (22) | 119 (27) | 114 (21) | 0.24 |
| Systolic BP (mmHg) | 84 (15) | 87 (15) | 83 (17) | 80 (13) | 82 (14) | 0.07 |
| Diastolic BP (mmHg) | 47 (9) | 48 (9) | 47 (8) | 47 (8) | 45 (8) | 0.32 |
| Respiratory rate (breaths per minute) | 35 (7) | 35 (7) | 34 (7) | 37 (7) | 34 (8) | 0.34 |
| Vasopressor use (%) | 87.2 | 88.2 | 85.4 | 93.3 | 82 | 0.55 |
| Weight (kg) | 87.5 (27.2) | 88.1 (24.3) | 89.7 (27.5) | 86.4 (29.3) | 83.4 (33.6) | 0.75 |
| Body-mass index (kg/m2) | 30.9 (8.3) | 31.5 (7.6) | 31.3 (8.9) | 29.6 (9.4) | 29.4 (8.9) | 0.5 |
| Height (cm) | 168.2 (9.7) | 167.2 (9.6) | 169.3 (9.4) | 170.8 (9.2) | 167.6 (10.3) | 0.27 |
| Tidal volume (mL) | 414 (66) | 402 (63) | 421 (64) | 434 (63) | 427 (71) | 0.07 |
| Positive end-expiratory pressure (cm H2O) | 12.4 (4.3) | 12.8 (4.0) | 12.7 (4.8) | 12.7 (3.5) | 10.5 (4.5) | 0.05 |
| Driving pressure (cmH2O) | 15.9 (18.2) | 14.5 (11.2) | 16.8 (16.0) | 21.4 (38.8) | 14.5 (13.3) | 0.44 |
| Lung compliance (mL/cm H2O) | 30.6 (15.4) | 29.9 (13.2) | 29.8 (12.8) | 33.4 (25.8) | 31.4 (14.5) | 0.78 |
| Minute ventilation (L/min) | 14.7 (23.2) | 13.2 (11.3) | 13.5 (12.7) | 13.9 (13.3) | 21.8 (51) | 0.33 |
| pH | 7.30 (0.11) | 7.31 (0.09) | 7.31 (0.13) | 7.27 (0.13) | 7.30 (0.13) | 0.46 |
| pCO2 (mmHg) | 49.5 (14.2) | 51.0 (13.2) | 49.0 (14.6) | 49.2 (13.5) | 46.1 (16.9) | 0.43 |
| pO2 (mmHg) | 73 (36) | 71 (28) | 75 (46) | 74 (44) | 75 (35) | 0.93 |
| PaO2/FIO2 ratio | 99 (75) | 100 (66) | 96 (86) | 89 (83) | 106 (79) | 0.88 |
| Creatinine (mg/dL) | 2.2 (2.8) | 1.3 (2.2) | 2.5 (3.0) | 3.0 (3.0) | 3.7 (3.2) | <0.0001 |
| Bilirubin (mg/dL) | 0.7 (0.5) | 0.7 (0.4) | 0.8 (0.6) | 0.6 (0.3) | 0.9 (0.9) | 0.03 |
| Lactate (mmol/L) | 2.6 (3.4) | 1.8 (2.2) | 2.6 (3.3) | 3.5 (4.2) | 4.2 (5.1) | 0.007 |
| Hemoglobin (g/dL) | 11.2 (2.2) | 11.5 (2.1) | 11.1 (2.3) | 11.4 (2.3) | 9.9 (2.1) | 0.0014 |
| Hematocrit (%) | 34.9 (6.6) | 35.7 (6.3) | 35.0 (6.9) | 35.7 (6.9) | 31.5 (6.1) | 0.0054 |
| Central venous saturation (%, N=105) | 55.6 (22.0) | 59.4 (21.8) | 51.2 (22.0) | 43.9 (24.6) | 55.4 (20.4) | 0.18 |
| White blood cell count (1000 cells/mm3) | 12.4 (5.9) | 12.0 (5.4) | 12.4 (4.6) | 12.7 (6.4) | 13.4 (8.3) | 0.59 |
| Platelets (100 cells/mm3) | 220 (95) | 242 (99) | 199 (80) | 209 (95) | 189 (88) | 0.003 |
| Interleukin-6 (pg/mL, N=44) | 249 (409) | 240 (395) | 320 (587) | 302 (251) | 141 (89) | 0.71 |
| Ferritin (ng/mL) | 3177 (8738) | 1376 (4469) | 2761 (5601) | 4374 (8514) | 8337 (16857) | 0.0011 |
| Fibrinogen (mg/dL, N=105) | 614 (171) | 656 (133) | 580 (211) | 665 (150) | 479 (184) | 0.0008 |
| D-dimer (mcg/mL) | 4.8 (8.1) | 4.2 (7.0) | 5.0 (8.5) | 5.8 (9.0) | 5.9 (9.7) | 0.69 |
| C-reactive protein (mg/dL) | 63.3 (101) | 52.9 (88) | 74.5 (115) | 97.9 (106) | 52.7 (112) | 0.16 |
| Death (%) | 36.2 | 22.7 | 41.8 | 46.7 | 61.5 | 0.0001 |
Data shown as mean for continuous variables and percent for categorical variables, using multiply imputed data.
Association of myocardial injury with mortality in COVID-19.
Overall mortality rate was 36.2%. Mortality was 22.7% among intubated COVID-19 patients with troponin < ULN, and was greater with higher troponin levels up to 61.5% among those with the highest troponin levels (P<0.001, Figure 1). The distribution of myocardial injury was also different comparing survivors to non-survivors: among survivors, 59% had troponin < ULN and under 10% troponin > 10x ULN while among non-survivors 31% had troponin < ULN and 27% troponin > 10x ULN (P<0.001, Figure 1). Troponin-I and Troponin-T were also higher in non-survivors when considered as continuous variables (Table 2).
Figure 1:
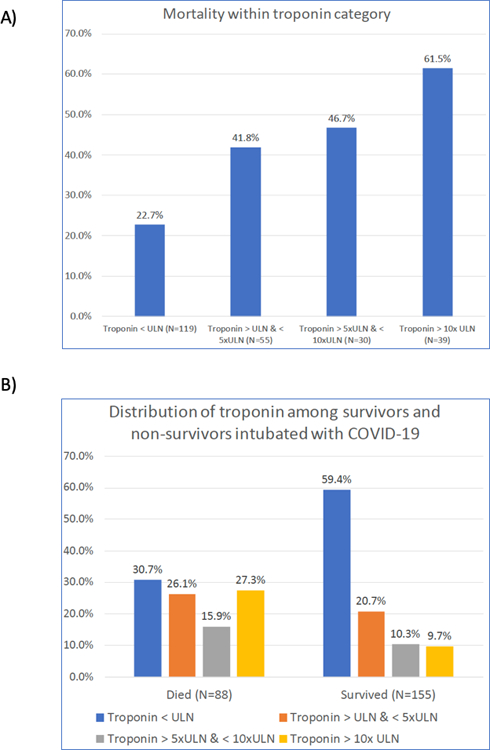
A) Mortality within each category of troponin level (P<0.001 for difference in proportions and for trend). B) Distribution of troponin stratified by survival status (P<0.001 for difference in proportions)
Table 2:
Characteristics of intubated COVID-19 patients by survival status.
| Total | Survived | Died | P | |
|---|---|---|---|---|
| Number | 243 | 155 (63.8) | 88 (36.2) | |
| Age (y) | 62.8 (14.9) | 58.1 (14.0) | 71.0 (12.7) | <0.001 |
| Female sex (%) | 39.1 | 36.8 | 43.2 | 0.33 |
| Hispanic ethnicity (%) | 22.2 | 27.1 | 13.6 | 0.017 |
| African-American race (%) | 35.4 | 36.1 | 34.1 | 0.75 |
| Chronic lung disease (%) | 22.2 | 18.7 | 28.4 | 0.082 |
| Congestive heart failure (%) | 28.8 | 25.1 | 35.2 | 0.097 |
| Hypertension (%) | 60.9 | 56.8 | 68.2 | 0.081 |
| Chronic immunodeficiency (%) | 23.9 | 19.4 | 31.8 | 0.03 |
| Diabetes with complication (%) | 19.3 | 18.7 | 20.5 | 0.74 |
| Chronic kidney disease (%) | 20.1 | 11.6 | 35.2 | <0.0001 |
| Temperature (C) | 38.2 (1.1) | 38.4 (1.0) | 38.0 (1.2) | 0.017 |
| Heart rate (beats per minute) | 113 (21) | 112 (20) | 116 (23) | 0.17 |
| Systolic BP (mmHg) | 84 (15) | 86 (14) | 81 (17) | 0.016 |
| Diastolic BP (mmHg) | 47 (9) | 48 (9) | 46 (8) | 0.066 |
| Respiratory rate (breaths per minute) | 35 (7) | 34 (7) | 36 (7) | 0.15 |
| Vasopressor use (%) | 87.2 | 90.3 | 81.8 | 0.06 |
| Weight (kg) | 87.5 (27.2) | 89.7 (25.0) | 83.6 (30.5) | 0.11 |
| Body-mass index (kg/m2) | 30.9 (8.3) | 31.5 (7.9) | 29.7 (8.9) | 0.13 |
| Tidal volume (mL) | 414 (66) | 409 (61) | 422 (72) | 0.18 |
| Positive end-expiratory pressure (cm H2O) | 12.4 (4.3) | 12.7 (4.2) | 11.8 (4.4) | 0.13 |
| Driving pressure (cmH2O) | 15.9 (18.2) | 14.5 (11.1) | 18.3 (26.4) | 0.18 |
| Lung compliance (mL/cm H2O) | 30.6 (15.4) | 29.1 (12.7) | 33.2 (18.9) | 0.11 |
| Minute ventilation (L/min) | 14.7 (23.2) | 12.7 (11.8) | 18.3 (35.1) | 0.1 |
| pH | 7.3 (0.1) | 7.31 (0.1) | 7.29 (0.1) | 0.38 |
| pCO2 (mmHg) | 49.5 (14.2) | 51.4 (13.2) | 46.3 (15.4) | 0.032 |
| pO2 (mmHg) | 73 (36) | 70 (28) | 78 (47) | 0.19 |
| PaO2/FIO2 ratio | 99 (75) | 97 (65) | 101 (90) | 0.78 |
| Creatinine (mg/dL) | 2.2 (2.8) | 1.8 (2.4) | 2.9 (3.4) | 0.004 |
| Bilirubin (mg/dL) | 0.7 (0.5) | 0.7 (0.5) | 0.8 (0.6) | 0.26 |
| Lactate (mmol/L) | 2.6 (3.4) | 2.0 (2.4) | 3.6 (4.6) | 0.005 |
| Hemoglobin (g/dL) | 11.2 (2.2) | 11.2 (2.3) | 11.1 (2.1) | 0.78 |
| Hematocrit (%) | 35 (7) | 35 (7) | 35 (6) | 0.57 |
| White blood cell count (1000 cells/mm3) | 12.4 (5.9) | 12.1 (5.7) | 13.0 (6.2) | 0.26 |
| Platelets (100 cells/mm3) | 220 (95) | 230 (94) | 201 (95) | 0.023 |
| Interleukin-6 (pg/mL, N=44) | 249 (409) | 286 (491) | 177 (84) | 0.5 |
| Ferritin (ng/mL) | 3177 (8738) | 1752 (4881) | 5687 (12649) | 0.003 |
| Fibrinogen (mg/dL, N=105) | 614 (171) | 643 (152) | 554 (194) | 0.011 |
| D-dimer (mcg/mL) | 4.8 (8.1) | 4.4 (7.3) | 5.5 (9.3) | 0.37 |
| C-reactive protein (mg/dL) | 63.3 (101) | 60.5 (92) | 68.3 (117) | 0.6 |
| Troponin T (ng/L) | 65.3 (12.2) | 43.8 (8.3) | 92.1 (154.6) | 0.037 |
| Troponin I (ng/L) | 692 (2373) | 371 (1524) | 1512 (3660) | 0.007 |
Data shown as mean for continuous variables and percent for categorical variables, using multiple imputed data.
Other factors associated with mortality in descriptive analyses included older age, chronic kidney disease and higher creatinine, lactate levels, lower fibrinogen and higher ferritin levels (Table 2). Positive troponin was associated with over 2-fold increased hazard for mortality in unadjusted models (HR 2.31, 95% CI 1.47–3.65) while the highest levels of troponin were associated with over 3 fold risk of mortality compared to troponin < ULN (HR 3.17 95% CI 1.80–5.56) as shown in Figures 2 and 3. The association of myocardial injury with mortality attenuated with progressive covariate adjustment and was no longer statistically significant after adjusting for age, sex, creatinine, bilirubin, PaO2/FIO2 ratio, vasopressor use and lactate levels (Figure 3).
Figure 2:
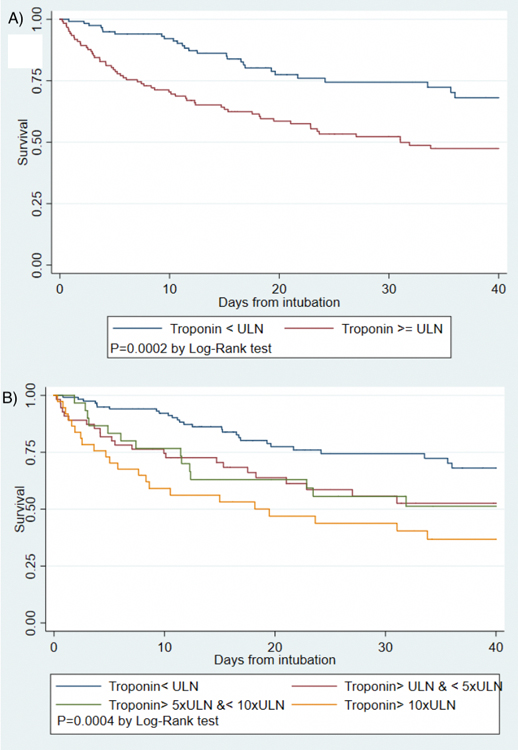
Kaplan-Meier survival curves for intubated COVID-19 patients by presence of any myocardial injury (A) and by category of troponin level (B)
Figure 3:
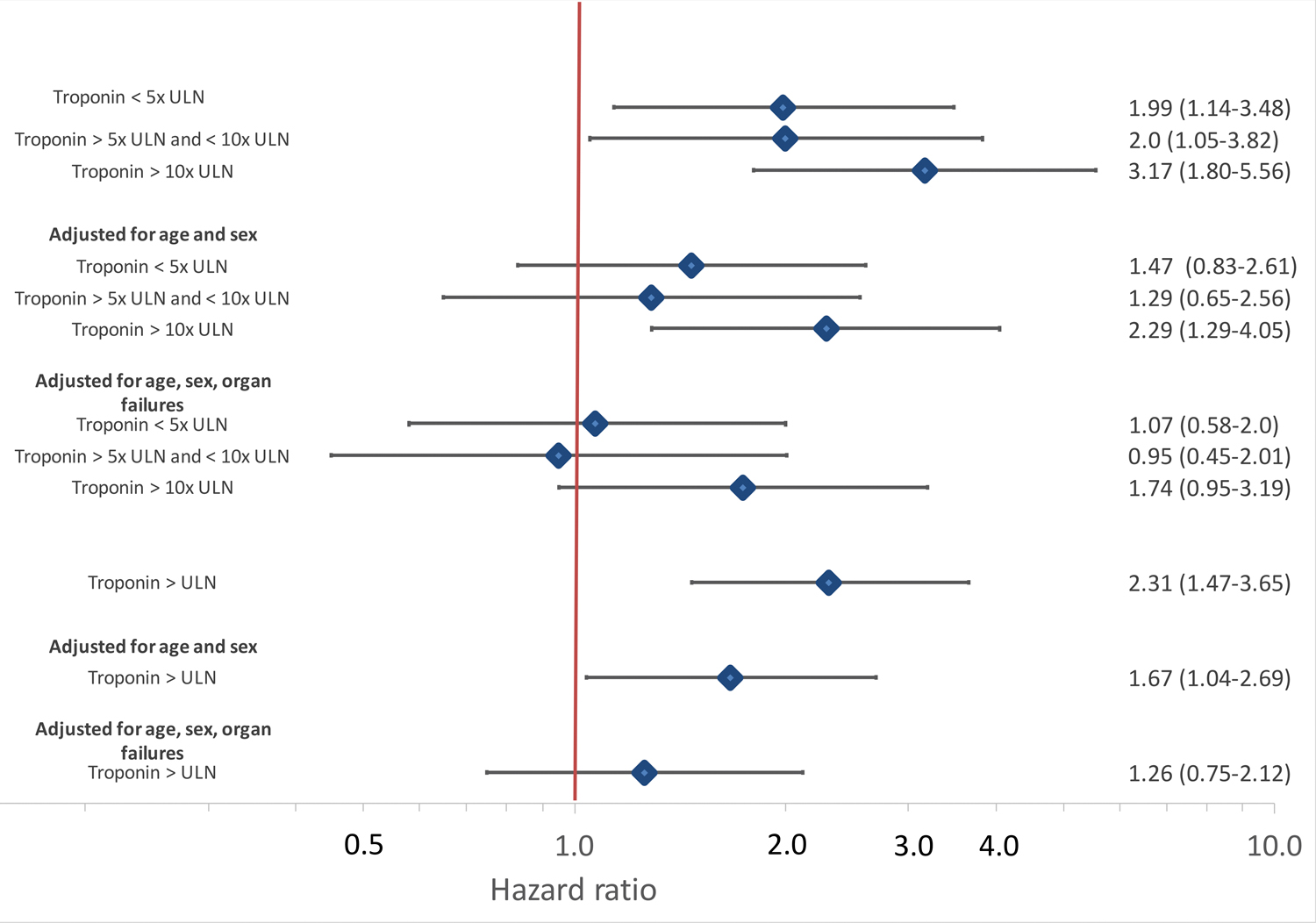
Univariable and adjusted hazard ratios for intubated COVID-19 patients by presence of any myocardial injury and by category of troponin level. Cox proportional hazard models adjusted first for age and sex and then for age, sex, creatinine, bilirubin, PaO2/FIO2 ratio, vasopressor use and lactate levels
Comparison of COVID-19 patients to ARDS patients
Table 3 displays demographics and clinical characteristics of 243 intubated COVID-19 patients to 506 subjects with ARDS due to pneumonia from the MI-ARDS study. Patients with COVID-19 have a different clinical profile compared to non-COVID ARDS patients including older age, fewer females and higher proportion of non-white race, higher BMI and creatinine, less favorable vital signs and worse oxygenation. The rate of any myocardial injury was similar between COVID-19 and ARDS- 51.0% in COVID-19 compared to 49.6% in ARDS (OR for myocardial injury 1.09 95% CI 0.78–1.44, P=0.72). The distribution of troponin levels were also similar between COVID-19 and general ARDS (Table 3, P=0.37). After adjusting for sex, age, creatinine, bilirubin, PaO2/FIO2 ratio, and vasopressor use, COVID-19 was associated with lower odds of myocardial injury compared to ARDS (OR 0.55 95% CI 0.36–0.84, P=0.005). COVID-19 patients had higher mortality than non-COVID ARDS- 36.2% v. 26.4%, P=0.007. In unadjusted analysis, mortality among ARDS patients with and without myocardial injury and COVID-19 patients without myocardial injury was similar, while COVID-19 patients with positive troponin had the highest mortality observed (Figure 4, P interaction = 0.012). After adjusting for age, sex, creatinine, bilirubin, PaO2/FIO2 ratio, and vasopressor use, the interaction was no longer significant (P interaction =0.082).
Table 3:
Characteristics of intubated COVID-19 patients compared to patients with ARDS secondary to pneumonia.
| ARDS | COVID-19 | P | |
|---|---|---|---|
| Number | 506 | 243 | |
| Age (y) | 50.2 (14.9) | 62.8 (14.9) | <0.001 |
| Female sex (%) | 51.3 | 39.0 | 0.0017 |
| African-American race (%) | 21.7 | 35.3 | 0.001 |
| Temperature (C) | 37.5 (1.0) | 38.2 (1.1) | <0.001 |
| Heart rate (beats per minute) | 101 (20) | 113 (21) | <0.001 |
| Systolic BP (mmHg) | 112 (20) | 84 (15) | <0.001 |
| Diastolic BP (mmHg) | 59 (12) | 47 (9) | <0.001 |
| Vasopressor use (%) | 28.1 | 87.2 | <0.001 |
| Weight (kg) | 77.5 (19.9) | 87.5 (27.2) | <0.001 |
| Body-mass index (kg/m2) | 27.2 (7.0) | 30.9 (8.3) | <0.001 |
| Tidal volume (mL) | 469 (106) | 414 (66) | <0.001 |
| Positive end-expiratory pressure (cm H2O) | 9.6 (3.9) | 12.4 (4.3) | <0.001 |
| Plateau pressure (cm H2O) | 26.5 (7.0) | 27.2 (18.6) | 0.58 |
| PaO2/FIO2 ratio | 143 (64) | 99 (75) | <0.001 |
| Lung compliance (mL/cm H2O) | 32.3 (18.3) | 30.6 (15.4) | 0.29 |
| pH | 7.36 (0.1) | 7.3 (0.11) | <0.001 |
| pCO2 (mmHg) | 40.3 (10.8) | 49.5 (14.2) | <0.001 |
| Creatinine (mg/dL) | 1.4 (1.4) | 2.2 (2.8) | <0.001 |
| Bilirubin (mg/dL) | 1.1 (1.5) | 0.7 (0.5) | 0.002 |
| White blood cell count (1000 cells/mm3) | 14.1 (8.4) | 12.4 (5.9) | 0.005 |
| Troponin positive (%) | 49.6 | 51.0 | 0.72 |
| Troponin category (%) | 0.37 | ||
| <ULN | 50.4 | 49.0 | |
| 1–5x ULN | 24.1 | 22.6 | |
| 5–10x ULN | 8.3 | 12.4 | |
| >10xULN | 17.2 | 16.1 | |
| Death (%) | 26.5 | 36.2 | 0.007 |
| Ventilator-free days (days) | 13.1 (9.9) | 13.0 (10.4) | 0.85 |
Data shown as mean for continuous variables and percent for categorical variables, using multiple imputed data.
Figure 4:
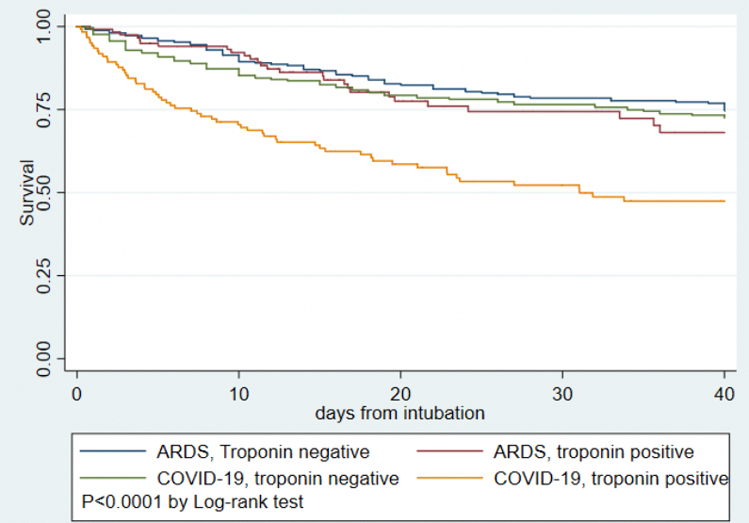
Kaplan-Meier survival curves for COVID-19 versus ARDS-pneumonia and presence or absence of myocardial injury
Discussion
Myocardial injury detected with cardiac biomarkers has garnered attention as a high risk marker in COVID-19. Yet the implications and pathogenesis of myocardial injury in COVID-19 remain unclear. In this study of myocardial injury in COVID-19 compared to traditional ARDS, we report several findings. First, half of intubated COVID-19 patients manifest myocardial injury assessed by clinical troponin assays which is associated with a graded increase in overall mortality. Yet, the magnitude of mortality risk is attenuated after adjustment for degree of critical illness suggesting that myocardial injury is reflective of baseline risk and comorbidities and underlying multisystem organ dysfunction. Age, comorbidities, ferritin, and fibrinogen are associated with myocardial injury in COVID-19. Finally, myocardial injury is actually less common in COVID-19 compared to conventional ARDS after adjusting for confounders of age, renal dysfunction and degree of critical illness. Our findings place myocardial injury in COVID-19 in context of that observed in general ARDS, add to the evidence base for the utility of troponin as a prognostic biomarker, and suggest several avenues to further elucidate the pathogenesis of troponin elevation in COVID-19 and construct a conceptual model.
Epidemiology of myocardial injury in COVID-19
Of intubated COVID-19 patients, we report approximately half with myocardial injury, which is consistent with other reports of up to 44% rates of myocardial injury 2, 3, 8. Our study included only the most severely impacted COVID-19 patients who were intubated, explaining the high percentage of myocardial injury observed. High rates of myocardial injury are also observed in non-COVID critical illnesses including ARDS 15, 17 and sepsis 26, and we demonstrate that the unadjusted profile of myocardial injury is similar between COVID-19 patients and a group of patients with ARDS secondary to pneumonia. However the clinical profile of COVID-19 patients compared to conventional ARDS patients was higher risk including more advanced age, more renal failure and more severe lung disease. Given worse critical illness, more myocardial injury would be expected. After adjusting for severity of disease, therefore, the odds of myocardial injury in COVID-19 were actually lower than conventional ARDS. The COVID-19 pandemic has resulted in a large number of critically ill patients presenting for care simultaneously, and it is debated which features of COVID-19 are unique to the virus versus facets of general critical illness 16, 27. Our results suggest that myocardial injury in severe COVID-19 is not substantially different in magnitude from that in general ARDS and in fact may be of lesser magnitude adjusting for age and comorbidities, while acknowledging that we are performing comparisons across disparate assays.
Contributors to myocardial injury in COVID-19
There are many potential mechanisms of elevated troponin in COVID-19 including thrombotic and plaque rupture events, supply-demand mismatch, and direct cardiac viral toxicity 28, 29. We found that older age, acute and chronic renal dysfunction, and serum lactate levels were strongly associated with myocardial injury, consistent with studies in non-COVID patients 15. The inflammatory and prothrombotic milieu of COVID-19 is hypothesized to also contribute to myocardial injury 30, 31. Higher ferritin and lower fibrinogen were associated with troponin levels in our study. Higher ferritin levels may simply reflect more active systemic inflammation, although ferritin levels have also been associated with myocardial infarction in case-control studies 32. Ferritin has also been proposed to participate in the myocyte response to ischemia 33. The lower fibrinogen may reflect consumption, microvascular thrombosis and endothelial dysfunction contributing to myocardial injury 34, 35. Therefore, our work reinforces inflammation as a factor associated with myocardial injury and suggests that pathways involving coagulation and iron metabolism may be fruitful mechanistic areas of inquiry.
Prognostic implications of myocardial injury in COVID-19
A growing evidence base supports that myocardial injury is associated with poor prognosis in COVID-19 8, 29, and our findings support prior findings- in our critically ill patient population, myocardial injury was associated with over two fold hazard for death. Yet the association of myocardial injury with mortality attenuated greatly after adjustment for age, sex, and multisystem organ dysfunction. We observed a similar pattern in the general ARDS population 15. These findings suggest that the association of myocardial injury with outcome in COVID-19 is a function of underlying critical illness and multisystem organ dysfunction, particularly concomitant renal dysfunction. The implication naturally follows that principles of critical care to optimize organ dysfunction would mitigate some of this risk and improve outcomes in COVID-19 patients with myocardial injury. This premise is further supported by the fact that autopsy studies have not shown widespread direct myocarditis from COVID-19 36. While MRI reports suggest abnormal myocardial signal in many patients with COVID-19 37–40, patients with myocardial injury due to sepsis also manifest significant and common abnormalities on cardiac MRI 41. We do report that COVID-19 patients with myocardial injury had worse prognosis than pneumonia-ARDS patients with myocardial injury; whether this incremental adverse prognosis related to baseline comorbidities or to differing pathogenesis of myocardial injury such as thrombotic complications 36 needs to be further clarified. The fact that the interaction of myocardial injury with COVID-19 status lessened after covariate adjustment supports the fact that much of the difference relates to degree of critical illness.
COVID-19 versus traditional ARDS
It is debated whether COVID-19 related lung disease represents a form of traditional ARDS or has a distinct pathophysiology, with advocates of both viewpoints 42, 43. We report crude rates of myocardial injury as similar to traditional ARDS, though after adjusting for clinical differences, the severely impacted COVID-19 population actually had lower odds of myocardial injury. This could represent survivor bias, with individuals most severely impacted suffering cardiac arrest 44–46 and death prior to surviving to assessment. Similar factors are associated with myocardial injury in both COVID-19 and traditional ARDS 15 including age, creatinine, and multisystem organ failure. This paradigm is supported by autopsy series suggesting virus involvement of pulmonary tissue with diffuse alveolar damage but rare microthrombi and endotheliitis; lymphocytic myocarditis was seen rarely but most patients had no evidence of direct cardiac involvement 36, 47. Whether the hypercoagulable state and increased system inflammation observed in COVID-19 are unique features causing myocardial injury should be further investigated. Considering myocardial injury broadly, most cases seem related to critical illness, however there are isolated reports of frank myocarditis and other severe direct cardiac manifestations 1, 48, and it is important to identify these rare and distinct manifestations.
Limitations
Limitations of our study include its observational nature; thus, hypotheses can be inferred but causal inference is not established. Our data are drawn from a single academic health system in a single state; in the context of a very heterogenous pandemic, reports from other health care systems are needed and anticipated. We chose a priori to focus only on intubated patients because of scientific interest and to enable our direct comparison to ARDS. Because our dataset is drawn from clinical care, there are missing data requiring imputation techniques, however results are similar in considering imputed and non-imputed data. In considering the comparison between ARDS and COVID-19, the MI ARDS study was a cross-sectional study with troponin checked in all patients while in COVID-19, the decision to check troponin was made clinically. Thus, patients without troponin assessed were not included in the COVID-19 group which could introduce bias. However baseline characteristics and outcomes of COVID-19 patients who did and did not have troponin checked were similar. An additional limitation relates to the fact that different assays were used within the hospitals admitting COVID-19 patients and in the ARDS cohort, which provides challenges for direct comparisons. Outcomes of COVID-19 are also variable across centers which could reflect local epidemiology of the pandemic, patient risk profile, and local resources among other factors. Finally, echocardiography and other detailed cardiac imaging is not available in our data set, and formal cardiac imaging is often deferred in COVID-19 in lieu of informal point-of-care ultrasound. Thus, an assessment of ventricular function and wall motion abnormalities in COVID-19 patients is not available in this study. This is an important area for future research.
Conclusion
Myocardial injury is common in severe COVID-19 as a function of baseline comorbidities, advanced age and multisystem organ dysfunction. The adverse prognosis of myocardial injury in COVID-19 is a function of multisystem organ involvement, similar to generic ARDS. Markers of inflammation, iron metabolism, and thrombotic activity are associated with myocardial injury. Future studies of myocardial injury in COVID-19 should investigate any novel mechanisms and identify focused treatment of both primary cardiac involvement and the multisystem organ dysfunction.
Supplementary Material
Clinical Perspective.
- What is new?
- Half of intubated COVID-19 patients manifest myocardial injury, however mortality risk associated with myocardial injury is attenuated after adjustment for degree of critical illness
- Myocardial injury is actually less common in COVID-19 compared to conventional ARDS after adjusting for confounders of age, renal dysfunction and degree of critical illness
- What are the clinical implications?
- Myocardial injury in COVID-19 is reflective of baseline risk and comorbidities and underlying multisystem organ dysfunction
- Most myocardial injury in COVID-19 is related to critical illness, however given isolated reports of frank myocarditis and other severe direct cardiac manifestations, it is important to identify these rare and distinct manifestations.
Acknowledgements:
The data utilized for this publication were part of the JH-CROWN: The COVID PMAP Registry which is based on the contribution of many patients and clinicians.
Funding:
Dr. Hays is supported by National Heart, Lung, and Blood Institute grant 1R01HL147660. Dr. Lowenstein is supported by NIH grants R01 HL134894 and R33 HL141791, a grant from Novartis, and the Michel Mirowski M.D. Professorship in Cardiology. Dr. Metkus is supported by the National Institutes of Health-funded Institutional Career Development Core at Johns Hopkins (project number 5KL2TR003099-02).
Disclosures:
Dr. Lowenstein receives research funding from Novartis for a clinical trial of treatment for patients with COVID-19 (NCT04435184). Abbott Laboratories provided reagents and financial support for MI-ARDS study (to PI Dr. Frederick Korley); the MI ARDS study was designed and executed solely by the study investigators without industry involvement. Dr. Post reports research support to an unrelated project from Abbott Laboratories for reagents. Dr. Sokoll’s institution received funding from Abbott Laboratories and disclosed reagent support from Abbott Laboratories.
Nonstandard Abbreviations and acronyms
- ARDS
Acute respiratory distress syndrome
- COVID-19
Coronavirus disease 2019
- ULN
Upper limit of normal
References
- 1.Santoso A, Pranata R, Wibowo A, Al-Farabi MJ, Huang I and Antariksa B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: A meta-analysis. Am J Emerg Med. 2020. 10.1016/j.ajem.2020.04.052. [Online ahead of print]. [DOI] [PMC free article] [PubMed]
- 2.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X and Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B and Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, Guo GY, Du J, Zheng CL, Zhu Q, Hu M, Li XY, Peng P and Shi HZ. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu L, Chen S, Fu Y, Gao Z, Long H, Wang JM, Ren HW, Zuo Y, Li H, Wang J, Xu QB, Yu WX, Liu J, Shao C, Hao JJ, Wang CZ, Ma Y, Wang Z, Yanagihara R and Deng Y. Risk Factors Associated with Clinical Outcomes in 323 COVID-19 Hospitalized Patients in Wuhan, China. Clin Infect Dis. 2020. 10.1093/cid/ciaa539. [Online ahead of print]. [DOI] [PMC free article] [PubMed]
- 6.Hendren NS, Drazner MH, Bozkurt B and Cooper LT Jr. Description and Proposed Management of the Acute COVID-19 Cardiovascular Syndrome. Circulation. 2020;141:1903–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H and Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Guan B, Su T, Liu W, Chen M, Bin Waleed K, Guan X, Gary T and Zhu Z. Impact of cardiovascular disease and cardiac injury on in-hospital mortality in patients with COVID-19: a systematic review and meta-analysis. Heart. 2020;106:1142–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J and Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng Y, Liu W, Liu K, Fang YY, Shang J, Zhou L, Wang K, Leng F, Wei S, Chen L and Liu HG. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin Med J (Engl). 2020;133:1261–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He XW, Lai JS, Cheng J, Wang MW, Liu YJ, Xiao ZC, Xu C, Li SS and Zeng HS. [Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 patients]. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:456–460. [DOI] [PubMed] [Google Scholar]
- 12.Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, Van Vleck T, Vaid A, Chaudhry F, De Freitas JK, Fayad ZA, Pinney SP, Levin M, Charney A, Bagiella E, Narula J, Glicksberg BS, Nadkarni G, Mancini DM, Fuster V and Mount Sinai CIC. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. J Am Coll Cardiol. 2020;76:533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A, LUNG SAFE Investigators, ESICM Trials Group. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315:788–800. [DOI] [PubMed] [Google Scholar]
- 14.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L and Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33. [DOI] [PubMed] [Google Scholar]
- 15.Metkus TS, Guallar E, Sokoll L, Morrow D, Tomaselli G, Brower R, Schulman S and Korley FK. Prevalence and Prognostic Association of Circulating Troponin in the Acute Respiratory Distress Syndrome. Crit Care Med. 2017;45:1709–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan E, Beitler JR, Brochard L, Calfee CS, Ferguson ND, Slutsky AS and Brodie D. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med. 2020;8:816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metkus TS, Guallar E, Sokoll L, Morrow DA, Tomaselli G, Brower R, Kim BS, Schulman S and Korley FK. Progressive myocardial injury is associated with mortality in the acute respiratory distress syndrome. J Crit Care. 2018;48:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT, National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. The New England journal of medicine. 2004;351:327–36. [DOI] [PubMed] [Google Scholar]
- 19.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr., Hite RD and Harabin AL. Comparison of two fluid-management strategies in acute lung injury. The New England journal of medicine. 2006;354:2564–75. [DOI] [PubMed] [Google Scholar]
- 20.Wilson JG and Calfee CS. ARDS Subphenotypes: Understanding a Heterogeneous Syndrome. Crit Care. 2020;24:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, Hibbert KA, Thompson BT and Hardin CC. Respiratory Pathophysiology of Mechanically Ventilated Patients with COVID-19: A Cohort Study. Am J Respir Crit Care Med. 2020;201:1560–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elixhauser A, Steiner C, Harris DR and Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 23.Azur MJ, Stuart EA, Frangakis C and Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall A, Altman DG, Holder RL and Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol. 2009;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM and Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. [DOI] [PubMed] [Google Scholar]
- 26.Masson S, Caironi P, Fanizza C, Carrer S, Caricato A, Fassini P, Vago T, Romero M, Tognoni G, Gattinoni L, Latini R and Albumin Italian Outcome Sepsis Study Investigators. Sequential N-Terminal Pro-B-Type Natriuretic Peptide and High-Sensitivity Cardiac Troponin Measurements During Albumin Replacement in Patients With Severe Sepsis or Septic Shock. Crit Care Med. 2016;44:707–716. [DOI] [PubMed] [Google Scholar]
- 27.Rice TW and Janz DR. In Defense of Evidence-based Medicine for the Treatment of COVID-19 Acute Respiratory Distress Syndrome. Ann Am Thorac Soc. 2020;17:787–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atri D, Siddiqi HK, Lang JP, Nauffal V, Morrow DA and Bohula EA. COVID-19 for the Cardiologist: Basic Virology, Epidemiology, Cardiac Manifestations, and Potential Therapeutic Strategies. JACC Basic Transl Sci. 2020;5:518–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandoval Y, Januzzi JL Jr. and Jaffe AS. Cardiac Troponin for Assessment of Myocardial Injury in COVID-19: JACC Review Topic of the Week. J Am Coll Cardiol. 2020;76:1244–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babapoor-Farrokhran S, Gill D, Walker J, Rasekhi RT, Bozorgnia B and Amanullah A. Myocardial injury and COVID-19: Possible mechanisms. Life Sci. 2020;253:117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quere I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH, Global Covid-19 Thrombosis Collaborative Group EbtINE, the Iua SbtESCWGoPC and Right Ventricular F. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claeys D, Walting M, Julmy F, Wuillemin WA and Meyer BJ. Haemochromatosis mutations and ferritin in myocardial infarction: a case-control study. Eur J Clin Invest. 2002;32 Suppl 1:3–8. [DOI] [PubMed] [Google Scholar]
- 33.Berenshtein E, Vaisman B, Goldberg-Langerman C, Kitrossky N, Konijn AM and Chevion M. Roles of ferritin and iron in ischemic preconditioning of the heart. Mol Cell Biochem. 2002;234–235:283–292. [PubMed] [Google Scholar]
- 34.Levi M and Opal SM. Coagulation abnormalities in critically ill patients. Crit Care. 2006;10:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Backer D, Donadello K and Favory R. Link between coagulation abnormalities and microcirculatory dysfunction in critically ill patients. Curr Opin Anaesthesiol. 2009;22:150–154. [DOI] [PubMed] [Google Scholar]
- 36.Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J and Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knight DS, Kotecha T, Razvi Y, Chacko L, Brown JT, Jeetley PS, Goldring J, Jacobs M, Lamb LE, Negus R, Wolff A, Moon JC, Xue H, Kellman P, Patel N and Fontana M. COVID-19: Myocardial Injury in Survivors. Circulation. 2020;142:1120–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M and Nagel E. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020. 10.1001/jamacardio.2020.3557. [Online ahead of print]. [DOI] [PMC free article] [PubMed]
- 39.Ho JS, Sia CH, Chan MY, Lin W and Wong RC. Coronavirus-induced myocarditis: A meta-summary of cases. Heart Lung. 2020;49:681–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang L, Zhao P, Tang D, Zhu T, Han R, Zhan C, Liu W, Zeng H, Tao Q and Xia L. Cardiac Involvement in Patients Recovered From COVID-2019 Identified Using Magnetic Resonance Imaging. JACC Cardiovasc Imaging. 2020;13:2330–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siddiqui Y, Crouser ED and Raman SV. Nonischemic myocardial changes detected by cardiac magnetic resonance in critical care patients with sepsis. Am J Respir Crit Care Med. 2013;188:1037–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S and Chiumello D. COVID-19 Does Not Lead to a “Typical” Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2020;201:1299–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haudebourg AF, Perier F, Tuffet S, de Prost N, Razazi K, Mekontso Dessap A and Carteaux G. Respiratory Mechanics of COVID-19- versus Non-COVID-19-associated Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2020;202:287–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai PH, Lancet EA, Weiden MD, Webber MP, Zeig-Owens R, Hall CB and Prezant DJ. Characteristics Associated With Out-of-Hospital Cardiac Arrests and Resuscitations During the Novel Coronavirus Disease 2019 Pandemic in New York City. JAMA Cardiol. 2020. 10.1001/jamacardio.2020.2488. [Online ahead of print]. [DOI] [PMC free article] [PubMed]
- 45.Mountantonakis SE, Saleh M, Coleman K, Kuvin J, Singh V, Jauhar R, Ong L, Qiu M and Epstein LM. Out-of-Hospital Cardiac Arrest and Acute Coronary Syndrome Hospitalizations During the COVID-19 Surge. J Am Coll Cardiol. 2020;76:1271–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paoli A, Brischigliaro L, Scquizzato T, Favaretto A and Spagna A. Out-of-hospital cardiac arrest during the COVID-19 pandemic in the Province of Padua, Northeast Italy. Resuscitation. 2020;154:47–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, Najafian B, Deutsch G, Lacy JM, Williams T, Yarid N and Marshall DA. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396:320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newton-Cheh C, Zlotoff DA, Hung J, Rupasov A, Crowley JC and Funamoto M. Case 24–2020: A 44-Year-Old Woman with Chest Pain, Dyspnea, and Shock. New Eng J Med. 2020;383:475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


