Abstract
There is a growing body of evidence supporting the association between immune processes and psychopathology, including major depressive disorder (MDD). However, lack of diagnostic specificity has given rise to a search for specific symptom types, as opposed to more heterogeneous categorical diagnoses, linked to increased inflammation. One such symptom could be anhedonia, which is not only a key feature of MDD, but also a pervasive and persistent transdiagnostic symptom. To evaluate the specific role of anhedonia as well as categorical MDD diagnoses, we examined endotoxin-evoked immune responses in vitro in relation to current levels of anhedonia and history of recurrent MDD (rMDD) in a sample of adults recruited from the community. A total of 39 participants either had a history of rMDD (n = 20) or no lifetime history of any MDD episodes (n = 19). The average age of participants was 36.81 years and the majority were women (87.2%) and Caucasian (76.3%). We found that higher levels of current anhedonia, but not history of rMDD, were associated with increased lipopolysaccharide-stimulated levels of inflammatory markers even after we statistically controlled for the potential influence of participants’ demographic (age, sex, ethnicity, income) and physiological (body temperature, BMI) characteristics, current symptoms of depression and anxiety, and the time of day of the sample collection. These findings highlight the relation of anhedonia specifically, rather than rMDD more generally, with inflammatory processes and identify endotoxin-stimulated cytokine production as a plausible biological marker of current anhedonia.
Keywords: Anhedonia, inflammation, cytokines, major depressive disorder, stimulated immune response
A rapidly growing body of research suggests that immune-derived signaling factors, such as pro-inflammatory cytokines, play an important role in the pathogenesis of depression. For example, otherwise healthy individuals with a major depressive disorder (MDD) display elevated peripheral and cerebrospinal fluid (CSF) levels of inflammatory immune markers, including interleukin-6 (IL-6), IL-1β, and C-reactive protein (CRP; Dowlati et al., 2010; Ford & Erlinger, 2004; Levine et al., 1999; Liu, Ho, & Mak, 2012). In addition, individuals suffering from chronic and acute inflammatory conditions demonstrate increased levels of inflammatory markers and depressive symptoms (Capuron et al., 2002; Musselman et al., 2001; Owen, Eccleston, Ferrier, & Young, 2001). Furthermore, up to 50% of patients undergoing cytokine immunotherapy develop MDD (Miller, Maletic, & Raison, 2009). Finally, higher levels of circulating inflammatory markers in childhood prospectively predicted greater risk of developing depression and psychopathology in adulthood (Khandaker, Pearson, Zammit, Lewis, & Jones, 2014). That said, inflammation has been implicated in a range of other psychiatric conditions, including anxiety disorders, schizophrenia, and post-traumatic stress disorder, among others (Bauer & Teixeira, 2019; Friedrich, 2014; Savitz & Harrison, 2018; Slavich & Cole, 2013). This lack of diagnostic specificity, likely resulting from phenotypic overlap and high co-morbidity, prompts the search for specific transdiagnostic symptoms linked to inflammation as opposed to categorical diagnoses. This line of inquiry is important, as there is a growing recognition of the need to study dimensional constructs underlying core features of psychopathology as opposed to focusing on specific diagnoses (Carcone & Ruocco, 2017; Zalta & Shankman, 2016).
Anhedonia–loss of pleasure from activities that one used to enjoy–has emerged as one such transdiagnostic symptom (Holtzheimer & Mayberg, 2011; Slavich & Irwin, 2014). Although anhedonia is regarded as a cardinal clinical feature of MDD, impaired motivation and reward processes and underlying brain circuitry have been noted across various psychiatric conditions (Barkus & Badcock, 2019; Husain & Roiser, 2018; Naguy, Alwetayan, & AlKhadhari, 2019; Whitton, Treadway, & Pizzagalli, 2015). Additionally anhedonia is strongly linked to poor outcomes and quality of life as well as treatment resistance among individuals with psychiatric and medical conditions ranging from schizophrenia to cardiovascular disease (Craske, Meuret, Ritz, Treanor, & Dour, 2016; Davidson et al., 2010; Ritsner, Arbitman, & Lisker, 2011; Rubin, 2012; Uher et al., 2012).
Building on previous findings that document effects of the immune system on the neurobiological substrates of reward circuitry, a small but growing body of research highlights the role of inflammation in reward processes and anhedonia (Dantzer, O’Connor, Lawson, & Kelley, 2011; Dantzer & Walker, 2014; Lee et al., 2018; Russo & Nestler, 2013). For instance, one study found that higher levels of inflammation, assessed via circulating levels of CRP, was linked to reduced functional connectivity within reward and motor neurocircuitry among patients with current MDD (Felger et al., 2016). This effect on neurocircuitry mediated the association between CRP and anhedonia, suggesting that inflammation may play an important role in motor deficits and decreased motivation observed in depressed individuals by modulating connectivity between brain areas underlying reward and motor processes (Felger et al., 2016). Furthermore, inducing inflammation via administration of endotoxin, a component of gram negative bacteria that robustly provokes an immune response, decreased responsivity of the ventral striatum, a key brain region involved in reward processing, to monetary rewards (Eisenberger et al., 2010). Additionally, higher levels of anhedonia were associated with higher circulating levels of T-cell-derived cytokines in adults with a current MDD diagnosis (Jha, Miller, Minhajuddin, & Trivedi, 2018). Similarly, greater levels of anhedonia, but not other symptoms (e.g., general depressive symptoms, anxiety), were linked to higher endotoxin-stimulated concentrations of 19 different immune molecules in a diagnostically heterogeneous sample of adolescents (Freed et al., 2018). Although together these findings strongly point to a role for inflammation in brain reward circuitry and anhedonia symptoms, it remains unclear whether evoked levels of the T-cell-derived cytokines were associated with anhedonia in adults with and without a lifetime diagnosis of recurrent MDD.
To further clarify the role of anhedonia specifically versus a diagnosis of rMDD, the present study examined potential differences in functional immune responses assessed via in vitro endotoxin-stimulated levels of cytokines based on adult participants’ lifetime histories of recurrent MDD and current levels of anhedonia. In doing so, we examined inflammatory markers of innate and adaptive immunity, including myeloid-cell-derived cytokines and T-cell-derived molecules. Innate immunity is a rapid, non-specific set of host’s defense mechanisms to injury and pathogen invasion. The adaptive immune response is slow-acting, antigen-specific, and its manifestation is much stronger upon a repeated exposure to the same pathogen (Berry, Watson, Jonjic, Degli-Esposti, & Rossjohn, 2020; Chaplin, 2010; Dempsey, Vaidya, & Cheng, 2003; Marshall, Warrington, Watson, & Kim, 2018). The cytokines involved in innate immune cell signaling were chosen due to their role in etiology and pathophysiology of psychiatric illness, including depression (Dowlati et al., 2010; Andrew Miller & Raison, 2016; Raison, Capuron, & Miller, 2006). Cytokines and receptors involved in T helper (Th) 17 cell signaling involved in adaptive immunity responses were selected due to their role in maintaining the balance between pro and anti-inflammatory immune responses, and their involvement in the pathogenesis of multiple inflammatory and autoimmune conditions characterized by increased anhedonia (Guglani & Khader, 2010). We hypothesized that a larger functional immune response would be observed among individuals with a history of rMDD and those with higher current levels of anhedonia compared to individuals with no rMDD or lower levels of anhedonia. Exploratory moderation analyses were also conducted to determine whether the link between current levels of anhedonia and immune responses differed between individuals with and without a history of rMDD.
Method
Participants and Procedure.
Participants were 39 adults recruited from the community as part of a larger study of depression and anxiety. Participants in this subsample were required to either have a history of recurrent MDD (rMDD; n = 20) or have no lifetime history of MDD (n = 19). The average age of participants was 36.81 years (SD = 7.55) and the majority were women (87.2%). In terms of race/ethnicity, the majority were Caucasian (76.3%) and the rest were African American (15.8%), Asian/Pacific Islander (2.6 %), or from other racial/ethnic groups (5.3%). The median annual family income was between $45,001 and $50,000. Other clinical and physiological participant characteristics can be found in Table 1. Upon arrival at the laboratory, participants were asked to provide informed consent and then administered the Structured Clinical Interview for DSM Disorders (SCID-I; First, Spitzer, Gibbon, & Williams, 2002) to assess lifetime diagnoses. Following this, participants completed questionnaires. Finally, peripheral blood samples, height, weight, and body temperature were collected.
Table 1.
Physiological and clinical characteristics of participants in our study.
| Participant Characteristics |
M (SD) |
|---|---|
| MASQ-AD | 61.02 (14.9) |
| BDI-II | 9.15 (7.45) |
| BAI | 8.51 (10.04) |
| BMI | 34.05 (7.33) |
| Body temperature (°F) | 98.21 (0.57) |
| IL-1β (pg/mL) | 5584.69 (1264.07) |
| IL-6 (pg/mL) | 35810.68 (15190.18) |
| IL-10 (pg/mL) | 1298.67 (769.10) |
| IL-17A (pg/mL) | 36.10 (5.57) |
| IL-17F (pg/mL) | 36.72 (3.23) |
| IL-21 (pg/mL) | 313.28 (28.48) |
| IL-22 (pg/mL) | 36.38 (5.97) |
| IL-23 (pg/mL) | 80.23 (18.01) |
| IL-25 (pg/mL) | 13.05 (1.01) |
| IL-31 (pg/mL) | 94.61 (9.01) |
| IL-33 (pg/mL) | 11.21 (0.98) |
| IFNγ (pg/mL) | 545.24 (457.15 |
| TNF-α (pg/mL) | 2011.92 (1198.42) |
| sCDL40 (pg/mL) | 401.33 (32.64) |
| Total Protein (μg/mL) | 4692.41 (1070.15) |
Note: MASQ-AD = anhedonic depression subscale of the Mood and Anxiety Symptom Questionnaire; BDI-II = Beck Depression Inventory-II; BAI= Beck Anxiety Inventory; BMI = Body Mass Index. The table lists untransformed values for all participant characteristics.
Clinical diagnoses.
The Structured Clinical Interview for DSM Disorders (SCID-I; First, Spitzer, Gibbon, & Williams, 2002) was used to assess for lifetime histories of DSM-IV psychiatric disorders. The SCID-I is a widely used diagnostic interview with well-established psychometric properties (First et al., 2002). A subset of 20 SCID-I interviews was coded by a second interviewer and interrater reliability for diagnoses of MDD was excellent (κ = 1.00). Out of the 39 participants in this study, 20 (51.3%) had a history of rMDD. The distribution of lifetime occurrences of other diagnoses was a follows: Alcohol Use Disorder = 14 (35.9%), Post-Traumatic Stress Disorder (PTSD) = 9 (23.1%), Panic Disorder (PD) = 8 (20.5%), Social Phobia (SP) = 6 (15.4%), Substance Use Disorder = 6 (15.4%), Obsessive Compulsive Disorder (OCD) = 4 (10.3%), and Generalized Anxiety Disorder (GAD) = 4 (10.3%). Of those, the distribution of participants who met criteria for current diagnoses was as follows: PD = 6 (15.4%), 5 of whom also had a history of rMDD; GAD = 4 (10.3%), 3 with a history of rMDD; SP = 3 (7.7%), all with a history of rMDD; OCD = 2 (5.1%), all with a history of rMDD; MDD = 2 (5.1%); and PTSD = 1 (5.1%), who also had a history of rMDD.
Anhedonia symptoms.
Levels of anhedonia were assessed using the anhedonic depression subscale of the Mood and Anxiety Symptom Questionnaire (MASQ; Watson et al., 1995). This is a well-established measure of anhedonia that demonstrated good reliability and validity in previous research (Bredemeier et al., 2010), with higher scores reflecting greater levels of symptomatology. In this sample, the MASQ-AD subscale exhibited excellent internal consistency (α = 0.92).
Current depression and anxiety symptoms.
We used Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996) and Beck Anxiety Inventory (BAI; Beck & Steer, 1993) to assess participant’s current symptoms of depression and anxiety. In this sample, both measures evidenced good to excellent internal consistency (α = 0.88 and 0.94, respectively). To reduce overlap with the MASQ-AD, we removed the BDI-II anhedonia items (items 4 and 12) when calculating the scores.
Evoked immune response.
We followed a well-established protocol for conducting an in vitro lipopolysaccharide (LPS) immune challenge (e.g., Schildberger, Rossmanith, Eichhorn, Strassl, & Weber, 2013). Whole blood was collected by certified phlebotomists using BD Vacutainer Blood Collection Sets into BD Vacutainer Cell Preparation silicon-coated 4.0 mL tubes with sodium citrate. PBMCs were isolated and stored using previously described methods (Mallone et al., 2011). Briefly, PBMCs were separated by centrifugation (1500 × g for 20 minutes at 23± 1°C) and washed three times in phosphate buffered saline (PBS) supplemented with 10% fetal bovine serum (FBS, Fisher Scientific, Pittsburg, PA). The cells were re-suspended in RPM Media-1640 with 10% dimethyl sulfoxide sterile solution (DMSO, Sigma-Aldrich, St. Louis, MO) and 20% FBS and placed into a Coolcell® freezing container (Biocision, San Rafael, CA), which was kept in a −80 °C freezer for 12h. After that, cryo vials with cells were transferred into liquid nitrogen storage until further testing. The average cell survival rate was > 98.00%, which is comparable or higher than the rates obtained in previous research (Mallone et al., 2011). On the day of testing, a fraction of the cells from each sample (10 μl) were stained with Trypan Blue Stain solution (Thermo Fisher Scientific, Waltham, MA) and the live/dead cells were counted via a hemocytometer. Cells were then pelleted by centrifugation at 250 × g and re-suspended in 10% cell-culture grade Fetal Bovine Serum (Seradigm Life Sciences, Philadelphia, PA) and LPS-enriched RPMI 1640 media (Invitrogen, Waltham, MA) containing at 1 × 106 cells/mL and incubated for 24h at 37 °C. The LPS concentration (10 ng/mL) and duration were selected based on recommendations from previous research (Schildberger et al., 2013). After 24h, the cells were pelleted by centrifugation (2000 rpm for 5 min) and the supernatants were removed and stored at −80°C. Concentrations of cytokines were measured subsequently using Bio-Plex Pro Human TH17 Cytokine assay (Bio-Rad, Philadelphia, PA). The panel contained the following 15 cytokines: IL-1β, IL-4, IL-6, IL-10, IL-17A, IL-17F, IL-21, IL-22, IL-23, IL-25, IL-31, IL-33, TNF-α, IFNγ, and sCDL40. As most of the IL-4 data were below the lowest limit of quantification, we excluded this cytokine from all further analyses and focused on the remaining 14 cytokines. The average intra- assay coefficient across all 14 cytokines was 3.9%. The average inter- assay coefficient of variation was 3.4%.
Total Protein Concentration.
Total supernatant protein concentrations were assessed via bicinchoninic acid (BCA) protein assay (Thermo Fisher Scientific, Waltham, MA). The lower limit of quantification was 20 μg/mL. The average inter and intra- assay coefficients of variation were both below 1%. All circulating and stimulated cytokine values were normalized to the total protein levels, as recommended by previous research (Ber et al., 2014; Collins, An, Peller, & Bowser, 2015; Hepworth et al., 2012).
Physiological Measures.
To control for potential confounding effects of participants’ Body Mass Index (BMI) and body temperature, participants’ height and weight were measured for BMI calculation. Body temperature was measured by sliding the probe of an infrared thermometer across the participant’s forehead (Exergen, Watertown, MA).
Statistical Analyses.
We first conducted a series of independent samples t tests to examine rMDD group differences in stimulated cytokine levels. Next, correlation analyses were used to examine whether current levels of anhedonia were associated with stimulated cytokine levels. To examine the robustness of significant findings, we conducted partial correlations to statistically control for the potential influence of participant demographic (age, sex, ethnicity, income) and physiological (body temperature, BMI) characteristics, as well as current mood (symptoms of depression and anxiety) and time of day when the sample was collected. Last, we conducted exploratory analyses using general linear models to examine whether history of rMDD moderated the link between current anhedonia and functional immune response. As before, we tested the robustness of significant findings by including participant characteristics mentioned above as covariates in the model.
Results
Due to missing data (BAI=3%, BDI-I =8%, MASQ-AD=10%), we examined whether the data were missing at random, thereby justifying the use of data imputation methods for estimating missing values (cf. Schafer & Graham, 2002). Little’s missing completely at random (MCAR) test, for which the null hypothesis is that the data are MCAR (Little & Rubin, 2002) was nonsignificant, χ2(19) = 17.76, p = 0.54, providing support for the imputation of missing values. Given these results, missing values were imputed using the expectation-maximization algorithm (Moon, 1996; Schafer & Graham, 2002).
Diagnosis-based group differences in evoked immune response.
First, we conducted a series of t tests to examine differences in levels of each of the stimulated cytokines based on participants’ lifetime history of rMDD (history of rMDD vs. no history of any MDD). There were no significant group differences in stimulated levels of any of the 14 cytokines in the panel (lowest p = 0.07). Thus, there was no support for the association between participants’ history of rMDD and functional immune response in our sample. However, given the relatively small sample sizes of the subgroups, these results should be interpreted with caution.
Association between anhedonia severity and evoked immune response.
Next, we examined whether current levels of anhedonia were associated with increased stimulated levels of the cytokines using correlations analysis. We found that higher levels of anhedonia symptoms were associated with higher stimulated levels of 11 of the 14 cytokines, including IL-17A (r = 0.37, p = 0.02), IL-17F (r = 0.38, p = 0.02), IL-21 (r = 0.45, p = 0.004), IL-22 (r = 0.35, p = 0.03), IL-23 (r = 0.33, p = 0.04), IL-25 (r = 0.41, p = 0.01), IL-31 (r = 0.38, p = 0.02), IL-33 (r = 0.44, p = 0.005), IFNγ (r = 0.42, p = 0.008), TNFα (r = 0.34, p =0.03), and sCDL40 (r = 0.43, p = 0.007). In contrast, the correlations were nonsignificant for the associations between anhedonia and stimulated levels of IL-1β (r = 0.25, p =0.12), IL-6 (r = 0.20, p = 0.22), and IL-10 (r = 0.24, p = 0.15). The significant relations are depicted visually in Figure 1.
Figure 1.
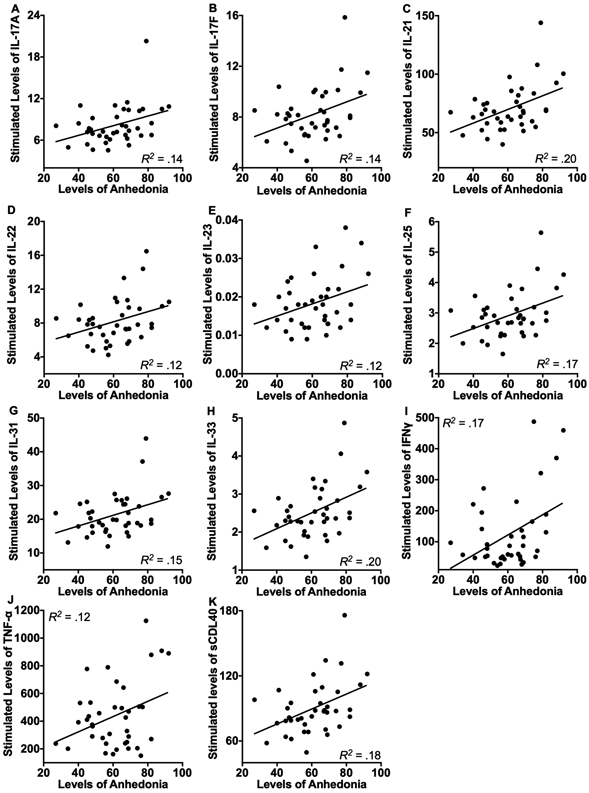
Levels of current anhedonia symptoms were significantly associated with increased endotoxin-stimulated levels of IL-17A (A), IL-17F (B), IL-21(C), IL-22 (D), IL-23 (E), IL-25 (F), IL-31 (G), IL-33 (H), IFNγ (I), TNF-α (J), and sCDL40 (K) prior to the tests of robustness. When we statistically controlled for the influence of participants’ demographic (age, sex, ethnicity, income) and physiological (body temperature, BMI) characteristics, current symptoms of depression and anxiety, and the time of day of the sample collection, the significant results were maintained for IL-17F, IL-21, IL-22, IL-25, IL-31, IL-33, IFNγ, and sCDL40. Values for stimulated cytokines were normalized to the total protein levels.
To examine the robustness of the significant findings, we examined whether they would be maintained after statistically controlling for the influence of participants’ demographics (age, sex, ethnicity, income) and physiological characteristics (body temperature, BMI), current depression and anxiety symptoms, and time of day of the sample collection. The findings were maintained for IL-17F, IL-21, IL-22, IL-25, IL-31, IL-33, IFNγ, and sCDL40 (all ps < 0.05). The significant findings for IL-17A and IL-23 remained significant when we statistically controlled for the influence of participants’ sex, ethnicity, body temperature, BMI, and time of day of the sample collection, or current depression and anxiety symptoms (all ps < 0.05), but were no longer significant when we included participants’ age and income as covariates (lowest p = 0.06). Additionally, findings for TNF-α approached but did not achieve significance when we statistically controlled for participants’ age, sex, income, body temperature, time of day, or current depression and anxiety symptoms (lowest p = 0.06). These results suggest the relations between levels of anhedonia and stimulated cytokine levels were the most robust for IL-17F, IL-21, IL-22, IL-25, IL-31, IL-33, IFNγ, and sCDL40.
Moderation effects of rMDD.
Last, exploratory analyses were conducted to examine whether participants’ history of rMDD moderated the relation between current levels of anhedonia and stimulated levels of cytokines. We found a significant rMDD × Anhedonia interaction for stimulated levels of IL-1β, t(38) = 2.87, p = 0.007, reffect size = 0.42, IL-6, t(38) = 2.80, p = 0.008, reffect size = 0.41, IL17F, t(38) = 2.08, p = 0.05, reffect size = 0.32, IFNγ, t(38) = 2.70, p = 0.01, reffect size = 0.40, and TNF-α, t(38) = 2.10, p = 0.03, reffect size = 0.35. Probing the form of these interactions, we found that, among participants with a history of rMDD, higher levels of anhedonia were associated with higher levels of IL-1β (r = 0.54, p = 0.02), IL-6 (r = 0.60, p = 0.005), IL17F (r = 0.52, p = 0.02), IFNγ (r = 0.56, p = 0.01), and TNF-α (r = 0.55, p = 0.01). In contrast, among participants with no lifetime history of any MDD episodes, levels of anhedonia were not significantly associated with any of these cytokines (lowest p > 0.29). When we statistically controlled for the influence of participants’ demographic (age, sex, ethnicity, income) and physiological (body temperature, BMI) characteristics, current depression and anxiety symptoms, and the time of day of the sample collection, the significant findings were maintained for IL-1β and IL-6 (all ps < 0.05), but not IL-17F, IFNγ, or TNF-α (lowest p = 0.06).
Discussion
The current investigation focused on examining the association between the immune response assessed via measuring in vitro lipopolysaccharide-evoked release of inflammatory markers and current anhedonia symptoms in individuals with and without a history of recurrent major depressive disorder (rMDD). Although previous research describes higher levels of peripheral inflammation among individuals with psychiatric conditions, including MDD (Dowlati et al., 2010; Gabbay et al., 2009; AH Miller et al., 2009), we did not find differences in the evoked immune response of individuals with and without a history of rMDD. That said, consistent with our findings, a number of previous studies failed to observe diagnosis-based group differences in inflammatory markers (Byrne et al., 2013; Cassano et al., 2017). These mixed findings suggest that depression may be associated with increased inflammation in a specific sub-population of individuals with rMDD and other psychiatric disorders, and emphasize the importance of focusing on dimensional trans-diagnostic psychiatric symptoms as opposed to categorical diagnoses.
Supporting this view, we found that higher current levels of anhedonia were associated with higher stimulated levels of 8 out of a panel of 14 Th17 cytokines, including IL-17F, IL-21, IL-22, IL-25, IL-31, IL-33, IFNγ, and soluble CD40 ligand (sCDL40) in the overall sample, after we statistically controlled for the influence of participants’ demographic (age, sex, ethnicity, income) and physiological (body temperature, BMI) characteristics, current symptoms of depression and anxiety, and the time of day of the sample collection. This is the first study, to our knowledge, that examined the link between current anhedonia and evoked immune response in adults with and without a history of rMDD. These findings are consistent with a growing body of research focusing on the role of inflammation in anhedonia (Felger et al., 2016; Freed et al., 2018; Jha et al., 2018) and in line with a call for focusing on dimensional trans-diagnostic phenotypes as opposed to diagnostic categories (Pizzagalli, 2014). Despite growing recognition of the need to study dimensional constructs underlying core features of psychopathology, including MDD, in addition to focusing on categorical diagnoses (Carcone & Ruocco, 2017; Zalta & Shankman, 2016), research examining depression symptoms beyond vegetative and somatic domains is scarce (Slavich & Irwin, 2014).
However, this growing body of literature highlights the role of inflammation in anhedonia. For example, increased circulating levels of C-reactive protein (CRP), which is a robust marker of inflammation, were associated with decreased connectivity in corticostriatal reward and motor neurocircuitry (Felger et al., 2016) underlying anhedonia symptoms (Heshmati & Russo, 2015). Moreover, increased levels of stimulated immune molecules involved in immune cell proliferation, maturation, migration, and activation of inflammatory pathways (e.g., granulocyte-colony stimulating factor, IL-2) were associated with greater levels of anhedonia among clinical adolescent population (Freed et al., 2018).
The current findings, therefore, provide promising evidence that supports and extends this research to T-cell-derived cytokines among adults with and without a recurrent MDD history, and points to new targets among inflammatory markers. As a trans-diagnostic symptom of many psychiatric and medical conditions, anhedonia may be impacted by common immune mechanisms that impact reward processing, including T-cell-mediated immunity (Contreras et al., 2016; Escalona & Fawcett, 2017; Swardfager, Rosenblat, Benlamri, & McIntyre, 2016). Along with B-lymphocytes, T-cells are mediators of an adaptive or acquired immunity that mounts a potent slow-acting pathogen-specific response (Punt, 2013). T-cell-derived cytokines produced by the activated T-cells, recruit and activate different types leukocytes and induce the production of antimicrobial molecules to eliminate invading pathogens (Chang & Dong, 2009; Duvallet, Semerano, Assier, Falgarone, & Boissier, 2011; Kleinschek et al., 2007; Rutz, Eidenschenk, & Ouyang, 2013). These cytokines are implicated in the etiology and pathogenesis of many inflammatory and autoimmune conditions including rheumatoid arthritis, psoriasis, and multiple sclerosis characterized by increased anhedonia (Guglani & Khader, 2010). Notably, these cytokines have been shown to affect dopaminergic, serotonergic, and glutamatergic functions in the reward circuitry of the brain, including ventral tegmental area (VTA), prefrontal and anterior cingulate cortices, and basal ganglia (Dantzer et al., 2011; Dantzer & Walker, 2014; Lee et al., 2018; Russo & Nestler, 2013). A member of the tumor-necrosis factor-receptor superfamily, soluble CD40 ligand (sCDL40) is released into circulation by activated T-cells and platelets, where it interacts with a co-stimulatory CD40 molecule along with CDL40, to exert regulatory effects on adaptive immunity responses (Chung & Lim, 2014; Noelle, 1996; van Kooten & Banchereau, 2000). Overactivation of CD40-CDL40 is a common neuropathological signature of major forms of dementia and has established links to cardiovascular diseases and cancer (Chen et al., 2017; Giunta, Figueroa, Town, & Tan, 2009; Kim et al., 2015). In the mental health context, sCDL40 has been inconsistently linked to MDD in previous literature (Leo et al., 2006; Piletz et al., 2009; Zeugmann, Quante, Heuser, Schwarzer, & Anghelescu, 2010). The current findings extend previous research that showed that activation of CD40-CDL40 in rodents decreased engagement in enjoyable activities (saccharin consumption; Cathomas et al., 2015), suggesting that increased sCDL40 is linked to greater anhedonia in rodent models.
Although there was no direct association between rMDD and higher evoked immune responses, rMDD history moderated the link between anhedonia and myeloid cell-derived cytokines, IL-1β and IL-6. Indeed, greater symptoms of anhedonia were associated with higher levels of these cytokines released by PBMCs in response to an in vitro stimulation by lipopolysaccharide, a common pathogen, among individuals with a history of rMDD, but not among participants with no history of rMDD, even after accounting for the potential influence of a number of other demographic and clinical variables. Myeloid cell-derived cells are a heterogeneous group of cells that play a key role in innate immune responses, including phagocytosis and release of inflammatory cytokines, in healthy individuals as well as pathophysiology of chronic health conditions (Gabrilovich & Nagaraj, 2009; Kawamoto & Minato, 2004). These findings suggest that individuals who experienced recurrent depression evidence greater functional immune response characterized by these specific cytokines in the presence of current anhedonia symptoms, with may constitute a vulnerability for depression recurrence.
Overall, increased lipopolysaccharide-evoked immune response was associated with greater anhedonia symptoms, either among participants with rMDD history or in the combined sample, highlighting the link between this transdiagnostic symptom and immune function. Specifically, greater current anhedonia levels were linked to increased levels of stimulated T-cell-derived cytokines that are typically involved in the adaptive immune responses (IL-17F, IL-21, IL-22, IL-25, IL-31, IL-33, IFNγ, and sCDL40) in the overall sample and to myeloid cell-derived cytokines involved in innate immunity (IL-1β and IL-6) in the rMDD group. The study’s strengths include using dimensional assessment of anhedonia in addition to a diagnostic category and examining evoked immune response as opposed to circulating levels of cytokines, which degrade quickly and report a multitude of conditions that may (or may not) relate to rMDD.
There are several limitations that provide important directions for future research. First, our sample size was small and predominantly female. Thus, future research using larger samples and examining potential sex differences is warranted, despite that we detected moderate effect sizes in our sample. Second, we used a self-report clinical measure of anhedonia symptoms. Although neuroimaging studies show that self-report measures of anhedonia are linked to reward processing (Felger et al., 2016), future studies that use multi-mode assessment of reward processing (e.g. neuroimaging paradigms) are needed to better characterize components of reward function most closely associated with evoked immune response. Lastly, although we statistically adjusted to a host of demographic and physiological factors in our analyses, we did not assess other potential confounders, including menstrual cycle phase and participants’ diet and physical activity level, which could impact immune functioning (Galland, 2010; Kajantie & Phillips, 2006; Kiecolt-Glaser et al., 2010; Puder et al., 2006). However, as mentioned above, assessing functional immune response vs. circulating levels of inflammatory markers minimizes the effects of these biological factors (Freed et al., 2018).
Given that this study was the first to examine the association between anhedonia severity and in vitro lipopolysaccharide stimulated T-cell-derived cytokines, conclusions must remain tentative and require further study. This said, the current findings suggest that an evoked immune response is associated specifically with anhedonia symptoms, as opposed to the more heterogeneous diagnoses of MDD. Additionally, the results highlight the utility of exploring the role of more novel inflammatory markers beyond those most commonly researched in this context, which may ultimately lead to personalized medicine based on clinical and biological patient profiles.
Acknowledgements
We would like to thank Max Owens, Cope Feurer, Eric Funk, Effua Sosoo, Sydney Meadows, Michael Van Wie, Devra Alper, Katie L. Burkhause, Mary L.Woody, Aliona Tsypes, Nathan Hall, and Aholibama Lopez for their help in conducting assessments for this project and Dr. Molly Deak for helping to conduct biological assays. We would also like to thank our participants for their valued contribution. This project was supported by National Institute of Mental Health grant MH098060 awarded to B.E. Gibb, T32 MH019927, and P50AA017823 and R01AG043467 awarded to T. Deak, as well as the Center for Development and Behavioral Neuroscience at Binghamton University. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the above stated funding agencies. The authors have no conflicts of interest to declare.
References
- Barkus E, & Badcock JC (2019). A Transdiagnostic Perspective on Social Anhedonia. Frontiers in Psychiatry, 10, 216. 10.3389/fpsyt.2019.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer ME, & Teixeira AL (2019). Inflammation in psychiatric disorders: what comes first? Annals of the New York Academy of Sciences, 1437(1), 57–67. 10.1111/nyas.13712 [DOI] [PubMed] [Google Scholar]
- Beck A, & Steer R (1993). Beck Anxiety Inventory Manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Beck A, Steer R, & Brown G (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Ber Y, Shiloh R, Gilad Y, Degani N, Bialik S, & Kimchi A (2014). DAPK2 is a novel regulator of mTORC1 activity and autophagy. Cell Death And Differentiation, 22, 465. Retrieved from 10.1038/cdd.2014.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry R, Watson GM, Jonjic S, Degli-Esposti MA, & Rossjohn J (2020). Modulation of innate and adaptive immunity by cytomegaloviruses. Nature Reviews Immunology, 20(2), 113–127. 10.1038/s41577-019-0225-5 [DOI] [PubMed] [Google Scholar]
- Bredemeier K, Spielberg JM, Silton RL, Berenbaum H, Heller W, & Miller GA (2010, September). Screening for depressive disorders using the MASQ anhedonic depression scale: A receiver-operator characteristic analysis. Psychological Assessment. 10.1037/a0019915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne ML, O’Brien-Simpson NM, Reynolds EC, Walsh KA, Laughton K, Waloszek JM, … Allen NB (2013). Acute phase protein and cytokine levels in serum and saliva: a comparison of detectable levels and correlations in a depressed and healthy adolescent sample. Brain, Behavior, and Immunity, 34, 164–175. 10.1016/j.bbi.2013.08.010 [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud a, Neveu PJ, Miller a H., Maes M, & Dantzer R (2002). Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Molecular Psychiatry, 7(5), 468–473. 10.1038/sj.mp.4000995 [DOI] [PubMed] [Google Scholar]
- Carcone D, & Ruocco AC (2017). Six Years of Research on the National Institute of Mental Health’s Research Domain Criteria (RDoC) Initiative: A Systematic Review . Frontiers in Cellular Neuroscience . Retrieved from https://www.frontiersin.org/article/10.3389/fncel.2017.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassano P, Bui E, Rogers AH, Walton ZE, Ross R, Zeng M, … Simon NM (2017). Inflammatory cytokines in major depressive disorder: A case-control study. The Australian and New Zealand Journal of Psychiatry, 51(1), 23–31. 10.1177/0004867416652736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathomas F, Fuertig R, Sigrist H, Newman GN, Hoop V, Bizzozzero M, … Pryce CR (2015). CD40-TNF activation in mice induces extended sickness behavior syndrome coincident with but not dependent on activation of the kynurenine pathway. Brain, Behavior, and Immunity, 50, 125–140. 10.1016/j.bbi.2015.06.184 [DOI] [PubMed] [Google Scholar]
- Chang SH, & Dong C (2009). IL-17F: regulation, signaling and function in inflammation. Cytokine, 46(1), 7–11. 10.1016/j.cyto.2008.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin DD (2010). Overview of the immune response. The Journal of Allergy and Clinical Immunology, 125(2 Suppl 2), S3–23. 10.1016/j.jaci.2009.12.980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li J-H, Zhao S-J, Wang D-Y, Zhang W-Z, & Liang W-J (2017). Clinical significance of costimulatory molecules CD40/CD40L and CD134/CD134L in coronary heart disease: A case-control study. Medicine, 96(32), e7634–e7634. 10.1097/MD.0000000000007634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HW, & Lim J-B (2014). Clinical significance of elevated serum soluble CD40 ligand levels as a diagnostic and prognostic tumor marker for pancreatic ductal adenocarcinoma. Journal of Translational Medicine, 12(1), 102. 10.1186/1479-5876-12-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MA, An J, Peller D, & Bowser R (2015). Total protein is an effective loading control for cerebrospinal fluid western blots. Journal of Neuroscience Methods, 251, 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras F, Prado C, González H, Franz D, Osorio-Barrios F, Osorio F, … Pacheco R (2016). Dopamine Receptor D3 Signaling on CD4+ T Cells Favors Th1- and Th17-Mediated Immunity. The Journal of Immunology, 196(10), 4143–4149. 10.4049/jimmunol.1502420 [DOI] [PubMed] [Google Scholar]
- Craske MG, Meuret AE, Ritz T, Treanor M, & Dour HJ (2016). Treatment for Anhedonia: A Neuroscience Driven Approach. Depression and Anxiety, 33(10), 927–938. 10.1002/da.22490 [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Lawson MA, & Kelley KW (2011). Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology, 36(3), 426–436. 10.1016/j.psyneuen.2010.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, & Walker AK (2014). Is there a role for glutamate-mediated excitotoxicity in inflammation-induced depression? Journal of Neural Transmission (Vienna, Austria : 1996), 121(8), 925–932. 10.1007/s00702-014-1187-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson KW, Burg MM, Kronish IM, Shimbo D, Dettenborn L, Mehran R, … Rieckmann N (2010). Association of anhedonia with recurrent major adverse cardiac events and mortality 1 year after acute coronary syndrome. Archives of General Psychiatry, 67(5), 480–488. 10.1001/archgenpsychiatry.2010.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey PW, Vaidya SA, & Cheng G (2003). The art of war: Innate and adaptive immune responses. Cellular and Molecular Life Sciences : CMLS, 60(12), 2604–2621. 10.1007/s00018-003-3180-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, & Lanctôt KL (2010). A meta-analysis of cytokines in major depression. Biological Psychiatry, 67(5), 446–457. 10.1016/j.biopsych.2009.09.033 [DOI] [PubMed] [Google Scholar]
- Duvallet E, Semerano L, Assier E, Falgarone G, & Boissier M-C (2011). Interleukin-23: a key cytokine in inflammatory diseases. Annals of Medicine, 43(7), 503–511. 10.3109/07853890.2011.577093 [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, & Irwin MR (2010). Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biological Psychiatry, 68(8), 748–754. 10.1016/j.biopsych.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalona R, & Fawcett J (2017, January). Pramipexole in Treatment Resistant-Depression, Possible Role of Inflammatory Cytokines. Neuropsychopharmacology. 10.1038/npp.2016.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, & Miller AH (2016). Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Molecular Psychiatry, 10, 1358–1365. 10.1038/mp.2015.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, & Williams J (2002). Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patient edition. (SCID-I/NP). New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Ford DE, & Erlinger TP (2004). Depression and C-Reactive Protein in US Adults. Archives of Internal Medicine, 164, 1010–1014. [DOI] [PubMed] [Google Scholar]
- Freed RD, Mehra LM, Laor D, Patel M, Alonso CM, Kim-Schulze S, & Gabbay V (2018). Anhedonia as a clinical correlate of inflammation in adolescents across psychiatric conditions. The World Journal of Biological Psychiatry : The Official Journal of the World Federation of Societies of Biological Psychiatry, 1–11. 10.1080/15622975.2018.1482000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MJ (2014, August). Research on psychiatric disorders targets inflammation. JAMA. United States. 10.1001/jama.2014.8276 [DOI] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Alonso CM, Babb JS, Nishawala M, De Jesus G, … Gonzalez CJ (2009). Immune system dysregulation in adolescent major depressive disorder. Journal of Affective Disorders, 115(1–2), 177–182. 10.1016/j.jad.2008.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, & Nagaraj S (2009). Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews. Immunology, 9(3), 162–174. 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland L (2010). Diet and Inflammation. Nutrition in Clinical Practice, 25(6), 634–640. 10.1177/0884533610385703 [DOI] [PubMed] [Google Scholar]
- Giunta B, Figueroa KP, Town T, & Tan J (2009). SOLUBLE CD40 LIGAND IN DEMENTIA. Drugs of the Future, 34(4), 333–340. 10.1358/dof.2009.034.04.1358595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglani L, & Khader SA (2010, March). Th17 cytokines in mucosal immunity and inflammation. Current Opinion in HIV and AIDS. 10.1097/COH.0b013e328335c2f6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth MR, Daniłowicz-Luebert E, Rausch S, Metz M, Klotz C, Maurer M, & Hartmann S (2012, April). Mast cells orchestrate type 2 immunity to helminths through regulation of tissue-derived cytokines. Proceedings of the National Academy of Sciences of the United States of America. 10.1073/pnas.1112268109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heshmati M, & Russo SJ (2015, September). Anhedonia and the brain reward circuitry in depression. Current Behavioral Neuroscience Reports. 10.1007/s40473-015-0044-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzheimer PE, & Mayberg HS (2011). Stuck in a rut: rethinking depression and its treatment. Trends in Neurosciences, 34(1), 1–9. 10.1016/j.tins.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M, & Roiser JP (2018). Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nature Reviews Neuroscience, 19(8), 470–484. 10.1038/s41583-018-0029-9 [DOI] [PubMed] [Google Scholar]
- Jha MK, Miller AH, Minhajuddin A, & Trivedi MH (2018). Association of T and non-T cell cytokines with anhedonia: Role of gender differences. Psychoneuroendocrinology, 95, 1–7. 10.1016/j.psyneuen.2018.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajantie E, & Phillips DIW (2006). The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology, 31(2), 151–178. 10.1016/j.psyneuen.2005.07.002 [DOI] [PubMed] [Google Scholar]
- Kawamoto H, & Minato N (2004). Myeloid cells. The International Journal of Biochemistry & Cell Biology, 36(8), 1374–1379. 10.1016/j.biocel.2004.01.020 [DOI] [PubMed] [Google Scholar]
- Khandaker GM, Pearson RM, Zammit S, Lewis G, & Jones PB (2014, October). Association of Serum Interleukin 6 and C-Reactive Protein in Childhood With Depression and Psychosis in Young Adult Life: A Population-Based Longitudinal Study. JAMA Psychiatry. 10.1001/jamapsychiatry.2014.1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Christian L, Preston H, Houts CR, Malarkey WB, Emery CF, & Glaser R (2010). Stress, inflammation, and yoga practice. Psychosomatic Medicine, 72(2), 113–121. 10.1097/PSY.0b013e3181cb9377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kim Y, Bae S, Kong JM, Choi J, Jang M, … Lee WJ (2015). Direct Interaction of CD40 on Tumor Cells with CD40L on T Cells Increases the Proliferation of Tumor Cells by Enhancing TGF-β Production and Th17 Differentiation. PloS One, 10(5), e0125742–e0125742. 10.1371/journal.pone.0125742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschek MA, Owyang AM, Joyce-Shaikh B, Langrish CL, Chen Y, Gorman DM, … Cua DJ (2007). IL-25 regulates Th17 function in autoimmune inflammation. Journal of Experimental Medicine, 204(1), 161–170. 10.1084/jem.20061738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Subramaniapillai M, Brietzke E, Mansur RB, Ho RC, Yim SJ, & McIntyre RS (2018). Anti-cytokine agents for anhedonia: targeting inflammation and the immune system to treat dimensional disturbances in depression. Therapeutic Advances in Psychopharmacology, 8(12), 337–348. 10.1177/2045125318791944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo R, Di Lorenzo G, Tesauro M, Razzini C, Forleo GB, Chiricolo G, … Romeo F (2006). Association between enhanced soluble CD40 ligand and proinflammatory and prothrombotic states in major depressive disorder: pilot observations on the effects of selective serotonin reuptake inhibitor therapy. The Journal of Clinical Psychiatry, 67(11), 1760–1766. 10.4088/jcp.v67n1114 [DOI] [PubMed] [Google Scholar]
- Levine J, Barak Y, Chengappa KNR, Rapoport A, Rebey M, & Barak V (1999). Cerebrospinal Cytokine Levels in Patients with Acute Depression. Neuropsychobiology, 40(4), 171–176. Retrieved from http://www.karger.com/DOI/10.1159/000026615 [DOI] [PubMed] [Google Scholar]
- Little RJA, & Rubin DB (2002). Statistical Analysis with Missing Data. Hoboken, NJ, USA: John Wiley & Sons, Inc. 10.1002/9781119013563 [DOI] [Google Scholar]
- Liu Y, Ho RC-M, & Mak A (2012). Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. Journal of Affective Disorders, 139(3), 230–239. 10.1016/j.jad.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Mallone R, Mannering SI, Brooks-Worrell BM, Durinovic-Belló I, Cilio CM, Wong FS, & Schloot NC (2011). Isolation And Preservation Of Peripheral Blood Mononuclear cells for analysis of islet antigen-reactive T cell responses: Position statement of the T-Cell Workshop Committee of the Immunology of Diabetes Society. Clinical and Experimental Immunology, 163(1), 33–49. 10.1111/j.1365-2249.2010.04272.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JS, Warrington R, Watson W, & Kim HL (2018). An introduction to immunology and immunopathology. Allergy, Asthma, and Clinical Immunology : Official Journal of the Canadian Society of Allergy and Clinical Immunology, 14(Suppl 2), 49. 10.1186/s13223-018-0278-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, & Raison C (2009). Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biological Psychiatry, 65(9), 732–741. 10.1016/j.biopsych.2008.11.029.Inflammation [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller Andrew, & Raison C (2016). The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature Reviews Immunology, 16(1), 22–34. 10.1038/nri.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon TK (1996). The expectation-maximization algorithm. IEEE Signal Processing Magazine, 13, 47–60. 10.1109/79.543975 [DOI] [Google Scholar]
- Musselman DL, Miller a H., Porter MR, Manatunga a, Gao F, Penna S, … Nemeroff CB (2001). Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. The American Journal of Psychiatry, 158(8), 1252–1257. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11481159 [DOI] [PubMed] [Google Scholar]
- Naguy A, Alwetayan S, & AlKhadhari S (2019, January). Anhedonia as a transdiagnostic construct. Asian Journal of Psychiatry. Netherlands. 10.1016/j.ajp.2019.01.001 [DOI] [PubMed] [Google Scholar]
- Noelle RJ (1996). CD40 and its ligand in host defense. Immunity, 4(5), 415–419. 10.1016/s1074-7613(00)80408-2 [DOI] [PubMed] [Google Scholar]
- Owen BM, Eccleston D, Ferrier IN, & Young AH (2001). Raised levels of plasma interleukin-1beta in major and postviral depression. Acta Psychiatrica Scandinavica, 103, 226–228. [DOI] [PubMed] [Google Scholar]
- Piletz JE, Halaris A, Iqbal O, Hoppensteadt D, Fareed J, Zhu H, … Devane CL (2009). Pro-inflammatory biomakers in depression: treatment with venlafaxine. The World Journal of Biological Psychiatry : The Official Journal of the World Federation of Societies of Biological Psychiatry, 10(4), 313–323. 10.3109/15622970802573246 [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA (2014). Depression, stress, and anhedonia: toward a synthesis and integrated model. Annual Review of Clinical Psychology, 10, 393–423. 10.1146/annurev-clinpsy-050212-185606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puder J, Blum C, Mueller B, DeGeyter C, Dye L, & Keller U (2006). Menstrual cycle symptoms are associated with changes in low-grade inflammation. European Journal of Clinical Investigation, 36(1), 58–64. 10.1111/j.1365-2362.2006.01591.x [DOI] [PubMed] [Google Scholar]
- Punt J (2013). Chapter 4 - Adaptive Immunity: T Cells and Cytokines. In Prendergast GC & E. M. B. T-CI (Jaffee Second E. (Eds.) (pp. 41–53). San Diego: Academic Press. [Google Scholar]
- Raison CL, Capuron L, & Miller AH (2006). Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in Immunology, 27(1), 24–31. 10.1016/j.it.2005.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritsner MS, Arbitman M, & Lisker A (2011). Anhedonia is an important factor of health-related quality-of-life deficit in schizophrenia and schizoaffective disorder. The Journal of Nervous and Mental Disease, 199(11), 845–853. 10.1097/NMD.0b013e3182349ce6 [DOI] [PubMed] [Google Scholar]
- Rubin DH (2012). Joy Returns Last: Anhedonia and Treatment Resistance in Depressed Adolescents. Journal of the American Academy of Child & Adolescent Psychiatry, 51(4), 353–355. 10.1016/j.jaac.2012.01.012 [DOI] [PubMed] [Google Scholar]
- Russo SJ, & Nestler EJ (2013). The brain reward circuitry in mood disorders. Nature Reviews. Neuroscience, 14(9), 609–625. 10.1038/nrn3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutz S, Eidenschenk C, & Ouyang W (2013). IL-22, not simply a Th17 cytokine. Immunological Reviews, 252(1), 116–132. 10.1111/imr.12027 [DOI] [PubMed] [Google Scholar]
- Savitz J, & Harrison NA (2018). Interoception and Inflammation in Psychiatric Disorders. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(6), 514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL, & Graham JW (2002). Missing data: Our view of the state of the art. Psychological Methods, 7, 147–177. 10.1037//1082-989X.7.2.147 [DOI] [PubMed] [Google Scholar]
- Schildberger A, Rossmanith E, Eichhorn T, Strassl K, & Weber V (2013). Monocytes, peripheral blood mononuclear cells, and THP-1 cells exhibit different cytokine expression patterns following stimulation with lipopolysaccharide. Mediators of Inflammation, 2013, 697972. 10.1155/2013/697972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, & Cole SW (2013). The Emerging Field of Human Social Genomics. Clinical Psychological Science : A Journal of the Association for Psychological Science, 1(3), 331–348. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3707393&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, & Irwin MR (2014). From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychological Bulletin, 140(3), 774–815. 10.1037/a0035302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swardfager W, Rosenblat JD, Benlamri M, & McIntyre RS (2016). Mapping inflammation onto mood: Inflammatory mediators of anhedonia. Neuroscience & Biobehavioral Reviews, 64, 148–166. [DOI] [PubMed] [Google Scholar]
- Uher R, Perlis RH, Henigsberg N, Zobel A, Rietschel M, Mors O, … McGuffin P (2012). Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychological Medicine, 42(5), 967–980. 10.1017/S0033291711001905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooten C, & Banchereau J (2000). CD40-CD40 ligand. Journal of Leukocyte Biology, 67(1), 2–17. 10.1002/jlb.67.1.2 [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, & McCormick RA (1995). Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology, 104, 3–14. [DOI] [PubMed] [Google Scholar]
- Whitton AE, Treadway MT, & Pizzagalli DA (2015). Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Current Opinion in Psychiatry, 28(1), 7–12. 10.1097/YCO.0000000000000122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalta AK, & Shankman SA (2016, March). Conducting Psychopathology Prevention Research in the RDoC Era. Clinical Psychology : A Publication of the Division of Clinical Psychology of the American Psychological Association. 10.1111/cpsp.12144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeugmann S, Quante A, Heuser I, Schwarzer R, & Anghelescu I (2010). Inflammatory biomarkers in 70 depressed inpatients with and without the metabolic syndrome. The Journal of Clinical Psychiatry, 71(8), 1007–1016. 10.4088/JCP.08m04767blu [DOI] [PubMed] [Google Scholar]


