Summary
Background
Inflammatory marker and hemoglobin levels (e.g. biomarkers) considered separately predict adverse events in selected populations.
Hypothesis
A multiple biomarker approach predicts adverse events in women referred for evaluation of ischemia.
Methods
We investigated associations between biomarkers (high sensitivity C-reactive protein, interleukin-6, serum amyloid-A and hemoglobin levels) with adverse outcomes in women referred for coronary angiography for suspected ischemia in WISE.
Results
Among 595 women (mean age 58 years, ejection fraction 65%, majority without coronary stenosis ≥50%) followed for 3.6±1.8 years (mean±SD), those without abnormal markers had fewer events (11.6%) compared to those with one (18.4%), two (20.9%), or three (37%) abnormal markers (p<0.001 for trend). Women without abnormal markers had fewer deaths (1.6%) than women with one (6.1%), two (9.1%), or three (17%) abnormal markers (p<0.001 for trend). Adding low hemoglobin was associated with higher adverse event and all-cause mortality rates. In multivariate analysis, as number of abnormal biomarkers increased risk increased. Women with three or four abnormal biomarkers were approximately 10–20 times more likely to die (p<0.05). Biomarkers added to predictive information provided by the Framingham Risk Score.
Conclusions
Among women undergoing coronary angiography for suspected ischemia, a multi-biomarker approach predicted adverse events. Biomarkers added prognostic information beyond that obtained from traditional risk factors.
Keywords: Inflammation, Anemia, Cardiovascular Events, Women, Multimarker
Introduction
Diagnosis of coronary artery disease (CAD) and identifying women at high risk is key to improving outcomes. Inflammation plays a key role in atherosclerosis,1 and biomarkers like high-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6) and serum amyloid A (SAA) predict adverse CV events.2,3 Recently, the combination of inflammatory markers with cardiac troponin and brain natiuretic peptide predicted adverse events in h acute coronary syndrome (ACS) patients.4 In acute plaque rupture/erosion syndromes, a multimarker approach presumably improves assessment of the complex pathobiology over a single marker.
Low hemoglobin (Hgb) level is also associated with adverse outcomes.5–7 In the Women’s Ischemia Syndrome Evaluation (WISE) Hgb levels were, inversely associated with inflammatory marker levels, and predicted adverse events independent of traditional risk factors.8
Using a multibiomarker approach we investigated associations between inflammatory marker and Hgb levels with adverse outcomes in women undergoing angiography for suspected ischemia.
Methods
Study Design
The WISE is a National Heart, Lung and Blood Institute-sponsored project aiming to improve diagnosis of ischemic heart disease in women.9 Institutional Review Boards at each site approved the study and participant consent was obtained. Briefly, women referred for clinically-indicated angiograms to further evaluate suspected ischemia were screened. Exclusion criteria included comorbidity compromising follow-up, pregnancy, contraindications to diagnostic testing, cardiomyopathy, New York Heart Association class III–IV CHF, recent MI, and significant valvular or congenital heart disease. Baseline evaluation included collection of clinical and laboratory data. Qualitative and quantitative coronary angiographic analyses were done by a core lab masked to clinical data and obstructive CAD was defined as stenosis ≥50% in ≥1 artery.9,10
Measurement of Hgb and Inflammatory Markers
Hgb levels were analyzed on site. Inflammatory markers were analyzed by core lab from plasma samples obtained at entry and frozen at −70° C. SAA and hs-CRP were measured on a Hitachi 911 analyzer by high-sensitivity methods using validated techniques.11 Reagents for measurement of hs-CRP were from Denka Seiken (Niigata, Japan). Interleukin-6 levels were measured from plasma collected at study entry using a commercially-available ELISA kit using validated techniques (Quantikine hs human IL-6, R&D Systems, Minneapolis, MN).11
Definitions
Abnormal thresholds were pre-specified and defined as <12 g/dl for Hgb: ≥0.85 mg/L for hs-CRP,2 ≥3.09 pg/mL for IL-6,12 and ≥0.59 mg/dL for SAA.13
Ascertainment of Events
Women were queried in person or by telephone interview for occurrence of adverse events by an experienced nurse and/or physician from each site at 6-weeks and yearly. When an adverse event was identified, the referring physician was contacted for documentation. In the event of death, a death certificate and/or hospital records were obtained, and an event committee reviewed available information to determine the likelihood of a CV cause. Deaths confirmed as clearly due to CV causes were classed as CV deaths. Adverse event was defined as a composite of all-cause death or hospitalization for non-fatal MI, CHF, stroke, or other vascular event. Other vascular events primarily included peripheral vascular-related events.
Statistical Analyses
Data are presented as means and standard deviations (SD) for continuous data and frequencies for categorical. Spearman rank correlation coefficients assessed relationships among inflammatory markers and between markers and Hgb. Mantel Haentzel chi-square tests for trends assessed associations of events with number of inflammatory markers and low Hgb. Univariate and multivariable Cox regression models were used to identify predictors of events. Univariate predictors of all-cause mortality included a history of hypertension and diabetes as well as age, creatinine, CAD, Hgb, and inflammatory markers. Univariate predictors of adverse events included a history of hypertension, diabetes, and dyslipidemia as well as age, CAD, race, creatinine, Hgb, and inflammatory markers. Multivariable Cox regression models were run as a stepwise procedure with variables chosen for entry based on significant univariate associations. As expected the inflammatory markers were correlated with each other so individual models were constructed with each marker entered into the basic model one at a time. The final model for adverse events and all-cause mortality was that containing the risk factors and four variables to show the relationship of each marker to no abnormal markers (HR=1.0). Sensitivity analyses were conducted using the upper quartile of each inflammatory marker. The Kaplan-Meier method was used to estimate the cumulative incidence rates of adverse events, with the log rank statistic used to assess differences by strata of Hgb/marker. The incremental value of multiple markers, beyond that of traditional risk factors (Framingham Risk Score [FRS]), was determined by receiver operating characteristics (ROC) analyses and the increment in area under the curve (AUC) determined when the markers were added to the FRS. All tests were two sided, and p-values <0.05 were considered statistically significant. All statistics were analyzed using SAS version 8.2 (Cary, N.C.).
Results
Demographics
Baseline characteristics for the 595 women (mean age 58 years) in this substudy are in Table 1. Approximately one-quarter were diabetic; over half had a history of hypertension or dyslipidemia; approximately 20% were current smokers; nearly two-thirds had a family history of premature heart disease; three-fourths were postmenopausal; approximately two-thirds were taking aspirin; and over one-quarter ACE-inhibitors and/or statins. Despite 8% reporting a history of CHF, the mean ejection fraction (EF) was 65.2±10.8%. The mean creatinine was 0.9±0.5 mg/dl. Angiographically, 65% had no obstructive CAD.
Table 1.
Baseline Characteristics of Women (N=595)
| Characteristics | Mean ± standard deviation |
|---|---|
| Age (y) | 58.0 ± 11.7 |
| Systolic blood pressure (mmHg) | 137 ± 22 |
| Diastolic blood pressure (mmHg) | 76 ± 11 |
| Pulse (beats per minute) | 74 ± 13 |
| BMI (kg/m2) | 29.8 ± 6.9 |
| Waist circumference (in) | 36.5 ± 7.3 |
| Hemoglobin (g/dl) | 12.9 ± 1.4 |
| Creatinine (mg/dl) | 0.9 ± 0.5 |
| Ejection fraction (%) | 65.2 ± 10.8 |
| Characteristics | Median (interquartile range) |
|---|---|
| Total cholesterol (mg/dl) | 187 (161–216) |
| Triglycerides (mg/dl) | 123 (81–189) |
| Fasting blood sugar (mg/dl) | 100 (88–127) |
| HDL-C (mg/dl) | 52 (44–60) |
| LDL-C (mg/dl) | 105 (84–130) |
| hs-CRP (mg/L) | 0.38 (0.17–0.88) |
| IL-6 (pg/ml) | 3.02 (1.82–5.56) |
| SAA (mg/dl) | 0.55 (0.31–1.02) |
| Characteristic | Percentage (%) |
|---|---|
| Non-white race | 18.3 |
| Obese (BMI ≥30) | 41.5 |
| History of | |
| Diabetes | 25.7 |
| (of those with diabetes, using insulin) | 43.8 |
| Dyslipidemia | 54.8 |
| Hypertension | 57.7 |
| Current smoking | 19.7 |
| Congestive heart failure | 8.4 |
| Premature CAD in family | 65.6 |
| Postmenopausal | 75.0 |
| Hysterectomy | 53.6 |
| Currently taking | |
| ACE inhibitors | 26.6 |
| Aspirin | 60.3 |
| Beta blockers | 40.9 |
| Statins | 27.6 |
| HRT (postmenopausal only) | 48.4 |
| Thyroid medication | 14.8 |
| Obstructive CAD | 35.5 |
BMI=body mass index; HDL-C=high-density lipoprotein; LDL-C=low-density lipoprotein; hs-CRP=high-sensitivity C-reactive protein; IL-6=interleukin-6; SAA= serum amyloid A; CAD-coronary artery disease; ACE=angiotensin-converting enzyme; HRT=hormone replacement therapy
Analysis of Markers and Hgb
At least one inflammatory marker was abnormal in 406 (68%): in 152 (26%) hs-CRP; 295 (50%) IL-6, and 269 (45%) SAA were abnormal. Of the women enrolled, 196 (33%), 110 (18%), and 100 (17%) had one, two, and three abnormal inflammatory markers, respectively. hs-CRP showed correlation with Il-6 (r=0.41, p<0.001) and SAA (r=0.57, p<0.001), while IL-6 showed modest correlation with SAA (r=0.32, p<0.001).
Abnormal Hgb occurred in 130 (22%); mean 12.9±1.4. Among women with low Hgb, 78% had at least one abnormal inflammatory marker. High-sensitivity CRP (r=−0.08, p=0.06), IL-6 (r=−0.14, p<0.001), and SAA (r=−0.09, p=0.03) were all inversely correlated with Hgb.
Markers and CV Events
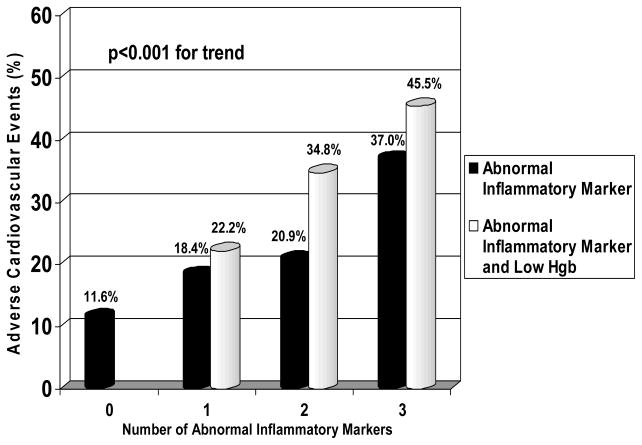
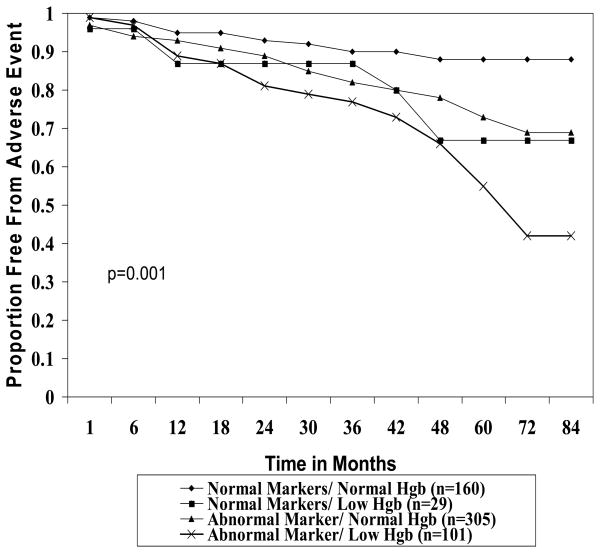
After a mean follow-up of 3.6±1.8 years, 118 (20%) women had an initial adverse CV event. Women with no abnormal inflammatory markers had significantly fewer CV events (11.6%) compared to those with one (18.4%), two (20.9%), and three (37%) abnormal inflammatory markers (p<0.001 for trend, Figure 1). Addition of low Hgb to each category of abnormal inflammatory markers was associated with an increased frequency of adverse events (Figure 1). For example, 45.5% of women with three abnormal inflammatory markers and a low Hgb had an adverse event. Moreover, women with low Hgb and at least one abnormal inflammatory marker had significantly less survival free from adverse events compared to those without low Hgb and/or abnormal inflammatory markers (p<0.001, Figure 2).
Figure 1. Proportion of women with adverse events by number of abnormal inflammatory markers and hemoglobin level.
Adverse event defined by death or hospitalization for myocardial infarction, heart failure, stroke, or other vascular event. Note the significant relationship between increase in events and number of abnormal inflammatory markers (black) and number of abnormal inflammatory markers plus low hemoglobin (Hgb, open)
Figure 2. Proportion of women free from adverse events during follow-up by inflammatory marker and hemoglobin level using Kaplan Meier methods.
Adverse events defined by death or hospitalization for myocardial infarction, stroke, congestive heart failure, or other vascular event. Note that adding low hemoglobin (Hgb) level is associated with reduction in the proportion free of events.
During follow-up, there were 42 (7%) deaths with 48% of definite CV etiology. Women with no abnormal inflammatory markers had fewer deaths (1.6%) than women with one (6.1%), two (9.1%), or three (17%) abnormal inflammatory markers (p<0.001 for trend). Addition of low Hgb to each category of abnormal markers was associated with an increase in all-cause mortality. For example, 24.2% of women with three abnormal inflammatory markers and a low Hgb died during follow-up.
Unadjusted Predictors of Adverse CV Events and All-Cause Mortality
On univariate analysis using dichotomous variables, abnormal hs-CRP (HR 2.09, 95%CI 1.44–3.03), abnormal IL-6 (HR 2.17, 95%CI 1.48–3.18), abnormal SAA (HR 1.81, 95%CI 1.25–2.62), and low Hgb (HR 2.05, 95% CI 1.40–3.01) predicted adverse CV events (all p<0.002). Moreover, abnormal hs-CRP (HR 2.80, 95% CI 1.53–5.13), abnormal IL-6 (HR 2.61, 95% CI 1.34–5.10), abnormal SAA (HR 4.53, 95% CI 2.17–9.46), and low Hgb (HR 2.69, 95% CI 1.45–4.98) predicted all-cause mortality (all p<0.01). Using the upper quartile for each inflammatory marker, similar results were seen for hs-CRP (HR 2.21, 95% CI 1.52–3.20), IL-6 (HR 2.52, 95% CI 1.75–3.64), and SAA (HR 2.22, 95% CI 1.54–3.22) in predicting adverse CV events. Similar results were also seen using the upper quartile for hs-CRP (HR 2.94, 95% CI 1.61–5.39), IL-6 (HR 3.55, 95% CI 1.94–6.51), and SAA (HR 3.13, 95% CI 1.71–5.72) in predicting all-cause mortality.
Adjusted Predictors of Adverse CV Events
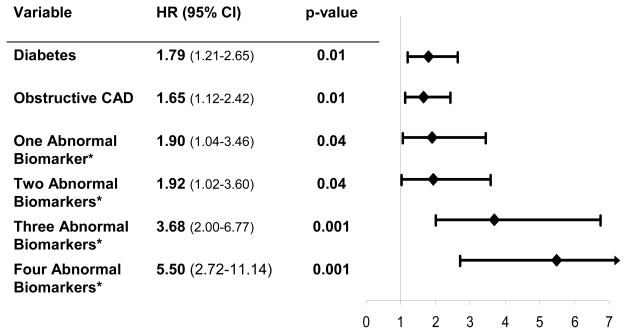
On multivariate analysis in separate Cox regression models, using dichotomous variables, hs-CRP (HR 1.86, 95% CI 1.28–2.71; p=0.001), IL-6 (HR 1.84, 95% CI 1.25–2.70; p=0.002), and SAA (HR 1.64, 95%CI 1.13–2.38; p=0.01) predicted adverse CV events. Again, using the upper quartile for each inflammatory marker in a multivariate analysis, similar results were found (data not shown). Since Hgb was associated with a higher rate of adverse events when added to abnormal inflammatory markers, we included Hgb as a fourth biomarker in addition to hs-CRP, IL-6, and SAA. On multivariate analysis (Figure 3), women with one (HR 1.90, 95% CI 1.04–3.46; p=0.04) two (HR 1.92, 95% CI 1.02–3.60; p=0.04), three (HR 3.68, 95%CI 2.00–6.77; p=0.001), or four (HR 5.50, 95%CI 2.72–11.14; p=0.001) abnormal biomarkers independently predicted adverse CV events. Using a multibiomarker approach was associated with higher adverse CV events than any single marker alone or other traditional CV risk factors including diabetes (HR 1.79, 95%CI 1.21–2.65; p=0.01) and obstructive CAD (HR 1.65, 95%CI 1.12–2.42; p=0.01).
Figure 3. Significant independent predictors of adverse events. Shown are hazards ratio (HR), 95% confidence intervals (95% CI), and p values.
*Abnormal biomarkers include hs-CRP, IL-6, SAA, and Hgb. Number of women with one, two, three and four abnormal biomarkers is 181, 131, 90, and 33, respectively compared to women with no abnormal markers (n=160).
Adjusted Predictors of All-Cause Mortality
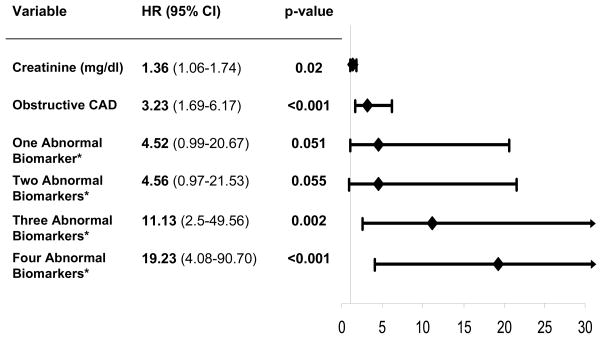
On multivariate analysis using dichotomous variables, hs-CRP (HR 2.67, 95% CI 1.44–4.96; p=0.002), IL-6 (HR 2.07, 95%CI 1.05–4.08; p=0.04), and SAA (HR 3.75, 95% CI 1.78–7.87; p<0.001) independently predicted all-cause mortality. Using the upper quartile for each inflammatory marker in a multivariate analysis, similar results were found (data not shown). When low Hgb was included as a fourth biomarker, women with three (HR 11.13, 95% CI 2.50–49.56; p=0.002) or four (HR 19.23 95% CI 4.08–90.70; p<0.001) abnormal biomarkers had significantly higher risk of all-cause mortality in comparison to women with no abnormal biomarkers (Figure 4). Using a multi-biomarker approach, women with three or four abnormal biomarkers had a higher all-cause mortality rate than women with a single abnormal biomarker or obstructive CAD (HR 3.23, 95%CI 1.69–6.17; p<0.001) and creatinine (HR 1.36, 95% CI 1.06–1.74; p=0.02).
Figure 4. Significant independent predictors of all-cause mortality. Shown are hazards ratio (HR), 95% confidence intervals (95% CI), and p values.
*Abnormal biomarkers include hs-CRP, IL-6, SAA, and Hgb. Number of women with one, two, three and four abnormal biomarkers was 181, 131, 90, and 33, respectively compared to women with no abnormal markers (n=160).
Traditional Risk Predictors to Predict CV events
Incremental value beyond the FRS, a traditional risk prediction tool that integrates most CV risk factors was 14.2 for the entire cohort corresponding to an overall 10 year risk of 4.6%. The FRS increased with increasing severity of CAD as would be expected for women with no, mild, and severe CAD having a corresponding FRS of 2.9%, 4.8%, and 6.4%, respectively. Higher FRS was associated with more abnormal inflammatory marker level. Women with zero, one, two, three, and four abnormal inflammatory markers had a corresponding FRS of 3.9%, 4.3%, 4.7%, 5.4%, and 6.9%, respectively. The number of abnormal biomarkers increased with increasing BMI, waist circumference, and waist/hip ratio (data not shown). However, neither BMI nor measures of abdominal obesity (waist circumference or waist hip ratio) were significant univariate predictors of adverse events and mortality.
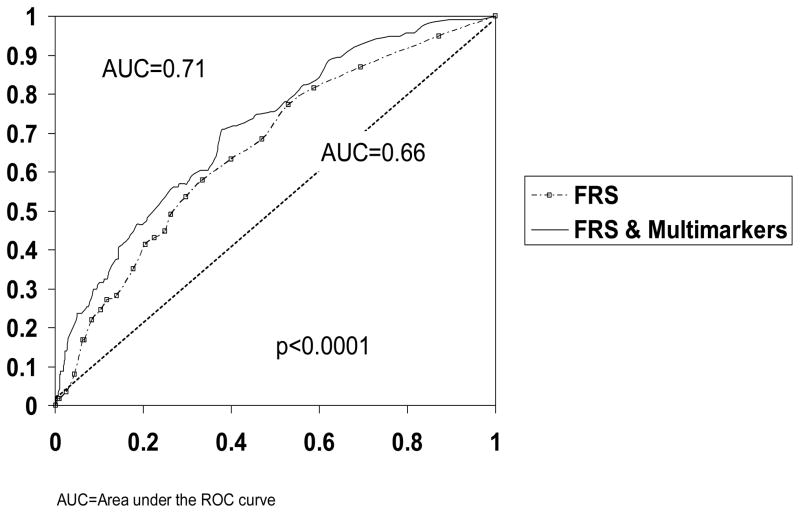
On univariate analysis, FRS was a predictor of adverse events (HR=1.08, p=0.0002) and all-cause mortality, however on multivariate analysis, the FRS was not selected. When FRS was forced into the model with the biomarkers, the multimarker approach independently predicted adverse events and all-cause mortality. With FRS forced into the model, women with three (HR 3.26, 95% CI 1.75–6.06, p=0.0002) or four (HR 4.86, 95% CI 2.38–9.92, p<0.0001) abnormal inflammatory markers were 3 to 5 times more likely to have an adverse CV event during follow-up. Furthermore, women with three (HR 10.42, 95% CI 2.33–46.52, p=0.002) or four (HR 16.47, 95% CI 3.44–78.70, p=0.0004) were >10 times more likely to die. Using ROC analysis, the FRS was associated with an overall accuracy predicting adverse events of 0.66 AUC, (Figure 5). However adding the combination of multiple biomarkers to the FRS improved the accuracy of predicting adverse events (AUC=0.71, p<0.0001). The combination of biomarkers plus FRS was also associated with higher predictive accuracy for all-cause mortality (AUC 0.78 vs 0.72, p<0.0001).
Figure 5.
Receiver operating characteristics (ROC) analysis for Framingham Risk Score (FRS) alone and FRS plus multimarkers for prediction of adverse cardiovascular events.
Discussion
A combination of multiple biomarkers, including hs-CRP, IL-6, SAA, and Hgb, is incrementally and independently associated with increased adverse events in the WISE cohort. Compared to women with no abnormal inflammatory markers, those with four abnormal biomarkers were >5 times more likely to have an adverse event and nineteen times more likely to die during follow-up. Over 40% of women with four abnormal biomarkers had an adverse event, and a quarter died within 4 years. Moreover, women with low Hgb and at least one abnormal inflammatory marker had significantly worse event-free survival.
The FRS, a commonly used traditional risk factor predicting tool, markedly underestimated risk in this population. When added to the FRS, the biomarkers provided incremental prognostic information over that ascertained from traditional risk factors.
Elevated inflammatory marker levels and anemia appear to predict adverse events in a variety of conditions.2,3,5–8 In patients presenting with AMI and decompensated heart failure, adverse events are typically related to atherothrombotic coronary occlusion and impaired left ventricular function. However, in this cohort of clinically stable women only 35% had a flow limiting coronary stenosis ≥50%, and mean ejection fraction was 65%. In ACS, women’s risk of events and response to invasive management are better predicted by inflammatory markers and brain natriuretic peptides than by traditional markers of myocardial necrosis (cardiac troponin or CK-MB).14 One proposed explanation for this finding is that women may have more inflammation and microvascular coronary dysfunction underlying ACS, as opposed to atherothrombotic occlusion. Support for this notion has also been provided in a preliminary report from Burke et al. showing that women dying suddenly have more area of microfibrosis and microvascular embolic than observed in men.15 In previous WISE analyses, women evaluated for suspected ischemia with non-obstructive CAD frequently had microvascular dysfunction.16 Adverse effects of inflammation on vascular function may contribute to microvascular dysfunction and the high event rate seen in the WISE cohort. Mediators of inflammation are under complex regulation and are produced at various sites including endothelial cells, macrophages, adipocytes, and atherosclerotic plaques.17,18 Each marker likely provides only a glimpse of the immunopathology underlying vascular disease so a multimarker approach likely provides more information about inflammation.
Furthermore, when added to traditional inflammatory markers, low Hgb levels added predictive value for risk. The cause for the low Hgb was not ascertained in the WISE cohort, it is likely that a high proportion of these cases were secondary to chronic disease. However, the additional prognostic information associated with low Hgb level, both alone and in a multimarker approach, suggests that Hgb mediates CV effects independent of the other markers.
Study Limitations
This prospective observational study has inherent limitations of such a design and case selection. Thresholds for abnormal biomarker levels were from previous studies in different cohorts, so the optimal thresholds for risk stratification in this population may be different. In addition, this was a relatively small population of women with markers measured only at baseline and the influence on outcomes of changes in biomarkers over time is unknown. Also, these markers were measured after approximately 300 women were enrolled in the WISE, so women enrolled prior to ascertainment of marker levels may have died or been lost to follow-up as result of an adverse event (e.g. survival bias); therefore an underestimation of the event rate is possible. Finally, before these markers can be used in practice, the results must be confirmed in independent populations particularly where the risk is lower.
Conclusion
In women undergoing coronary angiography for suspected ischemia, the cumulative number of abnormal biomarkers was associated with an incrementally increased risk of adverse events independent of traditional risk factors including diabetes and angiographic CAD. The multimarker approach provided more prognostic information than traditional risk factors. These markers relate to mediators of inflammation involved in development and progression of atherosclerosis and this information should stimulate interest in novel anti-inflammatory approaches to reducing residual risk.
Acknowledgments
Grant Support/Funding: This work was supported by NHLBI contracts NO1-HV-68161, NO1-HV-68162, NO1-HV-68163, and NO1-HV-68164, and grants UO1-HL64829-01, UO1-HL64914-01, and UO1-HL65924-01.
This work was supported by contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, a GCRC grant MO1-RR00425 from the National Center for Research Resources, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Denville, New Jersey, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, California, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, Pennsylvania, and The Edythe L. Broad Endowment for Women’s Heart Research, Los Angeles, California.
The authors thank Tjendimin Tjandrawan, BS for his laboratory assistance.
Footnotes
None of the authors have a conflict of interest to disclose
References
- 1.Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138:S419–20. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 3.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108:2317–22. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 4.Morrow DA, Braunwald E. Future of biomarkers in acute coronary syndromes: moving toward a multimarker strategy. Circulation. 2003;108:250–2. doi: 10.1161/01.CIR.0000078080.37974.D2. [DOI] [PubMed] [Google Scholar]
- 5.Kosiborod M, Smith GL, Radford MJ, Foody JM, Krumholz HM. The prognostic importance of anemia in patients with heart failure. Am J Med. 2003;114:112–9. doi: 10.1016/s0002-9343(02)01498-5. [DOI] [PubMed] [Google Scholar]
- 6.Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345:1230–6. doi: 10.1056/NEJMoa010615. [DOI] [PubMed] [Google Scholar]
- 7.Al Falluji N, Lawrence-Nelson J, Kostis JB, Lacy CR, Ranjan R, Wilson AC. Effect of anemia on 1-year mortality in patients with acute myocardial infarction. Am Heart J. 2002;144:636–41. doi: 10.1067/mhj.2002.124351. [DOI] [PubMed] [Google Scholar]
- 8.Arant CB, Wessel TR, Olson MB, Bairey Merz CN, Sopko G, et al. Hemoglobin level is an independent predictor for adverse cardiovascular outcomes in women undergoing evaluation for chest pain: results from the National Heart, Lung, and Blood Institute Women’s Ischemia Syndrome Evaluation Study. J Am Coll Cardiol. 2004;43:2009–14. doi: 10.1016/j.jacc.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 9.Merz CN, Kelsey SF, Pepine CJ, Reichek N, Reis SE, et al. The Women’s Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol. 1999;33:1453–61. doi: 10.1016/s0735-1097(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 10.Sharaf BL, Pepine CJ, Kerensky RA, Reis SE, Reichek N, et al. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation [WISE] Study Angiographic Core Laboratory) Am J Cardiol. 2001;87:937–41. A3. doi: 10.1016/s0002-9149(01)01424-2. [DOI] [PubMed] [Google Scholar]
- 11.Rifai N, Joubran R, Yu H, Asmi M, Jouma M. Inflammatory markers in men with angiographically documented coronary heart disease. Clin Chem. 1999;45:1967–73. [PubMed] [Google Scholar]
- 12.Pradhan AD, Manson JE, Rossouw JE, Siscovick DS, Mouton CP, et al. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: prospective analysis from the Women’s Health Initiative observational study. Jama. 2002;288:980–7. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1998;98:839–44. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- 14.Wiviott SD, Cannon CP, Morrow DA, Murphy SA, Gibson CM, et al. Differential expression of cardiac biomarkers by gender in patients with unstable angina/non-ST-elevation myocardial infarction: a TACTICS-TIMI 18 (Treat Angina with Aggrastat and determine Cost of Therapy with an Invasive or Conservative Strategy-Thrombolysis In Myocardial Infarction 18) substudy. Circulation. 2004;109:580–6. doi: 10.1161/01.CIR.0000109491.66226.26. [DOI] [PubMed] [Google Scholar]
- 15.Burke AP, Kolodgie FA, Virmani R. Gender differences in coronary plaque morphology in sudden coronary death: role of microembolization. Circulation. 2003;108:165A. [Google Scholar]
- 16.Reis SE, Holubkov R, Lee JS, Sharaf B, Reichek N, et al. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women’s Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 1999;33:1469–75. doi: 10.1016/s0735-1097(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 17.Barath P, Fishbein MC, Cao J, Berenson J, Helfant RH, Forrester JS. Detection and localization of tumor necrosis factor in human atheroma. Am J Cardiol. 1990;65:297–302. doi: 10.1016/0002-9149(90)90291-8. [DOI] [PubMed] [Google Scholar]
- 18.Yasojima K, Schwab C, McGeer EG, McGeer PL. Generation of C-reactive protein and complement components in atherosclerotic plaques. Am J Pathol. 2001;158:1039–51. doi: 10.1016/S0002-9440(10)64051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]