Abstract
Purpose of the review
To evaluate the relationship between infections with SARS-CoV-2 and autoimmunity.
Recent Findings
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome (SARS) associated coronavirus 2 (SARS-CoV-2). Although most of the infected individuals are asymptomatic, a proportion of patients with COVID-19 develop severe disease with multiple organ injuries. Evidence suggests that some medications used to treat autoimmune rheumatologic diseases might have therapeutic effect in patients with severe COVID-19 infections, drawing attention to the relationship between COVID-19 and autoimmune diseases (ADs). COVID-19 shares similarities with ADs in clinical manifestations, immune responses, and pathogenic mechanisms. Robust immune reactions participate in the pathogenesis of both disease conditions. Autoantibodies as a hallmark of ADs can also be detected in COVID-19 patients. Moreover, some patients have been reported to develop ADs, such as Guillain-Barré syndrome or systemic lupus erythematosus, after COVID-19 infection. It is speculated that SARS-CoV-2 can disturb self-tolerance and trigger autoimmune responses through cross-reactivity with host cells. The infection risk and prognosis of COVID-19 in patients with ADs remains controversial, but patient adherence to medication regimens to prevent autoimmune disease flares is strongly recommended.
Summary
We present a review of the association between COVID-19 and autoimmune diseases, focusing on similarities in immune responses, cross-reactivity of SARS-CoV-2, the development of ADs in COVID-19 patients, and the risk of COVID-19 infection in patients with pre-existing ADs.
Keywords: COVID-19, SARS-CoV-2, autoimmune diseases, cross-reactivity, molecular mimicry
Introduction
Since December 2019, a novel infection named Coronavirus disease 2019 (COVID-19) broke out in Wuhan, China and has been sweeping across the globe. COVID-19 was officially declared a pandemic by World Health Organization on March 11, 2020[1]. The disease is caused by a newly identified strain of severe acute respiratory syndrome (SARS) associated coronavirus which was named SARS-CoV-2 after SARS-CoV that caused the epidemic of SARS in 2002[2].
SARS-CoV-2 belongs to the coronavirus family which are enveloped viruses with a spherical morphology and a single-stranded RNA (ssRNA) genome[3]. The spike glycoproteins (S protein) cross through the peplos of the virus and form a crown-like surface[4]. Through the receptor binding domain (RBD) located in the S1 subunit of the S protein, the virus can ligate to the host cell receptor angiotensin-converting enzyme 2 (ACE2) and invade into the cell [5–7].
In most cases, hosts infected by SARS-CoV-2 present with flu-like symptoms, such as fever, fatigue, and dry cough. Headache, myalgia, sore throat, nausea, and diarrhea can also be seen in patients with COVID-19[8,9]. Shortness of breath and hypoxemia occurred in severe cases. In critical cases, the disease progresses rapidly and patients can develop septic shock and multi-organ dysfunction[10]. As such, COVID-19 can be a systemic disease affecting multiple organ systems, including the skin, kidneys, respiratory system, cardiovascular system, digestive system, nervous system, and hematological system[11]. The dysregulated immune response and increased pro-inflammatory cytokines induced by SARS-CoV-2 contribute to the disease pathogenesis and organ damage, which brought attention to immune-regulatory therapy in the treatment of COVID-19[12]. Medications used to treat autoimmune diseases are widely used in critical cases of COVID-19[13]. Further, some autoantibodies can be detected in patients with COVID-19[14]. These observations suggest that examining pathways known to contribute to the pathogenesis of autoimmunity might provide clues to better understand and treat COVID-19.
Similarities in Immune responses between SARS-CoV-2 infection and autoimmune diseases
Autoimmune diseases (ADs) are characterized by the existence of autoantibodies and perpetuated inflammatory reactions due to the loss of immune tolerance and dysregulated immune system, leading to target organ damage and malfunction[15]. These immune-mediated injuries also exist in COVID-19 (Figure 1). Infection with SARS-CoV-2 induces immune reactions which might have important implications in the development of vaccine strategies against this virus [16]. T cell immunity plays a central role in the control of SARS-CoV-2 infection. Antigen-specific CD4+ and CD8+ T cells and neutralizing antibody responses play protective roles against SARS-CoV-2, while impaired adaptive immune responses such as scarcity of naïve T cells may lead to poor disease outcomes[17].
Figure 1. Similar immune reactions in SARS-CoV-2 infection and autoimmune diseases.
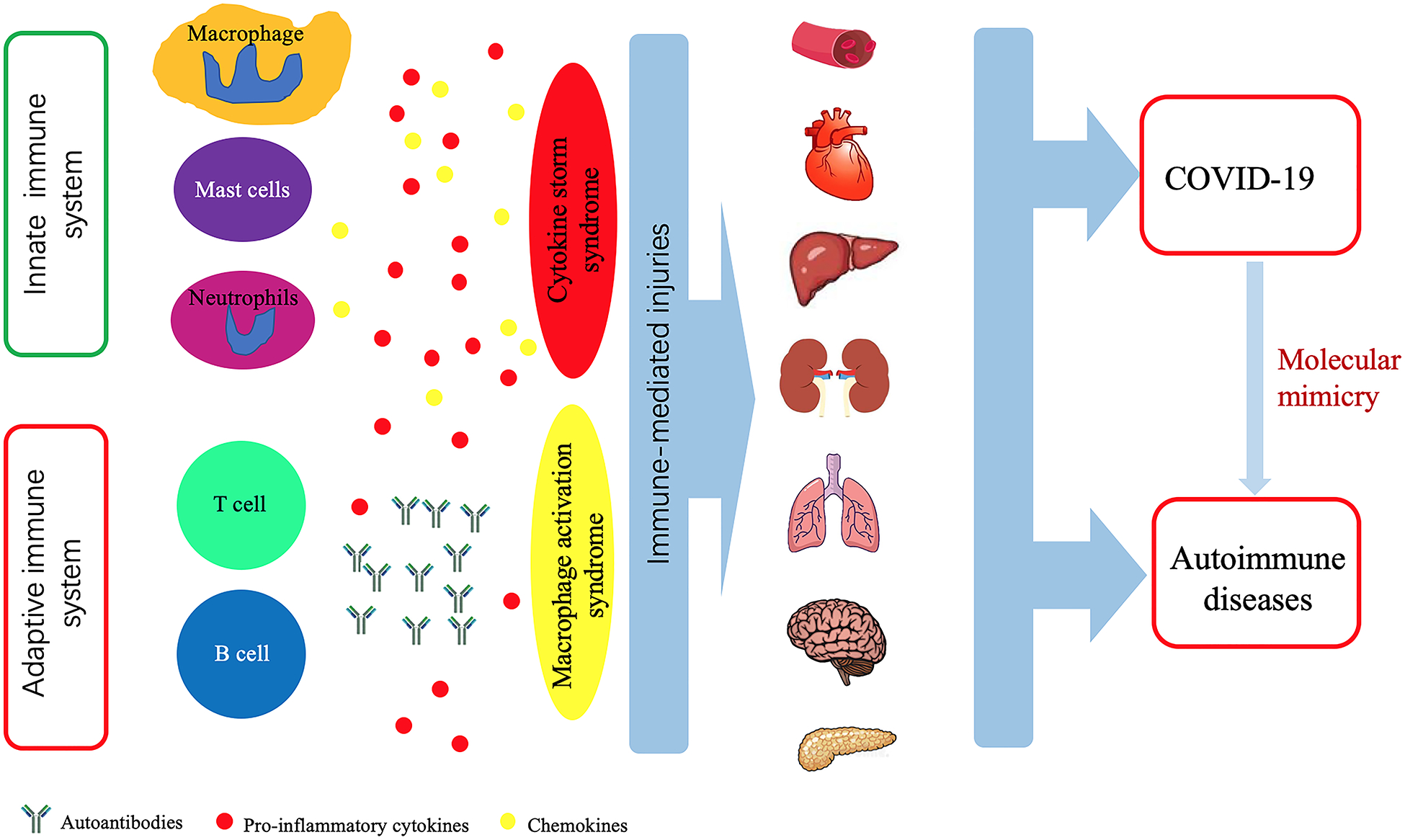
Both COVID-19 and autoimmune diseases present with various clinical symptoms involving different organs and systems, such as the hematological system, cardiovascular system, digestive system, kidneys, lungs, neurological system, and pancreas. Organ damage is caused by uncontrolled immune response characterized by excessive production of cytokines and over-activation of immune cells, and the break of immune tolerance leading to the production of autoantibodies. SARS-CoV-2 infection can trigger cross-reactivity through molecular mimicry, leading to autoimmunity in patients with COVID-19.
In clinical laboratory tests, lymphopenia (lymphocyte count ≤1.0 × 109 /L) is associated with severe illness in COVID-19 patients and might be a prognostic factor for disease severity and mortality[18–21]. Another notable hemocytological change is neutrophilia and associated excessive neutrophil extracellular traps which paralleled lung injury in severe COVID-19 patients[12]. Therefore, the immune response is a double-edged sword in COVID-19, with outcomes affected by the degree of cytokine imbalance and activation of immune cells. Excessive production and release of pro-inflammatory cytokines and chemokines can cause severe organ damage in critical cases, which is observed in autoimmune diseases as well. In COVID-19 patients, pro-inflammatory cytokines and chemokines, including IL-1, IL-2, IL-6, IL-8, IL-10, IL-17, IL-18, CXCL10, and CCL2, increased significantly and the expression levels of some of these cytokines, such as IL-1, IL-6, IL-10, and IL-18 have been demonstrated to be associated with the disease severity[22–25]. Similar to autoimmune diseases, damage-associated molecular patterns (DAMPs) also participate in the pathogenesis of COVID-19 and are related to disease outcome. Liting Chen, et al. revealed that serum levels of S100A8/A9 and HMGB1 increased significantly in patients with severe COVID-19 and that significant elevation of the two DAMPs was associated with higher mortality[26].
Activation and infiltration of immune cells participate in the pathogenesis of organ injuries in patients with COVID-19. Macrophage activation syndrome (MAS) could be a continuum of cytokine storm syndrome leading to life-threatening complications in COVID-19[27]. In this condition, activated macrophages will produce excessive pro-inflammatory cytokines, polarize into the inflammatory M1 phenotype, and exhibit cytotoxic dysfunction[28]. Recently, Conti P et al. proposed that SARS-CoV-2 activated mast cells could release histamine to increase IL-1 levels to initiate cytokine storm and aggravate lung injury[29]. Woodruff MC, et al. found extrafollicular B cell activation in critically ill patients with COVID-19, similar to what has been observed in autoimmunity. Further, extrafollicular B cell activation correlated strongly with the production of high concentrations of SARS-CoV-2-specific neutralizing antibodies and poor disease outcome [30]. Peripheral blood B-cell subpopulations are altered during COVID-19. In COVID-19 patients, atypical memory B-cells (CD21lo/CD27−/CD10−) expanded significantly while classical memory B-cells (CD21+/CD27+/CD10−) were significantly reduced [31]. Analysis of immune profiles of severe COVID-19 patients revealed an increased proportion of mature natural killer (NK) cells and decreased proportion of T-cell numbers[32].
Similar to some autoimmune and immune-mediated thrombo-inflammatory diseases, including lupus, antiphospholipid syndrome, and ANCA-associated vasculitis, neutrophil activation and neutrophil extracellular traps production (NETosis) appear to have a pathogenic role in COVID-19. Zuo et al. reported increased markers of NETs in sera from patients with COVID-19, and significantly more in patients requiring mechanical ventilation. In vitro experiments demonstrated that sera from COVID-19 patients triggered NETosis in normal neutrophils, similar to sera from patients with antiphospholipid syndrome [33,34].
In severe and critical cases, immunomodulatory drugs and biological agents targeting pro-inflammatory cytokines have been applied to contain the robust immune response in COVID-19. Corticosteroids, JAK inhibitors, IL-1 blockade, and IL-6 receptor antagonists, which are familial to rheumatologists, have been used to treat COVID-19 patients [35–38]. Similarities in immunopathogenesis of COVID-19 and autoimmune diseases are summarized in Table 1.
Table 1.
Similarities in immunopathogenesis of COVID-19 and autoimmune diseases
| Items | COVID-19 immunological features similar to autoimmune diseases | Refs |
|---|---|---|
| Innate immune cells | Overactivation of monocytes, macrophages, mast cells and neutrophils. Increased proportion of mature natural killer (NK) cells. | [12,27,29,32,33] |
| Adaptive immune cells | Decreased T-cell numbers, altered B-cell subsets, dysregulation of T cells and B cells. | [17,30,31] |
| Cytokines and chemokines | Increased levels of IL-1, IL-2, IL-6, IL-8, IL-10, IL-17, IL-18, CXCL10, CCL2. | [22–24] |
| Autoantibodies | ANA, APL, Lupus anticoagulant, Cold agglutinins, Anti-Ro/SSA antibodies, anti-Caspr2 antibody, anti- GD1b antibody, anti-MOG antibody | [14,50–57] |
| Clinical conditions | Immune-mediated hemolysis, decreased white blood cell counts, cytokine storm syndrome, macrophage activation syndrome, procoagulant condition | [25,28,56,73] |
| Other immunopathogenesis | Increased levels of DAMPs, molecular mimicry. | [26,45] |
Molecular mimicry of SARS-CoV-2
The production of autoantibodies is a key feature of ADs. However, the underlying mechanisms are complicated and still not fully understood. Molecular mimicry by infectious pathogens is believed to be one of the mechanisms[39]. Viral infection can disturb immunologic tolerance by exposure of antigen epitopes that elicit cross-reactive antibodies. There are a large number of reports indicating antigenic mimicry between viral and human proteins. Perhaps one of the most established examples of molecular mimicry in autoimmunity is the immune response to Epstein-Barr virus (EBV) in lupus patients [40]. An abnormal immune repose to Epstein-Barr virus Nuclear Antigen-1 (EBNA-1) can induce an autoimmune response targeting the Sm and Ro autoantigen systems [41]. Cross reactivity between anti-EBNA-1 antibodies and myelin basic protein in patients with multiple sclerosis has also been demonstrated (PMID: 31515129). Moreover, EBNA-1 showed structural similarity with β synuclein, a brain protein implicated in multiple sclerosis, and predicted to bind HLA class II DR2b (HLA-DRB1*15:01) [42]. In silico analysis revealed that an envelope protein of human endogenous retroviruses (HERV) shares similar sequence with three myelin proteins that induced an autoimmune response in multiple sclerosis and was predicted to bind to HLA-DRB1*15:01 (need reference here). Basavalingappa RH et al. demonstrated that Coxsackievirus B3 (CVB3) infection can induce the generation of autoreactive T cells for multiple antigens [43].
During this pandemic of COVID-19, some epitopes from SARS-CoV-2 revealed to exhibit cross-reactivity with autoantigens. Anand P et al. reported a unique S1/S2 cleavage site in SARS-CoV-2 identically mimicked a FURIN-cleavable peptide on the human epithelial sodium channel α-subunit (ENaC-α) which plays a critical role in the homeostasis of airway surface liquid[44]. Mimicry between SARS-CoV-2 and three proteins namely DAB1, AIFM, and SURF1 that are present in the human brainstem pre-Bötzinger complex (preBötC) may contribute to the respiratory failure in COVID-19[45]. Additionally, SARS-CoV-2 infection can elicit autoimmune responses through molecular mimicry. Marino Gammazza A et al. compared viral proteins with human molecular chaperones and postulated that the chaperones, most of which were heat shock proteins, could participate in molecular mimicry phenomena after SARS-CoV-2 infection[46]. Furthermore, Lucchese G et al. compared viral amino acid sequence with human autoantigens associated with immune-mediated polyneuropathies and showed that peptides embedded in immunoreactive epitopes of SARS-CoV-2 shared the same sequence with human heat shock proteins 90 and 60 that are associated with Guillain-Barré syndrome and other autoimmune diseases[47]. Venkatakrishnan AJ et al. reported 33 distinct 8-mer or 9-mer peptides with potential cross-reactivity between SARS-CoV-2 and the human reference proteome, among which 20 human peptides have not been observed in any previous coronavirus strains. Moreover, four of these human 8-mer/9-mer peptides mimicked by SARS-CoV-2 showed similarity with host pulmonary-arterial peptides and were predicted to bind with HLA-B*40:01, HLA-B*40:02, and HLA-B*35:01[48]. A recent study analyzed sharing between hexapeptides that define minimal epitopic sequences of the virus and the human proteome, and documented numerous immunoreactive epitopes shared with human proteins[49]. The results of this study imply the possibility that SARS-CoV-2 might induce cross-reactivity with host autoantigens and offer hints to possibly explain the various clinical manifestations and pathologies involving different organs and systems after SARS-CoV-2 infection.
Autoantibodies in patients with COVID-19
Autoantibodies known to occur in a number of autoimmune diseases have been detected in patients with COVID-19 (Table 2). Pascolini S et al. determined the presence of antinuclear antibodies (ANA), anti-cytoplasmic neutrophil antibodies (ANCA), and anti-antiphospholipid (APL) antibodies in 33 consecutive patients with COVID-19 [14]. The results showed that 45% of the patients were positive for at least one autoantibody and patients with positive autoantibodies tended to have a worse prognosis and a significantly higher respiratory rate at admission. The positive rate for ANA was 33%, the positive rate for anti-cardiolipin antibodies (IgG and/or IgM) was 24%, and 3 patients tested positive for anti-β2-glycoprotein-I antibodies (IgG and/or IgM) (9%). However, ANCA was negative in all patients[14]. Coagulopathy is a threatening complication of SARS-CoV-2 infection. Recently, a cohort study was performed in Montefiore Medical Center to assess lupus anticoagulant (LA) positivity in COVID-19 patients. The researchers found that patients with COVID-19 had an increased incidence of LA positivity compared with controls who tested negative by COVID-19 reverse transcriptase–polymerase chain reaction. In addition, COVID-19 patients with positive LA had an increased rate of thrombosis [50]. Amezcua-Guerra LM et al. also demonstrated a higher frequency of APL antibodies in patients with severe and critical COVID-19, and that the presence of APL antibodies seems to be associated with a hyperinflammatory state with extremely high levels of ferritin, C reactive protein, and IL-6, and with pulmonary thromboembolism [51]. The data discussed above provide a possible explanation for the hypercoagulable state in severe and critical COVID-19 cases and indicate that SARS-CoV-2 can induce autoimmune responses.
Table 2.
Autoantibodies detected in patients with COVID-19
| Autoantibodies | Clinical significance | Refs |
|---|---|---|
| ANA | Poor prognosis and a significant higher respiratory rate. | [14] |
| APL | Poor prognosis and a significant higher respiratory rate. Possible association with a hyperinflammatory state and thrombosis and thromboembolism. |
[14,51] |
| Lupus anticoagulant | A higher rate of thrombosis | [50] |
| Cold agglutinins | Hemolytic anemia. Complicating laboratory assessment and renal replacement therapy. |
[54,57] |
| Anti-Ro/SSA antibodies | Possible association with severe pneumonia. | [55] |
| anti-Caspr2 antibody | Unclear. | [53] |
| anti- GD1b antibody | Unclear. | [53] |
| Anti-MOG antibody | Unclear. | [52] |
| Red cell –bound antibodies | Associated with the severity of anemia. | [56] |
In COVID-19 patients presenting with neurological symptoms, the existence of autoantibodies against contactin-associated protein 2 (anti-Caspr2), ganglioside GD1b (anti-GD1b), and myelin oligodendrocyte glycoprotein (anti-MOG) has been shown in case reports or retrospective studies[52,53]. However, the clinical significance of these antibodies remain unclear. In addition, there are case reports demonstrating the presence of cold agglutinins and autoantibodies against RBC antigens in critically ill patients with COVID-19[54], and the presence of anti-Ro/SSA antibodies in patients with aggravated COVID-19 pneumonia[55]. A research including 113 samples studied red cell antibodies by direct and indirect antiglobulin test (DAT or IAT). A positive DAT was found in 46% of COVID-19 patients, which was significantly higher than that in non-COVID-19 controls. The presence of red cell membrane-bound immunoglobulins contributes to hemolytic anemia and is related to the severity of anemia in COVID-19 [56].
Development of autoimmune diseases after SARS-CoV-2 infection
Since SARS-CoV-2 infection could break immune tolerance and trigger autoimmune responses, it is likely to induce autoimmune diseases. Indeed, many reports have confirmed the development of autoimmune diseases after SARS-CoV-2 infection. Cold agglutinin syndrome (CAS) and autoimmune hemolytic anemia have been reported as a complication of COVID-19[54,57,58]. Meanwhile, Guillain-Barré syndrome (GBS) is also emerging as an autoimmune disease that may occur in COVID-19 patients. In most cases of COVID-19 associated GBS SARS-CoV-2 antibodies cannot be detected in the cerebrospinal fluid (CSF), however, Gigli GL et al. recently reported a case of GBS with a positive test for the SARS-CoV-2 antibodies in the CSF [59–61]. The mechanisms of how SARS-CoV-2 triggers GBS are debated. However, immune cross reaction between epitopes and host antigens may be a possible explanation[61]. Recently, a case of systemic lupus erythematosus has also been reported to be triggered by SARS-CoV-2 [62]. It is possible that additional autoimmune diseases induced by SARS-CoV-2 will be reported in the future.
Risk of patients with autoimmune diseases during the COVID-19 pandemic
Autoimmune diseases are heterogeneous and linked to a dysregulated immune system. Most of the patients with ADs have received or are receiving immunomodulatory medications or biological agents. During the pandemic of COVID-19, a proportion of the ADs patients suspended their medication due to the fear of the immunosuppressive effect of medications or lack of availabilities[63], and decreased medical visits because of concerns of the contagious nature of SARS-CoV-2[64]. However, disrupted continuity of medical care and medication non-adherence are associated with rheumatologic disease flares and worsened disease activity[65]. Therefore, building a reliable telemedicine platform and education on medication adherence should be strongly recommended.
Since the beginning of this pandemic, infection risk in patients with ADs has been a subject of interest [66–68]. The results of a cross-sectional study conducted in northeast Italy indicated that ADs patients had a similar rate of infection of SARS-CoV-2 compared with the general population[69]. Another Italian study performed in Milan also confirmed that autoimmune disease is not a risk factor of being positive to COVID-19[70]. To the contrary, the results of a multicenter retrospective study conducted in Hubei, China indicated that ADs patients might be more susceptible to SARS-CoV-2 infection compared with controls. Further, this study examined family members of the patients that resided at the same environment during the outbreak as controls[71]. It is to be observed that the study from Milan also indicated that patients with ADs do not have a worse prognosis compared to non-ADs subjects[70]. However, a Spanish study revealed that hospitalized patients with ADs have a more severe course of COVID-19[72]. At this time, until more data become available, it is crucial to emphasize the importance of physical distancing, wearing masks, and frequent hand washing, for everyone and especially in our patients with ADs. Adherence to medications is also very important to prevent flares of ADs that might result in organ damage.
Conclusion
COVID-19 is a novel pandemic that has had significant global health consequences. Similar to systemic autoimmune diseases, COVID-19 can present with heterogeneous and systemic clinical manifestations. To some extent, there are similarities in the immune response in both disease conditions, and organ damage in COVID-19 appears to be largely immune-mediated, similar to autoimmune diseases. The SARS-CoV-2 virus can disturb self-tolerance of the host at least in part through molecular mimicry. Indeed, the development of autoantibodies and sometimes organ-specific (e.g. GBS) or systemic (e.g. SLE-like disease) autoimmunity have been observed in COVID-19. Overall, more data are needed to further understand the relationship between COVID-19 and autoimmunity and characterize the risk and severity of COVID-19 in patients with pre-existing autoimmune diseases.
Key points:
COVID-19 infection can be complicated by involvement of multiple organ systems.
Immune-mediated injury contributes to the manifestations and complications of COVID-19.
Organ damage in COVID-19 is at least in part caused by perpetuated inflammatory responses, similar to autoimmune diseases.
SARS-CoV-2 might trigger autoimmune responses through molecular mimicry.
COVID-19 might be complicated by the development of autoantibodies and possibly de novo autoimmune diseases.
Funding
This study is supported by grants from the National Natural Science Foundation for Young Scientists of China (Grant No. 81502732) to YL, and an urgent grant of Hunan Province for fighting against coronavirus disease- 2019 epidemic (2020SK3005) to QL. AHS is supported by the National Institutes of Health (NIH) grants number R01AI097134 and R01AR070148, and the Lupus Research Alliance.
Footnotes
Conflicts of interest: There is no conflict of interest to declare.
References
• of special interest
- 1.Pollard C, Morran M, Nestor-Kalinoski A: The COVID-19 Pandemic: A Global Health Crisis. Physiological genomics 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Domingues R, Lippi A, Setz C, Outeiro T, Krisko A: SARS-CoV-2, immunosenescence and inflammaging: partners in the COVID-19 crime. Aging 2020, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hopfer H, Herzig M, Gosert R, Menter T, Hench J, Tzankov A, Hirsch H, Miller S: Hunting coronavirus by transmission electron microscopy - a guide to SARS-CoV-2-associated ultrastructural pathology in COVID-19 tissues. Histopathology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De P, Bhayye S, Kumar V, Roy K: In silico modeling for quick prediction of inhibitory activity against 3CL enzyme in SARS CoV diseases. Journal of biomolecular structure & dynamics 2020:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu F, Xiang R, Deng X, Wang L, Yu Z, Tian S, Liang R, Li Y, Ying T, Jiang S: Receptor-binding domain-specific human neutralizing monoclonal antibodies against SARS-CoV and SARS-CoV-2. Signal transduction and targeted therapy 2020, 5:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yi C, Sun X, Ye J, Ding L, Liu M, Yang Z, Lu X, Zhang Y, Ma L, Gu W, et al. : Key residues of the receptor binding motif in the spike protein of SARS-CoV-2 that interact with ACE2 and neutralizing antibodies. Cellular & Molecular Immunology 2020, 17:621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. : SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181:271–280 e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai Y, Xu Y, Wang X, Sun C, Guo Y, Qiu S, Ma K: Advances in SARS-CoV-2: a systematic review. European review for medical and pharmacological sciences 2020, 24:9208–9215. [DOI] [PubMed] [Google Scholar]
- 9.Rothan H, Byrareddy S: The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. Journal of autoimmunity 2020, 109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schettino M, Pellegrini L, Picascia D, Saibeni S, Bezzio C, Bini F, Omazzi B, Devani M, Arena I, Bongiovanni M, et al. : Clinical Characteristics of COVID-19 Patients With Gastrointestinal Symptoms in Northern Italy: A Single-Center Cohort Study. The American journal of gastroenterology 2020. [DOI] [PubMed] [Google Scholar]
- 11.Qian S, Hong W, Lingjie-Mao, Chenfeng-Lin, Zhendong-Fang, Pan J: Clinical Characteristics and Outcomes of Severe and Critical Patients With 2019 Novel Coronavirus Disease (COVID-19) in Wenzhou: A Retrospective Study. Frontiers in medicine 2020, 7:552002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Li Q, Yin Y, Zhang Y, Cao Y, Lin X, Huang L, Hoffmann D, Lu M, Qiu Y: Excessive Neutrophils and Neutrophil Extracellular Traps in COVID-19. Frontiers in immunology 2020, 11:2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esmaeilzadeh A, Elahi R: Immunobiology and immunotherapy of COVID-19: A clinically updated overview. Journal of cellular physiology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pascolini S, Vannini A, Deleonardi G, Ciordinik M, Sensoli A, Carletti I, Veronesi L, Ricci C, Pronesti A, Mazzanti L, et al. : COVID-19 and immunological dysregulation: can autoantibodies be useful? Clinical and translational science 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hejrati A, Rafiei A, Soltanshahi M, Hosseinzadeh S, Dabiri M, Taghadosi M, Taghiloo S, Bashash D, Khorshidi F, Zafari P: Innate immune response in systemic autoimmune diseases: a potential target of therapy. Inflammopharmacology 2020. [DOI] [PubMed] [Google Scholar]
- 16.Singh A, Thakur M, Sharma L, Chandra K: Designing a multi-epitope peptide based vaccine against SARS-CoV-2. Scientific reports 2020, 10:16219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rydyznski Moderbacher C, Ramirez S, Dan J, Grifoni A, Hastie K, Weiskopf D, Belanger S, Abbott R, Kim C, Choi J, et al. : Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lancman G, Mascarenhas J, Bar-Natan M: Severe COVID-19 virus reactivation following treatment for B cell acute lymphoblastic leukemia. Journal of hematology & oncology 2020, 13:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Setiati S, Harimurti K, Safitri E, Ranakusuma R, Saldi S, Azwar M, Marsigit J, Pitoyo Y, Widyaningsih W: Risk factors and laboratory test results associated with severe illness and mortality in COVID-19 patients: A systematic review. Acta medica Indonesiana 2020, 52:227–245. [PubMed] [Google Scholar]
- 20.Ziadi A, Hachimi A, Admou B, Hazime R, Brahim I, Douirek F, Zarrouki Y, El Adib A, Younous S, Samkaoui A: Lymphopenia in critically ill COVID-19 patients: A predictor factor of severity and mortality. International journal of laboratory hematology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciceri F, Castagna A, Rovere-Querini P, De Cobelli F, Ruggeri A, Galli L, Conte C, De Lorenzo R, Poli A, Ambrosio A, et al. : Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clinical immunology (Orlando, Fla.) 2020, 217:108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satış H, Özger H, Aysert Yıldız P, Hızel K, Gulbahar Ö, Erbaş G, Aygencel G, Guzel Tunccan O, Öztürk M, Dizbay M, et al. : Prognostic value of interleukin-18 and its association with other inflammatory markers and disease severity in COVID-19. Cytokine 2020, 137:155302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vassallo M, Manni S, Pini P, Blanchouin E, Ticchioni M, Seitz-Polski B, Puchois A, Sindt A, Lotte L, Fauque P, et al. : Patients with Covid-19 exhibit different immunological profiles according to their clinical presentation. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azar M, Shin J, Kang I, Landry M: Diagnosis of SARS-CoV-2 infection in the setting of cytokine release syndrome. Expert review of molecular diagnostics 2020. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y, Dong Y, Wang L, Xie H, Li B, Chang C, Wang F: Characteristics and prognostic factors of disease severity in patients with COVID-19: The Beijing experience. Journal of autoimmunity 2020, 112:102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, Long X, Xu Q, Tan J, Wang G, Cao Y, Wei J, Luo H, Zhu H, Huang L, et al. : Elevated serum levels of S100A8/A9 and HMGB1 at hospital admission are correlated with inferior clinical outcomes in COVID-19 patients. Cellular & Molecular Immunology 2020, 17:992–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conti P, Caraffa A, Gallenga C, Ross R, Kritas S, Frydas I, Younes A, Ronconi G: Coronavirus-19 (SARS-CoV-2) induces acute severe lung inflammation via IL-1 causing cytokine storm in COVID-19: a promising inhibitory strategy. Journal of biological regulators and homeostatic agents 2020, 34. [DOI] [PubMed] [Google Scholar]
- 28.Wampler Muskardin T: Intravenous Anakinra for Macrophage Activation Syndrome May Hold Lessons for Treatment of Cytokine Storm in the Setting of Coronavirus Disease 2019. ACR open rheumatology 2020, 2:283–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conti P, Caraffa A, Tetè G, Gallenga C, Ross R, Kritas S, Frydas I, Younes A, Di Emidio P, Ronconi G: Mast cells activated by SARS-CoV-2 release histamine which increases IL-1 levels causing cytokine storm and inflammatory reaction in COVID-19. Journal of biological regulators and homeostatic agents 2020, 34. [DOI] [PubMed] [Google Scholar]
- 30.Woodruff M, Ramonell R, Nguyen D, Cashman K, Saini A, Haddad N, Ley A, Kyu S, Howell J, Ozturk T, et al. : Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nature immunology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliviero B, Varchetta S, Mele D, Mantovani S, Cerino A, Perotti CG, Ludovisi S, Mondelli MU: Expansion of atypical memory B cells is a prominent feature of COVID-19. Cellular & Molecular Immunology 2020, 17:1101–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varchetta S, Mele D, Oliviero B, Mantovani S, Ludovisi S, Cerino A, Bruno R, Castelli A, Mosconi M, Vecchia M, et al. : Unique immunological profile in patients with COVID-19. Cellular & Molecular Immunology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair C, Weber A, Barnes BJ, Egeblad M, et al. : Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper demonstrates a possible role for neutrophils and NETs in the pathogeneis of COVID-19.
- 34.Ali RA, Gandhi AA, Meng H, Yalavarthi S, Vreede AP, Estes SK, Palmer OR, Bockenstedt PL, Pinsky DJ, Greve JM, et al. : Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat Commun 2019, 10:1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaminski M, Sunny S, Balabayova K, Kaur A, Gupta A, Abdallah M, Quale J: Tocilizumab Therapy of COVID-19: A Comparison of Subcutaneous and Intravenous Therapies. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Chang C, Lu Q: Management strategies for patients with autoimmune diseases during the COVID-19 pandemic: A perspective from China. European journal of rheumatology 2020, 7:S94–S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Canziani L, Trovati S, Brunetta E, Testa A, De Santis M, Bombardieri E, Guidelli G, Albano G, Folci M, Squadroni M, et al. : Interleukin-6 receptor blocking with intravenous tocilizumab in COVID-19 severe acute respiratory distress syndrome: A retrospective case-control survival analysis of 128 patients. Journal of autoimmunity 2020, 114:102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iglesias-Julián E, López-Veloso M, de-la-Torre-Ferrera N, Barraza-Vengoechea J, Delgado-López P, Colazo-Burlato M, Ubeira-Iglesias M, Montero-Baladía M, Lorenzo-Martín A, Minguito-de-la-Iglesia J, et al. : High dose subcutaneous Anakinra to treat acute respiratory distress syndrome secondary to cytokine storm syndrome among severely ill COVID-19 patients. Journal of autoimmunity 2020:102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reyes-Castillo Z, Valdés-Miramontes E, Llamas-Covarrubias M, Muñoz-Valle J: Troublesome friends within us: the role of gut microbiota on rheumatoid arthritis etiopathogenesis and its clinical and therapeutic relevance. Clinical and experimental medicine 2020. [DOI] [PubMed] [Google Scholar]
- 40.Harley JB, James JA: Everyone comes from somewhere: systemic lupus erythematosus and Epstein-Barr virus induction of host interferon and humoral anti-Epstein-Barr nuclear antigen 1 immunity. Arthritis Rheum 2010, 62:1571–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jog NR, Young KA, Munroe ME, Harmon MT, Guthridge JM, Kelly JA, Kamen DL, Gilkeson GS, Weisman MH, Karp DR, et al. : Association of Epstein-Barr virus serological reactivation with transitioning to systemic lupus erythematosus in at-risk individuals. Ann Rheum Dis 2019, 78:1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramasamy R, Mohammed F, Meier U: HLA DR2b-binding peptides from human endogenous retrovirus envelope, Epstein-Barr virus and brain proteins in the context of molecular mimicry in multiple sclerosis. Immunology letters 2020, 217:15–24. [DOI] [PubMed] [Google Scholar]
- 43.Basavalingappa R, Arumugam R, Lasrado N, Yalaka B, Massilamany C, Gangaplara A, Riethoven J, Xiang S, Steffen D, Reddy J: Viral myocarditis involves the generation of autoreactive T cells with multiple antigen specificities that localize in lymphoid and non-lymphoid organs in the mouse model of CVB3 infection. Molecular immunology 2020, 124:218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anand P, Puranik A, Aravamudan M, Venkatakrishnan A, Soundararajan V: SARS-CoV-2 strategically mimics proteolytic activation of human ENaC. eLife 2020, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lucchese G, Flöel A: Molecular mimicry between SARS-CoV-2 and respiratory pacemaker neurons. Autoimmunity reviews 2020, 19:102556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marino Gammazza A, Légaré S, Lo Bosco G, Fucarino A, Angileri F, Conway de Macario E, Macario A, Cappello F: Human molecular chaperones share with SARS-CoV-2 antigenic epitopes potentially capable of eliciting autoimmunity against endothelial cells: possible role of molecular mimicry in COVID-19. Cell stress & chaperones 2020, 25:737–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lucchese G, Flöel A: SARS-CoV-2 and Guillain-Barré syndrome: molecular mimicry with human heat shock proteins as potential pathogenic mechanism. Cell stress & chaperones 2020, 25:731–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Venkatakrishnan A, Kayal N, Anand P, Badley A, Church G, Soundararajan V: Benchmarking evolutionary tinkering underlying human-viral molecular mimicry shows multiple host pulmonary-arterial peptides mimicked by SARS-CoV-2. Cell death discovery 2020, 6:96. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This is one of several papers suggesting molecular mimickry as a possible mechnism underlying the development of immune-mediated manifestations in COVID-19.
- 49.Kanduc D: From Anti-SARS-CoV-2 Immune Responses to COVID-19 via Molecular Mimicry. Antibodies (Basel, Switzerland) 2020, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reyes Gil M, Barouqa M, Szymanski J, Gonzalez-Lugo J, Rahman S, Billett H: Assessment of Lupus Anticoagulant Positivity in Patients With Coronavirus Disease 2019 (COVID-19). JAMA network open 2020, 3:e2017539. [DOI] [PubMed] [Google Scholar]; • This paper and reference 51 below describe the presence of antiphospholipd antibodies in COVID-19 patients, which provides an insight to thrombo-inflammatory features observed in COVID-19
- 51.Amezcua-Guerra L, Rojas-Velasco G, Brianza-Padilla M, Vázquez-Rangel A, Márquez-Velasco R, Baranda-Tovar F, Springall R, Gonzalez-Pacheco H, Juárez-Vicuña Y, Tavera-Alonso C, et al. : Presence of antiphospholipid antibodies in COVID-19: case series study. Annals of the rheumatic diseases 2020. [DOI] [PubMed] [Google Scholar]; • This paper and reference 50 above describe the presence of antiphospholipd antibodies in COVID-19 patients, which provides an insight to thrombo-inflammatory features observed in COVID-19.
- 52.Pinto A, Carroll L, Nar V, Varatharaj A, Galea I: CNS inflammatory vasculopathy with antimyelin oligodendrocyte glycoprotein antibodies in COVID-19. Neurology(R) neuroimmunology & neuroinflammation 2020, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guilmot A, Maldonado Slootjes S, Sellimi A, Bronchain M, Hanseeuw B, Belkhir L, Yombi J, De Greef J, Pothen L, Yildiz H, et al. : Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. Journal of neurology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper summarizes immune-mediated neurological complications in 349 patients with COVID-19.
- 54.Jensen C, Wilson S, Thombare A, Weiss S, Ma A: Cold agglutinin syndrome as a complication of Covid-19 in two cases. Clinical infection in practice 2020, 7:100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fujii H, Tsuji T, Yuba T, Tanaka S, Suga Y, Matsuyama A, Omura A, Shiotsu S, Takumi C, Ono S, et al. : High levels of anti-SSA/Ro antibodies in COVID-19 patients with severe respiratory failure: a case-based review : High levels of anti-SSA/Ro antibodies in COVID-19. Clinical rheumatology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berzuini A, Bianco C, Paccapelo C, Bertolini F, Gregato G, Cattaneo A, Erba E, Bandera A, Gori A, Lamorte G, et al. : Red cell-bound antibodies and transfusion requirements in hospitalized patients with COVID-19. Blood 2020, 136:766–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maslov D, Simenson V, Jain S, Badari A: COVID-19 and Cold Agglutinin Hemolytic Anemia. TH open : companion journal to thrombosis and haemostasis 2020, 4:e175–e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patil N, Herc E, Girgis M: Cold agglutinin disease and autoimmune hemolytic anemia with pulmonary embolism as a presentation of COVID-19 infection. Hematology/oncology and stem cell therapy 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gigli G, Vogrig A, Nilo A, Fabris M, Biasotto A, Curcio F, Miotti V, Tascini C, Valente M: HLA and immunological features of SARS-CoV-2-induced Guillain-Barré syndrome. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Finsterer J, Scorza F, Fiorini A: SARS-CoV-2 associated Guillain-Barre syndrome in 62 patients. European journal of neurology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uncini A, Vallat J, Jacobs B: Guillain-Barré syndrome in SARS-CoV-2 infection: an instant systematic review of the first six months of pandemic. Journal of neurology, neurosurgery, and psychiatry 2020, 91:1105–1110. [DOI] [PubMed] [Google Scholar]
- 62.Bonometti R, Sacchi M, Stobbione P, Lauritano E, Tamiazzo S, Marchegiani A, Novara E, Molinaro E, Benedetti I, Massone L, et al. : The first case of systemic lupus erythematosus (SLE) triggered by COVID-19 infection. European review for medical and pharmacological sciences 2020, 24:9695–9697. [DOI] [PubMed] [Google Scholar]; • This case report suggests the development of new onset lupus as a complication of COVID-19.
- 63.Khabbazi A, Kavandi H, Paribanaem R, Khabbazi R, Malek Mahdavi A: Adherence to medication in patients with rheumatic diseases during COVID-19 pandemic. Annals of the rheumatic diseases 2020. [DOI] [PubMed] [Google Scholar]
- 64.Chuah S, Teh C, Wan Mohd Akbar S, Cheong Y, Sachdev Manjit Singh B: Impact of COVID-19 pandemic on hospitalisation of patients with systemic lupus erythematosus (SLE): report from a tertiary hospital during the peak of the pandemic. Annals of the rheumatic diseases 2020. [DOI] [PubMed] [Google Scholar]
- 65.Hassen L, Almaghlouth I, Hassen I, Daghestani M, Almohisen A, Alqurtas E, Alkhalaf A, Bedaiwi M, Omair M, Almogairen S, et al. : Impact of COVID-19 outbreak on rheumatic patients’ perceptions and behaviors: A cross-sectional study. International journal of rheumatic diseases 2020. [DOI] [PubMed] [Google Scholar]
- 66.Sawalha AH, Zhao M, Coit P, Lu Q: Epigenetic dysregulation of ACE2 and interferon-regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. Clin Immunol 2020, 215:108410. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper suggests that epigenetic dysregulation of host genes might be relavent to the pathogenesis of COVID-19.
- 67.Sawalha AH, Manzi S: Coronavirus Disease-2019: Implication for the care and management of patients with systemic lupus erythematosus. Eur J Rheumatol 2020, 7:S117–S120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sawalha AH: Patients with lupus are not protected from COVID-19. Ann Rheum Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zen M, Fuzzi E, Astorri D, Saccon F, Padoan R, Ienna L, Cozzi G, Depascale R, Zanatta E, Gasparotto M, et al. : SARS-CoV-2 infection in patients with autoimmune rheumatic diseases in northeast Italy: A cross-sectional study on 916 patients. Journal of autoimmunity 2020, 112:102502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murtas R, Andreano A, Gervasi F, Guido D, Consolazio D, Tunesi S, Andreoni L, Greco M, Gattoni M, Sandrini M, et al. : Association between autoimmune diseases and COVID-19 as assessed in both a test-negative case-control and population case-control design. Auto- immunity highlights 2020, 11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhong J, Shen G, Yang H, Huang A, Chen X, Dong L, Wu B, Zhang A, Su L, Hou X, et al. : COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. The Lancet Rheumatology 2020, 2:e557–e564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pablos J, Galindo M, Carmona L, Lledó A, Retuerto M, Blanco R, Gonzalez-Gay M, Martinez-Lopez D, Castrejón I, Alvaro-Gracia J, et al. : Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Annals of the rheumatic diseases 2020. [DOI] [PubMed] [Google Scholar]
- 73.Voicu S, Delrue M, Chousterman B, Stépanian A, Bonnin P, Malissin I, Deye N, Neuwirth M, Ketfi C, Mebazaa A, et al. : Imbalance between procoagulant factors and natural coagulation inhibitors contributes to hypercoagulability in the critically ill COVID-19 patient: clinical implications. European review for medical and pharmacological sciences 2020, 24:9161–9168. [DOI] [PubMed] [Google Scholar]


