Abstract
Background:
Coronavirus Disease 2019 (COVID-19) has caused over 1 200 000 deaths worldwide as of November 2020. However, little is known about the clinical outcomes among hospitalized patients with active COVID-19 after in-hospital cardiac arrest (IHCA).
Aim:
We aimed to characterize outcomes from IHCA in patients with COVID-19 and to identify patient- and hospital-level variables associated with 30-day survival.
Methods:
We conducted a multicentre retrospective cohort study across 11 academic medical centres in the U.S. Adult patients who received cardiopulmonary resuscitation and/or defibrillation for IHCA between March 1, 2020 and May 31, 2020 who had a documented positive test for Severe Acute Respiratory Syndrome Coronavirus 2 were included. The primary outcome was 30-day survival after IHCA.
Results:
There were 260 IHCAs among COVID-19 patients during the study period. The median age was 69 years (interquartile range 60–77), 71.5% were male, 49.6% were White, 16.9% were Black, and 16.2% were Hispanic. The most common presenting rhythms were pulseless electrical activity (45.0%) and asystole (44.6%). ROSC occurred in 58 patients (22.3%), 31 (11.9%) survived to hospital discharge, and 32 (12.3%) survived to 30 days. Rates of ROSC and 30-day survival in the two hospitals with the highest volume of IHCA over the study period compared to the remaining hospitals were considerably lower (10.8% vs. 64.3% and 5.9% vs. 35.7% respectively, p < 0.001 for both).
Conclusions:
We found rates of ROSC and 30-day survival of 22.3% and 12.3% respectively. There were large variations in centre-level outcomes, which may explain the poor survival in prior studies.
Keywords: COVID-19, in-hospital cardiac arrest, cohort study
Introduction:
The coronavirus disease 2019 (COVID-19) pandemic has infected almost 50 million people and, as of November 2020, has caused over 1 200 000 deaths worldwide [1]. Although many have mild symptoms, a significant proportion of patients develop more severe disease, often requiring hospitalization [2]. Once hospitalized, clinical deterioration is common; mortality in some U.S. hospitals has approached 25-30% [3, 4]. Despite the large numbers of critically ill COVID-19 patients in the last year, little is known about outcomes from patients who decompensate and progress to in-hospital cardiac arrest (IHCA).
International resuscitation guidelines have recommended that providers consider the likelihood of success and appropriateness of resuscitation when initiating or deciding whether to continue cardiopulmonary resuscitation (CPR) during IHCA of COVID-19 patients [5–7]. This decision process is complicated both by the direct risk of infection among healthcare workers during the resuscitation care of COVID-19 patients through aerosol-generating procedures such as CPR and by the substantial strain exerted on hospital resources during times of increased hospital occupancy [2]. However, a lack of robust data describing the outcome of COVID-19 patients who develop IHCA limits the ability of providers to weigh the likelihood of successful resuscitation in this population.
Few studies have described outcomes in the COVID-19 IHCA population. One single-centre cohort study of 136 IHCA in China at the beginning of the pandemic demonstrated extremely high rates of asystole (almost 90%) and 2.9% 30-day survival [8]. Two single-centre case series, one in New York and another in Michigan, both demonstrated 100% in-hospital mortality despite high rates of return of spontaneous circulation (ROSC) [9, 10]. More recently a multicentre collaborative published a report of 400 patients admitted across 86 ICUs who suffered IHCA, with a rate of ROSC of 33.8% and 12.0% survival to hospital discharge [11]. These studies have raised questions regarding the utility of resuscitating COVID-19 patients with IHCA, particularly when balancing risks to providers. Whether these early reports are broadly generalizable and should inform practice, particularly in patients outside of the ICU, remains unclear, nor is it known to what extent these results were confounded by conditions during the early phase of the pandemic.
Objective:
We aimed to characterize the 30-day survival after IHCA in patients with COVID-19, which was the primary outcome. Secondary outcomes included 30-day neurological status, measured according to the Cerebral Performance Category (CPC) scoring system, and ROSC rate.
Methods:
Setting and Study Design:
We conducted a multi-centre retrospective cohort study of patients hospitalized between March 1st and May 31st, 2020 at one of 11 academic medical centres in the U.S. that were part of the COVID-19 In-Hospital Cardiac Arrest (COVID IHCA) Study Group. A full list of participating sites is provided in Supplementary Table 1.
Patients were eligible for inclusion if they received CPR and/or defibrillation for IHCA and had a documented positive polymerase chain reaction test for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS CoV-2) during their hospitalization, either before or within 24 hours after IHCA. IHCA cases were identified by site investigator review of institutional quality improvement cardiac arrest databases, free-text searches of the Electronic Medical Record (EMR), and EMR billing and International Classification of Disease (ICD)-10 codes (codes I46.9, cardiac arrest, cause unspecified, and I49.01, ventricular fibrillation). Patients <18 years of age were excluded.
Data Collection:
Data were collected manually by chart review of the EMR. Data collected included patient characteristics (age, gender, race, ethnicity, hospital of admission, and ZIP code), pre-existing patient conditions (hypertension, hyperlipidaemia, diabetes mellitus, coronary artery disease, chronic kidney disease, metastatic or hematologic malignancy, or cirrhosis), and in-hospital conditions present 24 hours prior to IHCA (sepsis, hypotension, liver insufficiency, or renal insufficiency). IHCA event information included location of arrest, the initial presenting rhythm (pulseless electrical activity [PEA], ventricular fibrillation [VF], pulseless ventricular tachycardia [pVT], or asystole), interventions during IHCA (including dose of epinephrine and bicarbonate), whether ROSC was achieved, post-ROSC management including the use of targeted temperature management (TTM) and percutaneous coronary intervention (PCI), and 30-day survival after IHCA.
Data were collected according to the Utstein template, a commonly used consensus framework for reporting cardiac arrest outcomes [12]. To ensure consistency of data collection, each site was provided with a data dictionary. Acute conditions were determined by manual review of the EMR and recorded if they were present in the 24 hours prior to IHCA and met the following criteria: sepsis: a suspected infection plus a Sequential Organ Failure Assessment score change of greater than or equal to two [13]; hypotension: a documented mean arterial BP <65 mmHg for over 30 minutes or the need for vasopressor infusion; hepatic insufficiency: liver function tests ≥3x upper limit of normal and/or diagnosis of acute-on-chronic liver injury, cirrhosis, or liver failure; renal insufficiency: Chronic Kidney Disease (CKD) stage III or greater, need for dialysis, or acute kidney injury (defined as a creatinine increase of ≥0.3mg/dL or ≥1.5 x baseline) [14]. Neurological status was determined by manual review of the EMR and according to the Cerebral Performance Category (CPC) score; a CPC of 1-2 was considered a good neurological outcome, consistent with prior cardiac arrest studies [15, 16]. The most likely cause of IHCA as determined by the clinical team, if documented, was also recorded. Study data were collected and managed using a research database (REDCap, Vanderbilt University, TN) [17, 18]. REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for clinical research studies [17, 18].
The study was deemed exempt from full Institutional Review Board review at each site prior to data collection.
Statistical Methods:
Patient characteristics, comorbidities, pre-arrest conditions, and IHCA characteristics were tabulated and summarized using descriptive statistics and presented as mean with standard deviation, number (n) with percentage (%), or median with interquartile range (IQR). Continuous variables were analysed using Student’s t-test and categorical data using Chi-Square test. Stata Version 16 (StataCorp, College Station, Texas) was used for data analysis. Hospital volume of IHCA was plotted against rates of ROSC and 30-day survival for descriptive analysis.
Results:
A total of 260 COVID-19 patients with IHCA occurring between March 1, 2020 to May 31, 2020 met criteria for inclusion in the study cohort (Figure 1). Of these, 204 (78.5%) were from two hospitals in New York, and the remainder were from Pennsylvania (17 patients, 6.5%), Massachusetts (30 patients, 11.5%), New Jersey (7 patients, 2.7%), and Washington (2 patients, 0.8%). Sociodemographic characteristics of the study population are summarized in Table 1. The median age was 69 years (IQR 60-77), 71.5% were male, 49.6% were White, 16.9% were Black, and 16.2% were Hispanic. The most common presenting rhythm was PEA (45.0%), followed by asystole (44.6%) and pulseless VT or VF (8.5%). The initial rhythm was not known in five patients (1.9%). The majority (91.5%) of IHCA were witnessed and most IHCA occurred while the patient was on telemetry (97.3%).
Figure 1:
Flow diagram of number of participants at each stage of the study.
IHCA: in-hospital cardiac arrest, CPR: cardiopulmonary resuscitation.
Table 1:
Demographic features, IHCA features and outcomes of patients with COVID-19 IHCA.
| 30-Day Survival | |||||
|---|---|---|---|---|---|
| Total n=260 | No n=228 | Yes n=32 | p-value | ||
| Age, median (IQR) | 69 (60-77) | 69 (63-77) | 60 (52-72) | <0.001 | |
| Gender | Male | 186 (71.5%) | 162 (71.1%) | 24 (75.0%) | 0.64 |
| Female | 74 (28.5%) | 66 (28.9%) | 8 (25.0%) | ||
| Race/Ethnicity: | Black | 44 (16.9%) | 33 (14.5%) | 11 (34.4%) | 0.02 |
| Hispanic | 42 (16.2%) | 38 (16.7%) | 4 (12.5%) | ||
| White | 129 (49.6%) | 119 (52.2%) | 10 (31.2%) | ||
| Other | 38 (14.6%) | 31 (13.6%) | 7 (21.9%) | ||
| Unknown | 7 (2.7%) | 7 (3.1%) | 0 (0.0%) | ||
| Pre-Existing | |||||
| Comorbidities | |||||
| Hypertension | 171 (65.8%) | 148 (64.9%) | 23 (71.9%) | 0.44 | |
| Hyperlipidaemia | 100 (38.5%) | 92 (40.4%) | 8 (25.0%) | 0.10 | |
| Diabetes mellitus | 114 (43.8%) | 97 (42.5%) | 17 (53.1%) | 0.26 | |
| Coronary artery disease | 48 (18.5%) | 42 (18.4%) | 6 (18.8%) | 0.96 | |
| Chronic kidney disease | 24 (9.2%) | 18 (7.9%) | 6 (18.8%) | 0.05 | |
| Metastatic/haematological malignancy | 9 (3.5%) | 9 (3.9%) | 0 (0.0%) | 0.25 | |
| Cirrhosis | 4 (1.5%) | 2 (0.9%) | 2 (6.2%) | 0.02 | |
| Acute Conditions | Sepsis | 103 (39.6%) | 94 (41.2%) | 9 (28.1%) | 0.16 |
| Hypotension | 124 (47.5%) | 112 (49.1%) | 12 (37.5%) | 0.22 | |
| Hepatic insufficiency | 11 (4.2%) | 8 (3.5%) | 3 (9.4%) | 0.12 | |
| Renal insufficiency | 127 (48.8%) | 112 (49.1%) | 15 (46.9%) | 0.81 | |
| Location of IHCA | Non-ICU | 94 (36.2%) | 87 (38.2%) | 7 (21.9%) | 0.07 |
| ICU | 166 (63.9%) | 141 (61.8%) | 25 (78.1%) | ||
| Cardiac Arrest Witnessed | Yes | 238 (91.5%) | 209 (91.7%) | 29 (90.6%) | |
| No | 19 (7.3%) | 17 (7.5%) | 2 (6.2%) | 0.53 | |
| Unknown | 3 (1.2%) | 2 (0.9%) | 1 (3.1%) | ||
| IHCA Outcomes | ROSC | 58 (22.3%) | - | ||
| Survival to Hospital Discharge | 31 (11.9%) | - | |||
| 30-day Survival | 32 (12.3%) | - | |||
| CPC 1-2 | 16 (50.0%) | - | |||
| CPC 3-4 | 10 31.3%) | - | |||
| CPC 5 | 2 (6.3%) | - | |||
| Unknown | 4 (12.5%) | - | |||
Abbreviations: CPC: Cerebral Performance Category; ICU: Intensive Care Unit
At the time of IHCA, 82.7% required supplemental oxygen, 74.2% were mechanically ventilated, 75.8% of patients required either vasopressor or inotrope support, and 5.4% had been proned within 24 hours of the IHCA. Sustained ROSC was achieved in 58 patients(22.3%), 31 (11.9%) survived to hospital discharge, and 32 survived to30 days after IHCA (12.3%). CPC at 30 days was unavailable in four of these patients: 16 patients (50.0%) had a 30-day CPC of 1 or 2. After successful resuscitation and ROSC, TTM was initiated in 12 out of 58 patients (20.7%) and 4 patients received PCI after ROSC: 3 out of 8 (37.5%) patients with VF/pVT and none of the 50 patients with asystole or PEA. There was a significant difference in 30-day survival by subject ethnicity: 11 out of 44 Black subjects survived to 30-days (25.0%) compared to 4 out of 42 Hispanic subjects (9.5%) and 10 out of 129 White subjects (7.8%), p-value 0.02.
IHCA occurred in the intensive care unit (ICU) in 166 out of 260 patients (63.9%). Compared to those who had an IHCA outside of the ICU, patients who suffered cardiac arrest in critical care units were more likely to have sepsis (44.6% vs. 30.9%, p=0.03) and hypotension prior to their IHCA (57.2% vs. 30.9%, p<0.001). Patients in the ICU were less likely to have asystole as a presenting rhythm (35.5% vs. 60.6%, p<0.001), and were more likely to achieve sustained ROSC (29.5% vs 9.6%, p<0.001), but did not have significantly better 30-day survival compared to IHCA outside of the ICU (15.1% vs. 7.4%, p=0.07). Of the 14 patients who had their IHCA within 24 hours of requiring prone positioning, eight patients (57.1%) attained sustained ROSC and five (35.7%) survived for 30 days.
Rates of ROSC and survival both varied substantially between hospitals (Figure 2). The two hospitals in New York City (NYC) had the highest volume of COVID-19 IHCA and represented 78.5% of the cohort. Patients from NYC-based hospitals had lower rates of ROSC and 30-day survival, were older, were less likely to arrest in the ICU, and were more likely to have asystole as the presenting IHCA rhythm (Table 2).
Figure 2:
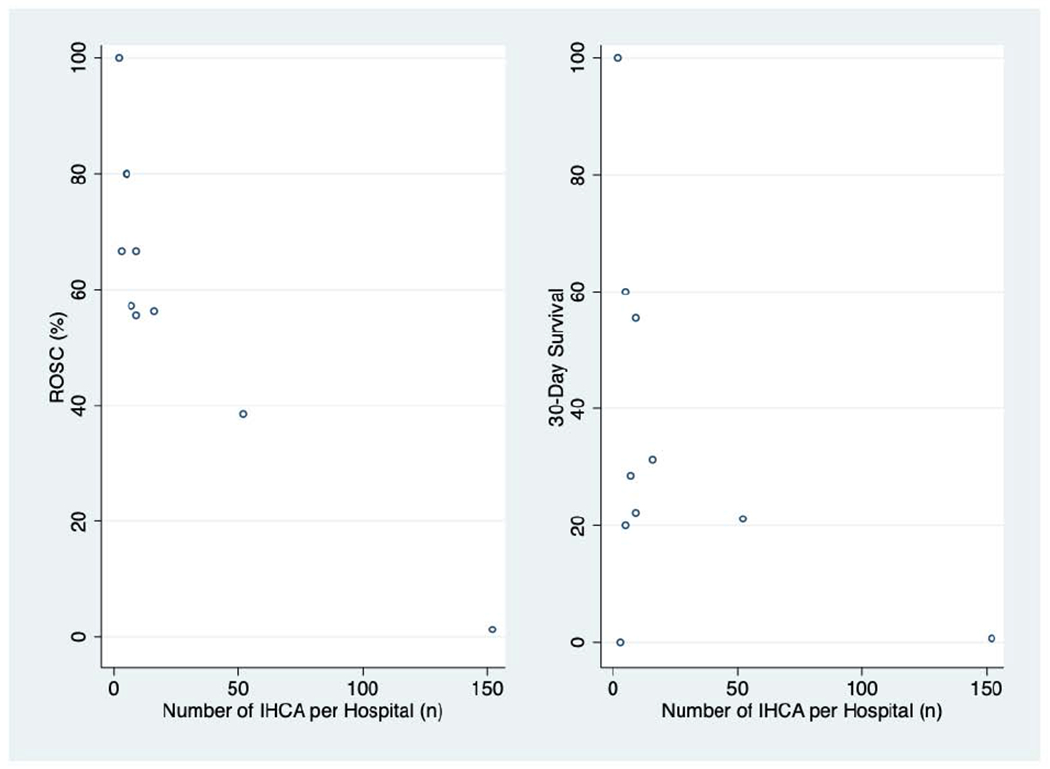
Rates of ROSC and 30-Day Survival by Hospital
Unadjusted rates of ROSC and 30-day survival by hospital against the volume of COVID-19 IHCA per hospital over the study period. Abbreviations: ROSC: Return of Spontaneous Circulation; IHCA: In-Hospital Cardiac Arrest.
Table 2:
Features of patients who suffered IHCA in the two New York City based hospitals compared with all other study hospitals.
| Non-NYC n=56 | NYC n=204 | p-value | ||
|---|---|---|---|---|
| Age, median (IQR) | 63 (54-73) | 70 (63-79) | <0.001 | |
| Gender | Male | 35 (62.5%) | 151 (74.0%) | 0.09 |
| Race/Ethnicity: | Black | 18 (32.1%) | 26 (12.7%) | <0.001 |
| Hispanic | 9 (16.1%) | 33 (16.2%) | ||
| White | 16 (28.6%) | 113 (55.4%) | ||
| Other | 8 (14.3%) | 30 (14.7%) | ||
| Unknown | 5 (8.9%) | 2 (1.0%) | ||
| Pre-IHCA Conditions | Sepsis | 19 (33.9%) | 84 (41.2%) | 0.33 |
| Hypotension | 23 (41.1%) | 101 (49.5%) | 0.26 | |
| Metastatic/haematological malignancy | 1 (1.8%) | 8 (3.9%) | 0.44 | |
| Hepatic insufficiency | 7 (12.5%) | 4 (2.0%) | <0.001 | |
| Renal insufficiency | 21 (37.5%) | 106 (52.0%) | 0.06 | |
| Location of Cardiac Arrest | Non-ICU | 12 (21.4%) | 82 (40.2%) | 0.01 |
| ICU | 44 (78.6%) | 122 (59.8%) | ||
| Initial Rhythm | VF | 4 (7.1%) | 3 (1.5%) | |
| pVT | 4 (7.1%) | 11 (5.4%) | <0.001 | |
| PEA | 41 (73.2%) | 76 (37.3%) | ||
| Asystole | 7 (12.5%) | 109 (53.4%) | ||
| Unknown | 0 (0.0%) | 5 (2.5%) | ||
| IHCA Outcomes | Sustained ROSC | 36 (64.3%) | 22 (10.8%) | <0.001 |
| Thirty Day Survival | 20 (35.7%) | 12 (5.9%) | <0.001 | |
Abbreviations: CCU: cardiac/coronary care unit; CPAP: continuous positive pressure ventilation; CPC: cerebral performance category; HHFNC: heated high flow nasal cannula; ICU: intensive care unit; IHCA: in-hospital cardiac arrest; NC: nasal cannula; NIV: non-invasive ventilation; NRB: non-rebreathe mask; PEA: pulseless electrical activity; pVT: pulseless ventricular tachycardia; ROSC: return of spontaneous circulation; VF: ventricular fibrillation.
The putative cause of cardiac arrest could be determined by hospital providers in 110 out of 260 events (42.3%). The most common presumed aetiology of IHCA was hypoxaemia (47 patients, 42.7%), followed by sepsis in 16 patients (14.5%), arrythmia in 12 patients (10.9%), and pulmonary embolism in 11 patients (10.0%) (Table 3).
Table 3:
Most likely cause of cardiac arrest as determined by the cardiac arrest team.
| Total** n=110 |
||
|---|---|---|
| % | n | |
| Arrythmia | 10.9% | (12) |
| PE | 10.0% | (11) |
| Hypoxaemia | 42.7% | (47) |
| Sepsis | 14.5% | (16) |
| Cardiogenic shock | 5.5% | (6) |
| ETT Malfunction | 6.4% | (7) |
| Haemorrhage | 0.9% | (1) |
| Other | 31.8% | (35) |
Multiple causes were possible for a single IHCA. Abbreviations: ETT: endotracheal tube, PE: Pulmonary Embolism.
Discussion:
In our multicentre analysis of IHCA among COVID-19 patients, we found average rates of ROSC and 30-day survival of 22.3% and 12.3%, respectively. Notably, these outcomes differed substantially by centre. Our findings contrast with the uniformly poor outcomes seen in singlecentre studies of IHCA in patients with COVID-19 but are consistent with those of another published multicentre IHCA study [11].
In our cohort, we have demonstrated rates of ROSC and 30-day survival that are numerically higher than previously published cohorts of survival after IHCA in COVID-19, but lower than recent studies of IHCA prior to COVID-19, which demonstrated rates of ROSC of 54-64% and survival of 22-28% [8–10, 19, 20]. Since early in the pandemic there has been speculation about poor outcomes from resuscitation of patients with COVID-19 [6, 21]. These concerns have been further validated by recent studies that described the clinical outcomes of IHCA in COVID-19. The first was a cohort of over 130 IHCA events in a single hospital in Wuhan. In this cohort, almost 90% of patients were found to be in asystole; the overall cohort had a ROSC rate of 13.2% and a 30-day survival of 2.9% [9]. Such a high rate of asystole as the presenting rhythm raises the question of delayed recognition of IHCA as a factor contributing to the extremely low survival in this cohort. Two recent case series have described outcomes from single centres in NYC and Michigan. In contrast to the cohort from Wuhan, the most common presenting rhythms were PEA (58-82%), followed by asystole (15-29%), with pVT/VF making up the minority (4-13%), with a ROSC rate of 42-52%, findings that are consistent with our cohort and other studies of non-COVID-19 IHCA [9, 10, 22, 23]. However, no patients in either cohort survived to hospital discharge. Unfortunately, no details were provided on the cause of cardiac arrest, cause of subsequent in-hospital death after ROSC, or on post-arrest management which limits interpretation of the cause of this notably high rate of in-hospital death [9, 10]. However, given that the median survival time after ROSC was only 2.8 hours (IQR 1.5-13.3) in the NYC cohort, withdrawal of life-sustaining measures and progressive critical illness likely played a role [9]. More recently, a multicentre observational study of 400 patients with COVID-19 who were admitted to the ICU demonstrated a 33.8% ROSC rate and a 12.0% survival to hospital discharge, with lower rates of survival in older patients [11]. The low survival rates in these studies and concern for stretched ICU resources have spurred discussions of expanded do-not-resuscitate orders for patients with COVID-19 and concern for futility of resuscitation in this population [24–26].
We found large variation in outcomes after IHCA within hospitals in our cohort: the hospital with the largest number of IHCA over the study period had only one patient out of 152 survive to 30 days after IHCA, rates that were similar to the previous cohorts of IHCA in COVID-19. However, many of the other hospitals in our cohort, including one hospital with over 50 IHCA, had rates of 30-day survival rates over 20%. Such inter-hospital variation may explain the low survival found in other studies of outcomes from IHCA in patients with COVID-19. The cause of this finding is still unclear, although variation in outcomes after IHCA has been well-described even prior to COVID-19 and is only partially explained by patient-level factors: differences in centre-level resuscitation practices, withdrawal of life-sustaining measures, and post-IHCA care likely contribute [27–29]. Additional research is needed to investigate the patient and hospital-level factors that contribute to the heterogeneity in IHCA outcomes described in our cohort.
NYC was particularly severely affected during the study period and subsequently had the highest volume of IHCA in our cohort. In these NYC-based hospitals, IHCA was more likely to occur outside of the ICU and to present with an initial rhythm of asystole. Hospitals outside of NYC had rates of ROSC and survival that were numerically similar to IHCA in prior studies of IHCA [19]. The interplay between IHCA case volume and outcomes is complex and prior studies have demonstrated a non-linear association, with increasing volume initially associated with improved outcomes, and then becoming negatively associated with outcomes [30]. A recently published study of all-cause IHCA during the COVID-19 pandemic in NYC showed that hospital survival decreased substantially during the pandemic when compared to the year before, with no reported difference in outcomes between COVID-19 patients and those without the virus, suggesting that centre-wide changes in IHCA management may have impacted outcomes [31]. It has been hypothesized that particularly high volumes of IHCA may be an indicator for hospitals with stretched resources and less developed capacities for identification of patient deterioration and post-arrest care [30]. As COVID-19 surges and overwhelms hospitals with a challenging volume of critically ill patients, patient care teams have been required to practice in unprecedented circumstances [2]. It seems likely that this might lead to delayed recognition of deterioration and critical illness, factors that have long been associated with worse outcomes in hospitalized patients [32]. It is possible that, in addition to centre-level differences in the management of IHCA, the volume of IHCA may have contributed to worse outcomes in the NYC-based hospitals.
Hypoxaemia was the leading potential causative factor in IHCA among those in our cohort with a documented cause. We did not detect a difference in the 30-day survival or rates of ROSC in patients who had IHCA while mechanically ventilated, nor in those who required prone positioning, suggesting that patients with severe respiratory dysfunction from COVID-19 have the potential to survive an IHCA event. Pulmonary embolism and cardiac arrhythmias were identified as potentially causative in over 20% of IHCA. These aetiologies, which are potentially acutely reversible, could represent areas for further improvement in outcomes.
Our study has several significant limitations that must be considered. The first is the lack of a control group, which limits the conclusions that can be derived from the cohort; we were unable to determine whether outcomes from IHCA in COVID-19 were different to those in patients without COVID-19 who suffered IHCA in the same time frame at these hospitals. The substantial centre-level variation in both ROSC and 30-day survival in our multicentre cohort limits the ability to identify patient-level factors that may contribute to IHCA outcomes, especially given that one hospital represented over half of patients in our cohort and this hospital had particularly low survival. Additionally, due to the low number of outcomes, we were unable to account for clustering by site. Given the retrospective nature of the study, we were unable to verify that all cases of IHCA during the study period were captured by each site, nor did we have any means of verifying the data that was documented in the EMR. We were also unable to determine the effect of hospital-level factors on patient outcomes, particularly the impact of delays for the donning of personalised protective equipment, changes in IHCA protocols, and hospital rates of do-not-resuscitate orders. Finally, the fact that varying methods were used at different sites and the proven inaccuracies of using EMR codes to identify IHCA make it unlikely that the entire population of COVID-19 IHCA was captured at study hospitals [33]. This may have contributed to the variation in outcomes between study sites.
Conclusion:
In our cohort, we found rates of ROSC and 30-day survival of 22.3% and 12.3% respectively. Half of the patients who survived to 30-days post-IHCA did so with a good neurological status. There were large variations in centre-level outcomes, which may explain the uniformly poor survival seen in prior studies. More research is needed to understand the factors that contribute to outcomes after IHCA in patients with COVID-19.
Supplementary Material
Acknowledgments:
Conflicts of interest: BA has received philanthropic funding for a randomized trial of COVID-19 therapeutics, and a City of Philadelphia grant for COVID-19 community testing. JH receives fees for consulting at Inari Medical, Penumbra Inc. and Abiomed. JL receives consultancy fees from Butterfly Inc. and grant support from the National Institute of Health (NIH), Department of Defense (DOD), Nihon-Kohden Corp, and Beckman Coulter Inc. OJLM is supported by an NIH T32 grant, 5T32HL007891-22. OJLM had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
A manuscript containing 55 patients from one hospital in the study cohort (as well as 55 controls from 2018/2019) is currently under consideration at Resuscitation Plus as a retrospective case-control study - RESPLU-D-20-00074R1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jordan Anderson, Department of Internal Medicine, Brigham and Women’s Hospital.
Katherine M. Berg, Department of Medicine, Division of Pulmonary and Critical Care, Beth Israel Deaconess Medical Center.
Mahlaqa Butt, Department of Emergency Medicine, Maimonides Medical Centera.
Donna S. Covin, Penn Medicine Princeton Health.
Aashka Damani, University of Washington School of Medicine.
Patrick J. Donnelly, Pennsylvania Hospital.
Haytham M.A Kaafarani, Division of Trauma, Emergency Surgery and Surgical Critical Care Massachusetts General Hospital.
Sarah Kabariti, Department of Emergency Medicine, Maimonides Medical Center.
Thomas C. Kingsley, Department of General Internal Medicine, Mayo Clinic Rochester.
Rachel Kohn, Division of Pulmonary, Allergy, and Critical Care Medicine, University of Pennsylvania.
Kevin C. Ma, Division of Pulmonary, Allergy, and Critical Care Medicine, University of Pennsylvania.
Margaret Mullen-Fortino, Division of Pulmonary, Allergy, and Critical Care Medicine, University of Pennsylvania.
Leon Naar, Division of Trauma, Emergency Surgery and Surgical Critical Care Massachusetts General Hospital.
Frances Mae West, Division of Pulmonary and Critical Care Medicine, Thomas Jefferson University.
Patrick Zeniecki, Thomas Jefferson University – Sidney Kimmel Medical College.
References:
- [1].Center for Systems Science and Engineering at Johns Hopkins Hospital. COVID-19 Dashboard. 2020.
- [2].Griffin KM, Karas MG, Ivascu NS, Lief L. Hospital Preparedness for COVID-19: A Practical Guide from a Critical Care Perspective. Am J Respir Crit Care Med. 2020;201:1337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in Critically Ill Patients in the Seattle Region - Case Series. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Maron BA, Gladwin MT, Bonnet S, et al. Perspectives on Cardiopulmonary Critical Care for COVID-19 Patients: From Members of the American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. J Am Heart Assoc. 2020:e017111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Edelson DP, Sasson C, Chan PS, et al. Interim Guidance for Basic and Advanced Life Support in Adults, Children, and Neonates With Suspected or Confirmed COVID-19: From the Emergency Cardiovascular Care Committee and Get With The Guidelines-Resuscitation Adult and Pediatric Task Forces of the American Heart Association. Circulation. 2020;141:e933–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Soar J, Böttiger BW, Carli P, et al. European Resuscitation Council COVID-19 Guidelines. In: Council ER, editor. www.erc.edu2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shao F, Xu S, Ma X, et al. In-hospital cardiac arrest outcomes among patients with COVID-19 pneumonia in Wuhan, China. Resuscitation. 2020;151:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sheth V, Chishti I, Rothman A, et al. Outcomes of in-hospital cardiac arrest in patients with COVID-19 in New York City. Resuscitation. 2020;155:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Thapa SB, Kakar TS, Mayer C. Clinical Outcomes of In-Hospital Cardiac Arrest in COVID-19. JAMA Internal Medicine. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hayek SS, Brenner SK, Azam TJ, et al. In-hospital cardiac arrest in critically ill patients with covid-19: multicenter cohort study. BMJ. 2020;371:m3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nolan JP, Berg RA, Andersen LW, et al. Cardiac Arrest and Cardiopulmonary Resuscitation Outcome Reports: Update of the Utstein Resuscitation Registry Template for In-Hospital Cardiac Arrest: A Consensus Report From a Task Force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia).. Resuscitation. 2019;144:166–77. [DOI] [PubMed] [Google Scholar]
- [13].Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Khwaja A KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–84. [DOI] [PubMed] [Google Scholar]
- [15].Ajam K, Gold LS, Beck SS, Damon S, Phelps R, Rea TD. Reliability of the Cerebral Performance Category to classify neurological status among survivors of ventricular fibrillation arrest: a cohort study. Scand J Trauma Resusc Emerg Med. 2011;19:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Phelps R, Dumas F, Maynard C, Silver J, Rea T. Cerebral Performance Category and long-term prognosis following out-of-hospital cardiac arrest. Crit Care Med. 2013;41:1252–7. [DOI] [PubMed] [Google Scholar]
- [17].Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Girotra S, Nallamothu BK, Spertus JA, et al. Trends in survival after in-hospital cardiac arrest. N Engl J Med. 2012;367:1912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wiberg S, Holmberg MJ, Donnino MW, et al. Age-dependent trends in survival after adult in-hospital cardiac arrest. Resuscitation. 2020;151:189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chan PS, Berg RA, Nadkarni VM. Code Blue During the COVID-19 Pandemic. Circ Cardiovasc Qual Outcomes. 2020;13:e006779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rohlin O, Taeri T, Netzereab S, Ullemark E, Djarv T. Duration of CPR and impact on 30-day survival after ROSC for in-hospital cardiac arrest-A Swedish cohort study. Resuscitation. 2018;132:1–5. [DOI] [PubMed] [Google Scholar]
- [23].Girotra S, Tang Y, Chan P, Nallamothu BK. Survival After In-Hospital Cardiac Arrest In Critically Ill Patients: Implications For The Covid-19 Pandemic? medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cha AE. Hospitals consider universal do-not-resuscitate orders for coronavirus patients. The Washington Post 2020.
- [25].Rosen RJ. Physician-discretion DNIC (Do Not Initiate Compressions) in the COVID era. Resuscitation. 2020;153:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Curtis JR, Kross EK, Stapleton RD. The Importance of Addressing Advance Care Planning and Decisions About Do-Not-Resuscitate Orders During Novel Coronavirus 2019 (COVID-19). JAMA. 2020. [DOI] [PubMed] [Google Scholar]
- [27].Girotra S, Nallamothu BK, Tang Y, Chan PS, American Heart Association Get With The Guidelines-Resuscitation I. Association of Hospital-Level Acute Resuscitation and Postresuscitation Survival With Overall Risk-Standardized Survival to Discharge for In-Hospital Cardiac Arrest. JAMA Netw Open. 2020;3:e2010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kolte D, Khera S, Aronow WS, et al. Regional variation in the incidence and outcomes of in-hospital cardiac arrest in the United States. Circulation. 2015;131:1415–25. [DOI] [PubMed] [Google Scholar]
- [29].Merchant RM, Berg RA, Yang L, et al. Hospital variation in survival after in-hospital cardiac arrest. J Am Heart Assoc. 2014;3:e000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Akintoye E, Adegbala O, Egbe A, Olawusi E, Afonso L, Briasoulis A. Association between Hospital volume of cardiopulmonary resuscitation for in-hospital cardiac arrest and survival to Hospital discharge. Resuscitation. 2020;148:25–31. [DOI] [PubMed] [Google Scholar]
- [31].Miles JA, Mejia Saldarriaga M, Rios S, et al. Characteristics and Outcomes of In-Hospital Cardiac Arrest Events During the COVID-19 Pandemic: A Single Center Experience from a New York City Public Hospital. Circ Cardiovasc Qual Outcomes. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Barwise A, Thongprayoon C, Gajic O, Jensen J, Herasevich V, Pickering BW. Delayed Rapid Response Team Activation Is Associated With Increased Hospital Mortality, Morbidity, and Length of Stay in a Tertiary Care Institution. Crit Care Med. 2016;44:54–63. [DOI] [PubMed] [Google Scholar]
- [33].DeZorzi C, Boyle B, Qazi A, et al. Administrative Billing Codes for Identifying Patients With Cardiac Arrest. J Am Coll Cardiol. 2019;73:1598–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


