Abstract
Interstitial inflammation is an important feature of cystic kidney disease. Renal macrophages are the most well-studied inflammatory cell in the kidney, and their involvement in cyst formation has been reported in different animal models and patients with cystic kidney disease. Originally, it was believed that renal macrophages were maintained from a constant supply of bone marrow–derived circulating monocytes, and could be recruited to the kidney in response to local inflammation. However, this idea has been challenged using fate-mapping methods, by showing that at least two distinct developmental origins of macrophages are present in the adult mouse kidney. The first type, infiltrating macrophages, are recruited from circulating monocytes and gradually develop macrophage properties on entering the kidney. The second, resident macrophages, predominantly originate from embryonic precursors, colonize the kidney during its development, and proliferate in situ to maintain their population throughout adulthood. Infiltrating and resident macrophages work together to maintain homeostasis and properly respond to pathologic conditions, such as AKI, cystic kidney disease, or infection. This review will briefly summarize current knowledge of resident macrophages in cystic kidney disease.
Macrophages in Kidney
Renal macrophages, the largest population of immune cells in kidney, play a critical role in homeostasis, surveillance, immune response, tissue injury, and repair (1–3). Macrophages are a major constituent of the renal mononuclear phagocyte system and were believed to arise from bone marrow–derived monocytes that could be polarized into different phenotypic subsets in response to environmental stimuli (4–6). However, they were often confused with dendritic cells due to their shared cell-surface expression of CD11c and MHCII (7,8). With the advent of new lineage tracing and single-cell RNA sequencing approaches, it is now possible to clearly delineate macrophages and dendritic cells in the kidney across mammalian species (9–11). In these studies, the authors demonstrated that renal macrophages were highly heterogenous and specialized populations and were derived from two different developmental origins (12–14) (Figure 1). One type, infiltrating macrophages, is derived from monocyte precursors in the bone marrow and recruited to the kidney in response to local inflammation (15,16). The other type, resident macrophages, maintain long-term residency in kidney with less mobility and arise primarily during organogenesis (17). They are derived in a Myb-independent manner from erythromyeloid progenitors that are first generated in the fetal yolk sac, colonize the fetal liver, and migrate into the kidney during early development (12,13,17).
Figure 1. |. The origins of macrophages in kidney.
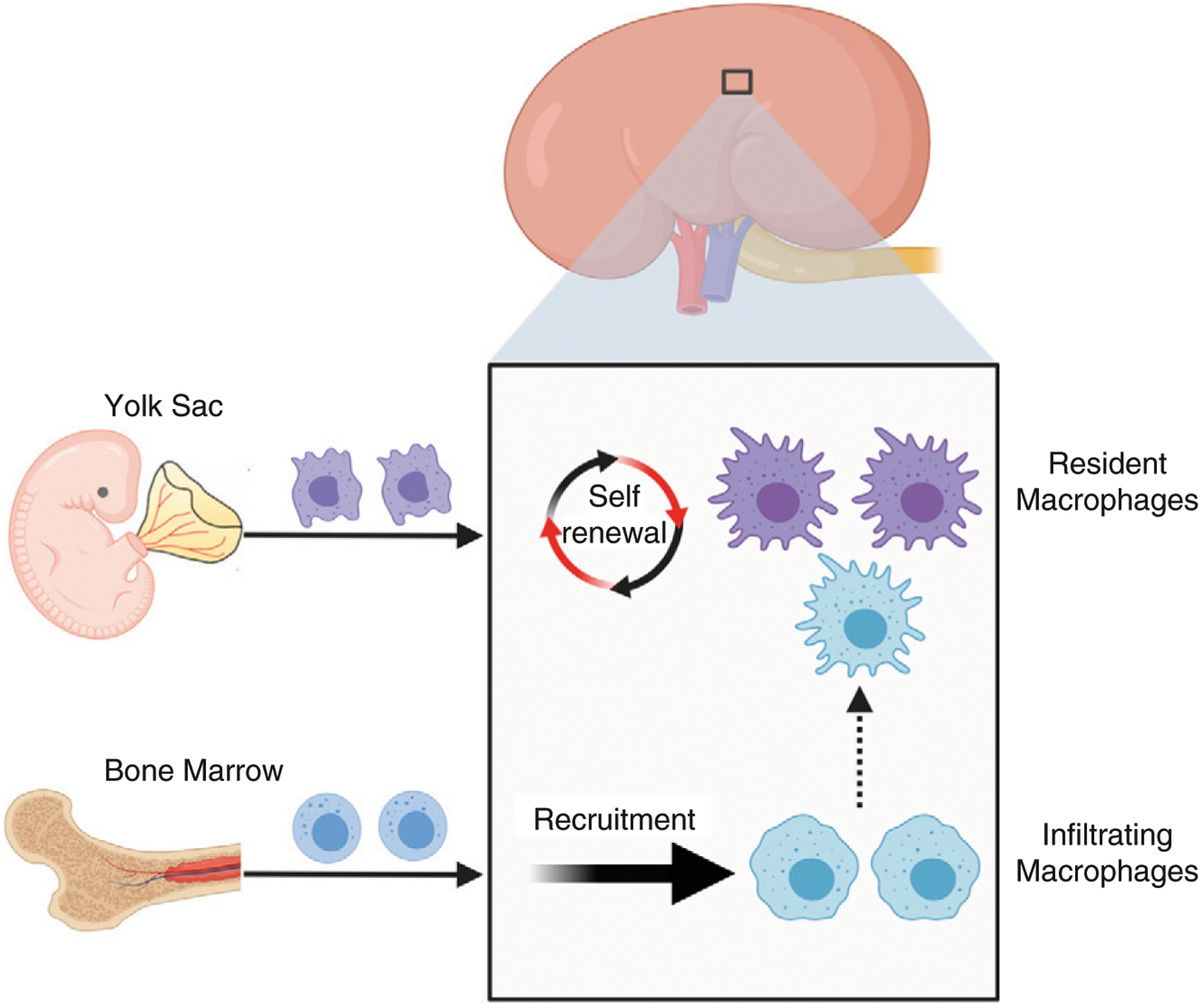
Two types of macrophages are present in the adult mouse kidney. Resident macrophages predominantly originate from embryonic precursors, migrate into the kidney during early development, and are maintained in the kidney through local proliferation. Infiltrating macrophages are derived from monocyte precursors in the bone marrow, and are recruited to the kidney in response to local inflammation. The dashed arrow indicates a limited but continuous contribution of monocyte-derived cells to the resident-macrophage pool in adult mouse kidneys.
The idea that resident macrophages are a homogenous population in the kidney and are exclusively derived from embryonic precursors has recently been challenged. Utilizing a newly generated cre-induced-hCD59 transgenic line, Liu et al. (18) traced the fate of resident macrophages in the kidney from birth to full maturity, and found that a portion of resident macrophages are actually derived from peripheral monocytes. The idea that some kidney-resident macrophages are derived from monocytes was supported by data from Ms4a3Cre-RosaTdT fate-mapping mice, which faithfully label all adult hematopoietic stem cell–derived monocytes (19). In these studies, the authors showed that a significant portion of kidney-resident macrophages were originated from adult hematopoietic stem cell–derived monocytes. Liu et al. (18) also demonstrated that both lineages of resident macrophages shared the feature of long-lived residency in the kidney, but had functional differences in aspects including immune response and metabolic profile in disease conditions. These data suggest kidney-resident macrophages can be derived from multiple precursor populations and their ontological origin may influence their function (6,17,18).
In mice, infiltrating and resident macrophages can be distinguished on the basis of the expression of surface markers F4/80 and CD11b, with resident macrophages being F4/80high, CD11blow and infiltrating macrophages being F4/80low, CD11bhigh (12,13,20). The exact function of resident macrophages in the kidney is not well known, although emerging evidence suggests they play an important role in kidney development, vascularization, and renal repair in response to AKI (17,21–24). Although there are limited data on the function of resident macrophages in the kidney, in part due to the nonspecific approaches used to study these cells in the past, we may be able to gain insight into their proposed functions due to similarities between M2-like macrophages and resident macrophages. M2 macrophages have an anti-inflammatory and profibrotic function (3,4,25); most resident macrophages also exhibit an M2-like phenotype, with intrinsic anti-inflammatory properties (3). In addition, renal resident macrophages share expression of CD206 and Arg1 with M2 macrophages (20,26), suggesting that M2 macrophages and resident macrophages are similar populations of cells. It has also been reported that CD206+ M2 macrophages can promote tubular regeneration by expressing growth factors during the reparative phase after AKI, which is similar to the function of resident macrophages in AKI (27–29). Although these cells share significant functional properties, direct evidence that M2 macrophages and resident macrophages are the same cell type is still lacking.
Overall, it is evident the renal macrophage niche in the kidney is more diverse than originally appreciated. To better understand the role of macrophages in kidney under normal and pathologic conditions, further studies are needed to characterize the respective functions of these macrophage subsets and the underlying molecular mechanisms involved.
Macrophages in Cystic Kidney Disease
Seminal studies in the field of cystic kidney disease highlighted an association between the number of renal macrophages and the severity of cystic disease (30–33). However, it was debated whether macrophages had a causative role in cyst formation or were a secondary consequence of cyst progression and expansion. Karihaloo et al. (34) provided the first evidence that macrophages could promote cyst progression in animal models utilizing a phagocytic poison, liposomal clodronate (LC), to deplete all macrophages in the kidney. The authors showed that treatment of cystic mice with LC not only significantly decreased the number of renal macrophages (95% reduction), but more importantly, reduced the cystic index and improved renal function when compared with vehicle-treated controls (Table 1). The conclusion that macrophages could promote cystic kidney disease was further supported by subsequent studies from Swenson-Fields et al. (35). In these studies, the authors showed that treatment with LC also reduced cystic disease in a recessive model of cystic kidney disease. These data suggested macrophages may be involved in promoting cyst progression in multiple forms of cystic kidney disease. However, because these studies removed all phagocytic cells in the kidney, which includes infiltrating macrophages, resident macrophages, and dendritic cells, the contribution of each subset to cyst progression was still unknown.
Table 1.
Effect of macrophage interventions on cystic kidney disease and their outcomes
| Animal Model | Intervention Method | Outcomes | Reference (Yr) |
|---|---|---|---|
| Pkd1fl/fl;Pkhd1-cre mice | LC was administrated on alternate d from PN10 to PN24. Mice were harvested at PN24. | LC treatment improved renal function and reduced cystic index by 24%. | 34 (2011) |
| cpk/cpk mice | LC was administrated at PN3 and PN6. Mice were harvested at PN10. | LC treatment improved renal function and reduced cortical cystic index by 29%. | 35 (2013) |
| Pck rats | Bindarit (MCP-1 synthesis inhibitor) was administrated daily from 5 to 15 wk. Mice were harvested at 15 wk. | No effect on cystic index or kidney function. | 40 (2015) |
| Hypomorphic Pkd1nl/nlmice or | ISO-1 (MIF inhibitor) was administrated daily from PN5 to PN27 in Pkd1nl/nl mice. Mice were harvested at PN28. | ISO-1 treatment improved renal function and reduced cystic index by 51%. | 38 (2015) |
| Mif−/−;Pkd1fl/fl;Ksp-cre mice (DKO) | Mif−/−;Pkd1fl/fl;Ksp-Cre mice (DKO) were harvested at PN7. | Mif−/−;Pkd1fl/fl;Ksp-cre mice had improved renal function and reduced cystic index by 58%. | |
| Pkd1fl/fl;Pax8-rtTA;TetO-cre or | INCB3344(CCR2 antagonist) was administrated daily from 6 wk to 12 wk of age in Pkd1fl/fl;Pax8- rtTA;TetO-Cremice. | INCB3344 treatment improved renal function and reduced cystic index by 31%. | 37 (2018) |
| Mcp1fl/fl;Pkd1fl/fl;Pax8-rtTA;TetO-cremice (DKO) | DKO mice were induced with doxycycline from PN28 to PN42. Mice were harvested at 18 wk. | Mcp1fl/fl;Pkd1fl/fl;Pax8-rtTA;TetO-Cre mice had improved renal function and reduced cystic index by 60%. | |
| Pkd1fl/fl;CAGG-creERT2 | LC was administrated daily from PN16 to PN23 (Stage 1) or from PN24 to PN32 (Stage 2) in PN10 induced Pkd1fl/fl;CAGG-CreERT2 mice. | LC treatment during Stage 1 did not affect renal cysts, but LC treatment during Stage 2 improved renal function and reduced cystic index by 60%. | 26 (2018) |
| Mcp1fl/fl;Pkd1fl/fl;Pax8-rtTA;TetO-cremice (DKO) | DKO were induced with doxycycline from PN28 to PN42. Mice were harvested at 13.5 wk. | Genetic deletion of Mcp1 in Pkd1fl/fl; Pax8-rtTA;TetO-Cre mice improved renal function and reduced cyst formation. | 36 (2018) |
| cpk/cpk;Ccl2−/− mice | cpk/cpk Ccl2−/− mice were harvested at PN18. | Genetic deletion of Ccl2 did not affect kidney cysts, but prolonged survival of mice. | 41 (2019) |
| Ift88fl/fl;CAGG-CreERT2 mice with ischemia-reperfusion (IR) injury | GW2580 (CSF1R kinase inhibitor) was administrated daily from d 1 to d 35 post IR injury in adult induced Ift88fl/fl;CAGGCre ERT2 mice. | GW2580 treatment reduced cystic index by 60%. | 20 (2019) |
| cpk/cpk mice | GW2580 was administrated daily from PN11 to PN21 in cpk/cpk mice. | GW2580 treatment on cpk mice reduced rate of growth in TKV (slope) by 37%. | |
| Pkd1fl/fl;CAGG-CreERT2 mice with UNX | IRF5 ASO was given weekly to Pkd1fl/fl;CAGG-CreERT2 mice for 6 wk post nephrectomy. | IRF5 ASO treatment reduced cystic index by 50%. | 51 (2020) |
LC, liposomal clodronate; MCP, monocyte chemoattractant; MIF, migration inhibitory factor; DKO, double knockout; INCB, International Narcotics Control Board; UNX, unilateral nephrectomy; TKV, total kidney volume; ASO, antisense oligo.
There was a long-held belief that renal macrophages are derived from, and continually replenished by, circulating monocytes. This has led to the idea that targeting the recruitment of monocytes to the kidney may be therapeutically beneficial in the context of cystic kidney disease. This hypothesis is supported by data indicating that genetic deletion or pharmacologic inhibition of Ccl2 (also known as monocyte chemoattractant-1 or MCP-1) and macrophage migration inhibitory factor reduce the severity of cystic kidney disease (36–38). More importantly, the level of cyst reduction using macrophage-targeted therapeutics was comparable to other inhibitors commonly used in the PKD field, such as tolvaptan or rapamycin (39,40). Whereas several studies indicate that inhibition of these macrophage ligand:receptor interactions restricts cyst growth, other studies did not report similar findings (41–43). The involvement of infiltrating macrophages in cystic disease remains controversial, and has been reviewed previously (44–47). For comparison, we include a table that summarizes the outcomes from multiple studies targeting macrophages in cyst severity (Table 1).
Resident-Macrophage Involvement in Cystic Kidney Disease
In retrospect, data suggested the presence and involvement of resident macrophages in cystic kidney disease for several decades. For example, multiple studies indicate that alternatively activated M2-like macrophages expressing CD206 (26,35,48), a newly identified cross-species marker of resident macrophages (11,49), could promote renal cyst formation. Microarray analysis from Mrug et al. (50) found that in a nonorthologous model of autosomal recessive polycystic kidney disease (Cys1cpk/cpk), the most highly expressed genes were those associated with the innate immune response. Of interest, several of these innate immune genes were recently identified as being specific for resident macrophages by single-cell RNA sequencing (C1qa, Cxcl16) (11). Also, Viau et al. (36) found increased numbers of both infiltrating and resident macrophages during cyst progression in inducible Lkb1 and Pkd1 mouse models. Although the authors emphasized the importance of infiltrating macrophages in controlling cyst progression in this study, they also observed an increased number of resident macrophages expressing CCR2 (receptor for MCP-1) in cystic kidneys, suggesting the MCP1-CCR2 axis may also be important for resident macrophages.
Zimmerman et al. (20) provided evidence for the involvement of resident macrophages in cystic disease using conditional Ift88 cilia mutant mice. These mutants develop a cystic kidney disease phenotype, where the severity is greatly influenced by the age at which cilia loss is induced (51). Ift88 loss induced in juvenile periods (before postnatal day 14) leads to rapid and severe cyst formation. In contrast, induction in adults results in slow cyst progression occurring in focal regions of the kidney. The rate of cyst formation in adult-induced mutants was found to be greatly increased and more widespread after ischemia-reperfusion injury. In correlation with these rates of cystogenesis, the authors showed that resident macrophages in wild-type mouse kidneys undergo a phenotypic switch (from CD11clow to CD11chigh) during the postnatal period. CD11clow resident macrophages are enriched during juvenile periods, negligible in adult mice, and reappeared after renal ischemia-reperfusion injury in adult cilia mutant mice. More importantly, the number of CD11clow resident macrophages was increased before and during cyst formation, suggesting a potential causative or at least contributing role for these cells in cyst formation. Analysis of confocal images indicated most resident macrophages coexpressed F4/80, Ki67, and CD206 in cystic regions, suggesting a reciprocal communication between resident macrophages and cyst-lining epithelial cells (Figure 2). To understand the mechanism through which resident macrophages were accumulating, the authors performed parabiosis experiments by joining the circulation of a CD45.2 control or cilia mutant mouse with a congenic CD45.1 wild-type mouse and found the accumulation of resident macrophages in cilia mutant kidneys was independent of peripheral blood input. Analysis of cell proliferation using Ki67 indicated that accumulation of resident macrophages in injured cilia mutant mice was largely driven by self-proliferation. To determine the cell type that was responsible for driving resident macrophage proliferation, the authors flow sorted epithelial populations and showed the expression of membrane-bound colony stimulation factor 1 was significantly increased in the proximal tubule cells of the injured cilia mutant kidneys compared with injured controls (Figure 2). Inhibition of CSF1R kinase signaling, using GW2580 (52), reduced resident macrophage proliferation, prevented the accumulation of CD11clow resident macrophages, and reduced the severity of cystic disease in the injured conditional Ift88 model and in the more rapidly progressing cpk mouse model. Interestingly, GW2580 treatment did not affect the infiltrating macrophage number, suggesting the effects of GW2580 were resident-macrophage specific.
Figure 2. |. Proposed functions of resident macrophages during cyst formation and expansion.
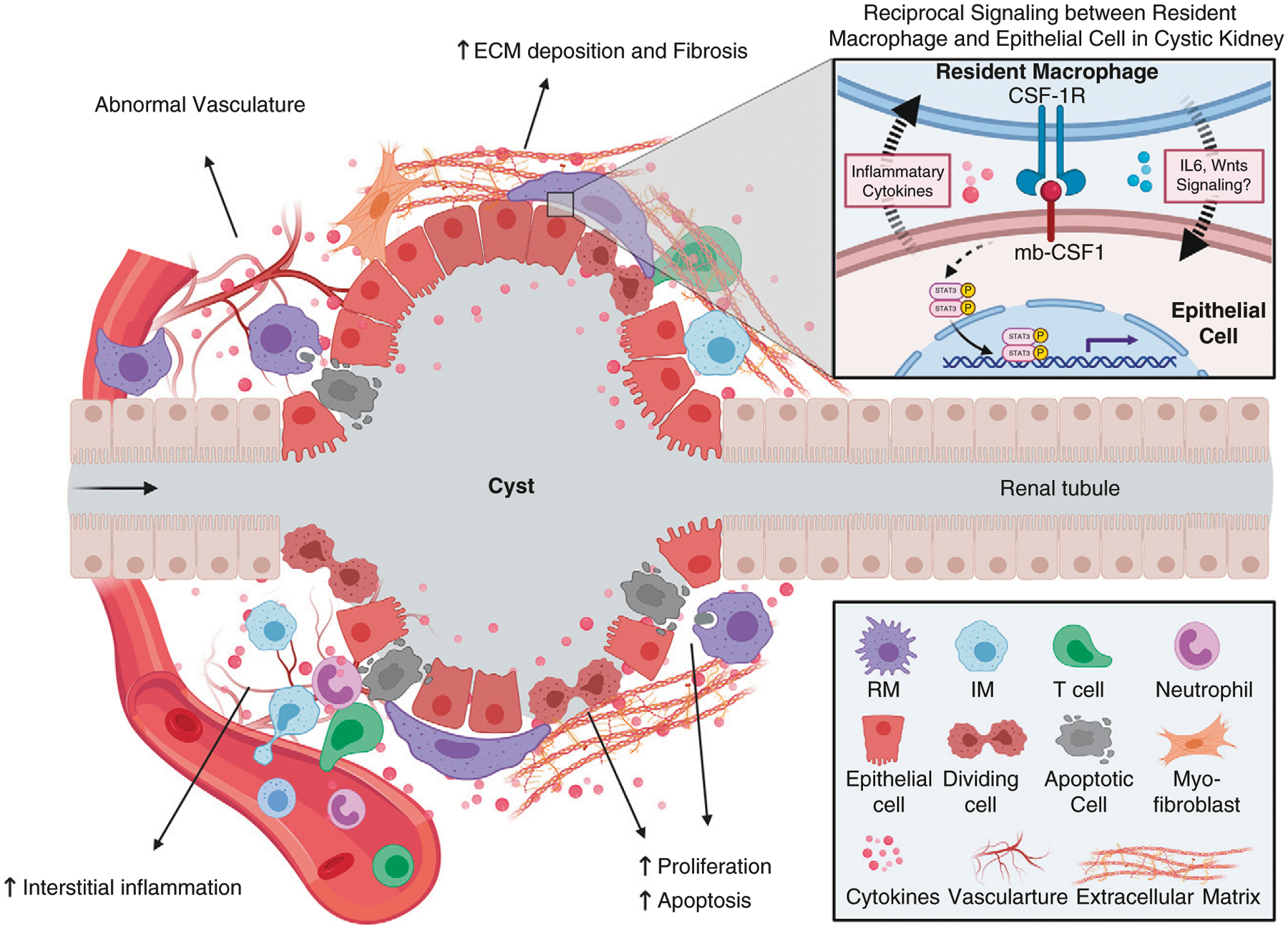
Multiple processes occur during cyst formation and expansion, including increased epithelial proliferation and apoptosis, interstitial inflammation, increased extracellular matrix deposition and renal fibrosis, and vasculature abnormalities. Resident macrophages may be involved in controlling several of these processes directly or indirectly. It has been proposed that resident macrophages can promote cystic epithelial proliferation by secretion of cytokines and phagocytosis of apoptotic epithelial cells. Also, renal-resident macrophages may drive interstitial myofibroblast activation and proliferation, leading to increased extracellular matrix deposition and renal fibrosis. Resident macrophages may serve as “first responders” in the kidney and control the accumulation and effector function of other immune cells, such as neutrophils, infiltrating macrophages, and T cells, to indirectly regulate cyst formation. Finally, renal resident macrophages may also regulate vasculature abnormalities through their proposed proangiogenic functions. The inset indicates the reciprocal communication between resident macrophages and the cilia mutant epithelium via mb-CSF1/CSF-1R. ECM, extracellular matrix; mb-CSF1, membrane-bound colony-stimulating factor-1; RM, resident macrophages; IM, infiltrating macrophages.
Data showing that resident macrophages could promote cystic disease were supported by follow-up studies investigating the involvement of macrophage subsets in an orthologous mouse model of autosomal dominant polycystic kidney disease (ADPKD) (53). Using conditional Pkd1 mice with unilateral nephrectomy, Zimmerman and colleagues showed the numbers of infiltrating and resident macrophages were increased in conditional Pkd1 mice with unilateral nephrectomy compared with sham-operated mice, and that the increase occurred before the onset of severe cystogenesis. Furthermore, the authors showed that IFN regulatory factor 5 (Irf5), a transcription factor known to induce inflammatory cytokine production in macrophages (54), was increased in infiltrating and resident macrophages in cystic kidneys. To identify the function of IRF5 in macrophages and its importance in cyst formation, the authors suppressed the expression of IRF5 with an antisense oligo (ASO) treatment and found that IRF5 suppression decreased the number of kidney-resident macrophages, reduced inflammatory gene expression, and reduced cyst growth. More careful characterization of infiltrating and resident macrophages showed that IRF5 ASO treatment specifically reduced Irf5 and Il6 expression in resident macrophages, but did not affect their expression in infiltrating macrophages. More importantly, the authors found that IRF5 ASO-treated mice have reduced STAT3 phosphorylation and expression of p-STAT3 target genes compared with vehicle treated mice suggesting that IRF5-expressing resident macrophages released inflammatory cytokines (IL-6) stimulating STAT3 phosphorylation in the epithelium thereby promoting cyst growth in mice lacking Pkd1.
The data presented in this review suggest the involvement of resident macrophages in cystic kidney disease. However, the exact mechanism by which resident macrophages influence cyst growth is largely unknown. Although Zimmerman and colleagues provided evidence that cytokines such as IL6 may influence cyst growth through a STAT-dependent mechanism, other direct or indirect mechanisms are likely involved in regulating cyst formation and disease progression (55) (Figure 2). For example, resident macrophages control injury and repair processes in the kidney by promoting tubular epithelial cell proliferation and de-differentiation, which are hallmarks of renal cyst formation (55). RNA sequencing of resident macrophages after AKI indicated transcriptional reprogramming of resident macrophages, including upregulation of several Wnt genes (Wnt4, Wnt7b, Wnt10a, and Wnt10b) (21). Wnt-induced β-catenin signaling can protect against epithelial apoptosis and promote proliferative repair (27,56,57). In addition, Wnt signaling can also drive interstitial myofibroblast activation and proliferation, leading to increased matrix-protein deposition and renal fibrosis (56,58). These data suggest that resident macrophage–derived Wnts promote the proliferation of cystic epithelium and drive interstitial fibrosis during cystic disease progression.
It is also possible that resident macrophages serve as “first responders” in the kidney and control the accumulation and effector function of other immune cells, such as neutrophils, infiltrating macrophages, and T cells, which have all been observed in patients and mouse models of cystic kidney disease (47,59–62). In fact, resident macrophages are well suited for this role as they are able to maintain a persistent residence in an adult kidney through self-proliferation and are located directly adjacent to the tubular epithelium (63). Thus, they may serve as sentinels in the kidney to regulate the accumulation of other immune cells that influence cyst growth and progression. Indeed, due to their residency advantage, resident macrophages can act more rapidly than neutrophils, which have always been regarded as first responders in kidney injury. Using a combination of intravital imaging and confocal multiplex microscopy, Uderhardt et al. (24) observed that resident macrophages exert a “cloaking” behavior by extending pseudopods around a local injury, which will prevent injury-induced neutrophil activation and neutrophil-driven inflammation. Thus, it is possible resident macrophages serve a similar role in cystic disease.
In addition, epithelial apoptosis is detected during cyst formation (64) and macrophages are known to be professional phagocytes. Thus, resident macrophages may control cyst growth through phagocytosis of damaged epithelial cells and subsequent activation of inflammatory signaling pathways. This is supported by data showing that resident macrophages can detect and scavenge immune complexes or foreign debris in the interstitium, and upregulate several inflammatory signaling pathways including NF-κB and JAK/STAT, both of which are associated with worsened cystic disease (22,65).
Moreover, resident macrophages could also contribute to cyst progression through other mechanisms, including regulation of vasculature abnormalities through their proposed proangiogenic functions (23,66,67). It is also possible resident macrophages directly or indirectly regulate fluid secretion as data suggest macrophage-derived cytokines mediate the localization and activity of multiple ion channels in kidney and other tissues (68–71). All of these processes have been reported to be involved in cystic kidney disease (72,73).
Targeting Resident Macrophages as a Potential Therapeutic Intervention
Inhibition or reduction of resident-macrophage numbers has beneficial effects both on cyst burden and disease progression in multiple preclinical models (20,53). However, because the mechanism of resident-macrophage involvement in cyst growth is unknown, and the approaches for identifying these cells across species are difficult, there have been significant limitations in targeting these cells in patients with cystic kidney disease. Despite these limitations, it should be noted that inhibition of two proinflammatory signaling pathways that are present in resident macrophages, the NF-κB and JAK/STAT pathway, has significantly ameliorated cystic severity in animal models (45,46). For example, a STAT3 inhibitor, S3I-201, significantly inhibited cyst formation and growth in a neonatal PKD mouse model (74). In addition, triptolide has well-known anti-inflammatory effects through inhibiting NF-κB transactivation and its beneficial effect on cystogenesis in ADPKD mouse models has been reported for decades (75,76). Results from a phase 3 clinical trial of triptolide in ADPKD (NCT02115659) are much anticipated (https://clinicaltrials.gov/ct2/show/NCT02115659).
Another caveat to understanding resident-macrophage involvement is the difficulty of translating resident-macrophage–focused animal studies to humans. This difficulty is due to the fact that markers used to delineate mouse macrophages (i.e., F4/80) are not expressed by their human counterparts, making it challenging to identify analogous populations between species. Also, due to the lack of an appropriate method, there is no way to distinguish monocyte-derived infiltrating macrophages from embryonically seeded resident macrophages in the human kidney. Utilizing the recently developed single-cell RNA sequencing technique, Zimmerman et al. (11) identified a cross-species kidney resident-macrophage–specific gene expression signature by sequencing CD45+ cells isolated from mouse, rat, pig, and human kidney tissue. As part of this signature, the authors identified C1QC, CD81, and CD74 as novel, cross-species markers of resident macrophages. The authors went on to show these markers were expressed in mouse resident macrophages at the protein level, and were coexpressed at the protein level in a population of CD45+ cells isolated from rats and humans. Thus, it is likely that resident macrophages are present in other species and C1q, CD81, and CD74 can be used to identify these cells.
The identification of resident macrophages in human kidneys will greatly facilitate clinically relevant translational research from murine models to human patients. Macrophage targeting as a potential therapeutic intervention has been implicated and has led to promising results in preclinical models of inflammatory diseases and cancer (77). However, specifically targeting resident macrophages in the kidney of patients is extremely challenging, due to the lack of precise approaches to deplete kidney resident macrophages from their useful counterparts in other tissues. Thus, any resident-macrophage inhibitors would deplete resident macrophages in all tissues where they are essential for basic biologic functions such as synapse pruning, cardiac electrical conduction, and preventing infections (78,79). Therefore, it is critical to develop kidney-specific approaches to selectively deplete resident macrophages.
Conclusions and Future Perspectives
In summary, studies have shown the involvement of renal resident macrophages in cyst progression and that targeting resident macrophages using genetic deletion or pharmacologic inhibition is a promising therapeutic target for reducing cyst growth. Understating the function of resident macrophages in physiologic and pathologic conditions is important to reveal their mechanism of action in cystic kidney disease and translate these novel mechanisms to benefit patients with cystic kidney disease.
Acknowledgments
The authors are thankful to Dr. Courtney Haycraft and members of Dr. B.K. Yoder’s laboratory for providing insightful comments during the preparation of this manuscript. Figures in this review were created with BioRender.com.
Funding
These studies were supported in part by University of Alabama at Birmingham (UAB) School of Medicine AMC21 grant (to B.K. Yoder); Polycystic Kidney Disease Research Foundation grant 214g16a (to B.K. Yoder); National Institute of Diabetes and Digestive and Kidney Diseases grants R01DK115752 (to B.K. Yoder) and K01DK119375 (to K.A. Zimmerman); a pilot and feasibility grant from the Baltimore PKD Center 2P30DK090868 (to K.A. Zimmerman); a pilot grant from the UAB Hepato/Renal Fibrocystic Disease Core Center 5P30DK074038 (to K.A. Zimmerman); a seed grant from the Presbyterian Health Foundation (to K.A. Zimmerman); and a pilot grant from Oklahoma Center for Microbial Pathogenesis and Immunity (1P20GM134973; to K.A. Zimmerman).
Footnotes
Disclosures
All authors have nothing to disclose.
References
- 1.Duffield JS: Macrophages and immunologic inflammation of the kidney. Semin Nephrol 30: 234–254, 2010. 10.1016/j.semnephrol.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baek J-H: The impact of versatile macrophage functions on acute kidney injury and its outcomes. Front Physiol 10: 1016, 2019. 10.3389/fphys.2019.01016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen T, Cao Q, Wang Y, Harris DCH: M2 macrophages in kidney disease: Biology, therapies, and perspectives. Kidney Int 95: 760–773, 2019. 10.1016/j.kint.2018.10.041 [DOI] [PubMed] [Google Scholar]
- 4.Italiani P, Boraschi D: From monocytes to M1/M2 macrophages: Phenotypical vs. functional differentiation. Front Immunol 5: 514, 2014. 10.3389/fimmu.2014.00514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray PJ: Macrophage polarization. Annu Rev Physiol 79: 541–566, 2017. 10.1146/annurev-physiol-022516-034339 [DOI] [PubMed] [Google Scholar]
- 6.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I: Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159: 1312–1326, 2014. 10.1016/j.cell.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George JF, Lever JM, Agarwal A: Mononuclear phagocyte sub-populations in the mouse kidney. Am J Physiol Renal Physiol 312: F640–F646, 2017. 10.1152/ajprenal.00369.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merad M, Sathe P, Helft J, Miller J, Mortha A: The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 31: 563–604, 2013. 10.1146/annurev-immunol-020711-074950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K: Development of monocytes, macrophages, and dendritic cells. Science 327: 656–661, 2010. 10.1126/science.1178331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salei N, Rambichler S, Salvermoser J, Papaioannou NE, Schuchert R, Pakalniškytė D, Li N, Marschner JA, Lichtnekert J, Stremmel C, Cernilogar FM, Salvermoser M, Walzog B, Straub T, Schotta G, Anders HJ, Schulz C, Schraml BU: The kidney contains onto-genetically distinct dendritic cell and macrophage subtypes throughout development that differ in their inflammatory properties. J Am Soc Nephrol 31: 257–278, 2020. 10.1681/ASN.2019040419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmerman KA, Bentley MR, Lever JM, Li Z, Crossman DK, Song CJ, Liu S, Crowley MR, George JF, Mrug M, Yoder BK: Single-cell RNA sequencing identifies candidate renal resident macrophage gene expression signatures across species. J Am Soc Nephrol 30: 767–781, 2019. 10.1681/ASN.2018090931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F: A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336: 86–90, 2012. 10.1126/science.1219179 [DOI] [PubMed] [Google Scholar]
- 13.Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, Beaudin AE, Lum J, Low I, Forsberg EC, Poidinger M, Zolezzi F, Larbi A, Ng LG, Chan JK, Greter M, Becher B, Samokhvalov IM, Merad M, Ginhoux F: C-Myb(1) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 42: 665–678, 2015. 10.1016/j.immuni.2015.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoeffel G, Ginhoux F: Ontogeny of tissue-resident macrophages. Front Immunol 6: 486, 2015. 10.3389/fimmu.2015.00486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang HS, Kim JI, Jung KJ, Kim J, Han KH, Park KM: Bone marrow-derived cells play a major role in kidney fibrosis via proliferation and differentiation in the infiltrated site. Biochim Biophys Acta 1832: 817–825, 2013. 10.1016/j.bbadis.2013.02.016 [DOI] [PubMed] [Google Scholar]
- 16.Sheng J, Ruedl C, Karjalainen K: Most tissue-resident macrophages except microglia are derived from fetal hematopoietic stem cells. Immunity 43: 382–393, 2015. 10.1016/j.immuni.2015.07.016 [DOI] [PubMed] [Google Scholar]
- 17.Munro DAD, Hughes J: The origins and functions of tissue-resident macrophages in kidney development. Front Physiol 8: 837, 2017. 10.3389/fphys.2017.00837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu F, Dai S, Feng D, Qin Z, Peng X, Sakamuri SSVP, Ren M, Huang L, Cheng M, Mohammad KE, Qu P, Chen Y, Zhao C, Zhu F, Liang S, Aktas BH, Yang X, Wang H, Katakam PVG, Busija DW, Fischer T, Datta PK, Rappaport J, Gao B, Qin X: Distinct fate, dynamics and niches of renal macrophages of bone marrow or embryonic origins. Nat Commun 11: 2280, 2020. 10.1038/s41467-020-16158-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Gu Y, Chakarov S, Bleriot C, Kwok I, Chen X, Shin A, Huang W, Dress RJ, Dutertre CA, Schlitzer A, Chen J, Ng LG, Wang H, Liu Z, Su B, Ginhoux F: Fate mapping via ms4a3-expression history traces monocyte-derived cells. Cell 178: 1509–1525.e19, 2019. 10.1016/j.cell.2019.08.009 [DOI] [PubMed] [Google Scholar]
- 20.Zimmerman KA, Song CJ, Li Z, Lever JM, Crossman DK, Rains A, Aloria EJ, Gonzalez NM, Bassler JR, Zhou J, Crowley MR, Revell DZ, Yan Z, Shan D, Benveniste EN, George JF, Mrug M, Yoder BK: Tissue-resident macrophages promote renal cystic disease. J Am Soc Nephrol 30: 1841–1856, 2019. 10.1681/ASN.2018080810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lever JM, Hull TD, Boddu R, Pepin ME, Black LM, Adedoyin OO, Yang Z, Traylor AM, Jiang Y, Li Z, Peabody JE, Eckenrode HE, Crossman DK, Crowley MR, Bolisetty S, Zimmerman KA, Wende AR, Mrug M, Yoder BK, Agarwal A, George JF: Resident macrophages reprogram toward a developmental state after acute kidney injury. JCI Insight 4: e125503, 2019. 10.1172/jci.insight.125503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stamatiades EG, Tremblay ME, Bohm M, Crozet L, Bisht K, Kao D, Coelho C, Fan X, Yewdell WT, Davidson A, Heeger PS, Diebold S, Nimmerjahn F, Geissmann F: Immune monitoring of trans-endothelial transport by kidney-resident macrophages. Cell 166: 991–1003, 2016. 10.1016/j.cell.2016.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munro DAD, Wineberg Y, Tarnick J, Vink CS, Li Z, Pridans C, Dzierzak E, Kalisky T, Hohenstein P, Davies JA: Macrophages restrict the nephrogenic field and promote endothelial connections during kidney development. eLife 8: e43271, 2019. 10.7554/eLife.43271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uderhardt S, Martins AJ, Tsang JS, Lämmermann T, Germain RN: Resident macrophages cloak tissue microlesions to prevent neutrophil-driven inflammatory damage. Cell 177: 541–555.e17, 2019. 10.1016/j.cell.2019.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rőszer T: Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm 2015: 816460, 2015. 10.1155/2015/816460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Chen M, Zhou J, Lv J, Song S, Fu L, Chen J, Yang M, Mei C: Interactions between macrophages and cyst-lining epithelial cells promote kidney cyst growth in Pkd1-deficient mice. J Am Soc Nephrol 29: 2310–2325, 2018. 10.1681/ASN.2018010074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS: Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A 107: 4194–4199, 2010. 10.1073/pnas.0912228107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao Q, Harris DC, Wang Y: Macrophages in kidney injury, inflammation, and fibrosis. Physiology (Bethesda) 30: 183–194, 2015. 10.1152/physiol.00046.2014 [DOI] [PubMed] [Google Scholar]
- 29.Ferenbach DA, Sheldrake TA, Dhaliwal K, Kipari TM, Marson LP, Kluth DC, Hughes J: Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/ reperfusion injury in mice. Kidney Int 82: 928–933, 2012. 10.1038/ki.2012.207 [DOI] [PubMed] [Google Scholar]
- 30.Cowley BD Jr, Gudapaty S, Kraybill AL, Barash BD, Harding MA, Calvet JP, Gattone VH 2nd: Autosomal-dominant polycystic kidney disease in the rat. Kidney Int 43: 522–534, 1993. 10.1038/ki.1993.79 [DOI] [PubMed] [Google Scholar]
- 31.Vogler C, Homan S, Pung A, Thorpe C, Barker J, Birkenmeier EH, Upadhya P: Clinical and pathologic findings in two new allelic murine models of polycystic kidney disease. J Am Soc Nephrol 10: 2534–2539, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Cowley BD Jr, Ricardo SD, Nagao S, Diamond JR: Increased renal expression of monocyte chemoattractant protein-1 and osteopontin in ADPKD in rats. Kidney Int 60: 2087–2096, 2001. 10.1046/j.1523-1755.2001.00065.x [DOI] [PubMed] [Google Scholar]
- 33.Phillips JK, Hopwood D, Loxley RA, Ghatora K, Coombes JD, Tan YS, Harrison JL, McKitrick DJ, Holobotvskyy V, Arnolda LF, Rangan GK: Temporal relationship between renal cyst development, hypertension and cardiac hypertrophy in a new rat model of autosomal recessive polycystic kidney disease. Kidney Blood Press Res 30: 129–144, 2007. 10.1159/000101828 [DOI] [PubMed] [Google Scholar]
- 34.Karihaloo A, Koraishy F, Huen SC, Lee Y, Merrick D, Caplan MJ, Somlo S, Cantley LG: Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 22: 1809–1814, 2011. 10.1681/ASN.2011010084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swenson-Fields KI, Vivian CJ, Salah SM, Peda JD, Davis BM, van Rooijen N, Wallace DP, Fields TA: Macrophages promote polycystic kidney disease progression. Kidney Int 83: 855–864, 2013. 10.1038/ki.2012.446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viau A, Bienaimé F, Lukas K, Todkar AP, Knoll M, Yakulov TA, Hofherr A, Kretz O, Helmstädter M, Reichardt W, Braeg S, Aschman T, Merkle A, Pfeifer D, Dumit VI, Gubler MC, Nitschke R, Huber TB, Terzi F, Dengjel J, Grahammer F, Köttgen M, Busch H, Boerries M, Walz G, Triantafyllopoulou A, Kuehn EW: Cilia-localized LKB1 regulates chemokine signaling, macrophage recruitment, and tissue homeostasis in the kidney. EMBO J 37: e98615, 2018. 10.15252/embj.201798615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cassini MF, Kakade VR, Kurtz E, Sulkowski P, Glazer P, Torres R, Somlo S, Cantley LG: Mcp1 promotes macrophage-dependent cyst expansion in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 29: 2471–2481, 2018. 10.1681/ASN.2018050518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L, Zhou X, Fan LX, Yao Y, Swenson-Fields KI, Gadjeva M, Wallace DP, Peters DJ, Yu A, Grantham JJ, Li X: Macrophage migration inhibitory factor promotes cyst growth in polycystic kidney disease. J Clin Invest 125: 2399–2412, 2015. 10.1172/JCI80467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HJ, Edelstein CL: Mammalian target of rapamycin inhibition in polycystic kidney disease: From bench to bedside. Kidney Res Clin Pract 31: 132–138, 2012. 10.1016/j.krcp.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torres VE: Vasopressin antagonists in polycystic kidney disease. Semin Nephrol 28: 306–317, 2008. 10.1016/j.semnephrol.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zimmerman KA, Song CJ, Gonzalez-Mize N, Li Z, Yoder BK: Primary cilia disruption differentially affects the infiltrating and resident macrophage compartment in the liver. Am J Physiol Gastrointest Liver Physiol 314: G677–G689, 2018. 10.1152/ajpgi.00381.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zoja C, Corna D, Locatelli M, Rottoli D, Pezzotta A, Morigi M, Zanchi C, Buelli S, Guglielmotti A, Perico N, Remuzzi A, Remuzzi G: Effects of MCP-1 inhibition by bindarit therapy in a rat model of polycystic kidney disease. Nephron 129: 52–61, 2015. 10.1159/000369149 [DOI] [PubMed] [Google Scholar]
- 43.Salah SM, Meisenheimer JD, Rao R, Peda JD, Wallace DP, Foster D, Li X, Li X, Zhou X, Vallejo JA, Wacker MJ, Fields TA, Swenson-Fields KI: MCP-1 promotes detrimental cardiac physiology, pulmonary edema, and death in the cpk model of polycystic kidney disease. Am J Physiol Renal Physiol 317: F343–F360, 2019. 10.1152/ajprenal.00240.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karihaloo A: Role of inflammation in polycystic kidney disease In: Polycystic Kidney Disease, edited by Li X, Brisbane (AU), Codon Publications, 2015. 10.15586/codon.pkd.2015.ch14 [DOI] [PubMed] [Google Scholar]
- 45.Ta MH, Harris DC, Rangan GK: Role of interstitial inflammation in the pathogenesis of polycystic kidney disease. Nephrology (Carlton) 18: 317–330, 2013. 10.1111/nep.12045 [DOI] [PubMed] [Google Scholar]
- 46.Song CJ, Zimmerman KA, Henke SJ, Yoder BK: Inflammation and fibrosis in polycystic kidney disease. Results Probl Cell Differ 60: 323–344, 2017. 10.1007/978-3-319-51436-9_12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zimmerman KA, Hopp K, Mrug M: Role of chemokines, innate and adaptive immunity. Cell Signal 73: 109647, 2020. 10.1016/j.cellsig.2020.109647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lakhia R, Yheskel M, Flaten A, Ramalingam H, Aboudehen K, Ferrè S, Biggers L, Mishra A, Chaney C, Wallace DP, Carroll T, Igarashi P, Patel V: Interstitial microRNA miR-214 attenuates inflammation and polycystic kidney disease progression. JCI Insight 5: e133785, 2020. 10.1172/jci.insight.133785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stewart BJ, Ferdinand JR, Young MD, Mitchell TJ, Loudon KW, Riding AM, Richoz N, Frazer GL, Staniforth JUL, Vieira Braga FA, Botting RA, Popescu DM, Vento-Tormo R, Stephenson E, Cagan A, Farndon SJ, Polanski K, Efremova M, Green K, Del Castillo Velasco-Herrera M, Guzzo C, Collord G, Mamanova L, Aho T, Armitage JN, Riddick ACP, Mushtaq I, Farrell S, Rampling D, Nicholson J, Filby A, Burge J, Lisgo S, Lindsay S, Bajenoff M, Warren AY, Stewart GD, Sebire N, Coleman N, Haniffa M, Teichmann SA, Behjati S, Clatworthy MR: Spatiotemporal immune zonation of the human kidney. Science 365: 1461–1466, 2019. 10.1126/science.aat5031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mrug M, Zhou J, Woo Y, Cui X, Szalai AJ, Novak J, Churchill GA, Guay-Woodford LM: Overexpression of innate immune response genes in a model of recessive polycystic kidney disease. Kidney Int 73: 63–76, 2008. 10.1038/sj.ki.5002627 [DOI] [PubMed] [Google Scholar]
- 51.Sharma N, Malarkey EB, Berbari NF, O’Connor AK, Vanden Heuvel GB, Mrug M, Yoder BK: Proximal tubule proliferation is insufficient to induce rapid cyst formation after cilia disruption. J Am Soc Nephrol 24: 456–464, 2013. 10.1681/ASN.2012020154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conway JG, McDonald B, Parham J, Keith B, Rusnak DW, Shaw E, Jansen M, Lin P, Payne A, Crosby RM, Johnson JH, Frick L, Lin MH, Depee S, Tadepalli S, Votta B, James I, Fuller K, Chambers TJ, Kull FC, Chamberlain SD, Hutchins JT: Inhibition of colony-stimulating-factor-1 signaling in vivo with the orally bioavailable cFMS kinase inhibitor GW2580. Proc Natl Acad Sci U S A 102: 16078–16083, 2005. 10.1073/pnas.0502000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zimmerman KA, Huang J, He L, Revell DZ, Li Z, Hsu J-S, Fitzgibbon WR, Hazard ES, Hardiman G, Mrug M, Bell PD, Yoder BK, Saigusa T: Interferon regulatory factor-5 in resident macrophage promotes polycystic kidney disease. Kidney360 1: 179–190, 2020. 10.34067/KID.0001052019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weiss M, Blazek K, Byrne AJ, Perocheau DP, Udalova IA: IRF5 is a specific marker of inflammatory macrophages in vivo. Mediators Inflamm 2013: 245804, 2013. 10.1155/2013/245804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harris PC, Torres VE: Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J Clin Invest 124: 2315–2324, 2014. 10.1172/JCI72272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou D, Tan RJ, Fu H, Liu Y: Wnt/β-catenin signaling in kidney injury and repair: A double-edged sword. Lab Invest 96: 156–167, 2016. 10.1038/labinvest.2015.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whyte JL, Smith AA, Helms JA: Wnt signaling and injury repair. Cold Spring Harb Perspect Biol 4: a008078, 2012. 10.1101/cshperspect.a008078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maarouf OH, Aravamudhan A, Rangarajan D, Kusaba T, Zhang V, Welborn J, Gauvin D, Hou X, Kramann R, Humphreys BD: Paracrine Wnt1 drives interstitial fibrosis without inflammation by tubulointerstitial cross-talk. J Am Soc Nephrol 27: 781–790, 2016. 10.1681/ASN.2014121188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prasad S, McDaid JP, Tam FW, Haylor JL, Ong AC: Pkd2 dosage influences cellular repair responses following ischemia-reperfusion injury. Am J Pathol 175: 1493–1503, 2009. 10.2353/ajpath.2009.090227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bernhardt WM, Wiesener MS, Weidemann A, Schmitt R, Weichert W, Lechler P, Campean V, Ong AC, Willam C, Gretz N, Eckardt KU: Involvement of hypoxia-inducible transcription factors in polycystic kidney disease. Am J Pathol 170: 830–842, 2007. 10.2353/ajpath.2007.060455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kleczko EK, Marsh KH, Tyler LC, Furgeson SB, Bullock BL, Altmann CJ, Miyazaki M, Gitomer BY, Harris PC, Weiser-Evans MCM, Chonchol MB, Clambey ET, Nemenoff RA, Hopp K: CD8+ T cells modulate autosomal dominant polycystic kidney disease progression. Kidney Int 94: 1127–1140, 2018. 10.1016/j.kint.2018.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zimmerman KA, Gonzalez NM, Chumley P, Chacana T, Harrington LE, Yoder BK, Mrug M: Urinary T cells correlate with rate of renal function loss in autosomal dominant polycystic kidney disease. Physiol Rep 7: e13951, 2019. 10.14814/phy2.13951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Franken L, Schiwon M, Kurts C: Macrophages: Sentinels and regulators of the immune system. Cell Microbiol 18: 475–487, 2016. 10.1111/cmi.12580 [DOI] [PubMed] [Google Scholar]
- 64.Zhou JX, Li X: Apoptosis in polycystic kidney disease: From pathogenesis to treatment In: Polycystic Kidney Disease, edited by Li X, Brisbane (AU), Codon Publications, 2015 [PubMed] [Google Scholar]
- 65.Roberts AW, Lee BL, Deguine J, John S, Shlomchik MJ, Barton GM: Tissue-resident macrophages are locally programmed for silent clearance of apoptotic cells. Immunity 47: 913–927.e6, 2017 10.1016/j.immuni.2017.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Puranik AS, Leaf IA, Jensen MA, Hedayat AF, Saad A, Kim KW, Saadalla AM, Woollard JR, Kashyap S, Textor SC, Grande JP, Lerman A, Simari RD, Randolph GJ, Duffield JS, Lerman LO: Kidney-resident macrophages promote a proangiogenic environment in the normal and chronically ischemic mouse kidney. Sci Rep 8: 13948, 2018. 10.1038/s41598-018-31887-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karasawa K, Asano K, Moriyama S, Ushiki M, Monya M, Iida M, Kuboki E, Yagita H, Uchida K, Nitta K, Tanaka M: Vascular-resident CD169-positive monocytes and macrophages control neutrophil accumulation in the kidney with ischemia-reperfusion injury. J Am Soc Nephrol 26: 896–906, 2015. 10.1681/ASN.2014020195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Norlander AE, Madhur MS: Inflammatory cytokines regulate renal sodium transporters: How, where, and why? Am J Physiol Renal Physiol 313: F141–F144, 2017. 10.1152/ajprenal.00465.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang J, Rudemiller NP, Patel MB, Karlovich NS, Wu M, McDonough AA, Griffiths R, Sparks MA, Jeffs AD, Crowley SD: Interleukin-1 receptor activation potentiates salt reabsorption in angiotensin II-induced hypertension via the NKCC2 Co-transporter in the nephron. Cell Metab 23: 360–368, 2016. 10.1016/j.cmet.2015.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Veit G, Bossard F, Goepp J, Verkman AS, Galietta LJ, Hanrahan JW, Lukacs GL: Proinflammatory cytokine secretion is suppressed by TMEM16A or CFTR channel activity in human cystic fibrosis bronchial epithelia. Mol Biol Cell 23: 4188–4202, 2012. 10.1091/mbc.e12-06-0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peteranderl C, Morales-Nebreda L, Selvakumar B, Lecuona E, Vadász I, Morty RE, Schmoldt C, Bespalowa J, Wolff T, Pleschka S, Mayer K, Gattenloehner S, Fink L, Lohmeyer J, Seeger W, Sznajder JI, Mutlu GM, Budinger GR, Herold S: Macrophage-epithelial paracrine crosstalk inhibits lung edema clearance during influenza infection. J Clin Invest 126: 1566–1580, 2016. 10.1172/JCI83931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang JL, Woolf AS, Kolatsi-Joannou M, Baluk P, Sandford RN, Peters DJ, McDonald DM, Price KL, Winyard PJ, Long DA: Vascular endothelial growth factor C for polycystic kidney diseases. J Am Soc Nephrol 27: 69–77, 2016. 10.1681/ASN.2014090856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jafree DJ, Moulding D, Kolatsi-Joannou M, Perretta Tejedor N, Price KL, Milmoe NJ, Walsh CL, Correra RM, Winyard PJ, Harris PC, Ruhrberg C, Walker-Samuel S, Riley PR, Woolf AS, Scambler PJ, Long DA: Spatiotemporal dynamics and heterogeneity of renal lymphatics in mammalian development and cystic kidney disease. eLife 8: e48183, 2019. 10.7554/eLife.48183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Takakura A, Nelson EA, Haque N, Humphreys BD, Zandi-Nejad K, Frank DA, Zhou J: Pyrimethamine inhibits adult polycystic kidney disease by modulating STAT signaling pathways. Hum Mol Genet 20: 4143–4154, 2011. 10.1093/hmg/ddr338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leuenroth SJ, Crews CM: Studies on calcium dependence reveal multiple modes of action for triptolide. Chem Biol 12: 1259–1268, 2005. 10.1016/j.chembiol.2005.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leuenroth SJ, Bencivenga N, Igarashi P, Somlo S, Crews CM: Triptolide reduces cystogenesis in a model of ADPKD. J Am Soc Nephrol 19: 1659–1662, 2008. 10.1681/ASN.2008030259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ponzoni M, Pastorino F, Di Paolo D, Perri P, Brignole C: Targeting macrophages as a potential therapeutic intervention: Impact on inflammatory diseases and cancer. Int J Mol Sci 19: 1953, 2018. 10.3390/ijms19071953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT: Synaptic pruning by microglia is necessary for normal brain development. Science 333: 1456–1458, 2011. 10.1126/science.1202529 [DOI] [PubMed] [Google Scholar]
- 79.Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A, Hucker WJ, Wülfers EM, Seemann G, Courties G, Iwamoto Y, Sun Y, Savol AJ, Sager HB, Lavine KJ, Fishbein GA, Capen DE, Da Silva N, Miquerol L, Wakimoto H, Seidman CE, Seidman JG, Sadreyev RI, Naxerova K, Mitchell RN, Brown D, Libby P, Weissleder R, Swirski FK, Kohl P, Vinegoni C, Milan DJ, Ellinor PT, Nahrendorf M: Macrophages facilitate electrical conduction in the heart. Cell 169: 510–522.e20, 2017. 10.1016/j.cell.2017.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]


