Abstract
G protein-coupled receptors (GPCRs) are the most structurally diverse family of signaling proteins and regulate a variety of cell function. For most GPCRs, the cell surface is their functional destination where they are able to respond a wide range of extracellular stimuli, leading to the activation of intracellular signal transduction cascades. Thus, the quantity of receptor expression at the cell surface is a crucial factor regulating the functionality of the receptors. Over the past decades, many methods have been developed to measure the cell surface expression of GPCRs. Here, we describe an intact live-cell radioligand binding assay to quantify the surface expression of GPCRs at the endogenous levels or after overexpression. In this assay, cell cultures will be incubated with specific cell-nonpermeable radioligands which selectively and stoichiometrically bind to individual GPCRs and the receptor numbers at the cell surface are quantified by the radioactivity of receptor-bound ligands. This method is highly specific for measuring the functional GPCRs at the surface of intact live cells and is particularly useful for endogenous, low-abundant GPCRs.
Keywords: G protein-coupled receptor, Adrenergic receptor, Angiotensin II receptor, Muscarinic receptor, Cell surface, Trafficking, Radioligand, Live cell
Background
G protein-coupled receptors (GPCRs) constitute the largest superfamily of cell surface receptors and modulate a variety of cell functions under physiological and pathological conditions (Hauser et al., 2017; Hilger et al., 2018; Weinberg and Puthenveedu, 2019). The precise functions of individual GPCRs at the cell surface are initiated by their binding to specific ligands which in turn activates cognate heterotrimeric G proteins or other signaling molecules, leading to the activation of intracellular signal transduction cascades. Although GPCR-mediated signaling and functioning are sophisticated processes which are coordinated by many factors, the net amount of receptor surface expression is undoubtedly a crucial element regulating the magnitude and duration (Zhang and Wu, 2019).
The quantity of GPCR expression at the cell surface is a balance of highly regulated, dynamic intracellular trafficking, including maturation, internalization, recycling, and degradation. Over the past decades, numerous methods have been well established to quantify the surface GPCRs, such as ELISA, flow cytometry, biotinylation, radioligand binding and imaging (Dunham et al., 2009; Qin et al., 2011; Zhu et al., 2015; Shiwarski et al., 2017 and 2019). Here, we describe an intact live-cell ligand binding assay to quantify their surface expression at steady state by using cell membrane-nonpermeable radioligands. In this assay, live cells will be incubated with individual receptor-specific antagonists or agonists labelled with radioactive isotopes at a saturating concentration. Since these radioligands are not able to penetrate the plasma membrane, the radioactivity of ligands bound to the cells will reflect the quantity of receptor expression at the cell surface. As the ligands used are radiolabeled and stoichiometrically bind to specific GPCRs, this method provides a very sensitive and highly specific approach to accurately quantify the surface functional GPCRs which are able to bind to their ligands in intact live cells and is particularly useful for endogenous, low-abundant GPCRs whose quantification by other methods is extremely difficult.
We have used this method to measure the surface expression of a number of family A GPCRs in different cell types at the endogenous levels or after overexpression (Filipeanu et al., 2004; Dong and Wu, 2006 and 2007; Dong et al., 2008; Duvernay et al., 2009a and 2009b; Dong et al., 2010a and 2010b; Duvernay et al., 2011; Zhang et al., 2011; Dong et al., 2012; Fan et al., 2012; Li et al., 2012; Zhang et al., 2016a and 2016b; Li et al., 2017; Wei et al., 2019; Zhang et al., 2019). Here, we first describe the procedures to measure the surface expression of α2B-adrenergic receptor (AR), which has long been used as a model GPCR in our studies, in NG108-15 neuroblastoma-glioma and MCF-7 breast cancer cells which express α2B-AR endogenously, and in human embryonic kidney 293 (HEK293) cells in which α2B-AR was overexpressed by transient transfection or inducible systems. We will then briefly discuss the quantification of surface expression of other GPCRs by using radioligand binding of intact live cells.
Materials and Reagents
6-well plates (Thermo Fisher Scientific, catalog number: 130184)
12-well plates (Thermo Fisher Scientific, catalog number: 130185)
HEK293 cells (ATCC, catalog number: CRL-1573)
NG108-15 cells (ATCC, catalog number: HB-12317)
MCF-7 cells (ATCC, catalog number: HTB-22)
[6,7-3H(N)]-RX821002 (PerkinElmer, catalog number: NET1153250UC)
[7-methoxy-3H]-Prazosin (PerkinElmer, catalog number: NET823025UC)
[5,7-3H]-CGP12177 (PerkinElmer, catalog number: NET1061250UC)
[125I]-Angiotensin II, [125I]-Ang II (PerkinElmer, catalog number: NEX105050UC)
[N-methyl-3H]-Scopolamine methyl chloride, [3H]-NMS (PerkinElmer, catalog number: NET636250UC)
Phentolamine hydrochloride (Sigma, catalog number: P7547)
Rauwolscine hydrochloride (Tocris, catalog number: 0891)
Alprenolol hydrochloride (Sigma, catalog number: A8676)
Angiotensin II, Ang II (Calbiochem, catalog number: 05-23-0101)
Atropine (Sigma, catalog number: A0132)
Doxycycline hyclate (Sigma, catalog number: D9891)
Dulbecco’s modified Eagle’s medium, DMEM (Sigma, catalog number: D6429-500ML)
Fetal bovine serum, FBS (HyClone, catalog number: SH30396.03)
Penicillin-streptomycin solution (HyClone, catalog number: SH40003.01)
Hypoxanthine (Sigma, catalog number: H9377)
Aminopterin (Sigma, catalog number: A3411)
Thymidine (Sigma, catalog number: T9250)
Poly-L-lysine hydrobromide (Sigma, catalog number: P1274)
Trypsin-EDTA (0.25%) (Sigma, catalog number: T4049-100ML)
Opti-MEM (Gibco, catalog number: 31985-070)
Lipofectamine 2000 (Invitrogen, catalog number: 11668-019)
10× Phosphate buffered saline, 10× PBS (Sigma, catalog number: P5493-4L)
NaOH (Sigma, catalog number: S5881-500G)
Scintillation solution, ScintiVerse™ BD cocktail (Fisher Chemical, catalog number: SX18-4)
High density polyethylene vials, 20 ml capacity (RPI, catalog number: 121043)
Poly-L-lysine (see Recipes)
NG108-15 growth medium (see Recipes)
DMEM complete medium (see Recipes)
3H-RX821002 binding solution (see Recipes)
3H-RX821002 binding solution containing 10 μM rauwolscine (see Recipes)
Equipment
Low speed orbital shaker (Southwest Science, catalog number: SBT30)
Hemocytometer (Hausser Scientific, catalog number: 1483)
Cell culture incubator (Thermo Scientific, Forma series II water jacketed CO2 incubator)
Tissue culture microscope (Nikon, model: Eclipse TS100)
Scintillation counter (Hitachi, model: AccuFLEX LSC-8000c)
Software
Microsoft Excel
Procedure
-
Quantification of endogenous α2B-AR at the cell surface
There are three different α2-AR subtypes: α2A-AR, α2B-AR and α2C-AR. NG108-15 neuroblastoma-glioma cells express only α2B-AR but not α2A-AR and α2C-AR, whereas MCF-7 breast cancer cells express both α2B-AR and α2C-AR but not α2A-AR.- Cell culture
- To pre-coat 6-well plates, add 2 ml of poly-L-lysine (Recipe 1) each well and incubate at 37 °C for 15 min. Wash the plate twice, each with 1 ml of sterilized MilliQ-water.
- Seed NG108-15 or MCF-7 cells on 6-well plates at a total of 5 × 105 cells per well. For each experiment, a total of 6 wells are needed; 3 wells are used to measure total radioligand binding and other 3 wells used to measure non-specific radioligand binding.
- Culture NG108-15 cells in NG108-15 growth medium (Recipe 2) under 95% air and 5% CO2 at 37 °C.
- Culture MCF-7 cells in DMEM complete medium (Recipe 3) under 95% air and 5% CO2 at 37 °C.
- When cells become 70–90% confluent, conduct radioligand binding.
- Radioligand binding
- Aspirate the growth medium completely.
- To measure total radioligand binding, add 500 μl of 3H-RX821002 at a concentration 2 nM diluted in DMEM (Recipe 4).
- To measure non-specific radioligand binding, add 500 μl of 3H-RX821002 at 2 nM containing nonradioactive rauwolscine at 10 μM (Recipe 5).
- Incubate cells for 90 min at room temperature (RT) on a low speed orbital shaker with constant shaking at 20–40 rpm.
- Aspirate radioligand binding solution and wash cells twice, each with 1 ml of 1× PBS (10× PBS diluted with MilliQ-water) for 5 min at RT with shaking to remove the excess radioligand.
- Remove PBS and add 500 μl of 1 M NaOH to digest cells for 2 h at RT with shaking.
- Add 4 ml of scintillation solution to each high density polyethylene vial, transfer cell lysates to vials, and mix vigorously by inverting the tubes or using a vortex.
- Count radioactivity in a scintillation counter.
- In a separate vial, add 4 ml of scintillation solution and 5 μl of radioligand binding solution, mix and count radioactivity. This number will be used as input to calculate receptor numbers.
- Quantification of transiently expressed α2B-AR at the cell surface
- Cell culture
- Seed HEK293 cells into 6-well plates pre-coated with poly-L-lysine at a total of 5 × 105 cells per well and culture cells in DMEM complete medium at 37 °C overnight.
- Check the cell density under a tissue culture microscope. When cells become 70–90% confluent, aspirate culture medium and add 1.8 ml of DMEM.
- Transient transfection
- α2B-AR plasmids in any mammalian expression vectors can be used. For example, we have used the pEGFP-N1 vector to generate α2B-AR tagged with GFP at the C-terminus (Dong et al., 2010a and 2010b). Based on our experience, transfection of 1 μg of α2B-AR plasmids in each well of 6-well plates is sufficient to achieve the maximal receptor expression.
- In an Eppendorf tube, add 125 μl of serum-free Opti-MEM and 1 μg of α2B-AR plasmids, mix gently, and incubate for 5 min at RT.
- In another tube, add 125 μl of Opti-MEM and 2.5 μl of Lipofectamine 2000, mix and incubate for 5 min at RT.
- Combine two solutions, mix gently by pipetting 3–4 times, and incubate for additional 20 min at RT.
- Add the mixture slowly into cells cultured in 1.8 ml of DMEM, mix gently, and culture cells for 6 h at 37 °C.
- Remove cell culture medium and wash cells once with 1 ml of sterilized 1× PBS.
- Treat cells with 100 μl of trypsin-EDTA for 2 min at 37 °C and add 1 ml of DMEM complete medium.
- Split cells from each well of 6-well plates into four wells of 12-well plates pre-coated with poly-L-lysine (0.27 ml each well). Two wells are used to measure total radioligand binding and other two wells used to measure non-specific radioligand binding.
- Culture cells in DMEM complete medium for 24–36 h at 37 °C.
- Radioligand binding
- Aspirate the growth medium completely.
- For measurement of total radioligand binding, add 200 μl of 3H-RX821002 at 20 nM diluted in DMEM (Recipe 4) into cells
- For measurement of non-specific radioligand binding, add 200 μl of 3H-RX821002 at 20 nM plus rauwolscine at 10 μM (Recipe 5) into cells.
- Incubate cells for 90 min at RT with constant shaking at 20–40 rpm.
- Aspirate radioligand binding solution and wash cells twice, each with 0.5 ml of 1× PBS for 5 min at RT with shaking.
- Remove PBS and add 500 μl of 1 M NaOH to digest cells for 2 h at RT with shaking.
- Transfer cell lysates to a vial containing 4 ml of scintillation solution, mix well and count radioactivity in a scintillation counter.
- Add 5 μl of radioligand binding solution and 4 ml of scintillation solution to a vial, mix and count radioactivity. This number will be used as input to calculate receptor numbers.
- Quantification of inducibly expressed α2B-AR at the cell surface
- Stable HEK293 cells inducibly expressing α2B-AR were generated by using Tet-On 3G tetracycline-inducible gene expression system as described previously (Zhang et al., 2016a and 2016b).
- Seed cells into 12-well plates pre-coated with poly-L-lysine at a density of 2 × 105 cells per well and culture cells in DMEM complete medium at 37 °C overnight.
- To measure doxycycline dose-dependent induction of α2B-AR expression, add doxycycline to the final concentrations of 0, 2.5, 5, 7.5, 10, 20, 40, 60, 80 and 100 ng/ml and incubate for 24 h at 37 °C.
- To measure time-dependent induction of α2B-AR expression, add doxycycline at the final concentration of 40 ng/ml and incubate cells for 2, 4, 6, 8, 10, 12, 14, 16, 20, 24, and 36 h.
- Conduct radioligand binding as described in Procedure B.
-
Measurement of the cell surface expression of other GPCRs
Over the past years, we have used intact live-cell ligand binding assays to quantify the cell surface expression of several family A GPCRs, including α1A-AR, α1B-AR, α2A-AR, α2B-AR, α2C-AR, β1-AR, β2-AR, Ang II type 1 (AT1R) and type 2 (AT2R) receptors, and M3-muscarinc receptor (M3-MR) using different radioligands (Table 1). One issue associated with intact cell radioligand binding assays is that radiolabeled ligands may be able to induce receptor internalization. For example, Ang II is an agonist of AT1R, thus incubation with 125I-Ang II may induce the internalization of AT1R from the cell surface to the endosomal compartment. One strategy to limit receptor internalization during incubation with radiolabeled agonists is to carry out ligand binding assays at low temperature (Filipeanu et al., 2004).
- Data analysis
- Calculate specific radioligand binding to receptors using the values of disintegrations per minute (DPM):
-
Calculate surface receptor numbers per cellIn order to calculate the receptor numbers at the surface in each cell, the total cell numbers used in ligand binding assays need to be determined. This can be done by preparing cell cultures in exactly the same way as for ligand binding and count the cell numbers in each well by using a hemocytometer.
-
Endogenous receptorsDetailed calculation:As 1 mole = 6.02 × 1023 molecules,Sample ligand number per well (molecules)= Sample DPM × 10−14 moles × 6.02 × 1023 ÷ Input DPM= Sample DPM × 6.02 × 109 ÷ total input DPMSample ligand number per cell (molecules)= Sample DPM × 6.02 × 109 ÷ total input DPM ÷ cell number
- Overexpressed receptors
-
- Experimental results
-
Inducible expression of the surface expression of α2B-ARBy using intact live-cell radioligand binding assays as described in the section C, we have measured the surface expression of α2B-AR in a stable HEK293 cell line which inducibly expresses α2B-AR. Our data have shown that doxycycline incubation induces the expression of α2B-AR at the cell surface in a dose- (Figure 1A) and time-dependent (Figure 1B) fashion. Doxycycline-induced α2B-AR expression reaches a plateau at a concentration of about 40 ng/ml (Figure 1A) and after 20 h of induction (Figure 1B), resulting in a total of 8.5 × 105 α2B-AR at the surface per cell (Zhang et al., 2016a and 2016b).
-
Structural determinants of α2B-AR transport to the cell surfaceOver the past decades, most studies on GPCR trafficking have focused on the events involved in receptor internalization from the cell surface to the endosomal compartments and recycling of internalized receptors back to the cell surface (Tan et al., 2004; Hanyaloglu and von Zastrow, 2008; Marchese et al., 2008; Kang et al., 2014). In contrast, the molecular mechanisms underlying the cell surface transport of newly synthesized receptors along the biosynthesis pathways are much less well understood. By using intact live cell radioligand binding assays as described above, we have successfully identified a number of motifs or specific residues which are essential for receptor transport to the cell surface either from the endoplasmic reticulum (ER) or from the Golgi apparatus. For example, we have demonstrated that mutation of the residues Y12/S13 in the N-terminus, L48 in the first intracellular loop, and I443/F444 and F436 in the C-terminus markedly reduces the cell surface expression of α2B-AR (Figure 2) (Dong and Wu, 2006; Duvernay, Dong et al., 2009a and 2009b), indicating important roles of these residues in α2B-AR biosynthesis.
-
Identification of α2B-AR surface transport regulatorsWe have also employed intact live cell radioligand binding assays in searching for proteins that regulate α2B-AR cell surface transport. For instance, in recent studies we have investigated the function of 48 Rab GTPases (Li et al., 2017) and 44 putative Rab GTPase-activating proteins (GAPs) (Wei et al., 2019) in the cell surface transport of α2B-AR. In these experiments, individual Rab mutants or Rab GAPs were transiently expressed in stable HEK293 cells expressing α2B-AR and their effects on the cell surface expression of α2B-AR were measured by radioligand binding of intact live cells as described above. Our results have shown that the expression of Rab43 mutants and six TBC domain-containing proteins, namely TBC1D5, TBC1D6, TBC1D8B, TBC1D20, TBC1D22A and RN-tre significantly reduces the cell surface numbers of α2B-AR (Figure 3) (Li et al., 2017; Wei et al., 2019). These results demonstrate important functions of Rab43 and the six TBC proteins in the forward trafficking of nascent GPCRs and reveal novel regulatory mechanisms underlying GPCR targeting to the functional destination.
-
Table 1.
Measurement of the surface expression of GPCRs by intact cell ligand binding assays
| Receptors | Radioligands | References |
|---|---|---|
| α1-AR (α1A- and α1B-AR) | [3H]-Prazosin (Phentolamine) | Duvernay, 2009a and 2009b; Zhang et al., 2011; Li et al., 2017 |
| α2-AR (α2A-, α2B- and α2C-AR) | [3H]-RX821002 (Rauwolscine) | Dong and Wu, 2007; Dong et al., 2008; Duvernay 2009a and 2009b; Dong et al., 2010a and 2010b; Zhang et al., 2011; Filipeanu et al., 2015; Li et al., 2017; Wei et al., 2019 |
| β-AR (β1- and β2-AR) |
[3H]-CGP12177 (Alprenolol) |
Dong and Wu, 2007; Dong et al., 2008; Duvernay 2009a and 2009b; Dong et al., 2010a and 2010b; Zhang et al., 2011; Filipeanu et al., 2015; Li et al., 2017; Wei et al., 2019 |
| AT1R and AT2R | [125I]-Ang II (Ang II) |
Filipeanu et al., 2004; Dong and Wu, 2007; Dong et al., 2008; Duvernay et al., 2009a and 2009b; Dong et al., 2010b; Zhang et al., 2011 |
| M3-MR | [3H]-NMS (Atropine) |
Dong et al., 2010b |
Drugs indicated in ( ) are used for measurement of non-specific radioligand binding.
Figure 1. Dose- (A) and time-dependent (B) induction of α2B-AR expression at the cell surface by doxycycline in HEK293 cells.
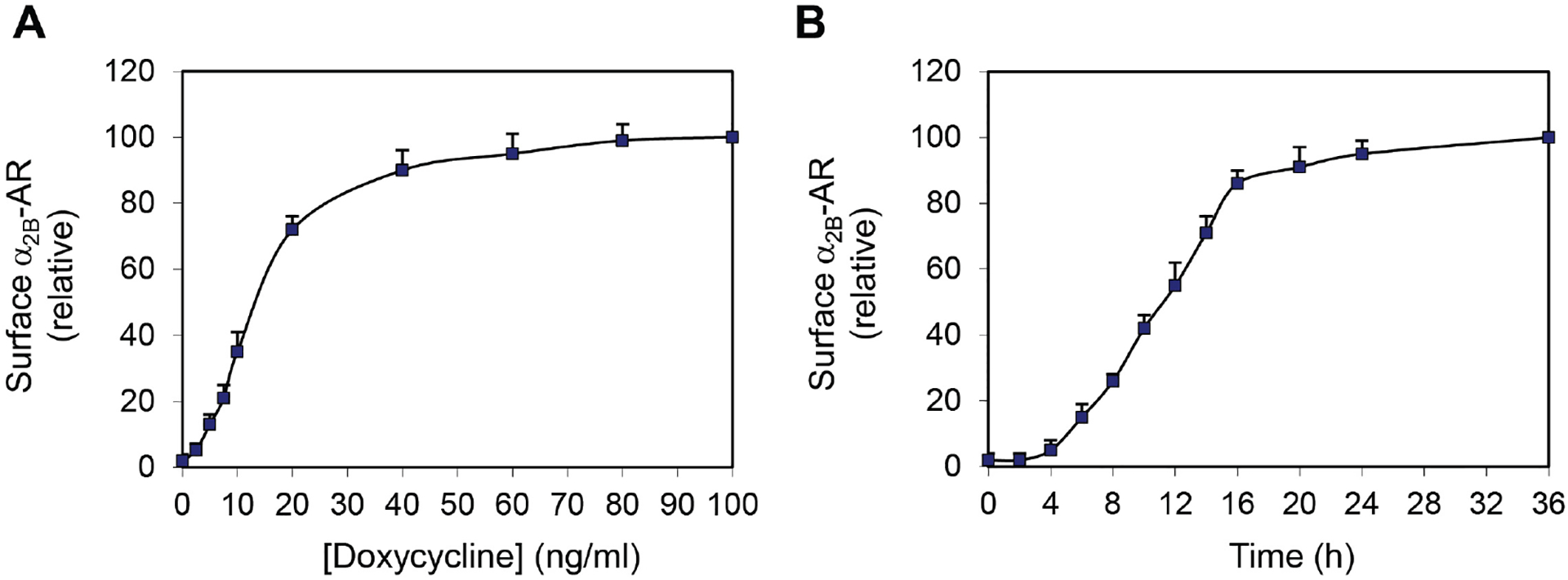
The data shown are percentages of specific binding obtained from cells treated with doxycycline at 100 ng/ml (A) or from cells after induction for 36 h (B) (Zhang et al., 2016a and 2016b).
Figure 2. Inhibition of the cell surface transport of α2B-AR by mutating residues Y12/S13, L48, F436 and I443/L444.
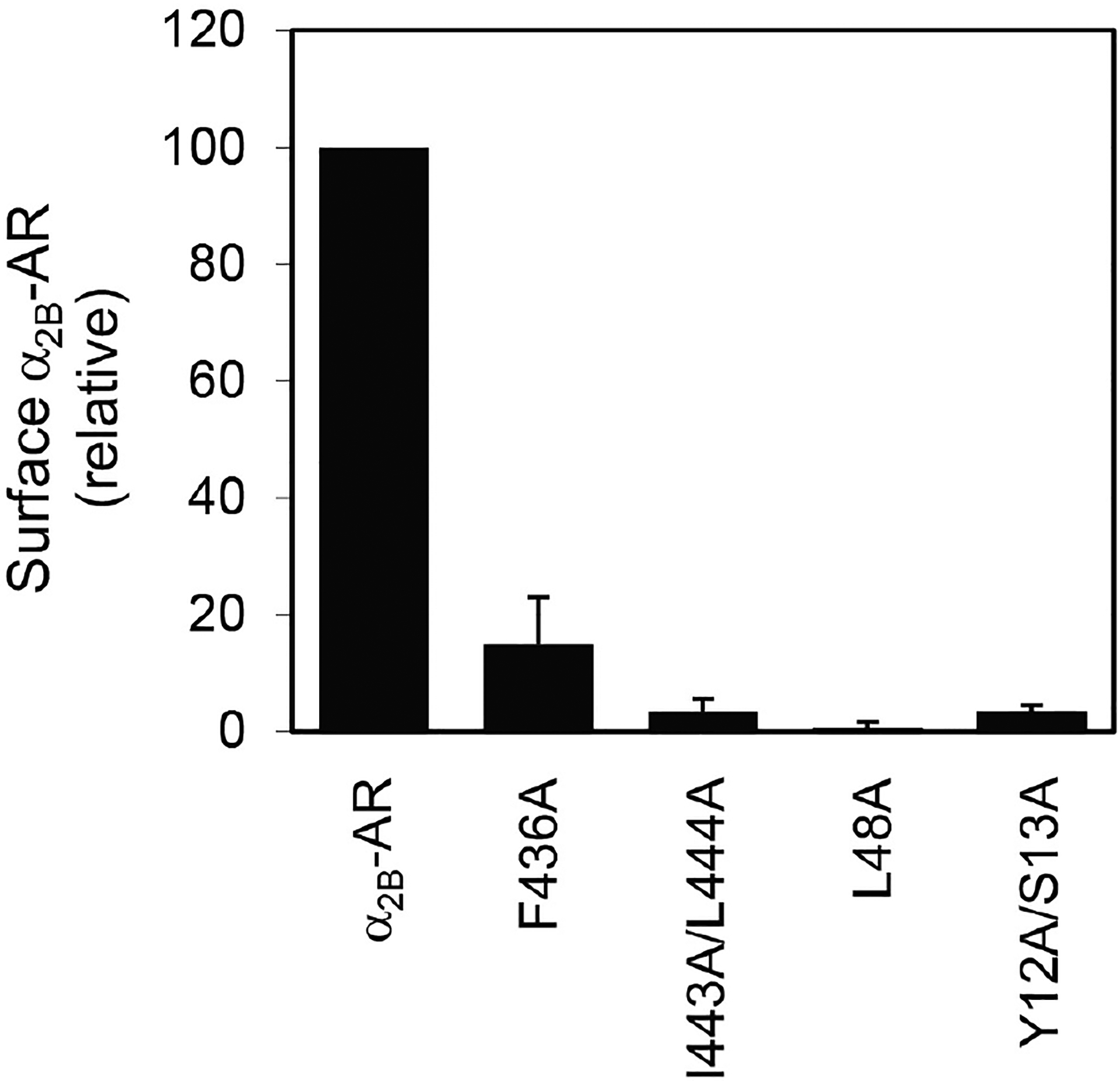
Wild-type and mutated α2B-AR were transiently expressed in HEK293 cells and their surface expression was measured by radioligand binding of intact live cells using [3H]-RX821002.
Figure 3. Inhibition of the cell surface transport of α2B-AR by mutated Rab43 and TBC domain-containing proteins.
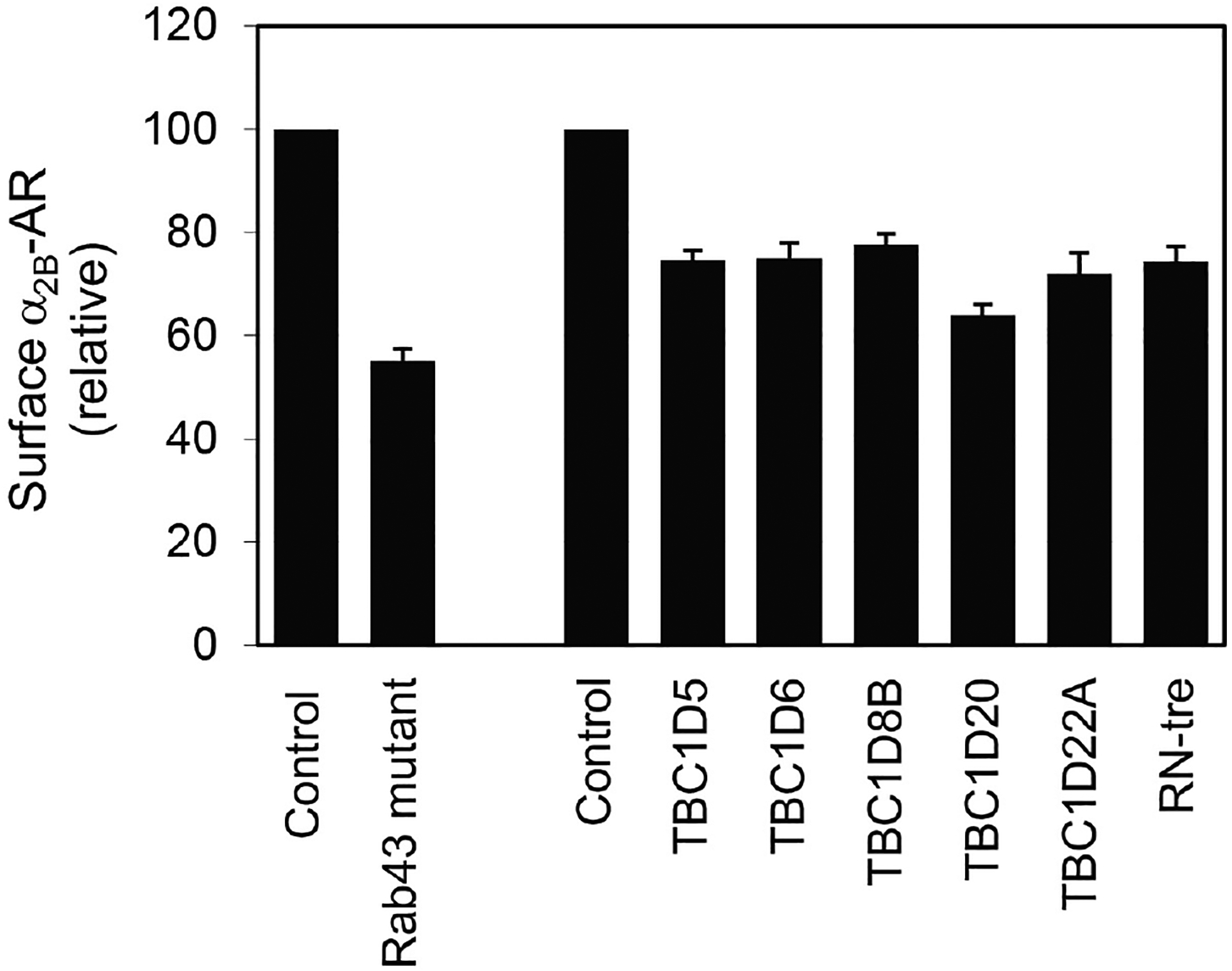
The Rab43 mutant or individual TBC proteins were transiently expressed in HEK293 cells stably expressing α2B-AR and α2B-AR expression at the surface was measured by intact cell ligand binding using [3H]-RX821002.
Recipes
-
Poly-L-lysine
Dissolve poly-L-lysine hydrobromide in sterilized Milli-Q water to a final concentration of 25 μg/ml
-
NG108-15 growth medium
DMDM
10% FBS
100 units/ml penicillin
100 μg/ml streptomycin
100 μM hypoxanthine
0.4 μM aminopterin
16 μM thymidine
-
DMEM complete medium
DMEM
10% FBS
100 units/ml penicillin
100 μg/ml streptomycin
-
3H-RX821002 binding solution
As the concentration of 3H-RX821002 varies in different batches, the following is just an example of concentration calculation of the radioligand in a vial containing 250 μCi in a total volume of 250 μl with specific activity of 63.9 Ci/mmol.Dilute 3H-RX821002 in DMEM to 2 nM for measurement of endogenous receptors or to 20 nM for measurement of overexpressed receptors.
- 3H-RX821002 binding solution containing 10 μM rauwolscine
- To prepare 5 mM rauwolscine stock solution, dissolve 19 mg of rauwolscine hydrochloride in 10 ml of Milli-Q water
- Dilute rauwolscine stock solution to 10 μM in radioligand binding solution prepared as Recipe 4
Acknowledgments
This work was supported by the National Institutes of Health grant R35GM136397.
Footnotes
Competing interests
The authors declare no conflict of interests.
References
- 1.Dong C, Nichols CD, Guo J, Huang W, Lambert NA and Wu G (2012). A triple arg motif mediates α2B-adrenergic receptor interaction with Sec24C/D and export. Traffic 13(6): 857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong C and Wu G (2006). Regulation of anterograde transport of alpha2-adrenergic receptors by the N termini at multiple intracellular compartments. J Biol Chem 281(50): 38543–38554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong C and Wu G (2007). Regulation of anterograde transport of adrenergic and angiotensin II receptors by Rab2 and Rab6 GTPases. Cell Signal 19(11): 2388–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong C, Yang L, Zhang X, Gu H, Lam ML, Claycomb WC, Xia H and Wu G (2010a). Rab8 interacts with distinct motifs in α2B- and β2-adrenergic receptors and differentially modulates their transport. J Biol Chem 285(26): 20369–20380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong C, Zhang X, Zhou F, Dou H, Duvernay MT, Zhang P and Wu G (2010b). ADP-ribosylation factors modulate the cell surface transport of G protein-coupled receptors. J Pharmacol Exp Ther 333(1): 174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong C, Zhou F, Fugetta EK, Filipeanu CM and Wu G (2008). Endoplasmic reticulum export of adrenergic and angiotensin II receptors is differentially regulated by Sar1 GTPase. Cell Signal 20(6): 1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunham JH, Meyer RC, Garcia EL and Hall RA (2009). GPR37 surface expression enhancement via N-terminal truncation or protein-protein interactions. Biochemistry 48(43): 10286–10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duvernay MT, Dong C, Zhang X, Robitaille M, Hebert TE and Wu G (2009a). A single conserved leucine residue on the first intracellular loop regulates ER export of G protein-coupled receptors. Traffic 10(5): 552–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duvernay MT, Dong C, Zhang X, Zhou F, Nichols CD and Wu G (2009b). Anterograde trafficking of G protein-coupled receptors: function of the C-terminal F(X)6LL motif in export from the endoplasmic reticulum. Mol Pharmacol 75(4): 751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duvernay MT, Wang H, Dong C, Guidry JJ, Sackett DL and Wu G (2011). Alpha2B-adrenergic receptor interaction with tubulin controls its transport from the endoplasmic reticulum to the cell surface. J Biol Chem 286(16): 14080–14089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan Y, Li C, Guo J, Hu G and Wu G (2012). A single lys residue on the first intracellular loop modulates the endoplasmic reticulum export and cell-surface expression of alpha2A-adrenergic receptor. PLoS One 7(12): e50416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filipeanu CM, Zhou F, Claycomb WC and Wu G (2004). Regulation of the cell surface expression and function of angiotensin II type 1 receptor by Rab1-mediated endoplasmic reticulum-to-Golgi transport in cardiac myocytes. J Biol Chem 279(39): 41077–41084. [DOI] [PubMed] [Google Scholar]
- 13.Hanyaloglu AC and von Zastrow M (2008). Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol 48: 537–568. [DOI] [PubMed] [Google Scholar]
- 14.Hauser AS, Attwood MM, Rask-Andersen M, Schioth HB and Gloriam DE (2017). Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov 16(12): 829–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilger D, Masureel M and Kobilka BK (2018). Structure and dynamics of GPCR signaling complexes. Nat Struct Mol Biol 25(1): 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang DS, Tian X and Benovic JL (2014). Role of β-arrestins and arrestin domain-containing proteins in G protein-coupled receptor trafficking. Curr Opin Cell Biol 27: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C, Fan Y, Lan TH, Lambert NA and Wu G (2012). Rab26 modulates the cell surface transport of α2-adrenergic receptors from the Golgi. J Biol Chem 287(51): 42784–42794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, Wei Z, Fan Y, Huang W, Su Y, Li H, Dong Z, Fukuda M, Khater M and Wu G (2017). The GTPase Rab43 controls the anterograde ER-Golgi trafficking and sorting of GPCRs. Cell Rep 21(4): 1089–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchese A, Paing MM, Temple BR and Trejo J (2008). G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol 48: 601–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin K, Dong C, Wu G and Lambert NA (2011). Inactive-state preassembly of Gq-coupled receptors and Gq heterotrimers. Nat Chem Biol 7(10): 740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiwarski DJ, Crilly SE, Dates A and Puthenveedu MA (2019). Dual RXR motifs regulate nerve growth factor-mediated intracellular retention of the delta opioid receptor. Mol Biol Cell 30(5): 680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiwarski DJ, Darr M, Telmer CA, Bruchez MP and Puthenveedu MA (2017). PI3K class IIα regulates δ-opioid receptor export from the trans-Golgi network. Mol Biol Cell 28(16): 2202–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan CM, Brady AE, Nickols HH, Wang Q and Limbird LE (2004). Membrane trafficking of G protein-coupled receptors. Annu Rev Pharmacol Toxicol 44: 559–609. [DOI] [PubMed] [Google Scholar]
- 24.Wei Z, Zhang M, Li C, Huang W, Fan Y, Guo J, Khater M, Fukuda M, Dong Z, Hu G and Wu G (2019). Specific TBC domain-containing proteins control the ER-Golgi-plasma membrane trafficking of GPCRs. Cell Rep 28(2): 554–566 e554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinberg ZY and Puthenveedu MA (2019). Regulation of G protein-coupled receptor signaling by plasma membrane organization and endocytosis. Traffic 20(2): 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang M, Davis JE, Li C, Gao J, Huang W, Lambert NA, Terry AV Jr. and Wu G (2016a). GGA3 interacts with a G protein-coupled receptor and modulates its cell surface export. Mol Cell Biol 36(7): 1152–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M, Huang W, Gao J, Terry AV and Wu G (2016b). Regulation of alpha2B-adrenergic receptor cell surface transport by GGA1 and GGA2. Sci Rep 6: 37921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang M and Wu G (2019). Mechanisms of the anterograde trafficking of GPCRs: Regulation of AT1R transport by interacting proteins and motifs. Traffic 20(2): 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, Xu X, Li C, Huang W, Xu N and Wu G (2019). A naturally occurring splice variant of GGA1 inhibits the anterograde Post-Golgi traffic of α2B-adrenergic receptor. Sci Rep 9(1): 10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Dong C, Wu QJ, Balch WE and Wu G (2011). Di-acidic motifs in the membrane-distal C termini modulate the transport of angiotensin II receptors from the endoplasmic reticulum to the cell surface. J Biol Chem 286(23): 20525–20535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu S, Zhang M, Davis JE, Wu WH, Surrao K, Wang H and Wu G (2015). A single mutation in helix 8 enhances the angiotensin II type 1a receptor transport and signaling. Cell Signal 27(12): 2371–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]


