Abstract
Background:
Motor-related brain activity in Parkinson’s disease has been investigated in a multitude of functional neuroimaging studies, which often yielded apparently conflicting results. Our previous meta-analysis did not resolve inconsistencies regarding cortical activation differences in Parkinson’s disease, which might be related to the limited number of studies that could be included. Therefore, we conducted a revised meta-analysis including a larger number of studies. The objectives of this study were to elucidate brain areas that consistently show abnormal motor-related activation in Parkinson’s disease and to reveal their functional connectivity profiles using meta-analytic approaches.
Methods:
We applied a quantitative meta-analysis of functional neuroimaging studies testing limb movements in Parkinson’s disease comprising data from 39 studies, of which 15 studies (285 of 571 individual patients) were published after the previous meta-analysis. We also conducted meta-analytic connectivity modeling to elucidate the connectivity profiles of areas showing abnormal activation.
Results:
We found consistent motor-related underactivation of bilateral posterior putamen and cerebellum in Parkinson’s disease. Primary motor cortex and the supplementary motor area also showed deficient activation, whereas cortical regions localized directly anterior to these areas expressed overactivation. Connectivity modeling revealed that areas showing decreased activation shared a common pathway through the posterior putamen, whereas areas showing increased activation were connected to the anterior putamen.
Conclusions:
Despite conflicting results in individual neuroimaging studies, this revised meta-analytic approach identified consistent patterns of abnormal motor-related activation in Parkinson’s disease. The distinct patterns of decreased and increased activity might be determined by their connectivity with different subregions of the putamen.
Keywords: Parkinson’s disease, functional neuroimaging, meta-analysis, motor
Parkinson’s disease (PD) is a common and disabling neurodegenerative disorder. Even though many patients develop nonmotor symptoms, such as depression or autonomic dysfunction, the disease is still considered a movement disorder and is defined by the hallmark presence of bradykinesia, that is, the slowing of movement initiation and progressive reduction in speed and amplitude of repetitive movements.1,2 Bradykinesia can be conceptualized as an impaired ability to “energize” or “charge” movements and has been attributed to an impaired modulation of movement vigor.3,4 To better understand the neural underpinning of this motor impairment, a multitude of studies have been conducted using neuroimaging techniques, such as functional magnetic resonance imaging (fMRI) and H2O15 positron emission tomography (PET) while patients perform a motor task. However, the results of these studies often seem conflicting. For example, several studies reported decreased activity in the medial prefrontal and frontal cortices in PD,5–8 whereas other studies reported activity in these areas to be increased.9–12 One approach to addressing these inconsistencies is to conduct meta-analyses to overcome some of the shortcomings of neuroimaging studies in PD, such as small sample size and heterogeneity of the studied patient group. Furthermore, it allows the generalization of findings beyond the precise experimental setup and task design of a specific study. Thus, meta-analyses allow assessing whether there are differences in neural activation in PD that are consistent across individual patient groups and motor tasks. We previously conducted a meta-analysis of neuroimaging studies in PD13 using a quantitative, coordinate-based approach termed activation likelihood estimation (ALE). This analysis pinpointed the motor territory of the striatum, the posterior putamen, as the brain region that was most consistently underactivated during motor tasks in PD. At the cortical level, the observed frontal and parietal activation differences were less consistent regarding the directionality of changes (ie, increased or decreased in PD relative to healthy controls) and appeared to rely more strongly on the applied motor task. This raises the question whether cortical activation changes in PD are task dependent rather than reflecting general disease-related neural dysfunction. An alternative explanation for the discordant results from our previous meta-analysis is the limited number of studies that could be included at the time, because meta-analyses with a small number of included studies have relatively low statistical power and can be strongly affected by results from individual experiments.14 To address this, we conducted a revised ALE meta-analysis, which included an additional 15 studies, reporting data from an additional 285 patients that were published after our previous meta-analysis. Furthermore, we computed functional connectivity profiles of the abnormally activated areas to further characterize the dysfunctional motor networks underlying PD.
Methods
Literature Search and Study Selection
We conducted a search on PubMed using the identical search strings as in our previous meta-analysis:13 (“Parkinson’s disease” OR “Parkinson disease” OR “Parkinsons disease”) AND (“functional magnetic resonance” OR “fMRI” OR “positron emission tomography” OR “PET”). The final search was conducted on June 30, 2020, and resulted in 3841 studies. We did not find any additional articles through review articles and reference tracing. We only screened studies using fMRI or H2O15-PET during motor paradigms that were written in the English language, resulting in 170 studies that were further assessed by reading the abstract and/or main text. The following exclusion criteria were then applied for all experiments:
Review articles reporting no original data or PET studies other than H2O15-PET (n = 20).
Studies testing passive movements, eye movements (saccades), speech, motor learning, or executive control, for example, task switching (n = 28).
Motor tasks were tested against each other rather than against baseline or a nonmotor control task, for example, fixation (n = 19).
Neither of the contrasts “PD OFF medication versus healthy controls,”, “PD ON medication versus healthy controls,” or “PD ON medication versus PD OFF medication” were statistically compared (n = 19).
Analyses were based on regions of interest (n = 29). These most commonly comprised the putamen and other basal ganglia areas, primary motor cortex, supplementary motor areas, cerebellum, and, less frequently, parietal or other cortical areas. Some studies in particular early publications did not cover the whole brain. These studies, however, were not excluded because they did not include regions based on a priori assumptions, and in many studies the field of view was not reported. Likewise we did not exclude studies that masked the between-group comparisons based on task-related activity in the control group because this was not based on a priori assumptions about the brain areas of interest.
Multivariate analyses or covariance analyses (n = 6).
Fewer than 6 PD patients were included (n = 2).
Studies in which PD patients were treated with deep brain stimulation or received acute challenges with drugs other than levodopa (eg, apomorphine), because these treatments induce distinct effects on the sensorimotor system in PD15,16 (n = 5).
As in our previous meta-analysis, another study17 was excluded because of a significant age difference between the PD and control groups. If coordinates were not reported, we contacted the corresponding author by email (coordinates could not be obtained in 3 studies). This procedure resulted in the exclusion of 131 studies, leaving 39 studies that were included.5,6,8–12,18–49 Fifteen of these studies were published after our previous meta-analysis and allowed us to conduct a well-powered meta-analysis. For an overview of the included studies, please see Table 1.
TABLE 1.
Studies included in the meta-analysis
| Study | Modality | PD, n | C, n | UPDRS-III OFF | UPDRS-III ON | Age of PD | Age of C | Foci, n | Contrast | |
|---|---|---|---|---|---|---|---|---|---|---|
| Baglio et al, 2011 | fMRI | 15 | 11 | 21.5 | 66.5 | 66.9 | 6 | HC vs ON | ||
| Task: | Button press with right index finger | |||||||||
| Buhmann et al, 2003 | fMRI | 8 | n/a | 54 | n/a | 2 | ON vs OFF | |||
| Task: | Random finger opposition task at 0.33 Hz with right and left hands | |||||||||
| Burciu et al, 2015 | fMRI | 20 | 20 | 31.9 | 65.8 | 64.8 | 20 | HC vs OFF | ||
| Task: | Grip force task with or without feedback with more affected hand | |||||||||
| Caproni et al, 2013 | fMRI | 11 | 11 | 20 | 65 | 65.1 | 7 | HC vs OFF | ||
| Task: | Tapping with right index finger | |||||||||
| fMRI | 11 | 11 | 20 | 65 | 65.1 | 15 | HC vs OFF | |||
| Task: | Sequence from dig I to V with right hand | |||||||||
| fMRI | 11 | 11 | 20 | 65 | 65.1 | 13 | HC vs OFF | |||
| Task: | Sequence with order dig I, III, V, II, IV with right hand | |||||||||
| Cerasa et al, 2006 | fMRI | 10 | 11 | 27.5 | 64.2 | 63.4 | 8 | HC vs OFF | ||
| Task: | Synchronized tapping with right index finger at 1.33 Hz | |||||||||
| fMRI | 10 | 11 | 27.5 | 64.2 | 63.4 | 3 | HC vs OFF | |||
| Task: | Continuation of the tapping with right index finger without stimulus | |||||||||
| Drucker et al, 2019 | fMRI | 22 | 19 | 33.9 | 67.7 | 64.7 | 4 | HC vs OFF | ||
| Task: | Externally cued foot-tapping sequence | |||||||||
| fMRI | 22 | 19 | 33.9 | 67.7 | 64.7 | 6 | HC vs OFF | |||
| Task: | Internally cued foot-tapping sequence | |||||||||
| Eckert et al, 2006 | fMRI | 9 | 9 | 20.6 | 10.7 | 63.3 | 60.6 | 18 | HC vs OFF | |
| fMRI | 9 | 9 | 20.6 | 10.7 | 63.3 | 60.6 | 9 | HC vs ON | ||
| fMRI | 9 | n/a | 20.6 | 10.7 | 63.3 | n/a | 4 | ON vs OFF | ||
| Task: | Opening and closing of right fist at ∼1 Hz | |||||||||
| Gonzalez-Garcia et al, 2011 | fMRI | 17 | 10 | 41 | 64.4 | 8 | HC vs ON | |||
| Task: | Button presses with right and left hands in predefined order | |||||||||
| fMRI | 17 | 10 | 41 | 64.4 | 5 | HC vs ON | ||||
| Task: | Button presses with right and left hands in random order | |||||||||
| Haslinger et al, 2001 | fMRI | 8 | 8 | 15.8 | 11.8 | 60.8 | 54.4 | 7 | HC vs OFF | |
| fMRI | 8 | 8 | 15.8 | 11.8 | 60.8 | 54.4 | 8 | HC vs ON | ||
| fMRI | 8 | n/a | 15.8 | 11.8 | 60.8 | n/a | 10 | ON vs OFF | ||
| Task: | Joystick movements with right hand with 4 spatial degrees of freddom (dof) | |||||||||
| Holiga et al, 2012 | fMRI | 12 | n/a | 33.5 | 9.6 | 56 | n/a | 5 | ON vs OFF | |
| Task: | Index-to-thumb opposition movements with right and left hands at 1 Hz | |||||||||
| Hughes et al, 2010 | fMRI | 16 | 15 | 31.3 | 18.9 | 63.9 | 66.5 | 10 | HC vs ON | |
| Task: | Specified and chosen button presses with right hand | |||||||||
| Jia et al, 2018 | fMRI | 22 | 22 | 16.45 | 61 | 60.6 | 8 | HC vs OFF | ||
| Task: | Self-initiated tapping with right index finger at approximately 0.5 Hz | |||||||||
| Katschnig et al, 2011 | fMRI | 20 | 20 | 37.9 | 66.8 | 62.3 | 2 | HC vs OFF | ||
| Task: | Dorsiflexion of right and left ankles at 1 Hz | |||||||||
| Kim et al, 2018 | fMRI | 16 | 15 | 36 | 63.1 | 64.1 | 6 | HC vs OFF | ||
| fMRI | 16 | 15 | 36 | 63.1 | 64.1 | 19 | HC vs ON | |||
| fMRI | 16 | n/a | 36 | 63.1 | n/a | 5 | ON vs OFF | |||
| Task: | Two-choice forced response task with fingers II and III of right hand | |||||||||
| Kraft et al, 2009 | fMRI | 12 | 12 | 21 | 13.9 | 60.8 | 53 | 12 | HC vs OFF | |
| fMRI | 12 | 12 | 21 | 13.9 | 60.8 | 53 | 8 | HC vs ON | ||
| fMRI | 12 | n/a | 21 | 13.9 | 60.8 | n/a | 4 | ON vs OFF | ||
| Task: | Grip-force task with right and left hands simultaneously | |||||||||
| fMRI | 12 | 12 | 21 | 13.9 | 60.8 | 53 | 13 | HC vs OFF | ||
| fMRI | 12 | 12 | 21 | 13.9 | 60.8 | 53 | 4 | HC vs ON | ||
| fMRI | 12 | n/a | 21 | 13.9 | 60.8 | n/a | 4 | ON vs OFF | ||
| Task: | Grip-force task with right and left hands alternating | |||||||||
| Maillet et al, 2012 | fMRI | 12 | n/a | 40.3 | 10 | 59.8 | n/a | 2 | ON vs OFF | |
| Task: | Joystick movements with right hand with 4 spatial dof at 0.5 Hz | |||||||||
| Mak et al, 2016 | fMRI | 26 | 21 | 29 | 61.4 | 60.9 | 3 | HC vs ON | ||
| Task: | Self-initiated index finger tapping at ∼0.2–0.3 Hz on most affected side | |||||||||
| fMRI | 26 | 21 | 29 | 61.4 | 60.9 | 3 | HC vs ON | |||
| Task: | Cued index finger tapping at ∼0.2–0.3 Hz on most affected side | |||||||||
| Mallol et al, 2007 | fMRI | 13 | 11 | 22.6 | 64.9 | 61.9 | 13 | HC vs OFF | ||
| Task: | Finger-to-thumb opposition and rotating movements of right hand | |||||||||
| Martin et al, 2019 | fMRI | 22 | 22 | 15.6 | 53 | 48.5 | 13 | HC vs OFF | ||
| Task: | Self-generated sequential button press with fingers I-IV of most affected hand | |||||||||
| fMRI | 22 | 22 | 15.6 | 53 | 48.5 | 10 | HC vs OFF | |||
| Task: | Self-generated sequential button press with fingers I-IV of less affected hand | |||||||||
| fMRI | 22 | 22 | 15.6 | 53 | 48.5 | 13 | HC vs OFF | |||
| Task: | Visually-cued sequential button press with fingers I-IV of most affected hand | |||||||||
| fMRI | 22 | 22 | 15.6 | 53 | 48.5 | 5 | HC vs OFF | |||
| Task: | Visually-cued sequential button press with fingers I-IV of less affected hand | |||||||||
| Mattay et al. 2002 | fMRI | 7 | n/a | 8.8 | 5 | 55 | n/a | 7 | ON vs OFF | |
| Task: | Button presses with right hand (0-back task) | |||||||||
| Mohl et al, 2017 | fMRI | 26 | 21 | 33 | 24 | 62.2 | 61.6 | 1 | HC vs OFF | |
| Task: | 1 Hz sequential tapping from fingers I-V and vice versa with right hand | |||||||||
| Payoux et al, 2011 | PET | 8 | 10 | 22 | 12 | 62 | 67 | 3 | HC vs OFF | |
| PET | 8 | n/a | 22 | 12 | 62 | n/a | 1 | ON vs OFF | ||
| Task: | Joystick movements with right hand with 4 spatial dof at 0.33 Hz | |||||||||
| Pinto et al, 2011 | fMRI | 9 | 15 | 33 | 59 | 55 | 6 | HC vs OFF | ||
| Task: | Joystick movements with right hand with 4 spatial dof at 0.5 Hz | |||||||||
| Planetta et al, 2015 | fMRI | 14 | 14 | 29.6 | 64 | 61.9 | 34 | HC vs OFF | ||
| Task: | Cued and memorized pinch grip force task with most affected hand (collapsed) | |||||||||
| Poisson et al, 2013 | fMRI | 6 | 10 | 16 | 65 | 53.6 | 13 | HC vs OFF | ||
| Task: | Finger-thumb tapping with right hand at 1 Hz | |||||||||
| Rottschy et al, 2013 | fMRI | 23 | 23 | 23.9 | 67.2 | 65 | 8 | HC vs ON | ||
| Task: | Direct repeat of sequence of 4 or 5 finger movements with both hands | |||||||||
| fMRI | 23 | 23 | 23.9 | 67.2 | 65 | 14 | HC vs ON | |||
| Task: | Delayed repeat of sequence of 4 or 5 finger movements with both hands | |||||||||
| Rowe et al. 2002 | fMRI | 12 | 12 | 33.7 | 62 | 62 | 2 | HC vs OFF | ||
| Task: | Sequential finger movements of right hand at 0.33 Hz | |||||||||
| Sabatini et al, 2000 | fMRI | 6 | 6 | 16 | 61 | 59 | 15 | HC vs OFF | ||
| Task: | Finger-to-thumb opposition movements and fist clenching with right hand | |||||||||
| Samuel et al, 1997 | PET | 6 | 6 | 17.7 | 70.2 | 64.3 | 7 | HC vs OFF | ||
| Task: | Sequential finger movements of right hand at 0.33 Hz | |||||||||
| PET | 6 | 6 | 17.7 | 70.2 | 64.3 | 10 | HC vs OFF | |||
| Task: | Bimanual sequential finger movements at 0.33 Hz | |||||||||
| Tessa et al, 2010 | fMRI | 15 | 11 | 16.1 | 70.1 | 69 | 12 | HC vs OFF | ||
| Task: | Continuous tapping of right hand | |||||||||
| Tessa et al, 2012 | fMRI | 15 | 13 | 16.3 | 68.1 | 64.2 | 4 | HC vs OFF | ||
| Task: | Continuous writing of the figure “8” with right hand | |||||||||
| Tessa et al, 2013 | fMRI | 11 | 10 | 13.5 | 67.7 | 64 | 6 | HC vs OFF | ||
| Task: | Continuous tapping of left hand | |||||||||
| Turner et al, 2003 | PET | 12 | 12 | 41.4 | 57 | 58 | 9 | HC vs OFF | ||
| Task: | Tracking task with right hand | |||||||||
| Wu et al, 2005 | fMRI | 12 | 12 | 25.5 | 61.2 | 61.8 | 12 | HC vs OFF | ||
| Task: | Sequential finger tapping with right hand at ∼0.5 Hz | |||||||||
| Wu et al, 2010 | fMRI | 15 | 15 | 20.7 | 59.7 | 60.3 | 15 | HC vs OFF | ||
| Task: | In-phase movements of both index fingers at ∼0.5 Hz | |||||||||
| fMRI | 15 | 15 | 20.7 | 59.7 | 60.3 | 20 | HC vs OFF | |||
| Task: | Antiphase movements of both index fingers at ∼0.5 Hz | |||||||||
| Wu et al, 2015 | fMRI | 26 | 26 | 13 | 59 | 58.9 | 7 | HC vs OFF | ||
| Task: | Tapping with right index finger at 0.3–0.5 Hz | |||||||||
| Wu et al, 2016 | fMRI | 18 | 18 | 20.4 | 60.4 | 59.9 | 11 | HC vs OFF | ||
| fMRI | 18 | n/a | 20.4 | 60.4 | n/a | 7 | ON vs OFF | |||
| Task: | Free writing in PD patients with consistent micrographia | |||||||||
| fMRI | 18 | 18 | 19.1 | 59.6 | 60 | 9 | HC vs OFF | |||
| fMRI | 18 | n/a | 19.1 | 59.6 | n/a | 4 | ON vs OFF | |||
| Task: | Free writing in PD patients with progressive micrographia | |||||||||
| Wurster et al, 2015 | fMRI | 10 | 10 | 20.7 | 66.4 | 64.9 | 2 | HC vs ON | ||
| Task: | Auditory-cued button press with right index finger at 1, 2.5, and 4 Hz (collapsed) | |||||||||
| Yan et al, 2015 | fMRI | 11 | 12 | 20.1 | 61.5 | 65.5 | 5 | HC vs OFF | ||
| Task: | Auditory-cued finger-to-thumb movement with left hand | |||||||||
| fMRI | 11 | 12 | 20.1 | 61.5 | 65.5 | 4 | HC vs OFF | |||
| Task: | Auditory-cued finger-to-thumb movement with right hand | |||||||||
HC, healthy control participants; OFF, Parkinson’s disease patients off dopaminergic medication; ON, Parkinson’s disease patients on dopaminergic medication; foci, number of activation foci reported in the respective study; n/a, not applicable.
Activation Likelihood Estimation Meta-Analysis
The meta-analyses were carried out using the revised version50 of the activation likelihood estimation approach for coordinate-based meta-analyses.51 Activation likelihood estimation (ALE) tests whether there is a significant convergence between activation foci from different experiments compared with a random distribution of foci. Because the term “experiment” refers to a contrast of interest (eg, PD-ON vs PD-OFF) for a given study, 1 study can contribute with several experiments to the ALE. A detailed description of the ALE technique can be found elsewhere.50,52 In short, activation foci from different experiments were modeled as spatial 3-dimensional Gaussian probability distributions, where the size of the distribution depends on the number of participants in the respective experiment (in case of different numbers of participants for the PD and healthy control groups, the lower number was used). If coordinates were reported in Talairach space, they were transformed to Montreal Neurological Institute space using the tal2icbm method.53 Combining probabilities for foci in each experiment resulted in a modeled activation (MA) map. Subsequently, voxel-wise ALE scores were computed by taking the union of the MA maps describing the convergence of results across experiments at each gray matter voxel. The nonparametric P values of ALE scores were derived by the proportion of equal or higher values obtained under the assumption of random spatial association and thresholded at a cluster level–corrected threshold of P < 0.05 family-wise error–corrected.
Since publication of our first meta-analysis,13 it has been demonstrated that the results of meta-analyses comprising only few experiments are driven by single studies. We therefore now only conducted meta-analyses for contrasts based on >20 experiments.14 Thus, no meta-analyses were conducted for the contrasts PD-ON versus healthy controls (13 experiments for healthy controls (HC) > PD-ON and 7 experiments PD-ON > HC) or PD-ON versus PD-OFF (10 experiments for PD-ON > PD-OFF and 5 experiments for PD-OFF > PD-ON). For the same reason we did not conduct analyses separately for motor tasks that were externally or internally cued (there were <20 experiments for all contrasts with internally timed and internally chosen movements). There were sufficient experiments to conduct meta-analyses for the contrasts PD-OFF > HC (34 experiments) and HC > PD-OFF (36 experiments). We also conducted meta-analyses comparing HCs and PD patients irrespective of medication (ie, irrespective of whether patients were ON or OFF medication), which included 41 experiments for the contrast PD > HC and 49 experiments for the contrast HC > PD.
Even though there is currently no optimal approach to conduct ALE correlation analyses across the whole brain, we attempted to relate the observed underactivation in PD to disease severity as indexed by the mean UPDRS scores of the individual studies. To this end, we computed how much individual studies contributed to a given cluster and then entered this variable into a nonparametric Spearman correlation with the mean UPDRS score.
We also computed the probability of experiments detecting abnormal activation of the putamen in PD. To this end, we assessed whether a given experiment activated the putamen in the control group (detected in 25 experiments) and whether this experiment found decreased putamen activity in PD. This additional analysis was motivated by the observation that in many experiments the motor task mainly induced activation in cortical areas and less frequently in the basal ganglia. Given the important role of the putamen in motor symptoms in PD,2 this lack of striatal engagement seemed surprising and might be because of the specific experimental design and data acquisition. This analysis thus tried to circumvent this problem by only looking at the subsample of studies revealing putamen activation in healthy participants. We could not perform the same analysis for cortical and cerebellar changes because the exact localization of activation in healthy controls was often not given and we could not distinguish between activation of, for example, pre-SMA versus SMA or rostral premotor versus precentral gyrus. On the other hand, in the case of basal ganglia activation, it was explicitly mentioned whether the putamen was activated in almost all studies.
Meta-Analytic Connectivity Modeling
After having established which foci showed consistent differences in activation between PD patients and healthy controls, we further analyzed these foci regarding their functional task-related connectivity profiles. Meta-analytic connectivity modeling (MACM) tests consistent coactivation patterns of a volume of interest (VOI) with the rest of the brain. In short, experiments in healthy subjects, which report activation at the VOI (here, the foci with consistent activation differences from the ALE analysis) were retrieved from the BrainMap database.54,55 A coordinate-based meta-analysis was then performed using ALE, which generates a coactivation pattern across the whole brain for each voxel in each VOI. In other words, the computed pattern reflects which brain areas a given region is commonly coactivated with in healthy subjects, reflecting its functional task-related connectivity profile. For more details, see reference 56. Because dopaminergic deafferentation of the putamen in PD shows a rostrocaudal gradient with the most pronounced deafferentation in the caudal (posterior) putamen and relatively preserved innervation of the rostral (anterior) putamen, we hypothesized that activity of cortical areas that are primarily connected with the posterior putamen might be more affected in PD compared with cortical areas that are connected to more anterior parts of the putamen. To test this, we analyzed where the coactivation patterns of the different VOIs overlapped, indicating common functional connectivity. To minimize lateralization (eg, left M1 is primarily connected with left putamen), we only used cortical VOIs from the hemisphere contralateral to the most frequently used right hand (only 5 and 6 experiments, respectively, used the left hand for the contrasts HC > PD and PD > HC) in case of bilateral VOIs. Thus, based on the results from the ALE analysis (see below), we used left M1, SMA, and right cerebellum as VOIs for the contrast HC > PD and left rostral precentral gyrus/middle frontal gyrus and pre-SMA as VOIs for the contrast PD > HC. We then computed 2 overlap images, one of the MACM maps of each of the VOIs for the contrast HC > PD and one for the VOIs for the contrast PD > HC. These 2 overlap images reflect which functional connectivity patterns are common for all VOIs of each contrast. Because we were mostly interested in the putamen (see above), we then used a mask of the bilateral putamen created using the automated anatomical labeling atlas57 to assess which areas of the putamen were consistently coactivated with areas that were more and less activated in PD.
Results
Thirty-nine publications (36 fMRI, 3 H2O15-PET) were included. Meta-analyses were conducted for contrasts comparing HCs with PD patients irrespective of medication as well as contrasts comparing HCs with PD patients OFF medication. Because only 3 studies used H2O15-PET, we also conducted the same meta-analyses without including H2O15-PET studies, which yielded identical results. The number of experiments was too low for comparing HCs with PD patients ON medication or comparing PD patients ON versus OFF medication (see Methods for more details).
Decreased Activation in Patients with PD
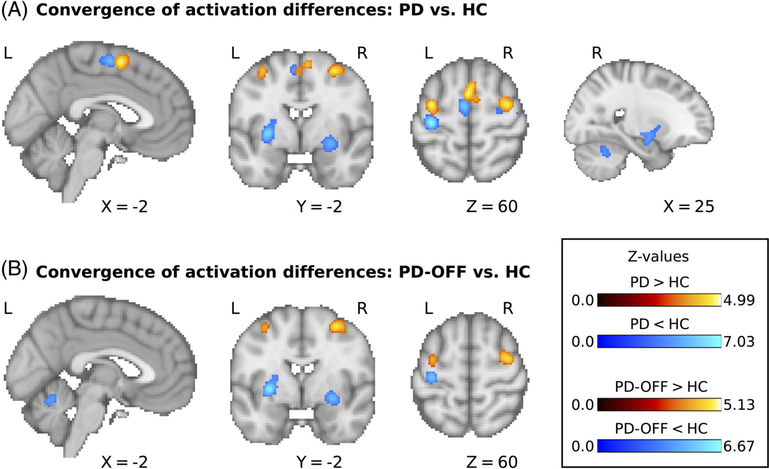
We first assessed areas that consistently showed decreased motor-related activation in PD. Forty-nine experiments (420 unique subjects; average sample size, 14.0) reported results for the contrast HC > PD. The meta-analysis revealed significant convergence of activation differences in the left and right posterior putamen (detected in 17 and 18 experiments, respecrively, corresponding to 35% and 37%, respectively, of all experiments), left and right precentral gyrus (12 and 10 experiments, respectively, corresponding to 24% and 20%, respectively), SMA (11 experiments, 22%) and right cerebellar lobule 6 (8 experiments, 16%); see Figure 1A and Table 2. When only considering studies in which PD patients were tested off dopaminergic medication, there were 36 experiments with 345 unique subjects and an average sample size of 14.0 that reported results for the contrast HC > PD-OFF. Activation differences converged in the left and right posterior putamen (detected in 13 and 14 experiments, respectively, corresponding to 36% and 39%, respectively), left precentral gyrus (8 experiments, 22%), and left cerebellar lobule 5/vermis (7 experiments, 19%); see Figure 1B and Table 2. None of the detected areas showing decreased activation in PD correlated with differences in disease severity across studies, as indexed by mean UPDRS scores (all Puncorrected > 0.05; see Methods for more details).
FIG. 1.
(A) Significant clusters for the comparison of motor-related activity between PD patients and HC. (B) Significant clusters for the comparison between PD patients off dopaminergic medication and HC. L, left; R, right; PD, Parkinson’s disease; HC, healthy control participants.
TABLE 2.
Results of ALE analyses for all between-group contrasts
| MNI coordinates (at peak) |
|||||
|---|---|---|---|---|---|
| Side | x | y | z | Z value (at peak) | |
| Decreased activation in PD compared with HC | |||||
| Putamen | Right | 30 | −10 | 6 | 5.78 |
| Putamen | Left | −30 | −8 | 2 | 6.87 |
| Precentral gyrus | Left | −34 | −22 | 62 | 7.03 |
| Precentral gyrus | Right | 36 | −20 | 72 | 5.18 |
| Supplementary motor area | Left | −4 | −6 | 58 | 5.68 |
| Cerebellum, lobule VI | Right | 26 | −54 | −30 | 4.27 |
| Decreased activation in PD-OFF compared with HC | |||||
| Putamena | Right | 30 | −10 | 6 | 5.26 |
| Putamen | Left | −30 | −4 | 0 | 6.67 |
| Precentral gyrus | Left | −34 | −22 | 62 | 5.68 |
| Cerebellum, lobule V/vermis | Left | −6 | −60 | −14 | 4.29 |
| Increased activation in PD compared with HC | |||||
| Pre-supplementary motor area | Left | −2 | 2 | 58 | 4.77 |
| Precentral gyrus/middle frontal gyrus | Left | −34 | −6 | 58 | 4.81 |
| Precentral gyrus/middle frontal gyrus | Right | 32 | −6 | 56 | 4.99 |
| Increased activation in PD-OFF compared with HC | |||||
| Precentral gyrus/middle frontal gyrus | Right | 30 | −4 | 56 | 4.77 |
| Precentral gyrus/middle frontal gyrus | Left | −34 | 2 | 52 | 4.33 |
Clusters with convergence of activation maxima are reported at a threshold of 0.05 family-wise error corrected at the cluster level.
The second peak of the cluster is listed because the first peak was localized in white matter.
Increased Activation in PD
We then analyzed which areas consistently showed increased motor-related activation in PD. Forty-one experiments with 369 unique subjects and an average sample size of 13.9 reported results for the contrast PD > HC. We found significant convergence of activation differences in pre–supplementary motor area (detected in 13 experiments, corresponding to 32%), as well as left and right rostral precentral gyrus/middle frontal gyrus (both detected in 13 experiments, corresponding to 32%); see Figure 1A and Table 2. When limiting the meta-analysis to studies of PD patients off medication there were 34 experiments with 300 unique subjects and an average sample size of 13.7 that reported results for the contrast PD-OFF > HC. This meta-analysis showed significant convergence of activation differences in the left and right rostral precentral gyrus/middle frontal gyrus (detected in 8 and 11 experiments, respectively, corresponding to 24% and 32%, respectively); see Figure 1B and Table 2.
Probability of Detecting Decreased Putamen Activation in PD
Even though the posterior putamen was the area that was most consistently underactivated in PD, it was only reported in roughly a third of all experiments (see above), which is somewhat surprising given the pivotal role of the putamen in pathophysiological models of PD.2 Because we observed that many of the included studies used motor tasks that primarily induced cortical activation, we hypothesized that some of these studies were not suited to detect decreased activation of the putamen in PD because the experimental task or study design was suboptimal for detecting task-related activity in the putamen. To test this, we analyzed whether a given experiment induced activation of the putamen in the control group and, if so, whether this experiment found abnormal putamen activation in PD. This analysis showed that 21 of the 25 experiments in which putamen activation was found in the healthy control group were able to detect decreased activation of the putamen in PD (corresponding to 84%), whereas only 4 of these experiments (ie, 16%) were not able to detect this difference. There were no experiments that found decreased activation of the putamen in PD without detecting putamen activity in the healthy control group. Thus, when using experimental paradigms that robustly activate the putamen, the probability of detecting hypoactivation in PD is much higher than reflected by the ALE analysis across all tasks (84% vs 35%–39%).
Meta-Analytic Connectivity Modeling
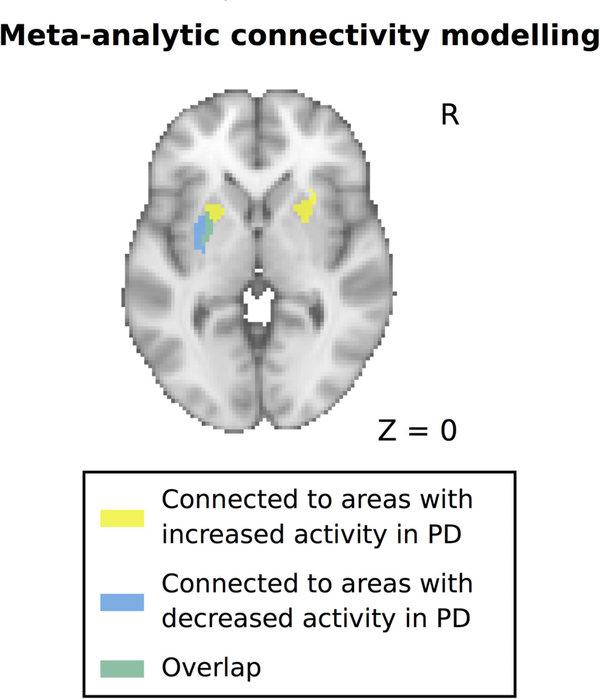
Because dopaminergic deafferentation of the putamen in PD shows a prominent caudal-to-rostral gradient, we hypothesized that areas showing decreased and increased activation in PD might be connected to distinct subareas of the putamen, with areas showing decreased activity being mainly connected to the more affected caudal (posterior) putamen, which contains the motor territory of the striatum. To test this, we computed functional connectivity profiles of the areas showing abnormal activation in PD using MACM (see Methods for more details). In line with our hypothesis, we found that areas that showed decreased activation in PD were mainly connected with the posterior putamen, whetrsd areas showing increased activation in PD were connected with more anterior parts of the putamen (Fig. 2).
FIG. 2.
Functional connectivity profiles of areas with decreased and increased activity in PD with the putamen. Functional connectivity was computed using meta-analytic connectivity modeling and revealed a rostrocaudal gradient for areas with increased versus decreased activity in PD. PD, Parkinson’s disease.
Discussion
Using a meta-analytic ALE approach, we found consistent patterns of motor-related hypo- and hyperactivation in several cortical and subcortical areas in PD. The area that most consistently showed decreased activation in PD was the posterior putamen (about 35%–39% of experiments). This finding is in good agreement with previous meta-analyses13,58 as well as single-photon emission computed tomography and PET studies showing marked dopaminergic denervation of the posterior putamen in PD.59 We also found consistent hypoactivation of bilateral M1 and SMA (between 20% and 24% of experiments). Although decreased activation of these areas was less often reported, both areas have long been implicated in the pathophysiology of PD.19,60,61 Finally, there was consistent hypoactivation in the cerebellum. Although only relatively few studies reported decreased cerebellar activation (between 16% and 19% of experiments), it should be noted that most of the early studies had a limited field of view, which did not include the cerebellum. Furthermore, several studies reported increased activation of the cerebellum in PD.62,63 These discrepancies might be related to differences in the applied motor tasks, different PD phenotypes, or different subareas of the cerebellum. Future meta-analyses comprising a larger number of studies testing cerebellar activation in PD might help to further clarify the role of altered cerebellar activation in PD. We did not find correlations between reduced activity in these areas and the mean UPDRS scores of the individual studies, suggesting that the observed activity changes do not closely reflect disease progression or, alternatively, that the group average UPDRS scores are not sensitive enough for elucidating this relationship.
Most included studies did not report activation changes in all, but only in a subset of these areas, and a common underactivation only became evident in this meta-analytic approach. However, there is evidence from multivariate analyses of neuroimaging data that PD is related to a network dysfunction rather than abnormal function of isolated neural areas.64 Overlaying meta-analytic functional connectivity maps of the hypoactivated cortical areas on an anatomical map of the putamen revealed that these areas share a common pathway through the posterior putamen, the striatal area that is most affected by dopaminergic denervation in PD.65 Dopaminergic denervation is thought to result in an imbalance between a net inhibitory (indirect) and net facilitatory (direct) pathway that connects the cortex with the basal ganglia in a closed-loop fashion.66 This results in abnormal inhibition of the cortex by the basal ganglia that can be further modified by the cerebellum, which shares reciprocal disynaptic connections with the basal ganglia.67 The loop running through the posterior putamen is often referred to as a “motor loop” because it is thought to be primarily involved in processes related to movement execution and habitual movements.66
Does the reduced task-related activation of this network in PD have a correlate at the behavioral level? Although reverse inference should be taken with caution,68 there is strong evidence from neuroimaging and electrophysiological studies for a critical role of this network in the modulation of movement vigor. This has been demonstrated for the posterior putamen,69–72 SMA,70,73 M1,72,74–77 and the cerebellum.71,72,77–79 Furthermore, it should be noted that this meta-analysis was conducted in studies using a variety of motor tasks implying that any detected difference should not be specific to a certain kind of movement, but rather a general process underlying motor execution. We speculate that the process that is probed in many of these neuroimaging studies in PD might be the modulation of movement vigor, which is a crucial aspect of motor control,80 and reduced movement vigor constitutes a core motor impairment in PD (clinically termed bradykinesia). This idea is supported by several neuroimaging studies in PD that directly tested movement vigor, for example, by recording force production, and found decreased activation in the posterior putamen, precentral gyrus, SMA, and cerebellum.5,7,36,79
We also detected areas that consistently showed increased activation in PD, a midline cluster primarily involving the pre-SMA (32% of experiments) and the bilateral rostral precentral gyrus/middle frontal gyrus (24%–32% of experiments). Interestingly, both the midline cluster and the more lateral clusters were localized directly anterior to areas that showed decreased activation in PD, namely, SMA and bilateral precentral gyrus (see Fig. 1). Anatomical studies have demonstrated a rostrocaudal gradient in both the medial prefrontal cortex (comprising the pre-SMA and SMA) and the premotor cortex, where the more rostral areas are connected to the prefrontal areas, whereas the more caudal areas are connected to the primary motor cortex and the spinal cord.81 This gradient is also reflected in distinct connectivity patterns with the basal ganglia, where more rostral cortical areas are connected to more rostral (and ventral) parts of the striatum.82 The more rostrally localized loop is often referred to as the “associative” loop and is thought to be primarily related to executive control of movements and goal-directed behavior.66,83 In line with these previous studies, the meta-analytic functional connectivity profiles of pre-SMA and premotor cortex in the MACM analysis showed common coactivation with the anterior putamen, which is relatively spared from dopaminergic denervation in PD. Of note, this coactivation was observed bilaterally, which might indicate less lateralization of this loop compared with the motor loop running through the posterior putamen. It has previously been suggested that PD patients might rely more on effortful or “goal-directed” behavior, which is related to the associative cortical–basal ganglia loop, because more “automatic” motor behavior, which has been related to the motor cortical–basal ganglia loop, is impaired.84 Similarly, it has been suggested that PD patients recruit areas that are involved in externally cued movements to compensate for impairments in internally generated movements.85 However, this remains speculative, and it should be noted that increased cortical activation of rostral motor areas in PD might not exclusively have compensatory effects but could also have deleterious effects. For example, increased activation of the pre-SMA in PD has been demonstrated in patients developing involuntary “dyskinesia” movements as a side effect of dopaminergic therapy.86,87 Elucidating the role of these areas in PD warrants further research.
In conclusion, we were able to detect distinct neural networks showing decreased and increased motor-related activation in PD using a meta-analytic approach. Meta-analyses should be continuously updated because the increasing number of studies that can be included further increases the sample size and reduces ambiguity of the results (see, eg, the current meta-analysis and our previous analysis from 2014). This might also allow analyzing contrasts that we were not able to test in the current analysis because of the limited number of individual experiments, such as PD-ON versus PD-OFF to elucidate effects of dopaminergic medication on neural activity in PD. To facilitate this, we will make all data from this meta-analysis publicly available on ANIMA (anima.inm7.de), including Excel sheets with the coordinates from all studies, the ALE software, and corresponding scripts. This allows replication of the results and will hopefully facilitate revised meta-analyses in the future.
Acknowledgments
Funding agencies: D.M.H. is supported by a postdoctoral grant from the Independent Research Fund Denmark (0168-00014B). D.M. is supported by a project grant of the NovoNordisk Foundation (NNF16OC0023090). S.B.E. and J.A.C. acknowledge funding by the National Institute of Mental Health (R01-MH074457), the Helmholtz Portfolio Theme “Supercomputing and Modeling for the Human Brain,” and the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement No. 945539 (HBP SGA3). H.R.S. holds a 5-year professorship in precision medicine at the Faculty of Health Sciences and Medicine, University of Copenhagen, which is sponsored by the Lundbeck Foundation (Grant No. R186-2015-2138).
Footnotes
Relevant conflicts of interest/financial disclosures: None.
References
- 1.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55(3):181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obeso JA, Stamelou M, Goetz CG, et al. Past, present, and future of Parkinson’s disease: a special essay on the 200th anniversary of the shaking palsy. Mov Disord 2017;32(9):1264–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hallett M, Khoshbin S. A physiological mechanism of bradykinesia. Brain 1980;103(2):301–314. [DOI] [PubMed] [Google Scholar]
- 4.Meder D, Herz DM, Rowe JB, Lehericy S, Siebner HR. The role of dopamine in the brain - lessons learned from Parkinson’s disease. Neuroimage 2019;190:79–93. [DOI] [PubMed] [Google Scholar]
- 5.Burciu RG, Ofori E, Shukla P, et al. Distinct patterns of brain activity in progressive supranuclear palsy and Parkinson’s disease. Mov Disord 2015;30(9):1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haslinger B, Erhard P, Kampfe N, et al. Event-related functional magnetic resonance imaging in Parkinson’s disease before and after levodopa. Brain 2001;124(Pt 3):558–570. [DOI] [PubMed] [Google Scholar]
- 7.Spraker MB, Prodoehl J, Corcos DM, Comella CL, Vaillancourt DE. Basal ganglia hypoactivity during grip force in drug naive Parkinson’s disease. Hum Brain Mapp 2010;31(12): 1928–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu T, Wang L, Hallett M, Li K, Chan P. Neural correlates of bimanual anti-phase and in-phase movements in Parkinson’s disease. Brain 2010;133(Pt 8):2394–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caproni S, Muti M, Principi M, et al. Complexity of motor sequences and cortical reorganization in Parkinson’s disease: a functional MRI study. PLoS One 2013;8(6):e66834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerasa A, Hagberg GE, Peppe A, et al. Functional changes in the activity of cerebellum and frontostriatal regions during externally and internally timed movement in Parkinson’s disease. Brain Res Bull 2006;71(1–3):259–269. [DOI] [PubMed] [Google Scholar]
- 11.Eckert T, Peschel T, Heinze HJ, Rotte M. Increased pre-SMA activation in early PD patients during simple self-initiated hand movements. J Neurol 2006;253(2):199–207. [DOI] [PubMed] [Google Scholar]
- 12.Turner RS, Grafton ST, McIntosh AR, DeLong MR, Hoffman JM. The functional anatomy of parkinsonian bradykinesia. Neuroimage 2003;19(1):163–179. [DOI] [PubMed] [Google Scholar]
- 13.Herz DM, Eickhoff SB, Lokkegaard A, Siebner HR. Functional neuroimaging of motor control in Parkinson’s disease: a meta-analysis. Hum Brain Mapp 2014;35(7):3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eickhoff SB, Nichols TE, Laird AR, et al. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage 2016;137:70–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradberry TJ, Metman LV, Contreras-Vidal JL, et al. Common and unique responses to dopamine agonist therapy and deep brain stimulation in Parkinson’s disease: an H(2)(15)O PET study. Brain Stimul 2012;5(4):605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko JH, Mure H, Tang CC, et al. Parkinson’s disease: increased motor network activity in the absence of movement. J Neurosci 2013;33(10):4540–4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwingenschuh P, Katschnig P, Jehna M, et al. Levodopa changes brain motor network function during ankle movements in Parkinson’s disease. J Neural Transm (Vienna) 2013;120(3): 423–433. [DOI] [PubMed] [Google Scholar]
- 18.Baglio F, Blasi V, Falini A, et al. Functional brain changes in early Parkinson’s disease during motor response and motor inhibition. Neurobiol Aging 2011;32(1):115–124. [DOI] [PubMed] [Google Scholar]
- 19.Buhmann C, Glauche V, Sturenburg HJ, Oechsner M, Weiller C, Buchel C. Pharmacologically modulated fMRI—cortical responsiveness to levodopa in drug-naive hemiparkinsonian patients. Brain 2003;126(Pt 2):451–461. [DOI] [PubMed] [Google Scholar]
- 20.Drucker JH, Sathian K, Crosson B, et al. Internally guided lower limb movement recruits compensatory cerebellar activity in people with Parkinson’s disease. Front Neurol 2019;10:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Garcia N, Armony JL, Soto J, Trejo D, Alegria MA, Drucker-Colin R. Effects of rTMS on Parkinson’s disease: a longitudinal fMRI study. J Neurol 2011;258(7):1268–1280. [DOI] [PubMed] [Google Scholar]
- 22.Holiga S, Moller HE, Sieger T, Schroeter ML, Jech R, Mueller K. Accounting for movement increases sensitivity in detecting brain activity in Parkinson’s disease. PLoS One 2012;7(5):e36271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes LE, Barker RA, Owen AM, Rowe JB. Parkinson’s disease and healthy aging: independent and interacting effects on action selection. Hum Brain Mapp 2010;31(12):1886–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia Q, Gao L, Zhang J, Wu T, Chan P. Altered functional connectivity of the subthalamic nucleus during self-initiated movement in Parkinson’s disease. J Neuroradiol 2018;45(4):249–255. [DOI] [PubMed] [Google Scholar]
- 25.Katschnig P, Schwingenschuh P, Jehna M, et al. Altered functional organization of the motor system related to ankle movements in Parkinson’s disease: insights from functional MRI. J Neural Transm (Vienna) 2011;118(5):783–793. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Zhang K, Cai W, et al. Dopamine-related dissociation of cortical and subcortical brain activations in cognitively unimpaired Parkinson’s disease patients OFF and ON medications.Neuropsychologia 2018;119:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraft E, Loichinger W, Diepers M, et al. Levodopa-induced striatal activation in Parkinson’s disease: a functional MRI study. Parkinsonism Relat Disord 2009;15(8):558–563. [DOI] [PubMed] [Google Scholar]
- 28.Maillet A, Krainik A, Debu B, et al. Levodopa effects on hand and speech movements in patients with Parkinson’s disease: a FMRI study. PLoS One 2012;7(10):e46541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mak MK, Cheung V, Ma S, et al. Increased cognitive control during execution of finger tap movement in people with Parkinson’s disease. J Parkinsons Dis 2016;6(3):639–650. [DOI] [PubMed] [Google Scholar]
- 30.Mallol R, Barros-Loscertales A, Lopez M, Belloch V, Parcet MA, Avila C. Compensatory cortical mechanisms in Parkinson’s disease evidenced with fMRI during the performance of pre-learned sequential movements. Brain Res 2007;1147:265–271. [DOI] [PubMed] [Google Scholar]
- 31.Martin JA, Zimmermann N, Scheef L, et al. Disentangling motor planning and motor execution in unmedicated de novo Parkinson’s disease patients: an fMRI study. Neuroimage Clin 2019;22:101784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattay VS, Tessitore A, Callicott JH, et al. Dopaminergic modulation of cortical function in patients with Parkinson’s disease. Ann Neurol 2002;51(2):156–164. [DOI] [PubMed] [Google Scholar]
- 33.Mohl B, Berman BD, Shelton E, Tanabe J. Levodopa response differs in Parkinson’s motor subtypes: a task-based effective connectivity study. J Comp Neurol 2017;525(9):2192–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Payoux P, Brefel-Courbon C, Ory-Magne F, et al. Motor activation in multiple system atrophy and Parkinson disease: a PET study. Neurology 2010;75(13):1174–1180. [DOI] [PubMed] [Google Scholar]
- 35.Pinto S, Mancini L, Jahanshahi M, et al. Functional magnetic resonance imaging exploration of combined hand and speech movements in Parkinson’s disease. Mov Disord 2011;26(12):2212–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Planetta PJ, Kurani AS, Shukla P, et al. Distinct functional and macrostructural brain changes in Parkinson’s disease and multiple system atrophy. Hum Brain Mapp 2015;36(3):1165–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poisson A, Ballanger B, Metereau E, et al. A functional magnetic resonance imaging study of pathophysiological changes responsible for mirror movements in Parkinson’s disease. PLoS One 2013;8(6): e66910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rottschy C, Kleiman A, Dogan I, et al. Diminished activation of motor working-memory networks in Parkinson’s disease. PLoS One 2013;8(4):e61786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rowe J, Stephan KE, Friston K, Frackowiak R, Lees A, Passingham R. Attention to action in Parkinson’s disease: impaired effective connectivity among frontal cortical regions. Brain 2002; 125(Pt 2):276–289. [DOI] [PubMed] [Google Scholar]
- 40.Sabatini U, Boulanouar K, Fabre N, et al. Cortical motor reorganization in akinetic patients with Parkinson’s disease: a functional MRI study. Brain 2000;123(Pt 2):394–403. [DOI] [PubMed] [Google Scholar]
- 41.Samuel M, Ceballos-Baumann AO, Blin J, et al. Evidence for lateral premotor and parietal overactivity in Parkinson’s disease during sequential and bimanual movements. A PET study. Brain 1997;120 (Pt 6):963–976. [DOI] [PubMed] [Google Scholar]
- 42.Tessa C, Diciotti S, Lucetti C, et al. fMRI changes in cortical activation during task performance with the unaffected hand partially reverse after ropinirole treatment in de novo Parkinson’s disease. Parkinsonism Relat Disord 2013;19(2):265–268. [DOI] [PubMed] [Google Scholar]
- 43.Tessa C, Lucetti C, Diciotti S, et al. Decreased and increased cortical activation coexist in de novo Parkinson’s disease. Exp Neurol 2010; 224(1):299–306. [DOI] [PubMed] [Google Scholar]
- 44.Tessa C, Lucetti C, Diciotti S, et al. Hypoactivation of the primary sensorimotor cortex in de novo Parkinson’s disease: a motor fMRI study under controlled conditions. Neuroradiology 2012;54(3): 261–268. [DOI] [PubMed] [Google Scholar]
- 45.Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain 2005;128(Pt 10): 2250–2259. [DOI] [PubMed] [Google Scholar]
- 46.Wu T, Hou Y, Hallett M, Zhang J, Chan P. Lateralization of brain activity pattern during unilateral movement in Parkinson’s disease. Hum Brain Mapp 2015;36(5):1878–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu T, Zhang J, Hallett M, Feng T, Hou Y, Chan P. Neural correlates underlying micrographia in Parkinson’s disease. Brain 2016; 139(Pt 1):144–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wurster CD, Graf H, Ackermann H, Groth K, Kassubek J, Riecker A. Neural correlates of rate-dependent finger-tapping in Parkinson’s disease. Brain Struct Funct 2015;220(3):1637–1648. [DOI] [PubMed] [Google Scholar]
- 49.Yan LR, Wu YB, Zeng XH, Gao LC. Dysfunctional putamen modulation during bimanual finger-to-thumb movement in patients with Parkinson’s disease. Front Hum Neurosci 2015;9:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage 2012;59(3): 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 2002;16(3 Pt 1):765–780. [DOI] [PubMed] [Google Scholar]
- 52.Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 2009;30(9): 2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lancaster JL, Tordesillas-Gutierrez D, Martinez M, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp 2007;28(11):1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fox PT, Lancaster JL. Opinion: mapping context and content: the BrainMap model. Nat Rev Neurosci 2002;3(4):319–321. [DOI] [PubMed] [Google Scholar]
- 55.Laird AR, Eickhoff SB, Fox PM, et al. The BrainMap strategy for standardization, sharing, and meta-analysis of neuroimaging data. BMC Res Notes 2011;4:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bzdok D, Laird AR, Zilles K, Fox PT, Eickhoff SB. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum Brain Mapp 2013;34(12):3247–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15(1):273–289. [DOI] [PubMed] [Google Scholar]
- 58.Xing Y, Tench C, Wongwandee M, Schwarz ST, Bajaj N, Auer DP. Coordinate based meta-analysis of motor functional imaging in Parkinson’s: disease-specific patterns and modulation by dopamine replacement and deep brain stimulation. Brain Imaging Behav 2020; 14(4):1263–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stoessl AJ. Neuroimaging in Parkinson’s disease: from pathology to diagnosis. Parkinsonism Relat Disord 2012;18(Suppl 1):S55–S59. [DOI] [PubMed] [Google Scholar]
- 60.Grafton ST. Contributions of functional imaging to understanding parkinsonian symptoms. Curr Opin Neurobiol 2004;14(6):715–719. [DOI] [PubMed] [Google Scholar]
- 61.Marsden CD. Slowness of movement in Parkinson’s disease. Mov Disord 1989;4(Suppl 1):S26–S37. [DOI] [PubMed] [Google Scholar]
- 62.Spay C, Meyer G, Welter ML, Lau B, Boulinguez P, Ballanger B. Functional imaging correlates of akinesia in Parkinson’s disease: still open issues. Neuroimage Clin 2019;21:101644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu T, Hallett M. The cerebellum in Parkinson’s disease. Brain 2013;136(Pt 3):696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holtbernd F, Eidelberg D. Functional brain networks in movement disorders: recent advances. Curr Opin Neurol 2012;25(4):392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med 1988;318 (14):876–880. [DOI] [PubMed] [Google Scholar]
- 66.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986;9:357–381. [DOI] [PubMed] [Google Scholar]
- 67.Bostan AC, Strick PL. The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci 2018;19(6):338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poldrack RA. Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. Neuron 2011;72(5): 692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Desmurget M, Grafton ST, Vindras P, Grea H, Turner RS. Basal ganglia network mediates the control of movement amplitude. Exp Brain Res 2003;153(2):197–209. [DOI] [PubMed] [Google Scholar]
- 70.Shirinbayan SI, Dreyer AM, Rieger JW. Cortical and subcortical areas involved in the regulation of reach movement speed in the human brain: an fMRI study. Hum Brain Mapp 2019;40(1): 151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Turner RS, Desmurget M, Grethe J, Crutcher MD, Grafton ST. Motor subcircuits mediating the control of movement extent and speed. J Neurophysiol 2003;90(6):3958–3966. [DOI] [PubMed] [Google Scholar]
- 72.Turner RS, Grafton ST, Votaw JR, Delong MR, Hoffman JM. Motor subcircuits mediating the control of movement velocity: a PET study. J Neurophysiol 1998;80(4):2162–2176. [DOI] [PubMed] [Google Scholar]
- 73.Tankus A, Yeshurun Y, Flash T, Fried I. Encoding of speed and direction of movement in the human supplementary motor area. J Neurosurg 2009;110(6):1304–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheney PD, Fetz EE. Functional classes of primate corticomotoneuronal cells and their relation to active force. J Neurophysiol 1980;44(4):773–791. [DOI] [PubMed] [Google Scholar]
- 75.Cherian A, Fernandes HL, Miller LE. Primary motor cortical discharge during force field adaptation reflects muscle-like dynamics. J Neurophysiol 2013;110(3):768–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Riehle A, Requin J. Monkey primary motor and premotor cortex: single-cell activity related to prior information about direction and extent of an intended movement. J Neurophysiol 1989;61(3): 534–549. [DOI] [PubMed] [Google Scholar]
- 77.Stark-Inbar A, Dayan E. Preferential encoding of movement amplitude and speed in the primary motor cortex and cerebellum. Hum Brain Mapp 2017;38(12):5970–5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fu QG, Mason CR, Flament D, Coltz JD, Ebner TJ. Movement kinematics encoded in complex spike discharge of primate cerebellar Purkinje cells. Neuroreport 1997;8(2):523–529. [DOI] [PubMed] [Google Scholar]
- 79.Spraker MB, Corcos DM, Kurani AS, Prodoehl J, Swinnen SP, Vaillancourt DE. Specific cerebellar regions are related to force amplitude and rate of force development. Neuroimage 2012;59(2): 1647–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dudman JT, Krakauer JW. The basal ganglia: from motor commands to the control of vigor. Curr Opin Neurobiol 2016;37: 158–166. [DOI] [PubMed] [Google Scholar]
- 81.Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol 2001;11(6):663–672. [DOI] [PubMed] [Google Scholar]
- 82.Kemp JM, Powell TP. The cortico-striate projection in the monkey. Brain 1970;93(3):525–546. [DOI] [PubMed] [Google Scholar]
- 83.Redgrave P, Vautrelle N, Reynolds JN. Functional properties of the basal ganglia’s re-entrant loop architecture: selection and reinforcement. Neuroscience 2011;198:138–151. [DOI] [PubMed] [Google Scholar]
- 84.Redgrave P, Rodriguez M, Smith Y, et al. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci 2010;11(11):760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brown RG, Marsden CD. Internal versus external cues and the control of attention in Parkinson’s disease. Brain 1988;111(Pt 2): 323–345. [DOI] [PubMed] [Google Scholar]
- 86.Cerasa A, Pugliese P, Messina D, et al. Prefrontal alterations in Parkinson’s disease with levodopa-induced dyskinesia during fMRI motor task. Mov Disord 2012;27(3):364–371. [DOI] [PubMed] [Google Scholar]
- 87.Herz DM, Haagensen BN, Christensen MS, et al. The acute brain response to levodopa heralds dyskinesias in Parkinson disease. Ann Neurol 2014;75(6):829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]