Abstract
Introduction:
Obesity has become increasingly prevalent worldwide and is a risk factor for many malignancies. We studied the correlation between body mass index (BMI) and the incidence of acute promyelocytic leukemia (APL), non-APL acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and control hospitalized patients without leukemia in the same community.
Methods:
Multi-center, retrospective analysis of 71,196 patients: APL (n=200), AML (n=437), ALL (n=103), non-leukemia hospitalized (n=70,456) admitted to University of Maryland and Johns Hopkins Cancer Centers, and University of Maryland Medical Center.
Results:
Patients with APL had a significantly higher unadjusted mean and median BMI (32.5 kg/m2 and 30.3 kg/m2) than those with AML (28.3 kg/m2 and 27.1 kg/m2), ALL (29.3 kg/m2 and 27.7 kg/m2), and others (29.3 kg/m2 and 27.7 kg/m2) (p<0.001). Log-transformed BMI multivariable models demonstrated that APL patients had a significantly higher adjusted mean BMI by 3.7 kg/m2 (p<0.001) or approximately 10% (p<0.01) compared to the other groups, when controlled for sex, race, and age.
Conclusions:
This study confirms that when controlled for sex, age, and race there is an independent association of higher BMI among patients with APL compared to patients with ALL, AML, and hospitalized individuals without leukemia in the same community.
Keywords: acute promyelocytic leukemia, APL, obesity, body mass index, BMI
1. Introduction:
The prevalence of overweight and obese individuals in developed western countries has been increasing at an alarming rate throughout the last few decades [1]. In addition to cardiovascular disease, kidney disease, diabetes, and musculoskeletal disorders, The International Agency for Research on Cancer (IARC) has acknowledged 13 cancers associated with overweight and obesity: gastrointestinal malignancies (esophageal adenocarcinoma, and cancers of gallbladder, liver, gastric cardia, pancreas, colon and rectum), gynecological cancers (ovary, corpus uteri), breast cancer in postmenopausal women, meningioma, multiple myeloma, and thyroid and renal cell cancers [2-8].
The association between excess body fat and different types of leukemias is not well characterized. In experimental animal models, an inverse association between caloric or dietary restriction and leukemia was observed [8,9]. A meta-analysis of 21 prospective cohort studies demonstrated that obesity was associated with increased incidence [relative risk (RR) 1.26 (95% confidence interval (CI) 1.17-1.37; p<0.001)] and mortality [RR 1.29 (95% CI 1.11-1.49; p = 0.001)] of leukemia in adults among all subtypes including acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML), and chronic lymphocytic leukemia (CLL) [10]. For AML, obesity was associated with an increased incidence, with RR of 1.53 (95% CI 1.26-1.85; p<0.001), compared to those with normal weight [10]. Once AML is categorized into acute promyelocytic leukemia (APL) vs non-APL, patients with APL had a significantly higher BMI than those with non-APL [7,11], with the BMI of patients with non-APL subtypes being similar to that of the general population when adjusted for age, sex, and race [11].
We performed a retrospective analysis of BMIs of patients with newly diagnosed APL, AML (non-APL subtypes), and ALL at the time of hospital admission, compared with patients without APL, AML, or ALL as a control, admitted to the University of Maryland Greenebaum Comprehensive Cancer Center (UMGCCC), the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center (SKCCC), and the University of Maryland Medical Center (UMMC) between 2002-2020.
2. Methods:
2.1. Study Population and Design
This two-center retrospective study was approved by the University of Maryland School of Medicine and the Johns Hopkins School of Medicine Institutional Review Boards. Patients age 18 years or older with newly diagnosed APL, AML, or ALL based on WHO 2008/2016 Criteria [12,13] initiating chemotherapy at UMGCCC, SKCCC between 2002 to 2020 were evaluated. Control patients were age 18 years or older without a history of APL/AML/ALL admitted (for the first admission) to UMMC from 2015 (when the EPIC electronic medical record was implemented hospital-wide) to May 2020.
Information on patient demographics including age and height/weight at diagnosis/upon admission, race/ethnicity as reported by patients on admission classified as Caucasian, African American, Hispanic, Asian, Indian, Middle Eastern, or unknown, and sex designated as male or female were collected. BMI was calculated as [BMI = weight (kilograms, kg)/(height (meters, m))2]. Patients were classified as underweight (<18.50 kg/m2), normal (18.50-24.99 kg/m2), overweight (25.00-29.99 kg/m2), or obese (≥30.00 kg/m2) according to WHO definitions [14].
2.2. Statistical Analysis
BMI was an independent variable in regression models. Patients with APL were further classified into low, intermediate, or high risk according to the Sanz score [15]. BMI was also compared to Breccia risk score, which predicts overall survival and disease-free survival, by using the Sanz score as a base, with 0 points for low risk, 1 point for intermediate, and 2 points for high risk; transcript type, 0 for bcr1/2 and 1 for bcr3; FLT3-ITD, 0 for absence and 1 for the presence; morphology, 0 for the classic form and 1 for the variant form; CD34 expression, 0 if absent and 1 if present [16]. Additional baseline characteristics compared with BMI were CD56 and CD15 expression at diagnosis, karyotype t(15;17) alone vs. with additional abnormalities, central nervous system (CNS) involvement at diagnosis, and relapse.
Descriptive statistics are presented using means with standard deviations and medians with interquartile ranges for continuous variables and percentages for categorical variables. Continuous variables were compared using t-test or ANOVA. Categorical variables were compared using Pearson chi-square or Fisher’s exact test. Multivariable linear models were used to estimate the adjusted average BMI of the comparison groups. To address potential non-linearity, we calculated multivariable linear regression models of log-transformed BMI. Regression model diagnostics were used to check for validity.
To account for missing data, we multiply-imputed missing data using the “mi” package [17]. All statistical tests were two-sided and p-values of less than 0.05 were considered significant. We used R statistical software (version 4.0.3) for analysis [18].
3. Results:
3.1. Baseline characteristics
The study included 71,196 patients, 200 with APL, 437 with AML, 103 with ALL, and 70,456 others. The median age was 55 years [inter quartile range (IQR) 37-68 years], and 35,744 (50.2%) were male. Median BMI was 27.7 kg/m2 (range 10.0-145.0 kg/m2, IQR 23.8 to 33.2 kg/m2). Racial distribution was 51.3% Caucasian, 41.5% African American, 5.2% unknown, and 1.8% Asian, with other races totaling less than 1% of the study population.
3.2. Characteristics of patients subdivided by type
Baseline patient characteristics divided by type are summarized in Table 1. Patients with APL had a significantly higher unadjusted mean (32.5 kg/m2, p<0.001) and median (30.3 kg/m2, p<0.001) BMI than those with AML (28.3 kg/m2 and 27.1 kg/m2), ALL (29.3 kg/m2 and 27.7 kg/m2), and all-comers (29.3 kg/m2 and 27.7 kg/m2) (Table 1, Figure 1). There was a significant difference in age and sex between the APL, AML, ALL, and other patients as well, with ALL and APL patients younger and APL patients being more frequently female (Table 1).
Table 1.
Baseline Characteristics
| Baseline Characteristics |
APL (n = 200) |
AML (n = 437) |
ALL (n = 103) |
All-comers (n = 70456) |
p value* |
|---|---|---|---|---|---|
| BMI, kg/m2, mean (SD) | 32.5 (8.8) | 28.3 (6.2) | 29.3 (8.7) | 29.3 (8.1) | <0.001 |
| BMI, kg/m2, median (range) | 30.3 (18.3-62.0) | 27.1 (16.0-56.3) | 27.7 (17.0-84.7) | 27.7 (10.0-145.0) | <0.001 |
| Age, years, mean (SD) | 49.9 (16.0) | 59.3 (15.2) | 43.1 (19.0) | 53.4 (19.0) | <0.001 |
| Sex, Male, n (%) | 90 (45.0) | 244 (55.8) | 68 (66.0) | 35342 (50.2) | <0.001 |
| Race, n (%) | |||||
| Caucasian | 107 (53.5) | 311 (71.2) | 55 (53.4) | 36043 (51.2) | <0.001 |
| African American | 51 (25.5) | 63 (14.4) | 25 (24.3) | 29401 (41.7) | |
| Asian | 6 (3.0) | 20 (4.6) | 5 (4.9) | 1264 (1.8) | |
| Middle Eastern | 1 (0.5) | 2 (0.5) | 1 (1) | 3 (0.004) | |
| Others | 16 (8.0) | 18 (4.1) | 17 (16.5) | 62 (0.1) | |
| Unknown | 19 (9.5) | 23 (5.3) | 0 (0) | 3683 (5.2) |
Abbreviations: APL, acute promyelocytic leukemia; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; BMI, body mass index; SD, standard deviation.
The p value depicted is for the comparison of all groups together.
Figure 1.
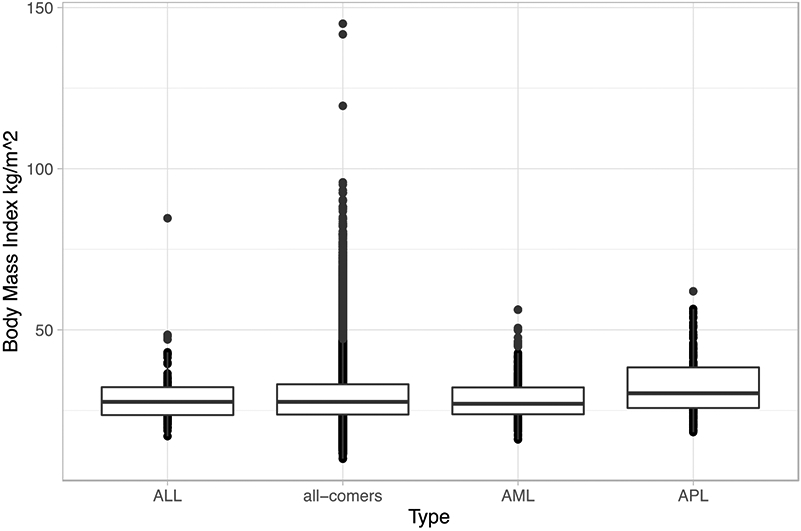
Box Plot of Unadjusted Body Mass Index Against Type
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; APL, acute promyelocytic leukemia
3.3. Multivariable models
We used multivariable linear regression to estimate the difference in BMI between the subgroups adjusted for gender, age, and race. Patients diagnosed with APL had a 3.0 kg/m2 higher adjusted BMI compared to patients diagnosed with ALL (95% CI: 1.08-4.92, P= 0.002) (Table 2). Similarly, patients with APL had a 3.5 kg/m2 higher adjusted BMI compared to patients diagnosed with AML (95% CI: 2.16-4.82, p<0.001), and 3.15 kg/m2 higher BMI compared to patients without acute leukemia diagnoses (95% CI: 2.05-4.26, p<0.001). Compared to all groups, patients with APL had a 3.7 kg/m2 higher BMI adjusted for age, gender and race (95% CI: 2.53-4.88, p<0.001).
Table 2.
Multivariable Analysis of Body Mass Index as Dependent Variable and Sex, Age, Race, and type as the Independent Variable.
| Estimate | Standard Error | T value | p Value | |
|---|---|---|---|---|
| Intercept | 32.195 | 0.892 | 36.094 | <0.001 |
| Sex, male | −1.310 | 0.596 | −2.199 | 0.028 |
| Age | −0.043 | 0.002 | −26.189 | <0.001 |
| Racea | ||||
| African American | 0.697 | 0.064 | 10.979 | <0.001 |
| Asian | −3.457 | 0.221 | −15.633 | <0.001 |
| Middle Eastern | −2.511 | 2.937 | −0.855 | 0.393 |
| Others | −1.111 | 0.783 | −1.419 | 0.157 |
| Typeb | ||||
| AML | −0.555 | 0.883 | −0.629 | 0.530 |
| APL | 3.007 | 0.980 | 3.069 | 0.002 |
| All-comers | −0.883 | 0.889 | −0.993 | 0.321 |
Abbreviations: AML, acute myeloid leukemia; APL, acute promyelocytic leukemia
Caucasian was used as the reference for each race.
Acute lymphoblastic leukemia was used as the reference for comparison.
On multivariable regression for log-transformed BMI, patients diagnosed with APL had a 9% higher adjusted-BMI compared to patients diagnosed with ALL (95% CI: 3%-14%, p=0.003). Similarly, patients diagnosed with APL had a higher adjusted-BMI compared to patients diagnosed with AML (delta: 10%, 95% CI: 6%-14%, p<0.001) and patients without acute leukemia diagnoses (delta:10%, 95% CI: 7%-14%, p=<0.001) (Table 3).
Table 3.
Multivariable Analysis of Log-Transformed Body Mass Index as Dependent Variable and Sex, Age, Race, and Type as the Independent Variable.
| Estimate | Standard Error | T value | p Value | |
|---|---|---|---|---|
| Intercept | 3.454 | 0.025 | 136.333 | <0.001 |
| Sex, male | −0.072 | 0.002 | −36.750 | <0.001 |
| Age | −0.001 | 5.28x10−5 | −23.110 | <0.001 |
| Racea | ||||
| African American | 0.012 | 0.002 | 6.346 | <0.001 |
| Asian | −0.118 | 0.007 | −16.563 | <0.001 |
| Middle Eastern | −0.071 | 0.095 | −0.745 | 0.456 |
| Others | −0.045 | 0.024 | −1.868 | 0.062 |
| Typeb | ||||
| AML | −0.015 | 0.028 | −0.554 | 0.579 |
| APL | 0.090 | 0.031 | 2.935 | 0.003 |
| All-comers | −0.012 | 0.025 | −0.486 | 0.627 |
Abbreviations: AML, acute myeloid leukemia; APL, acute promyelocytic leukemia
Caucasian was used as the reference for each race.
Acute lymphoblastic leukemia was used as the reference for comparison.
3.4. BMI and baseline characteristics among APL patients
In patients diagnosed with APL, we used regression models to check for statistical association between BMI (independent variable) and specific baseline characteristics (dependent variables). None of the univariable models showed statistically significant associations of higher BMI with the examined characteristics including risk status based on Sanz score or Breccia, WBC and platelet counts at diagnosis, FLT3-ITD, CD34/CD56/CD15 at diagnosis, t(15;17) alone vs. t(15;17) with additional abnormality, CNS involvement at diagnosis, and relapse status (Table 4).
Table 4.
Univariable Analysis of Body Mass Index as Independent Variable and baseline characteristics as the Dependent Variable.
| Estimate | Standard Error | p Value | |
|---|---|---|---|
| High risk, based on Sanz a | −0.002 | 0.018 | 0.895 |
| WBC at presentation b | −0.335 | 0.178 | 0.061 |
| Platelets at presentation b | −0.110 | 0.345 | 0.751 |
| FLT3-ITD mutation a | 0.010 | 0.021 | 0.650 |
| CD 34 expression a | −0.016 | 0.017 | 0.351 |
| CD 56 expression a | −0.023 | 0.026 | 0.380 |
| CD 15 expression a | −0.034 | 0.023 | 0.139 |
| Abnormal karyotype a | −0.021 | 0.029 | 0.465 |
| CNS involvement a | −0.025 | 0.065 | 0.701 |
| Relapse a | −0.049 | 0.056 | 0.379 |
| Brecia risk 1∣2 c | −0.646 | 0.715 | 0.366 |
| Brecia risk 2∣3 c | 0.916 | 0.719 | 0.203 |
Abbreviations: WBC: white blood cells. N.B. Intercepts and standard errors for logistic regression are un-exponentiated.
Calculated using logistic regression.
Calculated using linear regression.
Calculated using ordered logistic regression.
4. Discussion:
The purpose of this study was to assess the association between elevated BMI and the diagnosis of APL when compared to the non-APL acute leukemias and non-leukemic population in the same community. BMI of APL patients was significantly higher than those of patients with ALL, AML, and other diagnoses, with 3.2 to 4.2 kg/m2 and 2.6 to 3.2 kg/m2 higher mean and median values, respectively, in APL patients. Results of this study confirm that when controlled for sex, age, and race there is an independent association of higher BMI with APL compared ALL, AML, and other diagnoses.
The data in the current literature on the association of body fat and leukemias are somewhat conflicting and with this project we tried to clarify this more. Our observation aligns with previous literature describing higher BMI in APL patients compared to AML patients, with BMI of AML patients being similar to that of the general population averages [6,7,10,11]. Of note, other studies have utilized general population averages of BMI as a control; no study has directly used individual data of patients without leukemia admitted to the hospital in the same community as a control. On the other hand, in contrast to prior retrospective studies demonstrating an association between obesity and incidence of all leukemia subtypes (including both chronic and acute leukemias) [10,19], our study suggests that patients with AML and ALL are overweight but not obese, with BMI’s similar to those of the general hospitalized population in the community. Of note, all subtypes of leukemia were included in one group in one of the studies [19] and APL patients were included among AML patients in the other study [10]. Additionally, these studies often included self-reported height and weight values, which can leave room for error, whereas our study used directly measured values [10,19].
It has been demonstrated that among the US population, there is a higher proportion of obesity among those 40 years and older compared with those 20-39 years old, with the greatest proportion of obese individuals among non-Hispanic African Americans [20]. We attempted to mitigate these variations by age and race by correcting BMI for age, race, and sex. Our data suggest that regardless of age/race/sex, APL patients have higher BMI than those with AML and ALL. One limitation of our study is that our data are based solely on hospitalized patients, which may lead to an inherent selection bias; however, based on National Health and Nutrition Examination Survey data, the mean BMI of adults in the US is 29.1 kg/m2 [21], which is similar to the BMI of 29.3 kg/m2 found in hospitalized patients in our study.
The reason for the relationship between obesity and APL is unclear. Chronically increased insulin levels have been associated with cancers of breast, colon, pancreas, and endometrium [22-26]. Obesity can lead to insulin resistance, in turn causing an increase in insulin secretion. Chronic hyperinsulinemia can have tumorigenic effects thought to be due to the direct action of insulin on the insulin receptors in the pre-neoplastic target cells, or perhaps because hyperinsulinemia causes changes in endogenous hormone metabolism such as the promotion of insulin-like growth factor-1 (IGF-1) [25,27]. One study in mice demonstrated that increased fat intake leading to weight gain can promote leukemogenesis likely through the IGF-1 pathway [28]. While some ALL and AML cell lines express insulin and IGF-1 receptors (IGF-1R) [29-31], almost all of APL cell lines such as HL-60, NB4 and PL-21 express abundant IGF-1R protein and proliferate with IGF and IGF analogues stimulation and their growth and basal DNA synthesis decrease with monoclonal antibodies directed against the IGF-1R and other IGF antagonists [32-34]. Additionally, the leptin receptor, which is proliferative and bears anti-apoptotic effects when activated [35,36], is selectively up-regulated in the APL cells, whereas the normal promyelocytes lack its expression, suggesting a possible link between the leptin-rich environment in obese individuals and development of APL [35,36].
In conclusion, we highlight that there is an independent association of higher BMI in patients with APL compared to patients with ALL, AML, and control non-leukemic patients admitted to the hospital in the same community. On the basis of our findings, supported by the prior literature reports, we suggest adding APL to the list of the cancers that are associated with overweight and obesity.
Acknowledgments
Funding:
This work was partially supported by the University of Maryland Greenebaum Comprehensive Cancer Center Support grant (P30CA134274) and the State of Maryland’s Cigarette Restitution Funds.
Footnotes
Conflicts of Interest:
All authors declare that they do not have relevant competing interests.
References:
- 1.Di Cesare M, Bentham J, Stevens GA, Zhou B, Danaei G, Lu Y, et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Gonzalez AB, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363(23):2211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: A pooled analysis. PLoS One. 2013;8(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi EK, Park HB, Lee KH, Park JH, Eisenhut M, van der Vliet HJ, et al. Body mass index and 20 specific cancers: Re-analyses of dose-response meta-analyses of observational studies. Ann Oncol. 2018;29(3):749–57. [DOI] [PubMed] [Google Scholar]
- 5.Calle E, Rodriguez C, Walker-Thurmond K, Thun M. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults Eugenia. N Engl J Med. 2003;348(17):1625–38. [DOI] [PubMed] [Google Scholar]
- 6.Larsson SC, Wolk A. Overweight and obesity and incidence of leukemia: A meta-analysis of cohort studies. Int J Cancer. 2007;122(6):1418–21. [DOI] [PubMed] [Google Scholar]
- 7.Castillo JJ, Mulkey F, Geyer S, Kolitz JE, Blum W, Powell BL, et al. Relationship between obesity and clinical outcome in adults with acute myeloid leukemia: A pooled analysis from four CALGB (alliance) clinical trials. 2016;91(2):199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body Fatness and Cancer — Viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ray A, Cleary M. Animal models to study the interplay between cancer and obesity. In: Kolonin MG, editor. Adipose Tissue and Cancer. New York: Springer; 2013. p. 99–119. [Google Scholar]
- 10.Castillo JJ, Reagan JL, Ingham RR, Furman M, Dalia S, Merhi B, et al. Obesity but not overweight increases the incidence and mortality of leukemia in adults: A meta-analysis of prospective cohort studies. Leuk Res. 2012;36(7):868–75. [DOI] [PubMed] [Google Scholar]
- 11.Estey E, Thall P, Kantarjian H, Pierce S, Kornblau S, Keating M. Association between increased body mass index and a diagnosis of acute promyelocytic leukemia in patients with acute myeloid leukemia. Leukemia. 1997;11(10):1661–4. [DOI] [PubMed] [Google Scholar]
- 12.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–405. [DOI] [PubMed] [Google Scholar]
- 13.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. Lyon, France: IARC Press; 2008. 441 p. [Google Scholar]
- 14.WHO Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. WHO Tech Rep Ser 894 Geneva: World Heal Organ; 2000. [PubMed] [Google Scholar]
- 15.Sanz MA, Lo Coco F, Martin G, Avvisati G, Rayon C, Barbui T, et al. Definition of relapse risk and role of nonanthracycline drags for consolidation in patients with acute promyelocytic leukemia: A joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000;96(4):1247–53. [PubMed] [Google Scholar]
- 16.Breccia M, Stefania de Propris M, Molica M, Colafigli G, Minotti C, Diverio D, et al. Introducing biological features at diagnosis improves the relapse risk stratification in patients with acute promyelocytic leukemia treated with ATRA and chemotherapy. Am J Hematol. 2015;90(9):E181–2. [DOI] [PubMed] [Google Scholar]
- 17.Gelman A, Hill J, Su Y-S, Yajima M, Pittau M, Goodrich B, et al. R Foundation for Statistical Computing. Package ‘mi’. R CRAN. 2015. [Google Scholar]
- 18.R Development Core Team R. R: A language and environment for statistical computing. R foundation for statistical computing; Vienna, Austria; 2020. [Google Scholar]
- 19.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. [DOI] [PubMed] [Google Scholar]
- 20.Flegal KM, Carroll D, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA - J Am Med Assoc. 2012;307(5):491–7. [DOI] [PubMed] [Google Scholar]
- 21.Fryar CD, Kruszon-Moran D, Gu Q, Ogden CL. Mean body weight, height, waist circumference, and body mass index among adults: United States, 1999-2000 through 2015-2016. Natl Health Stat Report. 2018;2018(122):1999–2000. [PubMed] [Google Scholar]
- 22.Giovannucci E. Insulin, Insulin-Like Growth Factors and Colon Cancer: A Review of the Evidence. J Nutr. 2001;131(11):3109S–3120S. [DOI] [PubMed] [Google Scholar]
- 23.Carreras-Torres R, Johansson M, Gaborieau V, Haycock PC, Wade KH, Relton CL, et al. The Role of Obesity, Type 2 Diabetes, and Metabolic Factors in Pancreatic Cancer: A Mendelian Randomization Study. J Natl Cancer Inst. 2017;109(9):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: A meta-analysis. Int J Cancer. 2007;121(4):856–62. [DOI] [PubMed] [Google Scholar]
- 25.Tarasiuk A, Mosińska P, Fichna J. The mechanisms linking obesity to colon cancer: An overview. Obes Res Clin Pract. 2018;12(3):251–9. [DOI] [PubMed] [Google Scholar]
- 26.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: A synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1531–43. [PubMed] [Google Scholar]
- 27.Calle EE, Kaaks R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–91. [DOI] [PubMed] [Google Scholar]
- 28.Mazzarella L, Durfort T, Pelicci PG. Modelling The Influence Of Diet On APL Identifies Insulin-Growth Factor 1 As A Central Mediator and Provides A Mechanistic Rationale For Therapeutic Weight Loss. Blood. 2013;122(21):833–833. [Google Scholar]
- 29.Shimon I, Shpilberg O. The insulin-like growth factor system in regulation of normal and malignant hematopoiesis. Leuk Res. 1995;19(4):233–40. [DOI] [PubMed] [Google Scholar]
- 30.Doepfner KT, Spertini O, Arcaro A. Autocrine insulin-like growth factor-I signaling promotes growth and survival of human acute myeloid leukemia cells via the phosphoinositide 3-kinase/Akt pathway. Leukemia. 2007;21(9):1921–30. [DOI] [PubMed] [Google Scholar]
- 31.Esber EC, Buell DN, Leikin SL. Insulin binding of Acute Lymphocytic Leukemia Cells. Blood. 1976;48(1):33–9. [PubMed] [Google Scholar]
- 32.Holáň V, Minowada J. Selective enhancement of interleukin 1β production in myelomonocytic cell lines by insulin and its related cytokines. Immunol Lett. 1992;34(3):243–7. [DOI] [PubMed] [Google Scholar]
- 33.Verhagen HJMP, De Leeuw DC, Roemer MGM, Denkers F, Pouwels W, Rutten A, et al. IGFBP7 induces apoptosis of acute myeloid leukemia cells and synergizes with chemotherapy in suppression of leukemia cell survival. Cell Death Dis. 2014;5(6):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drexler HG, Quentmeier H, MacLeod RAF, Uphoff CC, Hu ZB. Leukemia cell lines: In vitro models for the study of acute promyelocytic leukemia. Leuk Res. 1995;19(10):681–91. [DOI] [PubMed] [Google Scholar]
- 35.Tabe Y, Konopleva M, Munsell MF, Marini FC, Zompetta C, McQueen T, et al. PML-RARα is associated with leptin-receptor induction: The role of mesenchymal stem cell-derived adipocytes in APL cell survival. Blood. 2004;103(5):1815–22. [DOI] [PubMed] [Google Scholar]
- 36.Konopleva M, Mikhail A, Estrov Z, Zhao S, Harris D, Sanchez-Williams G, et al. Expression and function of leptin receptor isoforms in myeloid leukemia and myelodysplastic syndromes: Proliferative and anti-apoptotic activities. Blood. 1999;93(5):1668–76. [PubMed] [Google Scholar]


