Abstract
Background:
Concurrent use of tobacco cigarettes and e-cigarettes (“dual use”) is common among tobacco users. Little is known about differences in demographics and toxicant exposure among subsets of dual users.
Methods:
We analyzed data from adult dual users (current every/someday users of tobacco cigarettes and/or e-cigarettes, n=792) included in the PATH Study Wave 1 (2013–2014) and provided urine samples. Samples were analyzed for biomarkers of exposure to nicotine and selected toxicants (tobacco specific nitrosamine NNK (NNAL), lead, cadmium, naphthalene (2-naphthol), pyrene (1-hydroxypyrene), acrylonitrile (CYMA), acrolein (CEMA), and acrylamide (AAMA)). Subsets of dual users were compared on demographic, behavioral, and biomarker measures to exclusive cigarette smokers (n=2,411) and exclusive e-cigarette users (n=247).
Results:
Most dual users were predominant cigarette smokers (70%), followed by daily dual users (13%), non-daily concurrent dual users (10%), and predominant vapers (7%). Dual users who smoked daily showed significantly higher biomarker concentrations compared to those who did not smoke daily. Patterns of e-cigarette use had little effect on toxicant exposure. Dual users with high toxicant exposure were generally older, female, and smoked more cigarettes per day. Dual users who had low levels of biomarkers of exposure were generally younger, male, and smoked non-daily.
Conclusions:
In this era, most dual users smoked cigarettes daily and used e-cigarettes occasionally. Cigarette smoking appears to be the primary driver of toxicant exposure among dual users, with little-to-no effect of e-cigarette use on biomarker levels. Results reinforce the need for dual users to stop smoking tobacco cigarettes to reduce toxicant exposure.
INTRODUCTION
In 2013–2014, approximately 5.5% of adults in the United States (U.S.) reported current e-cigarette use. Among these e-cigarette users, 70% reported current cigarette smoking.1 Dual use of tobacco cigarettes and e-cigarettes (herein referred to as “dual use”) is the most common poly-tobacco use behavior in the U.S., with nearly 23% of adult multiple tobacco product users reporting this poly-tobacco use pattern.1 Commonly indicated reasons for using e-cigarettes while continuing cigarette smoking include reducing health risks associated with smoking, circumventing smoke-free policies, reducing the number of cigarettes smoked per day (CPD), and as aids to help quit cigarettes.2 While dual use could represent a transitional behavior involving weaning smokers from tobacco cigarettes, dual use may also sustain continued cigarette use while substituting e-cigarettes in circumstances where conventional smoking is not permitted.3 Currently, data indicate that for most dual users, concurrent use of these products is a persistent behavior, or results in continued use of tobacco cigarettes alone.4
However, “dual use” is a broad label that is applied to a heterogeneous group of users with a wide range of behaviors, including variable frequency of smoking and vaping. Recently, data from an international observational study identified four distinct groups of dual users based on frequency of product use: “daily dual users” (concurrent users who report daily use of both products), “predominant smokers” (those who smoke cigarettes daily but use e-cigarettes less than daily), “predominant vapers” (those who use e-cigarettes daily but smoke cigarettes less than daily), and “non-daily concurrent dual users” (those who use both cigarettes and e-cigarettes non-daily.) These groups differed in nicotine dependence, attitudes toward smoking and vaping, and quit behaviors.5 In terms of psychosocial characteristics and behaviors, there are distinct differences within dual users that merit examination in other domains.
Although a significant proportion of dual users report that their primary reason for using e-cigarettes is to reduce harm from smoking tobacco cigarettes,2 at aggregate, studies have shown that dual users tend to exhibit similar levels of exposure to nicotine and toxicants when compared to exclusive cigarette smokers.6,7 However, different patterns of dual use (i.e., daily dual users, predominant smokers, predominant vapers, non-daily concurrent dual users)5 may result in differing levels of exposure to nicotine and toxicants,6 some of which may present greater or fewer negative health consequences. In a small international study of daily dual users, those who smoked fewer than five CPD exhibited lower levels of exposure to nicotine and toxicants when compared to daily dual users who smoked half a pack of cigarettes per day or more.8 Many biomarker studies employ small samples recruited using convenience-driven methods.7–12 Identifying differential exposures to nicotine and toxicants among dual users using population-based, nationally representative samples signifies an important gap in the research literature.4 Therefore, it is important to look further into the frequency and quantity of smoking and e-cigarette use among a nationally representative sample of dual users to better characterize tobacco-related exposures, and to understand the potential public health benefits and harms of e-cigarette use and dual use.
Using data from Wave 1 (2013–14) of the Population Assessment of Tobacco and Health (PATH) Study, we performed an analysis of dual users addressing two primary aims. First, we sought to characterize dual users based on their demographic, behavioral, and biomarker levels, with a primary focus on their frequency and intensity of smoking. Then, we compared the degree to which these dual users were similar to, or different from, exclusive users of e-cigarettes or tobacco cigarettes.
METHODS
Data Source
Data are from Wave 1 Restricted Use Files (RUF) and Biomarker Restricted-Use Files (BRUF) of the PATH Study (2013–14), a nationally representative, longitudinal cohort study designed to assess tobacco use patterns and associated health behaviors. Details on the study interview procedures, questionnaires, sampling, weighting, and information on accessing the data are available at https://doi.org/10.3886/Series606.13 At Wave 1, 32,320 adults aged 18 or older participated in the study. The weighted response rate for the household screener was 54.0%; among those who completed the adult interview, the weighted response rate for those providing a urine sample was 63.6%.13 Among adult respondents who provided a urine sample, a stratified probability sample of 11,522 adults was selected for laboratory analysis to ensure respondents represented diverse tobacco product use patterns, including users of multiple tobacco products and never users of any tobacco product; details are provided in the BRUF User Guide (http://doi.org/10.3886/ICPSR36840.userguide). Westat’s Institutional Review Board approved the study design and data collection protocol.
Biospecimen Collection and Laboratory Procedures
Consenting participants self-collected full-void spot urine samples in 500mL polypropylene containers. Samples were immediately placed in a Crēdo Cube shipper, which transported samples between 2°C and 8°C, and were shipped overnight to the PATH Study biorepository for storage and processing. Biomarkers were subsequently measured using highly selective mass spectrometric methods under a rigorous quality control/quality assurance program at the CDC Division of Laboratory Sciences.14–25
Analytic Sample
Our analysis built upon previous work6 and focused on “current product users”. To be included in the sample, respondents had to: 1) provide a urine sample for analysis, 2) report current every/someday use of tobacco cigarettes and/or e-cigarettes, 3) report no current (every/someday use) use of any other tobacco products, and 4) report no use of nicotine replacement therapies (NRT) in the last three days. The main group under study was “dual users” of tobacco cigarettes and e-cigarettes (n=792), stratified into four distinct groups developed by Borland et al 5 based on self-reported frequency of product use: daily cigarette smokers and daily e-cigarette users (“daily dual users”, n=90), daily cigarette smokers and non-daily e-cigarette users (“predominant smokers”, n=560), non-daily cigarette smokers and daily e-cigarette users (“predominant vapers”, n=55), and non-daily users of both products (“non-daily concurrent dual users”, n=87).
We compared the four subgroups of dual users to current exclusive cigarette smokers (n=2,411) and current exclusive e-cigarette users (n=247). In addition to the criteria above, exclusive cigarette smokers and dual users had to report smoking at least 100 cigarettes in their lifetime to be included in the study sample. The final analytic sample size was 3,450. In calculating adjusted geometric mean (GM) values, current exclusive e-cigarette users with urinary NNAL values in excess of 14.5 pg/mg creatinine were excluded (n=66) in order to rule out potential misclassification related to cigarette smoking status.26
User Characteristics and Tobacco Use Behaviors
User-related variables of interest included demographic information (including age, sex, race/ethnicity, and education level) and behavioral patterns of product use, including frequency, intensity, and time to first product. Frequency of product use was classified according to every day or some days use of cigarettes/e-cigarettes. Cumulative monthly exposure measures indicating intensity of cigarette/e-cigarette consumption were calculated by multiplying the number of cigarettes/e-cigarettes used per day by 30 (everyday product users) or by the number of days the product was used in the past 30 days (some days product users). This resulted in measures for the number of cigarettes smoked per month (CPM) and the number of e-cigarettes used per month (EPM). Time to first cigarette/e-cigarette use was also examined as an indicator of nicotine dependence. Due to data not missing at random for EPM (23%) and time to first e-cigarette (53%), these measures were categorized to allow the missing data to be considered in modelling to minimize the extent of bias in results. Both CPM/EPM measures were categorized using quartiles, with EPM having an additional category to indicate missing information. Time to first product was classified based on categories used in the Fagerstrom Test for Nicotine Dependence (FTND),27 with e-cigarette measures having an additional category to indicate missing information. Tests were performed to assess correlation between the predictors for potential collinearity issues and were determined to be within acceptable ranges.
Main Outcomes
Data for nine biomarkers of exposure selected from several classes of known tobacco constituents, including nicotine metabolites, tobacco-specific nitrosamines (TSNAs), metals, polycyclic aromatic hydrocarbons (PAHs), and volatile organic compounds (VOCs) served as the primary outcomes. References to analytic limits of detection (LOD) have been published elsewhere.6 Nicotine exposure was assessed using total nicotine equivalents-2, calculated by taking the molar sum of urinary cotinine and trans-3’-hydroxycotinine. Other selected biomarkers included those for tobacco-specific nitrosamine NNK (total NNAL), lead, cadmium, naphthalene (2-naphthol), pyrene (1-hydroxypyrene), acrylonitrile (CYMA), acrolein (CEMA), and acrylamide (AAMA). Representative biomarkers were chosen due to their documented association with tobacco-attributable illness or other adverse health effects, and their ability to discriminate between cigarette and e-cigarette use. The clinical significance of these biomarkers has been described elsewhere.6 In considering whether biomarker levels were linked to certain user demographics or tobacco use behavioral characteristics noted above, we estimated dual users’ likelihood of group membership into “low exposure”, “average exposure”, and “high exposure” groups. To create these measures, the person-weighted creatinine-adjusted biomarker distributions among dual users were split into quartiles. The lowest quartile (Q1) served as the “low exposure” group. The second and third quartiles (Q2 and Q3) were combined to represent as “average” exposure for dual users. The highest quartile (Q4) represented the “high exposure” group for a given biomarker.
Statistical Analysis
All questionnaire and biomarker data were initially examined using univariate and bivariate statistical procedures. We analyzed demographic and tobacco use characteristics according to dual use categories developed by Borland et al.5 For biomarker comparisons, preliminary analyses revealed that frequency of cigarette smoking appeared to be the primary driver of toxicant exposure. Therefore, adjusted GMs were calculated for each biomarker among dual users who smoked daily (i.e., predominant smokers and daily dual users) versus those who did not smoke daily (i.e., predominant vapers and non-daily concurrent dual users); these were compared to adjusted GMs for exclusive e-cigarette users and exclusive cigarette smokers with similar frequency of product use. Adjusted GMs were calculated by performing multiple linear regression using log-transformed biomarker values as outcomes, with controls for urinary creatinine, age, sex, race/ethnicity, exposure to secondhand smoke, and past 30-day use of cannabis. For all biomarkers other than nicotine, GMs also adjusted for TNE-2. TNE-2 was selected as a proxy adjustment variable for intensity of product use due to its ubiquitous presence across different nicotine-containing products and its documented relation to the number of cigarettes smoked, nicotine intake from e-cigarettes, and smoking topography (i.e., how an individual puffs on their product).28–30 Post-estimation procedures were run to obtain the adjusted marginal mean values of the natural log of the biomarker of interest and their respective 95% confidence intervals for each group (low/average/high dual user values, exclusive e-cigarette users, and exclusive cigarette smokers); these were exponentiated to produce the adjusted GM. Adjusted GMs were compared using Bonferroni-corrected pairwise contrasts following adjusted models.
In comparing demographic and behavioral correlates of dual users, multinomial logistic regression modeling was used to characterize dual users having low (Q1), average (Q2 + Q3), and high (Q4) biomarker levels. Three parallel sets of models were run to test associations 1 – among high and low dual users only (base referent category=average (Q2+Q3) exposure dual users), 2 – among all dual users compared to exclusive e-cigarette users (base referent category=exclusive e-cigarette users) and 3 - among all dual users compared to exclusive cigarette smokers (base referent category=exclusive cigarette smokers). Models were adjusted for relevant variables for the tobacco product of interest, - i.e., dual users were compared to each other on both tobacco cigarette and e-cigarette metrics, dual users vs. exclusive e-cigarette users were compared on e-cigarette metrics, and dual users vs. exclusive cigarette smokers were compared on tobacco cigarette metrics. All multinomial models were adjusted for age (continuous), sex, and race/ethnicity (White, non-Hispanic; non-white, non-Hispanic; Hispanic). Covariables for tobacco use behaviors included cigarettes/e-cigarettes used per month, frequency of cigarette/e-cigarette use (every day or some days), and time to first cigarette/e-cigarette.
Analyses were completed using svy commands in Stata version 14.0. All analyses were weighted using urine weights constructed specifically for the analyses of the PATH biomarker subsample data.31 Variance estimation was approached using balanced, repeated replications with Fay’s adjustment set to 0.3 to enhance the precision of the estimates. Estimates with relative standard errors greater than 30% have been flagged due to concerns of estimate stability. P-values <0.05 were considered statistically significant.
RESULTS
Demographic Characteristics of Dual Users
The distribution of demographic characteristics for dual users, exclusive cigarette smokers, and exclusive e-cigarette users has been described elsewhere.6 On average, all dual users tended to be similar in age to exclusive cigarette smokers and older than exclusive e-cigarette users (weighted χ2 F(2.91, 287.61)=6.35, p=0.0004), were more likely to be female than exclusive cigarette smokers (F(1,99)=9.20, p=0.003), identify as White compared to exclusive e-cigarette users and exclusive cigarette smokers (both p<0.05), and were generally more well educated than exclusive cigarette smokers (F(2.81,278.30)=8.80, p<0.001). All dual users and exclusive users were statistically similar in terms of cigarette and e-cigarette consumption.6
Cigarette Smoking and E-cigarette Use Patterns among Dual Users
The distribution of dual users according to frequency of product use was: predominant smokers (70%), followed by daily dual use (13%), non-daily concurrent dual use (10%), and predominant vapers (7%). Dual users who smoked daily (including predominant smokers and daily dual users) had similar cigarette and e-cigarette consumption to each other.6 Predominant smokers smoked 16.2 CPD on average and used 1.02 e-cigarettes per day on the days they used e-cigarettes, while daily dual users smoked 16.0 CPD and used 1.22 e-cigarettes per day. Predominant smokers and daily dual users exhibited small-medium positive associations with their CPD and urinary TNE-2 (Spearman ρ =0.24, p<0.001), NNAL (Spearman ρ =0.28, p<0.001), cadmium (Spearman ρ =0.17, p<0.001), 2-naphthol (Spearman ρ =0.14, p=0.002), CYMA (Spearman ρ =0.30, p<0.001), and CEMA (Spearman ρ =0.21, p<0.001) concentrations. These users differed in their history of e-cigarette use, with predominant vapers exhibiting the greatest propensity to have used e-cigarettes the previous year (20%). Daily dual users and predominant smokers exhibited the greatest propensity for smoking daily the previous year (84% and 94% respectively), with 69% of predominant vapers, and 32% of non-daily concurrent users smoking every day during the previous year.
Biomarkers of Exposure among Dual Users
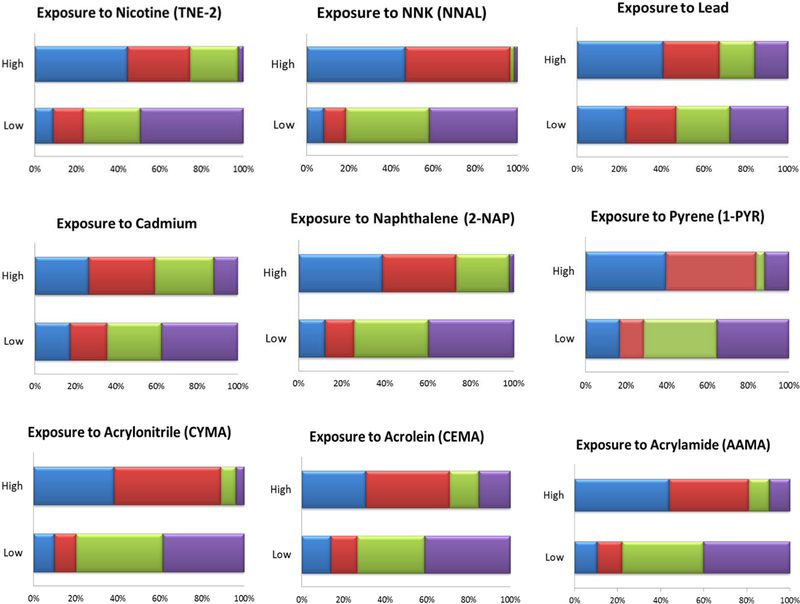
Figure 1 depicts the distribution of the four dual user subgroups into the low and high exposure categories for all nine biomarkers; average exposure dual users were omitted for presentation clarity. In general, classification into low and high exposure groups for each of the nine biomarkers followed a dose-response pattern correlated with self-reported frequency (daily/non-daily) of tobacco cigarette use.
Figure 1:
Weighted proportions of dual user subgroups accounting for low and high biomarker concentrations for total nicotine equivalents††, tobacco-specific nitrosamine NNK††, lead, cadmium, naphthalene, pyrene, acrylonitrile††, acrolein, and acrylamide (n=792)
††An estimate of the precision has been made, however it and the estimated statistic may not be valid due to skewness in the data.
The analytic sample size varies based on the specific biomarker (range: 761–792).
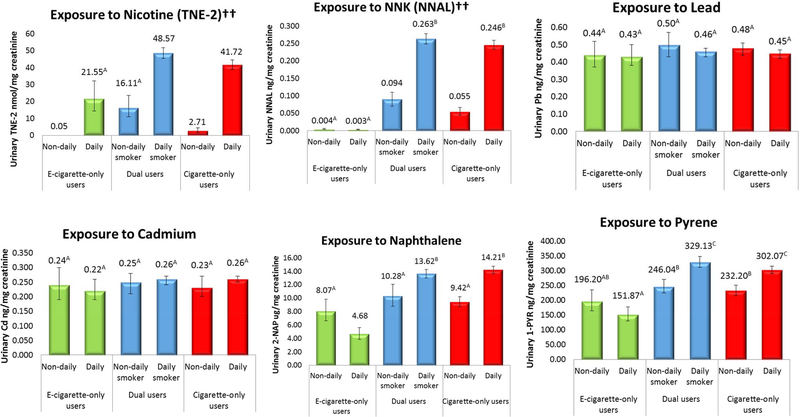
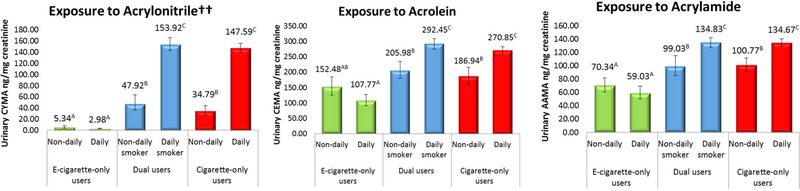
Comparisons of adjusted GMs for dual users in contrast to exclusive e-cigarette users and exclusive cigarette smokers can be viewed in Figure 2. Generally, dual users exhibited similar biomarker levels to exclusive cigarette smokers reporting the same frequency of smoking (daily or non-daily). GM toxicant concentrations for dual users mirrored those of exclusive cigarette smokers with the same reported frequency of smoking for NNAL, 2-naphthol, pyrene, acrylonitrile, and acrolein. Urinary lead and cadmium were statistically equivalent across all users. Nicotine exposure was significantly greater among dual users when compared to exclusive cigarette smokers with similar smoking frequency. There were no differences in nicotine exposure between dual users who were non-daily cigarette smokers and exclusive daily e-cigarette users. Dual users who smoked non-daily had significantly greater levels of biomarkers for naphthalene, NNK, naphthalene, pyrene, acrylonitrile, acrolein, and acrylamide than exclusive daily e-cigarette users. With few exceptions, dual users who smoked daily exhibited consistently greater adjusted GMs when compared to dual users who smoked non-daily.
Figure 2:
Weighted, adjusted geometric mean biomarker concentrations for total nicotine equivalents††, tobacco specific nitrosamine NNK††, lead, cadmium, naphthalene, pyrene, acrylonitrile††, acrolein, and acrylamide, by frequency of product use (n=3,384)
* “E-cigarette-only users”=exclusive e-cigarette users, “cigarette-only users”=exclusive cigarette smokers. Bars sharing a letter in the group label are not significantly different at the 5% level. Geometric means adjusted for urinary creatinine, age, sex, race/ethnicity, secondhand smoke exposure, past 30-day cannabis use, TNE-2 (for all biomarkers except nicotine). E-cigarette-only users with urinary NNAL concentrations exceeding 14.5 pg/mg creatinine were excluded from estimates.
††=An estimate of precision has been made, however the associated statistics may not be valid due to skewness in the data.
Association Between Users’ Demographics, Patterns of Product Use, and Biomarker Levels
Table 1 depicts a summary of results from 27 multinomial logistic regression models comparing demographic characteristics of dual users falling into low (Q1), average (Q2+Q3), and high (Q4) biomarker concentration groups. The full set of significant findings can be viewed in Supplemental Table 1. When comparing low and high exposure dual users to dual users with average exposure across all biomarkers, low exposure dual users were consistently younger, less likely to be female, tended to identify as racial/ethnic minorities (non-white, non-Hispanic or Hispanic), tended to smoke cigarettes some days rather than every day, and were less likely to consume a greater number of CPM. By contrast, high exposure dual users tended to be older, female, and have moderate EPM consumption, and smoked a high number of CPM.
Table 1:
Summary of findings from weighted multinomial logistic regression models comparing demographics and tobacco use behaviors among 1) low and high exposure to average exposure dual users, 2) low, average, and high exposure dual users to exclusive e-cigarette users, and 3) low, average, and high exposure dual users to exclusive cigarette smokers
| “Low Exposure” (Q1) | “Average exposure” (Q2 + Q3) | “High exposure” (Q4) | |
|---|---|---|---|
| 1: Low and high exposure dual users (base referent: average exposure dual users) | • Younger age (8/9 biomarkers) • Less likely to engage in low level monthly cigarette smoking (6/9 biomarkers) • Less likely to be female (5/9 biomarkers) • More likely to smoke cigarettes “some days” (4/9 biomarkers) • More likely to belong to a racial/ethnic minority group (2/9 biomarkers) • More likely to use moderate EPM (1/9 biomarkers) • Less likely to smoke 6–30 mins after waking (1/9 biomarkers) |
Base reference category for the outcome for multinomial model series 1 | • Older in age (7/9 biomarkers) • More likely to be female (6/9 biomarkers) • More likely to engage in moderate-heavy monthly cigarette smoking (6/9 biomarkers) • More likely to engage in moderate EPM (3/9 biomarkers) • More likely to be skipped out of EPM measures (2–9 biomarkers) • More likely to smoke first cigarette within first 30 minutes of the day (2/9 biomarkers) • Less likely to vape e-cigarettes “some days” (2/9 biomarkers) • Less likely to smoke cigarettes “some days” (1/9 biomarkers) • Less likely to have first e-cigarette within first five minutes of the day (1/9 biomarkers) |
| 2: Low, average, and high exposure dual users (base referent: exclusive e-cigarette users) | • More likely to use an e-cigarette “some days” (9/9 biomarkers) • Younger in age (5/9 biomarkers) • Less likely to be female (1/9 biomarkers) |
• Older in age (9/9 biomarkers) • Less likely to identify as racial/ethnic minority (9/9 biomarkers) • More likely to use e-cigarettes “some days” (9/9 biomarkers) |
• Older in age (9/9 biomarkers) • Less likely to identify as racial/ethnic minority (8/9 biomarkers) • More likely to use e-cigarettes “some days” (8/9 biomarkers) • More likely to be female (3/9 biomarkers) |
| 3: Low, average, and high exposure dual users (base referent: exclusive cigarette smokers) | • Younger in age (9/9 biomarkers) • More likely to smoke cigarettes “some days” (8/9 biomarkers) • Less likely to use high CPM (4/9 biomarkers) • Less likely to identify as racial/ethnic minority (3/9 biomarkers) |
• Less likely to identify as a racial/ethnic minority (9/9 biomarkers) • More likely to use moderate CPM (9/9 biomarkers) • More like to smoke cigarettes “some days” (3/9 biomarkers) • More likely to be female (2/9 biomarkers) |
• More likely to be female (9/9 biomarkers) • More likely to engage in moderate-heavy monthly cigarette smoking (9/9 biomarkers) • Older in age (5/9 biomarkers) • More likely to smoke cigarettes within first hour of waking (3/9 biomarkers) • More likely to smoke cigarettes “some days” (3/9 biomarkers) |
CPM = Cigarettes per month; EPM= E-cigarettes per month
Compared to exclusive e-cigarette users, low exposure dual users consistently tended to be younger and used e-cigarettes some days rather than every day. Average exposure dual users tended to be older than exclusive e-cigarette users, were less likely to be non-White, non-Hispanic or Hispanic rather than White, non-Hispanic, and were more likely to use e-cigarettes some days rather than every day. On average, high exposure dual users tended to be older than exclusive e-cigarette users, female, were less likely to be non-White, non-Hispanic or Hispanic rather than White, non-Hispanic, and were more likely to use e-cigarettes some days rather than every day. No statistically significant differences in intensity of e-cigarette use or time to first e-cigarette were detected between dual users and exclusive e-cigarette users.
Similar findings emerged when comparing dual users to exclusive cigarette smokers. Low exposure dual users tended to be younger than exclusive cigarette smokers, smoke some days rather than every day, and have high levels of monthly smoking. Average exposure dual users tended to be less likely to identify as non-white, non-Hispanic or Hispanic compared to exclusive cigarette smokers, were more likely to smoke some days rather than every day and were more likely to engage in moderate monthly smoking. High exposure dual users tended to be older than exclusive smokers, female, exhibit moderate-heavy levels of CPM, were more likely to smoke within the first hour of the day, and were more likely to smoke some days.
DISCUSSION
This study sought to characterize differences in tobacco-related constituent exposure profiles among dual users of tobacco cigarettes and e-cigarettes, and compare how these exposures, demographic characteristics, and tobacco-related behaviors compare to those of exclusive users of either product. Our findings suggest significant variability in nicotine and toxicant exposure among dual users, with continued, frequent cigarette smoking appearing to drive greater exposure to toxicants. Dual users with high toxicant exposure tended to be older in age, female, and to exhibit behaviors related to nicotine dependence and engrained cigarette smoking behaviors (including greater quantity of monthly smoking, infrequent vaping, and compared to exclusive cigarette smokers, evidence of a shorter time to first cigarette). By contrast, compared to dual users with average or high levels of toxicant exposure, dual users with lower levels of toxicant exposure tended to be younger in age, male, and to exhibit less frequent and lower quantity product use. These findings reinforce that dual users are a large and diverse group, which is evidenced not simply through their behaviors, but also by their toxicant exposure profiles.
Taken as a whole, our findings suggest that the majority of dual users mirror exposure profiles of exclusive cigarette smokers with similar smoking frequency.6 This reinforces findings by Borland et al that point toward the importance of product use frequency as a marker for delineation in subsets of dual users.5 Our findings extend this concept to note that, at the time of this data collection, the frequency of cigarette smoking served as an important demarcation for toxicant exposures, as well as select demographic characteristics. Specifically, select characteristics of low exposure dual users (i.e., younger, smoked fewer tobacco cigarettes) tended to mirror those for intermittent smokers. Akin to previous calls for research related to intermittent smoking,32 these data suggest the importance of conducting within-group assessments of dual users based on smoking frequency, as subsets of dual users with different smoking frequency likely exhibit distinct motives, quit intentions, and beliefs about their use that may correlate with exposure profiles and thus, potential negative health consequences arising from dual use.
The question of whether dual use serves as a bridge to cessation, or as a means to sustained tobacco cigarette smoking, remains important in light of increasing proliferation of e-cigarette use and e-cigarettes’ popularity among current smokers. Despite the significant, public emphasis toward the unknown long-term health effects of e-cigarette use, the health effects from cigarette smoking are well-documented.33 The majority of dual users in our study reported continued daily cigarette smoking in combination with their e-cigarette use; as such, many dual users exhibited high levels of toxicant exposure similar to exclusive cigarette smokers. It is important to communicate the need to completely quit using tobacco cigarettes to achieve any toxicant exposure reduction that e-cigarettes may provide. Recent data from the PATH Study indicate that cigarette smokers who reported daily use of e-cigarettes had 77% increased odds of achieving tobacco cigarette cessation 1–2 years later relative to cigarette smokers who did not use e-cigarettes.34 Conversely, the odds of cigarette smoking relapse were higher among former smokers who continued to use e-cigarettes more than one year after quitting cigarettes.35 The role of e-cigarettes as agents for cigarette smoking cessation warrants continued examination via longitudinal and randomized controlled trial designs, studies of contextual situations that may facilitate use of either product, and studies developing interventions to minimize negative health consequences among dual users.
Advantages of this study include the use of a nationally representative sample of never, former, and current tobacco product users from the U.S. non-institutionalized population to derive estimates of exposure and related demographic and behavioral correlates. The PATH Study includes detailed information related to tobacco use, including the ability to control for important confounders, such as secondhand smoke exposure and cannabis use. Limitations include the timeframe for analysis, which reflected widespread use of first generation e-cigarette devices that likely served as inferior sources of nicotine delivery. As the market continues to advance with newer-generation e-cigarette products (such as nicotine salt-based “pod-mod” products), continued surveillance of dual use patterns involving toxicant exposure, demographic characteristics, and related tobacco use behaviors will help inform whether and how the evolution of the e-cigarette market may facilitate or hinder continued dual use or cessation.
Further, several measured toxicant biomarkers have exposure sources other than tobacco smoke. For example, acrylamide is found in carbohydrate-rich foods that are cooked at high temperatures, as well as in tobacco smoke. Along these same lines, biomarkers of exposure to metals (lead and cadmium) accumulate in the body over years resulting from tobacco smoking and environmental exposures, and are slowly released in the urine over many years. Therefore, urinary metal concentrations are more driven by historical exposures (most typically from previous cigarette smoking) than from current tobacco use. Finally, there are currently no validated biomarkers specific to e-cigarette use, resulting in our analysis characterizing the presence of cigarette biomarkers in e-cigarette users and dual users. While new potential biomarkers of e-cigarette use have been proposed,36 those constituents were not measured in this study. Despite these limitations, these data add to our understanding of the diversity of exposures that occur among dual users, and can serve as a basis for other work that is required to improve understanding of toxicant exposures this large and important group of e-cigarette users may experience.
CONCLUSIONS
Most dual users smoke cigarettes daily and use e-cigarettes occasionally. Cigarette smoking appears to be the primary driver of toxicant exposure among dual users, with little-to-no effect of e-cigarette use on biomarker levels. Exclusive e-cigarette users have lower toxicant levels than exclusive cigarette smokers. These results reinforce the need for dual users to stop smoking tobacco cigarettes to reduce toxicant exposure.
Supplementary Material
ACKNOWLEDGEMENTS
Funding Statement: This manuscript is supported with Federal funds from the National Institute on Drug Abuse, National Institutes of Health, and the Center for Tobacco Products, Food and Drug Administration, Department of Health and Human Services, under a contract to Westat (Contract Nos. HHSN271201100027C and HHSN271201600001C) and through an interagency agreement between the FDA Center for Tobacco Products and the Centers for Disease Control and Prevention.
Footnotes
Conflict of Interest Statement: Maciej L. Goniewicz receives fees for serving on an advisory board from Johnson & Johnson and grant support from Pfizer. The other authors have no conflicts of interest to declare.
Disclaimer: The views and opinions expressed in this manuscript are those of the authors only and do not necessarily represent the views, official policy or position of the U.S. Department of Health and Human Services or any of its affiliated institutions or agencies.
REFERENCES
- 1.Kasza KA, Ambrose BK, Conway KP, et al. Tobacco-Product Use by Adults and Youths in the United States in 2013 and 2014. N Engl J Med. 2017;376(4):342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleman BN, Rostron B, Johnson SE, et al. Electronic cigarette use among US adults in the Population Assessment of Tobacco and Health (PATH) Study, 2013–2014. Tob Control. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Academies of Sciences, Engineering, and Medicine. Public Health Consequences of E-Cigarettes. Washington D.C.: National Academies Press; 2018: https://www.ncbi.nlm.nih.gov/books/NBK507187/. Accessed August 14, 2018. [PubMed] [Google Scholar]
- 4.Coleman B, Rostron B, Johnson SE, et al. Transitions in electronic cigarette use among adults in the Population Assessment of Tobacco and Health (PATH) Study, Waves 1 and 2 (2013–2015). Tob Control. 2019;28(1):50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borland R, Murray K, Gravely S, et al. A new classification system for describing concurrent use of nicotine vaping products alongside cigarettes (so-called ‘dual use’): findings from the ITC-4 Country Smoking and Vaping wave 1 Survey. Addiction. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goniewicz ML, Smith DM, Edwards KC, et al. Comparison of Nicotine and Toxicant Exposure in Users of Electronic Cigarettes and Combustible Cigarettes. JAMA Netw Open. 2018;1(8):e185937–e185937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shahab L, Goniewicz ML, Blount BC, et al. Nicotine, Carcinogen, and Toxin Exposure in Long-Term E-Cigarette and Nicotine Replacement Therapy Users: A Cross-sectional Study. Ann intern med. 2017;166(6):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith DM, Shahab L, Alwis KU, et al. Higher cigarette consumption among dual users of tobacco and electronic cigarettes is associated with greater levels of select tobacco-related toxicants: findings from an international observational study. Society for Research on Nicotine and Tobacco; 2018; Baltimore, MD [Google Scholar]
- 9.D’Ruiz CD, Graff DW, Robinson E. Reductions in biomarkers of exposure, impacts on smoking urge and assessment of product use and tolerability in adult smokers following partial or complete substitution of cigarettes with electronic cigarettes. BMC public health. 2016;16:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goniewicz ML, Gawron M, Smith DM, Peng M, Jacob P, Benowitz NL. Exposure to Nicotine and Selected Toxicants in Cigarette Smokers Who Switched to Electronic Cigarettes: A Longitudinal Within-Subjects Observational Study. Nicotine & Tobacco Research. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czoli CD, Fong GT, Goniewicz ML, Hammond D. Biomarkers of exposure among “dual users” of tobacco cigarettes and electronic cigarettes in Canada. Nicotine Tob Res. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piper ME, Baker TB, Benowitz NL, Kobinsky K, Jorenby DE. Dual Users Compared to Smokers: Demographics, Dependence, and Biomarkers. Nicotine Tob Res. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyland A, Ambrose BK, Conway KP, et al. Design and methods of the Population Assessment of Tobacco and Health (PATH) Study. Tob Control. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alwis KU, Bailey TL, Patel D, Wang L, Blount BC. Measuring urinary N-acetyl-S-(4-hydroxy-2-methyl-2-buten-1-yl)-L-cysteine (IPMA3) as a potential biomarker of isoprene exposure. Anal chim acta. 2016;941:61–66. [DOI] [PubMed] [Google Scholar]
- 15.Alwis KU, Blount BC, Britt AS, Patel D, Ashley DL. Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS). Anal chim acta. 2012;750:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caldwell KL, Jones RL, Verdon CP, Jarrett JM, Caudill SP, Osterloh JD. Levels of urinary total and speciated arsenic in the US population: National Health and Nutrition Examination Survey 2003–2004. J Expo Sci Environ Epidemiol. 2009;19(1):59–68. [DOI] [PubMed] [Google Scholar]
- 17.Caudill SP, Schleicher RL, Pirkle JL. Multi-rule quality control for the age-related eye disease study. Stat Med. 2008;27(20):4094–4106. [DOI] [PubMed] [Google Scholar]
- 18.Verdon CP, Caldwell KL, Fresquez MR, Jones RL. Determination of seven arsenic compounds in urine by HPLC-ICP-DRC-MS: a CDC population biomonitoring method. Anal Bioanal Chem. 2009;393(3):939–947. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Meng L, Pittman EN, et al. Quantification of urinary mono-hydroxylated metabolites of polycyclic aromatic hydrocarbons by on-line solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2017;409(4):931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei B, Feng J, Rehmani IJ, et al. A high-throughput robotic sample preparation system and HPLC-MS/MS for measuring urinary anatabine, anabasine, nicotine and major nicotine metabolites. Clin Chim Acta. 2014;436:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia B, Xia Y, Wong J, et al. Quantitative analysis of five tobacco-specific N-nitrosamines in urine by liquid chromatography-atmospheric pressure ionization tandem mass spectrometry. Biomed Chromatogr. 2014;28(3):375–384. [DOI] [PubMed] [Google Scholar]
- 22.Bernert JT, Harmon TL, Sosnoff CS, McGuffey JE. Use of continine immunoassay test strips for preclassifying urine samples from smokers and nonsmokers prior to analysis by LC-MS-MS. J Anal Toxicol. 2005;29(8):814–818. [DOI] [PubMed] [Google Scholar]
- 23.Caldwell KL, Hartel J, Jarrett J, Jones RL. Inductively coupled plasma mass spectrometry to measure multiple toxic elements in urine in NHANES 1999–2000. Atom Spectrosc. 2005;26(1):1–7. [Google Scholar]
- 24.Jarrett JM, Xiao G, Caldwell KL, Henahan D, Shakirova G, Jones RL. Eliminating molybdenum oxide interference in urine cadmium biomonitoring using ICP-DRC-MS. J. Anal. At. Spectrom. 2008;23(7):962–967. [Google Scholar]
- 25.Pirkle JL, Bernert JT, Caudill SP, Sosnoff CS, Pechacek TF. Trends in the exposure of nonsmokers in the U.S. population to paintsecondhand smoke: 1988–2002. Environ Health Perspect. 2006;114(6):853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goniewicz ML, Smith DM. Are Some E-Cigarette Users “Blowing Smoke”?: Assessing the Accuracy of Self-Reported Smoking Abstinence in Exclusive E-Cigarette Users. Nicotine Tob Res. 2018;21(5):699–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 28.Byrd GD, Chang KM, Greene JM, deBethizy JD. Evidence for urinary excretion of glucuronide conjugates of nicotine, cotinine, and trans-3’-hydroxycotinine in smokers. Drug Metab Dispos. 1992;20(2):192–197. [PubMed] [Google Scholar]
- 29.Patterson F, Benowitz N, Shields P, et al. Individual differences in nicotine intake per cigarette. Cancer Epidemiol Biomarkers Prev. 2003;12(5):468–471. [PubMed] [Google Scholar]
- 30.Strasser AA, Souprountchouk V, Kaufmann A, et al. Nicotine Replacement, Topography, and Smoking Phenotypes of E-cigarettes. Tob reg sci. 2016;2(4):352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.United States Department of Health and Human Services National Institutes of Health, National Institute on Drug Abuse, and United States Department of Health and Human Services, Food and Drug Administration, Center for Tobacco Products. Population Assessment of Tobacco and Health (PATH) Study [United States] Biomarker Restricted-Use Files, User Guide. Vol ICPSR36840-v1 Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor]; 2017. [Google Scholar]
- 32.Fagan P, Rigotti NA. Light and intermittent smoking: the road less traveled. Nicotine Tob Res. 2009;11(2):107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA)2010. [PubMed] [Google Scholar]
- 34.Kalkhoran S, Chang Y, Rigotti NA. Electronic cigarette use and cigarette abstinence over two years among U.S. smokers in the Population Assessment of Tobacco and Health Study. Nicotine Tob Res. 2019. July 11, doi: 10.1093/ntr/ntz114. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai H, Leventhal AM. Association of electronic cigarette vaping and subsequent smoking relapse among former smokers. Drug Alc Depend. 2019. June 1;199:10–17. doi: 10.1016/j.drugalcdep.2019.01.043. Epub 2019 Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.United States Food and Drug Administration. Harmful and Potentially Harmful Constituents in Tobacco Products; Established List; Proposed Additions; Request for Comments, 2019–16658 (August 2019). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.