Abstract
The SARS-CoV2 virus that causes COVID-19 binds to the angiotensin-converting enzyme 2 (ACE2) to gain cellular entry. Akt inhibitor triciribine (TCBN) has demonstrated promising results in promoting recovery from advanced-stage acute lung injury in preclinical studies. In the current study, we tested the direct effect of TCBN on ACE2 expression in human bronchial (H441) and lung alveolar (A549) epithelial cells. Treatment with TCBN resulted in the downregulation of both mRNA and protein levels of ACE2 in A549 cells. Since HMGB1 plays a vital role in the inflammatory response in COVID-19, and because hyperglycemia has been linked to increased COVID-19 infections, we determined if HMGB1 and hyperglycemia have any effect on ACE2 expression in lung epithelial cells and whether TCBN has any effect on reversing HMGB1 and hyperglycemia-induced ACE2 expression. We observed increased ACE2 expression with both HMGB1 and hyperglycemia treatment in A549 as well as H441 cells, which were blunted by TCBN treatment. Our findings from this study combined with our previous reports on the potential benefits of TCBN in the treatment of acute lung injury generate reasonable optimism on the potential utility of TCBN in the therapeutic management of COVID-19 patients.
Keywords: ACE2, Akt, COVID-19, SARS-CoV2, Triciribine
1. Introduction
December 2019 witnessed a series of pneumonia cases with unknown etiology in China later found to be associated with severe acute respiratory syndrome causing (Qin et al., 2020) novel coronavirus 2 (SARS-CoV2 or CoV2) (Bourgonje et al., 2020; Khulood, Adil, Sultana, & Nimra, 2020; Perrotta, Matera, Cazzola, & Bianco, 2020). The World Health Organization named the condition as the coronavirus disease-2019 (COVID-19) (Ahmad, Rehman, & Alkharfy, 2020; Yamin, 2020). The CoV2 express spike-protein on the surface (Hoffmann, Kleine-Weber, et al., 2020) that binds to the angiotensin-converting enzyme 2 (ACE2) on the target cells to gain cellular entry (Bourgonje et al., 2020; Cristelo, Azevedo, Marques, Nunes, & Sarmento, 2020; McKee, Sternberg, Stange, Laufer, & Naujokat, 2020). The CoV2 invasion requires priming of the spike-protein, which is reportedly facilitated by a transmembrane serine protease-2 (TMPRSS2) (Hoffmann, Kleine-Weber, et al., 2020; Lukassen et al., 2020; McKee et al., 2020).
ACE2 is a type I transmembrane glycoprotein, an ectoenzyme that hydrolyzes circulating peptides (Imai, Kuba, Ohto-Nakanishi, & Penninger, 2010). ACE2 is responsible for cleaving Ang II to angiotensin (1–7), which results in vasodilation and reduced inflammation (Bourgonje et al., 2020). ACE2 is abundantly expressed in the heart, blood vessels, lung, bronchus, gut, kidney, testis, and brain (Verdecchia, Cavallini, Spanevello, & Angeli, 2020), and also reported being expressed in lung alveolar epithelial cells (Bourgonje et al., 2020). Since ACE2 mediates CoV2 cellular entry it may constitute a pharmacological target to restrict the CoV-2 infections (Adil, Narayanan, & Somanath, 2020b; South, Diz, & Chappell, 2020; Wyganowska-Swiatkowska, Nohawica, Grocholewicz, & Nowak, 2020). In support of this theory, ACE2 knockout mice demonstrated reduced infection compared to the wild type (Kuba et al., 2005). On the contrary, over-expression of ACE2 in mice increased the susceptibility to CoV2 infection and resulted in severe pathological changes observed in humans (Yang et al., 2007). Although chloroquine was considered for COVID-19 therapy (Boulware et al., 2020)by inhibiting terminal phosphorylation of ACE2 (McKee et al., 2020), its potential side-effects and lack of efficacy led to its premature exit from the race (Group et al., 2020; Hoffmann, Mosbauer, et al., 2020).
Although the link between diabetes and COVID-19 is unclear, early reports indicate a higher susceptibility of diabetics to COVID-19 (Tadic, Cuspidi, & Sala, 2020; Wu et al., 2020). Interestingly, ACE2 glycosylation has been shown to increase its affinity for CoV2 binding [4] suggesting that hyperglycemia could favor the cellular entry of CoV2 and higher COVID-19 severity (Ceriello, 2020). Also, ACE2 is activated as part of inhibiting endoplasmic reticulum stress to improve glucose and lipid metabolism through the IKKβ/NFκB/IRS1/Akt pathway (Cao et al., 2019). The severity of COVID-19 also relies on inflammation (Hu, Huang, & Yin, 2020). High-mobility group box 1 (HMGB1) is a chromatin protein (Luft, 2016) and a mediator of inflammation when present extracellularly (Andersson, Yang, & Harris, 2018). In mice, HMGB1 induces lung neutrophil infiltration, cytokine production, and lung injury (Wyganowska-Swiatkowska et al., 2020). HMGB1, in high levels, induces cytokine release (Palmblad et al., 2015), which is a hallmark feature of COVID-19 (Street, 2020). HMGB1 facilitates the transport of extracellular CoV2 into the cytosol via lysosome leakage (Andersson, Ottestad, & Tracey, 2020). Extracellular HMGB1 secreted by activated innate immune cells or from the dying cells forms complexes with danger-associated molecular patterns (DAMPs) or pathogen-associated molecular (PAMPs) released post-lytic cell death. These complexes bind to lung-specific RAGE to get endocytosed through endosomes containing TLR4 receptor which in turn can be activated by HMGB1. The partner molecules translocate to the lysosomes, where HMGB1 acts as a detergent under acidic conditions and disrupts the lysosomal membrane enabling HMGB1-partner molecules access to the cytosol (Andersson et al., 2020). Moreover, HMGB1 is reportedly involved in regulation of autophagy which is one of the mechanisms linked to the COVID-19 viral entry and replication in cells (Street, 2020).
Akt is a serine-threonine kinase (Adil, Narayanan, & Somanath, 2020a) involved in the regulation of cell growth, survival, and proliferation (Adil, Khulood, & Somanath, 2020). Triciribine (TCBN) is an adenosine analog and a small molecule inhibitor of Akt (Abdalla et al., 2015; Gloesenkamp et al., 2012). It reportedly suppresses the phosphorylation level and kinase activity of all Akt isoforms without inhibiting known upstream activators, PDK1 and PI3-Kinase (Abeyrathna & Su, 2015). MK-2206 is another inhibitor of Akt that exerts its suppressive action by binding to the allosteric site of Akt and engaging the functionally influential residues in various interactions (Rehan, Beg, Parveen, Damanhouri, & Zaher, 2014). It is a highly potent, selective, and orally active agent effective against all three isoforms of Akt (Brown & Banerji, 2017; Rehan et al., 2014). We recently demonstrated that inhibiting the Akt pathway (Alwhaibi, Verma, Adil, & Somanath, 2019) using TCBN in the advanced stages of lung injury promotes recovery by increasing anti-inflammatory regulatory T cells (Artham et al., 2020), suggesting its potential benefits in treating advanced COVID-19 patients (Somanath, 2020). In the current study, we determined the effects of hyperglycemia, HMGB1, in the presence and absence of TCBN on ACE2 expression in human bronchial (H441) and alveolar (A549) epithelial cell lines. Our results indicated a significant increase in ACE2 expression in both cell types by hyperglycemia and HMGB1 and a robust decrease in ACE2 expression in all conditions by treatment with TCBN.
2. Materials and methods
2.1. Cell lines and reagents
The human A549 adenocarcinoma (ATCC® CCL-185™) and human H441 bronchial epithelial (ATCC® HTB-174™) cell lines were obtained from ATCC (Manassas, VA). A549 cells were cultured in DMEM high glucose and H441 cell were cultured in RPMI 1640 media obtained from Hyclone, Logan, UT. Media were supplemented with 10% heat-inactivated fetal bovine serum (FBS, Atlanta Biologicals, Atlanta, GA), 100 U/ml penicillin, and 100 mg/ml streptomycin in a humidified incubator at 37 °C and 5% CO2. Cells were routinely passaged when they reached 80–90% confluency. Compound inhibitors including MK2206 (Cat No. S1078) and TCBN (Cat No. S1117) were purchased from Selleckchem, Houston, TX. HMGB1 (Cat No. 1690-HMB-050) was obtained from R&D Systems® (Minneapolis, MN), and D-Glucose (Cat No. D16–500) was obtained from Fisher Scientific.
2.2. Western blotting
The cell lysates were prepared using 1X RIPA lysis buffer (Millipore, Temecula, CA) supplemented with protease and phosphatase inhibitor tablets (Roche Applied Science, Indianapolis, IN). Protein concentration was measured by the DC protein assay (Bio-Rad Laboratories, Hercules, CA) and approximately 40–50 μg of cell lysates in Laemmli buffer were used. Densitometry was performed using NIH ImageJ software. Primary antibodies against ACE2 (Cat No. MABN59, Millipore Sigma, Burlington, MA), β-actin (Cat No. A2228, Millipore Sigma), and β-tubulin (Cat No. 2118, Cell Signaling, Danvers, MA), pSer473 Akt (Cat No. 9271, Cell Signaling) and Akt (Cat No. 9272, Cell Signaling) were used in the study. Anti-mouse and anti-rabbit HRP conjugated secondary antibodies were obtained from Bio-Rad, Hercules, CA.
2.3. RNA isolation, cDNA preparation, and qRT-PCR
Cells were grown until confluent in six-well culture plates and treated with PBS or Akt inhibitors (MK, 10 μM; TCBN, 10μM) for 24h. RNA was extracted using an RNA isolation kit (RNeasy Plus, Qiagen, Valencia, CA) and RNA quality was confirmed using Nanodrop 2000 spectrophotometer (Thermo Scientific). Complementary DNA (cDNA) was synthesized from 700ng of RNA using RT2 First Strand kit (Qiagen) using a StepOnePlus™ thermal cycler and detection software (Applied Biosystems, Foster City, CA) and quantitative real-time PCR (qRT-PCR) was performed using the RT2 SYBR Green ROX qPCR Master Mix (Qiagen) in real-time PCR equipment (Applied Biosystems). Sample cDNA was amplified and quantified over many shorter cycles under the following conditions: an initial 10min 95°C period followed by 45 cycles of 95°C for 15s, 60°C for 1min, and 72°C for 15s. To analyze the fluorescence signal, a threshold cycle (Ct) was determined, using the exponential growth phase and the baseline signal from fluorescence vs cycle number plots. The following primers were used for the messenger RNA (mRNA) analysis: ACE2 (forward: 5′-GGACCCAGGAAATGTTCA GA-3′ and reverse: 5′-GGCTGCAGAAAGTGACATGA-3′), TMPRSS2 (forward: 5′-CACTGTGCATCAC CTTGA CC-3′ and reverse: 5′-ACACACCGATTCTCGTCCTC-3′), and β-actin (forward: 5′-GGACTTCGAGCAAGAGATGG-3′ and reverse: 5′-AGCACTGTGTTGGCGTACAG-3′). Ct values were normalized to the level of expression of the housekeeping gene (β-actin).
2.4. Statistical analysis
All the data are presented as mean ± SEM. The ‘n’ value for each figure implies the number of samples in each group. All band densitometry and mRNA analysis are presented as fold-changes compared to respective control groups. All the data were analyzed by parametric testing using the Student’s unpaired t-test or one-way ANOVA, followed by the posthoc test using the GraphPad Prism 6.01 software. Data with p<0.05 were considered significant.
3. Results
3.1. TCBN suppresses ACE2 expression in human alveolar epithelial cells.
Human alveolar epithelial (A549) cells were treated with MK2206 and TCBN, two well-known Akt inhibitors, for 24 hours, and the protein expression of phosphorylated and total Akt and ACE2 were analyzed. As expected, both compounds resulted in a statistically significant reduction of the phosphorylated form of Akt (Figure 1A and B). Although both compounds led to a statistically significant reduction in ACE2 expression, TCBN showed a more robust effect (Figure 1C). The mRNA analysis of ACE2 and TMPRSS2 in A549 cells revealed a significant reduction in ACE2 mRNA with TCBN treatment (Figure 1D) but the expression of TMPRSS2 at mRNA level remained unchanged with either MK2206 or TCBN treatment (Figure 1E).
Figure 1. Treatment with TCBN suppresses ACE2 expression in A549 cells.
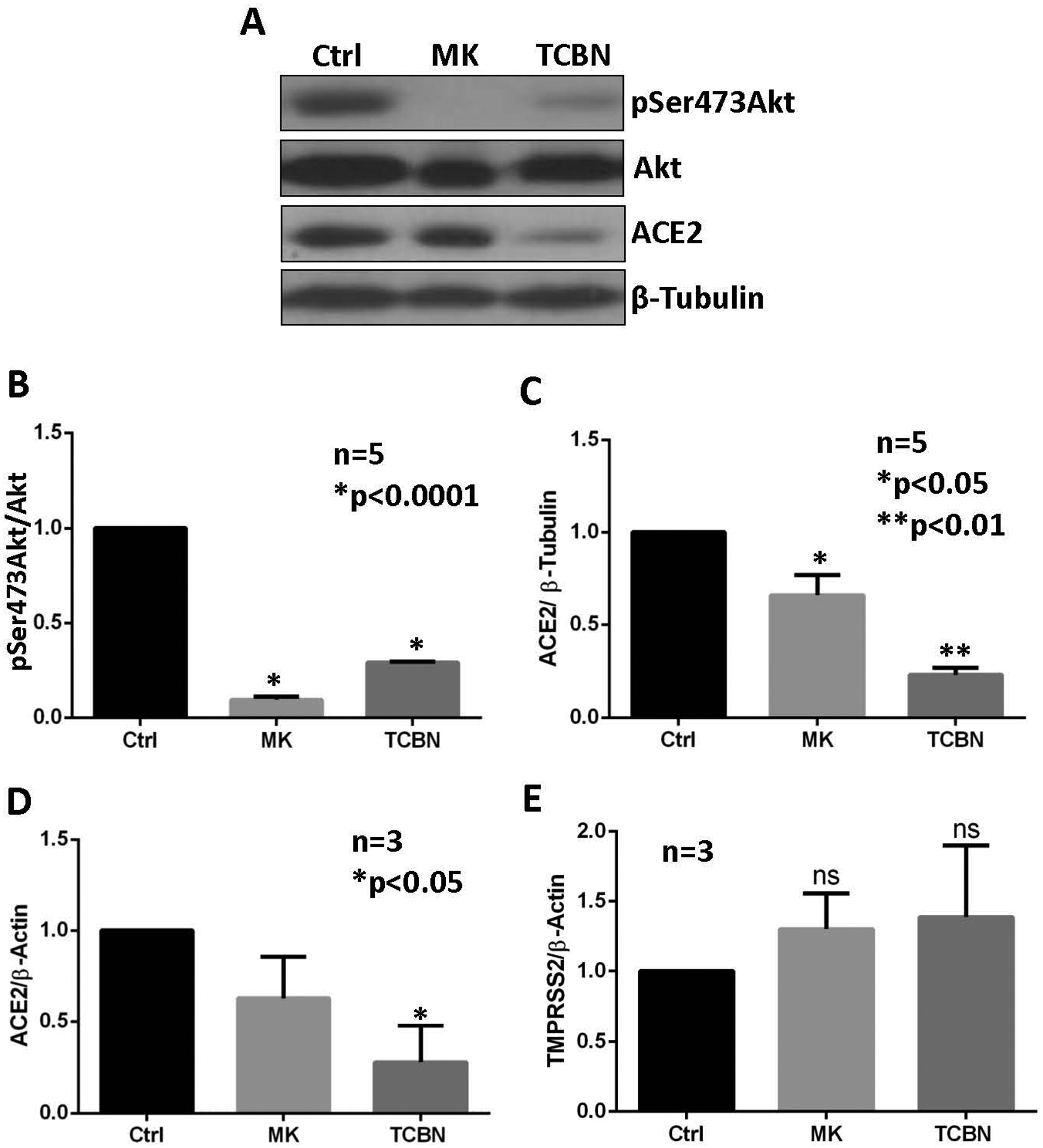
(A-C) Western blot images and bar graphs of band densitometry analysis of MK2206 (MK) and TCBN treated A549 cell lysates indicating changes in the expressions of ACE2, phosphorylated (Ser-473) Akt, and total Akt normalized to β-tubulin expression, respectively. (D-E) Bar graphs showing changes in the mRNA expression of ACE2 and TMPRSS2 normalized to the β-actin expression on MK2206 and TCBN-treated A549 cells compared to the vehicle (PBS) treated control. Data are shown as mean ± SEM.
3.2. TCBN inhibits HMGB1-induced ACE2 expression in A549 cells.
Human A549 epithelial cells were treated with various doses of HMGB1 for 24 hours to determine its effect on the ACE2 expression and activation of Akt. Our analysis indicated a statistically significant increase in ACE2 protein expression in A549 cells with 10 nM HMGB1 treatment (Figure 2A and B). Co-treatment of A549 cells with 10 nM HMGB1 and 10 μM TCBN resulted in significant inhibition of ACE2 protein expression in A549 cells compared to HMGB1-treated cells and vehicle-treated (PBS) control groups (Figure 2C and D). Although the treatment of A549 cells with HMGB1 did not increase the levels of phosphorylated (activated) Akt, treatment with TCBN significantly suppressed phosphorylated Akt in the HMGB1-treated group (Figure 2C and E).
Figure 2. TCBN suppresses HMGB1-induced ACE2 expression in A549 cells.
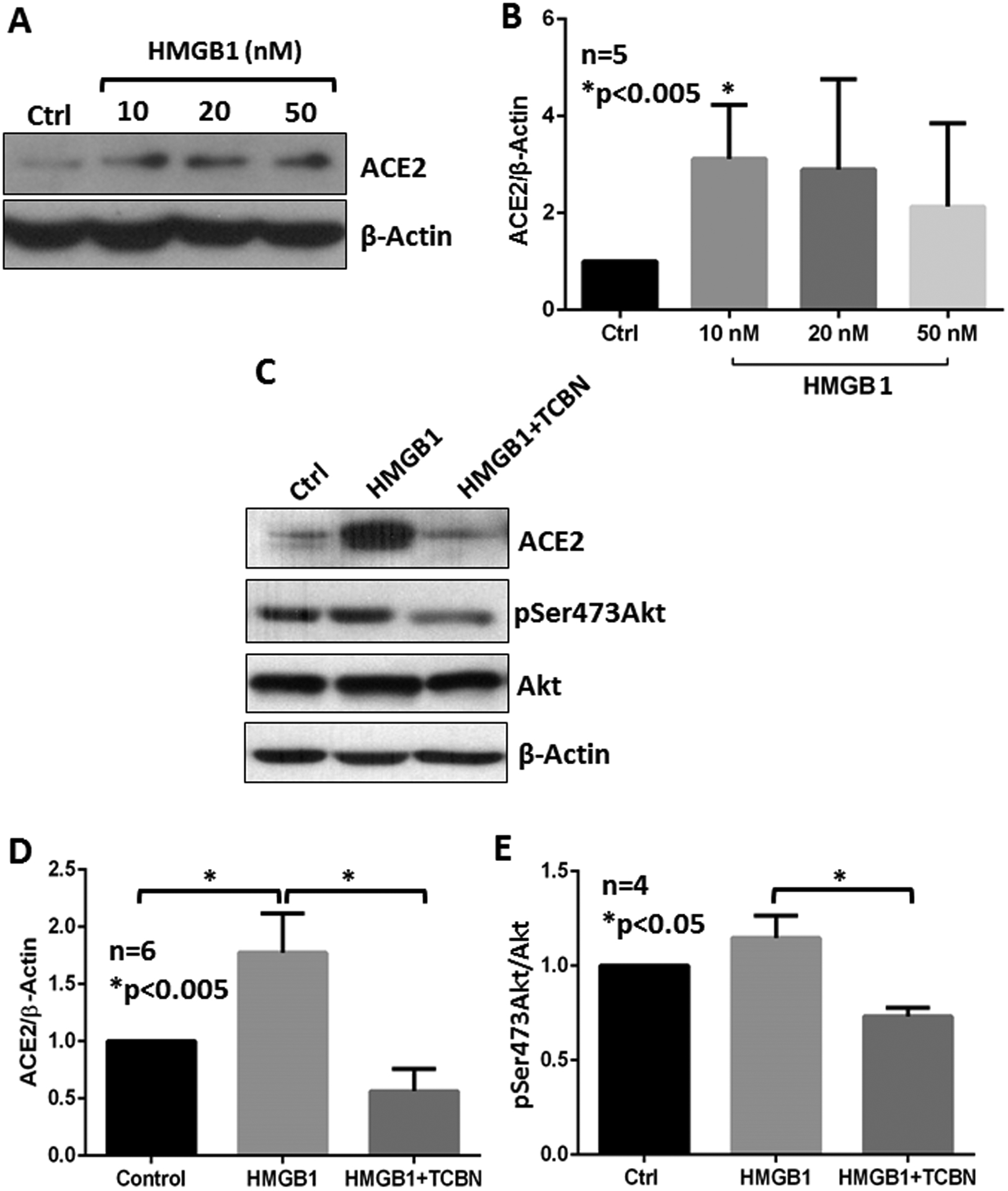
(A-B) Western blot images and bar graphs of band densitometry analysis of ACE2 expression induced by various doses of HMGB1 treatment in A549 cells. (C-E) Western blot images and bar graphs of band densitometry analysis of 10 nM HMGB1-induced ACE2 expression and Akt phosphorylation, and the effect of TCBN in A549 cells, respectively. Data are shown as mean ± SEM.
3.3. TCBN inhibits hyperglycemia-induced ACE2 expression in A549 and H441 cells.
Human alveolar epithelial cells were subjected to 50 mM and 100 mM D-Glucose for 48 hours with a change in 50% media at 24 hours and the expression of ACE2 protein was determined by immunoblotting. Although statistically not significant, a trend in increased ACE2 expression was observed in A549 cells with both 50 mM and 100 mM doses of hyperglycemia (Figure 3A and B). As a confirmation of the previous HMGB1 experiment, co-treatment with TCBN significantly inhibited ACE2 protein expression in A549 cells compared to the HMGB1-treated or vehicle-treated (PBS) control groups (Figure 3A and B). Interestingly, a dose-depended increase in the expression of phosphorylated Akt was observed with hyperglycemia treatment in A549 cells, which were inhibited by TCBN (Figure 3A and C).
Figure 3. TCBN suppresses hyperglycemia-induced ACE2 expression in A549 cells.

Western blot images (A) and bar graph of band densitometry analysis (B) of hyperglycemia (50 mM or 100 mM glucose) induced expression changes in ACE2 and phosphorylated Akt expression in A549 cells (C) in the presence and absence of TCBN, respectively. Data are shown as mean ± SEM.
Like the A549 cells, human bronchial (H441) epithelial cells also revealed a dose-dependent increase in ACE2 protein expression (Figure 4A–D) and Akt phosphorylation (Figure 4C and E) with high glucose treatment. Hyperglycemia-induced ACE2 expression and Akt phosphorylation were inhibited by TCBN treatment (Figure 4C and E).
Figure 4. Akt inhibition blunts hyperglycemia-induced increase in ACE2 expression in H441 cells.

(A-B) Western blot images and bar graphs of band densitometry analysis of hyperglycemia (HG) (50 mM or 100 mM glucose) induced expression changes in ACE2 in H441 cells. (C-E) Western blot images and bar graphs of band densitometry analysis of 50 mM glucose-induced changes in ACE2 phosphorylated Akt expression in H441 cells, respectively. Data are shown as mean ± SEM.
4. Discussion
In the current study, even though MK2206 is a more potent inhibitor of Akt as compared to TCBN, the latter was found to be a more powerful suppressor of ACE2 expression at the protein and mRNA level in the lung alveolar (A549) epithelial cells with no change in TMPRSS2 expression with either Akt inhibitors. Whereas treatment of A549 cells with HMGB1, a mediator of inflammation and cytokine release, did not have a robust effect on Akt phosphorylation, it significantly elevated ACE2 expression, which was blunted by cotreatment with TCBN. In sharp contrast, while hyperglycemia only modestly activated Akt, it robustly induced ACE2 expression in A549 and human bronchial (H441) epithelial cells, which were inhibited by cotreatment with TCBN. Collectively, the salient findings from this study are that HMGB1 is a potent inducer of ACE2 but not Akt activity inA549 cells, hyperglycemia treatment robustly increases Akt activity and modestly increases ACE2 expression in A549 cells and H441 cells, and that TCBN (not MK2206) suppresses ACE2 expression in A549 cells in an Akt independent manner.
COVID-19 can be better managed by a drug that can not only suppress inflammation but also resolve lung injury thereby preventing scar formation (Somanath, 2020). A recent study reported the favorable effects of anti-fibrotic therapies in the prevention of severe COVID-19 in patients with or without idiopathic pulmonary fibrosis (George, Wells, & Jenkins, 2020). In our previous studies, we have demonstrated that targeting Akt has potential antifibrotic (Abdalla, Goc, Segar, & Somanath, 2013; Abdalla et al., 2015; Goc, Choudhary, Byzova, & Somanath, 2011; Goc, Sabbineni, Abdalla, & Somanath, 2015; Ma, Kerr, Naga Prasad, Byzova, & Somanath, 2014; Somanath & Byzova, 2009; Somanath, Kandel, Hay, & Byzova, 2007) and anti-inflammatory (Fairaq, Goc, Artham, Sabbineni, & Somanath, 2015; Kerr et al., 2013) benefits, because of which pharmacological inhibition of Akt using compounds such as MK2206 and TCBN can increase the number of activated anti-inflammatory regulatory T-cells (Tregs) thus promoting the resolution of injury in the advanced stages of acute lung injury (Artham et al., 2020). Based on these observations, we recently commented that pharmacological suppression of Akt may have potential benefits in treating advanced-state COVID-19 patients with lung injury (Somanath, 2020). What is not currently known is whether the Akt pathway, directly or indirectly, has any effect on the expression of ACE2 and/or TMPRSS2, two major determinants of CoV2 infection in humans. In particular, targeting ACE2 can be a viable option in restricting the entry of the fatal virus (Michaud et al., 2020). The current study addresses this question and reveals that while Akt activity is not a primary mediator of either ACE2 or TMPRSS2 expression in A549 cells, treatment with HMGB1 and hyperglycemia induces ACE2 expression in lung epithelial cells, and treatment with TCBN suppresses ACE2 but not TMPRSS2 expression in lung epithelial cells (Figure 5).
Figure 5. Schematic representation of the potential effects of HMGB1, hyperglycemia, and TCBN on ACE2 expression and SARS-CoV2 binding to lung epithelial cells.
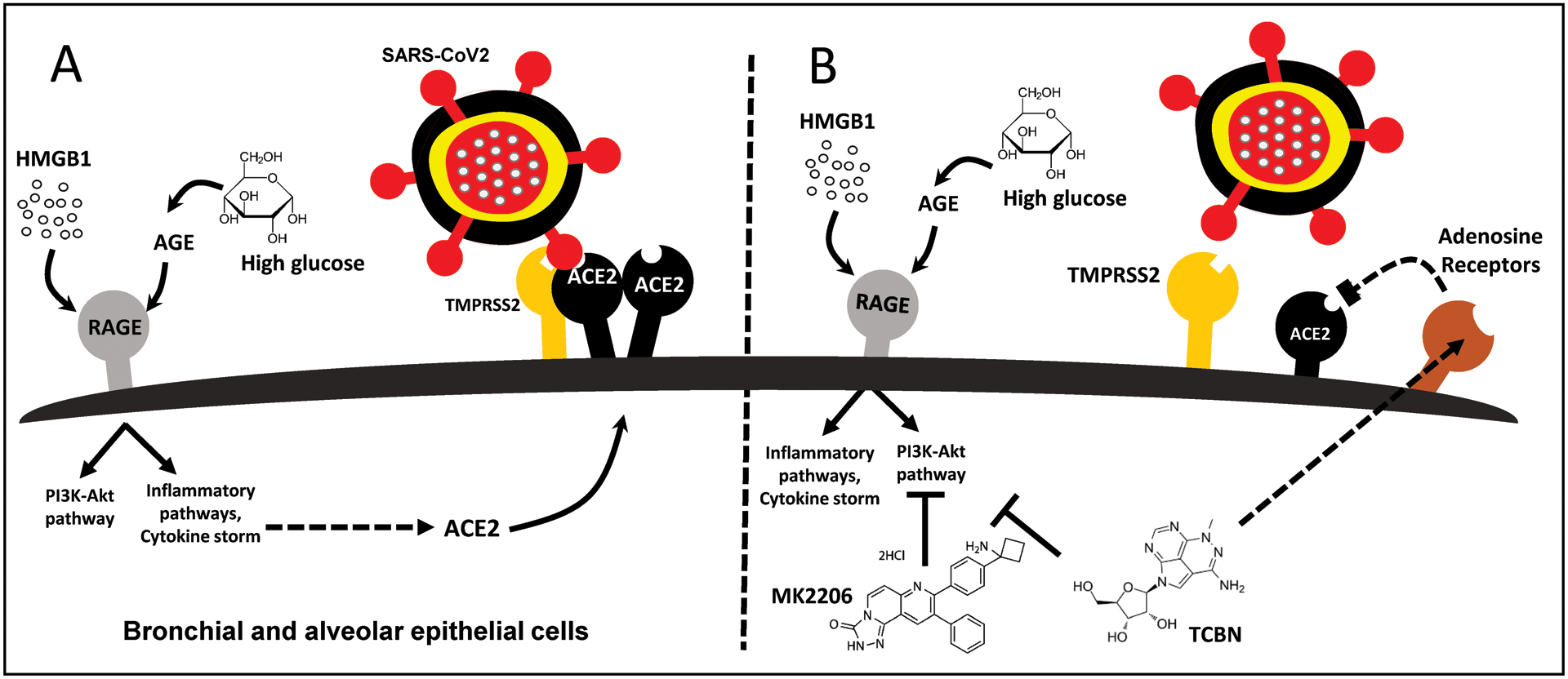
(A) HMGB1 or high glucose-induced AGE (advanced glycation end-products) promotes the expression of ACE2 receptors thereby increasing the risk of SARS-CoV-2 interaction with the lung epithelial cell surface ACE2 leading to their internalization and infection. (B) Whereas Akt inhibitor MK-2206 does not affect the expression of ACE2 receptors, treatment with TCBN blunts HMGB1 and hyperglycemia-induced ACE2 expression in lung epithelial cells potentially in an Akt-independent but adenosine-receptor dependent mechanism.
Several studies have tested the safety and tolerability of TCBN in humans (Garrett et al., 2011; Sampath et al., 2013). Since the Akt activity is not strongly correlated with ACE2 expression in lung epithelial cells, the suppression of ACE 2 expression by TCBN in these cells is likely independent of its ability to inhibit Akt activity. TCBN is a tricyclic adenosine analog that is phosphorylated to its active metabolite (TCBN-P) by adenosine kinase in certain cells such as the lung epithelial cells (Wotring, Crabtree, Edwards, Parks, & Townsend, 1986). TCBN can inhibit viral replication of HIV-1 and HIV-2 independent of Akt, including strains known to be resistant to azidothymidine or TIBO (Kucera et al., 1993). The phosphorylation of TCBN to TCBN-P has been reported to be essential for its anti-viral activity on HIV-1 (Porcari et al., 2003; Ptak et al., 2010). The ability of TCBN-P to function as an adenosine analog may likely have contributed to the suppression of ACE2 in A549 cells. In support of this theory, a study has reported that alveolar-epithelial A2B adenosine receptors confer pulmonary protection during acute lung injury (Hoegl et al., 2015). Extracellular adenosine generation and activation of the A1 adenosine receptor have also been very recently indicated to resist Streptococcus pneumoniae (pneumococcus) adherence to lung epithelial cells (Bhalla et al., 2020). Together, these reports support the idea that TCBN effects on ACE2 expression in lung epithelial cells may be independent of Akt suppression but likely reliant on its activation of A1 or A2B adenosine receptors.
The results related to the hyperglycemia-induced elevation of ACE2 expression in A549 cells explain the probable reason behind the higher incidence of COVID-19 in diabetic patients with high serum glucose (Tadic et al., 2020). This coincides with another study that suggests prognosis in people affected by CoV2 can be probably be improved by normalization of hyperglycemia as it may help in decreasing the release of the inflammatory cytokines thereby reducing the ACE2 binding capacity of the virus (Ceriello, 2020). Our findings reveal that hyperglycemia sets off increased ACE2 expression in lung epithelial cells, which might increase the risk of CoV2 entry and subsequent susceptibility to COVID-19. In conclusion, treatment with TCBN suppresses ACE2 expression in human lung epithelial cells in the presence or absence of hyperglycemia or HMGB1 thereby suggesting its potential utility in restricting the host cell entry of CoV2.
Acknowledgments
Funds were provided by the NHLBI grant R01HL103952, NCATS grant UL1TR002378, Wilson Pharmacy Foundation (intramural), and Translational Research Initiative grant (intramural) to PRS, and NEI grant R01EY028569 to SPN. This work has been accomplished using the resources and facilities at the VA Medical Center in Augusta, GA. The funders had no role in the study design, data collection, analysis, and decision to publish the data.
Footnotes
Conflict of interest
PR Somanath is a scientific advisory board member of Ayma Therapeutics Inc. NJ, USA. All other authors declare that they have no financial or other conflicts of interest exist.
Data availability statement: Research Data are not shared
References
- Abdalla M, Goc A, Segar L, & Somanath PR (2013). Akt1 mediates alpha-smooth muscle actin expression and myofibroblast differentiation via myocardin and serum response factor. J Biol Chem, 288(46), 33483–33493. doi: 10.1074/jbc.M113.504290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdalla M, Sabbineni H, Prakash R, Ergul A, Fagan SC, & Somanath PR (2015). The Akt inhibitor, triciribine, ameliorates chronic hypoxia-induced vascular pruning and TGFβ-induced pulmonary fibrosis. Br J Pharmacol, 172(16), 4173–4188. doi: 10.1111/bph.13203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeyrathna P, & Su Y (2015). The critical role of Akt in cardiovascular function. Vascul Pharmacol, 74, 38–48. doi: 10.1016/j.vph.2015.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adil MS, Khulood D, & Somanath PR (2020). Targeting Akt-associated microRNAs for cancer therapeutics. Biochem Pharmacol, 114384. doi: 10.1016/j.bcp.2020.114384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adil MS, Narayanan SP, & Somanath PR (2020a). Cell-cell junctions: structure and regulation in physiology and pathology. Tissue Barriers, 1848212. doi: 10.1080/21688370.2020.1848212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adil MS, Narayanan SP, & Somanath PR (2020b). Is amiloride a promising cardiovascular medication to persist in the COVID-19 crisis? Drug Discov Ther, 14(5), 256–258. doi: 10.5582/ddt.2020.03070 [DOI] [PubMed] [Google Scholar]
- Ahmad A, Rehman MU, & Alkharfy KM (2020). An alternative approach to minimize the risk of coronavirus (Covid-19) and similar infections. Eur Rev Med Pharmacol Sci, 24(7), 4030–4034. doi: 10.26355/eurrev_202004_20873 [DOI] [PubMed] [Google Scholar]
- Alwhaibi A, Verma A, Adil MS, & Somanath PR (2019). The unconventional role of Akt1 in the advanced cancers and in diabetes-promoted carcinogenesis. Pharmacol Res, 145, 104270. doi: 10.1016/j.phrs.2019.104270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson U, Ottestad W, & Tracey KJ (2020). Extracellular HMGB1: a therapeutic target in severe pulmonary inflammation including COVID-19? Mol Med, 26(1), 42. doi: 10.1186/s10020-020-00172-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson U, Yang H, & Harris H (2018). Extracellular HMGB1 as a therapeutic target in inflammatory diseases. Expert Opin Ther Targets, 22(3), 263–277. doi: 10.1080/14728222.2018.1439924 [DOI] [PubMed] [Google Scholar]
- Artham S, Verma A, Alwhaibi A, Adil MS, Manicassamy S, Munn DH, & Somanath PR (2020). Delayed Akt suppression in the lipopolysaccharide-induced acute lung injury promotes resolution that is associated with enhanced effector regulatory T cells. Am J Physiol Lung Cell Mol Physiol, 318(4), L750–l761. doi: 10.1152/ajplung.00251.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla M, Hui Yeoh J, Lamneck C, Herring SE, Tchalla EYI, Heinzinger LR, … Bou Ghanem EN (2020). A1 adenosine receptor signaling reduces Streptococcus pneumoniae adherence to pulmonary epithelial cells by targeting expression of platelet-activating factor receptor. Cell Microbiol, 22(2), e13141. doi: 10.1111/cmi.13141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, … Hullsiek KH (2020). A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N Engl J Med, 383(6), 517–525. doi: 10.1056/NEJMoa2016638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, … van Goor H (2020). Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. doi: 10.1002/path.5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JS, & Banerji U (2017). Maximising the potential of AKT inhibitors as anti-cancer treatments. Pharmacol Ther, 172, 101–115. doi: 10.1016/j.pharmthera.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Song LN, Zhang YC, Li Q, Shi TT, Yang FY, … Yang JK (2019). Angiotensin-converting enzyme 2 inhibits endoplasmic reticulum stress-associated pathway to preserve nonalcoholic fatty liver disease. Diabetes Metab Res Rev, 35(4), e3123. doi: 10.1002/dmrr.3123 [DOI] [PubMed] [Google Scholar]
- Ceriello A (2020). Hyperglycemia and the worse prognosis of COVID-19. Why a fast blood glucose control should be mandatory. Diabetes Res Clin Pract, 163, 108186. doi: 10.1016/j.diabres.2020.108186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristelo C, Azevedo C, Marques JM, Nunes R, & Sarmento B (2020). SARS-CoV-2 and diabetes: New challenges for the disease. Diabetes Res Clin Pract, 164, 108228. doi: 10.1016/j.diabres.2020.108228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairaq A, Goc A, Artham S, Sabbineni H, & Somanath PR (2015). TNFalpha induces inflammatory stress response in microvascular endothelial cells via Akt- and P38 MAP kinase-mediated thrombospondin-1 expression. Mol Cell Biochem, 406(1–2), 227–236. doi: 10.1007/s11010-015-2440-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett CR, Coppola D, Wenham RM, Cubitt CL, Neuger AM, Frost TJ, … Sebti SM (2011). Phase I pharmacokinetic and pharmacodynamic study of triciribine phosphate monohydrate, a small-molecule inhibitor of AKT phosphorylation, in adult subjects with solid tumors containing activated AKT. Invest New Drugs, 29(6), 1381–1389. doi: 10.1007/s10637-010-9479-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George PM, Wells AU, & Jenkins RG (2020). Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med, 8(8), 807–815. doi: 10.1016/s2213-2600(20)30225-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloesenkamp CR, Nitzsche B, Ocker M, Di Fazio P, Quint K, Hoffmann B, … Höpfner M (2012). AKT inhibition by triciribine alone or as combination therapy for growth control of gastroenteropancreatic neuroendocrine tumors. Int J Oncol, 40(3), 876–888. doi: 10.3892/ijo.2011.1256 [DOI] [PubMed] [Google Scholar]
- Goc A, Choudhary M, Byzova TV, & Somanath PR (2011). TGFbeta- and bleomycin-induced extracellular matrix synthesis is mediated through Akt and mammalian target of rapamycin (mTOR). J Cell Physiol, 226(11), 3004–3013. doi: 10.1002/jcp.22648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goc A, Sabbineni H, Abdalla M, & Somanath PR (2015). p70 S6-kinase mediates the cooperation between Akt1 and Mek1 pathways in fibroblast-mediated extracellular matrix remodeling. Biochim Biophys Acta, 1853(7), 1626–1635. doi: 10.1016/j.bbamcr.2015.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group RC, Horby P, Mafham M, Linsell L, Bell JL, Staplin N, … Landray MJ (2020). Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. doi: 10.1056/NEJMoa2022926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoegl S, Brodsky KS, Blackburn MR, Karmouty-Quintana H, Zwissler B, & Eltzschig HK (2015). Alveolar Epithelial A2B Adenosine Receptors in Pulmonary Protection during Acute Lung Injury. J Immunol, 195(4), 1815–1824. doi: 10.4049/jimmunol.1401957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, … Pöhlmann S (2020). SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell, 181(2), 271–280.e278. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Mosbauer K, Hofmann-Winkler H, Kaul A, Kleine-Weber H, Kruger N, … Pohlmann S (2020). Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature, 585(7826), 588–590. doi: 10.1038/s41586-020-2575-3 [DOI] [PubMed] [Google Scholar]
- Hu B, Huang S, & Yin L (2020). The cytokine storm and COVID-19. J Med Virol. doi: 10.1002/jmv.26232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Kuba K, Ohto-Nakanishi T, & Penninger JM (2010). Angiotensin-converting enzyme 2 (ACE2) in disease pathogenesis. Circ J, 74(3), 405–410. doi: 10.1253/circj.cj-10-0045 [DOI] [PubMed] [Google Scholar]
- Kerr BA, Ma L, West XZ, Ding L, Malinin NL, Weber ME, … Byzova TV (2013). Interference with akt signaling protects against myocardial infarction and death by limiting the consequences of oxidative stress. Sci Signal, 6(287), ra67. doi: 10.1126/scisignal.2003948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khulood D, Adil MS, Sultana R, & Nimra. (2020). Convalescent plasma appears efficacious and safe in COVID-19. Therapeutic Advances in Infectious Disease, 7, 2049936120957931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, … Penninger JM (2005). A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med, 11(8), 875–879. doi: 10.1038/nm1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera LS, Iyer NP, Puckett SH, Buckheit RW Jr., Westbrook L, Toyer BR, … et al. (1993). Activity of triciribine and triciribine-5’-monophosphate against human immunodeficiency virus types 1 and 2. AIDS Res Hum Retroviruses, 9(4), 307–314. doi: 10.1089/aid.1993.9.307 [DOI] [PubMed] [Google Scholar]
- Luft FC (2016). High-mobility group box 1 protein, angiotensins, ACE2, and target organ damage. J Mol Med (Berl), 94(1), 1–3. doi: 10.1007/s00109-015-1372-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukassen S, Chua RL, Trefzer T, Kahn NC, Schneider MA, Muley T, … Eils R (2020). SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. Embo j, 39(10), e105114. doi: 10.15252/embj.20105114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Kerr BA, Naga Prasad SV, Byzova TV, & Somanath PR (2014). Differential effects of Akt1 signaling on short- versus long-term consequences of myocardial infarction and reperfusion injury. Lab Invest, 94(10), 1083–1091. doi: 10.1038/labinvest.2014.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee DL, Sternberg A, Stange U, Laufer S, & Naujokat C (2020). Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol Res, 157, 104859. doi: 10.1016/j.phrs.2020.104859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud V, Deodhar M, Arwood M, Al Rihani SB, Dow P, & Turgeon J (2020). ACE2 as a Therapeutic Target for COVID-19; its Role in Infectious Processes and Regulation by Modulators of the RAAS System. J Clin Med, 9(7). doi: 10.3390/jcm9072096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmblad K, Schierbeck H, Sundberg E, Horne AC, Harris HE, Henter JI, … Andersson U (2015). High systemic levels of the cytokine-inducing HMGB1 isoform secreted in severe macrophage activation syndrome. Mol Med, 20(1), 538–547. doi: 10.2119/molmed.2014.00183 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Perrotta F, Matera MG, Cazzola M, & Bianco A (2020). Severe respiratory SARS-CoV2 infection: Does ACE2 receptor matter? Respir Med, 168, 105996. doi: 10.1016/j.rmed.2020.105996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcari AR, Ptak RG, Borysko KZ, Breitenbach JM, Drach JC, & Townsend LB (2003). Synthesis and antiviral activity of 2-substituted analogs of triciribine. Nucleosides Nucleotides Nucleic Acids, 22(12), 2171–2193. doi: 10.1081/ncn-120026873 [DOI] [PubMed] [Google Scholar]
- Ptak RG, Gentry BG, Hartman TL, Watson KM, Osterling MC, Buckheit RW Jr., … Drach JC (2010). Inhibition of human immunodeficiency virus type 1 by triciribine involves the accessory protein nef. Antimicrob Agents Chemother, 54(4), 1512–1519. doi: 10.1128/aac.01443-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, … Tian DS (2020). Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis, 71(15), 762–768. doi: 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehan M, Beg MA, Parveen S, Damanhouri GA, & Zaher GF (2014). Computational insights into the inhibitory mechanism of human AKT1 by an orally active inhibitor, MK-2206. PLoS One, 9(10), e109705. doi: 10.1371/journal.pone.0109705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath D, Malik A, Plunkett W, Nowak B, Williams B, Burton M, … Lancet JE (2013). Phase I clinical, pharmacokinetic, and pharmacodynamic study of the Akt-inhibitor triciribine phosphate monohydrate in patients with advanced hematologic malignancies. Leuk Res, 37(11), 1461–1467. doi: 10.1016/j.leukres.2013.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somanath PR (2020). Is targeting Akt a viable option to treat advanced-stage COVID-19 patients? Am J Physiol Lung Cell Mol Physiol, 319(1), L45–l47. doi: 10.1152/ajplung.00124.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somanath PR, & Byzova TV (2009). 14-3-3beta-Rac1-p21 activated kinase signaling regulates Akt1-mediated cytoskeletal organization, lamellipodia formation and fibronectin matrix assembly. J Cell Physiol, 218(2), 394–404. doi: 10.1002/jcp.21612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somanath PR, Kandel ES, Hay N, & Byzova TV (2007). Akt1 signaling regulates integrin activation, matrix recognition, and fibronectin assembly. J Biol Chem, 282(31), 22964–22976. doi: 10.1074/jbc.M700241200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- South AM, Diz DI, & Chappell MC (2020). COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol, 318(5), H1084–h1090. doi: 10.1152/ajpheart.00217.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street ME (2020). HMGB1: A Possible Crucial Therapeutic Target for COVID-19? Horm Res Paediatr, 93(2), 73–75. doi: 10.1159/000508291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadic M, Cuspidi C, & Sala C (2020). COVID-19 and diabetes: Is there enough evidence? J Clin Hypertens (Greenwich), 22(6), 943–948. doi: 10.1111/jch.13912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdecchia P, Cavallini C, Spanevello A, & Angeli F (2020). The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med, 76, 14–20. doi: 10.1016/j.ejim.2020.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotring LL, Crabtree GW, Edwards NL, Parks RE Jr., & Townsend LB (1986). Mechanism of activation of triciribine phosphate (TCN-P) as a prodrug form of TCN. Cancer Treat Rep, 70(4), 491–497. [PubMed] [Google Scholar]
- Wu J, Zhang J, Sun X, Wang L, Xu Y, Zhang Y, … Dong C (2020). Influence of diabetes mellitus on the severity and fatality of SARS-CoV-2 (COVID-19) infection. Diabetes Obes Metab. doi: 10.1111/dom.14105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyganowska-Swiatkowska M, Nohawica M, Grocholewicz K, & Nowak G (2020). Influence of Herbal Medicines on HMGB1 Release, SARS-CoV-2 Viral Attachment, Acute Respiratory Failure, and Sepsis. A Literature Review. Int J Mol Sci, 21(13). doi: 10.3390/ijms21134639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamin M (2020). Counting the cost of COVID-19. Int J Inf Technol, 1–7. doi: 10.1007/s41870-020-00466-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XH, Deng W, Tong Z, Liu YX, Zhang LF, Zhu H, … Qin C (2007). Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comp Med, 57(5), 450–459. [PubMed] [Google Scholar]


