Abstract
IMPORTANCE
Meta-analyses of randomized clinical trials have indicated that improved hypertension control reduces the risk for cognitive impairment and dementia. However, it is unclear to what extent pathways reflective of Alzheimer disease (AD) pathology are affected by hypertension control.
OBJECTIVE
To evaluate the association of intensive blood pressure control on AD-related brain biomarkers.
DESIGN, SETTING, AND PARTICIPANTS
This is a substudy of the Systolic Blood Pressure Intervention Trial (SPRINT MIND), a multicenter randomized clinical trial that compared the efficacy of 2 different blood pressure–lowering strategies. Potential participants (n = 1267) 50 years or older with hypertension and without a history of diabetes or stroke were approached for a brain magnetic resonance imaging (MRI) study. Of these, 205 participants were deemed ineligible and 269 did not agree to participate; 673 and 454 participants completed brain MRI at baseline and at 4-year follow-up, respectively; the final follow-up date was July 1, 2016. Analysis began September 2019 and ended November 2020.
INTERVENTIONS
Participants were randomized to either a systolic blood pressure goal of less than 120 mm Hg (intensive treatment: n = 356) or less than 140 mm Hg (standard treatment: n = 317).
MAIN OUTCOMES AND MEASURES
Changes in hippocampal volume, measures of AD regional atrophy, posterior cingulate cerebral blood flow, and mean fractional anisotropy in the cingulum bundle.
RESULTS
Among 673 recruited patients who had baseline MRI (mean [SD] age, 67.3 [8.2] years; 271 women [40.3%]), 454 completed the follow-up MRI at a median (interquartile range) of 3.98 (3.7–4.1) years after randomization. In the intensive treatment group, mean hippocampal volume decreased from 7.45 cm3 to 7.39 cm3 (difference, −0.06 cm3; 95% CI, −0.08 to −0.04) vs a decrease from 7.48 cm3 to 7.46 cm3 (difference, −0.02 cm3; 95% CI, −0.05 to −0.003) in the standard treatment group (between-group difference in change, −0.033 cm3; 95% CI, −0.062 to −0.003; P = .03). There were no significant treatment group differences for measures of AD regional atrophy, cerebral blood flow, or mean fractional anisotropy.
CONCLUSIONS AND RELEVANCE
Intensive treatment was associated with a small but statistically significant greater decrease in hippocampal volume compared with standard treatment, consistent with the observation that intensive treatment is associated with greater decreases in total brain volume. However, intensive treatment was not associated with changes in any of the other MRI biomarkers of AD compared with standard treatment.
TRIAL REGISTRATION
ClinicalTrials.gov Identifier: NCT01206062
Magnetic resonance imaging (MRI) data from the Systolic Blood Pressure Intervention Trial (SPRINT), the Action to Control Cardiovascular Risk in Diabetes (ACCORD) blood pressure trial, and the Intensive vs Standard Ambulatory Blood Pressure Lowering to Prevent Functional Decline in the Elderly (INFINITY) trial have all indicated less progression of white matter lesions (WML or leukoaraiosis), a biomarker for cerebrovascular injury, with more intensive blood pressure control.1–3 However, the effect of intensive treatment on other mechanistic markers of cognitive impairment and dementia has yet to be explored. The most common cause of dementia in older adults is Alzheimer disease (AD).4 Vascular disease is a very common comorbidity and may play a factor in initiating or accelerating AD neuropathology.5,6 Some studies have found that treating hypertension may reduce the incidence of AD7; however, it is unclear whether this could be a direct effect on AD-related neurodegeneration or comorbid vascular pathology. The neurodegeneration of AD results in brain atrophy, favoring but not limited to particular brain regions, and biomarkers measuring these changes may be more sensitive to early AD-related change than downstream cognitive or clinical variables, such as incident dementia.8 Both ACCORD and SPRINT showed small but statistically significant decreases in total brain volume (TBV) with intensive treatment compared with standard treatment,1,9 although it is unclear to what extent these differences reflect atrophy vs factors such as hydration status.10
There are multiple MRI biomarkers for AD-related brain changes. The most widely studied is hippocampal atrophy, a hallmark of typical mild cognitive impairment and AD.11 More recently, evaluation of patterns of cortical thinning and machine learning methods have identified patterns of AD-like atrophy that often have reported higher sensitivity and specificity than hippocampal atrophy.12 One method, termed the spatial pattern for recognition of AD (SPARE-AD),13 assigns a single global score based on overall similarity of the brain to an AD pattern vs normal. Prior studies have also identified AD-associated changes in white matter integrity, including decreased fractional anisotropy (FA) in the cingulum bundle14 and abnormalities of regional cerebral blood flow (CBF), such as decreased perfusion in AD signature regions.15
Owing to the potential synergistic effects of vascular and AD pathologies, we hypothesized that intensive treatment would be associated with AD-specific brain measurements from MRI (ie, would be associated with less atrophy as measured by hippocampal volume, AD regional cortical thickness, and SPARE-AD; a smaller decrease in FA in the cingulum bundle; and greater perfusion in the posterior cingulated gyrus). For comparison, we also evaluated changes in several putative brain MRI biomarkers for vascular disease, specifically frontal gray matter volume, frontal gray matter perfusion, and FA in the corpus callosum genu.16,17
Methods
Study Participants
The trial design, protocol, and primary cardiovascular outcome results were described previously.18,19 The trial protocol is also available in Supplement 1. The trial and MRI substudy were approved by the institutional review board at each site. Each participant provided written informed consent. Participants were older than 50 years and had systolic blood pressure levels between 130 and 180 mm Hg at the screening visit and increased cardiovascular risk, defined as having clinical or subclinical cardiovascular disease, chronic kidney disease (estimated glomerular filtration rate of <60 mL/min/1.73m2), a 10-year Framingham cardiovascular disease risk 15% or higher, or age 75 years and older. Inhabiting a nursing home, dementia diagnosis (by medical record review), diabetes, or stroke history were exclusions. Between November 2010 and March 2013, 9361 participants were randomized in a 1:1 allocation to an intensive treatment strategy with a systolic blood pressure goal of less than 120 mm Hg or a standard treatment strategy with a systolic blood pressure goal of less than 140 mm Hg.19 At the time of randomization, a subset of participants (n = 2921) were selected for a cognitive function substudy designed to examine associations of domain-specific cognitive function with outcomes,20 where participants were administered a comprehensive cognitive battery at baseline and during follow-up. MRI was performed in a subset of participants in the cognitive substudy.
MRI
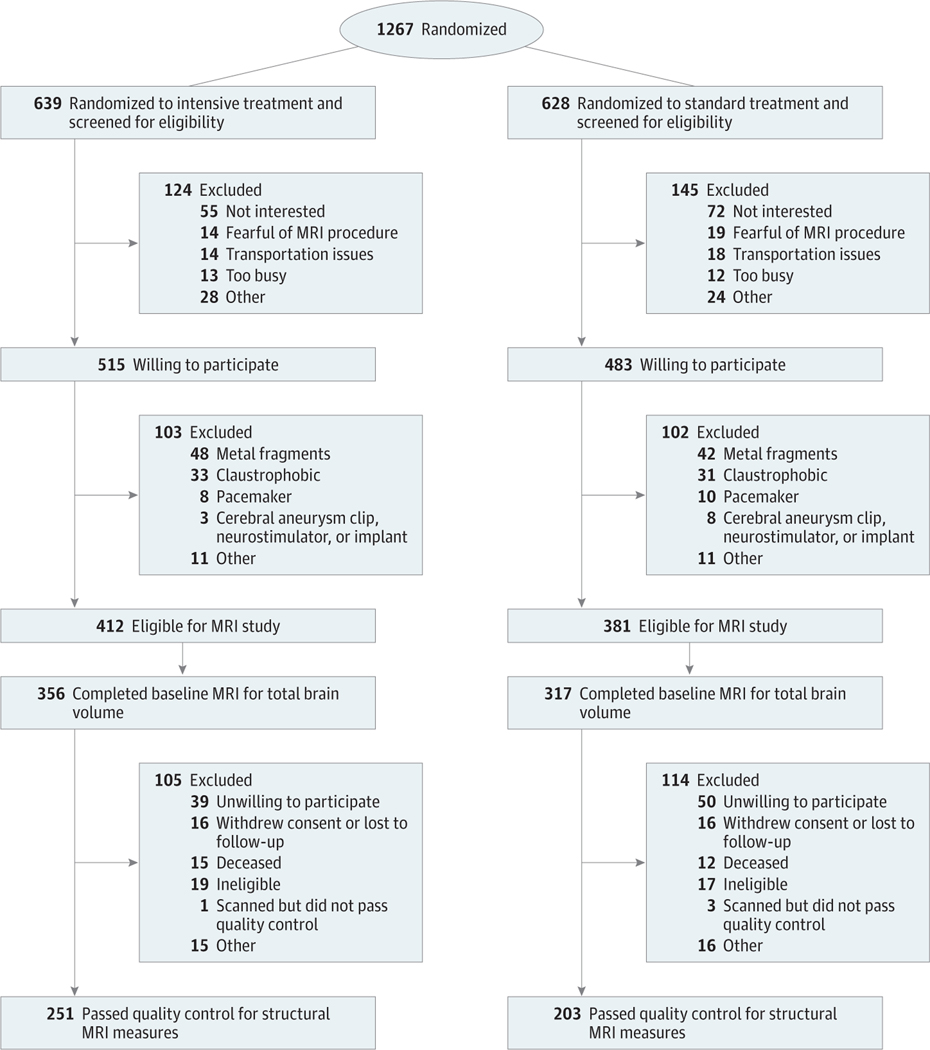
Brain MRIs were obtained at baseline with planned follow-up 4 years after randomization. All participants with access to 1 of 7 MRI sites were screened for the MRI study. Eligible participants provided written informed consent. Exclusion criteria for the MRI substudy have been previously described,1 and the MRI protocol is described in Supplement 1. Figure 1 summarizes recruitment, randomization, and follow-up.
Figure 1. Eligibility, Randomization, and Follow-up For Participants in the Magnetic Resonance Imaging (MRI) Substudy.
The total randomization number indicates the number of participants in the SPRINT MIND substudy who were within range of a study magnetic resonance imaging scanner.
MRI Processing
Image analysts were blinded to treatment group. Preprocessing included correction of magnetic field intensity in-homogeneity21 and multi-atlas skull stripping.22 Measurement of intracranial volume, TBV, and WML volume were previously described.1 We used a multi-atlas, multiwarp label-fusion method23 to segment T1 brains scan into 145 anatomic regions of interest (ROIs) spanning the entire brain. After preprocessing, we implemented 3 pipelines to obtain a set of structural imaging measures of AD-like atrophy: (1) hippocampal volumes using a longitudinal segmentation procedure, (2) a longitudinal cortical thickness measure from a meta-ROI comprising selected brain regions previously shown to differ between patients with AD and healthy controls, and (3) a machine learning–based AD classification score using data from the Alzheimer’s Disease Neuroimaging Initiative24 as training examples. Each pipeline is further detailed below.
Longitudinal Hippocampal Volumes
We measured longitudinal change in hippocampal volumes using an unbiased deformation-based morphometry pipeline.25 Inputs for the pipeline included cross-sectional hippocampal segmentations and baseline and follow-up T1 images. We excluded 2 participants for failed segmentations.
Longitudinal Meta-ROI Cortical Thickness
Cortical thickness maps were derived using the open-source Advanced Normalization Tools longitudinal cortical thick-nesspipeline26 with default parameters. Inputs included participant baseline and follow-up T1 images and group templates from the Desikan-Killiany-Tourville protocol.27 Three participants failed processing. The meta-ROI mean cortical thickness measure was computed from 12 brain regions previously shown to differentiate between patients with AD and healthy controls: bilateral entorhinal cortex, temporal cortex, precuneus, and fusiform gyrus.28
Machine Learning–Based AD Classifier Score
We applied a previously described machine learning–based likelihood score of AD vs healthy control classification, SPARE-AD (eMethods in Supplement 2).13 SPARE-AD had a cross-validated accuracy of 90.1% for classifying AD vs healthy controls in the Alzheimer’s Disease Neuroimaging Initiative. Greater positive scores indicate a more AD-like pattern, while more negative scores indicate similarity to the pattern seen in cognitively normal controls.
FA
FA is affected by axon tract structural integrity, with decreases in value associated with pathology. FA scalar maps were calculated from diffusion tensor imaging. Using the Johns Hopkins white matter atlas,29 we extracted mean FA from the right and left cingulum bundles14 and the right and left corpus callosum genu, which were averaged.
CBF Processing
CBF maps were calculated from pseudo-continuous arterial spin labeling using a custom data cleaning and processing pipeline.30 Arterial spin labeling processing followed recommendations of Alsop et al31 and consisted of motion correction, CBF quantification, and subsequent denoising based on a structural correlation with Robust Bayesian criteria.32,33 Mean CBF map quality was evaluated using an automated quality evaluation index,34 which ranges between 0 and 1, with a higher value indicating a better map. We excluded from analysis CBF maps with quality evaluation index less than 0.5, a threshold that was shown to have a good sensitivity/specificity for poor-quality data.34 Using a lower-quality evaluation index score cutoff of 0.35 did not appreciably change the results (data not shown). We assessed the change in absolute changes in posterior cingulated gyrus CBF and relative mean CBF in the posterior cingulated gyrus normalized to mean CBF in the putamen, a measurement sensitive to AD-related change.15 We also measured mean CBF in frontal gray matter.
Normative MRI Data
We compared hippocampal volume and SPARE-AD scores from SPRINT with data derived from a consortium of 4 studies with more than 5000 cognitively normal participants matching the age recruitment criteria for SPRINT.35,36 While cognitively normal, this composite cohort includes participants with variable comorbidities, including hypertension and cardiovascular disease.
Assessment of Cognitive Status
Methods for neuropsychological testing of cognitive function were previously described.20,37 Briefly, MRI substudy participants were administered a comprehensive cognitive battery at baseline and at 2 and approximately 4 years of follow-up, including measures of global cognitive function, learning and memory, processing speed, attention/concentration, verbal and nonverbal memory, language, and executive function. For these analyses, we used composite cognitive domain scores derived from raw cognitive test scores (eMethods in Supplement 2 provides additional detail on cognitive tests).
Statistical Analysis
Baseline correlations between brain MRI measures were estimated using Spearman rank correlations and partial correlation coefficients adjusting for age.38 Linear mixed models, including random effects for participant and MRI facility, were used to estimate the mean change in each MRI outcome between the treatment groups, including age, sex, and time since randomization as a covariate. Intracranial volume was additionally included as a covariate in the models for hippocampal volume, frontal gray matter volume, and SPARE-AD to control for differences in head size. As AD incidence strongly increases with age and previous results indicating a differential association of intensive treatment with changes in TBV by sex,1 we also evaluated whether there was any evidence of treatment effect heterogeneity by age (age <75 years vs ≥75 years) and sex. Analyses of longitudinal cognitive domain scores by treatment group for participants in the MRI subgroup was similarly performed using linear mixed models, including participant and clinic random effects. For these models, change over time was modeled via a linear group effect, ie, an annual slope. All analyses were performed using SAS statistical software version 9.4 (SAS Institute) and R version 3.4.2 (R Project for Statistical Computing). All hypothesis tests were 2-sided, and P values less than .05 were considered statistically significant. No explicit adjustments for multiple comparisons were made, so the interpretation of these analyses should be considered exploratory. Analysis began September 2019 and ended November 2020.
Results
A total of 673 participants completed baseline MRI with a measurement of TBV passing quality control and 454 of these completed the follow-up MRI. At baseline, the mean (SD) age of participants in the MRI substudy was 67.3 (8.2) years; 271 (40.3%) were female, 218 (32.4%) were Black, mean (SD) systolic blood pressure was 138.1 (16.7) mm Hg, and mean (SD) diastolic blood pressure was 77.9 (11.5) mm Hg (Table 1).1 Compared with overall trial participants, participants in the MRI substudy were younger, more likely to be female, less likely to be of Hispanic ethnicity, had lower systolic blood pressure, and higher cognitive test scores (eTable 1 in Supplement 2). Baseline hippocampal volume and SPARE-AD scores were comparable with values in a large, cognitively normal cohort (eFigure 1 in Supplement 2).36 Mean SPARE-AD scores across the cohort were negative at baseline, with only 43 participants (6.4%) having positive SPARE-AD scores, which is indicative of a pattern more AD-like than normal. As expected, older participants (age ≥75 years) showed lower baseline hippocampal volumes (mean [SD], 7.2 [0.8] cm3 vs 7.6 [0.8] cm3; P < .001) and worse baseline SPARE-AD scores (mean [SD], −0.9 [0.9] vs −1.7 [0.9]; P < .001), compared with younger participants. At baseline, the AD-related biomarkers were generally modestly correlated with WML volume or TBV, with a higher correlation between hippocampal volumes and TBV (partial Spearman correlation adjusting for age = 0.61; P < .001; eFigure 2 in Supplement 2). Hippocampal volume was also negatively correlated with the SPARE-AD score at baseline (partial Spearman correlation adjusting for age = −0.49; P < .001).
Table 1.
Characteristics of Participants in the Magnetic Resonance Imaging Substudy
| Variable | Completed baseline scan | Completed follow-up scan | ||
|---|---|---|---|---|
| Intensive (n = 356) | Standard (n = 317) | Intensive (n = 251) | Standard (n = 203) | |
| Age, mean (SD), y | 67.6 (8.0) | 66.9 (8.5) | 67.7 (7.7) | 66.4 (7.8) |
| Age ≥75 y, No. (%) | 83 (23.3) | 67 (21.1) | 54 (21.5) | 37 (18.2) |
| Montreal Congnitive Assessmenta | 24 (21 to 26) | 24 (22 to 26) | 24 (21 to 27) | 24 (22 to 27) |
| Total brain volume, mean (SD), cm3 | 1127.1 (113.5) | 1141.7 (114.7) | 1108.3 (106.7) | 1124.0 (114.8) |
| WML volume, cm3 | ||||
| No. | 355 | 315 | 249 | 200 |
| Median (IQR) | 3.0 (1.5 to 6.2) | 3.3 (1.6 to 6.1) | 2.9 (1.5 to 5.8) | 3.2 (1.7 to 6.2) |
| Hippocampal volume, cm3 | ||||
| No. | 353 | 316 | 251 | 203 |
| Mean (SD) | 7.4 (0.8) | 7.5 (0.8) | 7.4 (0.8) | 7.5 (0.8) |
| Frontal gray matter volume, cm3 | ||||
| No. | 356 | 317 | 251 | 203 |
| Mean (SD) | 171.0 (20.7) | 173.7 (20.8) | 165.3 (19.1) | 168.9 (20.6) |
| SPARE-ADb | ||||
| No. | 354 | 316 | 255 | 203 |
| Mean (SD) | −1.5 (0.9) | −1.6 (0.9) | −1.2 (1.0) | −1.4 (1.0) |
| Meta-ROI mean cortical thickness, mm | ||||
| No. | 353 | 315 | 252 | 202 |
| Mean (SD) | 2.8 (0.3) | 2.8 (0.3) | 2.6 (0.3) | 2.6 (0.3) |
| Mean FA in the cingulum bundlec | ||||
| No. | 347 | 308 | 252 | 200 |
| Mean (SD) | 0.29 (0.04) | 0.29 (0.03) | 0.29 (0.04) | 0.29 (0.04) |
| Mean FA in the corpus callosum genuc | ||||
| No. | 348 | 309 | 253 | 200 |
| Mean (SD) | 0.41 (0.04) | 0.40 (0.08) | 0.39 (0.08) | 0.38 (0.08) |
| CBF in the posterior cingulate gyrus, mL/100 mg/min |
||||
| No. | 309 | 284 | 174 | 136 |
| Mean (SD) | 62.4 (16.6) | 59.3 (14.3) | 64.0 (15.8) | 59.5 (14.1) |
| rCBF in posterior cingulate gyrus relative to putamen | ||||
| No. | 309 | 284 | 174 | 136 |
| Median (IQR) | 1.3 (1.2 to 1.5) | 1.3 (1.2 to 1.4) | 1.2 (1.1 to 1.4) | 1.2 (1.1 to 1.4) |
| Frontal gray matter CBF, mL/100 mg/min | ||||
| No. | 326 | 296 | 173 | 149 |
| Mean (SD) | 50.1 (14.4) | 48.7 (12.6) | 53.5 (12.5) | 49.0 (12.1) |
Abbreviations: CBF, cerebral blood flow; FA, fractional anisotropy; IQR, interquartile range; rCBF, regional cerebral blood flow; ROI, region of interest; SPARE-AD, spatial pattern for recognition of Alzheimer disease; WML, white matter lesion.
Scores range from 0 to 30, with higher scores denoting better cognitive function.
Higher positive scores indicate greater similarity to the Alzheimer disease pattern, while lower negative scores indicate greater similarity to a cognitively normal sample.
Values range from 0 to 1, with higher values indicating greater limitation of diffusion in a single direction.
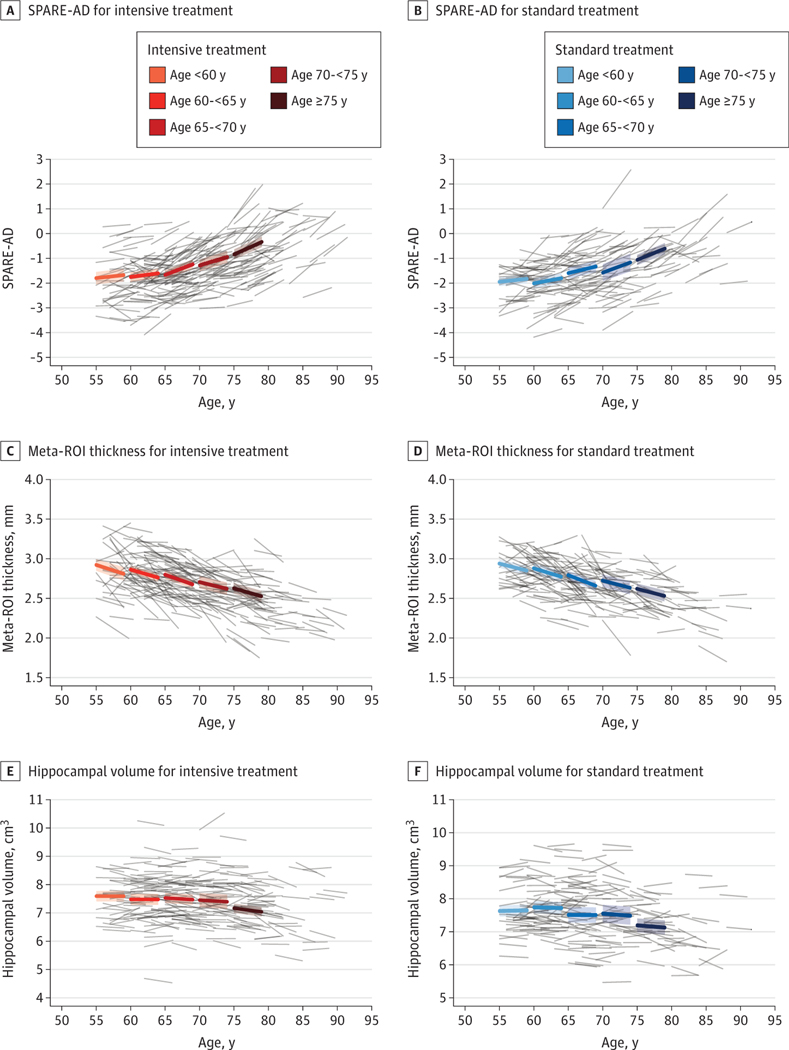
Intensive treatment was associated with larger declines in hippocampal volume during follow-up compared with standard treatment (mean difference = −0.033 cm3 [95% CI,−0.062 to −0.003]; P = .03; Table 2). There were not significant between-group differences for the other 2 structural biomarkers, SPARE-AD score and meta-ROI mean cortical thickness (Table 2 and Figure 2). Mean FA in the cingulum bundle showed no significant difference between groups. Mean absolute and relative CBF in the posterior cingulate gyrus declined during follow-up, but there were not significant treatment group differences (Table 2). Of the secondary biomarkers associated with vascular disease, frontal gray matter volume showed significantly greater decrease in the intensive treatment group (mean difference = −1.06 cm3 [95% CI, −1.95 to −0.16]; P = .02), but there were no between-group differences for the change in frontal gray matter CBF or mean FA in the corpus callosum genu.
Table 2.
Changes in Magnetic Resonance Imaging Outcomes by Treatment Groupa
| Outcome | Intensive treatment (95% CI) |
Standard treatment (95% CI) |
Difference in change (95% CI) | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Change | Baseline | Follow-up | Change | |||
|
| ||||||||
| Hippocampal volume, cm3 | 7.45 (7.37 to 7.53) | 7.39 (7.31 to 7.47) | −0.06 (−0.08 to −0.04) | 7.48 (7.40 to 7.57) | 7.46 (7.38 to 7.54) | −0.02 (−0.05 to 0) | −0.033 (−0.062 to −0.003) | .03 |
|
| ||||||||
| Frontal gray matter volume, cm3 | 172.4 (170.4 to 174.3) | 164.7 (162.7 to 166.6) | −7.74 (−8.34 to −7.13) | 172.3 (170.3 to 174.3) | 165.6 (163.6 to 167.6) | −6.68 (−7.34 to −6.01) | −1.06 (−1.95 to −0.16) | .02 |
|
| ||||||||
| SPARE-AD | −1.48 (−1.63 to −1.33) | −1.17 (−1.32 to −1.02) | 0.31 (0.27 to 0.36) | −1.63 (−1.79 to −1.48) | −1.36 (−1.51 to −1.20) | 0.28 (0.22 to 0.33) | 0.037 (−0.031 to 0.106) | .29 |
|
| ||||||||
| Meta-ROI mean cortical thickness, mm | 2.77 (2.75 to 2.80) | 2.67 (2.64 to 2.70) | −0.11 (−0.12 to −0.09) | 2.80 (2.77 to 2.82) | 2.69 (2.66 to 2.72) | −0.10 (−0.12 to −0.09) | −0.002 (−0.027 to 0.023) | .88 |
|
| ||||||||
| Mean FA in the cingulum bundle | 0.290 (0.277 to 0.303) | 0.289 (0.276 to 0.302) | −0.002 (−0.005 to 0.002) | 0.288 (0.275 to 0.301) | 0.289 (0.275 to 0.302) | 0.001 (−0.003 to 0.004) | −0.002 (−0.007 to 0.002) | .33 |
|
| ||||||||
| Mean FA in the corpus callosum genu | 0.41 (0.37 to 0.46) | 0.39 (0.35 to 0.43) | −0.022 (−0.026 to −0.018) | 0.41 (0.37 to 0.45) | 0.39 (0.35 to 0.44) | −0.018 (−0.023 to −0.014) | −0.003 (−0.01 to 0.003) | .29 |
|
| ||||||||
| CBF in the posterior cingulate gyrus, mL/100 mg/min | 61.9 (56.3 to 67.5) | 62.0 (56.2 to 67.7) | 0.04 (−2.18 to 2.26) | 59.2 (53.6 to 64.8) | 57.9 (52.0 to 63.7) | −1.33 (−3.75 to 1.09) | 1.37 (−1.89 to 4.64) | .41 |
|
| ||||||||
| rCBF in posterior cingulate gyrus relative to putamen | 1.35 (1.29 to 1.40) | 1.25 (1.19 to 1.31) | −0.10 (−0.14 to −0.06) | 1.31 (1.25 to 1.36) | 1.24 (1.17 to 1.30) | −0.07 (−0.12 to −0.03) | −0.024 (−0.085 to 0.037) | .44 |
|
| ||||||||
| Frontal gray matter CBF, mL/100 mg/min | 51.27 (46.16 to 56.38) | 52.59 (47.35 to 57.83) | 1.32 (−0.47 to 3.10) | 49.58 (44.44 to 54.71) | 48.72 (43.43 to 54.01) | −0.86 (−2.77 to 1.05) | 2.18 (−0.42 to 4.77) | .10 |
Abbreviations: CBF, cerebral blood flow; FA, fractional anisotropy; rCBF, regional cerebral blood flow; ROI, region of interest; SPARE-AD, spatial pattern for recognition of Alzheimer disease.
Estimates based on a linear mixed model adjusting for age, sex, intracranial volume (for hippocampal volume, frontal gray matter volume, and SPARE-AD), and days since randomization, with random effects for participant and magnetic resonance imaging facility. Change denotes estimated least square mean comparing follow-up (estimated at 3.98 years postrandomization); negative values denote decreases from baseline, while positive values indicate increases from baseline. Difference in change reflects intensive treatment group minus standard treatment group.
Figure 2. Longitudinal Change in Alzheimer Disease Magnetic Resonance Imaging Biomarkers by Age and Treatment Group.
Bold lines represent age-specific change for each measurement estimated from a treatment group specific linear mixed model, with associated 95%CIs. Gray lines represent individual participant trajectories. ROIs indicate regions of interest; SPARE-AD, spatial pattern for recognition of Alzheimer disease.
Older adults (age ≥ 75 years) tended to exhibit larger changes in most AD-related biomarker changes compared with those younger than 75 years, specifically larger decreases in hippocampal volumes and larger increases in SPARE-AD scores. However, there was no evidence of treatment effect heterogeneity across these age subgroups for either the AD or vascular biomarkers (Table 3). We did observe nominally significant interactions with respect to sex for mean cortical thickness (men: 0.035 [95% CI,−0.007 to 0.076]; women: −0.022 [95% CI, −0.054 to 0.010]; P = .03), relative mean CBF in the posterior cingulated gyrus relative to putamen (men: −0.11 [95% CI, −0.21 to −0.01]; women: 0.03 [95% CI, −0.05 to 0.11]; P = .03), and mean FA in the corpus callosum genu (men: 0.005 [95% CI, −0.006 to 0.016]; women: −0.009 [95% CI, −0.017 to −0.0004]; P = .05) (eTable 2 in Supplement 2). However, heterogeneity by sex was generally of a small magnitude and was in inconsistent directions across these measures.
Table 3.
Change in Magnetic Resonance Imaging Outcomes by Treatment Group and Stratified by Agea
| Outcome | Age, y | Change (SE) | Difference in change (95% CI) | P value for interaction | |
|---|---|---|---|---|---|
| Intensive treatment | Standard treatment | ||||
| Hippocampal volume, cm3 | <75 | −0.04 (0.01) | −0.02 (0.01) | −0.021 (−0.053 to 0.011) | .18 |
| ≥75 | −0.12 (0.02) | −0.05 (0.03) | −0.072 (−0.137 to −0.006) | ||
| Frontal gray matter volume, cm3 | <75 | −7.75 (0.35) | −6.73 (0.38) | −1.02 (−2.02 to −0.02) | .76 |
| ≥75 | −7.80 (0.67) | −6.43 (0.80) | −1.37 (−3.42 to 0.68) | ||
| SPARE-AD | <75 | 0.27 (0.03) | 0.24 (0.03) | 0.033 (−0.042 to 0.107) | .97 |
| ≥75 | 0.49 (0.05) | 0.45 (0.06) | 0.036 (−0.117 to 0.188) | ||
| Meta-ROI mean cortical thickness, mm | <75 | −0.10 (0.01) | −0.10 (0.01) | −0.002 (−0.031 to 0.026) | .91 |
| ≥75 | −0.11 (0.02) | −0.10 (0.02) | −0.006 (−0.063 to 0.052) | ||
| Mean FA in the cingulum bundle | <75 | −0.0004 (0.002) | 0.002 (0.002) | −0.003 (−0.008 to 0.002) | .58 |
| ≥75 | −0.006 (0.003) | −0.006 (0.004) | 0.0004 (−0.010 to 0.011) | ||
| Mean FA in the corpus callosum genu | <75 | −0.021 (0.003) | −0.017 (0.003) | −0.004 (−0.011 to 0.003) | .69 |
| ≥75 | −0.024 (0.005) | −0.023 (0.006) | −0.001 (−0.016 to 0.014) | ||
| CBF in the posterior cingulate gyrus, mL/100 mg/min | <75 | 0.03 (1.28) | −1.93 (1.36) | 1.96 (−1.70 to 5.63) | .41 |
| ≥75 | 0.13 (2.38) | 1.63 (2.88) | −1.50 (−8.84 to 5.85) | ||
| rCBF in the posterior cingulate gyrus relative to putamen | <75 | −0.10 (0.02) | −0.08 (0.03) | −0.021 (−0.090 to 0.047) | .64 |
| ≥75 | −0.07 (0.04) | −0.01 (0.05) | −0.058 (−0.195 to 0.079) | ||
| Frontal gray matter CBF, mL/100 mg/min | <75 | 1.49 (1.03) | −1.15 (1.08) | 2.64 (−0.27 to 5.56) | .40 |
| ≥75 | 0.57 (1.96) | 0.78 (2.28) | −0.21 (−6.12 to 5.70) | ||
Abbreviations: CBF, cerebral blood flow; FA, fractional anisotropy; rCBF, regional cerebral blood flow; ROI, region of interest; SPARE-AD, spatial pattern for recognition of Alzheimer disease.
Estimates based on a linear mixed model adjusting for sex, intracranial volume (for hippocampal volume, frontal gray matter volume, and SPARE-AD), and days since randomization, with random effects for participant and magnetic resonance imaging facility. Change denotes estimated least square mean comparing follow-up (estimated at 3.98 years postrandomization); negative values denote decreases from baseline, while positive values indicate increases from baseline. Difference in change reflects intensive treatment group minus standard treatment group.
We also investigated whether the between-group differences in hippocampal volume and frontal gray matter volume were associated with changes in cognitive function. Longitudinal declines across all cognitive domains were small (eTable 3 in Supplement 2). In this subgroup of participants, intensive treatment was associated with larger declines in the executive function domain (mean change per year: −0.019 [95% CI, −0.034 to −0.005] vs 0.005 [95% CI, −0.011 to 0.02]; P = .02), but other cognitive domains showed no significant between-group differences. While change in hippocampal volume weakly correlated with change in memory and global cognitive function, there was no evidence of a correlation with changes in executive function (eFigure 3 in Supplement 2). Change in frontal gray matter volume did not have a significant association with change for any of the cognitive domains (eFigure 4 in the Supplement 2).
Discussion
SPRINT has demonstrated beneficial associations of intensive vs standard treatment with cardiovascular morbidity and mortality18 and a composite outcome of mild cognitive impairment and probable dementia.37 Favorable effects on cerebral WML1 suggest that a lowering of vascular contributions to dementia may have contributed to the decreased rate of cognitive impairment. Prior observational and experimental studies suggest that treatment of vascular risk factors such as hypertension may lessen neurodegeneration in regions disproportionally affected in AD,39 suggesting the possibility of additional benefits. On the other hand, as seen in ACCORD,9 SPRINT showed a small but larger decline in TBV with intensive treatment that could be caused by an increase in neurodegeneration. Although specific markers of AD pathology were not measured in SPRINT, there are several MRI biomarkers examined here that are sensitive, although not specific, to AD-related neurodegeneration. Intensive treatment was associated with a small but statistically significant greater decrease in hippocampal volume compared with standard treatment, but we did not observe evidence of a treatment group difference for any of the other markers of AD-related neurodegeneration.
Early changes of AD are challenging to detect even with biomarkers that strongly correlate with disease severity, especially in populations consisting of largely cognitively normal individuals. SPRINT excluded patients with dementia, and 43.1% of participants in the MRI substudy were younger than 65 years at the time of randomization.37 Similarly, we found little MRI evidence of prevalent AD, as few participants had SPARE-AD scores more than 0. In addition, baseline SPARE-AD scores and hippocampal volumes were comparable with values from a large, multistudy cohort of cognitively normal individuals, and change in these 2 measures was comparable with other cognitively normal cohorts.13,40 Given this background, it is challenging to interpret the hippocampal volume result. While this result is comparable with observations from the Korean Brain Aging Study,41 a lack of treatment group differences for the other measured AD-related biomarkers would seem to suggest that the hippocampal volume result does not likely reflect differential development of AD-related neurodegeneration. However, it remains possible that the hippocampal volume result is due to a difference in early AD that is not detected by the other biomarkers. Alternatively, it could be a manifestation of the global effect of intensive treatment on TBV, related to volume status or another pathology.
The development of cognitive impairment and dementia depends on several factors, some of which may be affected by intensive treatment. Overall, there was little change in this subgroup of participants in terms of TBV, WML volume, and AD related and other vascular biomarkers over the 4 years of follow-up, paralleled by relative stability of cognitive test scores20 and low incidence of adjudicated cognitive impairment, with only 29 total cases among participants who completed the follow-up MRI.1 MRI was not performed in the entire trial population, and the MRI subgroup was somewhat younger and healthier than the overall trial population. Because of this, it is not possible to disentangle whether any of the observed biomarker differences underlie the adjudicated cognitive results in SPRINT or whether they reflect random variation due to sampling for the MRI substudy. Given the small between-group differences in MRI biomarkers, it is also possible that preserved functional status, conferred by improved overall cardiovascular health in the intensive group, may be a factor influencing the lower incidence of adjudicated cognitive impairment. Such resilience could potentially mitigate against the observed potentially unfavorable changes in TBV and hippocampal volume.
Strengths and Limitations
Strengths of this study include the randomized design, multisite imaging across the United States, and the interrogation of multiple biomarkers. There were several additional limitations. First, there was no ascertainment of amyloid or tau status or clinical categorization of dementia subtype. Second, this trial is not informative for understanding of the effect of hypertension treatment on the evolution of AD MRI biomarkers compared with uncontrolled hypertension or in populations with prevalent AD. As early AD-related neurodegeneration develops slowly, the 4-year follow-up may have been too short. Lastly, the small number of MRI participants with adjudicated cognitive impairment prevents meaningful evaluation of MRI biomarkers in this important group.
Conclusions
In conclusion, there was no consistent evidence of a difference in the progression of AD-related neurodegeneration between intensive and standard blood pressure control. Beyond the previously reported favorable effect on WML volume, there was also no consistent association with other putative biomarkers of cerebrovascular disease, and the differences between groups in all these MRI biomarkers were small. Overall, these MRI findings do not directly identify a specific pathologic mechanism for the observed associations of intensive blood pressure treatment with brain structure and the development of cognitive impairment.
Supplementary Material
Key Points.
Question Is intensive systolic blood pressure control associated with changes in the progression of Alzheimer disease–related magnetic resonance imaging biomarkers compared with standard blood pressure control?
Findings In this secondary analysis of a randomized clinical trial that included 454 adults with hypertension with follow-up magnetic resonance imaging, intensive treatment was associated with small but statistically significant larger decreases in hippocampal volume but was not associated with any other biomarker of Alzheimer disease neurodegeneration.
Meaning There is no consistent or clinically meaningful difference in magnetic resonance imaging biomarkers of Alzheimer disease between intensive and standard blood pressure treatment.
Acknowledgments
Funding/Support: The Systolic Blood Pressure Intervention Trial was funded by the National Institutes of Health (including the National Heart, Lung, and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; the National Institute on Aging; and the National Institute of Neurological Disorders and Stroke; grants HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, and HHSN268200900049C and interagency agreement A-HL-13-002-001). It was also supported in part with resources and use of facilities through the US Department of Veterans Affairs. Azilsartan and chlorthalidone (combined with azilsartan) were provided by Takeda Pharmaceuticals International. Computing resources were supported through 1S10OD023495-01 and additional support was provided through the following National Center for Advancing Translational Sciences (clinical and translational science awards UL1TR000439 [awarded to Case Western Reserve University]; UL1RR025755 [Ohio State University]; UL1RR024134 and UL1TR000003 [University of Pennsylvania]; UL1RR025771 [Boston University]; UL1TR000093 [Stanford University]; UL1RR025752, UL1TR000073, and UL1TR001064 [Tufts University]; UL1TR000050 [University of Illinois]; UL1TR000005 [University of Pittsburgh]; 9U54TR000017-06 [University of Texas Southwestern Medical Center]; UL1TR000105-05 [University of Utah]; UL1 TR000445 [Vanderbilt University]; UL1TR000075 [George Washington University]; UL1 TR000002 [University of California, Davis]; UL1 TR000064 [University of Florida]; and UL1TR000433 [University of Michigan]) and by National Institute of General Medical Sciences, Centers of Biomedical Research Excellence (award NIGMS P30GM103337 [Tulane University]). The work presented here was also supported by 1RF1AG054409, R01-AG055606 and funding from the Alzheimer’s Association.
Role of the Funder/Sponsor: The National Institutes of Health and the US Department of Veterans Affairs had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript, but not in the decision to submit the manuscript for publication. Takeda Pharmaceuticals did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Conflict of Interest Disclosures: Dr Nasrallah reports grants from the National Institutes of Health (NIH) during the conduct of the study and personal fees from Biogen outside the submitted work. Dr Gaussoin reports grants from NIH and Alzheimer’s Association during the conduct of the study. Dr Erus reports grants from NIH for processing of magnetic resonance imaging data during the conduct of the study. Dr Wright reports royalties from UpToDate outside the submitted work. Dr Wolk reports grants from Biogen, Merck, and Eli Lilly and Company and personal fees from Functional Neuromodulation DSMB and Neuronix Consultation outside the submitted work. Dr Davatzikos reports grants from NIH during the conduct of the study. Dr Williamson reports grants from NIH during the conduct of the study. Dr Pajewski reports grants from NIH and Alzheimer’s Association during the conduct of the study. Dr Bryan reports grants from NIH during the conduct of the study; nonfinancial support from Galileo CDS outside the submitted work; and has a patent licensed to Galileo CDS. No other disclosures were reported.
Group Information: The SPRINT Research Group members are listed in Supplement 3.
Group Information: The SPRINT Research Group members appear at the end of the article.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the US Department of Veterans Affairs, or the US government.
Footnotes
Author Contributions: Drs Nasrallah and Pajewski had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Nasrallah, Williamson, Pajewski, Bryan.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Nasrallah, Pomponio, Dolui, Launer, Pajewski.
Critical revision of the manuscript for important intellectual content: Nasrallah, Gaussoin, Dolui, Erus, Wright, Launer, Detre, Wolk, Davatzikos, Williamson, Pajewski, Bryan.
Statistical analysis: Nasrallah, Gaussoin, Pomponio, Dolui, Pajewski.
Obtained funding: Williamson, Bryan.
Administrative, technical, or material support: Nasrallah, Wright, Davatzikos, Williamson, Bryan.
Supervision: Nasrallah, Erus, Wright, Detre, Pajewski, Bryan.
Data Sharing Statement: See Supplement 4.
REFERENCES
- 1.Nasrallah IM, Pajewski NM, Auchus AP, et al. ; SPRINT MIND Investigators for the SPRINT Research Group. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA. 2019;322(6):524–534. doi: 10.1001/jama.2019.10551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White WB, Wakefield DB, Moscufo N, et al. Effects of intensive versus standard ambulatory blood pressure control on cerebrovascular outcomes in older people (INFINITY). Circulation. 2019;140(20):1626–1635. doi: 10.1161/CIRCULATIONAHA.119.041603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray AM, Hsu FC, Williamson JD, et al. ; Action to Control Cardiovascular Risk in Diabetes Follow-On Memory in Diabetes (ACCORDION MIND) Investigators. ACCORDION MIND: results of the observational extension of the ACCORD MIND randomised trial. Diabetologia. 2017;60(1):69–80. doi: 10.1007/s00125-016-4118-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alzheimer’s Association. 2012 Alzheimer’s disease facts and figures. Alzheimers Dement. 2012; 8(2):131–168. doi: 10.1016/j.jalz.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 5.Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37(1):56–74. doi: 10.1111/j.1365-2990.2010.01139.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12(12):723–738. doi: 10.1038/nrn3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haag MD, Hofman A, Koudstaal PJ, Breteler MM, Stricker BH. Duration of antihypertensive drug use and risk of dementia: a prospective cohort study. Neurology. 2009;72(20):1727–1734. doi: 10.1212/01.wnl.0000345062.86148.3f [DOI] [PubMed] [Google Scholar]
- 8.Jack CR Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013; 12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williamson JD, Launer LJ, Bryan RN, et al. ; Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes Investigators. Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels: a randomized clinical trial. JAMA Intern Med. 2014;174(3):324–333. doi: 10.1001/jamainternmed.2013.13656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duning T, Kloska S, Steinsträter O, Kugel H, Heindel W, Knecht S. Dehydration confounds the assessment of brain atrophy. Neurology. 2005;64 (3):548–550. doi: 10.1212/01.WNL.0000150542.16969.CC [DOI] [PubMed] [Google Scholar]
- 11.Jack CR Jr, Shiung MM, Weigand SD, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65(8):1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pettigrew C, Soldan A, Zhu Y, et al. ; BIOCARD Research Team. Cortical thickness in relation to clinical symptom onset in preclinical AD. Neuroimage Clin. 2016;12:116–122. doi: 10.1016/j.nicl.2016.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davatzikos C, Xu F, An Y, Fan Y, Resnick SM. Longitudinal progression of Alzheimer’s-like patterns of atrophy in normal older adults: the SPARE-AD index. Brain. 2009;132(pt 8):2026–2035. doi: 10.1093/brain/awp091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang H, Fan X, Weiner M, et al. Distinctive disruption patterns of white matter tracts in Alzheimer’s disease with full diffusion tensor characterization. Neurobiol Aging. 2012;33(9): 2029–2045. doi: 10.1016/j.neurobiolaging.2011.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolui S, Li Z, Nasrallah IM, Detre JA, Wolk DA. Arterial spin labeling versus 18F-FDG-PET to identify mild cognitive impairment. Neuroimage Clin. 2020; 25:102146. doi: 10.1016/j.nicl.2019.102146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vemuri P, Lesnick TG, Przybelski SA, et al. Development of a cerebrovascular magnetic resonance imaging biomarker for cognitive aging. Ann Neurol. 2018;84(5):705–716. doi: 10.1002/ana.25346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palesi F, De Rinaldis A, Vitali P, et al. Specific patterns of white matter alterations help distinguishing Alzheimer’s and vascular dementia. Front Neurosci. 2018;12:274. doi: 10.3389/fnins.2018.00274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright JT Jr, Williamson JD, Whelton PK, et al. ; SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ambrosius WT, Sink KM, Foy CG, et al. ; SPRINT Study Research Group. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11(5):532–546. doi: 10.1177/1740774514537404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rapp SR, Gaussoin SA, Sachs BC, et al. ; SPRINT Research Group. Effects of intensive versus standard blood pressure control on domain-specific cognitive function: a substudy of the SPRINT randomised controlled trial. Lancet Neurol. 2020;19 (11):899–907. doi: 10.1016/S1474-4422(20)30319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tustison NJ, Avants BB, Cook PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29(6):1310–1320. doi: 10.1109/TMI.2010.2046908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doshi J, Erus G, Ou Y, Gaonkar B, Davatzikos C. Multi-atlas skull-stripping. Acad Radiol. 2013;20 (12):1566–1576. doi: 10.1016/j.acra.2013.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doshi J, Erus G, Ou Y, et al. ; Alzheimer’s Neuroimaging Initiative. MUSE: MUlti-atlas region Segmentation utilizing Ensembles of registration algorithms and parameters, and locally optimal atlas selection. Neuroimage. 2016;127:186–195. doi: 10.1016/j.neuroimage.2015.11.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jack CR Jr, Bernstein MA, Fox NC, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27(4):685–691. doi: 10.1002/jmri.21049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das SR, Avants BB, Pluta J, et al. Measuring longitudinal change in the hippocampal formation from in vivo high-resolution T2-weighted MRI. Neuroimage. 2012;60(2):1266–1279. doi: 10.1016/j.neuroimage.2012.01.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tustison NJ, Holbrook AJ, Avants BB, et al. The ANTs longitudinal cortical thickness pipeline. bioRxiv. Preprint posted online August 16, 2018. doi: 10.1101/170209 [DOI] [Google Scholar]
- 27.Klein A, Tourville J. 101 labeled brain images and a consistent human cortical labeling protocol. Front Neurosci. 2012;6:171. doi: 10.3389/fnins.2012.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarz CG, Gunter JL, Wiste HJ, et al. ; Alzheimer’s Disease Neuroimaging Initiative. A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer’s disease severity. Neuroimage Clin. 2016;11:802–812. doi: 10.1016/j.nicl.2016.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230(1):77–87. doi: 10.1148/radiol.2301021640 [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Aguirre GK, Rao H, et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging. 2008;26(2):261–269. doi: 10.1016/j.mri.2007.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73(1):102–116. doi: 10.1002/mrm.25197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dolui S, Wang Z, Shinohara RT, Wolk DA, Detre JA; Alzheimer’s Disease Neuroimaging Initiative. Structural Correlation-based Outlier Rejection (SCORE) algorithm for arterial spin labeling time series. J Magn Reson Imaging. 2017;45 (6):1786–1797. doi: 10.1002/jmri.25436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dolui S, Wolk D, Detre J. SCRUB: a structural correlation and empirical robust bayesian method for ASL data. Paper presented at: International Society of Magnetic Resonance in Medicine; May 7–13, 2016; Singapore. [Google Scholar]
- 34.Dolui S, Wolf R, Nabavizadeh SA, Wolk D, Detre J. Automated quality evaluation index for 2D ASL CBF maps. Paper presented at: International Society of Magnetic Resonance in Medicine; April 22–27, 2017; Honolulu, Hawaii. [Google Scholar]
- 35.Bashyam VM, Erus G, Doshi J, et al. MRI signatures of brain age and disease over the lifespan based on a deep brain network and 14 468 individuals worldwide. Brain. 2020;143(7):2312–2324. doi: 10.1093/brain/awaa160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pomponio R, Erus G, Habes M, et al. Harmonization of large MRI datasets for the analysis of brain imaging patterns throughout the lifespan. Neuroimage. 2020;208:116450. doi: 10.1016/j.neuroimage.2019.116450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williamson JD, Pajewski NM, Auchus AP, et al. ; SPRINT MIND Investigators for the SPRINT Research Group. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321(6):553–561. doi: 10.1001/jama.2018.21442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Q, Li C, Wanga V, Shepherd BE. Covariate-adjusted Spearman’s rank correlation with probability-scale residuals. Biometrics. 2018;74 (2):595–605. doi: 10.1111/biom.12812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wahidi N, Lerner AJ. Blood pressure control and protection of the aging brain. Neurotherapeutics. 2019;16(3):569–579. doi: 10.1007/s13311-01900747-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiang GC, Insel PS, Tosun D, et al. ; Alzheimer’s Disease Neuroimaging Initiative. Hippocampal atrophy rates and CSF biomarkers in elderly APOE2 normal subjects. Neurology. 2010;75(22):1976–1981. doi: 10.1212/WNL.0b013e3181ffe4d1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeon SY, Byun MS, Yi D, et al. ; KBASE Research Group. Influence of hypertension on brain amyloid deposition and Alzheimer’s disease signature neurodegeneration. Neurobiol Aging. 2019;75:62–70. doi: 10.1016/j.neurobiolaging.2018.11.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.