Abstract
Objective:
Few studies of population-based cohorts have investigated prospective associations of lymphoid and myeloid cell subsets in cardiovascular disease onset and progression. The purpose of this analysis was to determine associations of pre-specified myeloid and lymphoid lineage cell subsets with common carotid artery intima-media thickness (IMT) progression.
Approach and Results:
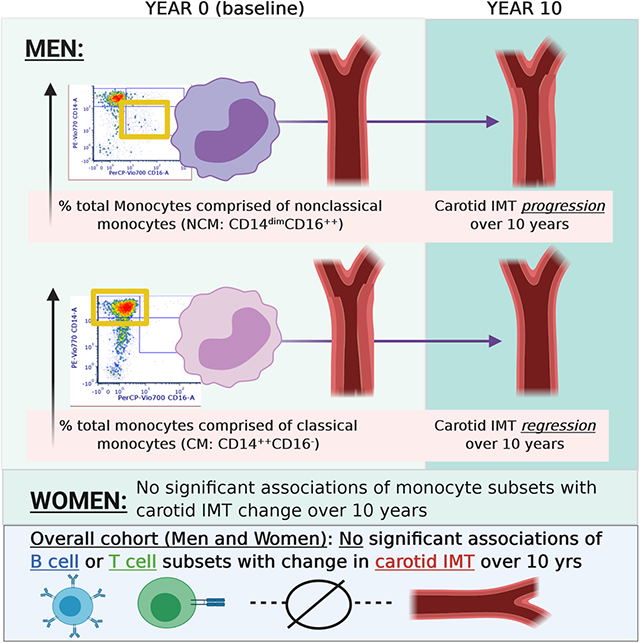
We performed a prospective case-cohort study of 1195 participants from the Multi-Ethnic Study of Atherosclerosis who had peripheral blood mononuclear cells (PBMCs) stored from the baseline examination. Key exposure variables were pre-specified subsets of lymphoid and myeloid lineage immune cells, phenotyped by multicolor flow cytometry. The primary outcome was progression from baseline (Exam 1) to year 10 (Exam 5) in common carotid IMT. Higher proportions of nonclassical monocytes (NCMs: CD14dimCD16++) were significantly associated with IMT progression over 10 years, but classical monocytes (CMs: CD14++CD16-), CD4+CD28- T cells, and T helper cells producing interleukin 17 (IL-17; Th17 cells) were not associated with significant changes in IMT over 10 years. There were significant interactions between monocyte subsets and sex with respect to IMT progression: in sex-stratified analyses, NCMs were associated with significant IMT progression and CMs were associated with significant IMT regression for men, whereas there were no significant associations of monocyte subsets with IMT change for women.
Conclusions:
NCMs were associated with progression of carotid IMT. There were significant sex differences in associations of monocyte subsets with IMT progression: for men, NCMs were associated with IMT progression and CMs were associated with regression, whereas these associations were null for women.
Keywords: Epidemiology, immune regulation, inflammation, carotid intima-media thickness, sex differences, Risk factors, biomarkers, inflammation, ultrasound, vascular disease
Graphical Abstract
Introduction:
Over the past two decades, evidence from animal models and clinical trials has clarified the importance of innate and adaptive immune responses in atherosclerosis onset, progression, and in some cases, resolution.1–7 Essentially, antigenic triggers, such as subendothelially retained and modified lipids, provide an initial nidus, releasing damage-associated molecular patterns; these in turn trigger innate, monocyte-macrophage-driven immune responses, followed by adaptive immune responses, which rely on a balance between effector and regulatory T lymphocytes.
The clinical and translational relevance of identifying such immunologic and inflammatory mediators of atherosclerosis is clear in light of recent landmark studies demonstrating significant cardiovascular risk-reducing benefits of canakinumab8 and colchicine9 for people with prior (and in the case of colchicine, recent) myocardial infarction. Importantly, although these trials tested cytokine blockade and a relatively broad antimitotic anti-inflammatory therapy with uncertain mechanisms of action, the role of further “upstream” targeting of innate and adaptive immune effector cells – which drive these inflammatory responses – is unclear.
Well-powered studies investigating associations of innate and adaptive immune phenotypes with clinical and subclinical cardiovascular disease (CVD) may identify additional therapeutic targets. Several studies have investigated associations of immunologic markers with atherosclerosis, but these studies have been small in size, focused on limited sets of circulating immune/inflammatory markers, and/or focused on highly selected populations.1–3, 10–14 In this study, we leveraged cryopreserved peripheral blood mononuclear cells (PBMCs) from a large, multicenter cohort (MESA: the Multi-Ethnic Study of Atherosclerosis) to investigate prospective associations of markers reflecting innate and adaptive immune phenotypes and functions with progression of carotid artery intima-media thickness (IMT). Our hypotheses were informed by literature linking monocyte and T lymphocyte activation with atherosclerosis3, 15–19 (and immunosenescence/inflamm-aging20). Our a priori hypotheses were that higher circulating proportions of “classical” monocytes (CM: CD14++CD16-), “nonclassical” monocytes (NCM: CD14dimCD16++), T helper 17 cells (Th17), and CD4+CD28- T cells are associated with larger increase in carotid IMT.
Materials and Methods:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study design
MESA is a multi-center, population-based cohort comprised of 6814 men and women aged 45–84 years at baseline, recruited from 6 field centers in the United States. Exclusion criteria include a self-reported history of clinical CVD (myocardial infarction, angina, cardiovascular procedures, heart failure, and/or cerebrovascular disease), active treatment for cancer, amputation, or pregnancy.21 Participants in MESA self-identified as White, Black, Hispanic, or Chinese, and additional details regarding design, recruitment, and objectives of the cohort are described elsewhere. As part of a case (participants with coronary heart disease, CHD)-cohort study, immune cell phenotypes were obtained on 1195 MESA participants from peripheral blood mononuclear cells obtained and stored at baseline (2000–2002).22 For this study, we leveraged immune cell phenotype data previously obtained to examine associations with IMT data though MESA examination 5 (2010–2012), yielding approximately 10 years of follow up.
Anthropometrics and clinical data
Standardized questionnaires and calibrated devices were used to obtain standardized data on demographics, medical conditions, tobacco use, medication usage, weight, and height at all baseline and follow-up examinations. Resting, seated blood pressure was measured 3 times at each examination using a Dinamap automated oscillometric sphygmomanometer (model Pro 100; Critikon, Tampa, Florida, USA), and the final 2 measurements were averaged for analysis. To account for treatment with antihypertensives (given 1/3rd of MESA participants were treated with antihypertensives at baseline), we added 10 mmHg of systolic blood pressure for treated participants as has been done previously.23, 24
Immune cell cryopreservation, thawing, and analysis of surface and functional markers
As previously reported,22 at the baseline MESA exam (2000–2002), a fasting 8 ml citrate CPT tube was drawn to collect peripheral blood mononuclear cells (PBMCs). The PBMCs were washed and cryopreserved in media containing 90% fetal bovine serum (FBS), 10% Dimethyl Sulfoxide (DMSO) at a controlled freezing rate. Cells (2 aliquots of 1 ml each) were stored in a −135 degree Celsius freezer. For the case-cohort study of CHD vs. controls (N=1195),22 in 2016, cryopreserved cells were thawed quickly at 37°C for 15 minutes. The cells were slowly diluted with media with gently mixing. The CD4+ T helper (Th) subsets, Th1, Th2 and Th17, and the corresponding CD8+ cytotoxic/effector (Tc) subsets, Tc1, Tc2 and Tc17, cells were activated with a broad spectrum activator, stained with live/dead stain, surface labeled for CD4/CD8, fixed and intracellularly stained for interferon-gamma (IFN-g), interleukin 4 (IL-4), and interleukin 17 (IL-17).25 For all other cell subtypes, samples were centrifuged and placed into phosphate buffer saline at pH 7.4 and subsequently labeled with live/dead stain for 20 minutes. The stain was removed by centrifugation, and cells were re-suspended and labeled with pre-specified surface markers for 20 minutes. Cells were washed and fixed in paraformaldehyde before analyses using a MACSQuant 10 flow cytometer (Miltenvi Biotec, Germany). Single-color controls were used for compensation, isotype controls were used for negative gate setting, and results were analyzed using MacsQuantify software (Miltenvi Biotec, Germany). Cellular phenotypes were reported as percentages, with natural killer (NK), B cells, CD4, and CD8 as percent of gated lymphocytes, CD4 subsets as percent of CD4+ cells, CD8 subsets as percent of CD8+ cells, monocytes subsets as percent of CD14+ gated monocytes, and B cell subsets as percent of B cells. Of note, NCMs were determined as having low expression of CD14 and are written as CD14dim rather than CD14++ or CD14-; previous studies have characterized NCMs as CD14- but, given the likelihood that truly CD14- cells may not be monocytes, we required CD14 expression as more rigorous method of characterizing the NCM subset.26, 27 Plots of flow cytometry gating strategies used by our group for immune cell phenotyping in this cohort have been published previously.22
Carotid artery measurements
Methods for carotid ultrasonography in MESA have been described in detail previously.28 At the MESA baseline examination, B-mode ultrasound images of the right and left common, bifurcation, and internal carotid artery segments were recorded on videotape, digitized at high resolution and frame rates, and converted into digital records. The same ultrasound system and digitizing equipment were used at examination 5, but the video output was directly digitized using the same recorder settings without videotape. Trained sonographers used preselected reference images from examination 1 to match scanning conditions of the initial study. Ultrasound images were reviewed and interpreted by the University of Wisconsin Atherosclerosis Imaging Research Program MESA Carotid Ultrasound Reading Center. Measurements of examination 1 and examination 5 carotid ultrasound images were performed simultaneously and images were matched side-by-side on a video monitor and measured contemporaneously. This analysis primarily focused on common carotid artery (CCA) IMT. As described previously, measurements of IMT were performed on the far wall of the distal common carotid artery over a longitudinal distance of the distal 10mm of the vessel, below the common carotid artery bulb. IMT was measured as the intima-media-thickness of the mean of the mean left and right far wall distal CCA wall thicknesses.28 The intraclass correlation coefficient for intrareader reproducibility for mean CCA IMT was 0.99 and the intraclass correlation coefficient for inter-reader reproducibility was 0.95.
Statistical analyses
To determine associations of lymphoid and myeloid subsets with change in IMT from Exam 1 to Exam 5, we used linear mixed with sampling weights to account for the case-cohort design of the original myocardial infarction study.22 We adjusted our models for potential confounders, which included baseline age, sex, race/ethnicity, smoking status, body-mass index, college education, diabetes status, systolic blood pressure, total cholesterol, high density lipoprotein (HDL) cholesterol, baseline antihypertensive and lipid-lowering medications, and log-transformed cytomegalovirus serostatus. Associations of each lymphoid or myeloid subset with change in IMT were assessed separately. We used the Bonferroni correction to account for multiple testing and the false discovery rate (FDR) was used in a sensitivity analysis as an alternative approach. Of note, not all immune subsets were measurable in all 1195 MESA participants included for analysis. The primary analyses therefore used all available data for each immune cell subset, assuming that missing data were missing at random; most missingness was due to technical errors which occur at random, therefore we assumed randomness.
Results:
Out of 1195 MESA participants for whom immune cell phenotyping was attempted, 1087 had at least one immune cell subset successfully measured (see Supplement), as others were excluded due to poor sample quality or technical issues with flow cytometry unrelated to underlying participant characteristics. Baseline characteristics of the 1195 and 1087 MESA participants with attempted and successful measurement of immune cell subsets, respectively, are included in Table 1. A separate comparison of baseline characteristics for cases (with CHD) versus controls analyzed in the MESA case-cohort study has been published previously.22 All baseline covariates were missing in <1% of participants analyzed (see Supplement), with the exception of data on alcohol use (missing in 16.7%), interleukin-6 levels (missing in 2.7%), and IMT measurement (missing in 1.4%).
Table 1.
Baseline (Exam 1) characteristics of MESA participants with immune cells measured subjects (n=1087)a or originally selected into the study (n=1195)b
| 1087 participants | 1195 participants | |||
|---|---|---|---|---|
| Variable | Mean / Number | Std Dev/Percent | Mean / Number | Std Dev/Percent |
| Age (years) | 63.9 | 10.1 | 63.8 | 10.17 |
| Male, n % | 578 | 53% | 624 | 52% |
| White, n % | 413 | 38% | 468 | 39% |
| Chinese, n % | 135 | 12% | 141 | 12% |
| Black, n % | 314 | 29% | 338 | 28% |
| Hispanic, n % | 227 | 21% | 248 | 21% |
| SBP (mmHG) | 130.0 | 21.675 | 129.7 | 21.6 |
| DBP (mmHG) | 72.7 | 10.4 | 72.6 | 10.4 |
| Cholesterol (mg/dL) | 193.9 | 35.8 | 193.8 | 35.4 |
| HDL Cholesterol (mg/dL) | 49.4 | 14.4 | 49.5 | 14.4 |
| BMI (kg/m2) | 28.3 | 5.4 | 28.3 | 5.4 |
| Diabetes, n % | 184 | 17% | 196 | 16% |
| Smoker, n % | 144 | 13% | 160 | 13% |
| Alcohol user | 557 | 64% | 615 | 64% |
| Statin user, n % | 191 | 18% | 212 | 18% |
| CCB user, n % | 159 | 15% | 172 | 14% |
| Diuretic user, n % | 173 | 16% | 214 | 18% |
| Beta Blocker user, n % | 110 | 10% | 121 | 10% |
| Vasodilator user, n % | 67 | 6% | 73 | 6% |
| ACE inhibitor user, n % | 171 | 16% | 182 | 15% |
| College education, n % | 378 | 35% | 416 | 35% |
| Intentional Exercise (met-min) | 1527 | 2293 | 1516 | 2240 |
| IL-6 (pg/mL) | 1.64 | 1.24 | 1.63 | 1.23 |
| CMV (EU/mL) | 240 | 266 | 241 | 266 |
| Baseline mean common carotid intima media thickness (mm)b | 0.796 | 0.237 | 0.794 | 0.232 |
| 10 year mean common carotid intima media thickness (mm)b | 0.894 | 0.242 | 0.892 | 0.239 |
Abbreviations: systolic blood pressure (SBP), diastolic blood pressure (DBP), high density lipoprotein (HDL), body-mass index (BMI), calcium channel blocker (CCB), angiotensin converting enzyme (ACE), interleukin-6 (IL-6), cytomegalovirus (CMV)
Among participants with both a baseline and follow-up measure of IMT
Regarding our a priori hypotheses (Table 2), NCMs were associated with a significant increase over time in IMT (0.018 mm increase per SD NCMs; 95% CI 0.0031, 0.033, p=0.019) whereas there was no significant association of CMs, Th17 cells, or CD4+CD28- T cells with IMT progression.
Table 2.
Change in mean common carotid artery thickness (mm) over 10 years of follow-up a
| Cells scaled per SD | Estimate (in mm) | 95% CI Lower | 95% CI Upper | p-value |
|---|---|---|---|---|
| th1 as %CD4 | 0.001841 | −0.01209 | 0.01577 | 0.7951 |
| th17 as %CD4 | 0.001317 | −0.01083 | 0.01347 | 0.8312 |
| CD4+CD38+ | −0.00298 | −0.01690 | 0.01094 | 0.6740 |
| CD8+CD38+ | −0.00248 | −0.01609 | 0.01114 | 0.7207 |
| NK %lymphs | 0.006968 | −0.00633 | 0.02027 | 0.3038 |
| CD3+gd+ | 0.000547 | −0.02107 | 0.02216 | 0.9604 |
| CD14++CD16- | −0.01333 | −0.02892 | 0.002270 | 0.0938 |
| th2 as %CD4 | 0.004064 | −0.01547 | 0.02360 | 0.6826 |
| CD14dimCD16++ | 0.01825 | 0.003089 | 0.03342 | 0.0185 |
| CD127-CD4+CD25+ | −0.02090 | −0.05400 | 0.01220 | 0.2153 |
| CD14+CD16+ | −0.01333 | −0.02892 | 0.002270 | 0.0938 |
| CD4+CD45RA+ | −0.00159 | −0.01775 | 0.01457 | 0.8467 |
| CD8+CD45RA+ | 0.006560 | −0.00769 | 0.02081 | 0.3661 |
| CD4+CD28- | −0.01169 | −0.02663 | 0.003250 | 0.1248 |
| CD8+CD28- | 0.006721 | −0.00926 | 0.02270 | 0.4089 |
| CD4+CD45RO+ | 0.002237 | −0.01376 | 0.01823 | 0.7835 |
| CD8+CD45RO+ | 0.008576 | −0.00459 | 0.02175 | 0.2013 |
| CD19+ B cells | −0.00105 | −0.00351 | 0.001418 | 0.4041 |
| CD19+CD5+ B cells | −0.00009 | −0.00069 | 0.000509 | 0.7628 |
| CD19+CD5+CD27- B cells | −0.00033 | −0.00110 | 0.000444 | 0.4035 |
| CD19+CD27+ B cells | 0.000408 | −0.00049 | 0.001305 | 0.3722 |
| CD19+CD5-CD27+ B cells | 0.000347 | −0.00077 | 0.001462 | 0.5416 |
Linear models adjusted for age, sex, race/ethnicity, current smoking, baseline exercise, body mass index, baseline college education, current diabetes, systolic blood pressure, total cholesterol, HDL cholesterol, baseline antihypertensive use, baseline statin use, and log transformed CMV. Confidence intervals use robust (sandwich) standard error estimates to ensure validity in the presence of weights that are used to account for case-cohort sampling. Pre-specified hypotheses are in red (and highlighted for B&W printers). All estimates are per standard deviation. Complete case data (numbers vary by subset).
In our pre-specified analyses of effect modification, we found significant qualitative interactions between sex and monocyte subsets (CMs and NCMs) with respect to change in IMT (p = 0.0002 and 0.0033 for the interaction terms of sex with CMs and NCMs, respectively: see Supplement). We therefore performed sex-stratified analyses (see Supplement) and found that, among men, NCMs were associated with significant increase in IMT (0.036 mm increase per SD NCMs; 95% CI 0.014, 0.058, p=0.0014) whereas CMs were associated with significant decrease in IMT (−0.040 mm; 95% CI −0.063, −0.016; p=0.0010). For women, associations of NCMs and CMs with IMT change were null. Given these sex-related differences, we performed sensitivity analyses displaying raw data of baseline and follow-up IMT stratified by sex and case (CHD)/control status; mean IMT progression by sex and case-control status was largely similar (see Supplement).
Regarding other analyses not specified a priori, there were no significant associations of other lymphoid or myeloid lineage cell subtypes with change in IMT. Additionally, there was no significant cross-sectional of baseline of NCMs, CMs, Th17 cells, or CD4+CD28- T cells with baseline IMT (see Supplement).
Discussion
This is the first study to our knowledge to investigate the prospective association of circulating monocyte and lymphocyte phenotypes with progression of carotid IMT. In a cohort with participants free of CVD at baseline, over a 10 year period of follow-up, a higher proportion of NCMs was associated with IMT progression (an association that was not present cross-sectionally for baseline NCMs and baseline IMT). Meanwhile, the associations of CMs, Th17 cells, and CD4+CD28- T cells with IMT progression were null. There was highly significant effect modification by sex for the association of NCMs with IMT progression; in sex-stratified analyses, NCMs were associated with significant IMT progression and CMs were associated with significant IMT regression for men, whereas monocyte subsets were not associated with IMT change for women.
The findings that NCMs were associated prospectively with IMT progression, whereas CMs were not (and were actually associated with regression in men), were not initially expected. Monocyte counts overall predict subclinical atherosclerosis and plaque formation,3, 17 but the roles of specific monocyte subsets are debated. A considerable body of literature from mouse models implicates CMs in atherogenesis and progression of atherosclerosis,29, 30 whereas the literature regarding NCMs is mixed. Mechanistically, NCMs are thought to play a generally atheroprotective role, patrolling along the endothelial surface on the vascular luminal side and removing debris from vasculature.31 Yet, observational studies in humans demonstrate that proportions of circulating NCMs are associated with atherogenic lipids32 and endothelial dysfunction,27 and that people with coronary artery disease (compared with controls without coronary artery disease) have higher proportions of NCMs.
Although NCMs and CMs are both recruited to vascular debris/fatty streaks/atherosclerosis, a central difference is that CMs readily migrate into the vascular wall and are effectors of atherosclerosis, whereas NCMs do not transmigrate across the endothelium. Instead, NCMs crawl along blood vessels with debris/fatty streaks/plaque but remain within the luminal compartment, serving a patrolling role.33 Therefore, if both NCMs and CMs are mobilized and recruited first to the vascular compartment and subsequently to the plaque (with CMs entering and NCMs remaining in the luminal compartment), it is plausible that: (1) proportions of circulating NCMs would be elevated as early indicators of subclinical (and future) plaque burden, whereas (2) peripheral CM proportions or levels may not rise and may actually be depleted if there is considerable plaque into which the CMs migrate. If this is the case, as we hypothesize based on our findings, NCMs may serve as an early biomarker of – but not a causal contributor to – pre-atherosclerotic vascular lesions that ultimately become atherosclerosis and plaque. Essentially, the more pre-atherosclerotic fatty streaks and debris, the more NCMs that may be mobilized to the surface of these lesions, which may take years before they are apparent on imaging. Therefore, it is possible that NCMs serve as a biomarker of total burden of early, pre-atherosclerotic lesions (as well as, of course, later lesions) throughout the vascular compartment. That the cross-sectional associations of NCMs and CMs with IMT were null may further support the plausibility of this hypothesis.
Another finding of interest relates to the sex differences in monocyte subset associations with IMT. We found significant sex-monocyte subset interactions with respect to IMT: for men, NCMs were associated with significant IMT progression and CMs were associated with IMT regression, whereas none of these associations were significant or near-significant in women. This stark difference may reflect underling differences in the respective roles of cell-mediated vs. humoral immunity for men and women. Estradiol is a major effector of the humoral immune response (whereas testosterone suppresses this response).34–37 Accordingly, women exert stronger humoral responses to vaccination and in the case of autoimmunity, whereas the testosterone-driven suppression of humoral immune responses makes men more dependent on strong cell-mediated immune responses. In this context, it is conceivable that factors activating cell-mediated immunity would drive especially potent responses – including monocytosis and chemoattraction toward vascular debris – in men.
Mechanistically, the differences we observed by sex in associations of monocyte subsets with IMT progression corroborate existing mechanistic literature outlining distinct effects of sex hormones on cell-mediated and humoral immunity. If, as discussed above, cell-mediated immunity predominates for men and is implicated to a greater extent in atherosclerosis onset and progression than humoral immunity, therapies targeting cell-mediated immunity and inflammation to prevent and treat atherosclerosis may be more effective in men than women. Given growing interest in modulating immunity and inflammation to prevent and treat CVD, further exploration of these hypotheses in clinical and mechanistic studies is warranted. Of note, the women analyzed in this study were predominantly post-menopausal; only 17 out of 1195 women (1.4%) in MESA who had PBMCs obtained at baseline were pre-menopausal; this prevented us from investigating potential influences of the menopause transition on immunity/IMT relationships and merits further investigation in cohorts with sufficient numbers of pre-, peri-, and post-menopausal women.
Strengths of the study include its large sample size, racial and ethnic diversity, rigorous measurement of immune cell phenotypic and functional markers, and longitudinal carotid IMT data. The study also has several limitations, including missing data, the use of cryopreserved PBMCs rather than fresh blood, and the lack of carotid IMT measurements between exams 1 and 5. We have attempted to minimize the effects of these limitations by imputing data for random errors, and comparing cryopreserved data from this study with data obtained from a previous study on whole blood performed in MESA examination 4,22,25 as well as literature on the use of cryopreserved cells.38 An additional limitation relates to variable timing of blood draws for PBMC processing, which generally occurred in the mornings but not at the same time for each individual (e.g. 8 AM vs. 11 AM). Given emerging data indicating intra-individual circadian variations in circulating immune cell subsets,39 there is potential unmeasured confounding related to the timing of blood draws. However, due to the largely random nature of blood draw timing unrelated to participant characteristics and IMT risk factors, if confounding is present it would most likely bias toward the null via regression-dilution rather than toward false-positive.40 Another potential cause of diluted exposure variables (and relatedly diluted, biased-toward-null exposure-outcome relationships) is the exposure measurements themselves, which merge admittedly heterogeneous cell subsets into over-arching groups; therefore, if a subset of NCMs were most strongly associated with an outcome of interest, the strength of this association would be diluted by our grouping of all NCMs together into a single exposure variable. Such dilution of exposure variables may especially bias toward the null when investigating outcomes further downstream from the subclinical (IMT) endpoint measured here, and may have contributed to the observed null associations between immune cell subsets and CHD.22
Future studies exploring heterogeneity among immune cell subsets and the biological relevance of these functionally distinct groups for cardiovascular disease onset and progression in humans would help clarify some of these questions. A separate, important limitation of our analyses is that intra-individual variation in immune cell subsets over longer periods of time is inevitable, limiting the extent to which a single-time point sampling of immune cells may reflect immune cell levels over the ensuing decade. This could be addressed with serial peripheral blood mononuclear cell sampling and analyses with respect to longitudinal outcomes in future studies.
In conclusion, we found that NCMs are associated with progression of carotid IMT in a large, multi-ethnic cohort study. Furthermore, we found significant sex differences in associations of monocyte subsets with IMT progression, which merit further investigation in future epidemiological and translational studies.
Supplementary Material
Highlights.
Higher circulating levels of nonclassical monocytes, defined as CD14dimCD16++, were associated with significant longitudinal increase in carotid intima-media thickness (IMT) over a 10 year period in a large, multi-ethnic, prospective cohort study.
We observed significant sex dimorphism in these associations. Among men, nonclassical monocytes were associated with IMT progression whereas classical monocytes, defined as CD14++CD16-, were associated with IMT regression. Among women, were no significant associations of monocyte subsets with IMT change over time.
The direction of associations observed in men – (1) circulating nonclassical (generally considered patrolling, or inflammation-resolving) monocytes being associated with IMT progression and (2) classical (“inflammatory”) monocytes being associated with IMT regression – were surprising. Rather than reflecting causal contributors to atherosclerosis, these differences may reflect differences in migration across the endothelium given evidence that CD14 mediates endothelial transmigration; in this case, nonclassical monocytes would remain in the lumen/circulation when attracted toward early atherosclerotic lesions whereas classical monocytes would migrate out of the lumen and cross the endothelium into the vascular intima.
More studies in humans investigating immune phenotypic and functional crosstalk between the circulation and organs/tissue beds of interest are needed.
a). Acknowledgements:
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
b) Sources of Funding: This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). The research was also supported by grant funding from the American Heart Association (Fellow-to-Faculty Award 16FTF312000010). This publication was also developed under the Science to Achieve Results (STAR) research assistance agreements, No. RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage), awarded by the U.S Environmental Protection Agency. It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Abbreviations:
- ApoB
Apolipoprotein B
- CM
Classical monocyte
- CVD
Cardiovascular disease
- IMT
Intima-media thickness
- MESA
Multi-Ethnic Study of Atherosclerosis
- NCM
Nonclassical monocyte
- PBMC
Peripheral blood mononuclear cell
Footnotes
c) Disclosure: None
References
- 1.Aristoteli LP, Moller HJ, Bailey B, Moestrup SK and Kritharides L. The monocytic lineage specific soluble CD163 is a plasma marker of coronary atherosclerosis. Atherosclerosis. 2006;184:342–347. [DOI] [PubMed] [Google Scholar]
- 2.Bekkering S, van den Munckhof I, Nielen T, Lamfers E, Dinarello C, Rutten J, de Graaf J, Joosten LA, Netea MG, Gomes ME and Riksen NP. Innate immune cell activation and epigenetic remodeling in symptomatic and asymptomatic atherosclerosis in humans in vivo. Atherosclerosis. 2016;254:228–236. [DOI] [PubMed] [Google Scholar]
- 3.Chapman AR, Shah ASV, Lee KK, Anand A, Francis O, Adamson P, McAllister DA, Strachan FE, Newby DE and Mills NL. Long-Term Outcomes in Patients With Type 2 Myocardial Infarction and Myocardial Injury. Circulation. 2018;137:1236–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cochain C, Vafadarnejad E, Arampatzi P, Pelisek J, Winkels H, Ley K, Wolf D, Saliba AE and Zernecke A. Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circulation research. 2018;122:1661–1674. [DOI] [PubMed] [Google Scholar]
- 5.Winkels H, Ehinger E, Ghosheh Y, Wolf D and Ley K. Atherosclerosis in the single-cell era. Curr Opin Lipidol. 2018;29:389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winkels H, Ehinger E, Vassallo M, et al. Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA-Sequencing and Mass Cytometry. Circulation research. 2018;122:1675–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf D and Ley K. Immunity and Inflammation in Atherosclerosis. Circulation research. 2019;124:315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. The New England journal of medicine. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 9.Tardif JC, Kouz S, Waters DD, et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. The New England journal of medicine. 2019;381:2497–2505. [DOI] [PubMed] [Google Scholar]
- 10.Carlucci PM, Purmalek MM, Dey AK, et al. Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus. JCI Insight. 2018;3(8):e99276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanna DB, Lin J, Post WS, et al. Association of Macrophage Inflammation Biomarkers With Progression of Subclinical Carotid Artery Atherosclerosis in HIV-Infected Women and Men. The Journal of infectious diseases. 2017;215:1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsue PY, Hunt PW, Schnell A, Kalapus SC, Hoh R, Ganz P, Martin JN and Deeks SG. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. Aids. 2009;23:1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKibben RA, Margolick JB, Grinspoon S, Li X, Palella FJ Jr., Kingsley LA, Witt MD, George RT, Jacobson LP, Budoff M, Tracy RP, Brown TT and Post WS. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. The Journal of infectious diseases. 2015;211:1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacre K, Hunt PW, Hsue PY, Maidji E, Martin JN, Deeks SG, Autran B and McCune JM. A role for cytomegalovirus-specific CD4+CX3CR1+ T cells and cytomegalovirus-induced T-cell immunopathology in HIV-associated atherosclerosis. Aids. 2012;26:805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg KE, Ljungcrantz I, Andersson L, Bryngelsson C, Hedblad B, Fredrikson GN, Nilsson J and Bjorkbacka H. Elevated CD14++CD16- monocytes predict cardiovascular events. Circulation Cardiovascular genetics. 2012;5:122–131. [DOI] [PubMed] [Google Scholar]
- 16.Hopfner F, Jacob M, Ulrich C, Russ M, Simm A, Silber RE, Girndt M, Noutsias M, Werdan K and Schlitt A. Subgroups of monocytes predict cardiovascular events in patients with coronary heart disease. The PHAMOS trial (Prospective Halle Monocytes Study). Hellenic J Cardiol. 2019;60:311–321. [DOI] [PubMed] [Google Scholar]
- 17.Johnsen SH, Fosse E, Joakimsen O, Mathiesen EB, Stensland-Bugge E, Njolstad I and Arnesen E. Monocyte count is a predictor of novel plaque formation: a 7-year follow-up study of 2610 persons without carotid plaque at baseline the Tromso Study. Stroke. 2005;36:715–719. [DOI] [PubMed] [Google Scholar]
- 18.Rogacev KS, Cremers B, Zawada AM, et al. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. Journal of the American College of Cardiology. 2012;60:1512–1520. [DOI] [PubMed] [Google Scholar]
- 19.Schlitt A, Heine GH, Blankenberg S, et al. CD14+CD16+ monocytes in coronary artery disease and their relationship to serum TNF-alpha levels. Thromb Haemost. 2004;92:419–424. [DOI] [PubMed] [Google Scholar]
- 20.Zanni F, Vescovini R, Biasini C, et al. Marked increase with age of type 1 cytokines within memory and effector/cytotoxic CD8+ T cells in humans: a contribution to understand the relationship between inflammation and immunosenescence. Exp Gerontol. 2003;38:981–987. [DOI] [PubMed] [Google Scholar]
- 21.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. American journal of epidemiology. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 22.Olson NC, Sitlani CM, Doyle MF, Huber SA, Landay AL, Tracy RP, Psaty BM and Delaney JA. Innate and adaptive immune cell subsets as risk factors for coronary heart disease in two population-based cohorts. Atherosclerosis. 2020;300:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy D, Ehret GB, Rice K, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui JS, Hopper JL and Harrap SB. Antihypertensive treatments obscure familial contributions to blood pressure variation. Hypertension. 2003;41:207–210. [DOI] [PubMed] [Google Scholar]
- 25.Tracy RP, Doyle MF, Olson NC, Huber SA, Jenny NS, Sallam R, Psaty BM and Kronmal RA. T-helper type 1 bias in healthy people is associated with cytomegalovirus serology and atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Journal of the American Heart Association. 2013;2:e000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, Kourilsky P and Wong SC. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118:e16–31. [DOI] [PubMed] [Google Scholar]
- 27.Urbanski K, Ludew D, Filip G, Filip M, Sagan A, Szczepaniak P, Grudzien G, Sadowski J, Jasiewicz-Honkisz B, Sliwa T, Kapelak B, McGinnigle E, Mikolajczyk T and Guzik TJ. CD14(+)CD16(++) “nonclassical” monocytes are associated with endothelial dysfunction in patients with coronary artery disease. Thromb Haemost. 2017;117:971–980. [DOI] [PubMed] [Google Scholar]
- 28.Tattersall MC, Gassett A, Korcarz CE, Gepner AD, Kaufman JD, Liu KJ, Astor BC, Sheppard L, Kronmal RA and Stein JH. Predictors of carotid thickness and plaque progression during a decade: the Multi-Ethnic Study of Atherosclerosis. Stroke. 2014;45:3257–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R and Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narasimhan PB, Marcovecchio P, Hamers AAJ and Hedrick CC. Nonclassical Monocytes in Health and Disease. Annu Rev Immunol. 2019;37:439–456. [DOI] [PubMed] [Google Scholar]
- 31.Franca CN, Izar MCO, Hortencio MNS, do Amaral JB, Ferreira CES, Tuleta ID and Fonseca FAH. Monocyte subtypes and the CCR2 chemokine receptor in cardiovascular disease. Clin Sci (Lond). 2017;131:1215–1224. [DOI] [PubMed] [Google Scholar]
- 32.Rothe G, Gabriel H, Kovacs E, Klucken J, Stohr J, Kindermann W and Schmitz G. Peripheral blood mononuclear phagocyte subpopulations as cellular markers in hypercholesterolemia. Arteriosclerosis, thrombosis, and vascular biology. 1996;16:1437–1447. [DOI] [PubMed] [Google Scholar]
- 33.McArdle S, Chodaczek G, Ray N and Ley K. Intravital live cell triggered imaging system reveals monocyte patrolling and macrophage migration in atherosclerotic arteries. J Biomed Opt. 2015;20:26005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giefing-Kroll C, Berger P, Lepperdinger G and Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging cell. 2015;14:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koenig A, Buskiewicz I and Huber SA. Age-Associated Changes in Estrogen Receptor Ratios Correlate with Increased Female Susceptibility to Coxsackievirus B3-Induced Myocarditis. Front Immunol. 2017;8:1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moulton VR. Sex Hormones in Acquired Immunity and Autoimmune Disease. Front Immunol. 2018;9:2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roved J, Westerdahl H and Hasselquist D. Sex differences in immune responses: Hormonal effects, antagonistic selection, and evolutionary consequences. Horm Behav. 2017;88:95–105. [DOI] [PubMed] [Google Scholar]
- 38.Thyagarajan B, Barcelo H, Crimmins E, Weir D, Minnerath S, Vivek S and Faul J. Effect of delayed cell processing and cryopreservation on immunophenotyping in multicenter population studies. J Immunol Methods. 2018;463:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beam CA, Wasserfall C, Woodwyk A, Akers M, Rauch H, Blok T, Mason P, Vos D, Perry D, Brusko T, Peakman M and Atkinson M. Synchronization of the Normal Human Peripheral Immune System: A Comprehensive Circadian Systems Immunology Analysis. Sci Rep. 2020;10:672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hutcheon JA, Chiolero A and Hanley JA. Random measurement error and regression dilution bias. BMJ. 2010;340:c2289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


