Brief Summary
A multiple biomarker strategy using CRP, D-dimer and troponin identifies COVID-19 patients at risk for in-hospital adverse events.
Infection with Severe Acute Respiratory Syndrome CoronaVirus-2 (SARS CoV-2) is responsible for the 2019 coronavirus disease (COVID-19) pandemic. Myocardial injury, thrombosis, and a systemic inflammatory response to SARS CoV-2 are common features of COVID-19, and biomarkers of each these processes (cardiac troponin [cTn], D-dimer and C-reactive protein [CRP], respectively) are associated with disease severity and mortality.1-3 The aim of this study was to determine whether a constellation of biomarkers at admission would predict in-hospital clinical outcomes in patients with COVID-19.
Consecutive adults age ≥18 years with COVID-19 admitted to the NYU Langone Health (NYULH) system between March 1st and April 16th, 2020 were identified and included if cTn, D-dimer, and CRP were measured. Routine multi-marker surveillance was standard of care for patients with COVID-19; electronic admission order sets included cTn, D-Dimer, and CRP laboratory tests. Myocardial injury was defined as an initial cTn above the site-specific upper limit of normal (ULN) for the assay (Siemens Dimension Vista [>0.045 ng/mL] / Centaur XPT [>0.5 ng/mL], Abbot Architect Troponin I [>0.04 ng/mL]). An elevated D-Dimer was defined based on the assay ULN (>230 ng/mL). Given that 98.5% of our hospitalized patients with COVID-19 had an initial CRP concentration above the ULN, we defined increased inflammation as a CRP (Siemens Dimension, Abbot Architect) >50 mg/L. This threshold was prognostically important in prior studies.4, 5 Demographics, comorbidities, medications, clinical presentation, and laboratory data were abstracted from the electronic health record. Comorbidities were defined by ICD-10 codes. All-cause, in-hospital mortality was recorded. Critical illness was defined by receipt of intensive care, mechanical ventilation, transfer to hospice, or death. Categorical variables are reported as frequencies and proportions and compared by chi-square tests. We used logistic regression models to estimate the odds of study endpoints based on the number of elevated biomarkers. We used Harrell's C-statistic to evaluate the survival model; AUCs were compared using Delong's test. The discriminative ability of the number of elevated biomarkers was characterized by the continuous net reclassification improvement (NRI). Adjusted risk models included demographics, clinical comorbidities, and characteristics at hospital presentation as covariates. Statistical analyses were performed using R (Vienna, Austria). Statistical tests were two-sided and p-values <0.05 were statistically significant. The study was approved by the NYULH Institutional Review Board with a waiver of informed consent; identifiable data will not be made publicly available.
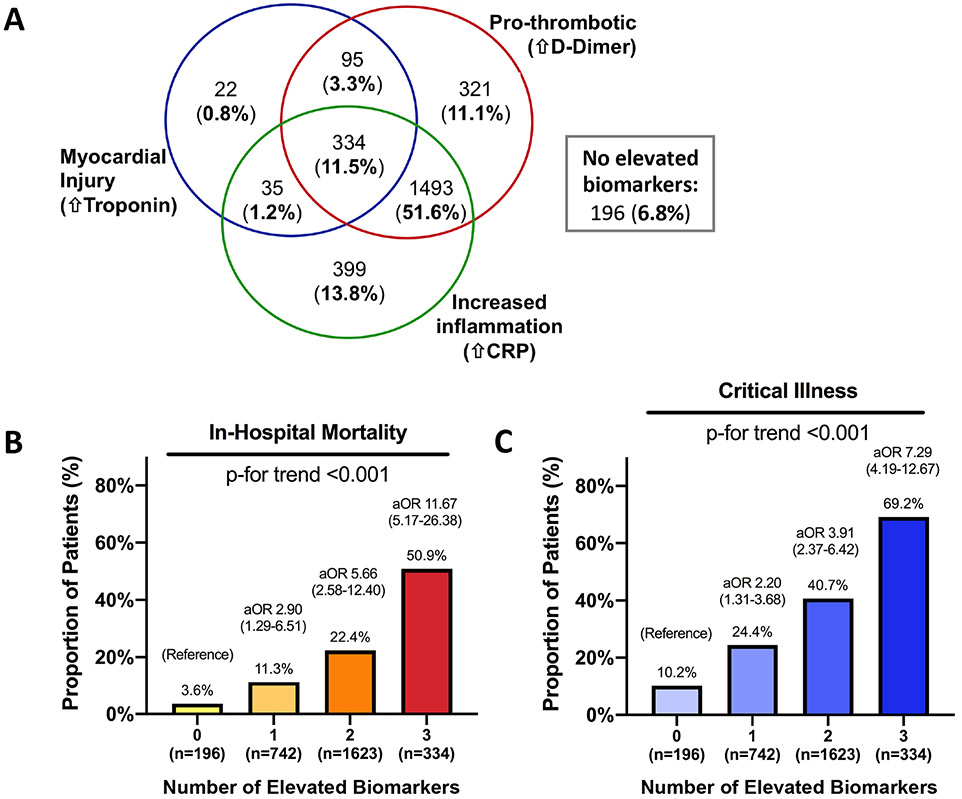
A total of 3,281 consecutive adults with COVID-19 were identified and 2,895 (88.2%) had measurement of all 3 biomarkers (cTn: median 0.015 ng/mL [IQR <0.015-0.03], D-Dimer: median 403 ng/mL [IQR 244-769], and CRP: median 113 mg/L [IQR 57-178]) at admission. Myocardial injury was present in 486 (16.8%) patients, D-Dimer level was elevated in 2,243 (77.5%), and CRP was >50 mg/L in 2,261 (78.1%). Only 196 (6.8%) patients had normal cTn, D-dimer, and had CRP<50 mg/L (“no elevated biomarkers”). Elevations of all 3 biomarkers, reflecting the pathobiological axes of myocardial injury, coagulation, and inflammation, were present in 334 (11.5%) (Figure). Patients with no elevated biomarkers were at low risk of critical illness and in-hospital mortality. Continuous CRP (C-statistic 0.611, 95% CI 0.587-0.634), D-dimer (C-statistic 0.639, 95% CI 0.615-0.664), and troponin (C-statistic 0.680, 95% CI 0.655-0.705) concentrations were each separately associated with mortality. Patients with 1, 2 or 3 elevated biomarkers had stepwise increases in the risk of adverse events (Figure). The number of elevated biomarkers alone yielded a C-statistic of 0.672 (95% CI 0.649-0.696) for mortality, with no difference in model performance compared to continuous data for all 3 biomarkers (0.657, 95% CI 0.633-0.681, p=0.17). Addition of the number of elevated biomarkers to an adjusted risk model yielded improvements in the C-statistic (from 0.765 [95% CI 0.745-0.785] to 0.789 [95% CI 0.770-0.807], p<0.001) and the appropriate reclassification of risk (NRI 0.227 [95% CI 0.141-0.313]) for mortality.
Figure.
Venn diagram illustrating overlap of elevated biomarkers at the time of initial hospital presentation in patients with COVID-19 and ≥1 elevated biomarker (n=2699); 196 patients had no elevated biomarkers. (Panel A). The proportion of patients with in-hospital mortality (Panel B) and critical illness (Panel C) are shown stratified by the number of elevated biomarkers measured at hospital presentation with COVID-19.
Odds ratios (versus 0 biomarkers elevated) shown in Panels B and C are adjusted for age, sex, race, body mass index, tobacco use, hypertension, hyperlipidemia, diabetes mellitus, chronic kidney disease, prior myocardial infarction, prior heart failure, atrial fibrillation, temperature at presentation, pulse oximetry at presentation, and outpatient prescriptions for anti-platelets, statin, and beta-blockers.
In this analysis of patients with COVID-19, 93% had myocardial injury, abnormalities in coagulation, or marked inflammation at hospital presentation. The combination of abnormalities across these pathobiological axes of disease provided incremental prognostic information for risk stratification. Patients with 1, 2, and 3 elevated biomarkers had 3-fold, 6-fold, and 11-fold higher adjusted odds of death compared to COVID-19 patients with no elevated biomarkers at presentation. These findings extend prior observations on the prognostic nature of these biomarkers in COVID-19 and provide a framework for rapid estimation of risk.
This study was retrospective and selection bias cannot be excluded. Only in-hospital events were recorded. We used dichotomous biomarker data to generate a simple scoring system for clinical use. A threshold higher than the ULN was selected for CRP, myocardial injury was defined by site-specific values for the cTn ULN, and high-sensitivity cTn assays were not employed. Finally, a separate validation cohort was not available. Still, a simple multi-marker strategy, in which patients are categorized based on the number of elevated biomarkers, was effective to identify patients at risk for in-hospital adverse events.
Acknowledgments
Funding Sources: Dr. Smilowitz is supported, in part, by the National Heart, Lung, And Blood Institute of the NIH (K23HL150315). Dr. Newman is funded, in part, by the National Heart and Lung Blood Institute of the NIH (K23HL125991). Dr. Berger is funded, in part, by the National Heart and Lung Blood Institute of the NIH (R01HL139909 and R35HL144993).
Footnotes
Conflict of Interest Disclosures: Dr. Smilowitz reports consulting for Abbott Vascular. The remaining authors have no disclosures to report.
References
- 1.Berger JS, Kunichoff D, Adhikari S, Ahuja T, Amoroso N, Aphinyanaphongs Y, Cao M, Goldenberg R, Hindenburg A, Horowitz J, et al. Prevalence and Outcomes of D-Dimer Elevation in Hospitalized Patients With COVID-19. Arteriosclerosis, thrombosis, and vascular biology. 2020:ATVBAHA120314872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smilowitz NR, Jethani N, Chen J, Aphinyanaphongs Y, Zhang R, Dogra S, Alviar CL, Keller N, Razzouk L, Quinones-Camacho A, et al. Myocardial Injury in Adults Hospitalized With COVID-19. Circulation. 2020;142:2393–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smilowitz NR, Kunichoff D, Garshick M, Shah B, Pillinger M, Hochman JS and Berger JS. C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniels JM, Schoorl M, Snijders D, Knol DL, Lutter R, Jansen HM and Boersma WG. Procalcitonin vs C-reactive protein as predictive markers of response to antibiotic therapy in acute exacerbations of COPD. Chest. 2010;138:1108–15. [DOI] [PubMed] [Google Scholar]
- 5.Komiya K, Ishii H, Teramoto S, Takahashi O, Eshima N, Yamaguchi O, Ebi N, Murakami J, Yamamoto H and Kadota J. Diagnostic utility of C-reactive protein combined with brain natriuretic peptide in acute pulmonary edema: a cross sectional study. Respir Res. 2011;12:83. [DOI] [PMC free article] [PubMed] [Google Scholar]