Abstract
Objective:
To determine if trophectoderm (TE) grade or inner cell mass (ICM) grade have predictive value after euploid frozen embryo transfer (euFET) among RPL patients.
Design:
Retrospective cohort study
Setting:
Single fertility center, 2012–2018.
Patients:
Patients with ≥ 2 prior pregnancy losses performing PGT-A with ≥1 euploid embryo for transfer.
Interventions:
All patients underwent ICSI, trophectoderm biopsy, blastocyst grading and vitrification, and single euFET. Outcome of the first transfer was recorded.
Main Outcome Measures:
Live birth (LB) and clinical miscarriage (CM) rates.
Results:
660 euFET were included. In a binomial logistic regression analysis accounting for age, BMI, AMH and day of blastocyst biopsy, ICM grade C was not significantly associated with odds of live birth (aOR 0.50, 95% CI 0.24–1.02 p=0.057), miscarriage (aOR 1.67, 95% CI 0.56–5.00, p=0.36) or biochemical pregnancy loss (aOR 1.58, 95% CI 0.53–4.75, p=0.42). TE grade C was significantly associated with odds of live birth (aOR 0.49, 95% CI 0.28–0.86, p=0.01) and was not associated with odds of miscarriage (aOR 2.00, 95% CI 0.89–4.47, p=0.09) or biochemical pregnancy loss (aOR 1.85, 95% CI 0.77–4.44, p=0.17). Blastocyst grade CC had significantly lower LB rate compared to all other blastocyst grades (p<0.05, chi-square analysis).
Conclusion:
Embryo grade CC and TE grade C are associated with decrease in odds of LB after euFET in RPL patients. Embryo grade is not associated with odds of CM in this cohort of RPL patients, suggesting that additional embryonic or uterine factors may influence risk of pregnancy loss.
Keywords: embryo grade, recurrent pregnancy loss, miscarriage, PGT-A, euploid transfer
Capsule:
Embryo grade CC and trophectoderm grade C are associated with significant decrease in live birth rate after single euploid frozen embryo transfer in recurrent pregnancy loss patients.
Introduction
Management of patients with recurrent pregnancy loss (RPL) continues to be a challenge for clinicians. The role of aneuploidy in miscarriage is well documented, with over 50% of pregnancy losses attributed to fetal chromosomal abnormalities and even higher aneuploidy rates reported among older patients (1,2). Due to the prevalence of aneuploidy in first trimester losses and in the RPL population, PGT-A has been utilized as a method for reducing miscarriage by selecting only euploid embryos for transfer (3,4). Causes of euploid miscarriage, particularly in the setting of RPL, remain a topic of great interest to both physicians and patients. In the absence of an explanation, patients often seek unproven testing and treatments.
Current literature on prognostic value of blastocyst assessment applies to a general infertile population. Blastocyst morphologic grading was first described by Gardner and Schoolcraft (5). Since then, studies have teased apart the relative contribution of trophectoderm (TE) and inner cell mass (ICM) grade on an embryo’s potential to implant and result in a live birth. After transfer of untested blastocysts, TE grade has been shown to be a superior predictor of live birth in fresh cycles (6,7) and a superior predictor of live birth and miscarriage in frozen cycles (8). Embryo morphology, however, is not consistently correlated with euploidy. ICM and TE grades have been reported by Capalbo et al to be unrelated to implantation outcomes after frozen euploid embryo transfer (euFET), however only 13 embryos were included in the poor blastocyst quality group (9). Conflicting results have subsequently been published using the same classification scheme but with a larger comparison group of 106 poor quality euploid embryos. In this study, Irani et al reports an approximately 2-fold higher pregnancy rate and 25-fold lower miscarriage rate among excellent quality euploid blastocysts compared to poor quality euploid blastocysts and that ICM morphology is a better predictor of pregnancy outcomes than TE morphology (10). A recent large study by Zhao et al show similar predictive value of TE and ICM grades in pregnancy outcome after euFET (11). The goal of this study was to determine if TE grade or inner cell mass (ICM) grade retain their established predictive values after euFET in a cohort of RPL patients.
Materials and Methods
Patient Selection
Patients with RPL performing PGT-A with at least one euploid embryo for transfer from 2012–2018 were included. RPL was defined as 2 or more prior pregnancy losses, inclusive of biochemical conceptions, independent of other infertility diagnoses. All patients had a complete RPL workup as recommended by the ASRM including blood work for parental karyotypes and to detect the presence of anti-phospholipid antibody syndrome (APLA) including anti-cardiolipin antibody, lupus anticoagulant and β−2-glycoprotein as well as a uterine cavity evaluation. Patients were also routinely screened for hypothyroidism and hyperprolactinemia with serum thyroid-stimulating hormone and prolactin, respectively. Patients who were known to be translocation carriers (either maternal or paternal) were excluded. Patients with the APLA syndrome were offered low dose aspirin and prophylactic heparin. Patients with uterine cavity anomalies including a uterine septum, intramural fibroids or uterine polyps underwent hysteroscopy and transection of the uterine septum, myomectomy or polypectomy, respectively, prior to embryo transfer.
Clinical Protocols
Controlled ovarian hyperstimulation (COH) was performed according to standard protocols per physician discretion. Stimulation protocols included microdose flare, Lupron down regulation, GnRH antagonist, and natural cycle. After transvaginal oocyte retrieval, all oocytes were fertilized with ICSI and all blastocysts underwent TE biopsy on day 5, 6 or 7 of embryo development once full expansion was achieved. All embryos were vitrified after TE biopsy. Embryos were graded at the time of cryopreservation using the Gardner grading scale. PGT-A was implemented using Next-Generation Sequencing (NGS), quantitative PCR (qPCR) or array comparative genomic hybridization (aCGH) platforms. All patients underwent frozen transfer of a single euploid blastocyst.
Endometrial lining was prepared using modified natural cycle or medicated cycle protocols per physician discretion. For natural cycles, patients used vaginal progesterone suppositories for luteal support until 8 weeks gestational age. For medicated cycles, the endometrium was primed with oral estradiol, estrogen patches, or intramuscular estrogen valerate and luteal support was provided through intramuscular progesterone in oil until 8 weeks gestational age, at which time support was transitioned to vaginal suppositories until 10 weeks. Blastocyst transfer took place on the sixth day after hCG administration in modified natural cycles, and on the sixth day of progesterone supplementation in programmed cycles. Embryo transfers were performed with transabdominal ultrasound guidance after confirmation of embryo survival after warming. Serum progesterone level was measured 2 days after transfer, and serum hCG level obtained 9 days after transfer. Transvaginal ultrasound to monitor for the presence of a gestational sac was performed during the fifth week of gestation, with subsequent pregnancy ultrasounds to monitor fetal heart rate and appropriate growth between 6 to eight weeks gestation. Outcome of the first euFET was recorded.
Outcome Variables
The main outcome measured was live birth, defined as birth of a neonate at or beyond 24 weeks gestation. The secondary outcome was clinical miscarriage, defined as loss of pregnancy after visualization of a gestational sac on ultrasound. Additional outcomes measured include implantation, defined as beta hcg > 5 mIU/mL, clinical pregnancy, defined as beta hcg > 5 mIU/mL and a visualized gestational sac, and biochemical pregnancy loss, defined as loss of pregnancy after conception (bHCG level >5mIU/mL) and prior to visualization of a gestational sac on transvaginal ultrasound. Implantation, clinical pregnancy, and live birth were calculated per embryo transfer. Biochemical pregnancy loss rate was calculated per implantation. Clinical miscarriage rate was calculated per clinical pregnancy.
Statistical Analysis
Baseline parameters of patients were compared using T tests or single factor ANOVA. Chi square analysis was performed to compare the proportion of ART outcomes within each morphologic grade. We modeled the association between TE grade or ICM grade (A, B or C, with A as reference) and pregnancy outcome (live birth, clinical miscarriage or biochemical pregnancy loss) after euploid frozen embryo transfer using logistic regression. The model was adjusted for age (continuous), BMI (categorical subdivided as BMI≥25 kg/m2 or BMI < 25 kg/m2), AMH (categorical subdivided as < 1 ng/mL or ≥1 ng/mL) and day of blastocyst biopsy (day 5, 6 or 7). AMH was included as a covariate in our analysis because it has been shown to be a predictor of live birth rate in RPL patients overall (12) and RPL patients with AMH < 1 ng/mL have a higher perentage of aneuploid blastocysts (13). Statistical analysis was performed using SPSS software version 25 (IBM, Armonk, New York). A P value of < 0.05 was considered to be statistically significant. This study was exempt from IRB approval as it was a retrospective analysis of de-identified data.
Results
660 euFETs were included in the analysis. Average patient age was 36.7 ± 3.4 years, average BMI was 25.7 ± 5.6 kg/m2, and patients had on average 3.5 ± 1.5 prior pregnancies, 0.5 ± 0.8 term deliveries, and 2.6 ± 1.1 prior pregnancy losses. Average AMH was 3.4 ± 3.8 ng/mL and average FSH was 7.5 ± 2.8 IU/mL prior to stimulation start. Patients with TE grade C (average age 38.3 years) were significantly older than patients with TE grade B (average age 37.2 years, p=0.02) and patients with TE grade A (average age 36.5 years, p<0.05). Patients with TE grade C had similar average AMH (3.4 ± 3.8 ng/mL) to patients with TE grade B (average AMH , p=0.95) and to patients with TE grade A (average AMH 3.9 ± 4.5 ng/mL, p=0.10). Patients with TE grade A, B and C had similar BMI (p=0.80). Patients with ICM grade C (average age 37.5 years) were significantly older than patients with ICM grade A (average age 36.0 years, p=0.01) and similar in age to patients with ICM grade B (average age 37.0 years, p=0.43). Patients with ICM grade A, B and C had similar BMI (p=0.55). Patients with embryo grade CC were on average 37.0 ± 4.3 years of age with AMH 2.7 ± 2.4 ng/mL. Compared to the overall cohort, these patients did not differ in terms of age (p=0.69) or AMH (p=0.52).
For COH, 82% of cycles utilized an antagonist protocol, 11% of cycles utilized a microdose flare protocol, 6% utilized a long Lupron protocol and 1% utilized a natural cycle. 54% of PGT-A was performed using real-time qPCR platform, 45% of PGT-A was performing using NGS and microarray platform was used for 1% of cycles. On average per oocyte retrieval, 15.5 ± 9.8 oocytes were retrieved, 5.4 ± 3.8 blastocysts were biopsied for PGT-A and 3.6 ± 2.9 embryos were euploid. Patients with TE grade C had on average per oocyte retrieval 11.1 ± 7.8 oocytes retrieved, 2.6 ± 1.7 embryos biopsied and 1.4 ± 0.9 euploid embryos. Compared to patients with TE grades A and B, patients with TE grade had fewer oocytes retrieved (p< 0.01), significantly fewer embryos biopsied (p<0.01) and significantly fewer euploid embryos (p<0.01). Patients with embryo grade CC had, on average per oocyte retrieval, 11.0 ± 5.8 oocytes were retrieved, 2.4 ± 1.6 embryos biopsied and 1.5 ± 1.0 euploid embryos. Compared to the overall cohort, patients with embryo grade CC did not differ in terms of number of oocytes retrieved (p=0.06) but had significantly fewer embryos biopsied (p=<0.01) and number of euploid embryos (p=<0.01).
The endometrial lining was prepared prior to transfer using a medicated cycle for 78% of transfers and the remaining cycles (22%) used a natural cycle protocol. Average endometrial thickness prior to ET was 9.4 ± 2.1 mm. Overall live birth rate per euFET was 62% (n=408).
Clinical outcomes stratified by ICM and TE grade are shown in Table 1. There were 217 ICM grade A euFETs, 405 ICM grade B euFETs and 38 ICM grade C euFETs. Live birth, clinical miscarriage and biochemical pregnancy loss rates were similar for ICM grades A, B and C (p=0.08, p=0.38, p=0.45, respectively, Chi-square analysis). In a logistic regression analysis accounting for age, BMI, AMH and day of blastocyst biopsy, ICM grade B was not significantly associated with odds of live birth (aOR 0.80, 95% CI 0.56–1.14, p=0.21), miscarriage (aOR 1.53, 95% CI 0.85–2.77, p=0.16) or biochemical pregnancy loss (aOR 0.87, 95% CI 0.47–1.62, p=0.66) (Table 2). ICM grade C was not significantly associated with odds of live birth (aOR 0.50, 95% CI 0.24–1.02 p=0.057), miscarriage (aOR 1.67, 95% CI 0.56–5.00, p=0.36) or biochemical pregnancy loss (aOR 1.58, 95% CI 0.53–4.75, p=0.42).
Table 1.
Comparison of outcomes after euploid frozen embryo transfer stratified by ICM and TE blastocyst grades.
| euFET (n) | Implantation1, n (%) | P valueψ | Clinical pregnancy2, n (%) | P valueψ | Live birth3, n (%) | P valueψ | Clinical miscarriage4, n (%) | P valueψ | Biochemical pregnancy loss5, n (%) | P valueψ | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inner Cell Mass Grade | |||||||||||
| A | 217 | 185 (85%) | 0.24 | 164 (76%) | 0.26 | 145 (67%) | 0.08 | 18 (11%) | 0.38 | 19 (10%) | 0.45 |
| B | 405 | 327 (81%) | 293 (72%) | 244 (60%) | 47 (16%) | 31 (9%) | |||||
| C | 38 | 29 (81%) | 24 (63%) | 19 (50%) | 5 (21%) | 5 (17%) | |||||
| Trophectoderm Grade | |||||||||||
| A | 312 | 259 (83%) | 0.38 | 232 (74%) | 0.09 | 203 (65%) | 0.01 | 28 (12%) | 0.25 | 24 (9%) | 0.37 |
| B | 277 | 228 (82%) | 205 (75%) | 172 (62%) | 31 (15%) | 22 (10%) | |||||
| C | 71 | 54 (76%) | 44 (62%) | 33 (46%) | 11 (25%) | 9 (17%) | |||||
Implantation was defined as beta hcg > 5 mIU/mL.
Clinical pregnancy was defined as a visualized gestational sac.
Live birth was defined as delivery of a neonate at or beyond 24 weeks
Clinical miscarriage rate was calculated per clinical pregnancy.
Biochemical pregnancy loss was defined as loss of pregnancy after conception and prior to visualization of a gestational sac on transvaginal ultrasound. Biochemical pregnancy loss rate was calculated per implantation.
Chi-square analysis.
Table 2.
Association between ICM or TE grade and pregnancy outcome after euploid frozen embryo transfer
| ICM Grade Bψ | TE Grade Bψ | |||||||
|---|---|---|---|---|---|---|---|---|
| Crude OR (95% CI) | P value | Adjusted OR (95% CI)* | P value | Crude OR (95% CI) | P value | Adjusted OR (95% CI)* | P value | |
| Live Birth | 0.75 (0.53–1.06) | 0.10 | 0.80 (0.56–1.14) | 0.21 | 0.86 (0.62–1.21) | 0.38 | 0.90 (0.63–1.27) | 0.53 |
| Clinical miscarriage | 1.12 (0.42–3.02) | 0.82 | 1.53 (0.85–2.77) | 0.16 | 1.33 (0.78–2.27) | 0.30 | 1.37 (0.79–2.39) | 0.27 |
| Biochemical pregnancy loss | 0.87 (0.48–1.57) | 0.64 | 0.87 (0.47–1.62) | 0.66 | 1.04 (0.57–1.90) | 0.90 | 1.08 (0.58–2.00) | 0.82 |
| ICM Grade Cψ | TE Grade Cψ | |||||||
| Crude OR (95% CI) | P value | Adjusted OR (95% CI)* | P value | Crude OR (95% CI) | P value | Adjusted OR (95% CI)* | P value | |
| Live Birth | 0.45 (0.22–0.90) | 0.02 | 0.50 (0.24–1.02) | 0.057 | 0.47 (0.28–0.79) | 0.004 | 0.49 (0.28–0.86) | 0.01 |
| Clinical miscarriage | 1.68 (0.58–4.82) | 0.34 | 1.67 (0.56–5.00) | 0.36 | 1.86 (0.88–3.94) | 0.11 | 2.00 (0.89–4.47) | 0.09 |
| Biochemical pregnancy loss | 1.58 (0.55–4.52) | 0.40 | 1.58 (0.53–4.75) | 0.42 | 1.74 (0.77–3.93) | 0.18 | 1.85 (0.77–4.44) | 0.17 |
Logistic regression model, ICM or TE grade A used as reference
Adjusted for age (continuous), overweight (categorical, BMI≥25 kg/m2 or BMI < 25 kg/m2), AMH (categorical, < 1 ng/mL or ≥1 ng/mL) and day of blastocyst biopsy (5, 6 or 7).
There were 312 TE grade A euFETs, 277 TE grade B euFETs, and 71 TE grade C euFETs. Live birth rates TE grades A, B and C differed significantly by chi-square analysis (p=0.01, Chi-square analysis) while clinical miscarriage and biochemical pregnancy loss rates were similar (p=0.25, p=0.37, respectively, Chi-square analysis). In a logistic regression analysis accounting for age, BMI, AMH and day of blastocyst biopsy, TE grade B was not associated with odds of live birth (aOR 0.90, 95% CI 0.63–1.27, p=0.53), miscarriage (aOR 1.37, 95% CI 0.79–2.39, p=0.27) or biochemical pregnancy loss (aOR 1.08, 95% CI 0.58–2.00, p=0.82). TE grade C was significantly associated with odds of live birth (aOR 0.49, 95% CI 0.28–0.86, p=0.01) and was not associated with odds of miscarriage (aOR 2.00, 95% CI 0.89–4.47, p=0.09) or biochemical pregnancy loss (aOR 1.85, 95% CI 0.77–4.44, p=0.17).
Live birth outcomes for embryos with combined ICM and TE grades AA, AB, BA, BB, BC, CA, CB and CC are shown in Figure 1. 16 blastocysts were grade CC, with implantation rate 69% (n=11), clinical pregnancy rate 50% (n=8), live birth rate 31% (n=5), clinical miscarriage rate 38% (n=3) and biochemical loss rate 27% (n=3). Blastocyst grade CC had significantly lower live birth rate compared to all other blastocyst grades AA, AB, BA, BB, BC, and CB (p<0.05, chi-square analysis).
Figure 1. Comparison of live birth rate per patient after euploid frozen embryo transfer stratified by combined ICM/TE blastocyst gradesψ.
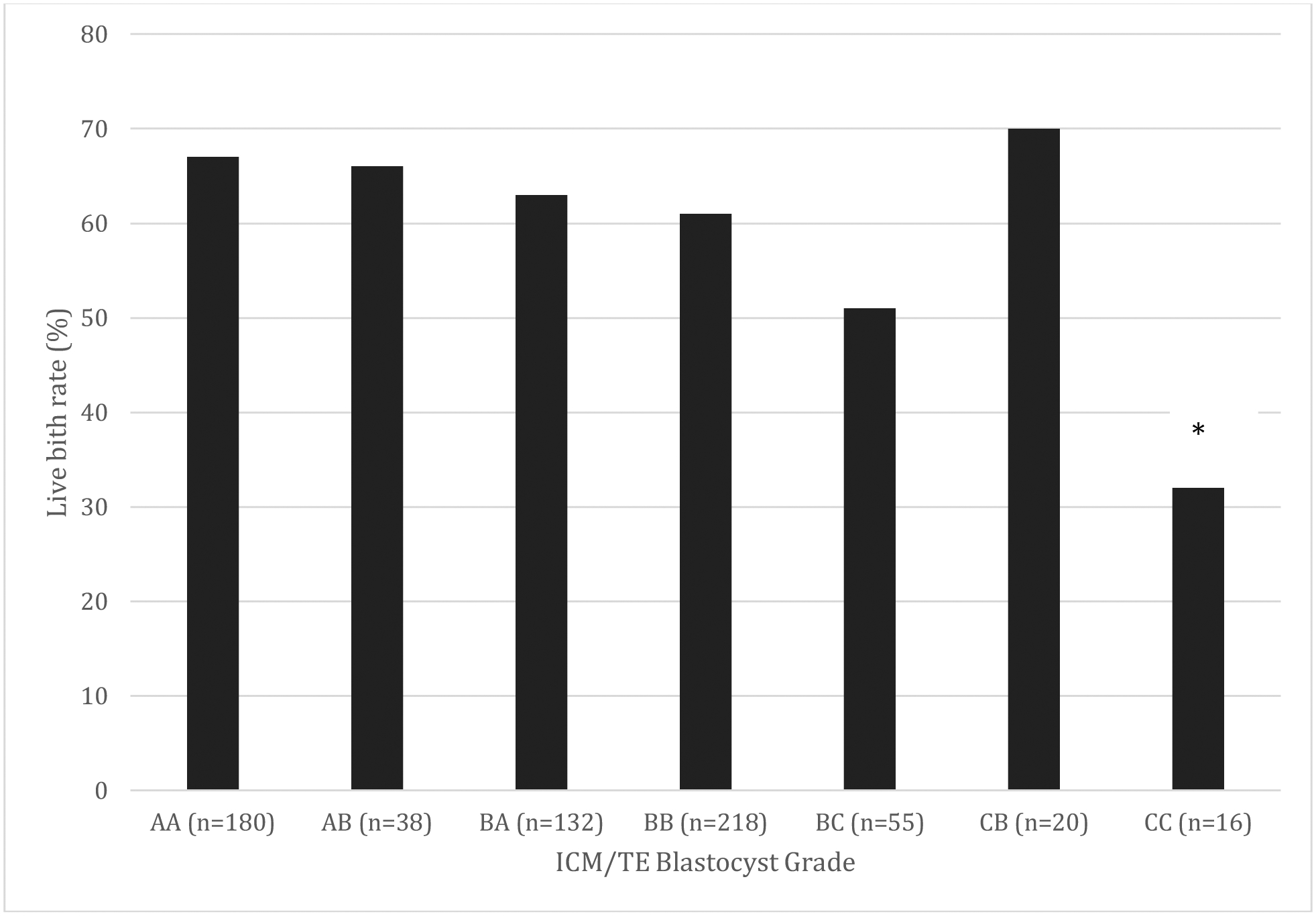
ψResults not shown in graph: One grade CA embryo was transferred, resulting in implantation and subsequent miscarriage.
*p<0.05, Chi-square analysis
Discussion
In this cohort of RPL patients, we report that TE grade C and blastocyst grade CC are associated with significant decrease in odds of live birth after euFET. After transfer of untested blastocysts in general infertile cohorts, TE grade has been shown to be a superior predictor of live birth in fresh cycles (6,7) and a superior predictor of live birth and miscarriage in frozen cycles (8).To our knowledge, this is the first report on the association of embryo grade and outcomes in RPL patients performing euFET and our data support previous reports that blastocyst morphology is associated with live birth outcome after euET (10,11). In any patient population, a myriad of factors influence reproductive outcomes. Among RPL patients in whom additional factors may impact outcomes, it is notable that blastocyst morphology retains its predictive value for live birth.
The association, if any, between embryo grade and pregnancy loss is particularly interesting in the RPL patient population. Irani et al reported a striking trend among euFET cycles in a general infertile cohort, with a 25% miscarriage rate after frozen transfer of poor quality euploid blastocysts (n=51) compared to 0% miscarriage rate after frozen transfer of excellent quality euploid blastocysts (n=32) and 6% miscarriage rate after frozen transfer of good quality euploid blastocysts (n=50). RPL patients are at higher risk of euploid miscarriage (14), and the varying contributions of embryonic and uterine factors are unknown.
In our study, when comparing outcomes for embryos with ICM or TE grade C compared to higher grades, there was a trend towards higher risk of miscarriage with poorer embryo grade but the differences were not statistically significant. This finding is likely multifactorial, due in part to the small size of the subgroups, but also may suggest that there is additional variability in reproductive efficiency among RPL patients that is not captured by euploidy or blastocyst morphology. The parameters that affect outcomes in a general infertile population may be less predictive among RPL patients, in whom uterine factors that are not accounted for or embryo factors that are yet to be understood may play a significant role in miscarriage. This information can be used as an aid to counsel RPL patients on risk of miscarriage after euploid transfer.
Strengths of this study include a relatively large sample size for studying an RPL population and use of single blastocyst euploid frozen transfer for all patients to minimize confounding due to number of embryos transferred and the effect of freeze/thaw cycles on the TE or ICM. Limitations of the study as previously noted in the literature is the intra- and inter-observer variability in embryo grading (15, 16). Our results, however, are from a single center with regular proficiency and consistency checks. Since our outcome was limited to a single embryo transfer, we are not able to identify overall egg or sperm quality. Furthermore, our analysis was limited to patients reaching transfer of at least a CC grade or higher quality euploid embryo, and may not be reflective of outcomes in poorer prognosis cycles. In addition, our findings may not be applicable to an unscreened population of embryos as morphologic assessment was only performed on embryos being designated of sufficient quality to merit cryopreservation. While all RPL patients underwent a complete evaluation per ASRM guidelines and all patients with translocations were excluded, granular data on the number of patients who received treatment of APLA or correction of uterine anomalies was not available and may present unmeasured confounding. As this study focused exclusively on patients with RPL, our findings cannot be used to differentiate prognostic value of embryos from other subgroups of patients. Our findings are, however, supported by prior studies in a general infertile population (10,11). Different PGT-A platforms were used for this study, which introduces heterogeneity and potential for unmeasured confounding of study results. However, qPCR, NGS and aCGH have been validated for use in large, prospective randomized clinical studies (17–19). In addition, many studies examining PGT-A are similarly limited by heterogeneity of testing methodology such that ASRM does not endorse one testing platform in particular (20). While day 7 blastocysts have been associated with lower pregnancy and live birth rates and could bias study results (21–24), of the 660 cycles included in this study, only 4 (0.6%) performed trophectoderm biopsy on day 7. Finally, the results are limited by the relatively small number of poorer quality embryos. Lack of difference in clinical outcomes between various embryo grades may be due to limited statistical power. Larger scale, prospective analyses accounting for total cycle potential are warranted to confirm the reported findings.
Conclusions
Embryo grade CC and TE grade C are associated with a significant decrease in odds of live birth after euFET in RPL patients. Furthermore, embryo grade is not predictive of clinical miscarriage rate in this cohort of RPL patients suggesting that additional embryonic or uterine factors may influence risk of pregnancy loss. Association between embryo grade and clinical outcomes has not been previously been reported in RPL patients.
References
- 1.Viaggi CD, Cavani S, Malcarne M, Floriddia F, Zerega G, Baldo C, et al. First trimester euploid miscarriages analyzed by array CGH. J Appl Genet 2013; 54: 353–359. [DOI] [PubMed] [Google Scholar]
- 2.Marquard K, Westphal LM, Milki AA and Lathi RB. Etiology of recurrent pregnancy loss in women over the age of 35 years. Fertil Steril 2010; 94: 1473–77. [DOI] [PubMed] [Google Scholar]
- 3.Shahine L and Lathi RB. Embryo selection with preimplantation chromosomal screening in patients with recurrent pregnancy loss. Semin Reprod Med 2014; 32: 093–099. [DOI] [PubMed] [Google Scholar]
- 4.Murugappan G, Shahine LK, Perfetto CO, Hickok LR, and Lathi RB. Intent to treat analysis of in vitro fertilization and preimplantation genetic screening versus expectant management in patients with recurrent pregnancy loss. Hum Reprod 2016; 31: 1668–74. [DOI] [PubMed] [Google Scholar]
- 5.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: toward a single blastocyst transfer. Fertil Steril 2000; 73: 1155–8. [DOI] [PubMed] [Google Scholar]
- 6.Ahlstom A, Westin C, Reismer E, Wikland M, Hardarson T. Trophectoderm morphology: an important parameter for predicting live birth after single blastocyst transfer. Hum Reprod 2011; 26: 3289–96. [DOI] [PubMed] [Google Scholar]
- 7.Hill MJ, Richter KS, Heitmann DO, Graham JR, Tucker MJ, DeCherney AH, et al. Trophectoderm grade predicts outcomes of single blastocyst transfers. Fertil Steril 2013; 99: 1283–89. [DOI] [PubMed] [Google Scholar]
- 8.Honnma H, Baba T, Sasaki M, Hashiba Y, Ohno H, Fukunaga T, et al. Trophectoderm morphology significantly affects the rates of ongoing pregnancy and miscarriage in frozen-thawed single-blastocyst transfer cycle in vitro fertilization. Fertil Steril 2012; 98:361–7. [DOI] [PubMed] [Google Scholar]
- 9.Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, et al. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod 2014; 29: 1173–1181. [DOI] [PubMed] [Google Scholar]
- 10.Irani M, Reichman D, Robles A, Melnick A, Davis O, Zaninovic N, et al. Morphologic grading of euploid blastocysts influences implantation and ongoing pregnancy rates. Fertil Steril 2017; 107:664–70. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y-Y, Yu Y, and Zhang X-W. Overall blastocyst quality, trophectoderm grade, and inner cell mass grade predict pregnancy outcome in euploid blastocyst transfer cycles. Chin Med J (Engl); 2018: 131:1261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murugappan G, Shahine L and Lathi RB. Antimullerian hormone is a predictor of live birth in patients with recurrent pregnancy loss. Fertil Res Pract 2019; 5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahine L, Marshall L, Lamb JD and Hickok LR. Higher rates of aneuploidy in blastocysts and higher risk of no embryo transfer in recurrent pregnancy loss patients with diminished ovarian reserve undergoing in vitro fertilization. Fertil Steril 2016; 106:1124–8. [DOI] [PubMed] [Google Scholar]
- 14.Ogasawara M, Aoki K, Okada S and Suzumori K. Embryonic karyotype of abortuses in relation to the number of previous miscarriages. Fertil Steril 2000; 73: 300–4. [DOI] [PubMed] [Google Scholar]
- 15.Filho ES, Noble JA, Poli M, Griffiths T, Emerson G, Wells D. A method for semi-automatic grading of human blastocyst microscopic images. Hum Reprod 2012; 27: 2641–8. [DOI] [PubMed] [Google Scholar]
- 16.Paternot G, Devroe J, Debrock S, d’Hooghe TM, Spiessens C. Intra-and inter-observer analysis in the morphological assessment of early-stage embryos. Reprod Biol Endocrinol 2009; 7:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Treff NR, Tao X, Ferry KM, Su J, Taylor D, Scott RT Jr. Development and validation of an accurate quantitative real-time polymerase chain reaction-based assay for human blastocyst comprehensive chromosomal aneuploidy screening. Fertil Steril. 2012;97:819–824. [DOI] [PubMed] [Google Scholar]
- 18.Treff NR, Krisher RL, Tao X, Garnsey H, Bohrer C, Silva E, et al. Next Generation Sequencing-Based Comprehensive Chromosome Screening in Mouse Polar Bodies, Oocytes, and Embryos. Biol Reprod. 2016;94:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capalbo A, Treff NR, Cimadomo D, Tao X, Upham K, Ubaldi FM, et al. Comparison of array comparative genomic hybridization and quantitative realtime PCR-based aneuploidy screening of blastocyst biopsies. Eur J Hum Genet 2015;23:901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Practice Committee of the American Society for Reproductive Medicine. The use of Preimplantation Genetic Testing for Aneuploidy (PGT-A). Fertil Steril 2018; 109:429–36. [DOI] [PubMed] [Google Scholar]
- 21.Hammond ER, Cree LM, Morbeck DE, Should extended blastocyst culture include Day 7?, Human Reproduction 2019; 3:991–997. [DOI] [PubMed] [Google Scholar]
- 22.Whitney JB, Balloch K, Anderson RE, Nugent N, Schiewe MC. Day 7 blastocyst euploidy supports routine implementation for cycles using preimplantation genetic testing. JBRA Assist Repro 2019; 2:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su Y, Li JJ, Wang C, Haddad G, Wang WH. Aneuploidy analysis in day 7 human blastocysts produced by in vitro fertilization. Reprod Biol Endocrinol 2016;14:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitney JB, Anderson RE, Schiewe MC. Day 7 blastocyst euploidy and implantation rates warrant implantation for all programs using preimplantation genetic screening (PGS). Fertil Steril 2016;106:e146. [Google Scholar]


