Abstract
Background:
Right ventricular failure is an underrecognized consequence of COVID-19 pneumonia. Those with severe disease are treated with extracorporeal membrane oxygenation (ECMO) but with poor outcomes. Concomitant right ventricular assist device (RVAD) may be beneficial.
Methods:
A retrospective analysis of intensive care unit patients admitted with COVID-19 ARDS (Acute Respiratory Distress Syndrome) was performed. Non-intubated patients, those with acute kidney injury, and age > 75 were excluded. Patients who underwent RVAD/ECMO support were compared with those managed via invasive mechanical ventilation (IMV) alone. The primary outcome was in-hospital mortality. Secondary outcomes included 30-day mortality, acute kidney injury, length of ICU stay, and duration of mechanical ventilation.
Results:
A total of 145 patients were admitted to the ICU with COVID-19. Thirty-nine patients met inclusion criteria. Of these, 21 received IMV, and 18 received RVAD/ECMO. In-hospital (52.4 vs 11.1%, p=0.008) and 30-day mortality (42.9 vs 5.6%, p=0.011) were significantly lower in patients treated with RVAD/ECMO. Acute kidney injury occurred in 15 (71.4%) patients in the IMV group and zero RVAD/ECMO patients (p<0.001). ICU (11.5 vs 21 days, p=0.067) and hospital (14 vs 25.5 days, p=0.054) length of stay were not significantly different. There were no RVAD/ECMO device complications. The duration of mechanical ventilation was not significantly different (10 vs 5 days, p=0.44).
Conclusions:
RVAD support at the time of ECMO initiation resulted in the no secondary end-organ damage and higher in-hospital and 30-day survival versus IMV in specially selected patients with severe COVID-19 ARDS. Management of severe COVID-19 ARDS should prioritize right ventricular support.
Keywords: extracorporeal membrane oxygenation, COVID-19, right ventricular assist device, acute respiratory distress syndrome
Introduction
Acute respiratory distress syndrome (ARDS) occurs in approximately 31–67% of patients hospitalized with SARS-CoV-2 pneumonia (COVID-19)1–3 and is associated with mortality rates upwards of 52%.1,2 Prior experience and recent epidemiologic reports suggest early intubation4, lung protective ventilation4,5, and prone-positioning6–9 have been effective strategies to manage COVID-19 ARDS in the early phases of the pandemic. Despite these strategies many patients progress to multisystem organ failure, with cardiogenic shock secondary to right ventricular (RV) dysfunction as the terminal event.1–3,10–16 Even with the routine use of echocardiography and pulmonary artery catheters, assessment of RV function can be challenging and unreliable. RV dysfunction or failure has been shown to occur in approximately 25% of patients with ARDS, and in our mechanical circulatory support practice we have observed that significant hemodynamic improvement can be achieved through the use of a percutaneous right ventricular assist device (RVAD) even in cases where pulmonary artery pulsatility index (PAPi) and tricuspid annular plane systolic excursion (TAPSE) are reassuring.17–20
In our institution’s early experience with managing COVID-19 ARDS and based on available literature regarding the pathophysiology of COVID-193, we hypothesized that concomitant RV support using a percutaneous RVAD cannula in patients referred for extracorporeal membrane oxygenation (ECMO) would result in superior outcomes compared to invasive mechanical ventilation (IMV) alone in similar patients not referred for ECMO.21 Although ECMO referral patterns varied between critical care providers, for those referred, we attempted to initiate therapy within 24 hours of intubation and subsequently pursue extubation and ambulation prior to decannulation from the ECMO circuit. In this report, we present our initial experience and a comparison of outcomes between patients receiving RVAD/ECMO and those in whom more traditional intensive care unit (ICU) protocols were utilized.
Methods
Institutional Review Board approval from the Medical College of Wisconsin, Milwaukee, WI was obtained prior to the start of the following study (IRB PRO 37951, 4/27/2020) and a waiver of informed consent was granted due to the low-risk nature and design. The study population consisted of adult COVID-19 patients admitted to medical and cardiovascular ICUs between March 1, 2020 and July 6, 2020 at a large urban teaching hospital in Southeastern Wisconsin. Patients with contraindications to ECMO according to Extracorporeal Life Support Organization (ELSO) guidelines as well as our institutional guidelines were excluded from analysis.22,23 Major criteria for exclusion included patients aged greater than 75 years old, those not intubated or declined intubation (and had thus not had exhausted medical therapy), and patients with acute kidney injury (defined as KDIGO stage 3) at the time of intubation (Figure 1). Patients treated with non-RVAD venovenous ECMO (VV ECMO) approaches were also excluded as this was not within the focus of the study. Patients of interest in the final cohort were included if they met Berlin criteria for severe ARDS and were therefore ECMO candidates according to ELSO criteria. The primary outcome was in-hospital mortality. Secondary outcomes included 30-day mortality, ICU length of stay (LOS), acute kidney injury, and duration of mechanical ventilation.
Figure 1.
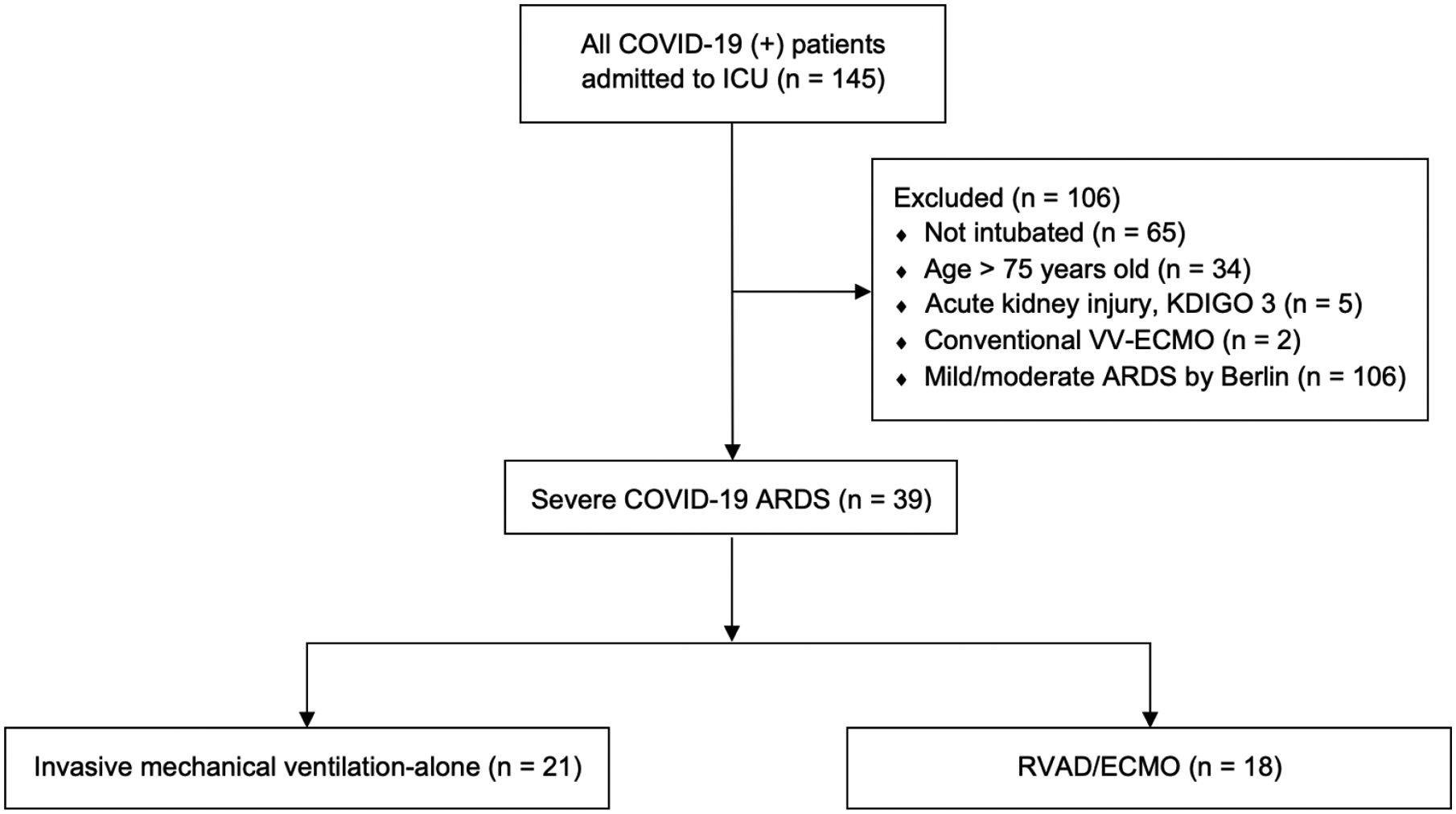
COVID-19 ICU cohort flow diagram. All patients admitted to the ICU were considered excluding patients based on specified exclusions criteria. Those with severe ARDS were considered in the final cohort and divided in those who received RVAD/ECMO and those treated with mechanical ventilation alone. ICU, intensive care unit; ARDS, acute respiratory distress syndrome; VV, venovenous; ECMO, extracorporeal membrane oxygenation; RVAD, right ventricular assist device
The decision to pursue internal referral for ECMO was made at the discretion of the medical ICU team according to general ELSO guidelines for Adult Respiratory Failure22. Because lung compliance in COVID-19 ARDS is relatively preserved and patients tolerate hypoxemia without excessive work of breathing, a strategy of high flow nasal cannula and awake prone-positioning was used. Intubation was performed only if the oxygen saturation was less than 88% on FiO2 of 1.0 for 30 minutes or more. The medical ICU team attempted to refer patients for ECMO as soon as oxygenation failure as defined by the above criteria were met, often at the time of intubation. Other patients were referred from outside institutions through our institution’s multidisciplinary “SHOCK” system at the discretion of the treated intensivist and accepted for ECMO therapy if deemed an appropriate candidate according to aforementioned criteria and review by our multidisciplinary team of surgeons, intensivist, and other specialists. For patients referred for ECMO, an attempt was made to cannulate within 24 hours of intubation. In each case, further management decisions were agreed upon by a multidisciplinary team. For a comparison control group, we applied the aforementioned inclusion/exclusion criteria for ECMO support to all patients who were admitted to the medical ICU and intubated, but who were not referred for ECMO during this same time period.
All ECMO procedures were performed using a 29 or 31 French dual-lumen RVAD cannula (TandemLife Protek Duo™, LivaNova, UK). The cannula (Figure 2) is inserted percutaneously via the right internal jugular vein. A Swan-Ganz pulmonary artery catheter is inserted and floated into the main pulmonary artery which is used to facilitate placement of a guidewire into the main pulmonary artery. Next, using the Seldinger technique, the cannula is passed into the main pulmonary artery. All procedures were performed by experienced cardiothoracic surgeons in a hybrid operating room under transesophageal echocardiographic and fluoroscopic guidance. The cannula was connected to either a Cardiohelp™ (Getinge, Gothenburg, SE) or Centrimag™ (Abbott, Abbott Park, IL) ECMO circuit.
Figure 2.
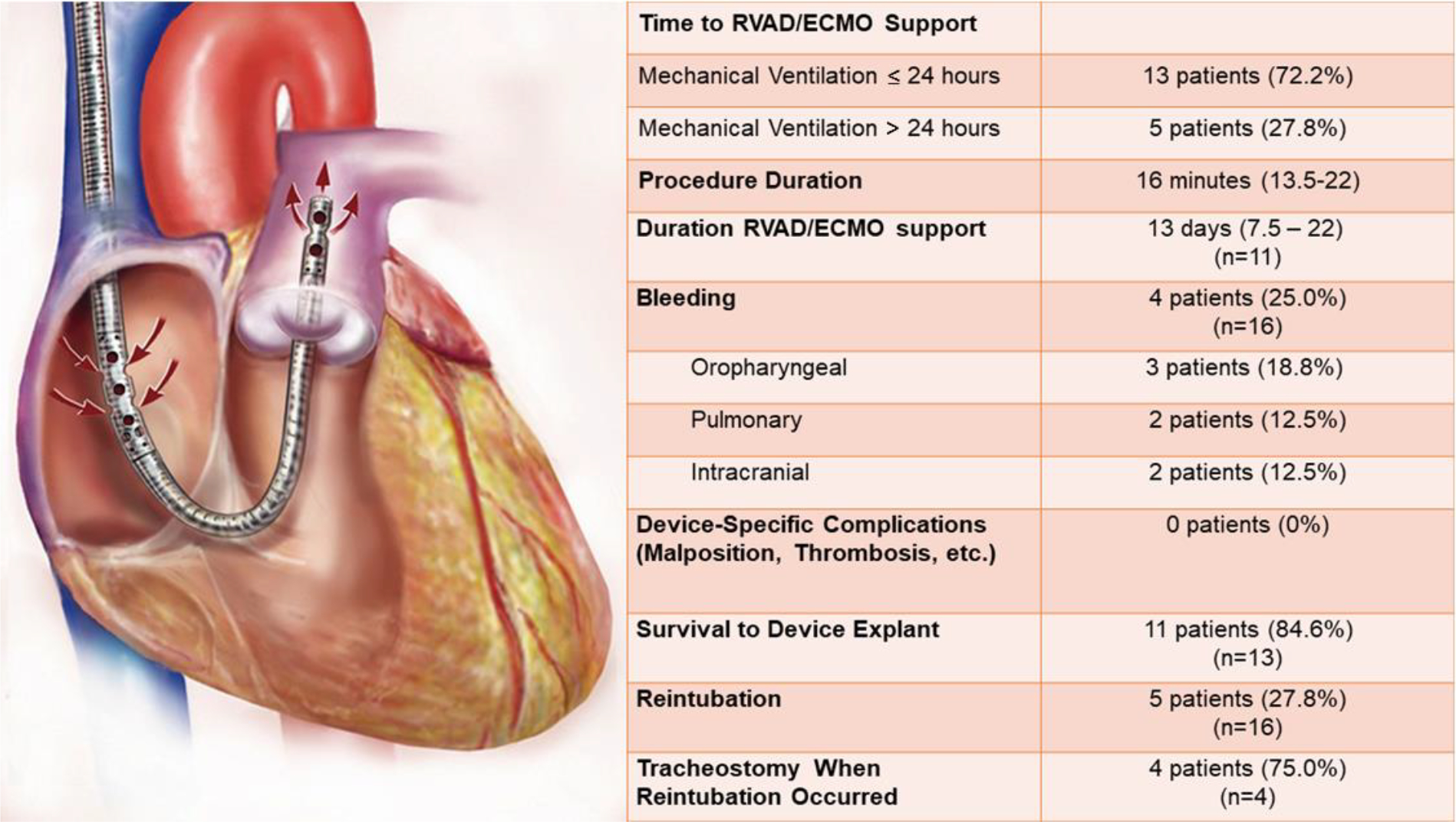
RVAD/ECMO specific outcomes. The TandemLife™ Protek Duo is a percutaneous right ventricular assist device (RVAD) which is inserted into the right internal jugular vein. Inflow to the extracorporeal circuit occurs via the outer, which is positioned in the right atrium, while outflow to the pulmonary artery occurs via the inner lumen. ECMO, extracorporeal membrane oxygenation
The decision to provide concomitant RV support utilizing a percutaneous RVAD as the preferred ECMO cannula was based upon our large institutional experience with this cannula for VV ECMO and our previously reported favorable outcomes17, but also several key observations about the clinical course and pathophysiology of COVID-19 from early in the pandemic. COVID-19 has been well documented to directly affect cardiac function both in the acute infection and long after illness recover.12–15,24 The myocardial involvement of COVID-19 is a unique feature of the disease with reported cases of cardiac failure, myocarditis, and right ventricular failure previously reported.10–14 Additionally, the time course of COVID-19 organ dysfunction in non-surviving patients has been detailed to follow a sequence of sepsis, ARDS, acute cardiac injury, acute kidney injury, and death.3 In the treatment of our institutions first COVID-19 ECMO patient utilizing a conventional VV ECMO strategy (without RV support), this sequence was observed in that despite suitable oxygenation support once ECMO was instituted, the patient eventually succumbed to cardiogenic shock and multisystem organ failure, particular acute kidney injury and ischemic hepatitis. This experience and knowledge of the unique pathophysiology of COVID-19 lead us to modify our treatment strategy early in the pandemic to focus exclusively on both RV and oxygenation support in our ECMO patients, which is reflected in the cohort reported here.
RVAD/ECMO management was performed with the goals of optimizing oxygen delivery, RV support, early extubation, and physical rehabilitation, balanced with standard ARDS care. ECMO flows were maintained at 3 to 4.5 liters per minute with adjustment of sweep gas, flow, and mechanical ventilation to achieve a goal PaO2 > 55 mmHg, SpO2 > 85%, and arterial pH 7.30–7.40. ECMO and ventilator FiO2 were initiated at 100% with a sweep of 5 liters per minute and weaned appropriately. Bilevel or pressure control ventilatory modes were used with driving pressure less than 15 mmHg to minimize ventilator-induced lung injury (VILI). Standard extubation criteria were followed, including ECMO FiO2 less than 60% and 21–30% FiO2 on mechanical ventilation. Patients who were unable to be extubated underwent tracheostomy within 14 days of cannulation. Patients were deemed candidates for decannulation from extracorporeal support when they were on 21% FiO2 on ECMO with a flow rate of 1 liter per minute while maintaining stable PaO2 and PaCO2 levels with supplemental nasal cannula oxygen. All patients were therapeutically anticoagulated with heparin monitored using unfractionated heparin levels adjusted according to emerging data on the thromboembolic risks associated with COVID-19.25
Categorical variables are reported as frequency (percentage) and compared using either a Chi-square or Fisher’s Exact test where appropriate; continuous variables are reported as median (interquartile range) and compared using a Wilcoxon-Mann-Whitney Rank Sum test. All time to events were calculated from time of intubation and censored at July 6, 2020. To compare survival between the two groups a Kaplan-Meier curve was created with comparison between the two groups using a Log-rank test for equity of survivor function. Cox Proportional Hazards model for survival and competing-risks regression models, for the competing risk of death, for outcomes of post-intubation ICU and hospital days and duration of mechanical ventilation were performed. Confounding variables treated as covariates in adjusted models included age, use of tocilizumab, and use of convalescent plasma. No interactions were included due to sample size. Analysis was performed using Stata 16.1 software with p<0.05 considered statistically significant.
Results
Between March 1 and July 6, 2020, 145 patients were admitted to the ICU with a confirmed diagnosis of COVID-19 pneumonia. A total of 39 patients met the inclusion/exclusion criteria, of which 18 underwent RVAD/ECMO cannulation (Figure 1). Patient demographics, comorbidities, and treatment characteristics are summarized in Table 1. Both groups were comparable with respect to age, gender, race, and medical comorbidities. All patients met Berlin criteria for severe ARDS. SpO2/FiO2 ratios were substituted for PaO2/FiO2 ratios if arterial blood gas data was absent for assessment of the severity of ARDS using previously reported cutoff values.26 All RVAD/ECMO PaO2/FiO2 and SpO2/FiO2 values were prior to cannulation. RVAD/ECMO patients had a significantly lower PaO2/FiO2 ratio (93.3 vs 71.9, p=0.039). Treatment regimens were similar except for the use of tocilizumab (23.8 vs 61.1%, p=0.025) and convalescent plasma (9.5 vs 66.7%, p=0.001) which occurred more frequently in the RVAD/ECMO group. Surrogate metrics for right ventricular function were lacking for the majority of patients at the time of intubation and/or RVAD/ECMO support due to institutional protocols designed to limit provider exposure to COVID-19 infected patients. Placement of invasive monitors and central venous catheters was often performed at the time of intubation by one provider to limit both PPE and the frequency of provider exposure. As a result, central venous pressure, pulmonary artery pressures, and PAPi were not routinely measured in either cohort. Echocardiographic data was available for 14 of 18 RVAD/ECMO patients and seven of 21 IMV-alone patients (supplementary material, sTable 1). The majority of patients demonstrated echocardiographic evidence of normal RV function and size without significant tricuspid regurgitation. Patients were hemodynamically stable at the time of cannulation for RVAD/ECMO patients and at the time of intubation for IMV-alone patients with minimal vasopressor requirements.
Table 1:
Characteristics and Demographics of COVID-19 Patients
| Total (N=39) | IMV-alone (N=21) | RVAD/ECMO (N=18) | p-value | |
|---|---|---|---|---|
| Age (years) | 53 (44 – 61) | 58 (42 – 67) | 51 (44 – 57) | .17 |
| Gender | .43 | |||
| Female | 20 (51.3%) | 12 (57.1%) | 8 (44.4%) | |
| Male | 19 (48.7%) | 9 (42.9%) | 10 (55.6%) | |
| PaO2/FiO2 ratio | 83 (68.4 – 96.7) (N=31) | 93.3 (71 – 106) (N=17) | 71.9 (62 – 85) (N=14) | .039 |
| SpO2/FiO2 ratio | 88.5 (82 – 99) (N=24) | 91.5 (83.5 – 101.4) (N=20) | 68.5 (50 – 86) (N=4) | .063 |
| Race | .49 | |||
| African American | 19 (48.7%) | 12 (57.1%) | 7 (38.9%) | |
| Non-Hispanic White | 12 (30.8%) | 6 (28.6%) | 6 (33.3%) | |
| Other Race/Ethnicity | 8 (20.5%) | 3 (14.3%) | 5 (27.8%) | |
| Comorbidity Count | .67 | |||
| < 4 | 33 (84.6%) | 17 (80.9%) | 16 (88.9%) | |
| 4 – 5 | 6 (15.4%) | 4 (19.1%) | 2 (11.1%) | |
| Comorbid Condition | ||||
| Chronic Lung Disease | 13 (33.3%) | 6 (28.6%) | 7 (38.9%) | .50 |
| Hypertension | 22 (56.4%) | 13 (61.9%) | 9 (50.0%) | .46 |
| Coronary Artery Disease | 3 (7.7%) | 0 (0%) | 3 (16.7%) | .089 |
| Chronic Kidney Disease | 3 (7.7%) | 2 (9.5%) | 1 (5.6%) | 1.00 |
| Preoperative Hemodialysis | 0 (0%) | 0 (0%) | 0 (0%) | |
| Diabetes Mellitus | 15 (38.5%) | 9 (42.9%) | 6 (33.3%) | .54 |
| Obesity | 25 (64.1%) | 14 (66.7%) | 11 (61.1%) | .72 |
| Therapies Given | ||||
| Hydroxychloroquine | 24 (75%) (N=32) | 16 (76.2%) (N=21) | 8 (72.7%) (N=11) | .83 |
| Azithromycin | 24 (75%) (N=32) | 18 (85.7%) (N=21) | 6 (54.6%) (N=11) | .053 |
| Doxycycline | 11 (34.4%) (N=32) | 6 (28.6%) (N=21) | 5 (45.5%) (N=11) | .44 |
| Tocilizumab | 16 (41.0%) (N=39) | 5 (23.8%) (N=21) | 11 (61.1%) (N=18) | .025 |
| Prednisone | 7 (21.9%) (N=32) | 4 (19.1%) (N=21) | 3 (27.3%) (N=11) | .67 |
| Hydrocortisone | 5 (15.6%) (N=32) | 4 (19.1%) (N=21) | 1 (9.1%) (N=11) | .64 |
| Dexamethasone | 7 (21.9%) (N=32) | 6 (28.6%) (N=21) | 1 (9.1%) (N=11) | .37 |
| Convalescent Plasma | 14 (35.9%) (N=39) | 2 (9.5%) (N=21) | 12 (66.7%) (N=18) | .001 |
| Remdesivir | 2 (6.3%) (N=32) | 2 (9.5%) (N=21) | 0 (0%) (N=11) | .53 |
| Proning | 37 (97.4%) (N=38) | 19 (95%) (N=20) | 18 (100%) (N=18) | 1.00 |
IMV, invasive mechanical ventilation; RVAD/ECMO, right ventricular assist device/extracorporeal membrane oxygenation
Overall, in-hospital mortality was 33.3% (Table 2). RVAD/ECMO patients demonstrated a significantly lower in-hospital mortality (52.4 vs 11.1%, p=0.008) compared to IMV-alone (Table 2.). Kaplan-Meier cumulative mortality was significantly lower for the RVAD/ECMO group (p=0.009) (Figure 3). Cox proportional hazard model demonstrated a durable survival advantage for RVAD/ECMO patients (HR 0.17, 0.03 – 0.91) even when including age, tocilizumab, and convalescent plasma as covariates, which were not significant (p>0.05) (sTable 2).
Table 2:
Outcomes of COVID-19 Patients
| Total (N=39) | IMV-alone (N=21) | RVAD/ECMO (N=18) | p-value | |
|---|---|---|---|---|
| In hospital mortality | 13 (33.3%) | 11 (52.4%) | 2 (11.1%) | .008 |
| 30-day Mortality | 10 (25.6%) | 9 (42.9%) | 1 (5.6%) | .011 |
| ICU LOS (days) | 13 (6 – 27) (N=37) | 11.5 (6 – 22.5) (N=20) | 21 (9 – 36) (N=17) | .067 |
| Post-intubation ICU days | 12 (6 – 27) (N=37) | 8.5 (5.5 – 22.5) (N=20) | 16 (9 – 27) (N=17) | .17 |
| Hospital LOS (days) | 18 (9 – 36) | 14 (8 – 29) | 25.5 (17 – 39) | .054 |
| Post-intubation hospital days | 16 (8 – 32) | 9 (6 – 27) | 20 (11 – 40) | .14 |
| Duration of mechanical ventilation (days) | 7.5 (1 – 22) (N=26) | 10 (5 – 20) (N=10) | 5 (1 – 34) (N=16) | .44 |
| Acute kidney injury | 15 (38.5%) | 15 (71.4%) | 0 (0%) | <.001 |
| Tracheostomy | 10 (27.0%) (N = 37) | 3 (14.3%) (N=21) | 7 (43.8%) (N=16) | .067 |
IMV, invasive mechanical ventilation; RVAD/ECMO, right ventricular assist device/extracorporeal membrane oxygenation; ICU, intensive care unit; LOS, length of stay
Figure 3.
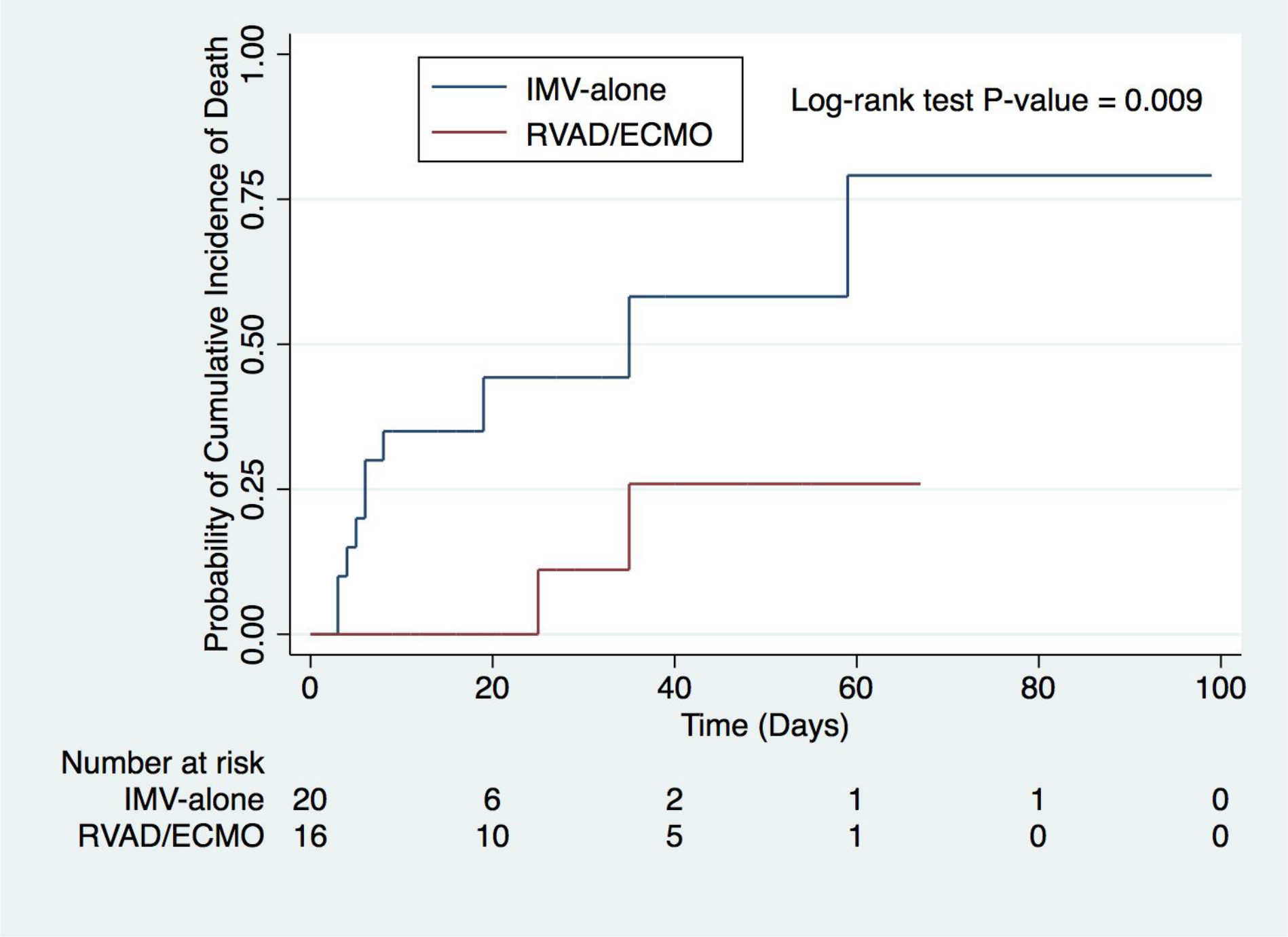
Kaplan-Meier cumulative mortality between RVAD/ECMO and IMV-alone. RVAD/ECMO, right ventricular assist device/extracorporeal membrane oxygenation; IMV, invasive mechanical ventilation.
Thirty-day mortality was significantly lower for RVAD ECMO patients (42.9 vs 5.6%, p=0.011) (Table 2.). ICU and hospital LOS (both overall and post-intubation) were longer for RVAD/ECMO patients, but not significantly different. Median duration of mechanical ventilation was numerically shorter for RVAD/ECMO patients (10 vs 5 days, p=0.44), but not significantly different. The rate of tracheostomy was numerically higher for RVAD/ECMO patients, but not significantly different (14.3 vs 43.8%, p=0.067). RVAD/ECMO patients had a significantly lower incidence of acute kidney injury with no patients meeting criteria versus 15 (71.4%) patients treated with IMV-alone (p<0.001). Competing risk cumulative incidences for post-intubation ICU and hospital LOS and duration of mechanical ventilation were not significantly different (p>0.05) even with consideration of covariates (sTable 3 & sFigure 1)
The majority (72.2%) of RVAD/ECMO patients underwent cannulation within 24 hours of intubation (Figure 2). Of the five patients cannulated greater than 24 hours after intubation, four were referred from outside facilities and thus time from intubation to cannulation was dependent upon the timing of initiating referral. By way of example, one patient initially achieved adequate oxygenation with conventional ARDS management, but subsequently developed progressive oxygenation failure several days later at which the patient was referred for ECMO consideration. Bleeding was the most frequent RVAD/ECMO complication (25.0%) including two instances of intracranial hemorrhage resulting in the death. There were no device-related complications observed. RVAD/ECMO patients were extubated a median of 3 days after cannulation. Following extubation, five (27.8%) patients required reintubation for behavioral reasons (i.e., agitation), but not respiratory compromise or airway protection. At the end of the study period, 11 patients had been successfully decannulated after an average of 13 days on device support, of which ten had been discharged. Two patients died without being decannulated. Six patients remained in the hospital, of which, five patients were still on device support and one decannulated, but still mechanically ventilated. Of the remaining cannulated patients, three were still intubated.
Discussion
Our findings suggest that patients who develop severe COVID-19 ARDS may benefit from early RVAD/ECMO after meeting criteria for intubation. This approach may represent an opportunity to improve clinical outcomes as compared to using prolonged invasive mechanical ventilation. COVID-19 ARDS is characterized by relatively preserved lung compliance, disproportionate hypoxemia to the degree of lung injury and non-injurious patterns of spontaneous breathing. While low tidal volume ventilation and prone positioning are cornerstones for managing non-COVID-19 ARDS, the optimal strategy for COVID-19 ARDS is unknown. Use of moderate to high positive end expiratory pressure (PEEP) usually accompanies other management strategies in ARDS; however, techniques to set optimal PEEP levels remain controversial. It is possible the excess PEEP in COVID-19 over-distends normal lung parenchyma, thereby increasing lung stress and strain which in turn increases VILI. In conjunction with hypoxemia, the these mechanisms may increase RV afterload, causing the RV to become dysfunctional. Injurious spontaneous breathing efforts and reverse triggering during mechanical ventilation may also cause harmful variations in regional trans-pulmonary pressures exacerbating VILI. Reversal of hypoxemia and mechanical unloading of the RV may allow for early extubation by mitigating these harmful effects of mechanical ventilation.
Our findings are incongruent with previously published data on the role for VV ECMO in the management of COVID-19 ARDS. Moreover, several landmark trials have failed to show a mortality benefit from early institution of VV ECMO when randomized against conventional ARDS treatment.27,28 In the management of severe COVID-19 ARDS, ECMO has generally shown poor outcomes. Early experience from Wuhan, China across three series reported a 90% mortality rate.1–3 Similarly, in Shanghai 50% of patients died on support.29 Data from North America have been more promising but highly variable. Osho et al. reported an 80% survival to decannulation, while Jacobs et al. a 33% survival to decannulation.30,31
Traditionally in ARDS, VV ECMO is used when impaired gas exchange occurs despite optimization of ventilatory support. ELSO guidelines recommend ECMO consideration in ARDS when the mortality risk is 50% or greater and is indicated when the mortality risk is 80% or greater.22 However, available data suggest that best outcomes are achieved with “early” ECMO intervention, typically within 1–2 days of disease onset.22,28 A 62% relative increase in mortality has been described in cases where delay in initiating ECMO support occurred (35 vs 57%).28 A deliberate effort on our part to cannulate early while lung compliance was still preserved and prior to the onset of multisystem organ failure may have contributed in part to the observed mortality benefit.
A unique and important feature of ECMO management in this study involves the surgical approach to cannulation. In the current era, there are three different cannulation options available to choose from. The first involves cannulation of both common femoral veins which has been the predominant method in previous efficacy trials.28 While many centers favor this approach, limitations include difficulties with extubating or ambulating patients. Innovation in cannula design has led to a second option which overcomes these challenges. The Avalon™ (Getinge, Gothenburg, SE) and Crescent™ (MC3 Cardiopulmonary, Dexter, MI) cannula allowing for right internal jugular vein insertion with inflow from the vena cava and return of oxygenated blood to the right atrium at the level of the tricuspid valve. In cases where gas exchange serves as the only limitation to recovering a patient, these approaches to cannulation are likely sufficient. However, COVID-19 is a multisystem disease and in severe cases adverse cardiac and thromboembolic phenomenon may occur.11–16,25,32 Therefore, we preferred to use a third cannulation strategy, one in which a large (29 or 31 French) dual lumen cannula was inserted percutaneously into the right internal jugular vein and passed into the main pulmonary artery, allowing for mechanical unloading of the RV in addition to gas exchange and oxygenation.
RV failure is often an underrecognized complication of critically ill patients, especially those with ARDS.20 In COVID-19, transmission risks to health care workers have tempered the enthusiasm for routine diagnostic maneuvers such as echocardiography or pulmonary artery catheters resulting in an under recognition of RV failure which has been described in up to 31% of hospitalized COVID-19 patients and shown to impact mortality.10 Thus we reasoned that isolated pulmonary support in the form of mechanical ventilation or conventional VV ECMO may be inadequate for these patients.10,18,19,33 Moreover, we did not experience any procedural complications. Bleeding complications from anticoagulation for the ECMO circuit were also no different from published experience.
Our report has important limitations. The small sample size significantly limits the power achievable and represents just the beginning of our institutions experience with treating this novel disease. The study design inherently renders this study susceptible to selection bias. Since the 21 patients in the control group were not referred for consideration of ECMO therapy, it is possible that these treatment decisions were due to differences in surgical candidacy rather than the management preferences of referring providers despite meeting institutional and ELSO guideline criteria for ECMO consideration. Differences in severity of illness from unmeasured confounders may also have influenced such decisions. There were differences in age and important comorbidities such as diabetes and hypertension favoring the RVAD/ECMO cohort. Although statistically insignificant, our small size precludes power to detect these differences accurately. These are known risk factors for worse outcomes in COVID-19 and may have confounded our results. Our approach did not involve the use of conventional ECMO cannulation strategies, limiting our ability to detect differences in outcomes between the two via direct comparison. However, published experiences with traditional VV ECMO report mortalities much higher than seen in this series, suggesting that an approach using concomitant RV support may be more effective.27–31 Regardless, the true benefit of concomitant RV support in COVID-19 ARDS remains unanswered as is the incidence of RV dysfunction given the lack of objective measures of RV function in this study. While our results paint a favorable picture for blanket RV support, they do so with limited evidence of RV dysfunction. Thus, the utility of routine RV support will need to be further investigated in future studies which include conventional VV ECMO for comparison with patients assigned according to RV function. Routine assessment of RV function for ECMO candidates using echocardiographic imaging prior to cannulation has now been incorporated into our institutional practices given our early experience in order to better identify patients with evidence RV dysfunction and make a more informed determination whether RVAD support is needed over conventional VV ECMO. Finally, the confounding effect of evolving COVID-19 treatments (remdesivir and convalescent plasma) may have also contributed to the improved outcomes in RVAD/ECMO patients. However, in a multivariate analysis these differences did not appear to bias the results. Despite these limitations, until a randomized trial can confirm or refute our results, consideration of early RVAD/ECMO should be given to COVID-19 patients at the point of intubation.
Conclusion
RVAD/ECMO may improve mortality in COVID-19 patients with severe ARDS who require mechanical ventilation or meet the criteria of intubation as used in our study. Referral to a multidisciplinary team should be considered at the time of intubation to properly weigh the risks and benefits of this approach for individual patients. As our experience grows, resources become less limited, and provider exposure and transmission are better understood, routine assessment of RV function with reliable surrogates of RV function will allow better determination of which patients may benefit for concomitant RV support and more clearly detail its utility in severe COVID-19 ARDS.
Supplementary Material
Acknowledgements
A special thank you to the Medical College of Wisconsin Clinical and Translational Sciences Institute, specifically David Zimmerman, for their support in facilitating a multidisciplinary approach to this study and other COVID-19 research initiatives.
Funding source statement:
This work was funded by using the following NIH Clinical and Translational Science Awards through the Medical College of Wisconsin Clinical and Translational Science Institute: 2UL1TR001436, 2TL1TR001437, 2KL2TR001438.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None of the listed authors have any conflicts of interest to disclose in the submission of this manuscript.
This manuscript does not describe utilization of any medical devices for unauthorized or unapproved indications. The TandemLife Protek Duo™ cannula is currently approved for usage as a veno-venous Extracorporeal Life Support (ELCS) cannula and recently received temporary expanded use authorization for novel coronavirus (i.e. SARS-CoV2, COVID-19) on April 6, 2020 by the U.S. Food and Drug Administration (FDA).
References
- 1.Wu C, Chen X, Cai Y, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Internal Medicine 2020;180(7):934–43 doi: 10.1001/jamainternmed.2020.0994 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8(5):475–81 doi: 10.1016/s2213-2600(20)30079-5 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395(10229):1054–62 doi: 10.1016/s0140-6736(20)30566-3 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meng L, Qiu H, Wan L, et al. Intubation and Ventilation amid the COVID-19 Outbreak: Wuhan’s Experience. Anesthesiology 2020;132(6):1317–32 doi: 10.1097/aln.0000000000003296 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. New England Journal of Medicine 2000;342(18):1301–08 doi: 10.1056/nejm200005043421801 published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 6.Thompson AE, Ranard BL, Wei Y, Jelic S. Prone Positioning in Awake, Nonintubated Patients With COVID-19 Hypoxemic Respiratory Failure. JAMA Internal Medicine 2020. doi: 10.1001/jamainternmed.2020.3030 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elharrar X, Trigui Y, Dols A-M, et al. Use of Prone Positioning in Nonintubated Patients With COVID-19 and Hypoxemic Acute Respiratory Failure. JAMA 2020;323(22):2336–38 doi: 10.1001/jama.2020.8255 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coppo A, Bellani G, Winterton D, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med 2020. doi: 10.1016/s2213-2600(20)30268-x published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368(23):2159–68 doi: 10.1056/NEJMoa1214103 published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 10.Argulian E, Sud K, Vogel B, et al. Right Ventricular Dilation in Hospitalized Patients with COVID-19 Infection. JACC Cardiovasc Imaging 2020. doi: 10.1016/j.jcmg.2020.05.010 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ullah W, Saeed R, Sarwar U, Patel R, Fischman DL. COVID-19 complicated by Acute Pulmonary Embolism and Right-Sided Heart Failure. JACC Case Rep 2020;2(9):1379–82 doi: 10.1016/j.jaccas.2020.04.008 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng JH, Liu YX, Yuan J, et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection 2020:1–5 doi: 10.1007/s15010-020-01424-5 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J 2020. doi: 10.1093/eurheartj/ehaa190 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tavazzi G, Pellegrini C, Maurelli M, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail 2020;22(5):911–15 doi: 10.1002/ejhf.1828 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020;5(7):1–6 doi: 10.1001/jamacardio.2020.1096 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi S, Qin M, Shen B, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol 2020;5(7):802–10 doi: 10.1001/jamacardio.2020.0950 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badu B, Cain MT, Durham LA 3rd, et al. A Dual-Lumen Percutaneous Cannula for Managing Refractory Right Ventricular Failure. Asaio j 2019. doi: 10.1097/mat.0000000000001099 published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 18.Boissier F, Katsahian S, Razazi K, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Medicine 2013;39(10):1725–33 doi: 10.1007/s00134-013-2941-9 published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 19.Bunge JJH, Caliskan K, Gommers D, Reis Miranda D. Right ventricular dysfunction during acute respiratory distress syndrome and veno-venous extracorporeal membrane oxygenation. J Thorac Dis 2018;10(Suppl 5):S674–s82 doi: 10.21037/jtd.2017.10.75 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zochios V, Parhar K, Tunnicliffe W, Roscoe A, Gao F. The Right Ventricle in ARDS. Chest 2017;152(1):181–93 doi: 10.1016/j.chest.2017.02.019 published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 21.Joyce DL. Mechanical Ventilation: An Unnecessary Evil. J Thorac Cardiovasc Surg 2020;Forthcoming [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ELSO. Extracorporeal Life Support Organization (ELSO) Guidelines for Adult Respiratory Failure. 2017
- 23.Bartlett RH, Ogino MT, Brodie D, et al. Initial ELSO Guidance Document: ECMO for COVID-19 Patients with Severe Cardiopulmonary Failure. Asaio j 2020;66(5):472–74 doi: 10.1097/mat.0000000000001173 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiology 2020;5(11):1265–73 doi: 10.1001/jamacardio.2020.3557 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020;135(23):2033–40 doi: 10.1182/blood.2020006000 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bilan N, Dastranji A, Ghalehgolab Behbahani A. Comparison of the spo2/fio2 ratio and the pao2/fio2 ratio in patients with acute lung injury or acute respiratory distress syndrome. J Cardiovasc Thorac Res 2015;7(1):28–31 doi: 10.15171/jcvtr.2014.06 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374(9698):1351–63 doi: 10.1016/s0140-6736(09)61069-2 published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 28.Combes A, Hajage D, Capellier G, et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med 2018;378(21):1965–75 doi: 10.1056/NEJMoa1800385 published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Guo Z, Li B, et al. Extracorporeal Membrane Oxygenation for Coronavirus Disease 2019 in Shanghai, China. Asaio j 2020;66(5):475–81 doi: 10.1097/mat.0000000000001172 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osho AA, Moonsamy P, Hibbert KA, et al. Veno-venous Extracorporeal Membrane Oxygenation for Respiratory Failure in COVID-19 Patients: Early Experience From a Major Academic Medical Center in North America. Ann Surg 2020. doi: 10.1097/sla.0000000000004084 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobs JP, Stammers AH, St Louis J, et al. Extracorporeal Membrane Oxygenation in the Treatment of Severe Pulmonary and Cardiac Compromise in Coronavirus Disease 2019: Experience with 32 Patients. Asaio j 2020;66(7):722–30 doi: 10.1097/mat.0000000000001185 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng P, Zhu H, Witteles RM, et al. Cardiovascular Risks in Patients with COVID-19: Potential Mechanisms and Areas of Uncertainty. Curr Cardiol Rep 2020;22(5):34 doi: 10.1007/s11886-020-01293-2 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ñamendys-Silva SA, Santos-Martínez LE, Pulido T, et al. Pulmonary hypertension due to acute respiratory distress syndrome. Braz J Med Biol Res 2014;47(10):904–10 doi: 10.1590/1414-431x20143316 published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


