Abstract
The renin-angiotensin system is of vital significance not only in the maintenance of blood pressure (BP) but also because of its role in the pathophysiology of different organ systems in the body. Of the two angiotensin II (Ang II) receptors, the Ang II type 1 receptor (AT1R) has been extensively studied for its role in mediating the classical functions of Ang II, including vasoconstriction, stimulation of renal tubular sodium reabsorption, hormonal secretion, cell proliferation, inflammation, and oxidative stress. The other receptor, Ang II type 2 receptor (AT2R), is abundantly expressed in both immune and non-immune cells in fetal tissue. However, its expression is increased under pathological conditions in adult tissues. The role of AT2R in counteracting AT1R function has been discussed in the last two decades. However, with the discovery of the non-peptide agonist C21, the significance of AT2R in various pathologies such as obesity, hypertension and kidney diseases have been examined. This review focuses on the most recent findings on the beneficial effects of AT2R by summarizing both gene knockout studies as well as pharmacological studies, specifically highlighting its importance in BP regulation, obesity/metabolism, organ protection, and relevance in the treatment of COVID-19.
Keywords: Angiotensin II type 2 receptor, pathophysiology, metabolism, organ protection, inflammation
Introduction
Angiotensin II (Ang II) type-2 receptor (AT2R) is a lesser-known component of the renin-angiotensin system (RAS). Although AT2R was discovered and cloned in the early 1990s,1 its role in pathophysiology continues to be unraveled, and the significance associated with these functions has been ever-evolving. AT2R is activated by the peptides Ang II/-III/-(1–7) and potentially by alamandine that acts as the endogenous agonist.2–7 Typically, AT2R is considered as a functional antagonist of the well-known and predominantly (relative to AT2R) expressed Ang II type-1 receptor (AT1R),8 which is involved in renal and cardiovascular diseases, hypertension, and tissue injury.9 With the availability of preferential agonists and antagonists, particularly in the recent past, researchers have been able to make strides in understanding the underlying AT2R biology in health and diseases at the molecular, cellular, and whole-organism levels. The results of the pharmacological studies are convincing and suggest that selective activation of AT2R may mediate pro-natriuretic10 and anti-inflammatory11–13 effects and improves insulin resistance and metabolism,14,15 which is associated with anti-adiposity/obesity, anti-hypertensive, and reno- and cardioprotective outcomes. Moreover, AT2R has been recently crystallized,16 which extends our insight into receptor chemistry. This is a critical step in developing more selective and potent drugs to target AT2R to exert desired effects. The purpose of this review is to summarize the recent discoveries covering various aspects of AT2R structure, signaling, and biology, from molecules to whole animals. Since AT2R is a part of the protective arm of the RAS, namely Ang-(1–7)/MasR and functional similarities and interdependence between AT2R and MasR exist,17 the COVID-19 relevance of the pharmacological activation of AT2R is also briefly discussed.
AT2R Chemistry and Signaling
AT2R is a member of the G protein-coupled receptor (GPCR) family. However, its unique chemistry and cell signaling are puzzling and yet to be fully understood. AT2R is coupled to stimulatory (Gαs) or inhibitory (Gαi/o) proteins or G protein-independent pathways that include AT2R binding protein.18–20 The activation of G protein is also associated with the activation of Tyr phosphatase (SH2 domain-containing phosphatase-1 (SHP-1),21 Ser/Thr phosphatases (mitogen-activated protein kinase phosphatase-1 (MKP-1)22 and protein phosphatase 2A (PP2A).23 Most of the cellular and biological effects such as vasodilatation and natriuresis associated with AT2R activation are mediated via nitric oxide (NO)/cyclic guanosine monophosphate (cGMP) activation by phosphatase-mediated activation of NO synthase (NOS). A recent study shows that these Ser/Thr and Tyr phosphatases serve as the regulating enzymes for AT2R to dephosphorylate (Tyr657, Thr495) and phosphorylate (Ser1177) eNOS, thereby stimulating its activity in human aortic endothelial cells.23 The AT2R-mediated activation of eNOS also involves activation of the PI3K/Akt pathway.23
We have observed opposing effects in response to AT2R activation.24 For instance, the AT2R agonist produced a natriuretic and anti-inflammatory response in obese Zucker rats (OZR) but no natriuresis or pro-inflammatory response in lean animals.24 A difference was observed in the signaling mechanism. AT2R activation inhibited cAMP accumulation in the proximal tubules of both lean and OZR, whereas NO/cGMP stimulation was observed in the proximal tubules of OZR.25 The reasons for such a discrepancy in signaling and function are unknown. However, according to a recently reported crystal structure of AT2R, the AT2R’s helix VIII may be responsible for such opposing effects. The helix VIII renders AT2R in an active state and blocks the recruitment of the G proteins and β-arrestin.16 This blocking of the G proteins and β-arrestin and other features such as fewer serine residues on the third intracellular loop of the receptor may additionally explain why AT2R, unlike typical GPCRs such as β-adrenoceptor, is not desensitized, internalized, and degraded in response to chronic agonist exposure.26,27 Such chemical features that render AT2Rs resistant to desensitization/internalization make AT2R a potential target of drug discovery, particularly in terms of pharmacological activation.
Blood Pressure Regulation
AT2R has been studied as a target to examine its role in renovascular function, particularly in BP regulation. AT2R is widely expressed in the vasculature (particularly endothelial cells),28,29 heart (myocytes),30 various kidney regions,31–34 brain,35,36 and immune/blood cells.37 Moreover, AT2R expression and functions are increased under pathological conditions in the vasculature,38 diabetes (in both humans and animals),39,40 heart failure,41,42 and diabetic/obese kidney (vide infra).43,44 Early gene knockout (KO) studies suggest that AT2R may (a modest) or may not decrease BP in rodents.45,46 Pharmacological studies suggest that AT2R activation alone may not be sufficient to lower BP. However, it enhanced the BP-lowering effects of AT1R antagonist in the spontaneously hypertensive rat model (SHR)47. Conversely, blockade of the AT2R reversed the blood-pressure-lowering effects of the AT1R antagonist in normotensive animals.48 However, these pharmacological studies were acute and short-term. A recent article49 reviewed various mechanisms of AT2R-mediated blood pressure regulation, including peripheral and central mechanisms. In this article, we briefly discuss the peripheral mechanisms of blood pressure regulation, particularly long-term BP control. Orally active AT2R preferential agonist compound-21 (C21) helped examine the role of AT2R in long-term blood pressure regulation. The C21 treatment administered via subcutaneously implanted osmotic pumps over a period of 2-weeks completely prevented salt-induced hypertension in OZR50 and Ang II-induced hypertension in Sprague-Dawley (SD) rats.51 In both the studies, prevention of the increase in BP by C21 was associated with an increase in the urinary sodium excretion, preventing salt and water retention and the buildup of body fluid. The Ang II infusion hypertension model provides more direct evidence of the role of kidney AT2R in BP regulation as C21 was specifically delivered to the kidney. In our studies,52 the two RAS axes, namely the classical Ang II/AT1R and the protective ACE2/Ang-(1–7)/MasR, were evaluated in the kidneys of OZR who were administered a high-salt diet (HSD) and AT2R agonist C21. The BP increase in HSD-fed OZR was associated with a remarkable increase in the Ang II levels (pro-hypertensive) and a decrease in the ACE2/Ang-(1–7) levels (anti-hypertensive) in the kidney.52 C21 treatment reversed this trend or normalized these changes, inducing a further reduction in AT1R expression, thus leaving open questions whether the BP decrease was a direct consequence of C21 treatment or secondary to the changes in the Ang II/AT1R vs ACE2/Ang-(1–7) balance or a combination of both. Nonetheless, studies are required to further evaluate the anti-hypertensive mechanisms of AT2R.
Natriuresis:
Renal function in maintaining sodium and fluid homeostasis plays a critical role in long-term BP regulation. Numerous studies have demonstrated that acute as well as chronic AT2R activation by the AT2R agonists C21 and CGP42112A promotes natriuresis under normal and pathological conditions.10,51,53–56 AT2R-mediated natriuresis seems to originate at the tubular level as the renal hemodynamics involving the GFR and renal blood flow are not affected by AT2R agonists.53,56 The highest density of AT2R is present in the proximal tubule,57 a site of major sodium and water reabsorption. However, the receptor is also expressed in other parts of the nephron, including the distal tubule, a site of fine-tuning of sodium reabsorption. An increase in the fractional sodium and lithium excretion in the presence and absence of distal tubule sodium transport (Na-Cl co-transporter (NCC) and epithelial sodium channel (ENaC)) inhibitors indicated that proximal tubule may be a primary site of AT2R action, leading to natriuresis.53 At the cellular and molecular level, proximal tubule AT2R activation inhibited the activities of two major sodium transporters, namely NaK-ATPase (sodium pump) and NaH-exchanger via NO/cGMP pathway,25,54,58 suggesting a potential mechanism of inhibition of tubular sodium transport, resulting in natriuresis. A study in the distal tubules isolated from K-channel KO and wild-type (WT) mice implicated AT2R in the inhibition of NCC and K-channel (Kir4.1/5.1), leading to an increase in Na and potassium excretion.59 All these studies highlight the significant role of AT2Rs and the potential mechanism involved in maintaining sodium hemostasis and long-term BP regulation. Recent discoveries of selective peptidic AT2R ligands β-Pro7-Ang III, β-Tyr4-Ang II, and β-Ile5-Ang II showed their in vitro vasorelaxant and in vivo depressor effects in conscious SHR. These observations have further strengthened the anti-hypertensive role of the AT2R; however, the substituted Ang II peptides required the presence of an AT1R blocker to obtain a decrease in mean arterial pressure (MAP).60–62 The Ang II-derived cyclic ligands of AT2R (LP2)63,64 and MasR (cAng-(1–7))64–66 (vide supra) are more stable and resistant to peptidases due to the presence of thioester linkage and are studied for their vasorelaxant and organ protective effects.67
Sex-specific differences and pregnancy:
Sex-specific differences in various RAS components in terms of their expression and function have been documented. Numerous studies suggest a positive feedback regulation between levels of estrogen, its receptors, and AT2R expression.68–72 Female rodent kidneys expressed higher AT2R,73 which was decreased in ovariectomized mice. Estrogen supplementation restored the AT2R expression to normal,68,69 likely via the activation of Erα, as demonstrated by the ERα-KO mice model and AT2R antagonist treatment.74,75 In contrast, ERβ (not ERα) is upregulated in pregnancy and binds with and transactivates ER-responsive elements in the AT2R promoter in the uterine arteries of pregnant mice; this effect was mediated by ERα in non-pregnant mice.72 This finding suggests a shift towards ERβ/AT2R-mediated BP regulation in pregnancy. In contrast, AT2R activation increased 17-β-estradiol levels, as shown in ovary cells in vitro71 and HFD-fed mice treated with AT2R agonist C21.70 The enhanced AT2R expression may be responsible for higher BP-lowering effects under the conditions of preeclampsia.76 The Denton group has particularly reported sex-specific differences in the role of AT2R in BP-regulating mechanisms such as pressure-natriuresis and tubuloglomerular feedback.77–81 In Ang II-induced hypertension developed in WT and AT2R-KO mice, AT2R-mediated protection against BP increase was only found in females and not in males.77 The study showed that WT (28 mmHg) and AT2R-KO (26 mmHg) males showed a similar increase in MAP in response to Ang II infusion. However, WT females demonstrated only a 12 mmHg rise in BP compared to 26 mmHg in AT2R-KO females. In another study,79 rise in MAP was lower (9 mmHg) in females than that in males. However, this difference was lost in AT2R-KO mice, demonstrating more potent effects of AT2R in protection against BP in females. Moreover, chronic pressure-natriuresis in female WT mice was shifted leftward compared to AT2R-KO mice. However, this shift was lost with age; female aging mice demonstrated lower renal AT2R expression, indicating an association between reduced AT2R expression and loss in pressure-natriuresis shift.79 Ovariectomy of aged mice did not result in a further difference in AT2R expression or pressure-natriuresis in females. This study highlights the fact that AT2R expression and function are dependent on changes in estrogen levels, which are reduced with age. However, it would be interesting to treat aged mice with an AT2R agonist or supplement with estrogen to examine whether positive feedback comes into play between AT2R and estrogen and improves pressure-natriuresis in these animals.
Augmented AT2R expression during pregnancy is responsible for an increase in the uterine blood flow induced by elevated Ang II levels. Estradiol exposure leads to the upregulation of AT2R expression, which is inhibited by the ER-α antagonist.74 This study suggests that AT2R expression and function are increased during pregnancy and may play a role in attenuating BP-related complications. A similar conclusion was revealed by another study in an AT1a knockout mouse model.82 This study showed mid-gestation systolic BP decline, which was abolished by treatment with the AT2R antagonist PD123319, suggesting that mid-gestation decline in BP is an outcome of AT2R activation mediated by Ang II.
Obesity, Metabolism, and Insulin Sensitivity
Obesity is a major cause of metabolic dysfunction, leading to hyperinsulinemia, hyperglycemia (diabetes), and dyslipidemia, which show inflammation and cell and organ injury, thereby affecting organ function. Other studies have revealed that AT2R expression in adipose and non-adipose tissues such as kidney and blood vessels is increased in obesity and diabetes 10,70,83–85 indicating an enhanced role in these pathologies. Hyperglycemia is one of the factors that lead to an increase in AT2R expression.86 Therefore, understanding the role of AT2R in obesity, metabolism, insulin sensitivity, and renal-cardiovascular function associated with these pathologies is crucial and currently being studied.
Insulin resistance:
Several studies have implicated AT2R activation in reducing adiposity/obesity and improving metabolism, resulting in improvement in insulin sensitivity, glucose disposal, and blood lipid profile.14,79,87–90 Insulin sensitivity/resistance and pancreatic function in relation to the role of AT2R have been a major focus in both normal and diabetic conditions. Chronic and acute blockade of AT2R with the antagonist PD123319 reduced glucose uptake in the muscles91 and insulin receptor signaling in terms of PI3K/Akt activation in the liver and adipose tissue,92 suggesting a physiological role of AT2R. The physiological role of AT2R was also confirmed by a study in AT2R-KO mice, which showed that STZ-induced glycemia was higher in AT2R-KO mice coupled with lower pancreatic insulin levels.87
Studies examining the pharmacological activation of AT2R with C21 have been particularly helpful to test the therapeutic potential of AT2R in improving insulin resistance and metabolism. A recent study in normal mice suggested that C21 treatment for 12 weeks reduced adipocyte size and enhanced insulin receptor signaling in terms of Akt activity in the liver and adipose tissues.88 Several studies performed in animal models of diabetes and insulin resistance indicated the relevance of AT2R in these pathologies. For instance, C21 administration promoted adipocyte differentiation and improved adipose insulin sensitivity in high-fat/high-fructose diet-induced insulin resistance in rats.15 These changes were associated with PI3K/Akt-mediated increase in PPARƴ expression,15 a transcription factor known to improve insulin sensitivity by altering the expression of various genes associated with insulin receptor signaling. Another study performed in type 2 diabetic mice shows that AT2R activation with C21 enhanced PPARƴ activation and ameliorated insulin resistance, partly due to adipocyte differentiation and protection of pancreatic β-cells.93 Previously, the role of AT2R in PPARƴ activation has been shown under in vitro conditions in PC12W cells.94 Anti-oxidative stress, anti-apoptosis, improved microvascular perfusion, and efficient delivery of insulin to tissues, such as muscles, and increase in adiponectin demonstrated by various studies may also be linked to AT2R-mediated improvement in insulin resistance, glycemia, and pancreatic function.88,89,95–97 AT2R activation also reduced the levels of inflammatory cytokines, such as TNF-α, in these aforementioned studies. Since inflammation is a causative factor for insulin resistance,98,99 anti-inflammatory activity of AT2R is likely another mechanism contributing to an improvement in insulin sensitivity and metabolism.
Obesity:
As insulin resistance is associated with obesity, the role of AT2R in obesity and adiposity has been documented in animals treated with AT2R agonist and AT2R-KO mice.100–102 Overall AT2R-KO models have shown inconclusive results. For example, deletion of AT2R-KO protected against HFD-induced obesity and insulin resistance, which involves altered lipogenic and lipolytic enzymes, increased total energy expenditure, whole-body lipid oxidation, and decreased food intake.102–104 In contrast, AT2R deletion in ApoE-null mice did not impact weight gain, per se, but increased adipose tissue mass, decreased the number of adipocytes and insulin-receptor substrate-1 levels, increased plasma cholesterol levels and free fatty acids upon intake of high cholesterol diet and worsened oxidative stress and atherosclerotic lesions.100 Our study in male and female AT2R-KO mice showed that males had higher calorie intake but less weight gain and gonadal adiposity as compared to females, suggesting decreased metabolic capacity in females.101 Nonetheless, these genetic studies show the involvement of AT2R in both preadipocytes differentiation and mature adipocyte metabolism and glucose utilization.102 However, recent pharmacological studies have explicitly suggested that AT2R activation reduces adiposity and obesity. For example, we have reported that C21 administration in male mice attenuated adiposity and improved lipid metabolism, likely by decreasing lipid synthesis and enhancing lipid degradation.14 Moreover, in vitro studies in freshly isolated adipocytes from mice suggested that AT2R activation inhibits fatty acid uptake via the NO/cGMP pathway.14 However, as we know that AT2R agonist treatment lowers FFAs and triglyceride levels in the plasma,14 the fate of FFAs, which are not internalized by the adipose tissue and are not circulating needs to be investigated.
Sex-specific differences in obesity:
We have reported that AT2 null mice were protected against HFD-induced weight gain for 16 weeks. However, this observation was only recorded in males; females, in contrast, gained more weight than WT, suggesting a sex-specific difference.101 The anti-obesity role of AT2R was also confirmed in our pharmacological studies.70 We have reported that HFD-induced (16-weeks) weight gain and adiposity in female mice were attenuated by treatment with systemic AT2R agonist C21, which was associated with reduced adipocyte size, plasma insulin, and plasma fatty acids, and improved glucose tolerance.70 Similar improvements in metabolic changes were observed after treatment of HFD-fed ovary-intact as well as ovariectomized mice with C21 for two weeks,70 suggesting an estrogen-independent role of AT2R. However, estrogen supplementation of ovariectomized mice treated with the AT2R agonist C21 caused a remarkable improvement in the metabolic indices. Since the estrogen dose administered to these mice was high, a definite conclusion regarding the synergism between AT2R activation and estrogen could not be established in this study. Consistent with KO and pharmacological studies in females, female diabetic db/db mice treated with C21 for one month demonstrated improvements in the glucose/pyruvate tolerance and fatty liver via the NO pathway.97
Overall, we conclude that while AT2R-KO studies are inconclusive in males but show a sex-specific difference. Pharmacological activation of AT2R improves metabolic dysfunction in various animal models irrespective of sex. However, the cellular signaling pathway responsible for the various beneficial effects of AT2R activation remains to be elucidated.
Organ protection
Cardio-vascular protection
Hypertension, myocardial ischemia, and metabolic dysfunction, including obesity and diabetes, are known risk factors for cardiac hypertrophy, leading to cardiac remodeling and heart disease. Numerous studies have explored the protective role of AT2R in cardiac hypertrophy/remodeling using pharmacological and genetic approaches in various animal models of myocardial infarction (MI), obesity, hypertension, and HSD feeding.105–110
Pharmacological studies: The first study to analyze the effect of direct stimulation of AT2R by C21 on cardiac function and molecular events was performed in Wistar rats subjected to left coronary artery ligation for the induction of MI.108 The results revealed that C21 treatment (for 7 days) reduced the scar size and improved systolic and diastolic ventricular functions. These changes were associated with reduced inflammation and apoptosis in C21 treated groups (particularly, reduction in the expressions of Fas ligand and caspase-3 in the peri-infarct area), and components of the mechanism associated with the regulation of cell survival (a rescue in MI-induced decrease in phosphorylation of p44/42 and p38 MAPK) in C21 treated animals. In another similar study, long-term (6 weeks) treatment of rats with MI showed improvements in arterial stiffness parameters and reduced collagen content in peri-infarct myocardium associated with improved heart function in terms of ejection fraction and fractional shortening. These results suggest a long-term beneficial effect of AT2R activation in preventing cardiac remodeling.111 Similar cardiac remodeling protective effects were reported in stroke-prone SHR and high-salt diet-fed animals.106,110 C21 treatment administered for six weeks showed a reduction in myocardial fibrosis, as evidenced by a decrease in myocardial interstitial collagen content and the expression of cardiac hypertrophy-related genes, namely, myosin heavy chain β and α-skeletal muscle actin (α-SMA). In addition to cardiac protection, vascular injury, indicated by a reduction in vascular stiffness, collagen content, and fibronectin, was reduced by C21 treatment. In addition, C21 prevented the development of cardiac hypertrophy in HSD-fed animals.106
Genetic studies:
Genetic studies also support the cardioprotective effects associated with AT2R. For instance, lack of AT2R during acute MI exacerbated heart failure and decreased the survival of mice compared to that in wild type within 7 days post-MI.105 Lack of AT2R was associated with increased expression of myocardial inflammatory prostaglandins and reduced collagen deposition, which chronically resulted in cardiac rupture.107 In contrast, AT2R overexpressing transgenic mice showed improved baseline LV systolic function and preservation of systolic function after MI compared to WT mice.112 This conclusion is further supported by another study showing that the moderate overexpression of AT2R in the myocardium either before or after induction of MI protected against ischemic injury by attenuating the decrease in fractional shortening and increase in LV end-diastolic pressure.109 In contrast, chronic expression of AT2R reduced myocardial contractility113 and high ventricular-specific expression of AT2R caused heart failure and dilated cardiomyopathy compared to that observed with low levels of AT2R expression and that in WT mice.114 Hence, it seems that the level of AT2R overexpression may be important to mediate protective vs opposing effects on cardiac function after MI.
Role of immune and stem cells:
Chronic inflammation is an important biological process that triggers tissue injury, resulting in tissue/organ remodeling. AT2R is expressed in immune cells, particularly T lymphocytes. In a rat model of MI, the infarcted myocardium was infiltrated with CD4+AT2R+ T cells and CD8+AT2R+ T cells, which were characterized by FoxP3 expression, upregulated interleukin-10 (IL-10) levels and downregulated interleukin-2 and interferon-gamma expression.115,116 These T cells have been implicated in the improvements in cardiac function post-MI by reducing ischemic injury, thus offering a promising approach for regenerative cardiac therapy via myocardial transplantation of these cells. A study focusing on mesenteric arteries reported that AT2R activation resulted in a release of interleukin-17 by memory T cells surrounding the high-flow arteries, suggesting that AT2R could play a possible therapeutic role in ischemic disorders by allowing flow-mediated outward remodeling of resistance arteries that are involved in revascularization.117 The experimental proof of this effect on the outward remodeling of coronary vessels is still lacking. Evidence also indicates the role of stem cells and their modulation by AT2Rs in cardiac repair. The preconditioning of bone marrow mononuclear cells (BMMNCs) by AT2R stimulation either by agonist CGP42112A or a combination of the Ang II and AT1R antagonist valsartan increased the activation of ERK and eNOS expression, thereby enhancing the generation of NO. This increase in NO enhanced cardiomyocyte function in vitro as well as improved survival of transplanted heart tissue in the ischemic region after intramyocardial transplantation of preconditioned BMMNCs. This suggests a new therapeutic strategy for the efficient utilization of stem cells in cardiac repair.118 Furthermore, AT2R stimulation of CD117+ stem cells showed improvements of cardiac cell morphology in vitro, but it did not translate to the beneficial effect of CD117+ stem cells.119
In contrast to the antihypertrophic effect of AT2R in cardioprotection, some studies have shown that AT2R may be responsible for or may not have any effect on cardiac hypertrophy and fibrosis. AT2R agonism mediated by 0.3 mg/kg/day C21 and receptor upregulation in MI mouse models was not beneficial against LV remodeling, but rather increased the LV end-systolic and diastolic volume, suggesting an adverse effect of direct AT2R stimulation post-MI.120 AT2R deletion prevented HFD-induced hypertrophy in mice. This observation was further supported by an in vitro study showing that blocking AT2R with the antagonist PD123319 prevented leptin-induced cardiomyocyte hypertrophy.121 This study is consistent with the finding that AT2R deletion protects against obesity.
Overall, only a few studies indicate no effect or adverse effects related to AT2R during MI or cardiac tissue remodeling. However, an overwhelming number of studies, both genetic and pharmacological, suggest that AT2R exerts cardiovascular protective effects in various animal models. However, an explanation of the opposing role of AT2R in cardiovascular protection would be helpful to ascertain what experimental conditions, if any, are responsible for the diverse effects.
Renoprotection
Obesity, hypertension, and diabetes are independent major risk factors for chronic kidney injury/disease.122 AT2R activation reduces adiposity/obesity, improves insulin resistance and glycemia, and lowers BP, as discussed in the previous sections of this review. Recent evidence suggests that AT2R activation protects the kidneys against injury in various animal models. Our group has shown that treatment of HSD-fed OZR with C21 improved the kidney conditions, including glomerulosclerosis, visceral epithelial cell hypertrophy, tubular atrophy, and interstitial fibrosis, along with a reduction in protein-to-creatinine ratio, as well as fractional excretion of protein and albumin in these animals. In addition to improvements in proteinuria, a decline in GFR, a hallmark of CKD, was prevented by C21 treatment.50 Another study performed in younger OZR (7 weeks old) without HSD administration showed that AT2R protective effects were independent of BP changes.123 In this study, C21 protected the kidney from early renal damage caused by obesity-linked renal pathology associated with enhanced mesangial matrix expansion (MME) in these animals. In addition, AT2R-KO mice exhibited increased MME and albuminuria, which worsened upon HFD feeding for 16 weeks,124 suggesting a protective role of AT2R against HFD-induced kidney injury.
In models of type 1 diabetes, AT2R plays a protective role under various experimental conditions. For instance, C21 treatment of ApoE−/− mice with type 1 diabetic nephropathy demonstrated protection effects by decreasing the mesangial area, glomerular injury, α-SMA expression, and collagen content in the ECM.125 C21 treatment was effective in lowering albuminuria in mouse models of diabetic nephropathy at 15 weeks of age; this effect was lost around 20 weeks of age. However, C21 in combination with losartan was more efficacious in attenuating tubulointerstitial fibrosis and albuminuria during the progression of diabetic nephropathy.126 In addition to pharmacological studies, genetic studies also support the protective effects of AT2R in diabetic nephropathy. For example, transgenic overexpression of mitochondrial AT2R in the renal tubular cells of streptozotocin-induced diabetic rats resulted in renoprotection in the early stages of diabetes by inhibiting the decrease in mitochondrial bioenergetics efficiency, increase in superoxide production, and increased cell proliferation.127 In contrast, mice lacking AT2R showed features of early diabetic nephropathy, including renal hypertrophy, tubular apoptosis, progressive ECM protein accumulation, and decreased GFR, indicating impaired kidney structure and function.128
Renal anti-inflammation effects:
Similar to the anti-inflammatory activity of AT2Rs in cardiac hypertrophy, several reports have shown the anti-inflammatory role of AT2R in various kidney injury models, including obesity and diabetes. The activation of AT2R by C21 treatment attenuated albuminuria and early inflammation by decreasing renal TNF-α and IL-6 levels and increasing NO and cGMP levels in renovascular hypertension and diabetes, independent of blood pressure.129,130 In our two studies, treatment of OZR with the AT2R agonist C21 or CGP42112A for two weeks reduced the levels of pro-inflammatory cytokines TNF-α, IL-6, and chemokine MCP-1, but increased the levels of anti-inflammatory cytokine IL-10 in the kidney and plasma along with a reduction in oxidative stress (heme oxygenase-1, gp-91phox) markers.24,123 These changes in cytokine levels were associated with a reduction in the renal infiltration of CD68+ cells, which may also differentiate into macrophages.123 Further in vitro studies performed in human kidney proximal tubule epithelial cells and monocytes (THP-1) confirmed that C21 decreased the LPS-induced increase in IL-6 and TNF-α; however, it increased the levels of IL-10. Furthermore, the studies revealed that the NO/IL-10 pathway may mediate the anti-inflammatory effect of AT2R activation on the production of TNF-α and IL-6. In support of this, neutralizing IL-10 antibody or the NO synthase inhibitor L-arginine methyl ester abrogated the C21-mediated reduction in TNF-α and IL-6 production.13 However, the involvement of the NO/IL-10 pathway in AT2-mediated anti-inflammatory effects in animals is still unknown. The mechanism might be more complex as AT2R is expressed in various cell types, including immune and non-immune cells, which are involved in an interplay to mediate a net anti-inflammatory response.
In light of the THP-1 studies,13 we performed an acute study in an LPS-induced acute kidney injury (AKI) mouse model.131 We have observed that prior, not concomitant, treatment with C21 prevented the LPS-induced renal infiltration of CD11b+ immune cells and ameliorated the increased levels of cytokines IL-6 and MCP-1, while preserving IL-10 levels, indicating that AT2R exerts prophylactic effects in preventing renal injury and dysfunction. The changes in cytokine profile occurred in parallel with the changes in kidney structure and function. Hence, C21 treatment prevented LPS-induced vacuolization and reduction in BUN and albuminuria.131
Overall, it is clear that the aforementioned pharmacological and genetic studies suggest that AT2R plays a renoprotective role under conditions of obesity, salt-induced injury, diabetic injury, and 5/6 kidney model of CKD 132,133 as well as in a mouse model of AKI.131 These renoprotective effects are associated with the anti-inflammatory activity of AT2R activation. However, significant research is required to understand the initial steps of immune cell infiltration and/or repair mechanisms associated with AT2R activation in immune and kidney cells.
AT2R-MasR Interaction and SARS-CoV-2
As mentioned earlier, AT2R is a GPCR with low expression. However, its pharmacological activation significantly affects various pathophysiological processes, some of which are reviewed in this article. Recently, we published a review37 showing that AT2R interacts with MasR and other GPCRs, namely bradykinin BK1, dopamine D1, and angiotensin AT1 receptors, and proposed that such an interaction could constitute a potential mechanism by which AT2 amplifies its cellular signaling and biological responses. AT2 and MasR are components of the protective arm of RAS and demonstrate similar beneficial effects in various renal-cardiovascular conditions, such as vasodilatation, natriuresis, and lowering of BP.37 Our group6,37and others134 have shown that AT2R and MasR are colocalized, dimerized, and functionally interdependent. The AT2R antagonist abolishes the Ang-(1–7) activation of MasR and vice versa. This AT2R-MasR functional interdependence is relevant to SARS-CoV-2 virus infection, which has been recently reviewed thoroughly.135 The pharmacological activation of AT2R via interaction with MasR may compensate for Ang-(1–7) deficiency induced by the inhibition of ACE2 mediated by binding to SARS-CoV-2. Currently, it is known that ACE2 is a receptor for SARS-CoV-2 for its entry into the cell, leading to an attenuation of ACE2 activity.136,137
ACE2 is a major enzyme that converts Ang II into Ang-(1–7), and the inhibition/attenuation of ACE2 leads to an increase in the Ang II accumulation and reduction in Ang-(1–7), which is an endogenous ligand for MasR. This change shifts the balance between deleterious effects associated with Ang II/AT1R and beneficial effects of Ang-(1–7)/MasR.138 We have reported that chronic treatment of OZR with AT2R agonist C21 caused changes in various components of the kidney RAS, such as a reduction in AT1R expression and increase in MasR expression and ACE2 activity, with a net decrease in Ang II levels and increase in Ang-(1–7) levels in the kidney.52 Moreover, despite the reduced production of Ang-(1–7) during SARS-CoV-2-mediated downregulation of ACE2, the AT2R may activate MasR and sustain MasR signaling without the need of Ang-(1–7) owing to its physical dimerization and functional interdependence with MasR. AT2R also exerts beneficial effects by reducing Ang II/AT1R signaling. Based on this rationale, the AT2R agonist C21 has been used in double-blind, randomized, placebo-controlled phase 2 clinical trials conducted in COVID-19 patients. The outcome of this study demonstrated that oral doses of 100 mg of C21 administered twice daily for 7 days reduced the requirement of supplemental oxygen by 90% on day 14 after the start of treatment. One patient in the C21 group required mechanical ventilation as compared to four in the placebo group and one death was observed in the C21 group compared to three deaths in the placebo group.139 This may indicate a scope of C21 in the potential treatment strategies for COVID-19 patients.
Conclusion
Sufficient reports suggest that the pharmacological activation of AT2R shows significant therapeutic benefits in natriuresis, vasorelaxation, insulin sensitivity, and inflammation, which results in anti-hypertensive, anti-obesity, and organ protective effects in various pre-clinical models. Moreover, the modulation of immune cells and stem cells by AT2R shows further promise for tissue repair and regeneration. The long-term and sex-specific responses to direct AT2R stimulation and whether estrogen therapy is amenable to enhance AT2R-mediated benefits in the postmenopausal period require further examination to reveal AT2R’s therapeutic potential. Since AT2R signaling is still understudied, it is important to explore how various pathways linked to AT2R may lead to various protective effects and, in some cases, opposite effects. The crystal structure of AT2R and its conformation in the presence of various agonists and antagonists could provide a lead to harness the full potential of AT2R activation in various pathologies. The beneficial effects of the AT2R agonist C21 in COVID-19 patients provide a direct rationale for further exploration of the therapeutic effects of receptor agonism.
Fig. 1: AT2R is advantageous in metabolism, organ protection, and inflammation.
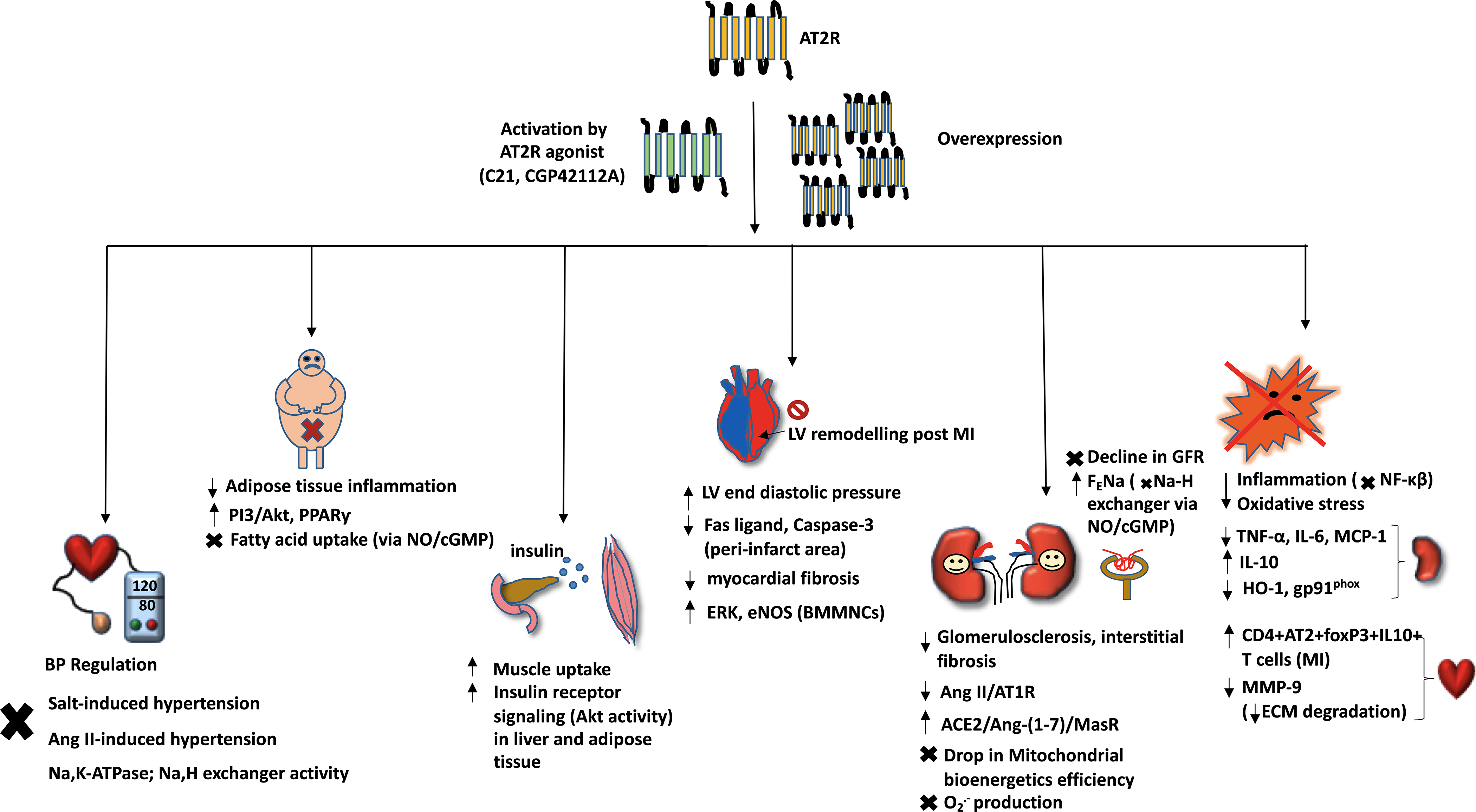
This figure highlights the main signaling molecules involved in AT2R-mediated beneficial effects in blood pressure regulation, obesity and metabolism, cardiovascular protection, renoprotection, and inflammation accompanying organ remodeling. The arrows pointing upwards (↑) indicate an increase or upregulation, the arrows pointing downward (↓) indicate decrease or downregulation, and the cross (X) symbol indicates inhibition.
Acknowledgments
All authors prepared, edited, and proofread the manuscript and approved the final version of the manuscript.
Sources of Funding
National Institutes of Health R01 DK117495 and R01 DK 061578
Footnotes
Disclosures
None.
References:
- 1.Mukoyama M, Nakajima M, Horiuchi M, Sasamura H, Pratt RE, Dzau VJ. Expression cloning of type 2 angiotensin II receptor reveals a unique class of seven-transmembrane receptors. J Biol Chem. 1993; 268:24539–24542 [PubMed] [Google Scholar]
- 2.Carey RM. Blood pressure and the renal actions of AT2 receptors. Curr Hypertens Rep. 2017; 19:21. doi: 10.1007/s11906-017-0720-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kemp BA, Bell JF, Rottkamp DM, Howell NL, Shao W, Navar LG, Padia SH, Carey RM. Intrarenal angiotensin III is the predominant agonist for proximal tubule angiotensin type 2 receptors. Hypertension. 2012; 60:387–395. doi: 10.1161/HYPERTENSIONAHA.112.191403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Padia SH, Howell NL, Siragy HM, Carey RM. Renal angiotensin type 2 receptors mediate natriuresis via angiotensin III in the angiotensin II type 1 receptor-blocked rat. Hypertension. 2006; 47:537–544. doi: 10.1161/HYPERTENSIONAHA.111.184788 [DOI] [PubMed] [Google Scholar]
- 5.Vasile S, Hallberg A, Sallander J, Hallberg M, Aqvist J, Gutierrez-de-Teran H. Evolution of angiotensin peptides and peptidomimetics as angiotensin II receptor type 2 (AT2) receptor agonists. Biomolecules. 2020; 10. doi: 10.3390/biom10040649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel SN, Ali Q, Samuel P, Steckelings UM, Hussain T. Angiotensin II type 2 receptor and receptor Mas are colocalized and functionally interdependent in obese Zucker rat kidney. Hypertension. 2017; 70:831–838. doi: 10.1161/HYPERTENSIONAHA.117.09679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hrenak J, Paulis L, Simko F. Angiotensin A/Alamandine/MrgD axis: Another clue to understanding cardiovascular pathophysiology. Int J Mol Sci. 2016; 17:1098. doi: 10.3390/ijms17071098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.AbdAlla S, Lother H, Abdel-tawab AM, Quitterer U. The angiotensin II AT2 receptor is an AT1 receptor antagonist. J Biol Chem. 2001; 276:39721–39726. doi: 10.1074/jbc.M105253200 [DOI] [PubMed] [Google Scholar]
- 9.Kaschina E, Unger T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press. 2003; 12:70–88. doi: 10.1080/08037050310001057 [DOI] [PubMed] [Google Scholar]
- 10.Hakam AC, Hussain T. Renal angiotensin II type-2 receptors are upregulated and mediate the candesartan-induced natriuresis/diuresis in obese Zucker rats. Hypertension. 2005; 45:270–275. doi: 10.1161/01.HYP.0000151622.47814.6f [DOI] [PubMed] [Google Scholar]
- 11.Menk M, Graw JA, von Haefen C, Sifringer M, Schwaiberger D, Unger T, Steckelings U, Spies CD. Stimulation of the angiotensin II AT2 receptor is anti-inflammatory in human lipopolysaccharide-activated monocytic cells. Inflammation. 2015; 38:1690–1699. doi: 10.1007/s10753-015-0146-9 [DOI] [PubMed] [Google Scholar]
- 12.Rompe F, Artuc M, Hallberg A, Alterman M, Stroder K, Thone-Reineke C, Reichenbach A, Schacherl J, Dahlof B, Bader M, et al. Direct angiotensin II type 2 receptor stimulation acts anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor kappaB. Hypertension. 2010; 55:924–931. doi: 10.1161/HYPERTENSIONAHA.109.147843 [DOI] [PubMed] [Google Scholar]
- 13.Dhande I, Ma W, Hussain T. Angiotensin AT2 receptor stimulation is anti-inflammatory in lipopolysaccharide-activated THP-1 macrophages via increased interleukin-10 production. Hypertension Res. 2015; 38:21–29. doi: 10.1038/hr.2014.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nag S, Patel S, Mani S, Hussain T. Role of angiotensin type 2 receptor in improving lipid metabolism and preventing adiposity. Mol Cell Biochem. 2019; 461:195–204. doi: 10.1007/s11010-019-03602-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shum M, Pinard S, Guimond MO, Labbe SM, Roberge C, Baillargeon JP, Langlois MF, Alterman M, Wallinder C, Hallberg A, Carpentier AC, Gallo-Payet N. Angiotensin II type 2 receptor promotes adipocyte differentiation and restores adipocyte size in high-fat/high-fructose diet-induced insulin resistance in rats. Am J Physiol Endocrinol Metab. 2013; 304:E197–210. doi: 10.1152/ajpendo.00149.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Han GW, Batyuk A, Ishchenko A, White KL, Patel N, Sadybekov A, Zamlynny B, Rudd MT, Hollenstein K, et al. Structural basis for selectivity and diversity in angiotensin II receptors. Nature. 2017; 544:327–332. doi: 10.1038/nature22035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel S, Hussain T. Dimerization of AT2 and Mas receptors in control of blood pressure. Curr Hypertens Rep. 2018; 20:41. doi: 10.1007/s11906-018-0845-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lara LdS, Cavalcante F, Axelband F, De Souza AM, Lopes AG, Caruso-Neves C. Involvement of the Gi/o/cGMP/PKG pathway in the AT2-mediated inhibition of outer cortex proximal tubule Na+-ATPase by Ang-(1–7). Biochemical J. 2006; 395:183–190. doi: 10.1042/BJ20051455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Souza AM, Lopes AG, Pizzino CP, Fossari RN, Miguel NC, Cardozo FP, Abi-Abib R, Fernandes MS, Santos DP, Caruso-Neves C. Angiotensin II and angiotensin-(1–7) inhibit the inner cortex Na+ -ATPase activity through AT2 receptor. Regul Pept. 2004; 120:167–175. doi: 10.1016/j.regpep.2004.03.005 [DOI] [PubMed] [Google Scholar]
- 20.Feng YH, Sun Y, Douglas JG. Gbeta gamma -independent constitutive association of Galpha s with SHP-1 and angiotensin II receptor AT2 is essential in AT2-mediated ITIM-independent activation of SHP-1. Proc Natl Acad Sci U S A. 2002; 99:12049–12054. doi: 10.1073/pnas.192404199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui T, Nakagami H, Iwai M, Takeda Y, Shiuchi T, Daviet L, Nahmias C, Horiuchi M. Pivotal role of tyrosine phosphatase SHP-1 in AT2 receptor-mediated apoptosis in rat fetal vascular smooth muscle cell. Cardiovasc Res. 2001; 49:863–871. doi: 10.1016/s0008-6363(00)00299-6 [DOI] [PubMed] [Google Scholar]
- 22.Horiuchi M, Hayashida W, Kambe T, Yamada T, Dzau VJ. Angiotensin type 2 receptor dephosphorylates Bcl-2 by activating mitogen-activated protein kinase phosphatase-1 and induces apoptosis. J Biol Chem. 1997; 272:19022–19026. doi: 10.1074/jbc.272.30.19022 [DOI] [PubMed] [Google Scholar]
- 23.Peluso AA, Bertelsen JB, Andersen K, Mortsensen TP, Hansen PB, Sumners C, Bader M, Santos RA, Steckelings UM. Identification of protein phosphatase involvement in the AT2 receptor-induced activation of endothelial nitric oxide synthase. Clin Sci (Lond). 2018; 132:777–790. doi: 10.1042/CS20171598 [DOI] [PubMed] [Google Scholar]
- 24.Sabuhi R, Ali Q, Asghar M, Al-Zamily NR, Hussain T. Role of the angiotensin II AT2 receptor in inflammation and oxidative stress: opposing effects in lean and obese Zucker rats. Am J Physiol Renal Physiol. 2011; 300:F700–706. doi: 10.1152/ajprenal.00616.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hakam AC, Hussain T. Angiotensin II type 2 receptor agonist directly inhibits proximal tubule sodium pump activity in obese but not in lean Zucker rats. Hypertension. 2006; 47:1117–1124. doi: 10.1161/01.HYP.0000220112.91724.fc [DOI] [PubMed] [Google Scholar]
- 26.Porrello ER, Pfleger KD, Seeber RM, Qian H, Oro C, Abogadie F, Delbridge LM, Thomas WG. Heteromerization of angiotensin receptors changes trafficking and arrestin recruitment profiles. Cell Signal. 2011; 23:1767–1776. doi: 10.1016/j.cellsig.2011.06.011 [DOI] [PubMed] [Google Scholar]
- 27.Turu G, Szidonya L, Gaborik Z, Buday L, Spat A, Clark AJ, Hunyady L. Differential beta-arrestin binding of AT1 and AT2 angiotensin receptors. FEBS Lett. 2006; 580:41–45. doi: 10.1016/j.febslet.2005.11.044 [DOI] [PubMed] [Google Scholar]
- 28.Banos M, Arellano-Mendoza MG, Vargas-Robles H, Avila-Casado MC, Soto V, Romo E, Rios A, Hernandez-Zavala A, de la Pena-Diaz A, Escalante B. Relationship between angiotensin II receptor expression and cardiovascular risk factors in Mexican patients with coronary occlusive disease. Exp Mol Pathol. 2011; 91:478–483. doi: 10.1016/j.yexmp.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 29.Toedebusch R, Belenchia A, Pulakat L. Cell-specific protective signaling induced by the novel AT2R-agonist NP-6A4 on human endothelial and smooth muscle cells. Front Pharmacol. 2018; 9:928. doi: 10.3389/fphar.2018.00928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Booz GW, Baker KM. Role of type 1 and type 2 angiotensin receptors in angiotensin II-induced cardiomyocyte hypertrophy. Hypertension. 1996; 28:635–640. doi: 10.1161/01.hyp.28.4.635 [DOI] [PubMed] [Google Scholar]
- 31.Kakuchi J, Ichiki T, Kiyama S, Hogan BL, Fogo A, Inagami T, Ichikawa I. Developmental expression of renal angiotensin II receptor genes in the mouse. Kidney Int. 1995; 47:140–147. doi: 10.1038/ki.1995.16 [DOI] [PubMed] [Google Scholar]
- 32.Ozono R, Wang ZQ, Moore AF, Inagami T, Siragy HM, Carey RM. Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension. 1997; 30:1238–1246. doi: 10.1161/01.hyp.30.5.1238 [DOI] [PubMed] [Google Scholar]
- 33.Siragy HM. The angiotensin II type 2 receptor and the kidney. J Renin Angiotensin Aldosterone Syst. 2010; 11:33–36. doi: 10.1177/1470320309347786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhuo J, Dean R, MacGregor D, Alcorn D, Mendelsohn FA. Presence of angiotensin II AT2 receptor binding sites in the adventitia of human kidney vasculature. Clin Exp Pharmacol Physiol Suppl. 1996; 3:S147–154. doi: 10.1111/j.1440-1681.1996.tb03077.x [DOI] [PubMed] [Google Scholar]
- 35.de Kloet AD, Wang L, Ludin JA, Smith JA, Pioquinto DJ, Hiller H, Steckelings UM, Scheuer DA, Sumners C, Krause EG. Reporter mouse strain provides a novel look at angiotensin type-2 receptor distribution in the central nervous system. Brain Struct Funct. 2016; 221:891–912. doi: 10.1007/s00429-014-0943-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front Neuroendocrinol. 1997; 18:383–439. doi: 10.1006/frne.1997.0155 [DOI] [PubMed] [Google Scholar]
- 37.Patel SN, Fatima N, Ali R, Hussain T. Emerging role of angiotensin AT2 receptor in anti-inflammation: an update. Curr Pharm Des. 2020; 26:492–500. doi: 10.2174/1381612826666200115092015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barker TA, Massett MP, Korshunov VA, Mohan AM, Kennedy AJ, Berk BC. Angiotensin II type 2 receptor expression after vascular injury: differing effects of angiotensin-converting enzyme inhibition and angiotensin receptor blockade. Hypertension. 2006; 48:942–949. doi: 10.1161/01.HYP.0000241061.51003.b7 [DOI] [PubMed] [Google Scholar]
- 39.Savoia C, Touyz RM, Volpe M, Schiffrin EL. Angiotensin type 2 receptor in resistance arteries of type 2 diabetic hypertensive patients. Hypertension. 2007; 49:341–346. doi: 10.1161/01.HYP.0000253968.95136.b8 [DOI] [PubMed] [Google Scholar]
- 40.Shao C, Yu L, Gao L. Activation of angiotensin type 2 receptors partially ameliorates streptozotocin-induced diabetes in male rats by islet protection. Endocrinology. 2014; 155:793–804. doi: 10.1210/en.2013-1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsutsumi Y, Matsubara H, Ohkubo N, Mori Y, Nozawa Y, Murasawa S, Kijima K, Maruyama K, Masaki H, Moriguchi Y, Shibasaki Y, Kamihata H, Inada M, Iwasaka T. Angiotensin II type 2 receptor is upregulated in human heart with interstitial fibrosis, and cardiac fibroblasts are the major cell type for its expression. Circ Res. 1998; 83:1035–1046. doi: 10.1161/01.res.83.10.1035 [DOI] [PubMed] [Google Scholar]
- 42.Wharton J, Morgan K, Rutherford RA, Catravas JD, Chester A, Whitehead BF, De Leval MR, Yacoub MH, Polak JM. Differential distribution of angiotensin AT2 receptors in the normal and failing human heart. J Pharmacol Exp Ther. 1998; 284:323–336. [PubMed] [Google Scholar]
- 43.Ali Q, Wu Y, Hussain T. Chronic AT2 receptor activation increases renal ACE2 activity, attenuates AT1 receptor function and blood pressure in obese Zucker rats. Kidney Int. 2013; 84:931–939. doi: 10.1038/ki.2013.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siddiqui AH, Ali Q, Hussain T. Protective role of angiotensin II subtype 2 receptor in blood pressure increase in obese Zucker rats. Hypertension. 2009; 53:256–261. doi: 10.1161/HYPERTENSIONAHA.108.126086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hein L, Barsh GS, Pratt RE, Dzau VJ, Kobilka BK. Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor in mice. Nature. 1995; 377:744–747. doi: 10.1038/377744a0 [DOI] [PubMed] [Google Scholar]
- 46.Ichiki T, Labosky PA, Shiota C, Okuyama S, Imagawa Y, Fogo A, Niimura F, Ichikawa I, Hogan BL, Inagami T. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature. 1995; 377:748–750. doi: 10.1038/377748a0 [DOI] [PubMed] [Google Scholar]
- 47.Barber MN, Sampey DB, Widdop RE. AT2 receptor stimulation enhances antihypertensive effect of AT1 receptor antagonist in hypertensive rats. Hypertension. 1999; 34:1112–1116. doi: 10.1161/01.HYP.34.5.1112 [DOI] [PubMed] [Google Scholar]
- 48.Duke LM, Evans RG, Widdop RE. AT2 receptors contribute to acute blood pressure-lowering and vasodilator effects of AT1 receptor antagonism in conscious normotensive but not hypertensive rats. Am J Physiol Heart Circ Physiol. 2005; 288:H2289–2297. doi: 10.1152/ajpheart.01096.2004 [DOI] [PubMed] [Google Scholar]
- 49.Assersen KB, Sumners C, Steckelings UM. The renin-angiotensin system in hypertension, a constantly renewing classic: focus on the angiotensin AT2-receptor. Can J Cardiol. 2020; 36:683–693. doi: 10.1016/j.cjca.2020.02.095 [DOI] [PubMed] [Google Scholar]
- 50.Patel SN, Ali Q, Hussain T. Angiotensin II type 2-receptor agonist C21 reduces proteinuria and oxidative stress in kidney of high-salt-fed obese Zucker rats. Hypertension. 2016; 67:906–915. doi: 10.1161/HYPERTENSIONAHA.115.06881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kemp BA, Howell NL, Gildea JJ, Keller SR, Padia SH, Carey RM. AT₂ receptor activation induces natriuresis and lowers blood pressure. Circulation Res. 2014; 115:388–399. doi: 10.1161/circresaha.115.304110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ali Q, Patel S, Hussain T. Angiotensin AT2 receptor agonist prevents salt-sensitive hypertension in obese Zucker rats. Am J Physiol Renal Physiol. 2015; 308:F1379–1385. doi: 10.1152/ajprenal.00002.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ali Q, Hussain T. AT2 receptor non-peptide agonist C21 promotes natriuresis in obese Zucker rats. Hypertens Res. 2012; 35:654–660. doi: 10.1038/hr.2012.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hakam AC, Hussain T. Angiotensin II AT2 receptors inhibit proximal tubular Na+-K+-ATPase activity via a NO/cGMP-dependent pathway. Am J Physiol Renal Physiol. 2006; 290:F1430–1436. doi: 10.1152/ajprenal.00218.2005 [DOI] [PubMed] [Google Scholar]
- 55.Hilliard LM, Denton KM. The angiotensin type 2 receptor weighs in on obesity: a promising therapeutic target? Hypertension Res. 2012; 35:582–584. doi: 10.1038/hr.2012.22 [DOI] [PubMed] [Google Scholar]
- 56.Sabuhi R, Asghar M, Hussain T. Inhibition of NAD(P)H oxidase potentiates AT2 receptor agonist-induced natriuresis in Sprague-Dawley rats. Am J Physiol Renal Physiol. 2010; 299:F815–820. doi: 10.1152/ajprenal.00310.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miyata N, Park F, Li XF, Cowley AW, Jr. Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol. 1999; 277:F437–F446. doi: 10.1152/ajprenal.1999.277.3.F437 [DOI] [PubMed] [Google Scholar]
- 58.Zhuo JL, Li XC. Angiotensin III/AT(2) receptor/NHE3 signaling pathway in the proximal tubules of the kidney: a novel natriuretic and antihypertensive mechanism in hypertension. J Am Heat Assoc. 2019; 8:e012644–e012644. doi: 10.1161/JAHA.119.012644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu P, Gao ZX, Duan XP, Su XT, Wang MX, Lin DH, Gu R, Wang WH. AT2R (Angiotensin II type 2 receptor)-mediated regulation of NCC (Na-Cl cotransporter) and renal K excretion depends on the K channel, Kir4.1. Hypertension. 2018; 71:622–630. doi: 10.1161/HYPERTENSIONAHA.117.10471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Del Borgo M, Wang Y, Bosnyak S, Khan M, Walters P, Spizzo I, Perlmutter P, Hilliard L, Denton K, Aguilar MI, Widdop RE, Jones ES. beta-Pro7Ang III is a novel highly selective angiotensin II type 2 receptor (AT2R) agonist, which acts as a vasodepressor agent via the AT2R in conscious spontaneously hypertensive rats. Clin Sci (Lond). 2015; 129:505–513. doi: 10.1042/CS20150077 [DOI] [PubMed] [Google Scholar]
- 61.Jones ES, Black MJ, Widdop RE. Influence of angiotensin II subtype 2 receptor (AT(2)R) antagonist, PD123319, on cardiovascular remodelling of aged spontaneously hypertensive rats during chronic angiotensin II subtype 1 receptor (AT(1)R) blockade. Int J Hypertens. 2012; 2012:543062. doi: 10.1155/2012/543062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones ES, Del Borgo MP, Kirsch JF, Clayton D, Bosnyak S, Welungoda I, Hausler N, Unabia S, Perlmutter P, Thomas WG, Aguilar MI, Widdop RE. A single beta-amino acid substitution to angiotensin II confers AT2 receptor selectivity and vascular function. Hypertension. 2011; 57:570–576. doi: 10.1161/HYPERTENSIONAHA.110.164301 [DOI] [PubMed] [Google Scholar]
- 63.Wagenaar GT, Sengers RM, Laghmani el H, Chen X, Lindeboom MP, Roks AJ, Folkerts G, Walther FJ. Angiotensin II type 2 receptor ligand PD123319 attenuates hyperoxia-induced lung and heart injury at a low dose in newborn rats. Am J Physiol Lung Cell Mol Physiol. 2014; 307:L261–272. doi: 10.1152/ajplung.00345.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wagenaar GT, Laghmani el H, Fidder M, Sengers RM, de Visser YP, de Vries L, Rink R, Roks AJ, Folkerts G, Walther FJ. Agonists of MAS oncogene and angiotensin II type 2 receptors attenuate cardiopulmonary disease in rats with neonatal hyperoxia-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2013; 305:L341–351. doi: 10.1152/ajplung.00360.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kluskens LD, Nelemans SA, Rink R, de Vries L, Meter-Arkema A, Wang Y, Walther T, Kuipers A, Moll GN, Haas M. Angiotensin-(1–7) with thioether bridge: an angiotensin-converting enzyme-resistant, potent angiotensin-(1–7) analog. J Pharmacol Exp Ther. 2009; 328:849–854. doi: 0.1124/jpet.108.146431 [DOI] [PubMed] [Google Scholar]
- 66.Kuipers A, Moll GN, Wagner E, Franklin R. Efficacy of lanthionine-stabilized angiotensin-(1–7) in type I and type II diabetes mouse models. Peptides. 2019; 112:78–84. doi: 10.1016/j.peptides.2018.10.015 [DOI] [PubMed] [Google Scholar]
- 67.Namsolleck P, Richardson A, Moll GN, Mescheder A. LP2, the first lanthipeptide GPCR agonist in a human pharmacokinetics and safety study. Peptides. 2020; 136:170468. doi: 10.1016/j.peptides.2020.170468 [DOI] [PubMed] [Google Scholar]
- 68.Armando I, Jezova M, Juorio AV, Terrón JA, Falcón-Neri A, Semino-Mora C, Imboden H, Saavedra JM. Estrogen upregulates renal angiotensin II AT2receptors. Am J Physiol Renal Physiol. 2002; 283:F934–F943. doi: 10.1152/ajprenal.00145.2002 [DOI] [PubMed] [Google Scholar]
- 69.Baiardi G, Macova M, Armando I, Ando H, Tyurmin D, Saavedra JM. Estrogen upregulates renal angiotensin II AT1 and AT2 receptors in the rat. Regul Pept. 2005; 124:7–17. doi: 10.1016/j.regpep.2004.06.021 [DOI] [PubMed] [Google Scholar]
- 70.Nag S, Khan MA, Samuel P, Ali Q, Hussain T. Chronic angiotensin AT2R activation prevents high-fat diet-induced adiposity and obesity in female mice independent of estrogen. Metabolism. 2015; 64:814–825. doi: 10.1016/j.metabol.2015.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoshimura Y, Karube M, Aoki H, Oda T, Koyama N, Nagai A, Akimoto Y, Hirano H, Nakamura Y. Angiotensin II induces ovulation and oocyte maturation in rabbit ovaries via the AT2 receptor subtype. Endocrinology. 1996; 137:1204–1211. doi: 10.1210/en.137.4.1204 [DOI] [PubMed] [Google Scholar]
- 72.Mishra JS, Te Riele GM, Qi QR, Lechuga TJ, Gopalakrishnan K, Chen DB, Kumar S. Estrogen receptor-beta mediates estradiol-induced pregnancy-specific uterine artery endothelial cell angiotensin type-2 receptor expression. Hypertension. 2019; 74:967–974. doi: 10.1161/HYPERTENSIONAHA.119.13429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sampson AK, Moritz KM, Jones ES, Flower RL, Widdop RE, Denton KM. Enhanced angiotensin II type 2 receptor mechanisms mediate decreases in arterial pressure attributable to chronic low-dose angiotensin II in female rats. Hypertension. 2008; 52:666–671. doi: 10.1161/hypertensionaha.108.114058 [DOI] [PubMed] [Google Scholar]
- 74.Mishra JS, Gopalakrishnan K, Kumar S. Pregnancy upregulates angiotensin type 2 receptor expression and increases blood flow in uterine arteries of rats. Biol Reprod. 2018; 99:1091–1099. doi: 10.1093/biolre/ioy130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brosnihan KB, Hodgin JB, Smithies O, Maeda N, Gallagher P. Tissue-specific regulation of ACE/ACE2 and AT1/AT2 receptor gene expression by oestrogen in apolipoprotein E/oestrogen receptor-alpha knock-out mice. Exp Physiol. 2008; 93:658–664. doi: 10.1113/expphysiol.2007.041806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takeda-Matsubara Y, Iwai M, Cui TX, Shiuchi T, Liu HW, Okumura M, Ito M, Horiuchi M. Roles of angiotensin type 1 and 2 receptors in pregnancy-associated blood pressure change. Am J Hypertens. 2004; 17:684–689. doi: 10.1016/j.amjhyper.2004.03.680 [DOI] [PubMed] [Google Scholar]
- 77.Brown RD, Hilliard LM, Head GA, Jones ES, Widdop RE, Denton KM. Sex differences in the pressor and tubuloglomerular feedback response to angiotensin II. Hypertension. 2012; 59:129–135. doi: 10.1161/HYPERTENSIONAHA.111.178715 [DOI] [PubMed] [Google Scholar]
- 78.Hilliard LM, Nematbakhsh M, Kett MM, Teichman E, Sampson AK, Widdop RE, Evans RG, Denton KM. Gender differences in pressure-natriuresis and renal autoregulation: role of the Angiotensin type 2 receptor. Hypertension. 2011; 57:275–282. doi: 10.1161/HYPERTENSIONAHA.110.166827 [DOI] [PubMed] [Google Scholar]
- 79.Mirabito KM, Hilliard LM, Kett MM, Brown RD, Booth SC, Widdop RE, Moritz KM, Evans RG, Denton KM. Sex- and age-related differences in the chronic pressure-natriuresis relationship: role of the angiotensin type 2 receptor. Am J Physiol Renal Physiol. 2014; 307:F901–907. doi: 10.1152/ajprenal.00288.2014 [DOI] [PubMed] [Google Scholar]
- 80.Hilliard LM, Chow CL, Mirabito KM, Steckelings UM, Unger T, Widdop RE, Denton KM. Angiotensin type 2 receptor stimulation increases renal function in female, but not male, spontaneously hypertensive rats. Hypertension. 2014; 64:378–383. doi: 10.1161/HYPERTENSIONAHA.113.02809 [DOI] [PubMed] [Google Scholar]
- 81.Hilliard LM, Jones ES, Steckelings UM, Unger T, Widdop RE, Denton KM. Sex-specific influence of angiotensin type 2 receptor stimulation on renal function: a novel therapeutic target for hypertension. Hypertension. 2012; 59:409–414. doi: 10.1161/HYPERTENSIONAHA.111.184986 [DOI] [PubMed] [Google Scholar]
- 82.Chen K, Merrill DC, Rose JC. The importance of angiotensin II subtype receptors for blood pressure control during mouse pregnancy. Reprod Sci. 2007; 14:694–704. doi: 10.1177/1933719107309060 [DOI] [PubMed] [Google Scholar]
- 83.Lee JH, Xia S, Ragolia L. Upregulation of AT2 receptor and iNOS impairs angiotensin II-induced contraction without endothelium influence in young normotensive diabetic rats. Am J Physiol Regul Integr Comp Physiol. 2008; 295:R144–154. doi: 10.1152/ajpregu.00191.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mezzano S, Droguett A, Burgos ME, Ardiles LG, Flores CA, Aros CA, Caorsi I, Vío CP, Ruiz-Ortega M, Egido J. Renin-angiotensin system activation and interstitial inflammation in human diabetic nephropathy. Kidney Int Suppl. 2003:S64–70. doi: 10.1046/j.1523-1755.64.s86.12.x [DOI] [PubMed] [Google Scholar]
- 85.Hakam AC, Siddiqui AH, Hussain T. Renal angiotensin II AT2 receptors promote natriuresis in streptozotocin-induced diabetic rats. Am J Physiol Renal Physiol. 2006; 290:F503–508. doi: 10.1152/ajprenal.00092.2005 [DOI] [PubMed] [Google Scholar]
- 86.Ali Q, Sabuhi R, Hussain T. High glucose up-regulates angiotensin II subtype 2 receptors via interferon regulatory factor-1 in proximal tubule epithelial cells. Mol Cell Biochem. 2010; 344:65–71. doi: 10.1007/s11010-010-0529-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Begorre MA, Dib A, Habchi K, Guihot AL, Bourreau J, Vessieres E, Blondeau B, Loufrani L, Chabbert M, Henrion D, Fassot C. Microvascular vasodilator properties of the angiotensin II type 2 receptor in a mouse model of type 1 diabetes. Sci Rep. 2017; 7:45625. doi: 10.1038/srep45625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Quiroga DT, Munoz MC, Gil C, Pffeifer M, Toblli JE, Steckelings UM, Giani JF, Dominici FP. Chronic administration of the angiotensin type 2 receptor agonist C21 improves insulin sensitivity in C57BL/6 mice. Physiol Rep. 2018; 6:e13824. doi: 10.14814/phy2.13824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Than A, Xu SH, Li R, Leow MS, Sun L, Chen P. Angiotensin type 2 receptor activation promotes browning of white adipose tissue and brown adipogenesis. Signal Transduct Target Ther. 2017; 2:17022. doi: 10.1038/sigtrans.2017.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Quiroga DT, Miquet JG, Gonzalez L, Sotelo AI, Munoz MC, Geraldes PM, Giani JF, Dominici FP. Mice lacking angiotensin type 2 receptor exhibit a sex-specific attenuation of insulin sensitivity. Mol Cell Endocrinol. 2019; 498:110587. doi: 10.1016/j.mce.2019.110587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chai W, Wang W, Dong Z, Cao W, Liu Z. Angiotensin II receptors modulate muscle microvascular and metabolic responses to insulin in vivo. Diabetes. 2011; 60:2939–2946. doi: 10.2337/db10-1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muñoz MC, Burghi V, Miquet JG, Cervino IA, Quiroga DT, Mazziotta L, Dominici FP. Chronic blockade of the AT2 receptor with PD123319 impairs insulin signaling in C57BL/6 mice. Peptides. 2017; 88:37–45. doi: 10.1016/j.peptides.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 93.Ohshima K, Mogi M, Jing F, Iwanami J, Tsukuda K, Min LJ, Ogimoto A, Dahlof B, Steckelings UM, Unger T, Higaki J, Horiuchi M. Direct angiotensin II type 2 receptor stimulation ameliorates insulin resistance in type 2 diabetes mice with PPARgamma activation. PLoS One. 2012; 7:e48387. doi: 10.1371/journal.pone.0048387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao Y, Foryst-Ludwig A, Bruemmer D, Culman J, Bader M, Unger T, Kintscher U. Angiotensin II induces peroxisome proliferator-activated receptor gamma in PC12W cells via angiotensin type 2 receptor activation. J Neurochem. 2005; 94:1395–1401. doi: 10.1111/j.1471-4159.2005.03275.x [DOI] [PubMed] [Google Scholar]
- 95.Yan F, Yuan Z, Wang N, Carey RM, Aylor KW, Chen L, Zhou X, Liu Z. Direct activation of angiotensin II type 2 receptors enhances muscle microvascular perfusion, oxygenation, and insulin delivery in male rats. Endocrinology. 2018; 159:685–695. doi: 10.1210/en.2017-00585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu M, Li X, Ha S, Wang A, Yin S, Mu Y. Angiotensin type 2 receptor agonist C21 ameliorates the high-fat diet-induced pancreatic beta-cell dysfunction partially by activation of antiapoptosis and autophagy. Pancreas. 2019; 48:250–256. doi: 10.1097/MPA.0000000000001241 [DOI] [PubMed] [Google Scholar]
- 97.Dominici FP, Veiras LC, Shen JZY, Bernstein EA, Quiroga DT, Steckelings UM, Bernstein KE, Giani JF. Activation of AT(2) receptors prevents diabetic complications in female db/db mice by NO-mediated mechanisms. Br J Pharmacol. 2020; 177:4766–4781. doi: 10.1111/bph.15241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008; 582:97–105. doi: 10.1016/j.febslet.2007.11.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Rooij SR, Nijpels G, Nilsson PM, Nolan JJ, Gabriel R, Bobbioni-Harsch E, Mingrone G, Dekker JM, Low-grade chronic inflammation in the relationship between insulin sensitivity and cardiovascular disease (RISC) population: associations with insulin resistance and cardiometabolic risk profile. Diabetes Care. 2009; 32:1295–1301. doi: 10.2337/dc08-1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Iwai M, Tomono Y, Inaba S, Kanno H, Senba I, Mogi M, Horiuchi M. AT2 receptor deficiency attenuates adipocyte differentiation and decreases adipocyte number in atherosclerotic mice. Am J Hypertens. 2009; 22:784–791. doi: 10.1038/ajh.2009.85 [DOI] [PubMed] [Google Scholar]
- 101.Samuel P, Khan MA, Nag S, Inagami T, Hussain T. Angiotensin AT(2) receptor contributes towards gender bias in weight gain. PLoS One. 2013; 8:e48425. doi: 10.1371/journal.pone.0048425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yvan-Charvet L, Even P, Bloch-Faure M, Guerre-Millo M, Moustaid-Moussa N, Ferre P, Quignard-Boulange A. Deletion of the angiotensin type 2 receptor (AT2R) reduces adipose cell size and protects from diet-induced obesity and insulin resistance. Diabetes. 2005; 54:991–999. doi: 10.2337/diabetes.54.4.991 [DOI] [PubMed] [Google Scholar]
- 103.Yvan-Charvet L, Even P, Lamandé N, Ferré P, Quignard-Boulangé A. Prevention of adipose tissue depletion during food deprivation in angiotensin type 2 receptor-deficient mice. Endocrinology. 2006; 147:5078–5086. doi: 10.1210/en.2006-0754 [DOI] [PubMed] [Google Scholar]
- 104.Yvan-Charvet L, Massiéra F, Lamandé Nl, Ailhaud Gr, Teboul Ml, Moustaid-Moussa N, Gasc J-M, Quignard-Boulangé A. Deficiency of angiotensin type 2 receptor rescues obesity but not hypertension induced by overexpression of angiotensinogen in adipose tissue. Endocrinology. 2009; 150:1421–1428. doi: 10.1210/en.2008-1120 [DOI] [PubMed] [Google Scholar]
- 105.Adachi Y, Saito Y, Kishimoto I, Harada M, Kuwahara K, Takahashi N, Kawakami R, Nakanishi M, Nakagawa Y, Tanimoto K, et al. Angiotensin II type 2 receptor deficiency exacerbates heart failure and reduces survival after acute myocardial infarction in mice. Circulation. 2003; 107:2406–2408. doi: 10.1161/01.CIR.0000072763.98069.B4 [DOI] [PubMed] [Google Scholar]
- 106.Dopona EPB, Rocha VF, Furukawa LNS, Oliveira IB, Heimann JC. Myocardial hypertrophy induced by high salt consumption is prevented by angiotensin II AT2 receptor agonist. Nutr Metab Cardiovasc Dis. 2019; 29:301–305. doi: 10.1016/j.numecd.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 107.Ichihara S, Senbonmatsu T, Price E Jr., Ichiki T, Gaffney FA, Inagami T. Targeted deletion of angiotensin II type 2 receptor caused cardiac rupture after acute myocardial infarction. Circulation. 2002; 106:2244–2249. doi: 10.1161/01.cir.0000033826.52681.37 [DOI] [PubMed] [Google Scholar]
- 108.Kaschina E, Grzesiak A, Li J, Foryst-Ludwig A, Timm M, Rompe F, Sommerfeld M, Kemnitz UR, Curato C, Namsolleck P, et al. Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction? Circulation. 2008; 118:2523–2532. doi: 10.1161/CIRCULATIONAHA.108.784868 [DOI] [PubMed] [Google Scholar]
- 109.Qi Y, Li H, Shenoy V, Li Q, Wong F, Zhang L, Raizada MK, Sumners C, Katovich MJ. Moderate cardiac-selective overexpression of angiotensin II type 2 receptor protects cardiac functions from ischaemic injury. Exp Physiol. 2012; 97:89–101. doi: 10.1113/expphysiol.2011.060673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rehman A, Leibowitz A, Yamamoto N, Rautureau Y, Paradis P, Schiffrin EL. Angiotensin type 2 receptor agonist compound 21 reduces vascular injury and myocardial fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension. 2012; 59:291–299. doi: 10.1161/HYPERTENSIONAHA.111.180158 [DOI] [PubMed] [Google Scholar]
- 111.Lauer D, Slavic S, Sommerfeld M, Thone-Reineke C, Sharkovska Y, Hallberg A, Dahlof B, Kintscher U, Unger T, Steckelings UM, Kaschina E. Angiotensin type 2 receptor stimulation ameliorates left ventricular fibrosis and dysfunction via regulation of tissue inhibitor of matrix metalloproteinase 1/matrix metalloproteinase 9 axis and transforming growth factor beta1 in the rat heart. Hypertension. 2014; 63:e60–67. doi: 10.1161/HYPERTENSIONAHA.113.02522 [DOI] [PubMed] [Google Scholar]
- 112.Yang Z, Bove CM, French BA, Epstein FH, Berr SS, DiMaria JM, Gibson JJ, Carey RM, Kramer CM. Angiotensin II type 2 receptor overexpression preserves left ventricular function after myocardial infarction. Circulation. 2002; 106:106–111. doi: 10.1161/01.cir.0000020014.14176.6d [DOI] [PubMed] [Google Scholar]
- 113.Nakayama M, Yan X, Price RL, Borg TK, Ito K, Sanbe A, Robbins J, Lorell BH. Chronic ventricular myocyte-specific overexpression of angiotensin II type 2 receptor results in intrinsic myocyte contractile dysfunction. Am J Physiol Heart Circ Physiol. 2005; 288:H317–327. doi: 10.1152/ajpheart.00957.2003 [DOI] [PubMed] [Google Scholar]
- 114.Yan X, Price RL, Nakayama M, Ito K, Schuldt AJ, Manning WJ, Sanbe A, Borg TK, Robbins J, Lorell BH. Ventricular-specific expression of angiotensin II type 2 receptors causes dilated cardiomyopathy and heart failure in transgenic mice. Am J Physiol Heart Circ Physiol. 2003; 285:H2179–2187. doi: 10.1152/ajpheart.00361.2003 [DOI] [PubMed] [Google Scholar]
- 115.Curato C, Slavic S, Dong J, Skorska A, Altarche-Xifro W, Miteva K, Kaschina E, Thiel A, Imboden H, Wang J, Steckelings U, Steinhoff G, Unger T, Li J. Identification of noncytotoxic and IL-10-producing CD8+AT2R+ T cell population in response to ischemic heart injury. J Immunol. 2010; 185:6286–6293. doi: 10.4049/jimmunol.0903681 [DOI] [PubMed] [Google Scholar]
- 116.Skorska A, von Haehling S, Ludwig M, Lux CA, Gaebel R, Kleiner G, Klopsch C, Dong J, Curato C, Altarche-Xifro W, Slavic S, Unger T, Steinhoff G, Li J, David R. The CD4(+) AT2R(+) T cell subpopulation improves post-infarction remodelling and restores cardiac function. J Cell Mol Med. 2015; 19:1975–1985. doi: 10.1111/jcmm.12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Caillon A, Grenier C, Grimaud L, Vessieres E, Guihot AL, Blanchard S, Lelievre E, Chabbert M, Foucher ED, Jeannin P, Beauvillain C, Abraham P, Loufrani L, Delneste Y, Henrion D. The angiotensin II type 2 receptor activates flow-mediated outward remodelling through T cells-dependent interleukin-17 production. Cardiovasc Res. 2016; 112:515–525. doi: 10.1093/cvr/cvw172 [DOI] [PubMed] [Google Scholar]
- 118.Xu Y, Hu X, Wang L, Jiang Z, Liu X, Yu H, Zhang Z, Chen H, Chen H, Steinhoff G, Li J, Jian’an W. Preconditioning via angiotensin type 2 receptor activation improves therapeutic efficacy of bone marrow mononuclear cells for cardiac repair. PLoS One. 2013; 8:e82997. doi: 10.1371/journal.pone.0082997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ludwig M, Tolk A, Skorska A, Maschmeier C, Gaebel R, Lux CA, Steinhoff G, David R. Exploiting AT2R to improve CD117 stem cell function in vitro and in vivo--perspectives for cardiac stem cell therapy. Cell Physiol Biochem. 2015; 37:77–93. doi: 10.1159/000430335 [DOI] [PubMed] [Google Scholar]
- 120.Jehle AB, Xu Y, Dimaria JM, French BA, Epstein FH, Berr SS, Roy RJ, Kemp BA, Carey RM, Kramer CM. A nonpeptide angiotensin II type 2 receptor agonist does not attenuate postmyocardial infarction left ventricular remodeling in mice. J Cardiovasc Pharmacol. 2012; 59:363–368. doi: 10.1097/FJC.0b013e3182444110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lima VM, Lino CA, Senger N, de Oliveira Silva T, Fonseca RIB, Bader M, Santos RAS, Junior JD, Barreto-Chaves MLM, Diniz GP. Angiotensin II type 2 receptor mediates high fat diet-induced cardiomyocyte hypertrophy and hypercholesterolemia. Mol Cell Endocrinol. 2019; 498:110576. doi: 10.1016/j.mce.2019.110576 [DOI] [PubMed] [Google Scholar]
- 122.Kazancioğlu R Risk factors for chronic kidney disease: an update. Kidney Int Suppl (2011). 2013; 3:368–371. doi: 10.1038/kisup.2013.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dhande I, Ali Q, Hussain T. Proximal tubule angiotensin AT2 receptors mediate an anti-inflammatory response via interleukin-10: role in renoprotection in obese rats. Hypertension. 2013; 61:1218–1226. doi: 10.1161/HYPERTENSIONAHA.111.00422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ali Q, Dhande I, Samuel P, Hussain T. Angiotensin type 2 receptor null mice express reduced levels of renal angiotensin II type 2 receptor/angiotensin (1–7)/Mas receptor and exhibit greater high-fat diet-induced kidney injury. J Renin Angiotensin Aldosterone Syst. 2016; 17. doi: 10.1177/1470320316661871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Koulis C, Chow BS, McKelvey M, Steckelings UM, Unger T, Thallas-Bonke V, Thomas MC, Cooper ME, Jandeleit-Dahm KA, Allen TJ. AT2R agonist, Compound 21, is reno-protective against type 1 diabetic nephropathy. Hypertension. 2015; 65:1073–1081. doi: 10.1161/HYPERTENSIONAHA.115.05204 [DOI] [PubMed] [Google Scholar]
- 126.Castoldi G, di Gioia CR, Bombardi C, Maestroni S, Carletti R, Steckelings UM, Dahlof B, Unger T, Zerbini G, Stella A. Prevention of diabetic nephropathy by compound 21, selective agonist of angiotensin type 2 receptors, in Zucker diabetic fatty rats. Am J Physiol Renal Physiol. 2014; 307:F1123–1131. doi: 10.1152/ajprenal.00247.2014 [DOI] [PubMed] [Google Scholar]
- 127.Micakovic T, Papagiannarou S, Clark E, Kuzay Y, Abramovic K, Peters J, Sticht C, Volk N, Fleming T, Nawroth P, Hammes HP, Alenina N, Grone HJ, Hoffmann SC. The angiotensin II type 2 receptors protect renal tubule mitochondria in early stages of diabetes mellitus. Kidney Int. 2018; 94:937–950. doi: 10.1016/j.kint.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 128.Chang SY, Chen YW, Chenier I, Tran Sle M, Zhang SL. Angiotensin II type II receptor deficiency accelerates the development of nephropathy in type I diabetes via oxidative stress and ACE2. Exp Diabetes Res. 2011; 2011:521076. doi: 10.1155/2011/521076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Matavelli LC, Huang J, Siragy HM. Angiotensin AT(2) receptor stimulation inhibits early renal inflammation in renovascular hypertension. Hypertension. 2011; 57:308–313. doi: 10.1161/HYPERTENSIONAHA.110.164202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Matavelli LC, Zatz R, Siragy HM. A nonpeptide angiotensin II type 2 receptor agonist prevents renal inflammation in early diabetes. J Cardiovasc Pharmacol. 2015; 65:371–376. doi: 10.1097/FJC.0000000000000207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Patel S, Dhande I, Gray EA, Ali Q, Hussain T. Prevention of lipopolysaccharide-induced CD11b(+) immune cell infiltration in the kidney: role of AT2 receptors. Biosci Rep. 2019; 39. doi: 10.1042/BSR20190429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Benndorf RA, Krebs C, Hirsch-Hoffmann B, Schwedhelm E, Cieslar G, Schmidt-Haupt R, Steinmetz OM, Meyer-Schwesinger C, Thaiss F, Haddad M, Fehr S, Heilmann A, Helmchen U, Hein L, Ehmke H, et al. Angiotensin II type 2 receptor deficiency aggravates renal injury and reduces survival in chronic kidney disease in mice. Kidney Int. 2009; 75:1039–1049. doi: 10.1038/ki.2009.2 [DOI] [PubMed] [Google Scholar]
- 133.Hashimoto N, Maeshima Y, Satoh M, Odawara M, Sugiyama H, Kashihara N, Matsubara H, Yamasaki Y, Makino H. Overexpression of angiotensin type 2 receptor ameliorates glomerular injury in a mouse remnant kidney model. Am J Physiol Renal Physiol. 2004; 286:F516–525. doi: 10.1152/ajprenal.00294.2003 [DOI] [PubMed] [Google Scholar]
- 134.Leonhardt J, Villela DC, Teichmann A, Munter LM, Mayer MC, Mardahl M, Kirsch S, Namsolleck P, Lucht K, Benz V, et al. Evidence for heterodimerization and functional interaction of the angiotensin type 2 receptor and the receptor MAS. Hypertension. 2017; 69:1128–1135. doi: 10.1042/BSR20190429 [DOI] [PubMed] [Google Scholar]
- 135.Steckelings UM, Sumners C. Correcting the imbalanced protective RAS in COVID-19 with angiotensin AT2-receptor agonists. Clin Sci (Lond). 2020; 134:2987–3006. doi: 10.1042/cs20200922 [DOI] [PubMed] [Google Scholar]
- 136.Datta PK, Liu F, Fischer T, Rappaport J, Qin X. SARS-CoV-2 pandemic and research gaps: Understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics. 2020; 10:7448–7464. doi: 10.7150/thno.48076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang J, Petitjean SJL, Koehler M, Zhang Q, Dumitru AC, Chen W, Derclaye S, Vincent SP, Soumillion P, Alsteens D. Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor. Nat Commun. 2020; 11:4541. doi: 10.1038/s41467-020-18319-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020; 126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tornling G, Batta R, Porter J, Bengtsson T, Parmar K, Kashiva R, Hallberg A, Cohrt AK, Westergaard K, Dalsgaard C-J, Raud J. The angiotensin type 2 receptor agonist C21 restores respiratory function in COVID19 - a double-blind, randomized, placebo-controlled Phase 2 trial. medRxiv. 2021; 2021.01.26.21250511. doi: 10.1101/2021.01.26.21250511 [DOI] [Google Scholar]


