Abstract
We used the RE-AIM framework to evaluate a Stroke Prevention Team’s readiness to prevent strokes in children with sickle cell anemia living in northern Nigeria. The NIH sponsored Stroke Prevention Trial in Nigeria included a goal of a sustainable stroke prevention program. The program’s one-year reach for transcranial Doppler (TCD) screening was 14.7% (4,710/32,000) of which 6.0% (281/4,710) had abnormal values (≥200 cm/sec). All participants with abnormal TCD measurements were started on hydroxyurea (effectiveness). All hospitals agreed to adopt the program. After one year, program-implementation and maintenance rates were 100%, demonstrating the program’s feasibility and short-term sustainability.
Keywords: sickle cell, stroke prevention, low-resource setting, Nigeria, RE-AIM
INTRODUCTION
In the American Society of Hematology (ASH) 2020 guidelines for preventing and treating cerebrovascular disease in sickle cell disease, the committee recommends screening for abnormal transcranial Doppler (TCD) measurements in children with sickle cell anemia (SCA) and treatment with regular blood transfusions for at least one year.1 After one year of regular blood transfusion therapy, hydroxyurea therapy may be used in children without cerebral vasculopathy.2,3 As a result of this effort in high-income countries, the stroke incidence rate has dropped in children with SCA by 10-fold.4,5 Among children with abnormal TCD measurements, approximately 20% will have a stroke over two years if not treated with regular blood transfusion or hydroxyurea therapy, based on an incidence rate of 10.9 strokes per 100 person-years.4 In low-income countries where blood transfusion is costly and not practical, such as Nigeria, the ASH guidelines recommend hydroxyurea therapy as an initial therapy for stroke prevention.1
As part of the capacity building component of the phase III Stroke Prevention Trial in Nigeria study (SPRING, NCT02560935) that tested low-dose and moderate-dose of hydroxyurea for primary stroke prevention, one aim focused on developing a sustainable solution for stroke prevention in SCA.6 Thus, we tested the hypothesis that implementing a primary stroke screening program would be a feasible and sustainable strategy for at least a year. This paper describes the evaluation of the stroke prevention program using the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework.7
MATERIALS AND METHODS
Study design, setting, and recruitment
A multi-center capacity-building program for establishing Sickle Cell Disease Stroke Prevention Teams was conducted from April 2016 to July 2019. Vanderbilt Institutional Review Board and the ethics review committees of participating clinical sites approved this observational study to build capacity. All participants were consented before enrollment. The clinical sites for implementing a regional stroke prevention effort included five major hospitals in Kano and Kaduna states, northern Nigeria, Table 1, providing medical care for an estimated 32,000 children with SCA ages 5 to 12.8 This number is an estimate due to the lack of electronic health records and newborn screening programs.
TABLE 1.
Primary Stroke Prevention Maintenance 12 months post-program implementation at the 5 participating sites in Kano and Kaduna, Nigeria
| Study Site | Children with SCA (age: 5–12 years) seen weekly, N | Average wait time to be seen in SCD clinic | TCD evaluation as standard of care 12m post-implementation on clinic days | Children receiving TCD services on clinic day, N | Children receiving TCD services on non-clinic days, N |
|---|---|---|---|---|---|
| Aminu Kano Teaching Hospital, Kano | 80 | 2 hours | Yes, Tuesday | 15 | 10 |
| Murtala Muhammad Specialist Hospital, Kano | 360 | 2 hours | Yes, Monday-Friday | 15 | 30 |
| Hasiya Bayero Pediatric Hospital, Kano* | 108 | 3 hours | Yes, Wednesday | 15 | 0 |
| Muhammad Abdullahi Wase Specialist Hospital, Kano* | 42 | 2 hours | Yes, Tuesday and Thursday | 3–4 | 5 |
| Barau Dikko Teaching Hospital, Kaduna | 45 | 2 hours | Yes, Thursday | 18 | 10 |
N= number; SCA = sickle cell anemia; TCD = transcranial doppler velocity
Aminu Kano Teaching Hospital, Murtala Muhammad Specialist Hospital, Barau Dikko Teaching Hospital are the clinical trial sites for the SPRING trial,
Hasiya Bayero Pediatric Hospital and Muhammad Abdullahi Wase Specialist Hospital in Kano were the referral sites for the trial. All sites provided TCD screening as standard of care for children with sicklek cell anemia ages 5 to 12 years old.
Intervention program
Each hospital’s leadership was initially contacted to assess their capacity to deliver primary stroke prevention strategies for children with SCA. After the assessment was completed, a uniform strategy was decided upon, and the leadership of the five collaborating hospitals signed a Memorandum of Understanding after approximately four years of discussions. The core components of the intervention program included:
Identifying physician medical officers and nurses willing to be trained to perform TCD screening
Before starting the trial, the institutional practice was that only radiologists could perform TCD assessment. We initially identified non-radiologists and radiologists to conduct TCD screening and, subsequently, nurses because only 142 radiologists are available for a population of 85 million in northern Nigeria.9 There is an insufficient number of radiologists to provide screening TCD evaluations in the region.
Capacity building: obtain TCD equipment and identify health care personnel to create a multidisciplinary team willing to commit to standard care TCD screening in SCD clinics and stroke detection in children.
Screening services for children at risk for an initial stroke with TCD evaluation: perform onsite screening using TCD ultrasonography as standard care by a certified TCD-trained physician or nurse.
TCD ultrasonography and neurological evaluation training program
Based on the STOP protocol,4 non-imaging TCD training and certification were initiated for the Primary Stroke Prevention in Children with SCA in Nigeria (SPIN) trial at AKTH in Kano, Nigeria.10 Two Nigerian radiologists recruited for the trial were trained and certified after demonstrating reproducibility in assessing velocities in the middle cerebral arteries with an experienced TCD ultrasonographer.10
For the training, both children with and without SCA were recruited, including those with known abnormal TCD measurements. The TCD-certified non-radiologist physicians and nurses were also trained and certified on the Pediatric NIH Stroke Scale (PedNIHSS), a validated, standardized neurological examination assessing and quantifying the severity of strokes in children.11 An experienced pediatric neurologist traveled to Nigeria (E.T.), and two pediatric neurologists in Nashville (F.K. and L.J.) observed the use of the PedNIHSS in physicians (medical officers) and nurses. Each participant performed a minimum of 10 observed neurological examinations in children. The PedNIHSS was used to confirm a normal neurological exam on the same day as TCD and assess children with suspected acute stroke.
Evaluation: using the RE-AIM framework
We selected the RE-AIM framework to evaluate and assess the impact of the Sickle Cell Disease Stroke Prevention Team program implementation in northern Nigeria.12 The RE-AIM framework includes 5 dimensions, i.e., Reach, Effectiveness, Adoption, Implementation, and Maintenance. In this study, we report the RE-AIM factors on a 0% to 100% scale.
Reach
The reach of the intervention measures the participation rates and representativeness of individuals who participate in the program at each hospital. We defined numerator of the reach as the proportion of children with SCA ages between birth to 12 years old that received a transcranial Doppler screening (primary stroke prevention) or were screened for a stroke (secondary stroke prevention) one year after program initiation. We defined the denominator as the number of children with SCA, from birth to 12 years of age, evaluated from April 2016 to July 2019, at the participating hospitals in Kano and Kaduna, Nigeria. Although, we recognize that TCD screening does not start until 2 years of age.
Effectiveness
Given that the Sickle Cell Disease Stroke Prevention Team program was a research-based program delivered daily by health care providers (physicians and nurses) as standard care in an outpatient clinic setting, effectiveness data are reported as part of RE-AIM. Our primary outcome variable was the number of children with abnormal TCD measurements at risk for an initial stroke who initiated hydroxyurea treatment for primary stroke prevention. TCD measurements were performed, collected pre-intervention and at 12 months post-intervention. All children with abnormal TCD measurements were informed about disease-modifying therapy and were offered regular blood transfusion therapy and hydroxyurea therapy for primary stroke prevention.
Adoption
Adoption includes an assessment of the delivery settings (i.e., intervention locations) and the participation rate of delivery agents involved in implementing the program. We defined adoption as the number of certified delivery agents (physicians and nurses) who agreed to adopt and initiate the program.
Implementation
Implementation was measured by determining whether the intervention was delivered as intended. Implementation of the Sickle Cell Disease Stroke Prevention Program was analyzed as follows: (1) After program initiation, each institution completed fidelity checks during weekly phone calls between team members in Nigeria and the Data Coordinating Center: a) number of eligible children seen and receiving TCD screening per week; b) number of abnormal TCD measurements; c) the number of children with abnormal TCD measurements referred for hydroxyurea therapy; d) number of children receiving hydroxyurea therapy. (2) The participants’ training attendance and TCD certification were recorded and analyzed.
Setting-Level Maintenance
Setting-level maintenance was defined as the extent to which the program became part of routine organizational processes and maintained effectiveness. Maintenance at 12-months post-implementation was assessed by computing the percentage of participating hospitals that continued participating in the intervention and if the delivery components of the intervention program were still maintained with the same rigor.
RESULTS
Prior to our program intervention, no systematic approach for primary stroke prevention was utilized in the region. As result of the program, children between 2 and 12 years of age received TCD free of charge as standard care in five hospitals. Children with abnormal TCD velocities hydroxyurea were provided free of charge for children with abnormal TCD velocities. Figure 1 shows the RE-AIM factors on a 0% to 100% scale and their application in the evaluation of the Sickle Cell Disease Stroke Prevention Team program. Fig. 2 is a flow diagram depicting the study reach and effectiveness.
FIGURE 1.
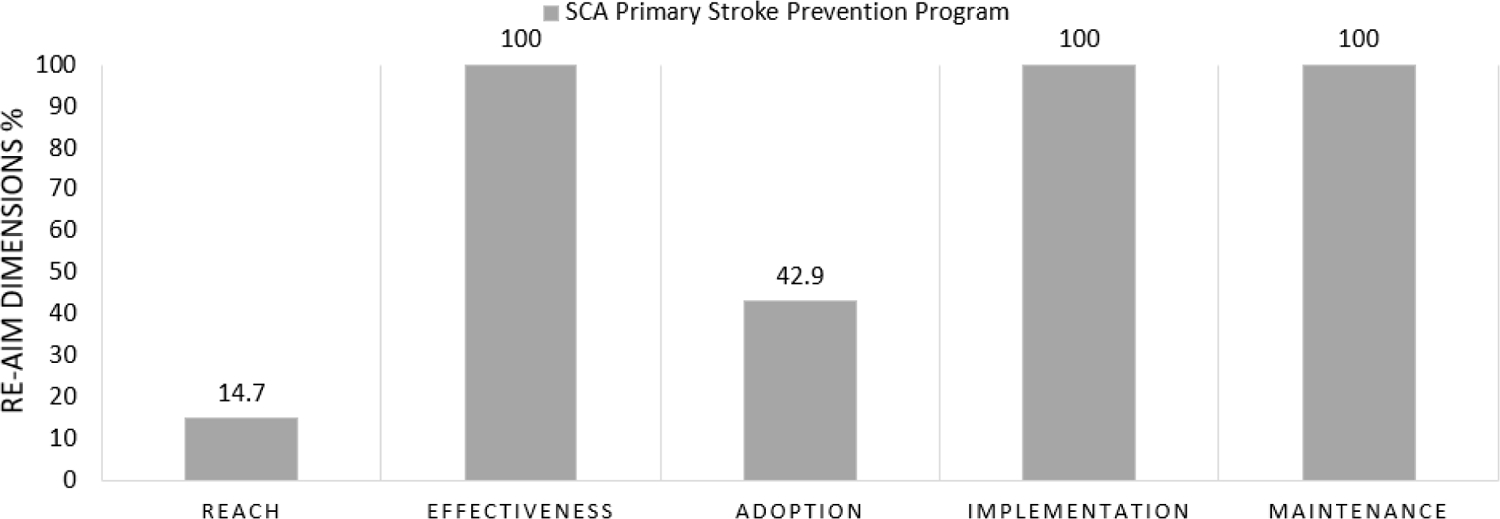
Performance of Sickle Cell Disease Stroke Prevention Team program on individual RE-AIM dimensions, combined from four hospitals in Kano and one hospital in Kaduna, Nigeria
FIGURE 2.
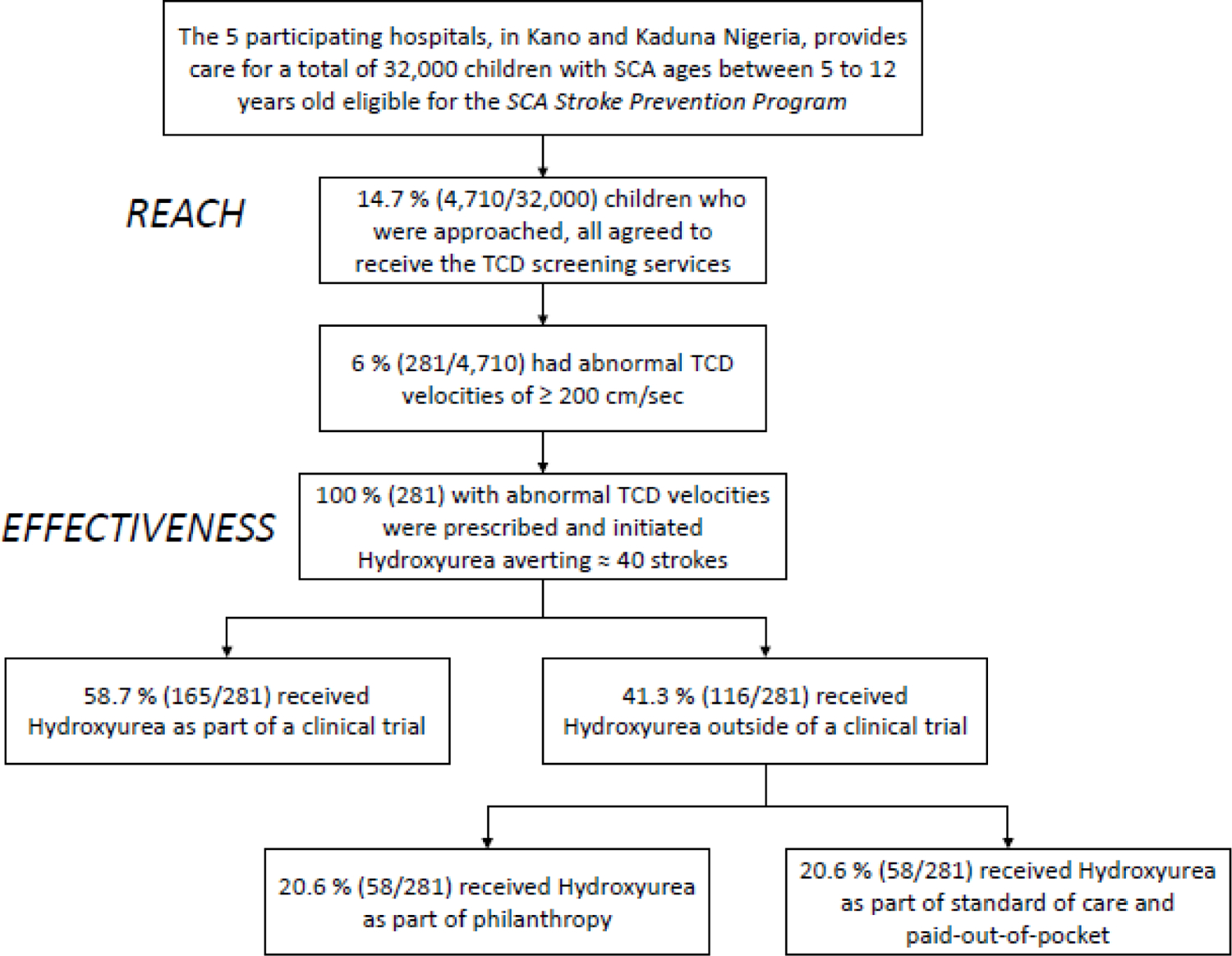
Flow Diagram describing: (1) the Program Reach as the proportion of children followed in the sickle cell disease clinics at the five hospitals receivingTCD screening as standard care; and (2) Program Effectiveness defined as the number of children with abnormal TCD measurements at risk for an initial stroke who initiated hydroxyurea treatment as form of primary stroke prevention, at the four hospitals in Kano and one hospital in Kaduna, Nigeria
Reach
Hospital leadership at the five eligible hospitals with established pediatric SCD clinics in metropolitan Kano and Kaduna, northern Nigeria, agreed to participate in the SCA Stroke Prevention Team program. From April 2016 to July 2019, approximately 32,000 children attending the SCD clinics were identified and found to be eligible for the Stroke Prevention program. All children with SCA between 2 and 12 years of age, who visited the participating hospitals and were approached, agreed to receive the TCD evaluation, Table 2. The program reach was 14.7%, i.e., 4,710 of 32,000 children with SCA received the TCD screening services. Of those children receiving TCD screening, 6% (281/4,710) had confirmed abnormal TCD values.
TABLE 2.
Baseline demographics and characteristics of children with sickle cell anemia ages between 2 to 12 years receiving TCD screening as standard care at the our hospitals in Kano and one hospital in Kaduna, Nigeria
| Variable | Children with SCA* (N=3,296) |
|---|---|
| Age, median (IQR) (years) | 7.0 (6) |
| Sex, male, % | 50.8 |
| TAMMV (cm/sec), mean (SD) | 140.13 (32.28) |
| Normal (<170 cm/sec), % | 79.7 |
| Conditional (170–199 cm/sec), % | 12.0 |
| Abnormal (≥200 cm/sec), % | 6.1 |
| No Signal, % | 1.9 |
TAMMV TCD, time-averaged mean of the maximum velocity of transcranial Doppler value; SCA, sickle cell anemia
Children with sickle cell anemia living in Kano Nigeria, who were approached and agreed to receive the TCD screening with complete data on age and sex
Children with pre-existing strokes, not eligible for primary stroke prevention but available for secondary stroke prevention, were evaluated at four, not five hospitals because the fifth hospital’s clinical staff was not set up to address the presence of pre-existing strokes. Among the children with SCA assessed for pre-existing strokes, 5.6% (172/3,049) of the children were identified as having an affirmative answer to the 10 screening stroke questions, a validated questionnaire for detecting moderate to severe neurological impairment in children living in Africa, and had a subsequent history and physical examination that confirmed they had had a stroke.13,14 Further, only four of the five participating hospitals asked the 10 stroke screening questions at the beginning of each clinic visit. Due to limited clinic space, the questions were asked in a small group format, often with 10–15 guardians with at least as many children in a group.
Effectiveness
Regarding the effectiveness outcome (primary stroke prevention), pre-program implementation, none of the children were prescribed moderate-fixed dose (20mg/kg/day) hydroxyurea therapy. Post-program implementation, all physicians prescribed hydroxyurea to children with abnormal TCD measurements. All guardians of children with confirmed abnormal TCD measurements rejected the initial offer of blood transfusion therapy for their children.
All children with abnormal TCD measurements received hydroxyurea manufactured by Bond Chemical Industries, Nigeria. Of the 281 children with confirmed abnormal TCD values, 20.6% (58/281) received hydroxyurea free-of-charge from philanthropic donations, 20.6% paid out-of-pocket,4,15 and the remaining children received hydroxyurea as part of the clinical trial (58.7%; SPRING trial). To address the inability of many families to pay out-of-pocket for hydroxyurea, recently, the Director-General of Hospital Management Board in Kano, Nigeria, and the Minister of Health in Kaduna, Nigeria, agreed to provide hydroxyurea free-of-charge to all children with SCA and abnormal TCDs.16 In the current cohort, treating 281 children with SCA with abnormal TCD presumably averted approximately 40 strokes using the number-needed-to-treat to prevent one stroke (NNT=7) from the STOP trial and assuming equal efficacy, approximately < 1 event per 100 person-years.4,15
For the effectiveness of secondary stroke prevention, prescribing disease-modifying therapy (hydroxyurea and regular blood transfusion) was also high (100%). No parent elected for transfusion therapy outside of the clinical trial setting; 98.8% (170/172) of participants identified as having a stroke received hydroxyurea therapy for secondary stroke prevention.
Adoption
All five delivery settings providing care for children with SCD in Kano and Kaduna in northern Nigeria were involved in the adoption. For primary stroke prevention, adoption at the provider level included the following: of the eligible 28 physicians (20 non-radiology physicians and 8 radiologists) at the participating sites, 60.7% (17/28) were approached and agreed to TCD ultrasonography training for primary stroke prevention; 42.9% (12/28) received certification.
Implementation
Overall, at 12-month evaluation, 100% of the originally planned components of the program for primary stroke prevention for children with SCA were implemented at all five sites. The following objectives were achieved: 1) all sites created a Sickle Cell Disease Stroke Prevention Team, and 42.9% (12 of 28) of radiology and non-radiology physicians were successfully certified in TCD ultrasonography; 2) all sites and delivery agents agreed to provide screening services in their outpatient SCD clinics.
Maintenance
At 12 months post-initiation of the program, all the initially planned program components were implemented per protocol, including hands-on, one-on-one TCD training and TCD certification, PedNIHSS training, and certification of physicians and nurses. All sites continued to maintain and participate in the program, financially supporting a certified TCD ultrasonographer, a certified nurse in standardized neurological examination, and routine screening of children with SCA, with TCD evaluation during outpatient clinic visits (Table 1).
Seven healthcare workers (two nurses and five physicians) at the five hospitals agreed to adopt the program. After four years of interaction, the Director-General of Hospital Boards in Kano and Kaduna states agreed to sign a Memorandum of Understanding to maintain program sustainability. In both states, a gap of approximately three months and six months, respectively, occurred between activation of the Memorandum of Understanding when the state leaders agreed to provide the hydroxyurea free of charge for children at high risk of an initial or subsequent stroke. Before the state provided hydroxyurea free of charge, the team members paid for the hydroxyurea out of pocket, and a health care administrator secured additional funds. The children receiving hydroxyurea are being monitored as part of the new standard of care by the multidisciplinary Sickle Cell Disease Stroke Prevention Teams at each site bimonthly, with CBC evaluation every six months. There is no hydroxyurea dose escalation based on the results of the SPIN Trial, showing adequate stroke prevention with fixed moderate dose hydroxyurea (20 mg/kg/day).10
Discussion
We used the RE-AIM framework to evaluate the impact of implementing the Sickle Cell Disease Stroke Prevention Teams program over 1 year in Kano and Kaduna states, Nigeria, to identify ‘why’ and ‘how’ this intervention works in this setting. Creating Sickle Cell Disease Stroke Prevention Teams, including a physician, a skilled TCD ultrasonographer, and a skilled nurse, all trained in performing appropriate neurological examinations, will allow for additional children to be reached. Understanding the barriers and facilitators of program adoption can inform its generalizability to other settings and scalability to other populations.17–19 To our knowledge, our Sickle Cell Disease Stroke Prevention Team program for children with SCA is one of the first intervention programs for a region in Africa, not an individual hospital. This program is feasible in northern Nigeria and may be generalizable to other areas of Africa.
Before our approach, primary stroke prevention strategies were not embedded as the standard of care for children with SCA in northern Nigeria. All five hospitals agreed to adopt the program strategies, and all components were implemented and maintained for at least 12 months post-implementation, as planned, Table 1. Reach was limited by the high volume of children with SCA, lack of trained personnel certified to perform TCD, and the paucity of TCD equipment. The training of the physicians and nurses was well-received. The program gained support and acceptability among the local leaders and health professionals before the signed Memorandum of Understanding.
All guardians of children with abnormal TCD measurements or pre-existing strokes refused initial regular blood transfusion therapy. Reasons guardians did not accept monthly blood transfusion therapy for primary and secondary stroke prevention include but are not limited to the regional practice of requiring families to regularly seek blood donors to replace the units used and the high cost of red blood cell units’ cost relative to income. The mean annual cost of chronic transfusion (without chelation) is $3,276 United States dollars; 20 Approximately 40% of the Nigerian population (~83 million people) live below Nigeria’s poverty, approximately $1.00 United States Dollars.21 The large discrepancy between the cost to prevent strokes and the annual income is a significant barrier to blood transfusion therapy. Our experience is identical to that of Lagunju et al. at a single site in Nigeria where 100% of families refused regular blood transfusion therapy for primary and secondary stroke prevention.20 Some Nigerians can afford monthly blood transfusion therapy and iron chelation, but most cannot.
Significant barriers for SCA stroke prevention included the lack of TCD machines in the region, the scarcity of TCD-certified sonographers, and the slow development of state leadership partnerships to activate the Memorandum of Understanding. Additionally, the cost of imported hydroxyurea is prohibitively high for most families in the region. Sickle Cell Disease Stroke Prevention Teams are feasible in northern Nigeria and elsewhere in Africa, with meaningful partnerships between regional public health care administrators, health care providers, state government leaders, and local philanthropists. Together these entities can ensure the sustainability of preventing strokes in children with SCA.
We have demonstrated the feasibility of implementing Sickle Cell Disease Stroke Prevention Teams in northern Nigeria. The cornerstone of the Stroke Prevention Team’s success was the guiding philosophy that Nigerians should provide sustainable stroke prevention services for Nigerian children with SCA. This philosophy led to two critical efforts that strengthened the team’s resolve. First, finding a Nigerian pharmaceutical company (Bond Chemical Industries, Nigeria) produces hydroxyurea at a subsidized cost ($0.16 United States dollar for 500 mg capsule per day per child). Second, the Nigerian team’s incremental belief that they could make a difference in children’s lives with SCA. The team’s raison d’etre was manifested in their enthusiasm to obtain TCD and stroke detection training, coupled with the team’s willingness to pay-out-of-pocket for hydroxyurea in children with abnormal TCD velocities before the state-supported free hydroxyurea therapy was available.
Acknowledgments:
We are posthumously thankful for Shehi Ali, MBBS, who was a radiologist certified to complete transcranial Doppler ultrasounds and later certified physicians and nurses to conduct the assessment in Nigeria. We are grateful to our research coordinators and study personnel who tirelessly coordinated the trial in Kano, Nigeria, and Kaduna, Nigeria. We appreciate Mr. Mustafa Nateqi and Mrs. Jennifer Beck-Smith for facilitating administrative tasks required for the successful conduct of this study. We appreciate Mrs. Khadija Bulama, Miss Gloria Bahago, and Fahad Usman for facilitating administrative and operational tasks required for the successful conduct of this study. We would like to thank the members of the DeBaun laboratory at Vanderbilt-Meharry Center of Excellence in Sickle Cell Disease for their support of this work.
Funding: Research reported in this publication was supported by the National Institutes of Health Grant #1R01NS094041–01, P50 CA-19–006, NCT 03380351, UL1 TR00234, 5U24HL136790, the Thrasher Foundation, and the generous donation of the Phillips family. The sponsor did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
List of Abbreviations
- SCA
Sickle cell anemia
- SCD
Sickle cell disease
- RE-AIM
Reach, effectiveness, adoption, implementation, maintenance
- TCD
Transcranial doppler
- TAMMV
Time-averaged mean of the maximum velocity
- SPIN trial
Primary Stroke Prevention in Children with SCA in Nigeria
- SPRING trial
Primary Prevention of Stroke in Children with SCD in Sub-Saharan Africa II
- AKTH
Aminu Kano Teaching Hospital
- MMSH
Murtala Muhammad Specialist Hospital
- BDTH
Barau Dikko Teaching Hospital
- MAWSH
Muhammad Abdullahi Wase Specialist Hospital
- HBPH
Hasiya Bayero Pediatric Hospital
Footnotes
Ethics Approval and Consent to Participate: The study that generated the data was IRB approved by Vanderbilt University Medical Center and the local sites in Nigeria, and all participants provided informed consent.
Conflict of Interests: The authors declare no competing financial interests.
References
- 1.Kwiatkowski JL, Kanter J, Fullerton HJ, et al. Ischemic Stroke in Children and Young Adults with Sickle Cell Disease (SCD) in the Post-STOP Era. Blood 2015; 126(23): 68-. [DOI] [PubMed] [Google Scholar]
- 2.Kanter J, Kwiatkowski J, Fullerton HJ, et al. Impact of TCD Screening Protocol on the Incidence of Hemorrhagic Stroke in Children and Young Adults with Sickle Cell Disease. Blood 2015; 126(23): 3402-. [Google Scholar]
- 3.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med 1998; 339(1): 5–11. [DOI] [PubMed] [Google Scholar]
- 4.Galadanci NA, Umar Abdullahi S, Vance LD, et al. Feasibility trial for primary stroke prevention in children with sickle cell anemia in Nigeria (SPIN trial). American journal of hematology 2018; 93(3): E83. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman SA, Schultz WH, Burgett S, Mortier NA, Ware RE. Hydroxyurea therapy lowers transcranial Doppler flow velocities in children with sickle cell anemia. Blood 2007; 110(3): 1043–7. [DOI] [PubMed] [Google Scholar]
- 6.DeBaun MR, Jordan LC, King AA, et al. American Society of Hematology 2020 guidelines for sickle cell disease: prevention, diagnosis, and treatment of cerebrovascular disease in children and adults. Blood Adv 2020; 4(8): 1554–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glasgow RE, Harden SM, Gaglio B, et al. RE-AIM Planning and Evaluation Framework: Adapting to New Science and Practice With a 20-Year Review. Frontiers in Public Health 2019; 7(64). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galadanci AA, Galadanci NA, Jibir BW, et al. Approximately 40 000 children with sickle cell anemia require screening with TCD and treating with hydroxyurea for stroke prevention in three states in northern Nigeria. Am J Hematol 2019; 94(11): E305–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galadanci NA, Umar Abdullahi S, Vance LD, et al. Feasibility trial for primary stroke prevention in children with sickle cell anemia in Nigeria (SPIN trial). Am J Hematol 2017; 92(8): 780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beslow LA, Kasner SE, Smith SE, et al. Concurrent validity and reliability of retrospective scoring of the Pediatric National Institutes of Health Stroke Scale. Stroke 2012; 43(2): 341–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. American journal of public health 1999; 89(9): 1322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ware RE, Davis BR, Schultz WH, et al. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia-TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial. Lancet 2016; 387(10019): 661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piel FB, Patil AP, Howes RE, et al. Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nature communications 2010; 1: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan CK, Oldenburg B, Viswanath K. Advancing the science of dissemination and implementation in behavioral medicine: evidence and progress. International journal of behavioral medicine 2015; 22(3): 277–82. [DOI] [PubMed] [Google Scholar]
- 15.Tabak RG, Sinclair KA, Baumann AA, et al. A review of diabetes prevention program translations: use of cultural adaptation and implementation research. Translational behavioral medicine 2015; 5(4): 401–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carroll C, Patterson M, Wood S, Booth A, Rick J, Balain S. A conceptual framework for implementation fidelity. Implementation science : IS 2007; 2: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrero DG. Evaluation of a Technology to Support a Translational Diabetes Prevention Intervention. Diabetes care 2016; 39(8): 1307–8. [DOI] [PubMed] [Google Scholar]
- 18.Oldenburg B, Absetz P. Lost in translation: overcoming the barriers to global implementation and exchange of behavioral medicine evidence. Translational behavioral medicine 2011; 1(2): 252–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ware RE, Helms RW, Investigators SW. Stroke With Transfusions Changing to Hydroxyurea (SWiTCH). Blood 2012; 119(17): 3925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oldenburg B, Absetz P. Lost in translation: overcoming the barriers to global implementation and exchange of behavioral medicine evidence. Transl Behav Med 2011;1:252–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagunju IA, Brown BJ, Sodeinde OO. Chronic blood transfusion for primary and secondary stroke prevention in Nigerian children with sickle cell disease: a 5-year appraisal. Pediatr Blood Cancer 2013;60:1940–1945. [DOI] [PubMed] [Google Scholar]


