SARS-CoV-2 infection relies on the binding of Spike glycoprotein (S protein) to angiotensin converting enzyme 2 (ACE2) in the host cells. Vascular endothelium can be infected by SARS-CoV-2,1 which triggers mitochondrial ROS production and glycolytic shift.2 Paradoxically, ACE2 is protective in the cardiovascular system, and SARS-CoV-1 S protein promotes lung injury by decreasing the level of ACE2 in the infected lungs.3 In the current study, we show that S protein alone can damage vascular endothelial cells (ECs) by downregulating ACE2 and consequently inhibiting mitochondrial function.
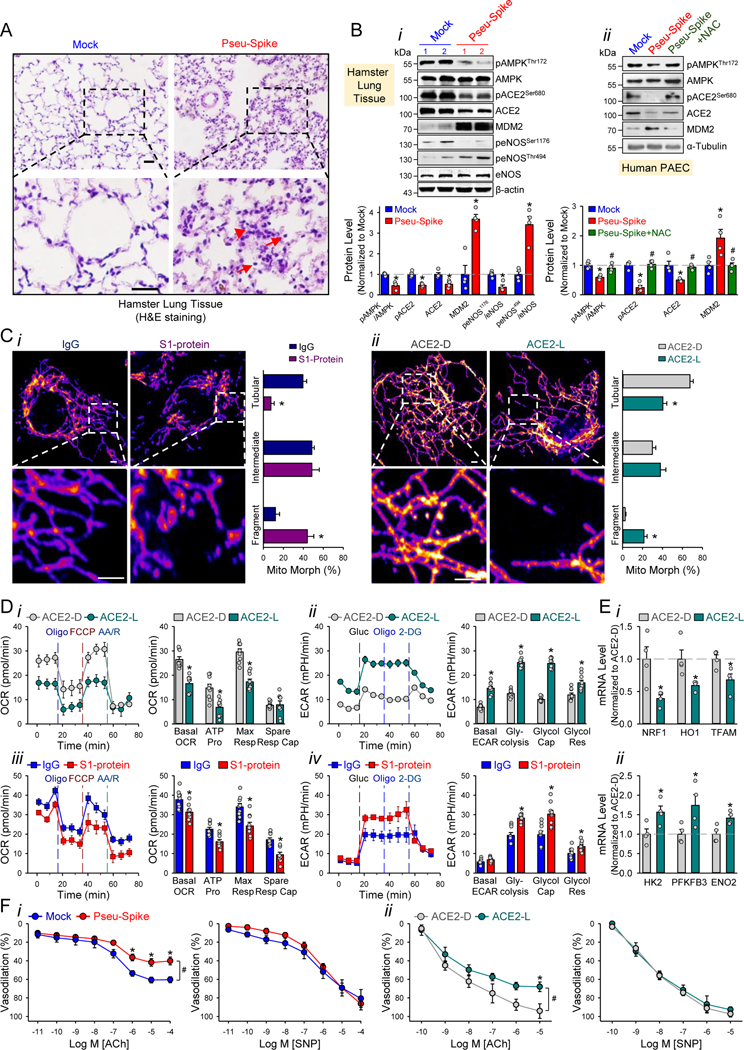
We administered a pseudovirus expressing S protein (Pseu-Spike) to Syrian hamsters intratracheally. Lung damage was apparent in animals receiving Pseu-Spike, revealed by thickening of the alveolar septa and increased infiltration of mononuclear cells (Figure 1A). AMPK phosphorylates ACE2 Ser-680, MDM2 ubiquitinates ACE2 Lys-788, and crosstalk between AMPK and MDM2 determines the ACE2 level.4 In the damaged lungs, levels of phospho-AMPK (pAMPK), phospho-ACE2 (pACE2), and ACE2 decreased but those of MDM2 increased (Figure 1Bi). Furthermore, complementary increased and decreased phosphorylation of eNOS Thr-494 and Ser-1176 indicated impaired eNOS activity. These changes of pACE2, ACE2, MDM2 expression, and AMPK activity in endothelium were recapitulated by in vitro experiments using pulmonary arterial ECs (PAECs) infected with Pseu-Spike which was rescued by treatment with N-acetyl-L-cysteine (NAC), a ROS inhibitor (Figure 1Bii).
Figure 1. SARS-CoV-2 Spike protein exacerbates EC function via ACE2 downregulation and mitochondrial impairment.
A, Representative H&E histopathology of lung specimens from 8–12 week-old male Syrian hamsters 5-day post administration of pseudovirus overexpressing Spike protein (Pseu-Spike) or mock virus in control group (n=3 mice per group, 1×108 PFU). Thickened alveolar septa (red arrowhead) and mononuclear cell (red arrow). Scale bar = 20 μm. B, Pseu-Spike (n=4) or mock virus (n=4)-infected hamster lungs were subjected to Western blot analysis for pAMPK T172, AMPK, pACE2 S680, ACE2, MDM2, peNOS S1176, peNOS T494, eNOS, and β-actin (Bi). Human PAECs were infected with Pseu-Spike or mock virus for 24 hr with or without NAC (5 mmol/L) pre-treatment for 2 hr. The protein extracts were analyzed by Western blot using antibodies against proteins as indicated (n=4) (Bii). C, Representative confocal images of mitochondrial morphology of ECs treated with human recombinant S1 protein or IgG (4 μg/ml) for 24 hr (Ci) or infected with human adenovirus ACE2 S680D (ACE2-D) or ACE2 S680L (ACE2-L) (10 MOI) for 48 hr (Cii). Mitochondria were visualized using TOM20 antibody (n=4, 50 cells counted for each replicate). Scale bar = 2.5 μm. “Tubular”: the majority of mitochondria in ECs was >10 μm in length; “Intermediate”: the mitochondria were less than ~10 μm; “Fragment”: the majority of mitochondria were spherical (no clear length or width). D, Measurement of oxygen consumption rate (OCR, Di and Diii) and extracellular acidification rate (ECAR, Dii and Div) in ECs infected with ACE2-D versus ACE2-L (10 MOI) for 48 hr (n=3) or treated with IgG versus S1 protein (4 μg/ml) for 24 hr (n=3). E, RT-qPCR analysis of the indicated mRNA levels in lung ECs from ACE2-D (n=4) and ACE2-L (n=4) knock-in mice. Eight-week-old ACE2-D and ACE2-L male mice with C57BL/6 background were used. F, Dose-response curves of acetylcholine (ACh, left panels)- and sodium nitroprusside (SNP, right panels)-mediated relaxation on the tension of phenylephrine (1 μmol/L) precontracted intrapulmonary artery stripes from Pseu-Spike-(ACh n=8, SNP n=5) or mock-(ACh n=6, SNP n=5) virus infected Syrian hamsters (1×108 PFU) (Fi) and ACE2-D (n=6) or ACE2-L (n=5) mice (Fii). The animal experiments were approved by the ethical committee of Xi’an Jiaotong University.
We next studied the impact of S protein on mitochondrial function. Confocal images of ECs treated with S1 protein revealed increased mitochondrial fragmentation, indicating altered mitochondrial dynamics (Figure 1Ci). To examine whether these mitochondrial changes were due, in part, to the decreased amount of ACE2, we overexpressed ACE2 S680D (ACE2-D, a phospho-mimetic ACE2 with increased stability) or S680L (ACE2-L, a dephospho-mimetic with decreased stability)4 in ECs. As shown in Figure 1Cii, ECs with ACE2-L had a higher number of fragmented mitochondria when compared to those with ACE2-D. Performing oxygen consumption rate and extracellular acidification rate assays, we found that ECs overexpressing ACE2-L had reduced basal mitochondrial respiration, ATP production, and maximal respiration compared to ECs overexpressing ACE2-D (Figure 1Di). Moreover, ACE2-L overexpression caused increased basal acidification rate, glucose-induced glycolysis, maximal glycolytic capacity, and glycolytic reserve (Figure 1Dii). Also, ECs incubated with S1 protein had attenuated mitochondrial function but increased glycolysis, when compared with control cells treated with IgG (Figure 1Diii,iv). We also compared the expressions of mitochondria- and glycolysis-related genes in lung ECs isolated from ACE2-D or ACE2-L knock-in mice4. Shown in Figure 1E, the mRNA levels of NRF1, HO1, and TFAM (mitochondria biogenesis-related genes) were increased, whereas those of HK2, PFKFB3, and ENO2 (glycolysis related-genes) were decreased in lung ECs in ACE2-D mice, as compared with those in ACE2-L mice.
SARS-CoV-2 infection induces EC inflammation, leading to endotheliitis.1,5 Because S protein decreased ACE2 level and impaired NO bioavailability, we examined whether S protein entry is indispensable for dysfunctional endothelium. As shown in Figure 1Fi, the endothelium-dependent vasodilation induced by acetylcholine (ACh) was impaired in pulmonary arteries isolated from Pseu-Spike-administered hamsters, whereas the endothelium-independent vasodilation induced by sodium nitroprusside (SNP) was not affected. We also compared the ACh- and SNP-induced vasodilation of pulmonary vessels from ACE2-D or ACE2-L mice. As anticipated, ACh-induced vasodilation was hindered in pulmonary arteries isolated from ACE2-L mice in comparison to ACE2-D mice (Figure 1Fii). There was, however, little difference of SNP-induced vasodilation between ACE2-D and ACE-L animals.
Although the use of a non-infectious pseudovirus is a limitation to this study, our data reveals that S protein alone can damage endothelium, manifested by impaired mitochondrial function and eNOS activity but increased glycolysis. It appears that S protein in ECs increases redox stress which may lead to AMPK deactivation, MDM2 upregulation, and ultimately ACE2 destabilization.4 While these findings need to be confirmed with the SARS-CoV-2 virus in the future study, it seems paradoxical that ACE2 reduction by S protein would decrease the virus infectivity, thereby protecting endothelium. However, a dysregulated renin-angiotensin system due to ACE2 reduction may exacerbate endothelial dysfunction, leading to endotheliitis. Collectively, our results suggest that the S protein-exerted EC damage overrides the decreased virus infectivity. This conclusion suggests that vaccination-generated antibody and/or exogenous antibody against S protein not only protects the host from SARS-CoV-2 infectivity but also inhibits S protein-imposed endothelial injury.
Acknowledgments
SOURCES OF FUNDING
This work was supported in part by grants from the NIH grants R01HL106579 and R01HL140898 (J.Y.S.); the NSFC grants 81870220 (S.P.W.), 81800328 (J.Z.), 81941005 (Z.Y.Y.); Shaanxi Natural Science Fund S2020-JC-JQ-0239 (S.P.W.); The National Key Research and Development Program (Grant No. 2018YFC1311500) (Z.Y.Y.); the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University (Grant No. XJTU1AF-CRF-2016–004) (Z.Y.Y.); Xi’an Jiaotong University Financial support.
Footnotes
DISCLOSURES
None.
This article is published in its accepted form. It has not been copyedited and has not appeared in an issue of the journal. Preparation for inclusion in an issue of Circulation Research involves copyediting, typesetting, proofreading, and author review, which may lead to differences between this accepted version of the manuscript and the final, published version.
Data Availability.
The data that support the findings of this study, including statistical analyses and reagents used, are available from the corresponding author upon request.
REFERENCES
- 1.Teuwen L-A, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Codo AC, Davanzo GG, Monteiro LdB, et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/Glycolysis-dependent axis. Cell Metab. 2020;32:437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen H, Zhang J, Wang C, et al. MDM2-mediated ubiquitination of angiotensin-converting enzyme 2 contributes to the development of pulmonary arterial hypertension. Circulation. 2020;142:1190–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]