Abstract
We compared the outcome of COVID-19 in immunosuppressed solid organ transplant (SOT) patients to a transplant naïve population. In total, 10 356 adult hospital admissions for COVID-19 from March 1, 2020 to April 27, 2020 were analyzed. Data were collected on demographics, baseline clinical conditions, medications, immunosuppression, and COVID-19 course. Primary outcome was combined death or mechanical ventilation. We assessed the association between primary outcome and prognostic variables using bivariate and multivariate regression models. We also compared the primary endpoint in SOT patients to an age, gender, and comorbidity-matched control group. Bivariate analysis found transplant status, age, gender, race/ethnicity, body mass index, diabetes, hypertension, cardiovascular disease, COPD, and GFR <60 mL/min/1.73 m2 to be significant predictors of combined death or mechanical ventilation. After multivariate logistic regression analysis, SOT status had a trend toward significance (odds ratio [OR] 1.29; 95% CI 0.99–1.69, p = .06). Compared to an age, gender, and comorbidity-matched control group, SOT patients had a higher combined risk of death or mechanical ventilation (OR 1.34; 95% CI 1.03–1.74, p = .027).
Keywords: clinical research/practice, immunosuppression/immune modulation, infection and infectious agents - viral, infectious disease, kidney (allograft) function/ dysfunction, organ transplantation in general
1 ∣. INTRODUCTION
Since December 2019, the novel coronavirus disease 2019 (COVID-19) has spread from a single city in Hubei, China to the rest of the world, overwhelming health-care systems. The World Health Organization has classified the resulting illness, COVID-19, as a global pandemic. Multinational studies have addressed the clinical characteristics of the virus, including risk factors and prognosis, in the general population.1,2 Patients found to be at higher risk for infection and adverse outcome include the elderly and patients with comorbid conditions—in particular, cardiovascular disease, diabetes, and obesity.1,3 An evolutionary genetic predisposition has also been reported.4 Whether immunosuppression constitutes a risk factor for poor outcome is not clear. Reports in immunocompromised cohorts, including solid organ transplantation (SOT), rheumatologic disease, and cancer, have revealed diverse outcomes.5-15
It has been postulated that an unregulated “cytokine storm” with features resembling hemophagocytic lymphohistiocytosis is pathologically tied to poor outcomes seen in COVID-19.16,17 Based on this rationale, multiple agents with immunomodulatory properties have been tested as potential therapeutic options.16 It has therefore been postulated that SOT patients, although at increased risk of infectious complications, may benefit from a diminished inflammatory response to the cytokine storm due to chronic immunosuppression. This has direct bearing on how to manage immunosuppression in SOT patients with COVID-19.
Therefore, the study was designed to compare the outcome of COVID-19 in SOT patients versus the nontransplant population. We assessed the association between SOT and death or mechanical ventilation, acute kidney injury (AKI), and prognostic variables, using regression models in a large cohort of COVID-19 hospitalizations across a multihospital health-care system with particular interest to SOT status. We also evaluated the same endpoints in a nested, matched case-control study of SOT patients.
2 ∣. MATERIALS AND METHODS
Data for this study were obtained from 12 Northwell Health hospitals in New York using the enterprise inpatient electronic health record (EHR) Sunrise Clinical Manager (Allscripts). All adult (age ≥18) patients who were admitted with a nasopharyngeal sample positive by polymerase chain reaction for SARS-CoV-2 during the study time period from March 1, 2020 to April 27, 2020 were eligible. Follow-up was through June 4, 2020. In cases of multiple qualifying hospital admissions, only the first hospitalization was included. Patients who were transferred between hospitals within the health system were treated as one hospital encounter. COVID-19 treatment guidelines were distributed throughout the health system with regular updates as new data emerged. Guidelines were the same for SOT patients and the general population. In brief, mechanical ventilation was utilized for patients with oxygen saturation <88% and/or increased work of breathing on 100% non-rebreather mask. Initially prone positioning was only used for refractory hypoxia. Later in the pandemic, early prone positioning became encouraged. Hydroxychloroquine was used in patients with oxygen saturation ≤94% and or radiographic pneumonia. Venous thromboembolism prophylaxis included low molecular weight heparin and unfractionated heparin for patients with low GFR. Immunosuppression management for SOT patients was left to the care team.
The following demographic information was collected: age, sex, race, Hispanic ethnicity, presence of comorbid conditions, and body mass index (BMI). Race and ethnicity data were collected by self-report in prespecified fixed categories. Renal function was categorized into three groups based on Kidney Disease Improving Global Outcomes (KDIGO) stages of chronic kidney disease (CKD) (GFR ≥60, 30–59, <30 mL/min/1.73 m2). Estimated GFR was calculated using the CKD EPI formula. Transplant medications and COVID-19 therapeutics (hydroxychloroquine, azithromycin, remdesivir, tocilizumab, sarilumab, anakinra, and corticosteroid use >5 mg/day) were recorded. In instances of variables with more than 5% missing data (BMI and race/ethnicity with 10.9% and 7.2%, respectively), the missing data were treated as separate groups. The first laboratory testing results available within 48 hours of presentation were computed as baseline values. Identification of SOT status was performed by querying hospital admission physician notes as well as specialty consultation notes for the following terms: “transplant,” “txp,” “OHT,” “OLT,” “DDRT,” “LURT,” “LRRT,” and “LDT.” Problem lists and past medical history were further searched for the following International Classification of Diseases 10 (ICD-10) codes that define patients with transplant: T86.1, T86.10, T86.11, T86.12, T86.13, T86.19, Z94.0, Z94.1, T86.20, T86.2, T86.290, T86.22, T86.21, T86.298, T86.23, Z94.4, T86.40, T86.4, T86.41, T86.42, T86.49, T86.43, Z94.2, T86.819, T86.810, T86.812, T86.811, T86.818, and Z94.83. The resulting list of patients was manually reviewed by members of the transplant team to validate the presence of a functioning SOT allograft. Patients with end-stage kidney disease (ESKD) on dialysis were excluded. This study was supported by the Northwell Health COVID-19 Research Consortium and was approved by the Institutional Review Board for the Feinstein Institutes of Medical Research at Northwell Health as minimal-risk research with waived requirement for informed consent.
3 ∣. STUDY POPULATION
3.1 ∣. Transplant group
All SOT recipients of kidney, liver, heart, pancreas, and lung who had a functioning allograft and were hospitalized with COVID-19 during the study period.
3.2 ∣. Total control group
All nontransplant patients who were hospitalized with COVID-19 during the same study period.
3.3 ∣. Matched control group
Exact matching was performed for gender, diabetes, hypertension, and cardiovascular disease (defined as any of coronary artery disease, peripheral arterial disease, or heart failure), while caliper matching was utilized for age (± 5 years) using the CALIPMATCH program in Stata® statistical analysis software.
4 ∣. OUTCOMES
Patients were followed from the date of initial hospitalization until the first outcome of either death, discharge from the hospital, transfer to another center, or end of the study (June 4, 2020). The primary outcome was the combined endpoint of either death or requirement of mechanical ventilation. Secondary outcomes included death, mechanical ventilation, AKI, and final disposition. AKI was defined according to KDIGO criteria.18 Methodology for ascertaining AKI has been described elsewhere.19
5 ∣. STATISTICAL ANALYSIS
Descriptive statistics for normally distributed continuous variables were expressed as mean ± standard deviation (SD) and compared using t-tests. Variables with nonnormal distribution were expressed as median (interquartile range [IQR]) and compared using Mann–Whitney–Wilcoxon test. As appropriate, categorical variables were expressed as percentages and compared using chi-squared or Fisher's exact tests.
For the primary and secondary outcome analysis, we performed bivariate and multivariate logistic regression on the total study group and multivariate conditional logistic regression on the matched cohort (matched control group plus SOT group). Covariates for the multivariate logistic regression were selected based on clinical importance as well as differences identified on bivariate analyses. In the matched cohort, covariates that could not be successfully included in the matching algorithm but were felt to be of clinical importance (BMI, race/ethnicity, and GFR) were added to the final multivariate conditional logistic regression. In both cohorts, the final multivariate logistic model was adjusted for cluster effect of admission hospital (transplant center vs. nontransplant center).
All tests were two tailed with a significance level of α = 0.05. Analyses were performed with Stata 16.0 for Mac, StataCorp LP (College Station, TX).
6 ∣. RESULTS
6.1 ∣. Baseline characteristics
From March 1, 2020 to April 27, 2020, there were 10 869 hospital admissions to 12 health system hospitals with a diagnosis of COVID-19. Four hundred thirty patients with ESKD and one liver transplant with allograft failure (cirrhosis) were excluded. The final study group was comprised of 10 356 patients in the total control group and 82 SOT patients.
Baseline demographics are depicted in Table 1. The majority of patients in the transplant and total control group were male (68.3% vs. 59.4%) and the mean age was 61.8 ± 11.7 years vs. 64.9 ± 16.2 years, respectively. There were 69 kidney transplants, six heart transplants, one kidney-liver transplant, two kidney-pancreas transplants, three liver transplants, and one lung transplant. The median time from transplantation to COVID-19 diagnosis was 2592 (IQR 599–4837) days. Most patients were on a calcineurin inhibitor (86.6%), mycophenolate (79.3%), and prednisone (67.1%). In the transplant group, the most frequent ethnicity was non-Hispanic black (31.7%) versus non-Hispanic white in the total control group (34%). BMI was lower in the transplant group (p = .041), while diabetes mellitus (62.2% vs. 35.6%; p < .001), hypertension (84.1% vs. 58.9%; p < .001), and coronary artery disease (24.4% vs. 12.6%; p = .004) were significantly more common among SOT patients. The majority of SOT patients had reduced GFR <60 mL/min/1.73 m2 (85.4% vs. 38.4%, p < .001). There was no difference in patients on a renin-angiotensin-aldosterone inhibitor (RAASi [ACE inhibitor, angiotensin receptor blocker]) at the time of hospital admission (22.4% vs. 31.5%; p = .11) or smoking status, peripheral vascular disease, heart failure, asthma, and COPD. A similar number of SOT patients and total control group patients were admitted to a tertiary hospital (73.2% vs. 66.3%; p = .19). More SOT patients were admitted to a transplant center (45.1% vs. 18.2%; p < .001).
TABLE 1.
Baseline characteristics
| Transplant |
Total control |
p value | Matched control |
p value | |
|---|---|---|---|---|---|
| N = 82 | N = 10 356 | N = 1625 | |||
| Age, mean ± SD | 61.8 ± 11.7 | 64.9 ± 16.2 | 0.081 | 62.7 ± 11.5 | 0.50 |
| Gender | |||||
| Male | 56 (68.3%) | 6151 (59.4%) | 0.11 | 1117 (68.7%) | 0.93 |
| Race/Ethnicity | |||||
| Non-Hispanic white | 19 (23.2%) | 3521 (34%) | 0.043 | 477 (29.4%) | 0.34 |
| Hispanic | 17 (20.7%) | 2185 (21.1%) | 335 (20.6%) | ||
| Non-hispanic black | 26 (31.7%) | 2075 (20%) | 385 (23.7%) | ||
| Other | 17 (20.7%) | 1824 (17.6%) | 312 (19.2%) | ||
| Unknown | 3 (3.7%) | 751 (7.3%) | 116 (7.1%) | ||
| BMI | |||||
| 18.5–29.9 | 51 (62.2%) | 5486 (53%) | 0.042 | 783 (48.2%) | <0.001 |
| <18.5 | 4 (4.9%) | 193 (1.9%) | 9 (0.6%) | ||
| ≥30.0 | 22 (26.8%) | 3544 (34.2%) | 684 (42.1%) | ||
| Unknown | 5 (6.1%) | 1133 (10.9%) | 149 (9.2%) | ||
| Diabetes | 51 (62.2%) | 3688 (35.6%) | <0.001 | 1005 (61.8%) | 0.95 |
| Hypertension | 69 (84.1%) | 6098 (58.9%) | <0.001 | 1365 (84%) | 0.97 |
| PVD/PAD | 3 (3.7%) | 238 (2.3%) | 0.44 | 59 (3.6%) | 0.99 |
| Heart failure | 10 (12.2%) | 818 (7.9%) | 0.15 | 163 (10%) | 0.51 |
| CAD | 20 (24.4%) | 1305 (12.6%) | 0.004 | 335 (20.6%) | 0.40 |
| Asthma | 3 (3.7%) | 869 (8.4%) | 0.12 | 154 (9.5%) | 0.099 |
| COPD | 2 (2.4%) | 640 (6.2%) | 0.16 | 117 (7.2%) | 0.96 |
| Initial serum creatinine (mg/dL) | 1.8 (1.3–2.9) | 1 (0.8–1.4) | <0.001 | ||
| GFR (mL/min/1.732) | |||||
| ≥ 60 | 12 (14.6%) | 6359 (61.4%) | <0.001 | 964 (59.3%) | <0.001 |
| 30–59 | 39 (47.6%) | 2630 (25.4%) | 447 (27.5%) | ||
| <30 | 31 (37.8%) | 1346 (13%) | 214 (13.2%) | ||
| Missing | 0 (0%) | 21 (0.2%) | |||
| Tobacco Status | |||||
| Smoker (current or former) | 15 (18.3%) | 2055 (19.8%) | 0.93 | 370 (22.8%) | 0.64 |
| Never smoked | 62 (75.6%) | 7646 (73.8%) | 1165 (71.7%) | ||
| Unknown | 5 (6.1%) | 655 (6.3%) | 90 (5.5%) | ||
| RAASi | 17 (22.4%) | 2969 (31.5%) | 0.11 | 740 (48.8%) | <0.001 |
| Admission hospital | |||||
| Nontransplant center | 45 (54.9%) | 8468 (81.8%) | <0.001 | 1327 (81.7%) | <0.001 |
| Transplant center | 37 (45.1%) | 1888 (18.2%) | 298 (18.3%) | ||
| Organ type | |||||
| Kidney | 69 (84.2%) | ||||
| Heart | 6 (7.3%) | ||||
| Liver | 3 (3.7%) | ||||
| Lung | 1 (1.2%) | ||||
| Kidney/Pancreas | 2 (2.4%) | ||||
| Kidney/Liver | 1 (1.2%) | ||||
| Induction agent | |||||
| Antithymocyte globulin (rabbit) | 8 (9.8%) | ||||
| Basiliximab | 14 (17%) | ||||
| None | 4 (4.9%) | ||||
| Unknown | 56 (68.3) | ||||
| Calcineurin inhibitor | 71 (86.6%) | ||||
| Mycophenolate | 65 (79.3%) | ||||
| Prednisone | 55 (67.1%) | ||||
| Belatacept | 2 (2.4%) | ||||
| mTOR inhibitor | 3 (3.7%) | ||||
| Immunosuppression reduced | 66 (80.5%) | ||||
| Transplant to COVID–19 infection, days (median [IQR]) | 2592 (599–4837) |
All data presented as n (%), unless otherwise noted.
Cardiovascular disease defined as coronary artery disease, peripheral vascular disease, or heart failure.
Abbreviations: CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; mTOR, mammalian target of rapamycin; PVD/PAD, peripheral vascular disease/peripheral artery disease; RAASi, renin-angiotensin-aldosterone receptor inhibitor.
In the matched control group, unmatched variables were similar in terms of race/ethnicity, asthma, chronic obstructive pulmonary disease (COPD), and smoking status to the SOT group. Within the cardiovascular disease category, groups had a similar percentage of PVD, heart failure, and coronary artery disease. SOT patients were less likely to be obese (26.8% vs. 42.1%; p < .001) and had lower use of RAASi (22.4% vs. 48.8%, p < .001). The majority of SOT patients had reduced GFR <60 mL/min/1.73 m2 (85.4% vs. 40.7%; p < .001).
6.2 ∣. Laboratory results in the SOT versus matched control group
Initial laboratory values at the time of hospital admission can be seen in Table 2. SOT patients had a lower lymphocyte count (0.7 cells/μL [IQR 0.3–0.9] vs. 0.9 cells/μL [IQR 0.6–1.3]; p < .001), hemoglobin, and lower alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels. There was no difference in D-dimer or C-reactive protein. Serum ferritin was higher in SOT patients (881.5 ng/mL [IQR 499.5–2115.5] vs. 753.1 ng/mL [IQR 395–1379]; p = .038). Mean admission serum creatinine was higher in SOT patients (2.4 ± 1.8 mg/dL vs. 1.5 ± 1.2; p < .001).
TABLE 2.
Initial laboratory values in SOT versus matched control
| Transplant |
Matched control |
p value |
|
|---|---|---|---|
| N = 82 | N = 1625 | ||
| Lymphocyte count (cells/μL) | .7 (0.3–0.9) | .9 (0.6–1.3) | <.001 |
| Neutrophil count (cells/μL) | 5.2 (4–6.6) | 5.7 (4–8.1) | .071 |
| Hemoglobin (g/dL) | 11.9 (10.6–13) | 13.3 (12–14.4) | <.001 |
| Platelet (K/μL) | 193 (155–275) | 212 (162–273) | .27 |
| Albumin (g/dL) | 3.4 (2.8–3.8) | 3.4 (3–3.8) | .59 |
| ALT (U/L) | 20 (14–29) | 35 (23–55) | <.001 |
| AST (U/L) | 32 (22–42) | 47 (32–70) | <.001 |
| Total bilirubin (mg/dl) | .4 (0.3–0.6) | .5 (0.4–0.7) | .055 |
| D-dimer assay (μg/mL) | 496 (280–1981) | 455 (263–874) | .25 |
| C-reactive protein (mg/L) | 10.1 (5–15.9) | 11.2 (5.9–19) | .074 |
| Ferritin (ng/mL) | 881.5 (499.5–2115.5) | 753.1 (395–1379) | .038 |
| Admission creatinine (mg/dL) | 2.4 ± 1.8 | 1.5 ± 1.2 | <.001 |
Data are presented as median (IQR) or mean ± SD.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
6.3 ∣. COVID-19–specific treatment in the SOT versus matched control group
Table 3 shows COVID-19–specific treatment received in the groups. There was no difference in treatment aside from corticosteroid use, which was higher in the transplant population (81.7% vs. 47.9%; p < .001). Fifty-five SOT patients were on prednisone 5 mg daily as part of their immunosuppression while 29 patients received methylprednisolone. The median maximum daily dose of methylprednisolone was 40 mg/day (IQR 40–50). No patient was treated for presumed or biopsy proven rejection. Steroid dosing for control patients was unable to be obtained due to limitations of our data warehouse and the large number of control patients. The majority of transplant patients (80.5%) had their immunosuppression reduced during treatment as defined by either stopping/lowering the calcineurin inhibitor, stopping the mTOR inhibitor, or stopping/reducing mycophenolate (Table).
TABLE 3.
COVID-19 treatment in SOT versus matched control
| Transplant |
Matched control |
p value |
|
|---|---|---|---|
| N = 82 | N = 1625 | ||
| HCQ | 60 (73.2%) | 1290 (79.4%) | .18 |
| Azithromycin | 36 (43.9%) | 839 (51.6%) | .17 |
| Remdesivir | 2 (2.4%) | 15 (0.9%) | .18 |
| Tocilizumab | 11 (13.4%) | 239 (14.7%) | .75 |
| Sarilumab | .(0%) | 1 (0.1%) | .82 |
| Anakinra | 9 (11%) | 220 (13.5%) | .51 |
| Corticosteroids | 67 (81.7%) | 779 (47.9%) | <.001 |
Abbreviation: HCQ, hydroxychloroquine.
6.4 ∣. Outcomes in the SOT versus matched control group
Primary and secondary outcomes can be seen in Table 4. Eighty-one SOT patients were matched to 20 controls each and one SOT patient was matched to eight controls. Thirty-five SOT patients (42.7%) reached the primary outcome of death or mechanical ventilation (31 kidney, three heart, and one lung transplant) versus 509 patients (31.3%) in the matched control group (OR = 1.63, 95% CI 1.04–2.56; p = .03). Twenty-six (31.7%) SOT patients died versus 391 (24.1%) in the matched control group (OR = 1.47; p = .12) and 28 (34.2%) SOT patients required mechanical ventilation versus 399 (24.6%) in the matched control group (OR = 1.59, 95% CI 0.99–2.55; p = .05). There was no significant difference between time to ventilation (5 vs. 2 days; p = .12) or time to death (12 vs. 8 days; p = .12) between SOT patients and matched controls. AKI was more common in SOT patients (58.5% vs. 42.8%; p = .009) and more SOT patients required renal replacement therapy as compared to matched controls (18.3% vs. 8.5%; [OR = 2.4, 95% 95% CI 1.34–4.34; p = .002]). There were significant differences in final disposition between the SOT and matched control group (p < .001). At the end of the study period, 49 (59.8%) SOT patients versus 1175 (72.3%) of the matched control group were discharged from the hospital alive, 26 (31.7%) versus 391 (24.1%) died, 2 (2.4%) versus 52 (3.2%) were transferred to another center, and 5 (6.1%) versus 7 (0.4%) remained hospitalized. Median time to discharge was the same in SOT and non-SOT patients (6 vs. 6.2 days; p = .79).
TABLE 4.
Outcomes in SOT versus matched control
| Transplant |
Matched control |
p value | |
|---|---|---|---|
| N = 82 | N = 1625 | ||
| Primary outcome | |||
| Death or mechanical ventilation | 35 (42.7%) | 509 (31.3%) | .03 |
| Secondary outcomes | |||
| Death | 26 (31.7%) | 391 (24.1%) | .1 |
| Mechanical ventilation | 28 (34.2%) | 399 (24.6%) | .05 |
| AKI stage | |||
| AKI 1 | 22 (26.8%) | 276 (17%) | .008 |
| AKI 2 | 5 (6.1%) | 154 (9.5%) | |
| AKI 3 | 21 (25.6%) | 266 (16.4%) | |
| No AKI | 34 (41.5%) | 892 (54.9%) | |
| Unknown AKI | .(0%) | 37 (2.3%) | |
| Need for RRT | 15 (18.3%) | 138 (8.5%) | .002 |
| Final dispositiona | |||
| Discharged | 49 (59.8%) | 1,175 (72.3%) | <.001 |
| Currently admitted | 5 (6.1%) | 7 (0.4%) | |
| Deceased | 26 (31.7%) | 391 (24.1%) | |
| Transfer | 2 (2.4%) | 52 (3.2%) |
All data presented as n (%) unless otherwise noted.
Abbreviations: AKI, acute kidney injury; RRT, renal replacement therapy.
Final disposition at the end of the study period.
6.5 ∣. Bivariate and multivariate analysis
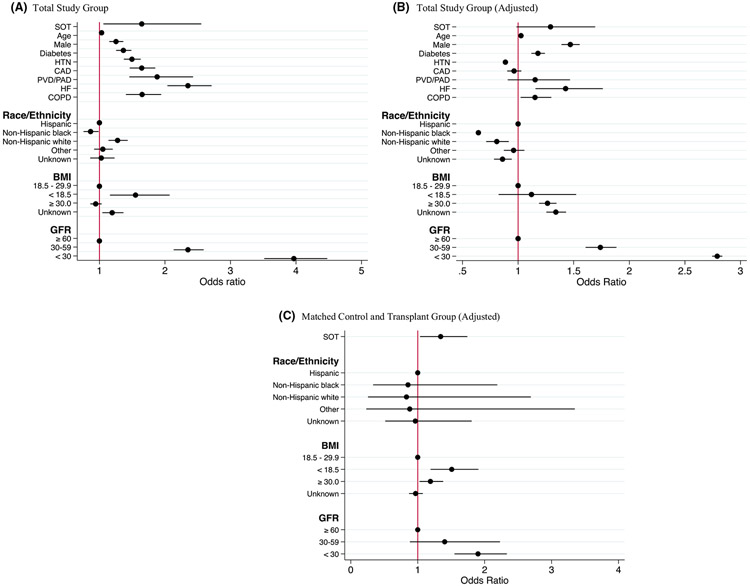
In the total study population (transplant and total control group), bivariate analysis found SOT status, older age, male gender, non-Hispanic white race/ethnicity, BMI <18.5, diabetes, hypertension, coronary artery disease, heart failure, peripheral vascular disease, GFR <60 mL/min/1.73 m2, and COPD to be significant predictors of death or mechanical ventilation (Table 5 and Figure 1). SOT status was also predictive of need for mechanical ventilation (OR 2.04, 95% CI 1.28–3.22; p = .002) and AKI requiring dialysis (OR 3.49, 95% CI 1.98–6.15; p < .001), but not death alone (OR 1.43, 95% CI 0.89– 2.27; p = .13).
TABLE 5.
Bivariate and multivariate analyses of risk factors associated with death or mechanical ventilation in patients hospitalized with COVID-19 infection
| Total study group unadjusted model |
Total study group adjusted model |
Matched cohort adjusted model |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | OR | 95% CI |
p value |
|
| SOT | 1.65 | 1.06–2.55 | .025 | 1.29 | .99–1.69 | .064 | 1.34 | 1.03–1.74 | .027 |
| Age | 1.03 | 1.03–1.04 | <.001 | 1.03 | 1.02–1.03 | <.001 | |||
| Male | 1.25 | 1.15–1.36 | <.001 | 1.47 | 1.39–1.55 | <.001 | |||
| Diabetes | 1.37 | 1.25–1.49 | <.001 | 1.18 | 1.12–1.24 | <.001 | |||
| Hypertension | 1.5 | 1.37–1.63 | <.001 | .89 | .88–0.89 | <.001 | |||
| CAD | 1.65 | 1.46–1.85 | <.001 | .96 | .9–1.03 | .27 | |||
| PVD/PAD | 1.88 | 1.46–2.43 | <.001 | 1.15 | .91–1.47 | .25 | |||
| Heart failure | 2.35 | 2.03–2.71 | <.001 | 1.43 | 1.16–1.76 | .001 | |||
| COPD | 1.65 | 1.4–1.94 | <.001 | 1.15 | 1.02–1.3 | .02 | |||
| Race/Ethnicity | |||||||||
| Hispanic | Ref | Ref | Ref | ||||||
| Non-Hispanic White | 1.28 | 1.14–1.43 | <.001 | .81 | .71–0.92 | .001 | .83 | .26–2.69 | .76 |
| Non-Hispanic Black | .86 | .76–0.99 | .03 | .64 | .63–0.66 | <.001 | .85 | .33–2.19 | .74 |
| Other | 1.05 | .92–1.2 | .54 | .96 | .87–1.52 | .4 | .88 | .23–3.35 | .85 |
| Unknown | 1.03 | .86–1.23 | .76 | .86 | .78–0.94 | .001 | .96 | .51–1.81 | .91 |
| BMI | |||||||||
| 18.5–29.9 | Ref | Ref | Ref | ||||||
| <18.5 | 1.55 | 1.16–2.07 | .003 | 1.12 | .82–1.52 | .47 | 1.51 | 1.19–1.91 | .001 |
| ≥30 | .94 | .86–1.03 | .21 | 1.26 | 1.19–1.34 | <.001 | 1.19 | 1.03–1.38 | .021 |
| Unknown | 1.19 | 1.04–1.37 | .01 | 1.34 | 1.25–1.43 | <.001 | .97 | .87–1.08 | .55 |
| GFR (mL/min/1.73 m2) | |||||||||
| ≥60 | Ref | Ref | Ref | ||||||
| 30–59 | 1.53 | 1.26–1.86 | <.001 | 1.74 | 1.61–1.88 | <.001 | 1.4 | .88–2.23 | .15 |
| <30 | 3.19 | 2.61–3.89 | <.001 | 2.79 | 2.74–2.84 | <.001 | 1.9 | 1.55–2.33 | <.001 |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; PVD/PAD, peripheral vascular disease/peripheral artery disease; SOT, solid organ transplant.
FIGURE 1.
Forest plot of bivariate (A) and multivariate (B, C) analyses demonstrating factors associated with death or mechanical ventilation in patients hospitalized with COVID-19 infection. SOT, solid organ transplant; HTN, hypertension; CAD, coronary artery disease; PVD/PAD, peripheral vascular disease/peripheral arterial disease; HF, heart failure; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate. Reference group for Race/Ethnicity = Hispanic. Reference group for BMI = BMI 18.5–29.9. Reference group for GFR = GFR ≥60 mL/min/1.73 m2
After multivariate logistic regression and adjusting for cluster effect of admission hospital, SOT status showed a trend toward the primary outcome of combined death or need for mechanical ventilation (OR 1.29, 95% CI 0.98–1.69; p = .06). Similar analysis performed on secondary outcome measures showed SOT status was a risk factor for requirement of mechanical ventilation (OR = 1.59, 95% CI 1.03–2.46; p = .036) and AKI requiring dialysis (OR = 1.96, 95% CI 1.37–2.81; p < .001) but not death alone (OR 1.17, 95% CI 0.78–1.88; p = .52). Additional independent risk factors for death or mechanical ventilation in the multivariate model were older age, male gender, obesity with BMI ≥30, diabetes, heart failure, COPD, Hispanic ethnicity, and low GFR <60 mL/min/1.73 m2 on admission. Multivariate conditional logistic regression on the matched cohort (transplant and matched control group) yielded an odds ratio of 1.34 for the primary outcome (combined death or mechanical ventilation) that was statistically significant after adjustment for BMI, race/ethnicity, GFR, and cluster effect of admission hospital (95% CI 1.03–1.74; p = .027). GFR below 30 mL/min/1.73 m2 and BMI ≥30 and <18.5 were also associated with the primary endpoint. SOT transplant status did not predict AKI requiring renal replacement therapy (OR = 1.7; p = .11), mechanical ventilation (OR = 1.41; p = .188), or death (OR = 1.12; p = .58) when tested separately.
7 ∣. DISCUSSION
Studies of SOT patients suggest worse outcome and higher mortality than the general hospitalized population, although biased by differences in comorbid conditions. In an attempt to better understand COVID-19 outcomes in SOT patients, we performed logistic regression in a large population of hospitalized patients and in a matched cohort. Upon adjusting for demographics and several comorbid conditions, our findings suggest a higher morbidity in SOT patients manifested by the need for mechanical ventilation in the total study population and a trend toward the combined endpoint of death or mechanical ventilation. When compared to the matched cohort (transplant and matched controls), SOT patients had an increased risk of the combined endpoint of death or mechanical ventilation. Mortality alone was not increased in either analysis.
Recent studies found transplant patients to be at increased risk for COVID-19 with a reported 20%–30% in-hospital mortality rate.5,6,8-10 This may be attributed to prevalent comorbidities in the transplant population such as diabetes, cardiovascular disease, hypertension, and obesity.1,3,20 Our study suggests that comorbid conditions account for much of the reported mortality risk, but SOT patients still appear to have a greater risk of a combined endpoint of death or mechanical ventilation. At this time it is unclear what role immunosuppression plays in these findings. Standard transplant immunosuppression targets the adaptive immune system by predominantly inhibiting interleukin-2 (IL-2), resulting in impaired T cell function and lymphopenia. T cell function is relevant in controlling viral replication, and prior studies have suggested worse COVID-19 outcomes in the setting of lymphopenia, a well-known side effect of immunosuppression.21 Alternatively, a recent publication (after our study period) demonstrated benefit with dexamethasone.22 Our database was not designed to differentiate among steroid dose and outcome, therefore could not confirm or disprove this finding. However, despite SOT patients being more likely to receive corticosteroids, outcomes were worse.
Several factors were found to impact ourprimary endpoint of death or mechanical ventilation. In the total study group, after regression analysis, age, male gender, BMI ≥30, diabetes, heart failure, COPD, and GFR <60 mL/min/1.73 m2 were all associated with worse outcomes. Although non-Hispanic ethnicity had a better outcome, race/ethnicity lost significance after regression analysis of the matched cohort. Unexpectedly HTN was associated with better outcome. In the matched cohort GFR less than 30 mL/min/1.73 m2 remained a strong predictor of death or mechanical ventilation along with either extremes of BMI.
The primary outcome of this study was death or mechanical ventilation. This choice was based on several factors unique to our study: (1) a diminished hyper-inflammatory reaction from immunosuppression may theoretically decrease pulmonary complications, including the need for mechanical ventilation; (2) based on the time frame we suspected a portion of the population would still be admitted; (3) previous COVID-19 studies had also included combined endpoints.23 In addition we also looked at death and mechanical ventilation separately as secondary endpoints.
The high incidence of AKI is an important secondary finding in our study. Recent study suggests AKI temporally occurs at the time of ventilation and is associated with worse outcomes.19 Higher disease severity and baseline renal dysfunction in SOT patients likely resulted in more AKI than controls, although the significance was lost after regression analysis of the matched cohort. Although no kidney transplant biopsies were obtained, manual chart review did not identify any SOT patient with suspected rejection after reduction of immunosuppression.
It should be acknowledged that our experience comes from an early and particularly hard hit region of the United States. Since then, treatment paradigms have shifted and several large studies have revealed disparate mortality rates among SOT patients. One large multicenter study had a similar morality rate as ours, 32%, while another had a lower rate of 20.5%.24,25 Two studies have compared SOT patients to a control group and found no difference in mortality.26,27 These studies along with ours have differed in terms of study design and volume. They are similar, however, as even our study did not reveal a difference in mortality alone, and suggested a higher incidence of AKI requiring dialysis.
Although the large volume of COVID-19 hospitalizations was a major strength of our study, it also has limitations. The study was retrospective and relied on chart review and ICD-10 coding, which may have led to misclassification of patients. We were unable to determine specific doses or duration of therapy for control patients treated with corticosteroids. Transplant patients were more likely to receive corticosteroids, though 67% were only on 5 mg of prednisone. We utilized admission creatinine to calculate baseline GFR, though manual review of 61 SOT patients suggested a small but significant lower baseline creatinine (data not shown). Since GFR was lower in SOT patients, GFR, BMI, and race were unable to fit into the matching algorithm. However, to account for these confounders, we performed logistic regression on the entire matched cohort (SOT patients plus matched controls). Follow-up stopped at hospital discharge and patients could have theoretically been readmitted or died later. In our large cohort of control patients it is possible that few were on immunosuppressants for other indications. In addition we were not able to manually extract symptoms before admission or determine the cause of death. Finally, renin-angiotensin-aldosterone system (RAAS) blockade has been postulated as potentially having an effect on COVID-19. Although it is not included in our analysis because of incomplete data, no study, to our best knowledge, has yet confirmed any benefit or harm associated with this drug class.25
In conclusion, we found an increased risk among hospitalized SOT patients of (1) the combined primary endpoint of death or mechanical ventilation than a matched control group and (2) mechanical ventilation and AKI requiring RRT compared to a large population of hospitalized COVID-19 patients. The role of immunosuppression in these findings is unclear, and although biopsies were not performed, there were no cases of suspected rejection. SOT patients infected with COVID-19 should be monitored closely with careful consideration to renal dysfunction.
ACKNOWLEDGMENTS
The authors acknowledge and honor all their Northwell team members who consistently put themselves in harm's way during the COVID-19 pandemic. They dedicate this article to them, as their vital contribution to knowledge about COVID-19 and sacrifices on the behalf of patients made it possible. This work was supported by grants R24AG064191 from the National Institute on Aging of the National Institutes of Health and R01LM012836 from the National Library of Medicine of the National Institutes of Health. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The views expressed in this paper are those of the authors and do not represent the views of the National Institutes of Health, the United States Department of Health and Human Services, or any other government entity. The corresponding author confirms that he had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding information
National Institute on Aging, Grant/Award Number: R24AG064191; National Institutes of Health, Grant/Award Number: R01LM012836
Abbreviations:
- AKI
acute kidney injury
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- CI
confidence interval
- CKD
chronic kidney disease
- COPD
chronic obstructive pulmonary disease
- COVID-19
coronavirus disease 2019
- EHR
electronic health record
- ESKD
end-stage kidney disease
- GFR
glomerular filtration rate
- HTN
hypertension
- ICD-10
International Classification of Diseases 10
- IL-2
interleukin-2
- IQR
interquartile range
- KDIGO
Kidney Disease Improving Global Outcomes
- OR
odds ratio
- RAAS
renin-angiotensin-aldosterone
- RAASi
renin-angiotensin-aldosterone inhibitor
- RRT
renal replacement therapy
- SD
standard deviation
- SOT
solid organ transplant system
- WHO
World Health Organization
APPENDIX
NORTHWELL HEALTH COVID-19 RESEARCH CONSORTIUM
Morgan Birabaharan1, Henry C. Bodenheimer1,2,6, Siavash Bolourani1,3,5, Vincenza A. Caruso3, Stuart L. Cohen1,3, Andrew J. Dominello3, Louise Falzon3, Jennifer Cookingham3, Jennifer C. Johnson3, Josue Minaya1, Trey Keel1, Akshay Khatri2,6, Robin V. Koshy2,6, Zachary M. Kozel1, Charlotte Kvasnovsky1, Martin Lesser1,3, Naomi I. Maria3, Jazmin N. Mogavero3, Rachel Monane3, Salem Najjar1, Jia Ng2,6, M. Wasif Saif1,2,6, Acacia Sheppard1, Cristina Sison3, Gerin R. Stevens1,2, Dylan Tan1, Sirish Vullaganti1,2,6
Footnotes
A complete list of Northwell Health COVID-19 Research Consortium investigators can be found in the Appendix.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from COVID19@northwell.edu. The data are not publicly available due to restrictions as it could compromise the privacy of research participants.
REFERENCES
- 1.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W-J, Ni Z-Y, Hu YU, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(24):759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segev DL, Muzaale AD, Caffo BS, et al. Perioperative mortality and long-term survival following live kidney donation. JAMA. 2010;303(10):959–966. [DOI] [PubMed] [Google Scholar]
- 5.Nair V, Jandovitz N, Hirsch JS, et al. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20(7):1819–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382(25):2475–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu L, Gong N, Liu B, et al. Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan. China. Eur Urol. 2020;77(6):748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández-Ruiz M, Andrés A, Loinaz C, et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;20(7):1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97(6):1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banerjee D, Popoola J, Shah S, et al. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;97(6):1076–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haberman R, Axelrad J, Chen A, et al. Covid-19 in immune-mediated inflammatory diseases - case series from New York. N Engl J Med. 2020;383(1):85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee LYW, Cazier J-B, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos-Casals M, Brito-Zerón P, López-Guillermo A, et al. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503–1516. [DOI] [PubMed] [Google Scholar]
- 18.Khwaja A KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. [DOI] [PubMed] [Google Scholar]
- 21.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. China. JAMA Intern Med. 2020;180(7):934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horby P, Lim WS, Emberson J, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020;NEJMoa2021436. published online July 17. [Google Scholar]
- 23.Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382(25):2411–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cravedi P, Mothi SS, Azzi Y, et al. COVID-19 and kidney transplantation: results from the TANGO international transplant consortium. Am J Transplant. 2020;20(11):3140–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kates OS, Haydel BM, Florman SS, et al. , COVID-19 in solid organ transplant: a multi-center cohort study. Clin Infect Dis, 2020; August 7;ciaa1097. Online ahead of print. [Google Scholar]
- 26.Chaudhry ZS, Williams JD, Vahia A, et al. Clinical characteristics and outcomes of COVID-19 in solid organ transplant recipients: a case-control study. Am J Transplant. 2020;20(11):3051–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molnar MZ, Bhalla A, Azhar A, et al. Outcomes of critically ill solid organ transplant patients with COVID-19 in the United States. Am J Transplant. 2020;20(11):3061–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from COVID19@northwell.edu. The data are not publicly available due to restrictions as it could compromise the privacy of research participants.