Abstract
Working memory capacity (WMC) measures the amount of information that can be maintained online in the face of distraction. Past work has shown that the efficiency with which the frontostriatal circuit filters out task-irrelevant distracting information is positively correlated with WMC. Recent work has demonstrated a role of posterior alpha oscillations (8 to 13 Hz) in providing a sensory gating mechanism. We investigated the relationship between memory-load modulation of alpha power and WMC in two verbal working memory experiments. In both experiments, we found that posterior alpha power increased with memory load during memory, in agreement with previous reports. Across individuals, the degree of alpha power modulation by memory load was negatively associated with WMC, namely, the higher the WMC, the less alpha power was modulated by memory load. After the administration of topiramate (TPM), a drug known to affect alpha oscillations and have a negative impact on working memory function, the negative correlation between memory load modulation of alpha power and WMC was no longer statistically significant but still somewhat detectable. These results suggest that (1) individuals with low WMC demonstrate stronger alpha power modulation by memory load, reflecting possibly an increased reliance on sensory gating to suppress task-irrelevant information in these individuals, in contrast to their high WMC counterparts who rely more on frontal areas to perform this function and (2) this negative association between memory-load modulation of alpha oscillations and WMC is vulnerable to drug-related cognitive disruption.
Keywords: Working memory capacity, Alpha power modulation, Sensory gating, Distractor filtering, Frontostriatal circuit
Introduction
Individual variability in the amount of information that can be stored in working memory with high fidelity can be quantified by working memory capacity (WMC) (Cowan, 2012; Engle et al., 1999). Higher WMC is linked to better performance on a variety of cognitive tasks, including attention, reading comprehension, planning, and problem solving (Adam and Hitch, 1997; Barrett et al., 2004; Daneman and Carpenter, 1980). It has even been demonstrated that higher WMC individuals can better overcome cognitive impairments resulting from aging and other brain disorders (Grenard et al., 2008; Otto et al., 2013).
As a measure of executive attention, WMC measures not only the capacity of the amount of information that can be stored, but also the capacity for sustaining attention to the stored information in the face of interference or distraction (Engle, 2002). Behaviorally, there is extensive evidence suggesting that the ability to inhibit task-irrelevant information is significantly higher in individuals with high WMC (Conway and Engle, 1994; Conway et al., 2001; Kane and Engle, 2000; Unsworth et al., 2004). Neuroscientifically, functional magnetic resonance imaging (fMRI) studies have shown that high WMC individuals, compared to their low capacity counterparts, have greater modulation of BOLD responses in the lateral prefrontal cortex (lateral PFC) when performing working memory tasks that require a high degree of attentional control, including n-back tasks (Burgess et al., 2011), Sternberg tasks with distraction (Minamoto et al., 2010), and complex span tests (Kondo et al., 2004a, 2004b; Osaka et al., 2003). Such working memory demand-dependent modulation of the lateral PFC is consistent with the notion that greater modulation of neural activation in higher WMC individuals reflects a higher capability at resolving interference from a competing task or task-irrelevant external/internal distractors to maintain goal-related information (Conway et al., 2003; D’Esposito and Postle, 2015). Moreover, McNab and Klingberg (2008) showed that distractor filtering activities in frontal cortex and basal ganglia were associated with individual differences in WMC, and proposed that the frontostriatal circuit is a key neural system that performs the function of excluding task-irrelevant information in high WMC individuals. Specifically, the basal ganglia helps accomplish this objective via a dynamic gating mechanism in which an inhibitory or disinhibitory signal is transiently transmitted to the prefrontal cortex to either suppress the processing of task-irrelevant information or enhance the processing of task-relevant information (Hazy et al., 2007).
In addition to the frontal filtering of distractor information, recent work has explored sensory gating mechanisms in the posterior sensory cortex, indexed by alpha oscillation (8 to 13 Hz) and its goal-oriented modulation. A frequently replicated finding is that during verbal working memory retention, alpha power increases as a function of working memory load (Jensen et al., 2002a; Klimesch et al., 2007; Sauseng et al., 2009), and such increases in alpha power, argued to reflect reduced sensory gain and excitability, serves to protect the information maintained online from external interference (Haegens et al., 2010; Jensen and Mazaheri, 2010; Jokisch and Jensen, 2007; Kaiser et al., 2007; Klimesch et al., 2007; Mathewson et al., 2011; Medendorp et al., 2007; Sauseng et al., 2009); the higher the memory load, the stronger the need for such sensory gating. In contrast, in the case of visual working memory tasks, in which subjects maintained visual items online after they were removed from the environment, alpha power decreases with increase in visual working memory load, reflecting increased visual cortex activation necessary for maintaining the representations of the increased amount of visual information. Importantly, Fukuda et al., (2015) showed that alpha power modulation by visual working memory load is associated with WMC; specifically, the larger the WMC, the more alpha power decreases as visual memory load increases. Is alpha power modulation by verbal working memory load related to WMC? This question has not been addressed. Addressing this question will help us better understand the relation between the frontal mechanisms of distractor suppression and the posterior mechanisms of sensory gating.
Many central nervous system (CNS) drugs can affect cognition as well as alter rhythmic brain activities. Topiramate (TPM), a second-generation antiepileptic drug with formal indications for partial and generalized seizures and migraine prophylaxis, has repeatedly been shown to have a pronounced negative impact on a wide range of cognitive functions (Javed et al., 2015), including verbal fluency (Marino et al., 2012; Thompson et al., 2000), language comprehension (Fritz et al., 2005), attention (de Araujo Filho et al., 2006), and short-term (Gomer et al., 2007) and working memory (Jung et al., 2010; Marino et al., 2012). While our previous work has examined the adverse behavioral effect of TPM on verbal working memory (Marino et al., 2012), and demonstrated a relationship between TPM-related behavioral detriments and WMC (Barkley et al., 2018), the neural correlates of TPM-mediated reduction in task performance remains to be understood. TPM is known to affect alpha oscillations (Neufeld et al., 1999). How does TPM affect the task-dependent modulation of alpha oscillations? Does TPM disrupt the relationship between memory-load modulation of alpha and WMC? Addressing these questions will help us better understand how alpha-mediated cognitive functions are affected by CNS drugs.
We analyzed high-density electroencephalogram (EEG) data (128 channels) from two separate experiments in which two groups of healthy human volunteers performed verbal working memory tasks. In the first experiment, conducted at the University of Florida, the subjects were shown a set of distinct digits (0 to 9) on a CRT monitor for 1s. Following a 3s retention period, a probe digit was presented, and a “yes” or “no” button press was required to indicate whether the probe digit belonged to the cue set. Memory load was controlled by the number of digits presented in the cue set (1, 3, or 5 digits). In the second experiment, conducted at the University of Minnesota, the subjects were presented a string of one, three, or five pronounceable syllables (load-1, load-3 and load-5) on a monitor for 1.5 s. This was followed by a 5 s retention period, during which subjects were instructed to retain the syllable string in memory. At the end of the retention period, a probe string was presented, and subjects were instructed to press a “yes” or “no” button to indicate whether or not the probe matched the cue string. In addition to the baseline session, the subjects participated in a follow-up session in which they were given different doses of TPM, and had their brain responses measured while completing the same verbal working memory task.
Materials and Methods
Overview
Two separate experiments employing similar verbal working memory paradigms were conducted at the University of Florida (UF dataset) and at the University of Minnesota (UM dataset). The UM dataset contained one additional manipulation in which the participants, on a follow-up visit, performed the same paradigm after taking the antiepilepsy drug topirimate (TPM), which is known to adversely impact cognition (Marino et al., 2012) and alter alpha oscillations (Neufeld et al., 1999). Analyses focusing on different aspects of the two datasets have been published (Barkley et al., 2018; Wang et al., 2016). This study is a reanalysis of the previously published data. The main purpose of including two experiments here is to test the replicability of the main findings and the robustness of the findings against drug-related cognitive impairments.
Participants and experimental paradigm
UF dataset:
The experimental protocol was approved by the University of Florida Institutional Review Board. Twenty-one healthy volunteers (ages 20–34, 3 women) gave written informed consent and participated in the study. All participants were right-handed, had normal or corrected-to-normal vision, and reported no history of psychiatric or neurological disorders. EEG was recorded from the participants while they performed a Sternberg working memory task (see below). One subject was excluded due to poor performance (accuracy less than 60%). The data from the remaining 20 individuals were analyzed and reported here.
The working memory paradigm is shown in Figure 1A. In each trial, a cue set of numerical digits (0–9) was displayed for 1 second (encoding), followed by a 3 second memory retention period. At the end of the retention period, a probe digit was presented, and the subject was instructed to indicate whether the probe digit was part of the cue set by a “yes” or “no” button press. Working memory load was determined by the number of digits in the cue set, which in this case, was either 1, 3, and 5 digits (load-1, load-3, and load-5). (Figure 1A depicts a load-5 trial in which ‘yes’ is the correct response.) The entire experiment consisted of 6 blocks with 60 trials in each block. The three memory loads were equally likely to occur. Breaks were given between blocks. Participants received a practice session prior to the experiment to familiarize themselves with the task and to minimize the effect of learning.
Figure 1.
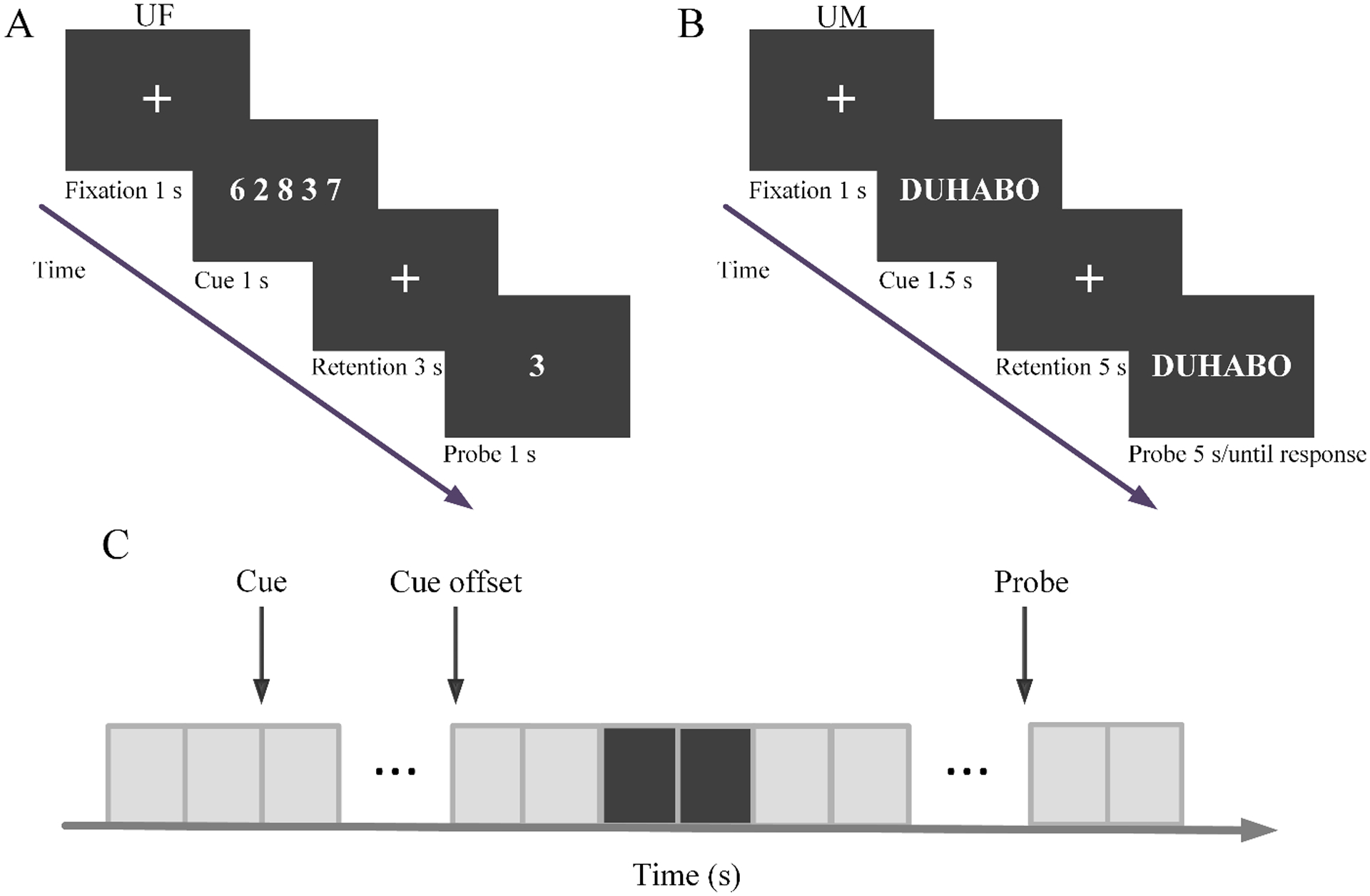
Paradigms for the two experiments. (A) Timeline of the verbal working memory task used at UF. (B) Timeline of the verbal working memory task used at UM. (C) Time period of interest during retention (black). Each rectangle represents 0.5 s.
UM dataset:
The experimental protocol was approved by the Institutional Review Board of the University of Minnesota prior to the commencement of the study. Forty-six healthy right-handed volunteers with no history of psychiatric or neurological disorders gave written informed consent and participated in the study. Seventeen subjects were excluded because of missing data resulting from technical issues with data acquisition or storage. Six participants were excluded from further analyses due to: 1) an overall accuracy rate below 60%; or 2) excessive head or body movements. Data from the remaining 23 subjects (mean age=25.60 ± 8.04; 14 women) were included in the analyses.
The experimental paradigm is shown in Figure 1B. At the beginning of each trial, a cue string of one, three, or five pronounceable syllables (load-1, load-3 and load-5) was displayed on a monitor for 1.5 s (encoding). This was followed by a 5 s retention period, during which subjects were instructed to retain the syllable string in working memory. At the end of the retention period, a probe string was presented, and subjects were instructed to press a “yes” or “no” button to indicate whether or not the probe matched the cue. (Figure 1B depicts a load-3 trial in which ‘yes’ is the correct response.) The next trial was triggered by either a response, or the absence of one, within 5 s of probe onset. The entire task consisted of 10 blocks of 36 trials each. There were an equal number of trials for each memory load (1, 3, or 5 syllables); trials were randomized as a function of memory load.
There were two sessions in the UM experiment. In addition to the baseline visit (BAS) in which the subjects completed the task in Figure 1B while their electroencephalogram (EEG) was recorded, in a follow-up visit separated by a 2-week interval, the subjects were randomized to receive either 100 mg (8 subjects), 150 mg (8 subjects), or 200 mg (7 subjects) of TPM to induce a wide range of TPM concentrations across individuals. Four hours after drug administration, the subjects’ EEG were recorded while they completed the same verbal working memory task. The study design was double-blind, randomized, and placebo-controlled with the drug being dispensed by the University of Minnesota Investigational Drug Services pharmacy.
Data acquisition
UF:
High density EEG data were recorded using a 128-channel BioSemi System at a sampling rate of 1 KHz. Eye movements and eye blinks were monitored using additional electrodes. Stimuli were presented using the BeriSoft Experimental Run-Time System (ERTS) and the subject’s responses were recorded with an EXKEY microprocessor logic pad (http://www.berisoft.com).
UM:
High density EEG data were recorded using a 128-channel EGI System (Electrical Geodesics Inc., USA) at a sampling rate of 1 KHz. Impedances were kept below 50 KΩ per manufacturer’s recommendation. Eye movements and eye blinks were monitored. Stimulus presentation and behavioral response recording were controlled with E-prime software (Psychology Software Tools, Inc., Pittsburgh, PA, USA).
Data analysis
Working memory capacity:
The participants’ individual WMC was quantified by Cowan’s K (Cowan, 2001; Pashler, 1988) which is defined as
where S is the size of the cue set, H is the hit rate, and F is the false alarm rate. To obtain a single WMC measure for each individual, K values for load 3 and load 5 were averaged (Fukuda et al., 2015; Vogel and Machizawa, 2004). To facilitate comparison across datasets, the WMC values so obtained within each dataset were transformed into z-scores.
EEG data preprocessing:
EEG data preprocessing was performed using BESA 6.0 (www.besa.de), EEGLAB (http://sccn.ucsd.edu/eeglab/index.html), and custom Matlab scripts. The continuous EEG data were bandpassed between 0.1 to 30 Hz, downsampled to 250 Hz, and re-referenced against the average reference. For the UF data, each trial was epoched from −500 to 4000 ms with 0 ms denoting the onset of the cue and −500 to 0 ms being the precue period. For each memory load, the data from the retention time period: 2000–3000 ms, defined as the time period of interest, were analyzed (Figure 1C). Here, the segment of 1000 ms to 2000 ms was excluded to avoid the negative impact of cue-offset evoked activities on the spectral analysis of ongoing neural oscillations and the segment of the 3000–4000 ms was excluded to avoid the negative impact of the anticipation of probe processing on alpha oscillations. For the UM dataset, each trial was epoched from −200 – 6500 ms with 0 ms denoting cue onset and −200 to 0 ms being the precue period. For similar reasons as above, the time period of interest was chosen to be 2500–3500 ms. For both datasets, trials with incorrect responses or contaminated by large movement-related artifacts were excluded from further analysis. For the remaining trials, independent components analysis (ICA) (Delorme and Makeig, 2004) was applied to remove artifacts due to eye movements, eye blinks, and other sources of noise that were not related to brain activity. To minimize the negative effects of volume conduction and common reference, the artifact-corrected scalp voltage data were converted to reference-free current source density (CSD) by calculating 2D surface Laplacian algorithm (Kayser and Tenke, 2006). All subsequent analyses were performed on the CSD data.
Power spectral density estimation (PSD):
For both datasets, Fast Fourier transforms (FFT) were applied to the data in the time period of interest to estimate the power spectra. Normalization by power in the precue baseline period was done on a subject-by-subject basis (1 to 30 Hz) (Jensen et al., 2002). This normalization procedure removed the influence of amplitude variability from subject to subject and allowed more straightforward averaging across participants. Alpha frequency bands were defined to be from 8 to 13 Hz.
Alpha modulation index:
To quantify the modulation of alpha power by memory load (L), we constructed a linear regression model
where Y is the alpha power and β is the fitted regression coefficient. Larger β values reflect stronger alpha modulation by memory load. A schematic illustration of large β versus small β was given in Figures 3A and 3B. L equals to 1, 3 and 5 for both UF and UM datasets. The posterior region of interest (ROI) used for alpha modulation analysis was defined according to our previous paper on memory-load modulation of alpha in verbal working memory (Wang et al., 2016). The channels included in the ROI such as PO7, P5, P3, P1, P03, O1, Oz, POz, Pz, P2, O2, P4, PO4, P6, and PO8 are similar to channels used in previous EEG alpha studies of verbal working memory (Haegens et al., 2010; Jensen and Mazaheri, 2010; Jensen et al., 2002; Jokisch and Jensen, 2007; Klimesch, 2012; Mathewson et al., 2011; Medendorp et al., 2007; Sauseng et al., 2009), which facilitate comparison across studies.
Figure 3.
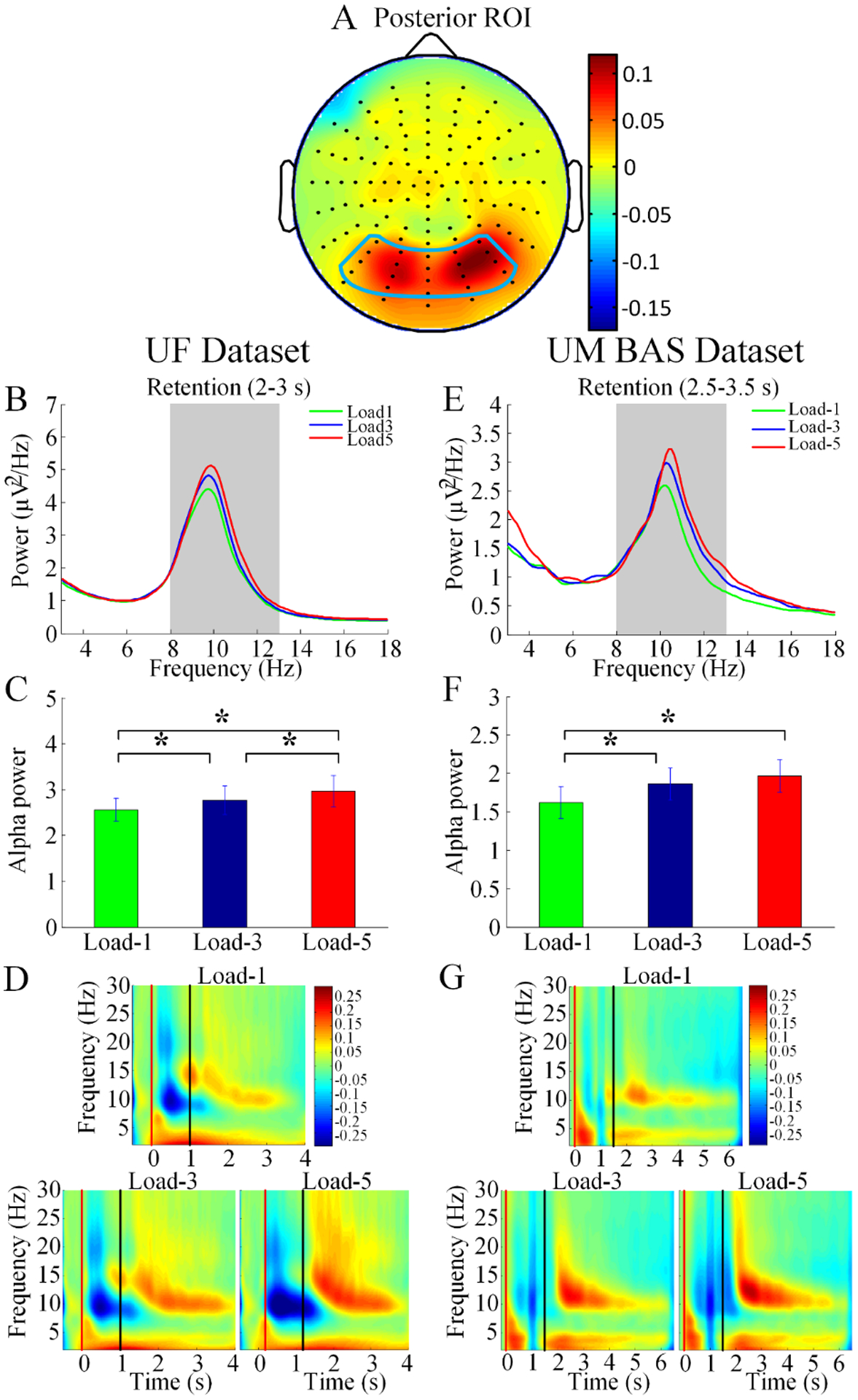
Alpha modulation by memory load. (A) Posterior region of interest (ROI) for the analysis of alpha activity was superimposed on the topographical map of alpha power modulation by working memory load (averaged across the UF and UM datasets). (B) Power spectra for different memory load and (C) modulation of alpha power (8–13Hz) by memory load during retention for UF dataset. (E) Power spectra for different memory load and (F) modulation of alpha power by memory load during retention for UM Baseline (BAS) dataset. Time-frequency analysis for (D) UF dataset and (G) UM BAS dataset. *p < 0.05.
Time-frequency analysis:
Time-frequency analysis was also performed to examine the evolution of neural activities under different working memory loads and across different stages of the working memory task. The time-frequency power changes were computed by calculating the percentage change in power from the precue baseline for different frequencies and different times using a complex Morlet wavelet transform (Herrmann et al., 1999; Tallon-Baudry et al., 1997). The number of cycles was selected according to the frequency (scale) and was increased from 0.5 at 1 Hz to 13.8 at 30 Hz. It has been suggested that this approach provides better frequency resolution at higher frequencies than a conventional wavelet approach that uses constant cycle length (Delorme and Makeig, 2004). The same wavelet transform as the one used for the whole trial analysis was applied to the two datasets. All the time-frequency calculations for a given working memory load were performed after subtracting the ensemble mean (ERP) of that load condition from all trials, in order to minimize the influence of stimulus-evoked response on spectral estimation (Kalcher and Pfurtscheller, 1995). This was done for each electrode, each condition and each subject separately.
Results
Behavioral results
As shown in Figure 2, reaction time increased as a function of memory load: UF dataset (F2,57 = 17.22, p < 0.001, Figure 2A) and UM BAS dataset (F2,66 = 42.63, p < 0.001, Figure 2C), and accuracy decreased with increasing memory load: UF dataset (F2,57 = 6.35, p < 0.005, Figure 2B) and UM baseline (BAS) dataset (F2,66 = 86.39, p < 0.001, Figure 2D). These results are in line with previous studies using similar paradigms (Bashivan et al., 2014; Jensen et al., 2002a; Leiberg et al., 2006; Michels et al., 2008; Nenert et al., 2012; Stokić et al., 2015; Tuladhar et al., 2007).
Figure 2.
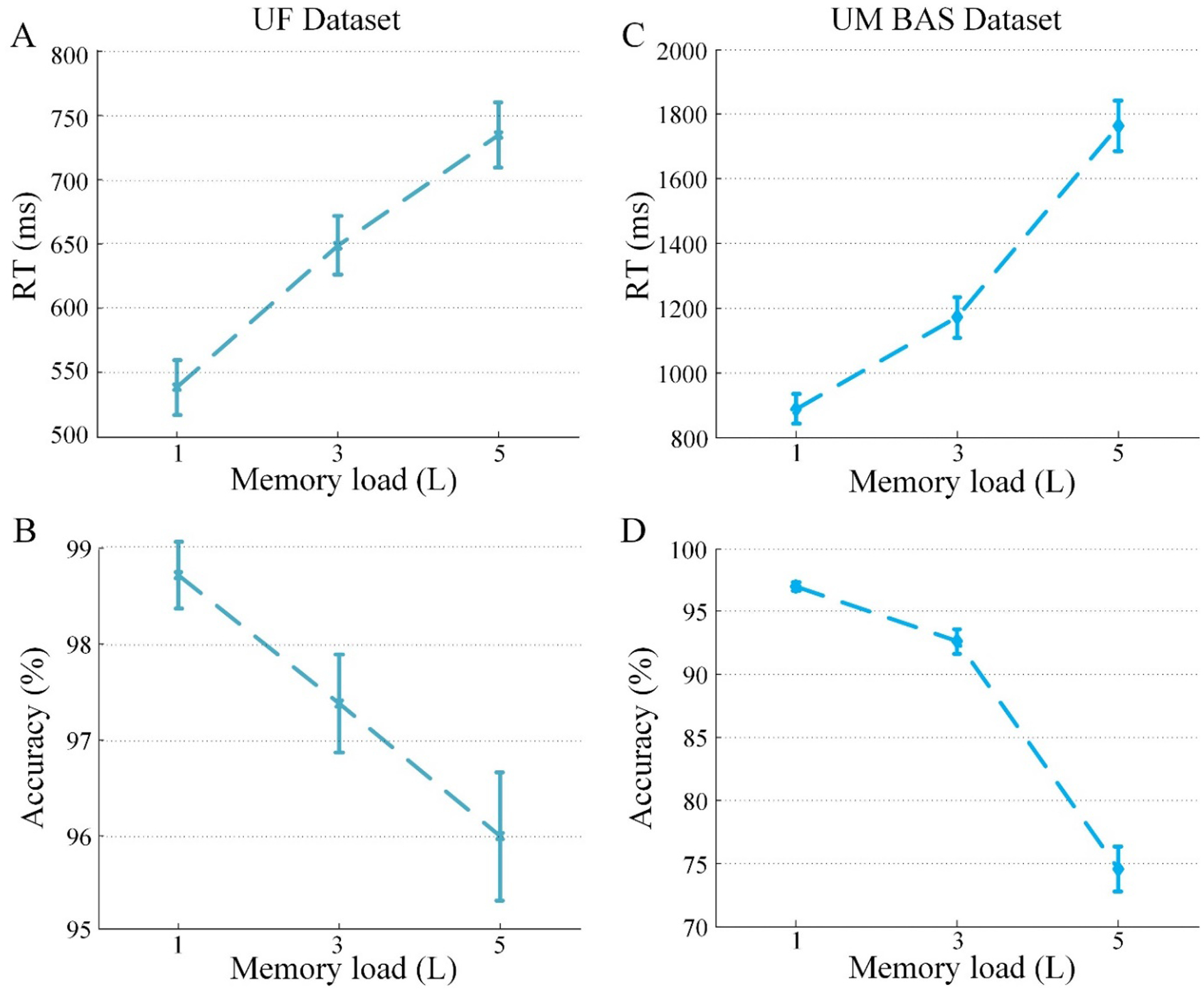
Behavioral results. (A) Reaction time (RT) and (B) accuracy as a function of memory load for the UF dataset. (C) RT and (D) accuracy as a function of memory load for the UM BAS dataset. Error bars are standard errors of the mean (SEM).
Alpha power modulation by memory load during retention
Posterior ROI for the analysis of alpha activity, superimposed on the topographical map of alpha power modulation by working memory load, was shown in Figure 3A. For the UF data, during the retention period, alpha power increased as a function of memory load (load-5 > load-1, t19 = −2.67, p = 0.015; load-3 > load-1, t19 = −2.21, p = 0.039; load-5 > load-3, t19 = −2.15, p = 0.048; Figure 3B–C); a similar pattern was also observed for the UM BAS data (load-5 > load-1, t25 = −2.14, p = 0.043; load-3 > load-1, t25 = −2.58, p = 0.017; load-5 > load-3, t25 = −0.92, p = 0.37; Figure 3E–F). A time-frequency analysis was carried out to examine the evolution of oscillatory activities across different stages of the working memory task under different memory loads. As shown in Figure 3D and 3G, in both datasets, the increase of alpha power during retention with increase in working memory load is clearly visible.
Memory-load modulation of alpha power and WMC
Load-dependent modulation of alpha power at the individual subject level can be quantified using the alpha modulation index (see Materials and Methods). An example of large alpha power modulation and an example of small alpha power modulation were schematically shown in Figures 4A and 4B. The relationships between the alpha modulation index and individual WMC for both datasets were shown in Figure 4C and 4D. For the UF dataset, there was a significant negative correlation between the alpha modulation index and WMC (r = −0.53, p = 0.016, d = −1.25; Figure 4C); in other words, the higher the WMC, the lower the alpha modulation index. Here, in addition to calculating p values, effect sizes were also reported where appropriate in terms of Cohen’s d. According to established conventions, effect size was considered small if 0.2<|d|<0.5, medium if 0.5<|d|<0.8, and large if |d|>0.8 (Rosenthal, 1984). For the UM BAS dataset, the same negative correlation between alpha modulation index and WMC was observed (r = −0.56, p = 0.006, d = −1.35; Figure 4D). In addition to WMC, we also considered behavioral accuracy. There was a significant positive correlation between accuracy and WMC in both UF (r = 0.99, p < 0.001, d = 15.72) and UM BAS dataset (r = 0.97, p < 0.001, d = 9.16), suggesting that the averaged K values can explain most of the variance of the working memory task performance in both datasets. Moreover, alpha modulation index and accuracy were significantly negatively correlated in both UF (r = −0.55, p < 0.012, d = −1.32) and UM BAS dataset (r = −0.59, p < 0.003, d = −1.44).
Figure 4.
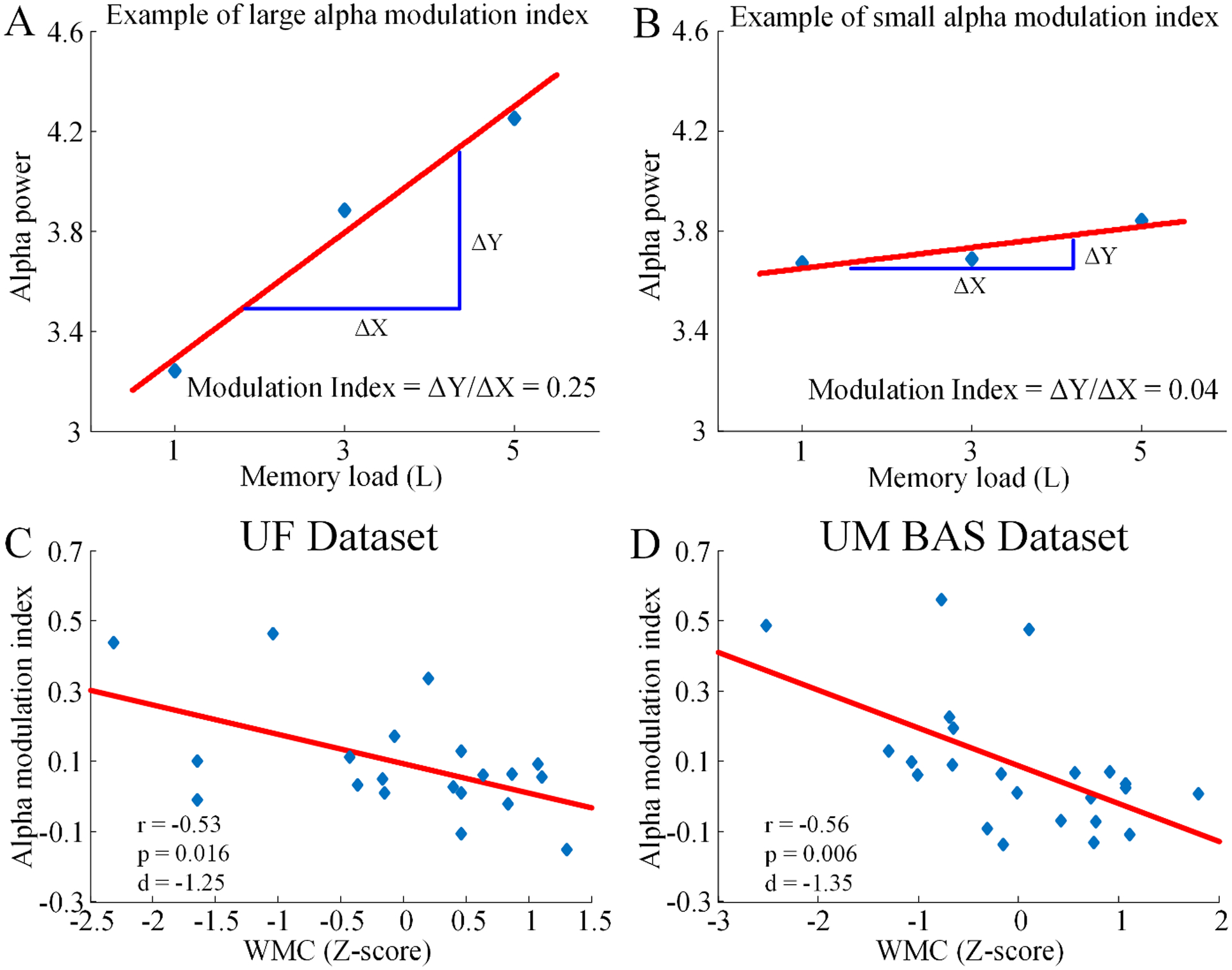
Alpha modulation index and WMC. Schematic examples of a large (A) and a small (B) alpha modulation index. Correlation between WMC and alpha modulation index for (C) the UF dataset and (D) the UM BAS dataset. Each point in the scatter plots represents an individual subject.
Alpha power and WMC
In visual working memory, Fukuda et al. (2015) found that only alpha power modulation by memory load, not alpha power per se, was correlated with WMC. In the present verbal working memory paradigms, we examined the relationship between alpha power and WMC as well as task performance, and presented the results in Table 1. Consistent with the findings in visual working memory, there were no significant correlation between alpha power and WMC for any of the memory load conditions, suggesting that it is the magnitude of task-demand based alpha power modulation rather than the magnitude of alpha power that is characteristic of an individual’s working memory function.
Table 1.
Correlation coefficient between alpha power and Accuracy, RT, and Cowan’s K for each memory load (p-value in parenthesis).
| Loads | Accuracy | RT | Cowan’s K | |
|---|---|---|---|---|
| UF Dataset | Load-1 | .183 (0.44) | .203 (0.39) | .182 (0.45) |
| Load-3 | −.091 (0.70) | .042 (0.86) | −.091 (0.70) | |
| Load-5 | −.194(0.41) | .171 (0.47) | −.193 (0.42) | |
| UM BAS Dataset | Load-1 | −.105 (0.63) | .003 (0.99) | −.106 (0.63) |
| Load-3 | −.066 (0.76) | .081 (0.71) | −.065 (0.76) | |
| Load-5 | −.246 (0.26) | −.189 (0.39) | −.247 (0.25) |
Effects of TPM on task performance
Effects of memory load (levels: load-1, load-3, or load-5) and treatment (BAS, TPM-100mg, TPM-150mg, or TPM-200mg) on accuracy (Figure 5A) and RT (Figure 5B) were analyzed using 3 × 4 repeated-measures analyses of variance. Regarding accuracy, there were significant main effects of both memory load (F2,126 = 129.30, p < 0.001) and treatment (F3,126 = 25.89, p < 0.001); the memory load × treatment interaction was not significant (F6,126 = 0.54, p = 0.76). Regarding RT, there were significant main effects of both memory load (F2,126 = 27.66, p < 0.001) and treatment (F3,126 = 4.74, p = 0.0036); the memory load × treatment interaction was not significant (F6,126 = 0.97, p = 0.45).
Figure 5.
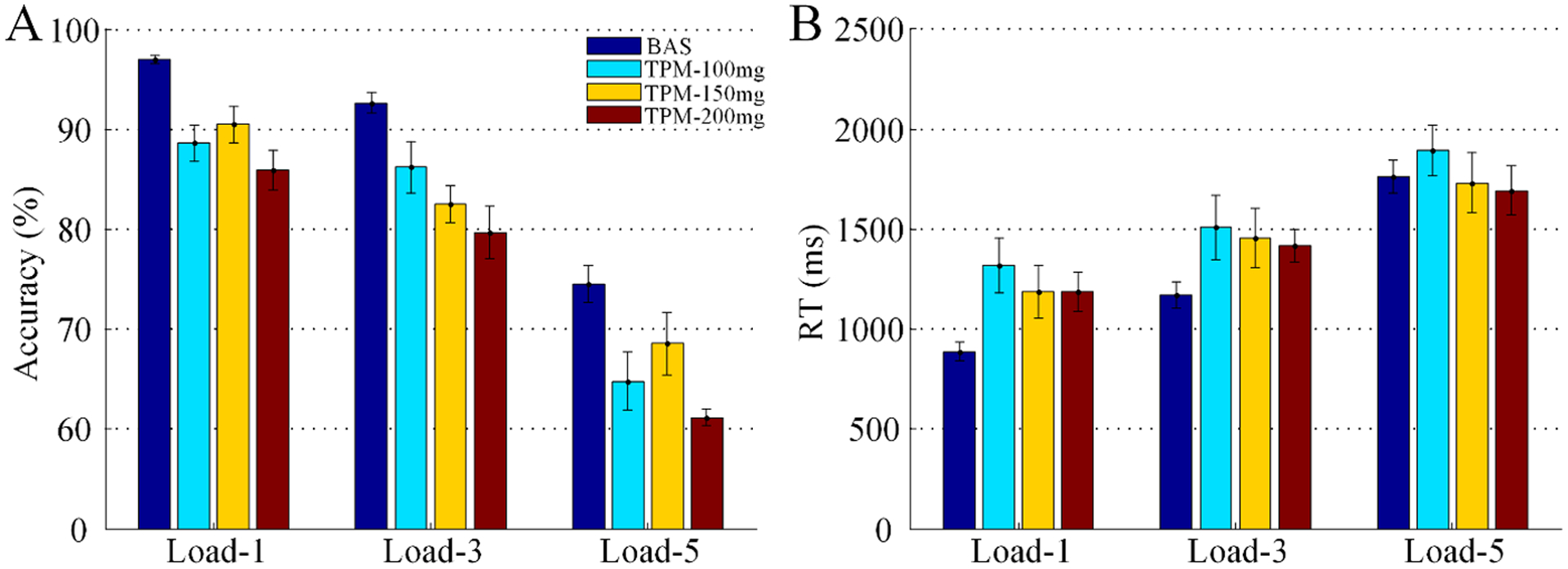
Effects of TPM on working memory task performance (UM TPM dataset). Data were shown separately for the 3 doses of TPM on load-1, load-3, and load-5 conditions. Error bars represent SEM.
Effects of TPM on relation between memory-load modulation of alpha and WMC
Under TPM, alpha power increased from load-1 to load-3 and from load-1 to load-5 but it was not significantly different between load-3 and load-5 (load-5 > load-1, t19 = −2.24, p = 0.035; load-3 > load-1, t19 = −2.29, p = 0.032; load-5 < load-3, t19 = −0.12, p = 0.91; Figures 6A and 6B). Alpha modulation index under different doses of TPM was not significantly different (p>0.1; Figure 6C) and the data from different dose groups were therefore combined for the next analysis. After the administration of TPM, the alpha modulation index and WMC were no longer significantly correlated (r = −0.20, p = 0.35, d = −0.41; Figure 6D), although at effect size of d=−0.41, it could be said that the negative correlation is still somewhat preserved with a small effect size.
Figure 6.
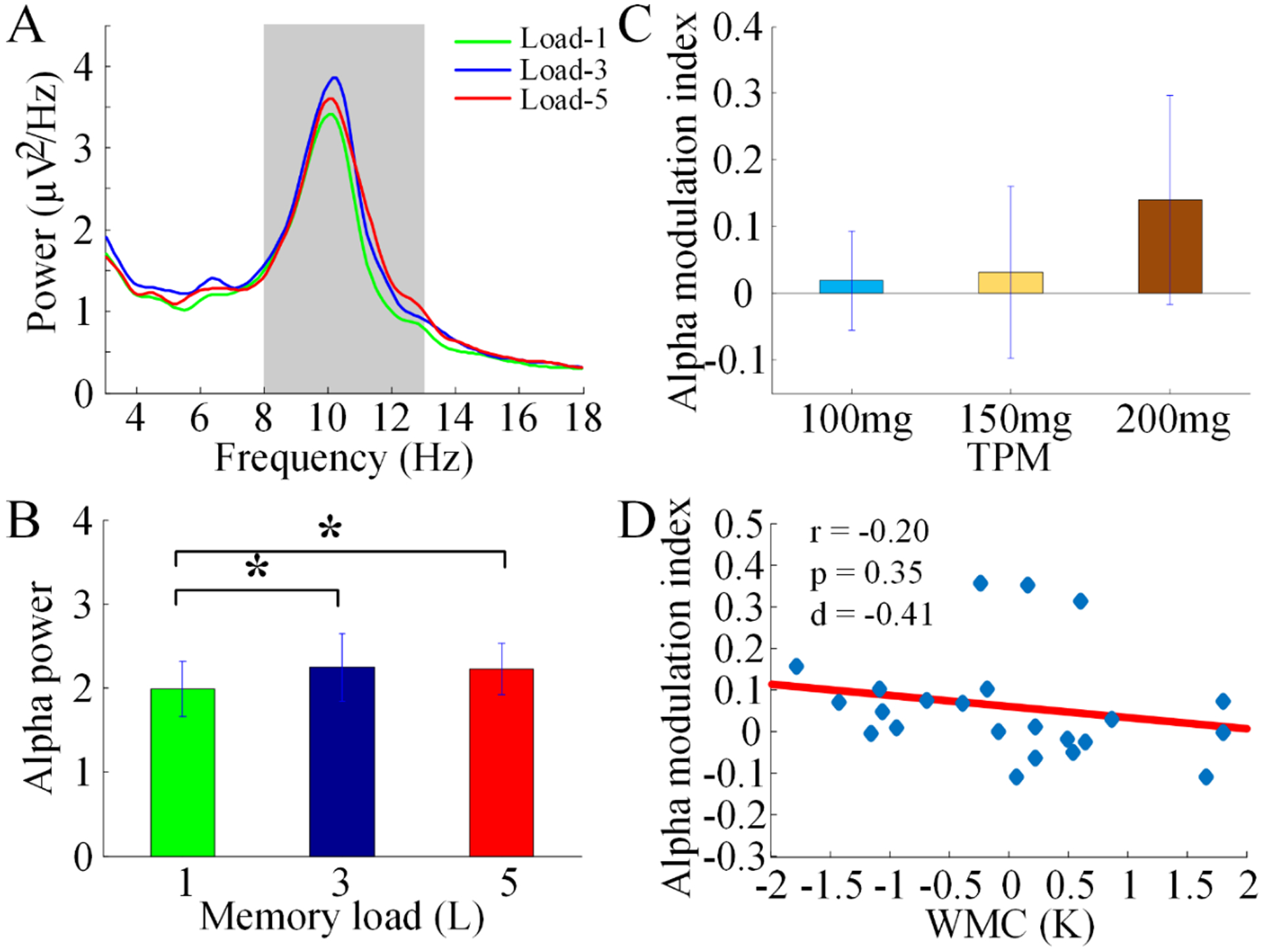
Effects of TPM on modulation of alpha power by memory load for UM TPM dataset. (A) Power spectra over the posterior ROI. (B) Alpha power for each memory load condition (*p < 0.05). (C) Alpha modulation index under the 3 doses of TPM. (D) The correlation between WMC and alpha modulation index.
Discussion
Two high-density EEG datasets recorded from healthy human volunteers performing verbal working memory tasks were analyzed to examine the relationship between memory-load modulation of posterior alpha power and WMC. The following results were found. First, in agreement with previous reports, posterior alpha power increased with memory load during working memory retention, irrespective of whether the remembered verbal information was numerical digits or pronounceable syllables. Second, across individuals, the degree of alpha power modulation by memory load was negatively associated with WMC, namely, the higher the WMC, the less the alpha power modulation by memory load. These two results were consistent across the two datasets. Third, in the UM dataset, after the administration of TPM, a drug known to adversely impact working memory and alter brain’s rhythmic activities, the negative correlation between memory-load modulation of alpha power and WMC became weaker (no longer statistically significant) but was still somewhat detectable with a small effect size (d=0.41).
Increase of alpha power as a function of verbal working memory load during memory retention is a widely replicated finding. In light of the inverse relationship between alpha power and cortical excitability, this finding is thought to reflect a GABA-mediated increase in functional inhibition of visual cortex, which could serve as a sensory gating mechanism to protect information held online from sensory interference (Jensen and Mazaheri, 2010; Jones et al., 2000; Klimesch et al., 2007; Lőrincz et al., 2009; Wang et al., 2016). One line of corroborating evidence supporting the alpha sensory gating hypothesis comes from simultaneous recordings of EEG and fMRI during working memory tasks (Michels et al., 2010; Scheeringa et al., 2009), which showed that alpha band power in posterior regions is negatively correlated with blood oxygenation level-dependent (BOLD) signal in the visual cortex during working memory retention. Furthermore, several studies have found that when to-be-remembered and to-be-ignored items are displayed simultaneously in separate hemifields, alpha activity increases over the hemisphere ipsilateral to the relevant hemifield and that this effect increases with memory load (Sauseng et al., 2009; Vissers et al., 2016). Using a Sternberg working memory task in which distractors were presented in the retention interval, whose strength and exact timing could be anticipated, Bonnefond and Jensen found a stronger alpha power increase in occipitotemporal areas prior to strong compare to weak distractor onsets, and observed a significant negative correlation between alpha power modulation and reaction time difference between weak and strong distractors. In other words, the stronger the difference in alpha power between anticipated distractor types, the smaller the difference in reaction time. These data suggest that alpha oscillations are modulated according to task conditions and the degree of alpha power modulation, rather than the alpha power itself, is linked to the effectiveness of the sensory suppression of distracting information. In visual working memory, Fukuda, Mance, and Vogel (2015) reported similar finding, namely, it is the alpha power modulation by memory load (i.e., degree of alpha decrease with increase in memory load) that predicts WMC rather alpha power per se. Our findings that there were no significant correlation between alpha power and WMC extends their finding to the verbal working memory domain where the alpha is modulated in the opposite direction (i.e., alpha increase with memory load).
As a measure of executive attention, WMC is not simply a measure of the capacity for storing information, but also a measure of the ability to sustain attention to the stored information in the face of interference or distraction (executive attention). Higher WMC individuals may be more efficient at filtering out task-irrelevant distractors in addition to having higher capacity to store more information. Indeed, studies have suggested that individuals with low WMC tend to be more easily distracted by task-irrelevant information, whereas high WMC individuals tend to excel at focusing attention on task-relevant information (Vogel et al., 2005). Neurophysiologically, it has been suggested that distractor filtering in working memory may be carried out by the frontostriatal circuit, principally consisting of the prefrontal cortex (Vogel et al., 2005) and the basal ganglia (Alexander et al., 1986). In this circuit, the basal ganglia provides a dynamic gating mechanism by transiently providing either an inhibitory or disinhibitory signal to the prefrontal cortex (Hazy et al., 2007), which suppresses the processing of task-irrelevant information or enhances task-relevant information. This role for the basal ganglia in working memory is thought to be highly similar to its involvement in gating the selection of actions in motor regions of the prefrontal cortex (Mink, 1996). In addition, the involvement of the basal ganglia in selecting items to be remembered is consistent with the evidence that this structure is important for a person’s ability to shift between task sets (Hayes et al., 1998), a process in which the active inhibition of irrelevant task sets is important (Mayr and Keele, 2000). Together, the frontostriatal circuit may determine what information is actively kept online for the current task, which then determines which items will gain admittance to the limited working memory. A recent study provides direct evidence supporting a role of the basal ganglia and prefrontal cortex in controlling the flow of task-relevant information into working memory (McNab and Klingberg, 2008). Consistent with the theory that an individual’s WMC is determined by their ability to selectively filter task-irrelevant distractors (Vogel et al., 2005), basal ganglia and prefrontal cortical activity was a significant predictor of WMC, manifesting a positive association between WMC and the ability of the frontostriatal circuit to filter distracting information.
The observed negative correlation between alpha power modulation by memory load and WMC over two experiments adds a new dimension to the current literature: individuals with low WMC demonstrate stronger alpha power modulation by memory load. We speculate that this may reflect the fact that individuals with a weaker ability to filter out distraction in the frontostriatal circuit relies more on sensory gating to achieve this function. High WMC individuals, on the other hand, having stronger frontal mechanisms for distractor suppression, is not as dependent on sensory gating. This phenomenon is reminiscent of the neural compensation phenomenon observed in cognitive aging. Neuroimaging studies have shown that in older adults, compared with young adults, neural activity increases in a variety of brain areas including the prefrontal cortex and posterior parietal cortex (Greenwood, 2007; Lighthall et al., 2014; Park and Reuter-Lorenz, 2009; Rajah and D’Esposito, 2005; Steffener et al., 2009). These increased activities, reflecting reorganized brain functioning, are thought to be compensatory, whose function is to counteract neural decline and to maintain task performance (Chanraud and Sullivan, 2014).
In visuo-spatial working memory tasks, in which subjects were given a set of visual items to remember, alpha power decreases with increase in memory load. This is in contrast to verbal working memory tasks, in which alpha increase with increase in memory load (Haegens et al., 2010; Jensen and Mazaheri, 2010; Jensen et al., 2002; Jokisch and Jensen, 2007; Klimesch, 2012; Mathewson et al., 2011; Medendorp et al., 2007; Sauseng et al., 2009a). Functionally, alpha decrease in visual working memory are thought to reflect increased activation of visual cortex necessary for maintaining the neural representations of remembered visual items (Fukuda et al., 2015). Recent studies on phase-coding models of visual working memory suggest that items in visual working memory are represented by synchronous low frequency activities and these synchronous activities coding remembered items are phase-shifted in order to avoid accidental synchronizations that could lead to misrepresentation or contamination of remembered information (Raffone and Wolters, 2001; Siegel et al., 2009). As a result, the measured scalp EEG data as the sum of the phase-shifted signals in the cortex shows a set-size-dependent decrease in alpha power. Although in visual working memory, WMC also predicts alpha power modulation by memory load (Fukuda et al., 2015), the underlying neural basis may be different from what we reported in this work.
What alternative hypotheses might explain the findings reported in this study? Regarding the fact that high WMC individuals exhibited smaller alpha modulation by memory load, one may argue that these individuals utilized visual working memory to represent the verbal stimuli. In the classic Sternberg paradigm, the verbal items are presented sequentially, and sequential presentation of memory items is thought to minimize the use of visual working memory for information retention. Previous work has shown that regardless of whether memory items are presented sequentially or presented on a single screen, alpha power increases with memory load during memory retention (Hwang et al., 2005; Jensen et al., 2002; Tuladhar et al., 2007). No studies, however, have directly compared the amount of alpha power modulation by memory load under the two different ways of presenting verbal stimuli. It is thus not possible to rule out that visual working memory may have played a role in representation verbal information. Regarding the fact that low WMC individuals exhibited more increase with increase in memory load, one may argue that this reflects a disengagement from the task. We reason that if the subject is disengaged from the task, then task performance will suffer as the result. Closer examination of task performance data in low WMC individuals reveals that accuracy remained quite high at about 94% for load 5 in the UF dataset. Moreover, we did a within-subject analysis in which single-trial alpha power within a given memory load was estimated and sorted into high and low alpha trials, and the task performance between the high and low alpha groups was compared. The accuracy and RT were not different between the high alpha group and the low alpha group; in fact, the accuracy was actually a little higher in the high alpha group under the high memory load conditions, but the effects didn’t reach significant level of p<0.05 (results not shown). Thus, the disengagement hypothesis may not provide an adequate explanation of the data.
In both datasets, we used Cowan’s K to estimate WMC (Cowan, 2001). If the performance accuracy is high, as is the case for the UF dataset, the values of K may underestimate the true WMC (Rouder et al., 2011). In the UM dataset, for comparable working memory loads, the performance accuracy is lower, and the underestimation of WMC is less of a concern. Yet, in both datasets, we observed the same relationship between WMC and load-dependent modulation of alpha, suggesting that the potential underestimation of WMC did not hamper our ability to uncover the relationship. In addition, we correlated alpha modulation index with accuracy instead of K values, and found that there was a significant negative correlation between accuracy and alpha modulation index in both UF (r = −0.55, p < 0.012, d = −1.32) and UM BAS dataset (r = −0.59, p < 0.003, d = −1.44), meaning that the higher the accuracy, the less the alpha power modulation by memory load, in agreement with our WMC results. Thus, whether using K values as estimation of WMC or behavioral accuracy, the same relation with alpha power modulation was observed.
In summary, in this study, we considered the relationship between memory-load modulation of alpha power and WMC in two verbal working memory experiments. Consistent between the two experiments, individuals with low WMC were found to have a stronger alpha power modulation by memory load, indicating possibly an increased dependence on sensory gating for coping with task-irrelevant information in these individuals. The negative association between memory-load modulation of alpha oscillations and WMC is vulnerable to drug-related cognitive disruption. These findings contribute to our understanding of the neural substrate of WMC, an important measure of executive functioning of the brain, point to the need to further study the relation between the frontal mechanisms of distractor suppression and the posterior mechanisms of sensory gating, and shed light on how CNS drugs can disrupt important neural mechanisms, resulting in impaired cognition.
Acknowledgments
This work was supported by the National Institute of Health grants MH112206 and NS076665.
Footnotes
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Adam J, and Hitch G (1997). Working memory and children’s mental addition. Working memory and arithmetic. Journal of Experimental Child Psychology 67, 21–38. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, and Strick PL (1986). Parallel Organization of Functionally Segregated Circuits Linking Basal Ganglia and Cortex. Annual Review of Neuroscience 9, 357–381. [DOI] [PubMed] [Google Scholar]
- de Araujo Filho GM, Pascalicchio TF, Lin K, Sousa PS, and Yacubian EMT (2006). Neuropsychiatric profiles of patients with juvenile myoclonic epilepsy treated with valproate or topiramate. Epilepsy & Behavior 8, 606–609. [DOI] [PubMed] [Google Scholar]
- Barkley CM, Hu Z, Fieberg AM, Eberly LE, Birnbaum AK, Leppik IE, and Marino SE (2018). Individual Differences in Working Memory Capacity Predict Topiramate-Related Cognitive Deficits. Journal of Clinical Psychopharmacology 38, 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Tugade MM, and Engle RW (2004). Individual Differences in Working Memory Capacity and Dual-Process Theories of the Mind. Psychological Bulletin 130, 553–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashivan P, Bidelman GM, and Yeasin M (2014). Spectrotemporal dynamics of the EEG during working memory encoding and maintenance predicts individual behavioral capacity. Eur J Neurosci 40, 3774–3784. [DOI] [PubMed] [Google Scholar]
- Burgess GC, Gray JR, Conway ARA, and Braver TS (2011). Neural Mechanisms of Interference Control Underlie the Relationship Between Fluid Intelligence and Working Memory Span. J Exp Psychol Gen 140, 674–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, and Sullivan EV (2014). Compensatory recruitment of neural resources in chronic alcoholism. Handb Clin Neurol 125, 369–380. [DOI] [PubMed] [Google Scholar]
- Conway ARA, and Engle RW (1994). Working memory and retrieval: A resource-dependent inhibition model. Journal of Experimental Psychology: General 123, 354–373. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Cowan N, and Bunting MF (2001). The cocktail party phenomenon revisited: The importance of working memory capacity. Psychonomic Bulletin & Review 8, 331–335. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Kane MJ, and Engle RW (2003). Working memory capacity and its relation to general intelligence. Trends in Cognitive Sciences 7, 547–552. [DOI] [PubMed] [Google Scholar]
- Cowan N (2001). The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci 24, 87–114; discussion 114–185. [DOI] [PubMed] [Google Scholar]
- Cowan N (2012). Working Memory Capacity (Psychology Press; ). [Google Scholar]
- Daneman M, and Carpenter PA (1980). Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior 19, 450–466. [Google Scholar]
- Delorme A, and Makeig S (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods 134, 9–21. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, and Postle BR (2015). The Cognitive Neuroscience of Working Memory. Annual Review of Psychology 66, 115–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle RW (2002). Working memory capacity as executive attention. Current Directions in Psychological Science 11, 19–23. [Google Scholar]
- Engle RW, Kane MJ, and Tuholski SW (1999). Individual differences in working memory capacity and what they tell us about controlled attention, general fluid intelligence, and functions of the prefrontal cortex. In Models of Working Memory: Mechanisms of Active Maintenance and Executive Control, dividual differences in working memory capacity and what they tell us about controlled attention, general fluid intelligence, Miyake A, and Shah P, eds. (New York, NY, US: Cambridge University Press; ), pp. 102–134. [Google Scholar]
- Fritz N, Glogau S, Hoffmann J, Rademacher M, Elger CE, and Helmstaedter C (2005). Efficacy and cognitive side effects of tiagabine and topiramate in patients with epilepsy. Epilepsy & Behavior 6, 373–381. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Mance I, and Vogel EK (2015). α Power Modulation and Event-Related Slow Wave Provide Dissociable Correlates of Visual Working Memory. J Neurosci 35, 14009–14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomer B, Wagner K, Frings L, Saar J, Carius A, Härle M, Steinhoff BJ, and Schulze-Bonhage A (2007). The influence of antiepileptic drugs on cognition: A comparison of levetiracetam with topiramate. Epilepsy & Behavior 10, 486–494. [DOI] [PubMed] [Google Scholar]
- Greenwood PM (2007). Functional plasticity in cognitive aging: review and hypothesis. Neuropsychology 21, 657–673. [DOI] [PubMed] [Google Scholar]
- Grenard JL, Ames SL, Wiers RW, Thush C, Sussman S, and Stacy AW (2008). Working memory capacity moderates the predictive effects of drug-related associations on substance use. Psychology of Addictive Behaviors 22, 426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Osipova D, Oostenveld R, and Jensen O (2010). Somatosensory working memory performance in humans depends on both engagement and disengagement of regions in a distributed network. Hum Brain Mapp 31, 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AE, Davidson MC, Keele SW, and Rafal RD (1998). Toward a functional analysis of the basal ganglia. J Cogn Neurosci 10, 178–198. [DOI] [PubMed] [Google Scholar]
- Hazy TE, Frank MJ, and O’Reilly RC (2007). Towards an executive without a homunculus: computational models of the prefrontal cortex/basal ganglia system. Philos Trans R Soc Lond B Biol Sci 362, 1601–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CS, Mecklinger A, and Pfeifer E (1999). Gamma responses and ERPs in a visual classification task. Clin Neurophysiol 110, 636–642. [DOI] [PubMed] [Google Scholar]
- Hwang G, Jacobs J, Geller A, Danker J, Sekuler R, and Kahana MJ (2005). EEG correlates of verbal and nonverbal working memory. Behavioral and Brain Functions 1, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed A, Cohen B, Detyniecki K, Hirsch LJ, Legge A, Chen B, Bazil C, Kato K, Buchsbaum R, and Choi H (2015). Rates and predictors of patient-reported cognitive side effects of antiepileptic drugs: An extended follow-up. Seizure 29, 34–40. [DOI] [PubMed] [Google Scholar]
- Jensen O, and Mazaheri A (2010). Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front. Hum. Neurosci 4, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, and Lisman JE (2002a). Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cerebral Cortex 12, 877. [DOI] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, and Lisman JE (2002b). Oscillations in the Alpha Band (9–12 Hz) Increase with Memory Load during Retention in a Short-term Memory Task. Cereb. Cortex 12, 877–882. [DOI] [PubMed] [Google Scholar]
- Jokisch D, and Jensen O (2007). Modulation of Gamma and Alpha Activity during a Working Memory Task Engaging the Dorsal or Ventral Stream. J. Neurosci 27, 3244–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Pinto DJ, Kaper TJ, and Kopell N (2000). Alpha-Frequency Rhythms Desynchronize over Long Cortical Distances: A Modeling Study. J Comput Neurosci 9, 271–291. [DOI] [PubMed] [Google Scholar]
- Jung K-Y, Cho J-W, Joo EY, Kim SH, Choi KM, Chin J, Park K-W, and Hong SB (2010). Cognitive effects of topiramate revealed by standardised low-resolution brain electromagnetic tomography (sLORETA) of event-related potentials. Clinical Neurophysiology 121, 1494–1501. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Heidegger T, Wibral M, Altmann CF, and Lutzenberger W (2007). Alpha synchronization during auditory spatial short-term memory. Neuroreport 18, 1129–1132. [DOI] [PubMed] [Google Scholar]
- Kalcher J, and Pfurtscheller G (1995). Discrimination between phase-locked and non-phase-locked event-related EEG activity. Electroencephalography and Clinical Neurophysiology 94, 381–384. [DOI] [PubMed] [Google Scholar]
- Kane MJ, and Engle RW (2000). Working-memory capacity, proactive interference, and divided attention: Limits on long-term memory retrieval. Journal of Experimental Psychology: Learning, Memory, and Cognition 26, 336–358. [DOI] [PubMed] [Google Scholar]
- Kayser J, and Tenke CE (2006). Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clinical Neurophysiology 117, 348–368. [DOI] [PubMed] [Google Scholar]
- Klimesch W (2012). Alpha-band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences 16, 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, and Hanslmayr S (2007). EEG alpha oscillations: The inhibition–timing hypothesis. Brain Research Reviews 53, 63–88. [DOI] [PubMed] [Google Scholar]
- Kondo H, Osaka N, and Osaka M (2004a). Cooperation of the anterior cingulate cortex and dorsolateral prefrontal cortex for attention shifting. NeuroImage 23, 670–679. [DOI] [PubMed] [Google Scholar]
- Kondo H, Morishita M, Osaka N, Osaka M, Fukuyama H, and Shibasaki H (2004b). Functional roles of the cingulo-frontal network in performance on working memory. NeuroImage 21, 2–14. [DOI] [PubMed] [Google Scholar]
- Leiberg S, Lutzenberger W, and Kaiser J (2006). Effects of memory load on cortical oscillatory activity during auditory pattern working memory. Brain Research 1120, 131–140. [DOI] [PubMed] [Google Scholar]
- Lighthall NR, Huettel SA, and Cabeza R (2014). Functional Compensation in the Ventromedial Prefrontal Cortex Improves Memory-Dependent Decisions in Older Adults. J. Neurosci 34, 15648–15657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lőrincz ML, Kékesi KA, Juhász G, Crunelli V, and Hughes SW (2009). Temporal Framing of Thalamic Relay-Mode Firing by Phasic Inhibition during the Alpha Rhythm. Neuron 63, 683–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino SE, Pakhomov SVS, Han S, Anderson KL, Ding M, Eberly LE, Loring DW, Hawkins-Taylor C, Rarick JO, Leppik IE, et al. (2012). The effect of topiramate plasma concentration on linguistic behavior, verbal recall and working memory. Epilepsy Behav 24, 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson KE, Lleras A, Beck DM, Fabiani M, Ro T, and Gratton G (2011). Pulsed Out of Awareness: EEG Alpha Oscillations Represent a Pulsed-Inhibition of Ongoing Cortical Processing. Front Psychol 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr U, and Keele SW (2000). Changing internal constraints on action: The role of backward inhibition. Journal of Experimental Psychology: General 129, 4–26. [DOI] [PubMed] [Google Scholar]
- McNab F, and Klingberg T (2008). Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience 11, 103–107. [DOI] [PubMed] [Google Scholar]
- Medendorp WP, Kramer GFI, Jensen O, Oostenveld R, Schoffelen J-M, and Fries P (2007). Oscillatory activity in human parietal and occipital cortex shows hemispheric lateralization and memory effects in a delayed double-step saccade task. Cereb. Cortex 17, 2364–2374. [DOI] [PubMed] [Google Scholar]
- Michels L, Moazami-Goudarzi M, Jeanmonod D, and Sarnthein J (2008). EEG alpha distinguishes between cuneal and precuneal activation in working memory. NeuroImage 40, 1296–1310. [DOI] [PubMed] [Google Scholar]
- Michels L, Bucher K, Lüchinger R, Klaver P, Martin E, Jeanmonod D, and Brandeis D (2010). Simultaneous EEG-fMRI during a Working Memory Task: Modulations in Low and High Frequency Bands. PLOS ONE 5, e10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamoto T, Osaka M, and Osaka N (2010). Individual differences in working memory capacity and distractor processing: Possible contribution of top–down inhibitory control. Brain Research 1335, 63–73. [DOI] [PubMed] [Google Scholar]
- Mink JW (1996). THE BASAL GANGLIA: FOCUSED SELECTION AND INHIBITION OF COMPETING MOTOR PROGRAMS. Progress in Neurobiology 50, 381–425. [DOI] [PubMed] [Google Scholar]
- Nenert R, Viswanathan S, Dubuc DM, and Visscher KM (2012). Modulations of ongoing alpha oscillations predict successful short-term visual memory encoding. Front. Hum. Neurosci 6, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld MY, Kogan E, Chistik V, and Korczyn AD (1999). Comparison of the effects of vigabatrin, lamotrigine, and topiramate on quantitative EEGs in patients with epilepsy. Clin Neuropharmacol 22, 80–86. [DOI] [PubMed] [Google Scholar]
- Osaka M, Osaka N, Kondo H, Morishita M, Fukuyama H, Aso T, and Shibasaki H (2003). The neural basis of individual differences in working memory capacity: an fMRI study. NeuroImage 18, 789–797. [DOI] [PubMed] [Google Scholar]
- Otto AR, Raio CM, Chiang A, Phelps EA, and Daw ND (2013). Working-memory capacity protects model-based learning from stress. Proceedings of the National Academy of Sciences 110, 20941–20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, and Reuter-Lorenz P (2009). The Adaptive Brain: Aging and Neurocognitive Scaffolding. Annu Rev Psychol 60, 173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler H (1988). Familiarity and visual change detection. Perception & Psychophysics 44, 369–378. [DOI] [PubMed] [Google Scholar]
- Raffone A, and Wolters G (2001). A Cortical Mechanism for Binding in Visual Working Memory. Journal of Cognitive Neuroscience 13, 766–785. [DOI] [PubMed] [Google Scholar]
- Rajah MN, and D’Esposito M (2005). Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain 128, 1964–1983. [DOI] [PubMed] [Google Scholar]
- Rosenthal R (1984). Essentials of behavioral research: methods and data analysis (McGraw-Hill Higher Education; ). [Google Scholar]
- Rouder JN, Morey RD, Morey CC, and Cowan N (2011). How to measure working memory capacity in the change detection paradigm. Psychon Bull Rev 18, 324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Heise KF, Gruber WR, Holz E, Karim AA, Glennon M, Gerloff C, Birbaumer N, and Hummel FC (2009). Brain Oscillatory Substrates of Visual Short-Term Memory Capacity. Current Biology 19, 1846–1852. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Petersson KM, Oostenveld R, Norris DG, Hagoort P, and Bastiaansen MCM (2009). Trial-by-trial coupling between EEG and BOLD identifies networks related to alpha and theta EEG power increases during working memory maintenance. Neuroimage 44, 1224–1238. [DOI] [PubMed] [Google Scholar]
- Siegel M, Warden MR, and Miller EK (2009). Phase-dependent neuronal coding of objects in short-term memory. PNAS 106, 21341–21346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffener J, Brickman AM, Rakitin BC, Gazes Y, and Stern Y (2009). The impact of age-related changes on working memory functional activity. Brain Imaging Behav 3, 142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokić M, Milovanović D, Ljubisavljević MR, Nenadović V, and Čukić M (2015). Memory load effect in auditory–verbal short-term memory task: EEG fractal and spectral analysis. Experimental Brain Research 233, 3023–3038. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Wienbruch C, Ross B, and Pantev C (1997). Combined EEG and MEG recordings of visual 40 Hz responses to illusory triangles in human. Neuroreport 8, 1103–1107. [DOI] [PubMed] [Google Scholar]
- Thompson PJ, Baxendale SA, Duncan JS, and Sander JW a. S. (2000). Effects of topiramate on cognitive function. J Neurol Neurosurg Psychiatry 69, 636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuladhar AM, ter Huurne N, Schoffelen J-M, Maris E, Oostenveld R, and Jensen O (2007). Parieto-occipital sources account for the increase in alpha activity with working memory load. Human Brain Mapping 28, 785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth N, Schrock JC, and Engle RW (2004). Working Memory Capacity and the Antisaccade Task: Individual Differences in Voluntary Saccade Control. Journal of Experimental Psychology: Learning, Memory, and Cognition 30, 1302–1321. [DOI] [PubMed] [Google Scholar]
- Vissers ME, van Driel J, and Slagter HA (2016). Proactive, but Not Reactive, Distractor Filtering Relies on Local Modulation of Alpha Oscillatory Activity. Journal of Cognitive Neuroscience 28, 1964–1979. [DOI] [PubMed] [Google Scholar]
- Vogel EK, and Machizawa MG (2004). Neural activity predicts individual differences in visual working memory capacity. Nature 428, 748–751. [DOI] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, and Machizawa MG (2005). Neural measures reveal individual differences in controlling access to working memory. Nature 438, 500–503. [DOI] [PubMed] [Google Scholar]
- Wang C, Rajagovindan R, Han S-M, and Ding M (2016). Top-Down Control of Visual Alpha Oscillations: Sources of Control Signals and Their Mechanisms of Action. Front. Hum. Neurosci 10. [DOI] [PMC free article] [PubMed] [Google Scholar]


