Abstract
Purpose:
Social determinants of health and racial inequalities impact healthcare access and subsequent coronavirus testing. Limited studies have described the impact of these inequities on rural minorities living in Appalachia This study investigates factors affecting testing in rural communities.
Methods:
PCR testing data were obtained for March through September 2020. Spatial regression analyses were fit at the census tract level. Model outcomes included testing and positivity rate. Covariates included rurality, percent Black population, food insecurity, and area deprivation index (a comprehensive indicator of socio-economic status).
Results:
Small clusters in coronavirus testing were detected sporadically, while test positivity clustered in mid-eastern and southwestern WV. In regression analyses, percent food insecurity (IRR = 3.69*109, (796, 1.92*1016)), rurality (IRR=1.28, (1.12, 1.48)), and percent population Black (IRR = 0.88, (0.84, 0.94)) had substantial effects on coronavirus testing. However, only percent food insecurity (IRR = 5.98 * 104, (3.59, 1.07*109)) and percent Black population (IRR = 0.94, (0.90, 0.97)) displayed substantial effects on the test positivity rate.
Conclusions:
Findings highlight disparities in coronavirus testing among communities with rural minorities. Limited testing in these communities may misrepresent coronavirus incidence.
Keywords: COVID-19 Testing, Health Disparities, Rural, Spatial Analysis
INTRODUCTION
COVID-19, novel coronavirus SARS-CoV-2, is a highly infectious virus transmitted primarily by respiratory particles [1]. In the United States alone, there have been 29,769,325 total cases and 541,289 deaths as of March 24, 2021 [2]. In the absence of a vaccine, preventive measures, such as social distancing, are routinely applied to mitigate risk of infection and lower financial impact of the pandemic [3]. While effective, communities maybe unable to practice social distancing when faced with higher disparities in social determinants of health [4–6]. In particular, studies have suggested that communities with lower socio-economic status and higher racial/ethnic diversity are at increased risk of inability to social distance resulting in overall higher risk of infections [7–8].
Adverse impacts of socio-economic and racial inequalities are potentially worsened in rural areas. Rural communities often have more limited access to healthcare, higher levels of food insecurity and poverty, and observe overall higher prevalence of pre-existing conditions which can impact severity of COVID-19 infection [4–5, 9–11]. Among U.S. COVID-19 cases requiring hospitalization, 57.2% had hypertension, 48% were classified as obese, and 32.2% had cardiovascular disease [12]. Historically, rural Appalachian states, such as West Virginia, have not been regarded as having highly diverse racial/ethnic populations. Recent 2019 American Community Survey (ACS) estimates indicate that approximately 3.7% of West Virginia’s resident population is Black/African American [13]. Despite lower diversity across the state as a whole, specific communities are experiencing racial/ethnic growth in rural areas [14]. These clusters of diverse rural communities face unique challenges regarding social vulnerability to COVID-19. For example, McDowell County in the southern coalfields of West Virginia has the second highest percent Black population at 8.4%, and is ranked as the most socially vulnerable county in WV to COVID-19 as of October 10, 2020, according to CDC [13,15].
Geographic information systems (GIS) and spatial analyses are ideally suited to identify geographic variation and spatial drivers of SARS-CoV-2 infection risk [16–20]. Studies have compared spatial autoregressive model performance to ordinary least squares approaches and found that incorporating spatial effects improves model fit [21]. While these basic approaches provide a foundation for more rigorous studies, they lack the technical complexity to accurately assess risk of disease when case sizes are small, thereby limiting their use in rural communities [16]. More robust methods, such as Bayesian Hierarchical Spatial Modeling, have been employed for more accurate risk estimation in rural versus urban areas [22]. Paul et al. 2020 found that prevalence of coronavirus increased in rural areas over time, particularly in counties with higher Black, obese, and/or cigarette smoker populations [22]. While these results support previous data regarding the association between risk of coronavirus and social determinants of health, they are highly aggregate, potentially masking community-level associations. Additionally, the use of case data potentially introduces significant time lags between testing and reporting.
The objective of this study is to perform a granular spatial model to identify factors adversely affecting SARS-CoV-2 testing among rural communities. Findings will identify high risk communities and vulnerable populations in West Virginia in whom SARS CoV-2 testing should be intensified. Additionally, this study provides new insights related to coronavirus testing coverage in rural minority populations.
METHODS
Data Management
Zip code level coronavirus polymerase chain reaction (PCR) testing data were obtained for March to September 2020 from the West Virginia Health and Human Resources (WVDHHR). Testing data contained fields for unique patient identifier, zip code of residence, date of collection, type of test, and test result. Inconclusive testing results were removed from analyses. Raw data were imported into R software [23] and aggregated such that each row represented a unique person who tested either positive or negative. Patients who received multiple tests were regarded as negative if no test was positive. Alternatively, patients who had at least one positive test were regarded as positive, and not counted in persons who had always tested negative to ascertain total unique persons tested and proportion which tested positive. Data were consolidated once more to aggregate unique patient testing data to zip code of residence over the study period. Zip code level data were visualized in ArcMap 10.5 (ESRI Redlands, CA) as centroids and spatially joined to a 2010 West Virginia Census Tracts shapefile [24–25].
Census tract human testing data were joined with 2018 census tract population estimates, and percent population Black/African American, as well as percent population food insecure, Area Deprivation Index (ADI), and Rural-Urban Communizing Area (RUCA) codes by tract identification number (GEOID) [26–29]. Percent food insecure data were de-aggregated from the county-level such that every census tract in a county was assigned the same county level percent food insecure. Area deprivation indices (ADI) available at the census block group level, were aggregated to census tract level using spatial join in ArcMap to estimate average ADI within tracts. Area deprivation indices ranged from 1–10 for within state comparisons, with higher scores indicating more disadvantage [29]. As a result, higher values of the average ADI for a tract also indicated more disadvantage. State and national ADI scores have been incorporated to describe neighborhood disadvantage in past health studies [30–31]. Tract level RUCA codes were regarded as continuous data ranging from 1–10, with higher scores indicating increasing rurality [27].
Statistical Analysis
Separate census tract level Bayesian hierarchical models were employed to identify associations between the dependent variables (SARS-CoV-2 testing and positivity rate) and model covariates using the R-INLA package [32]. In both instances, models employed a BYM model for Poisson log-normal approximation for our outcome, and adjustment for correlated and uncorrelated spatial heterogeneity [33]. Mathematical specifications for spatial BYM Poisson regression models have been described elsewhere in detail [22, 34–35]. Model offsets included census tract level population (testing rate model) and total unique tests (positivity rate model) respectively. Effects of covariates were assessed using 95% credible intervals (CrI). An interval including zero indicates the corresponding covariate is not influential. Priors for both models conducted in R-INLA were fixed (mean = 0, precision = 0.001). Model parameter estimates were exponentiated to calculate incidence rate ratios for interpretation of substantial effects from covariates on coronavirus testing and positivity rates [22]. Bivariate maps were created to visualize associations between outcomes and statistically significant variables using choropleth and color graduated symbols. The study protocol was approved by the West Virginia University Institutional Review Board (Protocol # 2010137835).
RESULTS
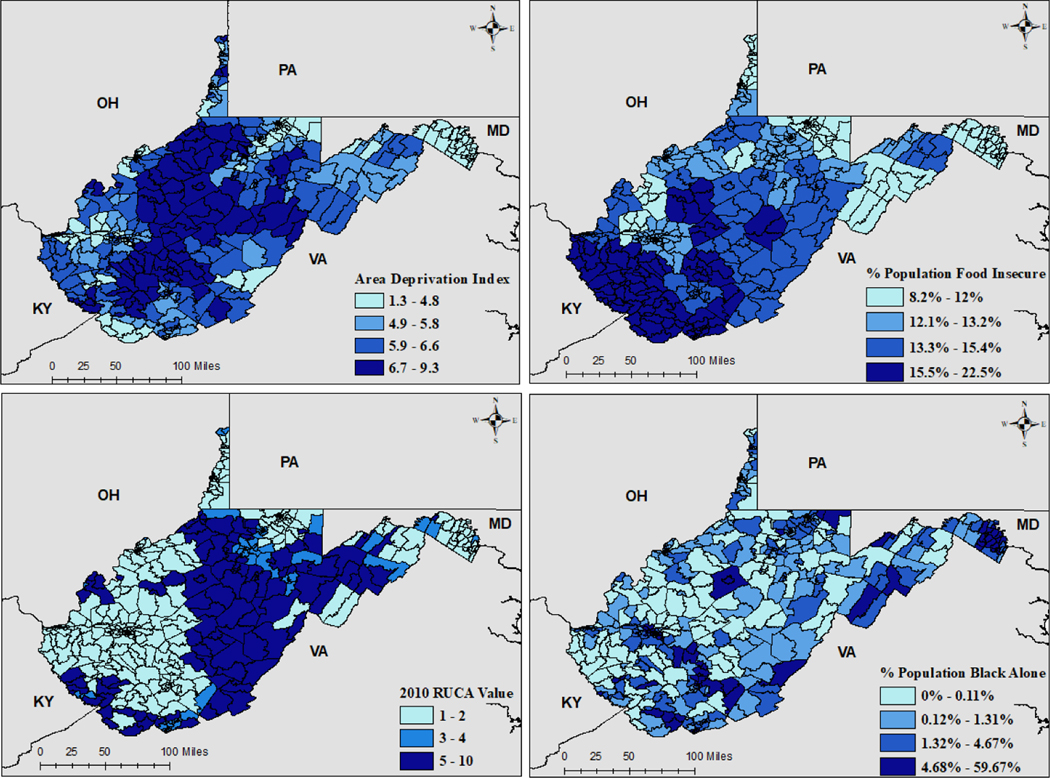
Raw number of unique tests conducted by census tract ranged from 0 to 9,834, with a mean and standard deviation of 624.6 ± 1,167.5. Number of unique positive tests ranged from 0 to 549, with a mean and standard deviation of 21 ± 45.4. Average Area Deprivation Index (ADI) for census tracts ranged from 1.33 to 9.28, with a mean and standard deviation of 5.6 ± 1.48. Percent population food insecure ranged from 8 to 22.5%, with a mean and standard deviation of 13.7% ± 2.7%. Rural-Urban Community Area codes (RUCA) at the census tract level ranged from 1 to 10, with a mean and standard deviation of 3.1 ± 2.8. Percent population Black ranged from 0 to 59.7%, with a mean and standard deviation of 4.1% ± 7.2%. Geographic distributions of model covariates are displayed in Figure 1, where darker shades of grey indicate higher values.
Figure 1.
Census Tract Level Model Covariates
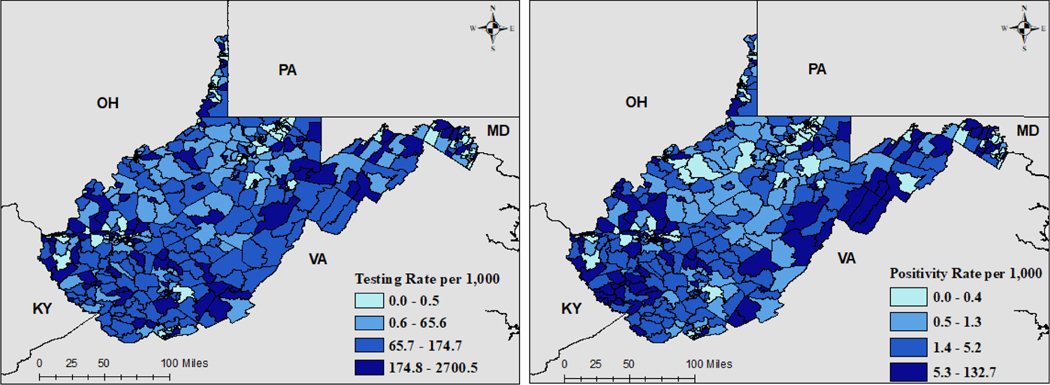
SARS-CoV-2 testing and positivity rates are shown in Figure 2. Overall, testing rates per 1,000 people appear sporadic with small clusters of high testing spread throughout the state. Census tracts with abnormally large testing rates (above 1,000 tests per 1,000 persons) (n = 16) are indicative of communities with populations not counted in census estimates (e.g. college towns). The coronavirus positivity rate per 1,000 persons clustered primarily in mid-eastern and southwestern West Virginia. However, other high risk communities were also identified sporadically throughout the state. In regression analyses, percent food insecurity (IRR = 3.69*109, 95%Crl = (796, 1.92*1016), 2010 RUCA score (IRR=1.28, 95%Crl = (1.12, 1.48)), and percent population Black (IRR = 0.88, 95%Crl = (0.84, 0.94)) were all significantly associated with coronavirus testing. However, only percent food insecurity (IRR = 5.98 * 104, 95%Crl = (3.59, 1.07*109)) and percent Black population (IRR = 0.94, 95%Crl = (0.90, 0.97)) displayed substantial effects on the positivity rate of coronavirus per 1,000 persons tested. A complete listing of parameter estimates for individual model covariates is provided in Table 1.
Figure 2.
Model Fitted Estimates of Testing and Positivity Rate per 1,000 People
Table 1.
Incidence Rate Ratio (IRR) and 95% Credible Intervals (Crl) from the Testing and Positivity Rate Models
| Variable | IRR | 95%Crl |
|---|---|---|
| Testing Rate Model | ||
| Average ADI (within state) | 1.31 | (0.99, 1.75) |
| Percent Food Insecure | 3.69*109 | (796, 1.92*1016) ** |
| 2010 RUCA score | 1.29 | (1.12, 1.48) ** |
| Percent Population Black | 0.88 | (0.84, 0.94) ** |
| Positivity Rate Model | ||
| Average ADI (within state) | 0.98 | (0.81, 1.18) |
| Percent Food Insecure | 5.98*104 | (3.59, 1.07*109) ** |
| 2010 RUCA score | 1.08 | (0.99, 1.18) |
| Percent Population Black | 0.94 | (0.90, 0.97) ** |
Indicates substantially influential credible intervals for model covariates.
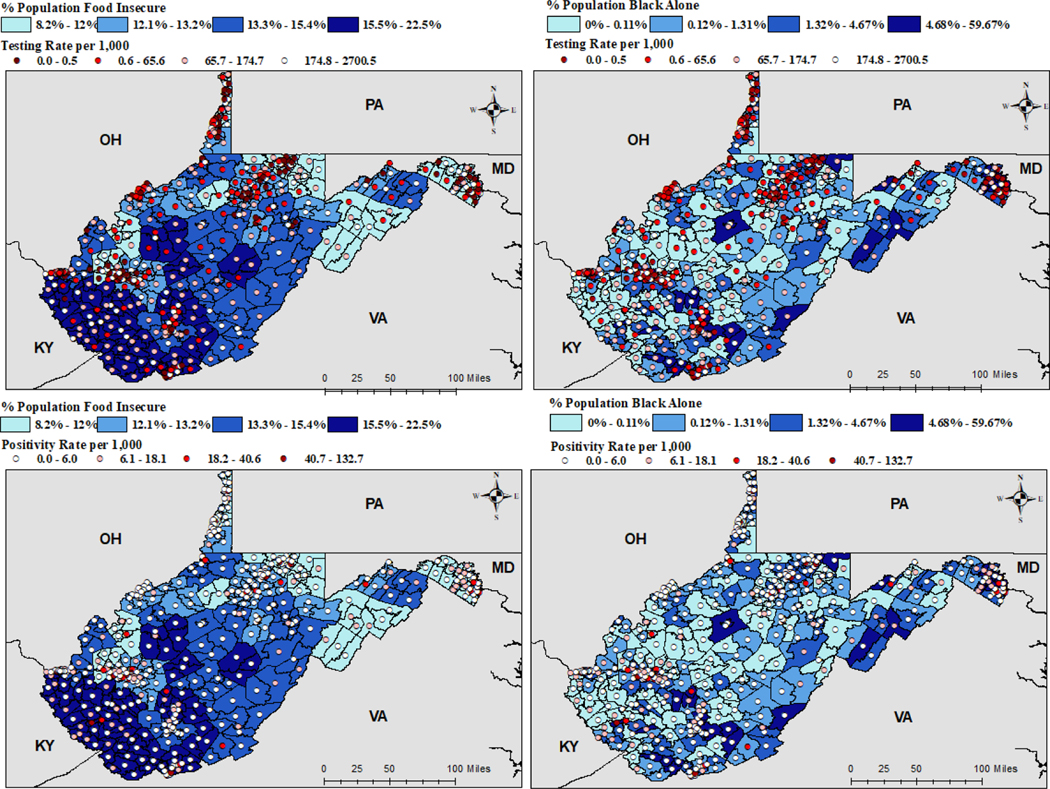
Bivariate relationships between coronavirus testing and positivity rates and percent food insecure and percent Black population are shown in Figure 3. For bivariate maps, higher testing rates are indicated by lighter shades of red to emphasize areas with limited testing resources. Alternatively, higher positivity rates are displayed in darker red to indicate high risk areas. With regard to bivariate relationships, more testing is being conducted in communities which have a higher percent food insecure and lower percent Black population. Similarly, more positive tests (darker shade of red) were found in communities with higher food insecurity (dark blue), and lower percent Black population (lighter blue).
Figure 3.
Bivariate Maps for testing and positivity rate and covariates were statistically significant in both modeling approaches.
DISCUSSION
This study identified significant variation between SARS-CoV-2 testing and positivity with respect to community level food insecurity and percent Black population in West Virginia. More specifically, results suggest that West Virginia communities with more white residents living in remote areas had higher test volume and positivity. Recent 2019 American Community Survey estimates indicated that approximately 12.8% of the U.S. population identifies as Black Alone [36–37]. In West Virginia, 39 of 485 (8%) of census tracts have communities exceeding the percent Black Alone national estimates. For these 39 census tracts the median testing and positivity rate was 10.3 and 0.53 per 1,000 people, which was lower than the state median testing and positivity rate of 65.7 and 1.26 per 1,000 people respectively.
Our conclusion of race as a significant predictor of coronavirus testing within communities is synonymous with previous research [38–39]. Interestingly, Lieberman-Cribbin et al. 2020 and Millet et al. 2020 also found that Black communities and those with lower socio-economic status had higher coronavirus positivity. This is in contrast to our findings which suggest no significant association between socio-economic status and a negative association between percent Black population and test positivity. Differences are potentially attributable to differences in geographic region, racial/ethnic population composition, or time frame considered. Another potential explanation for this difference is attributable to screening biases and the way socio-economic status was regarded. Historically, rural racial minorities have utilized health services less frequently than their white counterparts [40–41]. Fewer encounters with Black residents combined with overall lower testing rates could obscure true positivity rates in rural communities with higher percentages of Black populations. With regards to socio-economic status (SES), Lieberman-Cribbin et al. 2020 incorporated seven individual SES variables and Millet et al. 2020 considered three to four. Our study incorporated the area deprivation index proposed by Singh 2007 to characterize widening inequalities in U.S. mortality which more comprehensively describes socio-economic status using twenty-one SES variables. Most importantly, similar conclusions regarding racial disparities in testing, with differing results on social economic status supports the notion that adjusting for disparities in income and education does not control for racial inequalities in healthcare usage or access [42].
Use of testing data as an outcome in regression analyses provided a unique opportunity to examine test utilization and positivity as opposed to just incidence. However, limitations to our approach exist. Here we aggregate data such that each person contributes one test result. Cases were defined as any person who tested positive at least once, and these people were removed from the number of people who tested negative. This case definition was potentially prone to misclassification of negative tests due to improperly handled specimens or if patients were tested too early [43]. While this served the purposes of this ecological study, lack of longitudinal data, make it difficult to discern whether temporal variation in testing impacted vulnerable communities. Additionally, these data provide limited insight into the available testing infrastructure for individual patients who may have been tested many times. While this is a potential limitation, total tests in a zip code were not included as a covariate as testing volume was likely associated with individual level differences such as occupation and medical comorbidities which were not collected as part of routine state testing efforts. Other potential limitations are associated with targeted testing in specific community settings (e.g. universities) as opposed to random or systematic testing across the state. Biased testing campaigns could lead to higher infection rates among vulnerable communities given the 30% of people with COVID-19 who appear asymptomatic throughout infection [44].
CONCLUSION
Despite potential limitations this study identifies significant gaps in testing for vulnerable and underrepresented groups in West Virginia. Additionally, findings highlight how lower testing rates among rural minorities potentially bias overall coronavirus test positivity. Uncertainty regarding true positivity rates among rural minorities adversely impacts public health response and subsequent delivery of health services to control the ongoing pandemic. To date the National Guard, WV Department of Health and Human Resources and local health departments have held targeted testing events in counties with populations of rural racial minorities. Efforts to expand testing among rural racial minorities have been ongoing since the start of the WV epidemic [45]. This has included specific testing events for minorities and other vulnerable populations and development of a COVID-19 Advisory Commission on African American Disparities [46–47]. Nonetheless, this study suggests that intensified testing is indicated among Black communities in West Virginia. Further research is needed to determine the underlying barriers restricting coronavirus testing among Black communities, and to better understand the role food insecurity in isolated areas has on risk of SARS-CoV-2 infection.
Footnotes
Declaration of Competing Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].CDC “How COVID-19 Spreads” Accessed October 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html
- [2].CDC “COVID Data Tracker” Accessed March 25, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_casesinlast7days
- [3].Xie K; Liang B; Dulebenets MA; Mei Y. The Impact of Risk Perception on Social Distancing during the COVID-19 Pandemic in China. Int. J. Environ. Res. Public Health 2020, 17, 6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jones J, Sullivan PS, Sanchez TH, Guest JL, Hall EW, Luisi N, … & Siegler AJ (2020). Similarities and differences in COVID-19 awareness, concern, and symptoms by race and ethnicity in the United States: cross-sectional survey. Journal of medical Internet research, 22(7), e20001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].van Dorn A, Cooney RE, & Sabin ML (2020). COVID-19 exacerbating inequalities in the US. Lancet (London, England), 395(10232), 1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hake M, Engelhard E, Dewey A, Gundersen C. (2020). The Impact of the Coronavirus on Food Insecurity [Brief series]. Available from Feeding America: https://www.feedingamerica.org/research/coronavirus-hunger-research. [Google Scholar]
- [7].Weill JA, Stigler M, Deschenes O, & Springborn MR (2020). Social distancing responses to COVID-19 emergency declarations strongly differentiated by income. Proceedings of the National Academy of Sciences, 117(33), 19658–19660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Abedi V, Olulana O, Avula V, Chaudhary D, Khan A, Shahjouei S, … & Zand R. (2020). Racial, Economic, and Health Inequality and COVID-19 Infection in the United States. Journal of racial and ethnic health disparities, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].COVID-19 in Racial and Ethnic Minority Groups. US Centers for Disease Control and Prevention. 2020. Jun 25, [2020–06-23]. https://www-cdcgov.www.libproxy.wvu.edu/coronavirus/2019-ncov/need-extra-precautions/racial-ethnic-minorities.html.
- [10].Burr JA, Mutchler JE, Gerst K. Patterns of residential crowding among Hispanics in later life: immigration, assimilation, and housing market factors. J Gerontol B Psychol Sci Soc Sci. 2010. Nov;65(6):772–82. doi: 10.1093/geronb/gbq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Harlem G, Lynn M. Descriptive analysis of social determinant factors in urban communities affected by COVID-19. J Public Health (Oxf). 2020;42(3):466–469. doi: 10.1093/pubmed/fdaa078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].CDC “COVIDView Weekly Summary Week 40 October 3, 2020. ” Accessed September 24, 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html
- [13].U.S. Census Bureau “West Virginia Populations and People Table 2019. ” Accessed September 24, 2020. https://data.census.gov/cedsci/table?t=Populations%20and%20People&g=0400000US54&tid=ACSST1Y2019.S0101&hidePreview=false
- [14].CDC “Coronavirus Disease 2019 (COVID-19) – Rural Communities” Accessed September 24, 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/other-at-risk-populations/rural-communities.html
- [15].CDC Agency for Toxic Substances and Disease Registry “ CDC’s Social Vulnerability Index; ” Accessed October 10, 2020. https://svi.cdc.gov/map.html [Google Scholar]
- [16].Zhou C, Su F, Pei T, Zhang A, Du Y, Luo B, … & Song C. (2020). COVID-19: Challenges to GIS with big data. Geography and Sustainability. [Google Scholar]
- [17].Kathe N, & Wani RJ (2020). Determinants of COVID-19 Incidence and Mortality in the US: Spatial Analysis. medRxiv. [Google Scholar]
- [18].Andersen LM, Harden SR, Sugg MM, Runkle JD, & Lundquist TE (2021). Analyzing the spatial determinants of local Covid-19 transmission in the United States. Science of the Total Environment, 754, 142396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Franch-Pardo I, Napoletano BM, Rosete-Verges F, & Billa L. (2020). Spatial analysis and GIS in the study of COVID-19. A review. Science of The Total Environment, 739, 140033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cordes J, & Castro MC (2020). Spatial analysis of COVID-19 clusters and contextual factors in New York City. Spatial and Spatio-temporal Epidemiology, 34, 100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mollalo A, Vahedi B, & Rivera KM (2020). GIS-based spatial modeling of COVID-19 incidence rate in the continental United States. Science of The Total Environment, 138884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Paul R, Arif AA, Adeyemi O, Ghosh S, & Han D. (2020). Progression of COVID 19 From Urban to Rural Areas in the United States: A Spatiotemporal Analysis of Prevalence Rates. The Journal of Rural Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. [Google Scholar]
- [24].S. Census Bureau Tiger/Line Shapefiles “WV Census tracts 2010. ” Accessed August 2020. https://www.census.gov/geographies/mapping-files/time-series/geo/tiger-line-file.html
- [25].ESRI “USA Zip Code Points” Accessed August 2020. https://www.arcgis.com/home/item.html?id=1eeaf4bb41314febb990e2e96f7178df.
- [26].U.S. Census Bureau “West 2018 Virginia Census Tract Level Black Population Alone Table” Accessed August 2020. https://data.census.gov/cedsci/
- [27].USDA Economic Research Services “Rural –Urban Commuting Area Codes revised 2019. ”. Accessed August 2020. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/
- [28].Feeding America “U.S. County Level Percent Food Insecurity” June 2020. Public Data Request; Can be made at https://www.feedingamerica.org/ [Google Scholar]
- [29].University of Wisconsin “Neighborhood Atlas – State Level Area Deprivation Index”. Accessed August 2020. https://www.neighborhoodatlas.medicine.wisc.edu/
- [30].Lantos PM, Hoffman K, Permar SR, et al. Neighborhood disadvantage is associated with high cytomegalovirus seroprevalence in pregnancy. J Racial Ethn Health Disparities 2018; 5(4): 782–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hu J, Kind AJH, Nerenz D. Area Deprivation Index predicts readmission risk at an urban teaching hospital. Am J Med Qual 2018; (33):493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rue H, Martino S, & Chopin N. (2009). Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. Journal of the royal statistical society: Series b (statistical methodology), 71(2), 319–392. [Google Scholar]
- [33].Morris M, Wheeler-Martin K, Simpson D, Mooney SJ, Gelman A, & DiMaggio C. (2019). Bayesian hierarchical spatial models: Implementing the Besag York Mollié model in stan. Spatial and spatio-temporal epidemiology, 31, 100301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lawson AB (2018). Bayesian disease mapping: hierarchical modeling in spatial epidemiology. CRC press. [Google Scholar]
- [35].Rockett IR, Caine ED, Banerjee A, Ali B, Miller T, Connery HS, … & Jia H. (2021). Fatal self-injury in the United States, 1999–2018: Unmasking a national mental health crisis. EClinicalMedicine, 100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].U.S. Census Bureau “United States Age and Sex Table 2019. ” Accessed March 9, 2021.
- [37].U.S. Census Bureau “United States Population Black Alone Table 2019. ” Accessed March 9, 2021.
- [38].Lieberman-Cribbin W, Tuminello S, Flores RM, & Taioli E. (2020). Disparities in COVID-19 Testing and Positivity in New York City. American journal of preventive medicine, 59(3), 326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Millett GA, Jones AT, Benkeser D, Baral S, Mercer L, Beyrer C, … & Sherwood J. (2020). Assessing differential impacts of COVID-19 on Black communities. Annals of Epidemiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mueller KJ, Patil K, & Boilesen E. (1998). The role of uninsurance and race in healthcare utilization by rural minorities. Health Services Research, 33(3 Pt 1), 597. [PMC free article] [PubMed] [Google Scholar]
- [41].Parrish Deidra D.; Kent Charlotte K. Access to Care Issues for African American Communities: Implications for STD Disparities, Sexually Transmitted Diseases: December 2008 - Volume 35 - Issue 12 - p S19–S22 doi: 10.1097/OLQ.0b013e31818f2ae1 [DOI] [PubMed] [Google Scholar]
- [42].Williams DR, Lawrence JA, & Davis BA (2019). Racism and health: evidence and needed research. Annual review of public health, 40, 105–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kucirka LM, Lauer SA, Laeyendecker O, Boon D, & Lessler J. (2020). Variation in false-negative rate of reverse transcriptase polymerase chain reaction–based SARS-CoV-2 tests by time since exposure. Annals of Internal Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Oran DP, & Topol EJ (2021). The proportion of SARS-CoV-2 infections that are asymptomatic: a systematic review. Annals of internal medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Office of the Governor “COVID-19 UPDATE: Gov. Justice schedules reopenings for gyms, other recreational activities; announces plan to test vulnerable and minority populations” Accessed October 2020. https://governor.wv.gov/News/press-releases/2020/Pages/COVID-19-UPDATE-Gov.-Justice-announces-additional-reopenings.aspx
- [46].WV Department of Health and Human Resources (WV DHHR) “Testing Opportunities for Minorities and Other Vulnerable Populations Announced for Fayette County” Accessed October 2020. https://dhhr.wv.gov/News/2020/Pages/Testing-Opportunities-for-Minorities-and-Other-Vulnerable-Populations-Announced-for-Fayette-County.aspx
- [47].Herbert Henderson Office of Minority Affairs “COVID-19 Advisory Commission on African American Disparities” Accessed October 2020. https://minorityaffairs.wv.gov/COVID-19/Pages/default.aspx