Abstract
Office, home, and ambulatory blood pressure (BP) demonstrate variable associations with outcomes. The authors sought to compare office BP (OBP), home BP (HBP), and ambulatory BP (ABP) for measuring responses to hydrochlorothiazide (HCTZ), atenolol, and their combination. After completing washout, eligible patients were randomized to atenolol 50 mg or HCTZ 12.5 mg daily. Doses were doubled after 3 weeks and the alternate drug was added after 6 weeks if BP was >120/70 mm Hg (chosen to allow maximum opportunity to assess genetic associations with dual BP therapy in the parent study). OBP (in triplicate), HBP (twice daily for 5 days), and 24-hour ABP were measured at baseline, after monotherapy, and after combination therapy. BP responses were compared between OBP, HBP, and ABP for each monotherapy and combination therapy. In 418 patients, OBP overestimated BP response compared with HBP, with an average 4.6 mm Hg greater reduction in systolic BP (P<.0001) and 2.1 mm Hg greater reduction in diastolic BP (P<.0001) across all therapies. Results were similar for atenolol and HCTZ monotherapy. ABP response was more highly correlated with HBP response (r=0.58) than with OBP response (r=0.47; P=.04). In the context of a randomized clinical trial, the authors have identified significant differences in HBP, OBP, and ABP methods of measuring BP response to atenolol and HCTZ monotherapy.
Office, home, and ambulatory blood pressure (BP) are significantly associated with cardiovascular and patient outcomes. However, different BP measures have different associations. Office BP (OBP), while used most often to guide therapeutic decision making, is less closely related to target organ damage and may have less prognostic value than ambulatory BP (ABP) or home BP (HBP).1–5 In fact, a recent prospective study comparing OBP and ABP found no prognostic value of OBP among resistant hypertensive patients, with only ABP predicting mortality.6
A recent meta-analysis of more than 6000 patients found that antihypertensive response to therapy measured by HBP was 20% less than OBP.7 In addition, in a subset of patients with ABP measurements, BP response by ABP was 6% less than HBP response and 28% less than OBP response and was independent of the class of antihypertensive agent used including calcium channel blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers. However, whether these same differences in antihypertensive response by measurement method exist for diuretics or β-blockers is not known. Further, whether differences between BP measurement methods persist when a second drug is added has not been well described. This question is important given the common clinical use of these 2 drug classes and the frequent need for multiple medications to control BP.
The purpose of this study was to compare antihypertensive responses using OBP and HBP measurement from a randomized clinical trial for: (1) hydrochlorothiazide (HCTZ) and atenolol monotherapies, as well as (2) HCTZ+atenolol and atenolol+HCTZ combination therapies. In particular, we evaluated how the different methods of measuring BP response might clinically influence treatment decisions (assuming the standard BP goals for HBP and OBP as described in the methods). Finally, we sought to compare ABP responses with responses measured by the other 2 methods.
Methods
Participants
This report represents an interim analysis of a larger pharmacogenomic study (Pharmacogenomic Evaluation of Antihypertensive Responses [PEAR]). The detailed rationale and methods of this study have been previously described.8 Patients aged 17 to 65 years with mild to moderate essential hypertension were eligible for enrollment. BP inclusion was determined using HBP and OBP after a washout from all antihypertensive medications lasting a minimum of 18 days, but 4 weeks on average (mean 29±16 days). HBP was determined using a Microlife model 3AC1-PC monitor (Minneapolis, MN) measured in triplicate, then averaged, morning and evening over a period of 1 week. The HBP data was electronically stored then downloaded at clinic visits. A minimum of 5 morning and 5 evening determinations was required during the 1-week period to be included in the analysis. OBP was determined as the average of triplicate measurements using the same HBP monitor at the study center. Inclusion in the trial required an average seated home diastolic BP (DBP) >85 mm Hg and an average seated (>5 minutes) office DBP >90 mm Hg at baseline. Study participants were excluded if DBP by either method was >110 mm Hg or systolic BP (SBP) was >180 mm Hg. Other initial screening exclusion criteria included use of ≥3 antihypertensive drugs, SBP >170 mm Hg on active treatment, other diseases requiring treatment with antihypertensive drugs, known cardiovascular disease, diabetes, renal insufficiency, pregnancy or lactation, Raynaud's syndrome, liver dysfunction, or chronic treatment with medications known to increase BP such as nonsteroidal anti-inflammatory agents or oral contraceptives. The study is registered at ClinicalTrials.gov (# NCT00246519; http://clinicaltrials.gov/ct2/show/NCT00246519).
Study Protocol
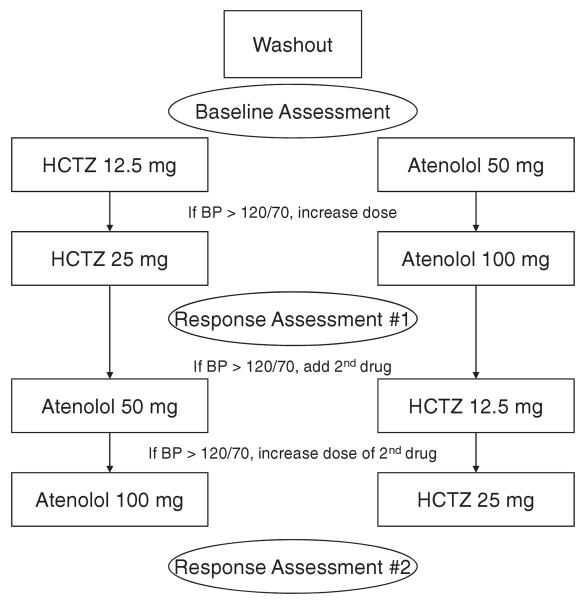
A diagram describing the overall study protocol is shown in Figure 1. After meeting eligibility requirements, patients had ABP monitors (Spacelabs model 90207, Redmond, WA) placed and were instructed to conduct their usual daily activities. Patients returned to the clinic after completion of 24-hour ABP monitoring. The ABP monitor was preprogrammed to record BPs 4 times per hour during the day (6 AM to 10 PM) and twice per hour at night (10 PM to 6 AM). After completion of baseline measurements, patients were randomized to receive either HCTZ 12.5 mg daily or atenolol 50 mg daily. After 3 weeks, the initial dose was doubled in all patients with average HBP or OBP > 120/70 mm Hg. Patients were maintained on this dose for a minimum of 6 additional weeks, after which additional studies (HBP, ABP, and OBP measurement as described) to assess response to the first study drug (response assessment #1) were performed (Figure 1). Those with BP values ≤120/70 mm Hg proceeded directly to response assessment #1 after a minimum of 6 weeks on the initial dose of antihypertensive therapy.
Figure 1.
Study flow diagram. HCTZ indicates hydrochlorothiazide; BP, blood pressure.
After completion of response assessment #1, the alternate drug was added (atenolol 50 mg daily or HCTZ 12.5 mg daily) in patients with HBP and OBP >120/70 mm Hg. This regimen was continued for at least 3 weeks, at which time HBP and OBP were assessed. The dose of the second drug was doubled if BP remained >120/70 mm Hg. This regimen was continued for a minimum of 6 weeks before repeating the response assessment studies (response assessment #2; Figure 2). For patients with BP by both methods <120/70 mm Hg, the initial dose was continued for at least 3 more weeks before response assessment #2 was completed. The rationale for titrating to <120/70 mm Hg was for the purposes of the pharmacogenomics study in which we wanted to ensure that most patients achieved maximum dose of the first drug and addition of the second drug so that genetic associations with combination therapy could be assessed.
Figure 2.
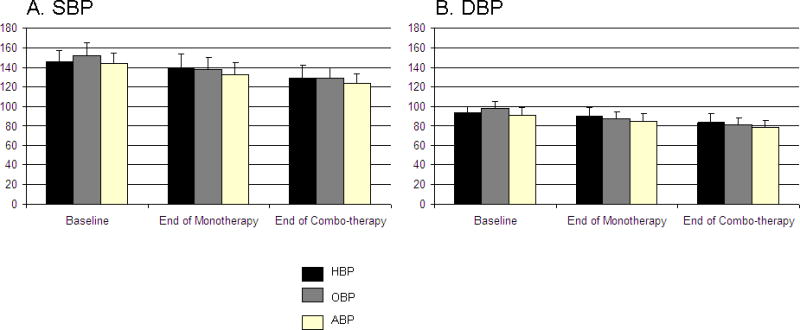
Blood pressure (BP) by office, home, and ambulatory methods at each study time point. Panel A represents average systolic blood pressure (SBP) and panel B displays average diastolic blood pressure (DBP). Error bars are the standard deviation. Baseline office BP (OBP) (n=418), home BP (HBP) (n=414), ambulatory BP (ABP) (n=391). End of monotherapy OBP (n=418), HBP (n=404), ABP (n=322). End of combination therapy OBP (n=363), HBP (n=341), and ABP (n=289). *Overall P<.0001.
All procedures were performed in accordance with the ethical standards of the Helsinki Declaration of 1975 (as revised in 1983), and each site's institutional review board approved the protocol.
Statistical Analysis
Mean ± standard deviation (SD) of BP was determined at each study visit and the change in BP with each treatment was analyzed. Baseline demographic characteristics were compared by treatment group using chi-square or t tests. Comparison of OBP, HBP, and ABP responses were determined using 95% confidence intervals (CIs). Pearson correlation coefficients between BP responses measured by each method were calculated. BP changes were corrected for baseline BP using the Oldham method (corrected change=actual change/average pretreatment and post-treatment BP).9 PROC GLM was used including the variables age, sex, race, body mass index, and baseline BP to determine adjusted BP responses. The adjusted BP response was calculated by adding the residual from the GLM regression and adding it to the mean delta BP. Paired t tests were used to compare BP responses between HBP and OBP methods, OBP and daytime ABP methods, and HBP and daytime ABP methods. Using cut-offs of <140/90 mm Hg for OBP and <135/85 mm Hg for HBP, we assessed the proportion of patients who would have had their treatment continued at the current dose or increased with OBP vs HBP and how well these agreed.
Results
The sample was comprised of 418 patients who had completed at least response assessment #1, 363 of whom also completed response assessment #2 (Table I). The HBP and ABP was significantly lower in those randomized to atenolol than HCTZ. These differences appear to have occurred by chance given that the study design was randomized, with randomization stratified by center. The average baseline SBP and DBP were significantly lower in HBP (146.0/93.6±10.8/6.4 mm Hg) vs OBP (152.2/98.6±13.0/6.7 mm Hg) (P<.0001). Of the 418 patients who completed response assessment #1, 387 (92.6%) had the dose of the first drug titrated upward from 50 to 100 mg of atenolol and from 12.5 to 25 mg of HCTZ. Of the patients who completed response assessment #2, 352 (97.0%) had the second drug added and 279 (76.9%) had the dose of the second drug uptitrated. Average HBP, OBP, and ABP at each major study activity are shown in Figure 2.
Table I.
Baseline Characteristics
| Atenolol (n=210) | HCTZ (n=208) | P Value | |
|---|---|---|---|
| Age, y | 49.7±9.0 | 50.5±8.6 | .38 |
| Female sex | 125 (60%) | 111 (53%) | .20 |
| White race | 121 (58%) | 116 (56%) | .40 |
| BMI, kg/m2 | 31.2±6.3 | 30.9±5.0 | .54 |
| Years since hypertension diagnosis | 8.3±7.7 | 7.8±7.7 | .51 |
| Home BP, mm Hg | 144.5/92.6±10.3/6.3 | 147.5/94.5±11.2/6.4 | .005/.002 |
| Office BP, mm Hg | 151.4/98.2±12.8/6.9 | 153.0/99.0±13.2/6.4 | .20/.23 |
| Daytime ABP, mm Hg | 142.4/90.7±9.8/7.8 | 144.7/92.1±11.6/7.9 | .03/.07 |
| 24-h ABP, mm Hg | 139.5/88.2±10.0/7.8 | 141.8/89.6±11.4/7.7 | .03/.07 |
Abbreviations: ABP, ambulatory blood pressure (BP); BMI, body mass index. Data are mean ± standard deviation or No. (%).
P values are comparing atenolol and hydrochlorothiazide (HCTZ) groups.
OBP and HBP Response to Monotherapy
Among all patients completing the monotherapy phase of the study, the mean reduction in OBP was 13.3/8.7±14.7/9.0 mm Hg (corresponding to an 8.4%/8.7% change) and in HBP was 8.5/6.4± 9.9/6.3 mm Hg (corresponding to a 6.1%/6.8% change). The mean reductions were similar when adjusted for age, sex, race, body mass index, and baseline BP (data not shown). The difference in response between OBP and HBP (ΔOBP – ΔHBP) was 4.6 mm Hg SBP (OBP greater than HBP) (95% CI, 3.3–5.9 mm Hg; P<.0001) and 2.1 mm Hg DBP (OBP greater than HBP) (95% CI, 1.3–2.9 mm Hg; P<.0001). The correlation coefficient for SBP reduction of OBP with HBP was r=0.51 and P<.0001 and for DBP, r=0.47 and P<.0001 (Figure 3). The BP changes corrected for baseline BP were 9.4% for office SBP, 9.5% for office DBP, 6.1% for home SBP, and 7.3% for home DBP.
Figure 3.
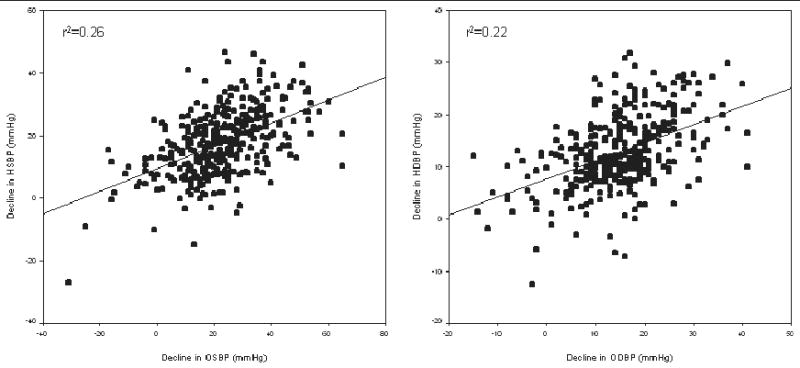
Correlations between change in blood pressure (BP) using office and home measurement. Plot on left represents systolic BP (SBP) decline and plot on right represents diastolic BP (DBP) decline. Both lines are regression lines. r2 for SBP change=0.26, r2 for DBP change=0.22. HSBP indicates home systolic blood pressure; OSBP, office systolic blood pressure; HDBP, home diastolic blood pressure; ODBP, office diastolic blood pressure.
For atenolol monotherapy, the mean reductions were 12.4/10.1±16.4/9.9 mm Hg (8.0%/10.1%) for OBP and 7.0/7.1±10.3/6.6 mm Hg (4.8%/7.7%) for HBP. The difference between OBP and HBP (ΔOBP – ΔHBP) response to atenolol was 5.2 mm Hg for SBP (95% CI, 3.3–7.1 mm Hg; P<.0001) and 2.8 mm Hg for DBP (95% CI 1.6 to 4.1 mm Hg; P<.0001).
For HCTZ, the mean reductions were 14.2/7.2±12.7/7.8 mm Hg (9.0%/7.2%) for OBP and 10.1/5.7±9.2/6.0 mm Hg (6.7%/6.0%) for HBP. The difference between OBP and HBP response to HCTZ was 4.0 mm Hg for SBP (95% CI, 2.2–5.8 mm Hg; P<.0001) and 1.3 mm Hg for DBP (95% CI, 0.2–2.4 mm Hg; P=.018).
The overestimation of response measured by OBP compared with HBP was not significantly greater for atenolol than for HCTZ (difference of 1.2 mm Hg for SBP [P=.36] and 1.5 mm Hg for DBP [P=.08]).
OBP and HBP Response to Combination Therapy
Among the 363 patients with data complete through response assessment #2, the overall mean reduction in OBP and HBP (baseline – response assessment #2) and the separate reductions for the atenolol+HCTZ and HCTZ+atenolol treatment arms are shown in Table II.
Table II.
Dual-Therapy BP Reductions Measured With OBP and HBP
| OBP Decreasea | HBP Decreasea | OBP % Decrease | HBP % Decrease | OBP% Decrease Corrected for Baseline | HBP % Decrease Corrected for Baseline | |
|---|---|---|---|---|---|---|
| Atenolol+HCTZ | 24.5/16.5±13.2/8.1 | 19.8/14.5±9.7/6.5 | 13.9/14.3 | 10.5/12.1 | 15.6/16.1 | 8.3/13.2 |
| HCTZ+atenolol | 21.7/14.2±16.2/10.2 | 15.5/11.3±11.2/7.2 | 15.7/16.5 | 13.3/15.3 | 17.4/18.4 | 10.7/16.8 |
| Pooled | 22.9/15.3±14.8/9.3 | 17.7/13.0±10.5/6.9 | 14.6/15.4 | 11.9/13.8 | 16.3/17.2 | 17.2/9.5 |
Abbreviations: BP, blood pressure; HBP, home BP; HCTZ, hydrochlorothiazide; OBP, office BP.
Baseline – response assessment #2.
The differences between measurement methods were largely eliminated with the addition of a second drug, regardless of the treatment order. Overall, the difference between OBP and HBP response (ΔOBP – ΔHBP) at response assessment #2 (response assessment #1 – response assessment #2, ie, contribution of second drug) was 0.8 mm Hg for SBP (95% CI, −0.5 to 2.0 mm Hg; P=.22) and 0.1 mm Hg for DBP (95% CI, −0.8 to 0.9 mm Hg; P=.87). When HCTZ was added to atenolol, the differences between OBP and HBP was 1.2 mm Hg SBP (95% CI, −0.64 to 3.1 mm Hg; P=.20) and 0.1 mm Hg DBP (95% CI, −1.3 to 1.5 mm Hg; P=.91). With the addition of atenolol to HCTZ, the difference between OBP and HBP response was 0.5 mm Hg SBP (95% CI, 2.1 to −1.1 mm Hg; P=.54) and 0.2 mm Hg DBP (95% CI 1.2 to −0.8 mm Hg; P=.64).
Influence of OBP and HBP on Treatment Decisions
In order to compare how OBP and HBP might lead to different clinical decisions, we assessed the percentage of patients who achieved an OBP of <140/90 mm Hg and HBP <135/85 mm Hg and the agreement between the 2 methods for each treatment group (Table III). For both atenolol and HCTZ monotherapy, OBP would have resulted in less aggressive treatment than HBP. For atenolol, OBP would have led to a “continue” decision (no dose increase or addition of a second drug based on the above criteria) in 68% of cases, whereas HBP would have led to a continue in 60%. For HCTZ, OBP would have led to a continue in 63%, whereas HBP would have led to a continue in 50%. The agreement between the 2 methods was similar for atenolol and for HCTZ, 75% compared with 69% (P=.16). Combination therapy led to much more similar treatment decisions between OBP and HBP. OBP would have suggested continuing current treatment in 83% and HBP in 86%, with an 83% agreement between the 2 methods (P=.96).
Table III.
Decision to Continue Current Treatment or Titrate for Home and Office BP
| OBP, % | HBP, % | Agreement, % | |
|---|---|---|---|
| Atenolol | |||
| Continue | 143 (68) | 127 (60) | 109 (52) |
| Increase treatment | 67 (32) | 83 (40) | 49 (23) |
| Total | 158 (75) | ||
| Hydrochlorothiazide | |||
| Continue | 130 (63) | 103 (50) | 84 (40) |
| Increase treatment | 78 (38) | 105 (50) | 59 (28) |
| Total | 143 (69) | ||
| Combination | |||
| Continue | 322 (83) | 331 (86) | 320 (77) |
| Increase treatment | 65 (17) | 56 (14) | 25 (6) |
| Total | 345 (83) |
Abbreviations: BP, blood pressure; HCTZ, hydrochlorothiazide. Decision to continue current treatment using office BP (OBP) <140/90 mm Hg or home BP (HBP) <135/85 mm Hg. P<.02 for continue/increase vs treatment group (atenolol/HCTZ) in HBP.
P=.23 for continue/increase vs treatment group (atenolol/HCTZ) in OBP.
ABP Response Comparisons
ABP response to monotherapy was intermediate to OBP and HBP responses and more highly correlated with HBP than OBP. The daytime monotherapy ABP response was 11.0/7.1±10.5/7.3 mm Hg (corresponding to a 7.4%/7.6% change) and 24-hour ABP response was 10.6/7.0±10.3/7.2mm Hg (corresponding to a 7.4%/7.7% change). The difference between OBP and daytime ABP was 2.3 mm Hg for SBP and 1.5 mm Hg for DBP, with correlation coefficients of r=0.47 for SBP and r=0.48 for DBP. The difference between HBP and daytime ABP was −2.5 mm Hg for SBP and −0.7 mm Hg for DBP, with correlation coefficients of r=0.58 for SBP and r=0.55 for DBP. The correlation coefficients for HBP and daytime ABP were statistically higher than those for OBP and daytime ABP for SBP (P=.04). The correlations were not significantly different for DBP (P=.20).
For atenolol monotherapy, the difference between OBP and daytime ABP (ΔOBP – Δday-time ABP) was 1.4 mm Hg SBP (95% CI, −0.7 to 3.6; P=.19) and 1.1 mm Hg DBP (95% CI, −0.2 to 2.4; P=.09). For HBP and daytime ABP (ΔHBP – Δdaytime ABP) the difference was −4.4 mm Hg SBP (95% CI, −6.0 to −2.8; P<.0001) and −1.8 mm Hg DBP (95% CI, −3.0 to −0.72; P=.002). For HCTZ monotherapy, the difference between OBP and daytime ABP was 3.1 mm Hg (95% CI, 1.2–5.1; P=.002) and 1.9 mm Hg DBP (95% CI, 0.6–3.1; P=.003). For HBP and daytime ABP the difference was −0.5 mm Hg for SBP (95% CI, −2.1 to 1.1; P=.54) and 0.4 mm Hg for DBP (95% CI, −0.7 to 1.6; P=.44).
Discussion
In the context of a randomized trial, we have identified significant differences in measuring BP response to atenolol and HCTZ using home, office, and ambulatory BP. Our results suggest significant overestimation of the BP response to monotherapy using OBP compared with HBP (8% vs 6%). HBP and ABP responses were more strongly correlated than either with OBP. These findings are important given that numerous studies have found home and ambulatory BP to be more closely related to the adverse cardiovascular outcomes that BP treatment is ultimately aimed at preventing. Given the close association with ABP, it may be possible to use HBP for management decisions in essential hypertension, particularly given the relative ease of incorporating HBP into daily activities (compared with 24-hour ABP). In fact, the American Heart Association, American Society of Hypertension, and the Preventive Cardiovascular Nurses Association have recently released a statement suggesting that HBP monitoring be incorporated into usual care.10 As HBP monitoring becomes more common and new treatment paradigms are created, it will be very important for clinicians to be aware of these differences in treatment response with out-of-office measurements compared with office measurements so that appropriate clinical decisions can be made.
Strengths and Limitations
One potential explanation for the differences in BP response by HBP, OBP, and ABP that should be addressed is the difference in the number of BP measurements. HBP was measured in triplicate twice daily for 1 week (42 readings) compared with OBP, which was measured in triplicate during 1 office visit. Therefore, the inherent nature of HBP and ABP to acquire many more BP readings than OBP and hence have narrower confidence limits is one of the intrinsic benefits of HBP and ABP that may contribute to the closer correlation with outcomes by closer approaching the “true BP.” Another potential explanation that should be addressed is the fact that HBP readings are made in the morning and late in the evening before bed, whereas OBP is measured in the morning in most cases. Given that HCTZ and atenolol are short-acting drugs and that patients were instructed to take their medications in the morning, the evening BP measures of HBP might reflect the waning of BP coverage in the evening with these therapies and could account for the lesser response with HBP compared with OBP. In order to assess this possibility, we compared the HBP and OBP responses using only the morning HBP readings and found nearly identical results as those presented. The differences between OBP and morning HBP were 5.7 mm Hg for SBP and 2.6 mm Hg for DBP. The correlations between morning HBP and OBP were even lower than those including morning and evening HBP (r2=0.19 for SBP and r2=0.14 for DBP), suggesting that the inclusion of evening HBP did not drive the differences between HBP and OBP. Last, white coat hypertension has been identified by others to be a primary determinant of treatment-induced changes in OBP (ie, patients with white coat hypertension have less treatment-induced reduction in OBP).11 Our protocol enrolled patients based on both home and office BP, thereby minimizing the inclusion of persons with white coat hypertension in our study. In fact, when 48 patients with missing or 24-hour ABP <130/80 mm Hg were excluded, the correlations between home and office BP did not change appreciably.
We found no difference in the overestimation of OBP response vs HBP between atenolol and HCTZ. Furthermore, the agreement rates for treatment decisions were similar for atenolol and HCTZ monotherapy. The differences we saw between OBP response vs ABP response were similar to those previously reported.12,13 Interestingly, however, we did identify differences between treatment response measurements when comparing ABP with HBP between the two drugs. In particular, atenolol was associated with significant differences in treatment response when comparing HBP with daytime ABP response (with greater response measured by ABP than HBP), whereas for HCTZ monotherapy, there was no difference in treatment response measured by HBP vs daytime ABP.
A novel finding of our study was that the differences between measurement methods were reduced when a second drug was added. The differences between OBP and HBP response were no longer evident with dual therapy. Whether this is a result of the new “baseline values” being normalized after the first drug before adding the second, acclimatization of patients as they become more comfortable with having their BP measured throughout the trial, or some other mechanism, is unclear. However, in contrast to the meta-analysis by Ishikawa and colleagues, our differences in OBP and HBP response did not seem to be entirely due to differences in baseline BP.7 Also in contrast to the recent meta-analysis, in our study, the HBP reductions were generally smaller overall than in the meta-analysis and they were smaller than the ABP reductions. One explanation for the HBP reductions being smaller than the ABP reductions is the different antihypertensive agents evaluated in our study vs those included in the meta-analysis. For example, the meta-analysis did not include β-blocker mono-therapy–treated patients and we found significantly greater treatment responses as measured by daytime ABP compared with HBP for atenolol-treated patients, but not for HCTZ-treated patients. These findings suggest that perhaps there are differences when comparing BP response measurement methods between drug classes that have not been noted before.
An important strength of our study compared with many other studies is that the OBP and HBP measurements were taken using the same device, eliminating potential equipment differences that could contribute to differences in response measurements between home and office.
Conclusions
Our findings of differences in measuring BP response using office, home, and ambulatory methods have important implications for both clinical care and research. From a clinical perspective, these results are important in that target organ damage and mortality are more closely associated with home and ambulatory BP than OBP. Therefore, OBP response to antihypertensive therapy might provide a false sense of security with regard to the degree of BP control achieved with monotherapy. The decision to continue current treatment rather than add a second drug or increase the dose of the first drug would have occurred (assuming a goal BP of <140/90 mm Hg for office and <130/85 mm Hg for home) in 63% to 68% using OBP compared with only 35% to 55% with HBP. Considering the high worldwide prevalence of hypertension, these differences suggest that great improvement in hypertension outcomes might be possible through use of HBP data to assess treatment response and in decisions about need for additional treatment. Our findings also have important research implications for the larger pharmacogenomic study from which these analyses were drawn. Our results suggest that different clinical phenotypes could have different genetic associations with drug response.
Acknowledgments
The authors acknowledge and thank the valuable contributions of the study participants, support staff, and study physicians: Drs George Baramidze, Carmen Bray, Kendall Campbell, R. Whit Curry, Karen Hall, Frederic Rabari-Oskoui, Dan Rubin, and Siegfried Schmidt. This work is supported by a grant from the National Institutes of Health (Bethesda, MD), grant No. U01 GM074492, funded as part of the Pharmacogenetics Research Network. Additionally, this work is supported by the following grants from the National Institutes of Health, National Center for Research Resources: grant M01 RR00082 to the University of Florida, grants UL1 RR025008 and M01 RR00039 to Emory University, UL1 RR024150 to Mayo Clinic, and K23HL091120 to Dr Beitelshees. The study sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Dr Johnson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Fagard RH, Van Den Broeke C, De Cort P. Prognostic significance of blood pressure measured in the office, at home and during ambulatory monitoring in older patients in general practice. J Hum Hypertens. 2005;19(10):801–807. doi: 10.1038/sj.jhh.1001903. [DOI] [PubMed] [Google Scholar]
- 2.Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26(10):1919–1927. doi: 10.1097/HJH.0b013e32830c4368. [DOI] [PubMed] [Google Scholar]
- 3.Sega R, Facchetti R, Bombelli M, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111(14):1777–1783. doi: 10.1161/01.CIR.0000160923.04524.5B. [DOI] [PubMed] [Google Scholar]
- 4.Stergiou GS, Argyraki KK, Moyssakis I, et al. Home blood pressure is as reliable as ambulatory blood pressure in predicting target-organ damage in hypertension. Am J Hypertens. 2007;20(6):616–621. doi: 10.1016/j.amjhyper.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Stergiou GS, Kalogeropoulos PG, Baibas NM. Prognostic value of home blood pressure measurement. Blood Press Monit. 2007;12(6):391–392. doi: 10.1097/MBP.0b013e32824958d1. [DOI] [PubMed] [Google Scholar]
- 6.Salles GF, Cardoso CR, Muxfeldt ES. Prognostic influence of office and ambulatory blood pressures in resistant hypertension. Arch Intern Med. 2008;168(21):2340–2346. doi: 10.1001/archinte.168.21.2340. [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa J, Carroll DJ, Kuruvilla S, et al. Changes in home versus clinic blood pressure with antihypertensive treatments: a meta-analysis. Hypertension. 2008;52(5):856–864. doi: 10.1161/HYPERTENSIONAHA.108.115600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson JA, Boerwinkle E, Zineh I, et al. Pharmacogenomics of antihypertensive drugs: Rationale and design of the Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) study. Am Heart J. 2009;157(3):442–449. doi: 10.1016/j.ahj.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill JS, Zezulka AV, Beevers DG, et al. Relation between initial blood pressure and its fall with treatment. Lancet. 1985;1(8428):567–569. doi: 10.1016/s0140-6736(85)91219-x. [DOI] [PubMed] [Google Scholar]
- 10.Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society Of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52(1):10–29. doi: 10.1161/HYPERTENSIONAHA.107.189010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palatini P, Dorigatti F, Mugellini A, et al. Ambulatory versus clinic blood pressure for the assessment of anti hypertensive efficacy in clinical trials: insights from the Val-Syst Study. Clin Ther. 2004;26(9):1436–1445. doi: 10.1016/j.clinthera.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Beitelshees AL, Zineh I, Yarandi HN, et al. Discordant beta-blocker effects on clinic, ambulatory, resting, and exercise hemodynamics in patients with hypertension. Pharmacotherapy. 2006;26(9):1247–1254. doi: 10.1592/phco.26.9.1247. [DOI] [PubMed] [Google Scholar]
- 13.Finkielman JD, Schwartz GL, Chapman AB, et al. Lack of agreement between office and ambulatory blood pressure responses to hydrochlorothiazide. Am J Hypertens. 2005;18(3):398–402. doi: 10.1016/j.amjhyper.2004.10.021. [DOI] [PubMed] [Google Scholar]