Abstract
Since the ongoing coronavirus disease 2019 (COVID-19) pandemic is linked to chronic inflammation, people with initial lower inflammatory status could have better outcomes from exposure to this disease. Because dietary habits are one of the most important modifiable risk factors for inflammation, identification of dietary components associated with inflammation could play a significant role in controlling or reducing the risk of COVID-19. We investigated the inflammatory potential of diets consumed by African American (AA) and Caucasian American (CA) women of childbearing age (n = 509) who are at high risk for exposure to COVID-19 by being residents of Birmingham, Alabama, a city severely affected by this pandemic. The overall pro and anti-inflammatory scores were calculated using dietary intake data gathered using Block food frequency questionnaire. The proinflammatory potential of diets consumed by AAs was significantly higher compared to CAs. Several anti- and proinflammatory nutrients and food groups consumed differed by race. With consumption of a greater number of antioxidants and B-vitamins, CAs switched toward an anti-inflammatory score more effectively than AAs while AAs performed better than CAs in improving the anti-inflammatory score with the consumption of a greater number of minerals and vitamin D. Effective race-specific dietary modifications or supplementation with nutrients identified will be useful to improve proinflammatory diets toward anti-inflammatory. This approach could aid in controlling the current COVID-19 pandemic and future pandemics of a similar nature in women at risk for exposure.
Keywords: Child-bearing age women, Race, COVID 19 risk, Diet, Inflammatory score
1. Introduction
A substantial body of evidence indicates that chronic inflammation has an impact on overall health and well-being of individuals as it is one of the major causes of a variety of chronic diseases that include cancer [1,2], diabetes [3], cardiovascular diseases [4,5] and metabolic syndrome [6,7]. The prevalence of these inflammation-associated diseases is anticipated to increase persistently over the next 30 years in the United States as indicated by the finding that only 21% of people had more than one such condition in 2000, while 14 years later nearly 42% had more than one such condition [8] and studies have documented racial disparities in the prevalence of such diseases [9–11]. Studies have also documented that risk factors for those chronic diseases such as age, race, excess body weight (EBW), dietary habits, socioeconomic status, parity and smoking are associated with inflammation [12–17]. Therefore, factors that reduce inflammation in those affected by these commonly observed diseases are beneficial to improve the overall health of many individuals.
The ongoing coronavirus disease 2019 (COVID-19) pandemic, caused by infections with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is also linked to chronic inflammation since at a certain stage of the infection, the virus becomes a powerful stimulator of inflammation, leading to higher severity or adverse outcomes from COVID-19 [18]. Reports also suggest that the susceptibility of getting infected or the severity of COVID-19 is dictated by some of the same factors associated with inflammation (age, race, EBW), being pregnant [19] and related non communicable diseases (NCDs) such as diabetes, cardiovascular diseases and cancers [20]. Therefore, people with initial lower inflammatory status may have better outcomes from COVID-19. This indicates the importance of evaluating modifiable factors of inflammation for reducing the severity of the current COVID-19 pandemic and future pandemics of a similar nature, with a goal of providing personalized and adjusted treatment approaches to protect against or combat diseases caused by these novel viruses as well as other infectious agents.
Dietary habits are one of the most important modifiable risk factors of inflammation and associated diseases [21–23]. Therefore, identification of dietary components and nutrient-related biomarkers associated with inflammation could play a significant role not only for identifying those at higher risk for COVID-19 disease but also for reducing the risk by implementing effective dietary habit modification regimens or supplementation with such nutrients. A recent perspective has highlighted the importance of repositioning nutrition research for the prevention and control of viral pandemics of this nature [24].
Studies have shown that a Mediterranean diet rich in fruits and vegetables is associated with lower inflammation [25] whereas Western-style diets rich in carbohydrates and fats are associated with higher inflammation [26]. In addition, specific nutrients such as omega-3 fatty acids [27], vitamins A and C [28], beta-carotene [29], magnesium [30], phytochemicals [31], moderate alcohol consumption [32], and higher dietary fiber intake [33] are consistently associated with anti-inflammatory effects. Previous studies that used dietary inflammatory indices (DIIs) or empirical dietary inflammatory indices (eDIIs) to assess the inflammatory potential of food items have shown their association with ageing [34], inflammatory biomarkers [35–37], cancer [38] and cardiovascular diseases [39].
Even though the importance of dietary influence on COVID-19 is beginning to be appreciated [40], there have been no comprehensive studies conducted in this area of research at this point. A recent study suggested that well-controlled blood glucose (BG) in COVID-19 patients was associated with lower mortality rates compared to individuals with poorly controlled BG [41]. This may be due to the fact that high blood sugar levels may increase the number of inflammatory immune cells and suppress the anti-inflammatory cells, throwing the immune system out of balance resulting in a cytokine storm, seen in many people who have died from COVID-19. It is known that low-glycemic index (GI) diets have the potential to positively affect measures of BG control [42].
The purpose of the current explorative study was to investigate the following in socio-economically disadvantaged African American (AA) and Caucasian American (CA) women of childbearing age at risk for exposure to COVID-19 who are residents of Birmingham, Alabama, a city that was severely affected by the COVID-19 pandemic initially and become progressively worse; (1) assess the inflammatory potential of the overall diet consumed by AAs and CAs; (2) assess the racial differences in the intakes of the number, amount and the frequencies of specific macronutrients, energy adjusted micronutrients and minerals, dietary fiber and servings of food groups contributing to the overall inflammatory score; (3) assess the race-specific determinants (demographic/lifestyle factors, health indices) of the overall dietary inflammatory score.
2. Methods and materials
2.1. Study population
The present analysis is based on 509 women (AA, n = 327 and CA, n = 182) of childbearing age enrolled by a previous study [43]. All women included in the study were well characterized for socio-demographic information (age, race, source of payment of health insurance), lifestyle factors (parity, smoking, physical activity), excess body weight (EBW)-related markers (body mass index [BMI], waist circumference [WC] and % body fat [%BF]) and dietary intake information. All study protocols of the study complied with the Helsinki Declaration as revised in 1983 and the study has been approved the University of Alabama at Birmingham Institutional Review Board (protocol number IRB-040126002)
2.2. Assessment of the dietary intake and the calculation of the dietary inflammatory score are described in our data in brief publication
2.3. Statistical analyse
The statistical analysis focused on testing the following: (1) The differences in the distribution of women according to anti-inflammatory and proinflammatory scores of each food item based on their amount and the frequency of consumption by race; (2) Average DIS based on moderate-high level of consumption of 19 proinflammatory food items in categories of 0, 1–2, 4–6, and ≥7 by race; (3) The association between race and the overall DIS after controlling for other demographic and lifestyle factors using a regression model; (4) Differences in demographic and lifestyle factors, indicators of EBW (BMI, % BF,or WC) by race and by the median DIS using the Pearson χ2; (5) The racial differences in the average intakes of macronutrients and energy adjusted micronutrients and minerals, dietary fiber and servings of food groups by the median DIS using ANOVA; (6) The racial differences in the DIS by the number of antioxidant nutrients, B-vitamins or minerals along with vitamin D consumed at higher than the median intakes of the population and by age, smoking status, parity and indicators of EBW within each race; (7) The associations between age, source of payment of health insurance, indicators of EBW, physical activity level, smoking status, parity, dietary indices (health eating index (HEI), GI, and GL) and DIS by using regression models stratified by race.
3. Results
The study population consisted of 64% AA and 35% of CA women between the age ranges of 19 −50 years. A higher percentage of AAs (60%) reported that they were unable to pay for their own health insurance compared to CAs (33%). Approximately 60% had EBW based on BMI, WC or % BF and 37% were current smokers. The overall DIS for the study population ranged from −36 to + 33 with a median of + 2 while it ranged from −36 to + 24 with a median of 1 for CAs and −24 to 33 with a median of 3 for AAs. Those who had a score ≥ + 2 and < + 2 were considered to have consumed an overall proinflammatory or an anti-inflammatory diet, respectively. We observed that AAs were 2.3 times more likely to have consumed a diet with proinflammatory potential compared to CAs, independent of other demographic and lifestyle factors (OR = 2.3, 95%CI = 1.5–3.4, P <.0001) suggesting the need to test food consumption habits and determinates of the inflammatory potential of the diet by race.
The racial differences in the distribution of women according to the anti-inflammatory and proinflammatory score of selected food items based on the amount and the frequency of consumption are given in Table 1.
Table 1 –
Racial differences in the distribution of women according to anti-inflammatory and proinflammatory scores of selected food items based on the amount and frequency of consumption of food items.
| Anti-inflammatory food items | African American | Caucasian American | P value | ||
|---|---|---|---|---|---|
| Moderate to high consumption(score −1 or −2) | Low consumption (score 0) | Moderate to high consumption(score −1 or −2) | Low consumption(score 0) | ||
| Vegetable/fruit juices | |||||
| Real 100% orange juice or grapefruit juice including fresh, frozen or bottled | 106 (32%) | 221 (68%) | 22 (12%) | 160 (88%) | <.0001 |
| Other real juices like apple juice, prune juice, lemonade | 90 (28%) | 237 (72%) | 17 (9%) | 165 (91%) | <.0001 |
| Milk, coffee and tea beverages | |||||
| Coffee regular or decaf | 38 (12%) | 289 (88%) | 42 (23%) | 140 (77%) | .0007 |
| Tea or iced tea | 43 (13%) | 284 (87%) | 74 (41%) | 108 (59%) | <.0001 |
| Milk | 56 (17%) | 271 (83%) | 55 (30%) | 127 (70%) | .0006 |
| Fruits and berries | |||||
| Raw peaches, apricots, nectarines (in season) | 60 (18%) | 267 (82%) | 16 (9%) | 166 (91%) | .0037 |
| Orange or tangerines | 33 (10%) | 294 (90%) | 8 (4%) | 174 (96%) | .0236 |
| Grapes, white, red or green | 80 (24%) | 247 (76%) | 28 (15%) | 154 (85%) | .0163 |
| Canned fruits like applesauce, fruit cocktail or dried fruit like raisins | 22 (7%) | 305 (93%) | 2 (1%) | 180 (99%) | .0036 |
| Vegetables and green leafy vegetables | |||||
| Mustard greens, turnip greens, collards | 24 (7%) | 303 (93%) | 2 (1%) | 180 (99%) | .0013 |
| Cereal and cereal products with high fiber | |||||
| Miscellaneous | |||||
| Catsup, salsa or chili peppers | 36 (11%) | 291 (89%) | 32 (18%) | 150 (82%) | .0367 |
| Proinflammatory food items | African American Moderate to high consumption(score + 1 or + 2) | Low consumption (score 0) | Caucasian American Moderate to high consumption(score + 1 or + 2) | Low consumption (score 0) | P value |
|---|---|---|---|---|---|
| Beverages | |||||
| Drinks with some juice in them like sunny delight, Juice squeeze | 46 (14%) | 281 (86%) | 10 (5%) | 172 (95%) | .0031 |
| Kool aid, HI C or other drinks with added vitamin C | 157 (48%) | 170 (52%) | 28 (15%) | 154 (85%) | <.0001 |
| Corn and related items | |||||
| Corn bread or corn muffins | 14 (4%) | 313 (96%) | 1(1%) | 181 (99%) | .0144 |
| Tortillas | 1 (0%) | 326 (100%) | 6 (3%) | 176 (97%) | .0097 |
| Refined foods – cakes, pastries, cookies, biscuits | |||||
| Cakes, sweet rolls, coffee cake | 27 (8%) | 300 (92%) | 4 (2%) | 178 (98%) | .0060 |
| Cookies | 86 (26%) | 241 (74%) | 24 (9%) | 158 (87%) | .0006 |
| Pancakes, waffles, French toast, pop tarts | 38 (12%) | 289 (88%) | 7 (4%) | 175 (96%) | .0031 |
| White bread or toast including French, Italian or in sandwiches | 69 (21%) | 258 (79%) | 23 (13%) | 159 (87%) | .0174 |
| Meat red, processed and organs | |||||
| Bacon | 101 (31%) | 226 (69%) | 33 (18%) | 149 (82%) | .0017 |
| Hamburgers, cheeseburgers, meat loafs, at home or in restaurant | 58 (18%) | 269 (82%) | 12 (7%) | 170 (93%) | .0005 |
| Hot dogs, hamburgers, sausages | |||||
| Hot dogs or sausages like polish, Italian or chorizos | 24 (7%) | 303 (93%) | 5 (3%) | 177 (97%) | .0441 |
| Breakfast sausages including sausage biscuits | 46 (14%) | 281 (86%) | 8 (4%) | 174 (96%) | .0007 |
| Fried foods | |||||
| Snacks like Potato chips, corn chip, popcorns (not pretzels) | 78 (24%) | 249 (76%) | 21 (12%) | 161 (88%) | .0008 |
| French fries, fried potatoes or hash browns | 55 (17%) | 272 (83%) | 16 (9%) | 166 (91%) | .0122 |
| Fried chicken at home or restaurant | 93 (28%) | 234 (72%) | 10 (5%) | 172 (95%) | <.0001 |
| Fried fish or fish sandwich at home or in restaurant | 41 (13%) | 286 (87%) | 1 (2%) | 178 (98%) | .0001 |
| Candy and bars | |||||
| Chocolate candy or candy bars | 66 (20%) | 261 (80%) | 22 (12%) | 160 (88%) | .0206 |
| Other candy, not chocolate, like hard candy, caramel, jelly beans | 72 (22%) | 255 (78%) | 22 (12%) | 160 (88%) | .0057 |
| Sugar in tea or coffee | 61 (19%) | 266 (81%) | 64 (35%) | 118 (65%) | <.0001 |
Values are number and percentage of the population.
P values are for Pearson χ2 test and Fischer exact test was used when the number were small in any one the groups.
A higher percentage of AAs were moderate to high consumers of 100% fruit juices or other real juices that contributed to a moderate to high anti-inflammatory scores (subsequently referred to as “higher score”) compared to CAs (P ≤.0001 for both). A higher percentage of CAs were higher consumers of coffee, regular tea/iced tea or brewed black tea contributing to a higher anti-inflammatory score compared to AAs (P =.0007, <.0001,and <.0001). A higher percentage of CAs also were higher consumers of milk contributing to a higher anti-inflammatory score compared to AAs (P =.0006). With regard to consumption of fruits, we observed that a higher percentage of AAs were higher consumers of fruits such as peaches/apricots/nectarines, oranges/tangerines, grapes and canned fruits contributing to a higher anti-inflammatory score compared to CAs (P =.0037,.0036,.0364,.0044). Green leafy vegetables were also consumed at higher amounts by AAs contributing to a higher anti-inflammatory score compared to CAs (0.0013).
Several proinflammatory food items except for added sugar and tortillas were consumed at higher amounts by AAs, contributing to a higher proinflammatory score compared to CAs. The amount and the frequency of consumption of soft drinks and vitamin C-fortified drinks was higher in AAs contributing to higher proinflammatory scores in AAs compared to CAs (P =.0031, <.0001). While the consumption of corn bread/muffins were higher in AAs, contributing to a higher proinflammatory score compared to CAs (P =.0144), the consumption of tortillas was higher in CAs contributing to a higher proinflammatory scores compared to AAs (P =.0097). Refined food items such as cakes/sweet rolls, cookies, pancakes/waffles/French toast/pop tarts and white bread were consumed at higher amounts by AAs contributing to a higher proinflammatory scores compared to the CAs (P =.0060,.0006,.0031, and.0174). A higher percentage of AAs consumed higher amounts of items such as bacon, hamburgers/cheese burgers/meat loaf, hot dogs and breakfast sausages, contributing to a higher proinflammatory score compared to CAs (P =.0017,.0005,.0441, and.0007). A higher percentage of AAs also consumed higher amounts of fried food items such as chips, fries or fried potatoes, fried chicken and fried fish, contributing to a high proinflammatory score compared to the CAs (P =.0008,.0122, <.0001, and.0001). Interestingly, although a higher percentage of AAs consumed higher amounts of chocolate candies and other candies, contributing to a higher proinflammatory score compared to CAs (P =.0206 and.0057), a higher percentage of CAs consumed greater amounts of sugar in tea or coffee that resulted in a higher proinflammatory score compared to AAs (P <.0001).
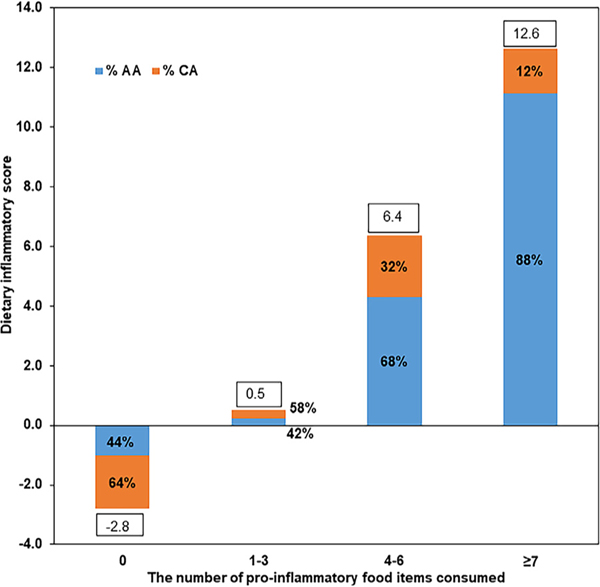
Fig. 1 depicts the stepwise increase in the DIS based on the consumption of higher amounts of 19 proinflammatory food items in categories of 0, 1 to 3, 4 to 6, and ≥7 and the percentages of AAs and CAs in each category. Those who did not consume higher amounts of any of the 19 proinflammatory food items had an anti-inflammatory score of −2.8 and the percentage of CAs in this category was higher than in AAs. There was a stepwise increase in the proinflammatory score as the number of proinflammatory items consumed increased along with an increase in the percentage of AAs in those categories. A similar analysis stratified by EBW indicated that the number of these food items consumed in higher amounts did not differ by higher or lower EBW status (data not shown).
Fig. 1 –
Dietary inflammatory score by the number of proinflammatory food items consumed at higher amounts.
Table 2 shows the racial differences in the demographic, EBW measurements and lifestyle factors by the inflammatory score categories. A higher percentage of AAs women with a proinflammatory score were smokers (30%) compared to AAs with an anti-inflammatory score (20%) and this difference was reaching significance (P =.0518). A higher percentage of CAs with an anti-inflammatory score (52%) had BMI ≥25kg/m 2 compared to those with proinflammatory score (32%) (P =.0321). None of the other demographic variables, EBW measurements and lifestyle factors were different by the median DIS in either race.
Table 2 –
Demographic and risk factors of the study population by the median dietary inflammatory score and by race.
| Variables | African American | Caucasian American | ||||
|---|---|---|---|---|---|---|
| DIS | P value | DIS | P value | |||
| <2 ( n = 137) | ≥2 ( n = 190) | <2 ( n = 105) | ≥2 ( n = 77) | |||
| Median age (y) | ||||||
| < 23 | 68 (50%) | 99 (52%) | .6593 | 52 (50%) | 36 (47%) | .7117 |
| ≥ 23 | 69 (50%) | 91 (48%) | 53 (50%) | 41 (53%) | ||
| Health insurance paid on their own | ||||||
| Yes | 56 (41%) | 75 (39%) | .7985 | 67(64%) | 55 (71%) | .2800 |
| No | 81 (59%) | 115 (61%) | 38 (36%) | 22 (29%) | ||
| BMI a (kg/m 2 ) | ||||||
| < 25 | 39 (28%) | 67 (35%) | .1952 | 50 (48%) | 49 (64%) | .0321 |
| ≥ 25 | 98 (72%) | 151 (65%) | 55 (52%) | 28 (36%) | ||
| % Body fat | ||||||
| < 33% | 40 (29%) | 63 (33%) | .4468 | 53 (50%) | 45 (58%) | .2869 |
| ≥ 33% | 97 (71 %) | 127 (67%) | 52 (50%) | 32 (42%) | ||
| Waist circumference (cm) | ||||||
| < 88 | 45 (33%) | 74 (39%) | .2579 | 47 (45%) | 42 (55%) | .1921 |
| ≥ 88 | 92 (67%) | 116 (61%) | 58 (55%) | 35 (45%) | ||
| Moderate physical activity (minutes/week) | ||||||
| < 150 | 107 (78%) | 158 (83%) | .2498 | 80 (76%) | 63 (82%) | .3606 |
| ≥ 150 | 30 (22%) | 32 (17%) | 25 (24%) | 14 (18%) | ||
| Smoking status | ||||||
| Non current | 109 (80%) | 133 (70%) | .0518 | 45 (43%) | 33 (43%) | 1.0000 |
| Current | 28 (20%) | 57 (30%) | 60 (57%) | 44 (57%) | ||
| Parity | ||||||
| 0 live births | 54 (39%) | 60 (32%) | .1423 | 33 (31%) | 23 (30%) | .8219 |
| ≥1 live births | 83 (61%) | 130 (68%) | 72 (69%) | 54 (70%) | ||
Values are number and percentage of the population.
P values are for Pearson χ2 test.
BMI-body mass index.
Table 3 shows the racial differences in the average intakes of macronutrients, energy adjusted micronutrients, minerals and dietary fiber and servings of food groups by the DIS. In both AA and CA women, those with a proinflammatory score consumed higher amounts of calories, proteins, carbohydrates, and total fat (P <.0001 for all). AAs and CAs with a higher anti-inflammatory score were more likely to have higher average intakes of antioxidant nutrients vitamins A, E, C, alpha and beta carotene, cryptoxanthin and lutein, (P <.01) while only AAs with an anti-inflammatory score were more likely to have higher intakes of antioxidant lycopene and total flavonoids compared to those with a proinflammatory score (P =.0467 and <.0001, respectively). Both AAs and CAs with a higher anti-inflammatory score were more likely to have higher average intakes of B-vitamins (thiamin, riboflavin, pyridoxine, folate, and pantothenic acid) and minerals (calcium, phosphorous, potassium, iron, copper, manganese and magnesium) and vitamin D (P <.01 for all) while only AAs with an anti-inflammatory score were more likely to have higher average intakes of zinc compared to AAs with proinflammatory score (P =.0214). Further, a higher percentage of AAs and CAs with an anti-inflammatory score were more likely to consume a greater number of servings of vegetables excluding servings of salads or potatoes, fruits with or without fruit juice and a lower number of servings of meat, fish, poultry eggs, and beans, fat and sugar (P <.01). None of the other variables were significantly different by the inflammatory score either race.
Table 3 –
Differences in the average intakes of macronutrients, energy adjusted micronutrients and minerals intakes, dietary fiber and servings of food groups by the overall dietary inflammatory score by race.
| Nutrient/food group serving/indices | African American | Caucasian American | ||||
|---|---|---|---|---|---|---|
| DIS | P value | DIS | P value | |||
| <2 | ≥2 | <2 | ≥2 | |||
| Nutrients | ||||||
| Energy Kcal/day | 1928.8 | 3126.4 | <.0001 | 1786.45 | 2443.8 | <.0001 |
| Protein (g)/day | 61.0 | 97.0 | <.0001 | 56.8 | 80.1 | .0001 |
| Fat (g)/day | 74.6 | 133.3 | <.0001 | 71.2 | 104.0 | <.0001 |
| Carbohydrate (g)/day | 254.5 | 383.5 | <.0001 | 227.9 | 297.3 | .0003 |
| Retinol (preformed vit A) ug/day | 207.8 | 181.2 | .1120 | 216.8 | 180.3 | .008 |
| Provitamin A (μg)/day | 1661.7 | 839.6 | <.0001 | 1743.9 | 1026.2 | .0030 |
| Alpha carotene (μg)/day | 220.2 | 87.7 | <.0001 | 258.3 | 134.0 | .0052 |
| Beta carotene (μg)/day | 1341.5 | 694.9 | <.0001 | 1307.8 | 815.4 | .0041 |
| Cryptoxanthin (μg)/day | 108.4 | 50.1 | <.0001 | 72.0 | 28.1 | <.0001 |
| Lutein (μg)/day | 922.2 | 440.9 | <.0001 | 692.5 | 375.9 | .0002 |
| Lycopene (μg)/day | 2085.1 | 1734.3 | .0467 | 2694.7 | 2301.2 | .2305 |
| Total flavonoids/day | 60.4 | 23.3 | <.0001 | 101.5 | 75.3 | .2252 |
| Vitamin C (mg)/day | 94.4 | 71.4 | <.0001 | 67.0 | 38.7 | <.0001 |
| Vitamin E (a-TE)/day | 4.4 | 3.8 | .0047 | 4.6 | 3.9 | <.0001 |
| Vitamin B1 (mg)/day | 0.7 | 0.6 | <.0001 | 0.7 | 0.6 | .0234 |
| Riboflavin (mg)/day | 0.8 | 0.7 | <.0001 | 0.9 | 0.7 | <.0001 |
| Vitamin B6 (mg)/day | 0.8 | 0.7 | <.0001 | 0.8 | 0.7 | .0005 |
| Vitamin B12 (μg)/day | 1.8 | 1.6 | .4161 | 1.8 | 1.7 | .0957 |
| Folate DFE (DFE)/day | 222.4 | 177.8 | <.0001 | 215.3 | 184.4 | .0040 |
| Pantothenic acid (mg)/day | 2.2 | 1.8 | <.0001 | 2.3 | 1.8 | .0004 |
| Vitamin D (IU)/day | 71.3 | 40.9 | <.0001 | 83.5 | 57.2 | .0007 |
| Calcium (mg)/day | 362.6 | 262.9 | <.0001 | 400.6 | 317.2 | .0002 |
| Phosphorous (mg)/day | 520.7 | 475.1 | <.0001 | 566.0 | 523.5 | .0137 |
| Potassium (mg)/day | 1336.5 | 1042.9 | <.0001 | 1395.8 | 1081.0 | <.0001 |
| Zinc (mg)/day | 4.6 | 4.1 | .0214 | 4.9 | 4.6 | .2969 |
| Iron (mg)/day | 6.8 | 5.8 | <.0001 | 6.5 | 5.8 | .0272 |
| Magnesium (mg)/day | 118.0 | 93.3 | <.0001 | 130.8 | 105.5 | <.0001 |
| Copper (mg)/day | 0.5 | 0.4 | <.0001 | 0.6 | 0.5 | <.0001 |
| Manganese(mg)/day | 1.3 | 1.1 | <.0001 | 1.7 | 1.3 | <.0001 |
| Total isoflavones (mg)/day | 0.6 | 0.6 | .4852 | 0.8 | 0.6 | .1677 |
| Total dietary fiber (g)/day | 7.1 | 5.3 | <.0001 | 7.2 | 5.8 | <.0001 |
| Servings of food groups | ||||||
| Vegetable servings/day a | 2.4 | 2.3 | .6954 | 2.6 | 2.3 | .3343 |
| Fruit servings/day b | 1.5 | 1.0 | <.0001 | 1.1 | 0.5 | <.0001 |
| Grain servings/day c | 4.0 | 6.5 | <.0001 | 3.6 | 5.3 | <.0001 |
| Whole grain servings/day d | 1.2 | 1.4 | .213 | 0.8 | 0.8 | .502 |
| Dairy servings/day e | 1.1 | 1.1 | .9163 | 1.4 | 1.4 | .7585 |
| Meat, fish, poultry, egg and bean | 1.9 | 3.3 | <.0001 | 1.6 | 2.5 | <.0001 |
| servings/day | ||||||
| Fat and sugar servings/day f | 2.8 | 4.7 | <.0001 | 3.4 | 4.5 | .0001 |
| Global vegetable servings/week g | 4.7 | 3.1 | .0019 | 7.3 | 4.9 | .0054 |
| Global fruit servings/week h | 5.3 | 2.7 | <.0001 | 4.1 | 1.7 | <.0001 |
| Global cereal servings i | 2.6 | 1.9 | .0632 | 2.5 | 2.1 | .5034 |
Abbreviations: DFE, dietary folate equivalent; TE, tocopherol equivalents.
Values are means; P-values are for ANOVA test for continuous variables.
Serving of vegetables including salads or potatoes.
Frequency of fruits including fruit juices.
Servings of breads, cereals, rice, pasta.
Servings of grains excluding pasta and refined cereal products.
Serving of milk, yoghurt, cheese.
Serving of fats and oils, sweets and sodas.
Servings of vegetables excluding salad or potatoes.
Servings of fruit excluding fruit juices.
Servings of cold cereals.
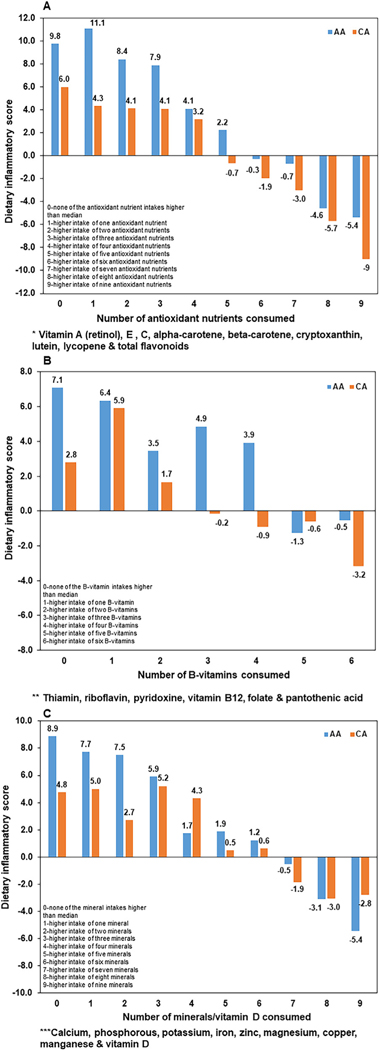
Fig.2 depicts the racial differences in the DIS by the number of antioxidant nutrients, B-vitamins or minerals along with vitamin D consumed at higher than the median intakes of the population (subsequently referred to as “higher amount”). We observed a gradual decrease in the proinflammatory score and an increase in anti-inflammatory score in both AAs and CAs when they consume higher amounts of multiple antioxidant nutrients, B-vitamins or minerals/vitamin D. However, given the same number of nutrient intakes (0–6 antioxidants, 0–4 B-vitamins, 0–6 minerals/vitamin D), AAs had a tendency to have a higher proinflammatory score compared to CAs. With a greater number of nutrients consumed at higher amounts (7–9 antioxidants, 5–6 B-vitamins), there was a tendency for CAs to switch toward anti-inflammatory score more effectively than AAs while with higher number of intakes of minerals/vitamin D, AAs performed better than CAs in improving the anti-inflammatory score. However, a sub analysis within each race by factors such as age, smoking, parity, and indicators of EBW that could influence the intakes of nutrients and minerals demonstrated that this pattern varied by those factors. That is, older AAs and CAs who consumed higher amounts of multiple antioxidant nutrients had a higher anti-inflammatory score compared to younger AAs or CAs. However, with the consumption of higher amounts of multiple B-vitamins or minerals, younger AAs had a higher anti-inflammatory score compared to older AAs while older CAs had a higher score compared to younger CAs. AA smokers who consumed higher amounts of multiple antioxidant nutrients had a higher anti-inflammatory score compared to AA non smokers while there was no difference in the anti-inflammatory score between CA by smoking status. Both AA and CA non–smokers who consumed higher amounts of multiple B-vitamins or minerals had higher anti-inflammatory score compared to smokers. CAs with no live births and AAs with ≥1 live births who consumed higher amounts of multiple antioxidant nutrients had a higher anti-inflammatory score compared to CAs with ≥1 live birth and AAs with no live births, respectively. The inflammatory score did not differ with the consumption of higher amounts of multiple Bvitamins by parity in both races. Both AA and CA women with greater WC, BMI or % BF had higher anti-inflammatory scores with the consumption of higher amounts of antioxidants or minerals compared to women with lower values for those indicators. With regard to the consumption of higher amounts of multiple B-vitamins, CAs with greater WC, BMI or % BF had a higher anti-inflammatory score compared to CAs with lower values for those indicators while there were no differences in the score in AAs by EBW status.
Fig. 2 –
Dietary inflammatory score by higher intakes of varying numbers of (A) antioxidant nutrients * (B) B-vitamin nutrients ** (C) minerals/vitamin D *** among AAs and Cas.
Table 4 shows the racial differences in the associations between demographic and lifestyle factors, health indices (HEI or GI or GL), and the DIS. Models were run replacing the indicators of EBW and health indices, one at a time since those were correlated. In both AAs and CAs, those with a higher GI or GL were more likely to have a higher proinflammatory scores compared to women with lower GI or GL (for AA, P =.0003 and <.0001, respectively and for CA P =.0022 and.0004 restively) while those with a higher HEI were less likely to have a higher proinflammatory score (P <.0001 for both AA and CA). None of the other variables, including indicators of EBW, were associated with the DIS in either race.
Table 4 –
Relationship between demographic variables, lifestyle factors, indicators of EBW a dietary indices and dietary inflammatory score by race.
| Variables | African American | Caucasian American | ||
|---|---|---|---|---|
| DIS ≥2 vs < 2 | P value | DIS ≥2 vs < 2 | P value | |
| OR (95% CI) | OR (95% CI) | |||
| Age | ||||
| < median | 1.00 | .4545 | 1.00 | .6213 |
| ≥median | 0.8 (0.5–1.4) | 1.2 (0.6–2.3) | ||
| Heath insurance paid on their own | ||||
| Yes | 1.00 | .8628 | 1.00 | .8216 |
| No | 1.0 (0.6–1.7) | 0.7 (0.5–1.9) | ||
| Waist circumference (cm) | ||||
| < 88 | 1.00 | .3295 | 1.00 | .3076 |
| ≥88 | 0.8 (0.5–1.3) | 0.7 (0.4–1.4) | ||
| Moderate physical activity (minutes/week) | ||||
| < 150 | 1.00 | .2247 | 1.00 | .6048 |
| ≥150 | 0.7 (0.4–1.3) | 0.8 (0.4–1.8) | ||
| Smoking status | ||||
| Non current | 1.00 | .1330 | 1.00 | .6015 |
| Current | 1.5 (0.9–2.6) | 0.8 (0.4–1.6) | ||
| Parity | ||||
| 0 live births | 1.00 | .1330 | 1.00 | .2372 |
| ≥1 live births | 1.5 (0.9–2.6) | 1.2 (0.8–3.0) | ||
| HEI b | ||||
| < 54 | 1.00 | <.0001 | 1.00 | .0002 |
| ≥54 | 0.2 (0.2–0.4) | 0.3 (0.2–0.6) | ||
| Glycemic index c | ||||
| < 54 | 1.00 | .0003 | 1.00 | .0022 |
| ≥54 | 2.7 (1.7–4.4) | 2.7 (1.4–5.1) | ||
| Glycemic load c | ||||
| < 139 | 1.00 | <.0001 | 1.00 | .0004 |
| ≥139 | 3.9 (2.4–6.3) | 3.2 (1.7–6.1) | ||
Unconditional logistic regression analysis used to test the relationships.
EBW-excess body weight.
HEI-health eating index.
Because of their significant correlation with each other, 3 indices were tested in separate models, one at a time.
4. Discussion
This study that focused on the inflammatory potential of diets consumed by AA and CA women who are at high risk for exposure to COVID-19 documented that the proinflammatory potential of diets consumed by AAs was significantly higher compared to CAs and several anti- and proinflammatory nutrients and food groups consumed differed by race. With consumption of a greater number of antioxidants and B-vitamins, CAs switched toward an anti-inflammatory score more effectively than AAs while AAs performed better than CAs in improving the anti-inflammatory score with the consumption of a greater number of minerals and vitamin D. Therefore, effective race-specific dietary modifications or supplementation with nutrients identified will be useful to improve proinflammatory diets toward anti-inflammatory. This approach could aid in controlling the current COVID-19 pandemic and future pandemics of a similar nature in women at risk for exposure to such viral infections.
The handling of the COVID-19 pandemic in the US and worldwide has been challenging since clinically approved COVID-19 medications or vaccines will not be available for at least 12 months after the onset of the pandemic. This underscores the importance of evaluating other factors that may reduce the burden of disease due to this virus as well as exposure to other viruses of similar nature in the future. We begin to understand that there are wide racial and individual variations in response to COVID-19 [16], indicating the possibility that treatments can be personalized or adjusted to protect or combat COVID-19 infections. Dietary factors should be consid ered in this approach since the consumption of a healthy diet provides nutrients that play a significant role in controlling the likelihood of getting infected as well as inflammatory re sponse to such viral infections. We have previously shown that in a cohort of women similar to the current study, those with higher circulating concentrations of folate were significantly less likely to get infected with carcinogenic or high-risk (HR) genotypes of human papillomaviruses (HPVs), and less likely to have a persistent infection and more likely to clear the in fection [44]. In addition, we also reported that Indian women with higher circulating concentrations of folate and vitamin B12 were significantly less likely to be infected with HR-HPVs [45]. Such nutrient-regulated mechanisms that control epige netic changes [46,47] could have a significant effect on in flammatory response to viral infections. Other micronutrients are also crucial to epigenetic modification to strengthen the immune system [48]. Several studies have documented that restoring or improving the nutritional status may enhance the ability to ward off NCDs and infectious diseases [49,50]. There fore, it is important to understand dietary habits or patterns that favor proinflammatory potential in all people who risk exposure to COVID-19 because of their lack of knowledge or lifestyle. We focused on women of childbearing age at risk for exposure to COVID-19, since recent studies have shown that pregnant women and their fetuses represent a high-risk population for this disease [51]. It is also clear that AAs are disproportionately affected by this pandemic with regard to the rate of transmission as well as adverse outcomes [52,53]. Even though the differences with respect to socio-economic status, knowledge or lack of access to appropriate methods to prevent infection by the COVID-19 may explain some of these racial disparities [54], the role played by other lifestyle factors such as diet-related inflammatory status should not be overlooked in a long-term plan for reducing the burden of viral infections of this nature. Several studies have shown that AAs have a poor diet and a higher prevalence of food insecurity than CAs, contributing to lower intakes of several nutrients with overall health benefits [55–58]
In this explorative study, both AA and CA women could be considered to have lower socio-economic status in general as they were all referred by health departments in the Birmingham area. However, we noted that a larger percentage of AAs were unable to pay for their health insurance on their own and needed assistance, suggesting that AAs are likely to have lower socio-economic status than CAs. We observed that the overall proinflammatory potential of diets consumed by AAs was significantly higher compared to CAs despite their consumption of more anti-inflammatory food items compared to CAs. This could be explained by the fact that AAs also consumed significantly higher amounts of proinflammatory food items compared to CAs, negating the beneficial effects of anti-inflammatory food items consumed.
A lack of significant association between EBW and the proinflammatory potential of diets consumed was somewhat unexpected. We, however, noted that a greater percentage of women with a higher EBW consumed ≥ 7 micronutrients and minerals but similar amounts of proinflammatory food items compared to those with a lower EBW, and this may explain this finding.
Our results also indicated that with targeted dietary advice, it is possible to change a proinflammatory diet toward an anti-inflammatory diet in both races but CAs appear to benefit more from incorporating higher amounts of several antioxidant nutrients and B-vitamins while AAs may benefit from higher intakes of minerals/vitamin D provided both races consume nutrient-dense food items rather than caloriedense food items. We noted that with a given number of both micronutrient and B-vitamin intakes, AAs have a higher proinflammatory score or lower anti-inflammatory score. This could be due to racial differences in food preparation methods such as over-cooking that diminishes the nutritive value of food. Future developments in diet questionnaires that capture details of food preparation methods and make a correction for nutrient losses and tailored advice based on this aspect is required to address this racial disparity. We also noted that certain factors that could influence the consumption of food items containing antioxidant nutrients, B-vitamins and minerals such as age and smoking habits have race-specific differential effects on improvements in the inflammatory score, suggesting that those factors may influence food choices or food preparation methods in different ways in AAs and CAs. We were unable to evaluate some factors that may influence the intakes of those nutrients and minerals such as the physical activity because of the smaller number of women in the comparison category. Therefore, in order to provide effective tailored dietary guidance to AAs and CAs, future studies with larger sample size are needed to confirm our results in this aspect and also evaluate other factors that could modify the intakes or bioavailability of such nutrients that are not considered in this study.
In this study, we have identified several anti- and proinflammatory nutrients and food groups consumed by this population in general and their race specific differences, thus allowing implementation of successful targeted dietary interventions. Further, we also noted that several health indices such as HEI, GI and GL are associated with the inflammatory potential of overall diet in this population. GL is especially important since lowering the GL of the diet may be an effective method to improve glycemic control, a factor that place people at higher risk for COVID-19 severity. These indices will be useful for monitoring the effectiveness of dietary interventions by calculating the indices before and after such interventions. Future discovery and validation of omic-based molecular markers that are related to dietary habits identified in this study in easily obtainable biological materials will strengthen such efforts.
Overwhelming evidence indicates that inflammatory state in humans is a feature of a wide range of chronic conditions including several important ones that increase the severity of exposure to COVID-19. There is also a substantial evidence to suggest that many macroand micronutrients as well as nonnutrient food components modulate both acute and chronic inflammation. [59,60] Therefore, effective race-specific modifications based on dietary components identified by our study in this high-risk population will not only be beneficial to improve overall health but also to prevent, control or lower the severity of the current COVID-19 pandemic and future pandemics of a similar nature. The influence of diet on COVID-19 risk has not yet been explored in any detail, and our study results introduce the concept that dietary effects on inflammation may have a possibly profound impact on response to this virus, which will likely be with us for many years to come. Our next step will be to couple these concepts with biomarker analyses in a population with known COVID-19 status in a setting of targeted dietary advice to improve their diets toward an anti-inflammatory potential.
Supplementary Material
Acknowledgment
This work was supported by National Cancer Institute (R01 105448 ) and Minority Health Research Training (T37MD001448) grant, National Institute on Minority Health and Health Disparities. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations:
- AA
African American
- ANOVA
analysis of variance
- BMI
body mass index
- BF
body fat
- BG
blood glucose
- CA
Caucasian American
- COVID-19
corona virus disease 2019
- DIIs
dietary inflammatory indices
- DIS
dietary Inflammatory Score
- EBW
excess body weight
- eDII
empirical dietary inflammatory index
- FFQ
food frequency questionnaire
- GI
glycemic Index
- GL
glycemic Load
- HEI
health eating index
- HR-HPV
high-risk human papillomavirus
- NCDs
noncommunicable diseases
- SARS, CoV-2
severe acute respiratory syndrome coronavirus 2
- WC
waist circumference
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.nutres.2021.04.004.
REFERENCES
- [1].Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology 2010;138:2101–14. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- [2].Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer 2013;13:759–71. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- [3].Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol 2019;11:45–63. [PMC free article] [PubMed] [Google Scholar]
- [4].Pearson TA, Mensah GA, Alexander RW, Anderson JL 3rd Cannon RO, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- [5].Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, Creactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA 1998;279:1477–82. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PubMed] [Google Scholar]
- [6].Welty FK, Alfaddagh A, Elajami TK. Targeting inflammation in metabolic syndrome. Transl Res 2016;167:257–80. doi: 10.1016/j.trsl.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Syauqy A, Hsu CY, Rau HH, Chao JC. Association of dietary patterns with components of metabolic syndrome and inflammation among middle-aged and older adults with metabolic syndrome in Taiwan. Nutrients 2018;10:143. doi: 10.3390/nu10020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pahwa R, Goyal A, Bansal P, Jialal I. Chronic inflammation. StatPearls, Treasure Island (FL): StatPearls Publishing; 2020. Accessed November 20, 2020 https://www.ncbi.nlm.nih.gov/books/NBK493173/. [Google Scholar]
- [9].Graham G. Disparities in cardiovascular disease risk in the United States. Curr Cardiol Rev 2015;11:238–45. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep. 2013;13:814–23. doi: 10.1007/s11892-013-0421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Holmes L, Hossain J, Ward D, Opara F. Racial/ethnic variability in diabetes mellitus among United States residents is unexplained by lifestyle, socio demographics and prognostic factors. ISRN Public Health 2012;2012:1–8. [Google Scholar]
- [12].Saltiel AR, Jerrold MO. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest 2017;127:1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Clendenen TV, Koenig KL, Arslan AA, Lukanova A, Berrino F, Gu Y, et al. Factors associated with inflammation markers, a crosssectional analysis. Cytokine 2011;56:769–78. doi: 10.1016/j.cyto.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stepanikova I, Bateman LB, Oates GR. Systemic inflammation in midlife: race, socioeconomic status, and perceived discrimination. Am J Prev Med 2017;52:S63–76. doi: 10.1016/j.amepre.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gardener SL, Rainey-Smith SR, Martins RN. Diet and inflammation in Alzheimer’s disease and related chronic diseases: a review. J Alzheimers Dis 2016;50:301–34. doi: 10.3233/JAD-150765. [DOI] [PubMed] [Google Scholar]
- [16].Coronavirus Disease 2019. (COVID 19) [internet] https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/racial-ethnic-minorities.html.
- [17].Lee J, Taneja V, Vassallo R. Cigarette smoking and inflammation: cellular and molecular mechanisms. J Dent Res 2012;91:142–9. doi: 10.1177/0022034511421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Prete M, Favoino E, Catacchio G, Racanelli V, Perosa F. SARS-CoV2 inflammatory syndrome. clinical features and rationale for immunological treatment. Int J Mol Sci 2020;21:3377. doi: 10.3390/ijms21093377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bahat PY, Talmac MV, Bestel A, Selcuki NT, Aydin Z, Polat I. Micronutrients in COVID-19 positive pregnancies. Cureus 2020;12:e10609. doi: 10.7759/cureus.10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 2020;12:6049–57. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Effects of ginger supplementation on inflammation in individuals of varying activity levels. Accessed May 7, 2020 https://scholarsrepository.llu.edu/cgi/viewcontent.cgi?article=1014&context=rr.
- [22].Darooghegi Mofrad M, Milajerdi A, Koohdani F, Surkan PJ, Azadbakht L. Garlic supplementation reduces circulating Creactive protein, tumor necrosis factor, and interleukin-6 in adults: a systematic review and meta-analysis of randomized controlled Trials. J Nutr 2019;149:605–18. doi: 10.1093/jn/nxy310. [DOI] [PubMed] [Google Scholar]
- [23].Smidowicz A, Regula J. Effect of nutritional status and dietary patterns on human serum C-reactive protein and interleukin-6 concentrations. Adv Nutr 2015;6:738–47. doi: 10.3945/an.115.009415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jaggers GK, Watkins BA, Rodriguez R. COVID-19: repositioning nutrition research for the next pandemic. Nutr Res 2020;81:1–6 10.1016/j.nutres.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sureda A, Bibiloni MDM, Julibert A, Bouzas C, Argelich E, Llompart I, et al. Adherence to the Mediterranean diet and inflammatory markers. Nutrients 2018;10:62. doi: 10.3390/nu10010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Christ A, Lauterbach M, Latz E. Western diet and the immune system: an inflammatory connection. Immunity 2019;51:794–811. doi: 10.1016/j.immuni.2019.09.020. [DOI] [PubMed] [Google Scholar]
- [27].Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, et al. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab 2006;91:439–46. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- [28].Wannamethee SG, Lowe GD, Rumley A, Bruckdorfer KR, Whincup PH. Associations of vitamin C status, fruit and vegetable intakes, and markers of inflammation and hemostasis. Am J Clin Nutr 2006;83:567–74. doi: 10.1093/ajcn.83.3.567. [DOI] [PubMed] [Google Scholar]
- [29].Erlinger TP, Guallar E, Miller ER 3rd, Stolzenberg-Solomon R, Appel LJ. Relationship between systemic markers of inflammation and serum beta-carotene levels. Arch Intern Med 2001;161:1903–8. doi: 10.1001/archinte.161.15.1903. [DOI] [PubMed] [Google Scholar]
- [30].Nielsen FH. Magnesium deficiency and increased inflammation: current perspectives. J Inflamm Res 2018;11:25–34. doi: 10.2147/JIR.S136742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ghanim H, Sia CL, Abuaysheh S, Korzeniewski K, Patnaik P, Marumganti A, et al. An antiinflammatory and reactive oxygen species suppressive effects of an extract of Polygonum cuspidatum containing resveratrol. J Clin Endocrinol Metab 2010;95:E1–8. doi: 10.1210/jc.2010-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Imhofa A, Woodwardb M, Doeringc A, Helbecqued N, Loewelc H, Amouyeld P, et al. Overall alcohol intake, beer, wine, and systemic markers of inflammation in western Europe: results from MONICA sample (Augsburg: Glasgow, Lille). Eur Heart J. 2004;25:2092–100. doi: 10.1016/j.ehj.2004.09.032. [DOI] [PubMed] [Google Scholar]
- [33].Ma Y, Griffith JA, Chasan-Taber L, Olendzki BC, Jackson E, Stanek EJ, et al. Association between dietary fiber and serum C-reactive protein. Am J of Clin Nutr 2006;83:760–6. doi: 10.1093/ajcn/83.4.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kanauchi M, Shibata M, Iwamura M. A novel dietary inflammatory index reflecting for inflammatory ageing: technical note. Ann Med Surg (Lond) 2019;47:44–6. doi: 10.1016/j.amsu.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shivappa N, Hebert JR, Marcos A, Diaz LE, Gomez S, Nova E, et al. Association between dietary inflammatory index and inflammatory markers in the HELENA study. Mol Nutr Food Res 2017;61:10. doi: 10.1002/mnfr.201600707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Corley J, Shivappa N, Hébert JR, Starr JM, Deary IJ. Associations between dietary inflammatory index scores and inflammatory biomarkers among older adults in the Lothian Birth Cohort 1936 study. J Nutr Health Aging 2019;23:628–36. doi: 10.1007/s12603-019-1221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Almeida-de-Souza J, Santos R, Barros R, Abreu S, Moreira C, Lopes L, et al. Dietary inflammatory index and inflammatory biomarkers in adolescents from LabMed physical activity study. Eur J Clin Nutr 2018;72:710–19. doi: 10.1038/s41430-017-0013-x. [DOI] [PubMed] [Google Scholar]
- [38].Vahid F, Shivappa N, Hatami M, Sadeghi M, Ameri F, Jamshidi Naeini Y, et al. Association between dietary inflammatory index (DII) and risk of breast cancer: a case-control study. Asian Pac J Cancer Prev 2018;19:1215–21. doi: 10.22034/APJCP.2018.19.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lee Y-N, Kang P. Relationship between the 10-Year Risk for atherosclerotic cardiovascular disease and the dietary inflammatory index among Korean adults based on the Seventh Korea National Health and Nutrition Examination Survey (KNHANES). BioMed Res Int 2020;2020:8196798. doi: 10.1155/2020/8196798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Singh V. Can vitamins, as epigenetic modifiers, enhance immunity in COVID-19 patients with non-communicable disease? Curr Nutr Rep 2020;9:202–9. doi: 10.1007/s13668-020-00330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–77. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zafar MI, Mills KE, Zheng J, Regmi A, Hu SQ, Gou L, et al. Lowglycemic index diets as an intervention for diabetes: a systematic review and meta-analysis. Am J Clin Nutr 2019;110:891–902. doi: 10.1093/ajcn/nqz149. [DOI] [PubMed] [Google Scholar]
- [43].Piyathilake CJ, Macaluso M, Alvarez RD, Bell WC, Heimburger DC, Partridge EE. Lower risk of cervical intraepithelial neoplasia in women with high plasma folate and sufficient vitamin B12 in the post-folic acid fortification era. Cancer Prev Res (Phila) 2009;2:658–64. 10.1158/19406207.CAPR-08-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Piyathilake CJ, Henao OL, Macaluso M, Cornwell PE, Meleth S, Heimburger DC, et al. Folate is associated with the natural history of high-risk human papillomaviruses. Cancer Res 2004;64:8788–93. doi: 10.1158/0008-5472.CAN-04-2402. [DOI] [PubMed] [Google Scholar]
- [45].Piyathilake CJ, Badiga S, Paul P, Vijayaraghavan K, Vedantham H, Sudula M, et al. Indian women with higher serum concentrations of folate and vitamin B12 are significantly less likely to be infected with carcinogenic or high-risk (HR) types of human papillomaviruses (HPVs). Int J Women’s Health 2010;2:7–12. doi: 10.2147/ijwh.s6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Herceg Z Epigenetics and cancer: towards an evaluation of the impact of environmental and dietary factors. Mutagenesis 2007;22:91–103. doi: 10.1093/mutage/gel068. [DOI] [PubMed] [Google Scholar]
- [47].Kouzarides T. Chromatin modifications and their function. Cell 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- [48].Ong TP, Perusse L. Impact of nutritional epigenomics on disease risk and prevention: introduction. J Nutrigenet Nutrigenomics 2011;4:245–7. doi: 10.1159/000334813. [DOI] [PubMed] [Google Scholar]
- [49].Meydani A, Ahmed T, Meydani SN. Aging, nutritional status, and infection in the developing world. Nutr Rev 2005;63:233–46. doi: 10.1111/j.1753-4887.2005.tb00379.x. [DOI] [PubMed] [Google Scholar]
- [50].Abba A, Fokam J, Kamgaing RS, Yimga JF, Kae AC, Nka AD, et al. Correlation between the immuno-virological response and the nutritional profile of treatment-experienced HIV-infected patients in the East region of Cameroon. Accessed November 10, 2020 10.1101/2020.02.11.943621v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Dashraath P, Wong JLJ, Lim MXK, Lim LM, Li S, Biswas A, et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol 2020;222:521–31. doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Laurencin CT, McClinton A. The COVID-19 pandemic: A call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities 2020;7:398–402. doi: 10.1007/s40615-020-00756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Holmes L Jr, Enwere M, Williams J, Ogundele B, Chavan P, Piccoli T, et al. Black-white risk differentials in COVID-19 (SARS-COV2) transmission, mortality and case fatality in the United States: translational epidemiologic perspective and challenges. Int J Environ Res Public Health 2020;17:4322. doi: 10.3390/ijerph17124322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Jones J, Sullivan PS, Sanchez TH, Guest JL, Hall EW, Luisi N, et al. Similarities and differences in COVID-19 awareness, concern, and symptoms by race and ethnicity in the United States: crosssectional survey. J Med Internet Res 2020;22:e20001. doi: 10.2196/20001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Byrd DA, Agurs-Collins T, Berrigan D, Lee R, Thompson FE. Racial and ethnic differences in dietary intake, physical activity, and body mass index (BMI) among cancer survivors: 2005 and 2010 National Health Interview Surveys (NHIS). J Racial Ethn Health Disparities 2017;4:1138–46. doi: 10.1007/s40615-016-0319-8. [DOI] [PubMed] [Google Scholar]
- [56].Gans KM, Burkholder GJ, Risica PM, Lasater TM. Baseline fatrelated dietary behaviors of white, Hispanic, and black participants in a cholesterol screening and education project in New England. J Am Diet Assoc 2003;103:699–706. doi: 10.1053/jada.2003.50135. [DOI] [PubMed] [Google Scholar]
- [57].Wu JR, Song EK, Moser DK, Lennie TA. Racial differences in dietary antioxidant intake and cardiac event-free survival in patients with heart failure. Eur J Cardiovasc Nurs 2018;17:305–13. doi: 10.1177/1474515118755720. [DOI] [PubMed] [Google Scholar]
- [58].Rehm CD, Monsivais P, Drewnowski A. The quality and monetary value of diets consumed by adults in the. Am J Clin Nutr 2011;94:1333–9. doi: 10.3945/ajcn.111.015560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr 2011;106(Suppl 3):S1–S78. doi: 10.1017/S0007114511005460. [DOI] [PubMed] [Google Scholar]
- [60].Calder PC, Albers R, Antoine JM, Blum S, Bourdet-Sicard R, Ferns GA, et al. Inflammatory disease processes and interactions with nutrition. Br J Nutr 2009;101(Suppl 1):S1–45. doi: 10.1017/S0007114509377867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.